Abstract
Cognitive impairment is one of the earliest, most common, and most disabling non-motor symptoms in Parkinson’s disease (PD). Thus, routine screening of global cognitive abilities is important for the optimal management of PD patients. Few global cognitive screening instruments have been developed for or validated in PD patients. The Mini-Mental State Examination (MMSE), Montreal Cognitive Assessment (MoCA), and Dementia Rating Scale-2 (DRS-2) have been used extensively for cognitive screening in both clinical and research settings. Determining how to convert the scores between instruments would facilitate the longitudinal assessment of cognition in clinical settings and the comparison and synthesis of cognitive data in multicenter and longitudinal cohort studies. The primary aim of this study was to apply a simple and reliable algorithm for the conversion of MoCA to MMSE scores in PD patients. A secondary aim was to apply this algorithm for the conversion of DRS-2 to both MMSE and MoCA scores. The cognitive performance of a convenience sample of 360 patients with idiopathic PD was assessed by at least two of these cognitive screening instruments. We then developed conversion scores between the MMSE, MoCA, and DRS-2 using equipercentile equating and log-linear smoothing. The conversion score tables reported here enable direct and easy comparison of three routinely used cognitive screening assessments in PD patients.
Keywords: Parkinson’s disease, Mini-Mental State Examination, Montreal Cognitive Assessment, Dementia Rating Scale-2, cognitive screening scales
Non-motor symptoms, including cognitive impairment, are common in patients with Parkinson’s disease (PD). Cognitive impairment is one of the earliest, most common, and most disabling non-motor symptoms in PD. A range of cognitive domains are impaired in PD patients, including visuospatial, executive, attention, and memory abilities.1–5 The long-term prevalence of PD dementia (PDD) is approximately 80%.6,7 Mild cognitive impairment (MCI) has been reported in 20% to 30% of nondemented established PD patients,8 and MCI is observed in 15% to 20% of newly diagnosed untreated PD patients.9
For these reasons, routine cognitive screening is important for the optimal management of PD patients. Although detailed neuropsychological testing is the gold standard for assessing specific neuropsychological functions, such extensive assessment is time consuming, and the use of briefer screening instruments for global cognition is a more practical approach in clinical care. Few global cognitive screening instruments have been developed for or validated in PD patients.10 The ideal screening instrument for cognitive impairment in PD should be brief, simple to administer, sensitive to subtle changes in cognition, unaffected by motor and visual problems, and able to evaluate a full range of cognitive domains.2,10
The 30-point Mini-Mental State Examination (MMSE)11 traditionally has been the most commonly used cognitive screening instrument in both clinical and research settings, because it can be administered in 5 minutes. However, the use of the MMSE in PD has been questioned10,12 for lacking sensitivity to detect subtle cognitive deficits and providing inadequate assessment of executive abilities.2,13–16
The Montreal Cognitive Assessment (MoCA)17 is also a brief 30-point cognitive screening instrument that can be administered within 10 minutes. The MoCA was shown to be more sensitive in detecting MCI in a group of healthy controls, MCI patients, and Alzheimer’s disease (AD) patients (sensitivity of 90%) compared with the MMSE (sensitivity of 18%).17 Among PD patients who scored above the MMSE cutoff point for cognitive impairment (only those with a population-based age- and education-adjusted MMSE score in the top 75th percentile were included), 52% were impaired according to the recommended MoCA cutoff score (<26).18 This is likely because the MoCA more thoroughly assesses executive abilities and is more challenging (ie, more items on memory recall subtest). Thus, the MoCA appears to be a superior screening instrument for cognitive impairment in PD compared with the MMSE.12,18–20
Another widely used and more comprehensive cognitive screening instrument is the Dementia Rating Scale-2 (DRS-2),21 which takes 20 to 30 minutes to administer. The DRS-2 is divided into five subscales: Attention, Initiation/Perseveration, Construction, Conceptualization, and Memory.21 The original DRS demonstrated better convergent and divergent validity compared with the MMSE or a battery of cognitive tests selected to assess specific deficits reported in PD,22 and a more recent study of the DRS-2 demonstrated high sensitivity (92%) and specificity (91%) to discriminate PD patients with from those without dementia.23
Because the MMSE, MoCA, and DRS-2 are all still widely used in PD, we perceived the need to develop a method for converting the score from one instrument to the other two. Such conversion will facilitate both the longitudinal assessment of cognition in clinical settings and the comparison and synthesis of cognitive data from multicenter and longitudinal cohort studies. A recent study calculated a simple algorithm for the conversion of MoCA to MMSE scores in MCI patients and patients with AD.24 Such an algorithm has not been applied in PD patients. This must be done separately for PD, because the cognitive profile in PD differs from that of AD,25 and the relative performance on the MMSE and MoCA will likely differ in the two disease states. The primary aim of this study was to develop and apply a simple and reliable algorithm for the conversion of MoCA to MMSE scores in PD patients, and a secondary aim was to apply the algorithm for the conversion of DRS-2 to both MMSE and MoCA scores.
Methods
Study Population
A convenience sample (ie, those patients willing and able to complete a time-consuming research protocol) of 360 patients with idiopathic PD,26 with a focus on non-demented patients, were recruited prospectively from the Parkinson’s Disease and Movement Disorders Center at the University of Pennsylvania and the Parkinson’s Disease Research, Education and Clinical Center at the Philadelphia Veterans Affairs Medical Center from 2007 through 2014 as part of ongoing studies examining cognitive performance in PD. From this cohort, patients were assigned to three different cohorts, based on which cognitive screening tests were assessed at similar time points (ie, within 6 months of each other); 197 PD patients underwent both the MMSE and the MoCA, 254 both the MMSE and the DRS-2, and 256 both the MoCA and DRS-2. The Institutional Review Board at each participating institution approved the study, and written informed consent was obtained from subjects before study participation.
Procedures
Basic demographic and clinical information, including the Unified Parkinson’s Disease Rating Scale motor examination and Hoehn & Yahr stage (higher score indicating greater severity of motor symptoms or disease severity),27 were obtained. In addition, the 15-item Geriatric Depression Scale was administered to measure severity of depressive symptoms.28
Trained research staff administered the MMSE, MoCA, and DRS-2, without randomized or consistent ordering. Both the MMSE and MoCA scores range from 0 to 30, with higher scores indicating better cognitive functioning. The raw MoCA scores include the 1-point education correction for patients with 12 years or less education. The DRS-2 scores range from 0 to 144, with a higher score indicating better cognition.
In addition, data concerning PD medication use were collected, including daily dose of levodopa (l-dopa), dopamine agonist use, or l-dopa equivalent daily dose for l-dopa plus other PD medications,29 depending on the cohort. For the MMSE-MoCA cohort, l-dopa equivalent daily dose data were not available, and information about the daily dose of l-dopa was not available for the MMSE-DRS-2 cohort nor for the MoCA-DRS-2 cohort. Patients were encouraged to take their regularly scheduled PD medications during the assessment process so they would be evaluated in their “on” state.
Statistical Analysis
Statistical analysis for the demographic characteristics was performed in IBM SPSS (version 22).30 Data are expressed as means ± standard deviation or median for the continuous variables and as a percentage for the categorical variables.
To convert from one test score to another, we used the equipercentile equating method.31 This method has been used to equate numerous standardized tests, such as the conversion of the Telephone Interview for Cognitive Status score to MMSE score32 and MoCA score to MMSE score in MCI and AD patients.24 A comprehensive explanation of equipercentile equating is described elsewhere24,31; in summary, scores from two different measures are considered as equivalent if their corresponding percentile ranks are equal. The strength of this method is that the equated scores always fall within the range of possible scores; a limitation is that this method can lead to an irregular distribution of scores. A log-linear transformation33 of each instrument’s raw score before the equipercentile equating is required to smooth the raw scores and to create a normal distribution without irregularities that are attributable to sampling. Log-linear transformation enhances the equating accuracy.31 The equitation calculations and log-linear smoothing were performed in the R statistical program, using the “equate” library.34
Results
Demographic and Clinical Characteristics
Demographic and clinical details for the three cohorts are listed in Table 1. In general, the three cohorts were representative of PD patients seen in specialty care settings (ie, mild to moderate disease severity) in that they were predominately male and elderly.
TABLE 1.
Demographic and clinical characteristics, and performance on cognitive tests
| Cohorts | |||
|---|---|---|---|
| Clinical Characteristics | MMSE-MoCA (n = 197) | MMSE-DRS-2 (n = 254) | MoCA-DRS-2 (n = 256) |
| Age, y | 67.1 ± 9.5 | 71.3 ± 7.5 | 70.4 ± 8.1 |
| Sex, % male | 67.0% | 70.5% | 69.1% |
| Education, y | 16.4 ± 2.8 | 15.9 ± 2.5 | 16.1 ± 2.4 |
| PD duration, y | 6.9 ± 5.1 | 6.7 ± 5.3 | 8.0 ± 7.9 |
| UPDRS-2 Motora | 24.1 ± 11.2 | 23.6 ± 11.5 | 26.3 ± 14.4 |
| Hoehn & Yahr stageb | 2.0 | 2.0 | 2.5 |
| 1 | 10.8% | 4.8% | 3.5% |
| 1.5 | 14.9% | 1.2% | 2.7% |
| 2 | 37.9% | 48.0% | 41.8% |
| 2.5 | 14.9% | 17.9% | 21.9% |
| 3 | 16.4% | 21.0% | 19.1% |
| 4 | 4.6% | 5.6% | 8.6% |
| 5 | 0.5% | 1.6% | 2.3% |
| Treatment | |||
| Levodopa dosage, mg/d | 524.4 ± 415.1 | — | — |
| Dopamine agonist use, % yes | 50.8% | 49.0% | 49.0% |
| LEDD, mg/d | — | 716.7 ± 487.9 | 728.3 ± 495.9 |
| GDS-15 score | 3.1 ± 3.2 | 3.3 ± 3.1 | 3.0 ± 3.0 |
| Cognition | |||
| MMSE score [range] | 27.7 ± 2.7 [12–30] | 26.8 ± 3.5 [10–30] | — |
| MoCA score [range] | 24.7 ± 4.2 [10–30] | — | 23.7 ± 5.0 [6–30] |
| DRS-2 score [range] | — | 132.1 ± 12.0 [74–144] | 133.0 ± 13.9 [20–144] |
| Time between assessments, d | 43.4 ± 38.8 | 7.3 ± 27.5 | 13.9 ± 24.9 |
Values are mean ± SD or median for continuous variables and % for categorical variables.
MMSE, Mini-Mental State Examination; MoCA, Montreal Cognitive Assessment; DRS-2, Dementia Rating Scale-2; UPDRS-2, Unified Parkinson’s Disease Rating Scale; LEDD, L-dopa Equivalent Daily Dose; GDS-15, Geriatric Depression Scale.
MMSE-MoCA: n = 197; MMSE-DRS-2: n = 252; MoCA-DRS-2: n = 256.
MMSE-MoCA: n = 195; MMSE-DRS-2: n = 252; MoCA-DRS-2: n = 256.
MMSE, MoCA, and DRS-2 Distribution
The distribution of the test scores for all of the cognitive screening tests in all three cohorts was negatively skewed, but the distribution profiles for each test in different cohorts were approximately the same (Supplemental Data Fig. S1).
MoCA to MMSE Conversion
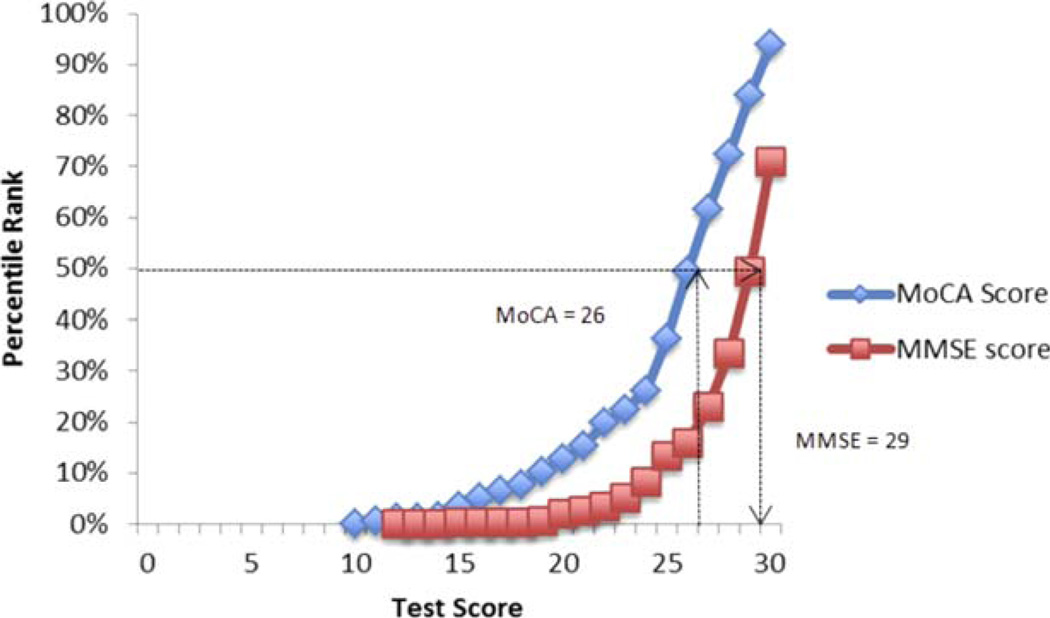
Figure 1 presents the MoCA score with the equivalent MMSE score. For example, a score of 26 on the MoCA is equivalent to a score of 29 on the MMSE, with both of these sores falling at approximately the 50th percentile rank.
FIG. 1.
Corresponding raw scores and percentages. [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
Table 2 presents the conversion results for each possible MoCA score in a table. Because the lowest raw MoCA score was 10 in the MMSE-MoCA cohort, the equivalent MMSE scores for raw MoCA scores lower than 10 are extrapolations and not based on actual data. A lower MoCA score was equivalent to a higher MMSE score, suggesting that the MoCA has greater sensitivity, less of a ceiling effect, but more of a floor effect, compared with the MMSE.
TABLE 2.
Conversion table for possible MoCA score to MMSE scorea
| Raw MoCA score | Equivalent MMSE score |
|---|---|
| 1 | 6 |
| 2 | 9 |
| 3 | 11 |
| 4 | 12 |
| 5 | 13 |
| 6 | 14 |
| 7 | 15 |
| 8 | 15 |
| 9 | 16 |
| 10 | 17 |
| 11 | 18 |
| 12 | 18 |
| 13 | 19 |
| 14 | 20 |
| 15 | 21 |
| 16 | 22 |
| 17 | 22 |
| 18 | 23 |
| 19 | 24 |
| 20 | 25 |
| 21 | 26 |
| 22 | 26 |
| 23 | 27 |
| 24 | 28 |
| 25 | 28 |
| 26 | 29 |
| 27 | 29 |
| 28 | 30 |
| 29 | 30 |
| 30 | 30 |
Scores in gray are extrapolated data; Scores in black are real data.
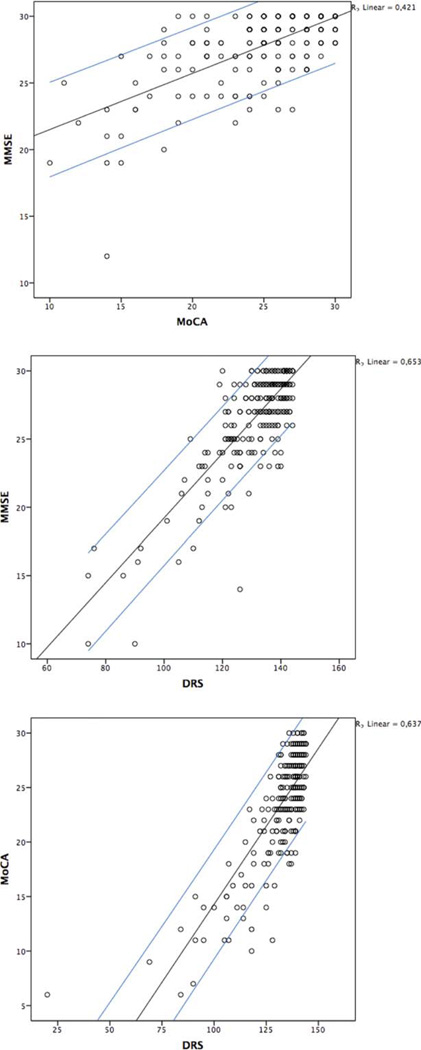
Figure 2 presents the scatterplot for MoCA and MMSE scores in individual patients. For example, a raw individual score of 20 on the MoCA is equal to a range of raw scores (24–30) on the MMSE.
FIG. 2.
Scatterplots of raw Mini-Mental State Examination (MMSE), Montreal Cognitive Assessment (MoCA), and Dementia Rating Scale-2 (DRS-2) scores. Blue lines represent 90% Confidence Intervals for individual values. Outer lines represent 90% Confidence Intervals for individual values. [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
DRS-2 to MMSE and DRS-2 to MoCA Conversion
A conversion score between the DRS-2 and MMSE (Supplemental Data Table S1) and between the DRS-2 and MoCA (Supplemental Data Table S2) was calculated in the same fashion. For example, a score of 120 on the DRS-2 is equivalent to a score of 23 on the MMSE and to a score of 18 on the MoCA. Figure 2 represents the scatterplots for MMSE, MoCA, and DRS-2 scores in individual patients.
Discussion
In this study, we developed an algorithm for the interconversion of MoCA, MMSE, and DRS-2 scores for PD patients, using equipercentile equating and log-linear smoothing. Our conversion tables enable direct and easy comparison of scores on the three instruments. Because the MMSE, MoCA, and DRS-2 are all extensively used in the cognitive assessment of PD patients in both clinical and research settings, conversion scores may facilitate the continuity of cognitive tracking in the clinic and the comparability of data on global cognition in longitudinal and multicenter studies.
To our knowledge, this is the first study on the conversion of cognitive screening instruments in a cohort of patients with PD. A recent study calculated a simple algorithm for the conversion of MoCA to MMSE scores in MCI patients and patients with AD,24 but the cognitive profile in PD differs from that of AD,25,35 so a specific effort to do the same for PD was needed. For instance, AD patients have more pronounced memory and language impairments, whereas PD patients show a “subcortical profile” with more severe impairment in executive function, attention, and visuospatial skills than AD patients.35–37 The MMSE primarily assesses memory and language abilities, whereas the MoCA assesses a broader range of cognitive domains, including executive and visuospatial functioning.
For these reasons, we expected a difference in the MoCA-MMSE conversion scores for PD and MCI/AD patients. However, the conversion scores are rather similar. For example, a MoCA score of 23 is equivalent to an MMSE score of 27 in PD patients and an MMSE score of 28 in AD/MCI patients. To our knowledge, no studies exist that examine the conversion between the DRS-2 and the MoCA or MMSE.
Several limitations should be noted. First, the distribution of the scores on all three cognitive screening tests was negatively skewed, suggesting that the number of patients with marked cognitive impairment was relatively low. Therefore, the interpretation of the conversion of lower scores should be done with caution, particularly because the minimum observed score on the MoCA was 10. This may have an adverse impact on the ability to generalize our data to patients with PDD and dementia with Lewy bodies. Additional studies are necessary to confirm our results and to explore the conversion scores in a sample with more severe cognitive dysfunction. Second, patients were recruited from specialty care centers, where most were male, highly educated, had mild to moderate PD, and were mostly without dementia (although some patients with dementia were included), which limits generalization to the overall PD population and to less well educated and more severe disease cohorts. Educational level is important, because this variable is known to impact both MoCA and MMSE performance.38,39 Third, the cognitive screening tests used to assess the cognition of the PD patients were all English versions, and, as a result, the generalizability of our conversion scores is limited. Fourth, analysis of MoCA and MMSE subcategories might provide more insight into the cognitive profile of PD patients, although these data were not available for the cohorts in this study. Finally, we did not have sufficient detailed cognitive tests or apply diagnostic criteria for MCI or dementia to most patients in this cohort, so comparing the discriminant validity of the three instruments was not possible. However, this was not the stated goal of these analyses, it has been done previously in PD,19,20 and our analyzing participants as a single cohort is similar to what was done previously, when healthy controls and MCI and AD patients were examined as a single group.24
The high prevalence of cognitive impairment in PD8,9 and its evolution to PDD long-term in most patients,40 profoundly affects functioning,41 quality of life,42 caregiver burden,43 and health economics.44 Therefore, the assessment of global cognitive abilities on a regular basis, starting at the time of diagnosis, is important for the optimal management of patients with PD. Indeed, annual assessment of cognition has been proposed as a quality care indicator for the management of patients with PD45 and adopted by the American Academy of Neurology.46 In addition, multiple ongoing longitudinal multicenter cohort studies in PD include global cognitive tests. In these studies, a variety of instruments can be used, and in some cases over time one instrument can substituted for another. The results of our study showing how scores of three commonly used cognitive assessments can be converted should help standardize the longitudinal assessment of cognitive function in a clinical setting and also facilitate the comparison and synthesis of cognitive data from multicenter and longitudinal cohort research.
Supplementary Material
Acknowledgments
Funding agencies: No financial support was received for this work.
Footnotes
Relevant conflicts of interest/financial disclosures: Nothing to report. Author roles may be found in the online version of this article.
Supporting Data
Additional Supporting Information may be found in the online version of this article at the publisher’s web-site.
References
- 1.Dubois B, Pillon B. Cognitive deficits in Parkinson’s disease. J Neurol. 1996;244:2–8. doi: 10.1007/pl00007725. [DOI] [PubMed] [Google Scholar]
- 2.Dubois B, Burn D, Goetz C, et al. Diagnostic procedures for Parkinson’s disease dementia: recommendations from the movement disorder society task force. Mov Disord. 2007;22:2314–2324. doi: 10.1002/mds.21844. [DOI] [PubMed] [Google Scholar]
- 3.Emre M, Aarsland D, Brown R, et al. Clinical diagnostic criteria for dementia associated with Parkinson’s disease. Movement Disorders. 2007;22:1689–1707. doi: 10.1002/mds.21507. [DOI] [PubMed] [Google Scholar]
- 4.Litvan I, Goldman JG, Tröster AI, et al. Diagnostic criteria for mild cognitive impairment in Parkinson’s disease: Movement Disorder Society Task Force guidelines. Mov Disord. 2012;27:349–356. doi: 10.1002/mds.24893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dalrymple-Alford JC, Livingston L, MacAskill MR, et al. Characterizing mild cognitive impairment in Parkinson’s disease. Mov Disord. 2011;26:629–636. doi: 10.1002/mds.23592. [DOI] [PubMed] [Google Scholar]
- 6.Aarsland D, Andersen K, Larsen J, Lolk A, Kragh-Sorensen P. Prevalence and characteristics of dementia in Parkinson disease: an 8-year prospective study. Arch Neurol. 2003;60:387–392. doi: 10.1001/archneur.60.3.387. [DOI] [PubMed] [Google Scholar]
- 7.Hely Ma, Reid WGJ, Adena Ma, Halliday GM, Morris JGL. The Sydney multicenter study of Parkinson’s disease: the inevitability of dementia at 20 years. Mov Disord. 2008;23:837–844. doi: 10.1002/mds.21956. [DOI] [PubMed] [Google Scholar]
- 8.Aarsland D, Bronnick K, Williams-Gray C, et al. Mild cognitive impairment in Parkinson disease: a multicenter pooled analysis. Neurology. 2010;75:1062–1069. doi: 10.1212/WNL.0b013e3181f39d0e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Aarsland D, Brønnick K, Larsen JP, Tysnes OB, Alves G. Cognitive impairment in incident, untreated Parkinson disease: the Norwegian ParkWest study. Neurology. 2009;72:1121–1126. doi: 10.1212/01.wnl.0000338632.00552.cb. [DOI] [PubMed] [Google Scholar]
- 10.Kulisevsky J, Pagonabarraga J. Cognitive impairment in Parkinson’s disease: tools for diagnosis and assessment. Mov Disord. 2009;24:1103–1110. doi: 10.1002/mds.22506. [DOI] [PubMed] [Google Scholar]
- 11.Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”: a practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 12.Zadikoff C, Fox SH, Tang-Wai DF, et al. A comparison of the mini mental state exam to the Montreal cognitive assessment in identifying cognitive deficits in Parkinson’s disease. Mov Disord. 2008;23:297–299. doi: 10.1002/mds.21837. [DOI] [PubMed] [Google Scholar]
- 13.Wind AW, Schellevis FG, Van Staveren G, Scholten RJ, Jonker C, Van Eijk JM. Limitations of the Mini-Mental State Examination in diagnosing dementia in general practice. Int J Geriatr Psychiatry. 1997;12:101–108. doi: 10.1002/(sici)1099-1166(199701)12:1<101::aid-gps469>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- 14.Tang-Wai DF, Knopman DS, Geda YE, et al. Comparison of the short test of mental status and the mini-mental state examination in mild cognitive impairment. Arch Neurol. 2003;60:1777–1781. doi: 10.1001/archneur.60.12.1777. [DOI] [PubMed] [Google Scholar]
- 15.Athey RJ, Porter RW, Walker RW. Cognitive assessment of a representative community population with Parkinson’s disease (PD) using the Cambridge Cognitive Assessment–Revised (CAMCOG-R) Age Ageing. 2005;34:268–273. doi: 10.1093/ageing/afi098. [DOI] [PubMed] [Google Scholar]
- 16.Riedel O, Klotsche J, Spottke A, et al. Cognitive impairment in 873 patients with idiopathic Parkinson’s disease. J Neurol. 2008;255:255–264. doi: 10.1007/s00415-008-0720-2. [DOI] [PubMed] [Google Scholar]
- 17.Nasreddine ZS, Phillips NA, Bédirian V, et al. The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc. 2005;53:695–699. doi: 10.1111/j.1532-5415.2005.53221.x. [DOI] [PubMed] [Google Scholar]
- 18.Nazem S, Siderowf AD, Duda JE, et al. Montreal cognitive assessment performance in patients with Parkinson’s disease with “normal” global cognition according to Mini-Mental State Examination score. J Am Geriatr Soc. 2009;57:304–308. doi: 10.1111/j.1532-5415.2008.02096.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hoops S, Nazem S, Siderowf A, et al. Validity of the MoCA and MMSE in the detection of MCI and dementia in Parkinson disease. Neurology. 2009;73:1738–1745. doi: 10.1212/WNL.0b013e3181c34b47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dalrymple-Alford J, MacAskill M, Nakas C, et al. The MoCA well-suited screen for cognitive impairment in Parkinson disease. Neurology. 2010;75:1717–1725. doi: 10.1212/WNL.0b013e3181fc29c9. [DOI] [PubMed] [Google Scholar]
- 21.Jurica P, Leitten C, Mattis S. Dementia Rating Scale-2: Professional manual. Lutz: Psychological Assessment Resources; 2001. [Google Scholar]
- 22.Brown GG, Rahill AA, Gorell JM, et al. Validity of the Dementia Rating Scale in assessing cognitive function in Parkinson’s disease. J Geriatr Psychiatry Neurol. 1999;12:180–188. doi: 10.1177/089198879901200403. [DOI] [PubMed] [Google Scholar]
- 23.Llebaria G, Pagonabarraga J, Kulisevsky J, et al. Cut-off score of the Mattis Dementia Rating Scale for screening dementia in Parkinson’s disease. Mov Disord. 2008;23:1546–1550. doi: 10.1002/mds.22173. [DOI] [PubMed] [Google Scholar]
- 24.Roalf DR, Moberg PJ, Xie SX, Wolk DA, Moelter ST, Arnold SE. Comparative accuracies of two common screening instruments for classification of Alzheimer’s disease, mild cognitive impairment, and healthy aging. Alzheimer’s & Dementia. 2013;9:529–537. doi: 10.1016/j.jalz.2012.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bronnick K, Emre M, Lane R, Tekin S, Aarsland D. Profile of cognitive impairment in dementia associated with Parkinson’s disease compared with Alzheimer’s disease. J Neurology Neurosurg Psychiatry. 2007;78:1064–1068. doi: 10.1136/jnnp.2006.108076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gelb DJ, Oliver E, Gilman S. Diagnostic criteria for Parkinson disease. Arch Neurol. 1999;56:33–39. doi: 10.1001/archneur.56.1.33. [DOI] [PubMed] [Google Scholar]
- 27.Fahn S, Elton RL. UPDRS Program Members. Unified Parkinson’s disease rating scale. In: Fahn S, Marsden CD, Goldstein M, Calne DB, editors. Recent developments in Parkinson’s disease. Vol. 2. Florham Park, NJ: Macmillan Healthcare Information; 1987. pp. 153–163.pp. 293–304. [Google Scholar]
- 28.Sheikh J, Yesavage J. Geriatric Depression Scale (GDS): recent evidence and development of a shorter version. In: Brink T, editor. Clinical Gerontology: A Guide to Assessment and Intervention. New York: The Haworth Press; 1986. pp. 165–173. [Google Scholar]
- 29.Tomlinson CL, Stowe R, Patel S, Rick C, Gray R, Clarke CE. Systematic review of levodopa dose equivalency reporting in Parkinson’s disease. Mov Disord. 2010;25:2649–2653. doi: 10.1002/mds.23429. [DOI] [PubMed] [Google Scholar]
- 30.IBM Corp. IBM SPSS Statistics for Macintosh, Version 22.0. Armonk, NY: Author; 2013. [Google Scholar]
- 31.Kolen MJ, Brennan RL. Test Equating, Scaling, and Linking: Methods and Practices. New York, NY: Springer; 2004. [Google Scholar]
- 32.Fong TG, Fearing MA, Jones RN, et al. Telephone interview for cognitive status: Creating a crosswalk with the Mini-Mental State Examination. Alzheimer’s & Dementia. 2009;5:492–497. doi: 10.1016/j.jalz.2009.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Moses T, vonDavier A. An SAS Macro for Loglinear Smoothing: Applications and Implications. Montreal: American Educational Research Association; 2005. [Google Scholar]
- 34.Albano A. Equate: Observed-Score Linking and Equating. R package version 2.0-2. 2014 [Google Scholar]
- 35.Litvan I, Aarsland D, Adler CH, et al. MDS task force on mild cognitive impairment in Parkinson’s disease: Critical review of PD-MCI. Mov Disord. 2011;26:1814–1824. doi: 10.1002/mds.23823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Caviness JN, Driver-Dunckley E, Connor DJ, et al. Defining mild cognitive impairment in Parkinson’s disease. Mov Disord. 2007;22:1272–1277. doi: 10.1002/mds.21453. [DOI] [PubMed] [Google Scholar]
- 37.Muslimović D, Post B, Speelman JD, Schmand B. Cognitive profile of patients with newly diagnosed Parkinson disease. Neurology. 2005;65:1239–1245. doi: 10.1212/01.wnl.0000180516.69442.95. [DOI] [PubMed] [Google Scholar]
- 38.Freitas S, Simões MR, Alves L, Santana I. Montreal Cognitive Assessment: influence of sociodemographic and health variables. Arch Clin Neuropsychol. 2012;27:165–175. doi: 10.1093/arclin/acr116. [DOI] [PubMed] [Google Scholar]
- 39.O’Connor D, Pollitt P, Treasure F, Brook C, Reiss B. The influence of education, social class and sex on Mini-Mental State scores. Psychol Med. 1989;19:771–776. doi: 10.1017/s0033291700024375. [DOI] [PubMed] [Google Scholar]
- 40.Janvin CC, Larsen JP, Aarsland D, Hugdahl K. Subtypes of mild cognitive impairment in Parkinson’s disease: progression to dementia. Mov Disord. 2006;21:1343–1349. doi: 10.1002/mds.20974. [DOI] [PubMed] [Google Scholar]
- 41.Rosenthal E, Brennan L, Xie S, et al. Association between cognition and function in patients with Parkinson disease with and without dementia. Mov Disord. 2010;25:1170–1176. doi: 10.1002/mds.23073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Marras C, McDermott MP, Rochon PA, Tanner CM, Naglie G, Lang AE. Predictors of deterioration in health-related quality of life in Parkinson’s disease: results from the DATATOP trial. Mov Disord. 2008;23:653–659. doi: 10.1002/mds.21853. [DOI] [PubMed] [Google Scholar]
- 43.Aarsland D, Larsen J, Karlsen K, Lim N, Tandberg E. Mental symptoms in Parkinson’s disease are important contributors to caregiver distress. Int J Geriatr Psychiatry. 1999;14:866–874. [PubMed] [Google Scholar]
- 44.Vossius C, Larsen JP, Janvin C, Aarsland D. The economic impact of cognitive impairment in Parkinson’s disease. Mov Disord. 2011;26:1541–1544. doi: 10.1002/mds.23661. [DOI] [PubMed] [Google Scholar]
- 45.Cheng EM, Siderowf A, Swarztrauber K, Eisa M, Lee M, Vickrey BG. Development of quality of care indicators for Parkinson’s disease. Mov Disord. 2004;19:136–150. doi: 10.1002/mds.10664. [DOI] [PubMed] [Google Scholar]
- 46.American Academy of Neurology (AAN) Parkinson’s disease physician performance measurement set. St. Paul, MN: American Academy of Neurology (AAN); 2009. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.