Abstract
Background
In the current study, the authors report the results of 39 patients with mantle cell lymphoma (MCL) who were treated with chemotherapy and high-dose rituximab-containing autologous stem cell transplantation (ASCT) during their first disease remission.
Methods
The median age of the patients was 54 years. At the time of diagnosis, 87% of patients had Ann Arbor stage IV disease, and 77% had bone marrow involvement. A Ki-67 level of >30% was found in 11 of 27 patients (40%), and SOX11 (SRY [sex determining region Y)-box 11] expression was found to be positive in 17 of 18 patients (94%). Twenty-seven patients (69%) underwent induction therapy with high-dose cytarabine-containing chemotherapy. Rituximab was administered during stem cell collection at a dose of 1000 mg/m2 on days +1 and +8 after ASCT.
Results
The estimated 4-year overall survival and progression-free survival rates were 82% and 59%, respectively. Twelve patients experienced disease recurrence. Fifteen of 16 patients who were alive and in complete remission at 36 months remained so at a median follow-up of 69 months (range, 38 months-145 months). The only determinant of recurrence risk found was a Ki-67 level of >30%. Seven of 11 patients with a Ki-67 level >30% experienced disease recurrence within the first 3 years versus only 3 of 16 patients with a Ki-67 level ≤ 30% (P = .02). Patients who received high-dose cytarabine did not have a significantly different risk of developing disease recurrence compared with other patients (P = .7).
Conclusions
Administering ASCT with rituximab during stem cell collection and immediately after transplantation may induce a continuous long-term disease remission in patients with MCL with a Ki-67 level of ≤ 30%.
Keywords: Ki-67, autologous stem cell transplantation, SOX11, mantle cell lymphoma
Introduction
The 2008 World Health Organization classification system defines mantle cell lymphoma (MCL) as a B-cell neoplasm with a CCND1 translocation.1 MCL has long been known for its chemoresistance, high rates of disease recurrence and progression, and relatively short median survival rate. Poorer outcomes have been associated with advanced patient age (> 65 years), leukemic phase, hepatosplenomegaly, advanced or bulky disease, poor performance status, anemia, and elevated serum lactate dehydrogenase and β-2 microglobulin levels.2,3
The MCL International Prognostic Index (MIPI) was recently introduced4; however, its prognostic significance appears to depend on the treatment regimen.5-8 Blastoid or pleomorphic morphologic characteristics and a high proliferation index, the latter evaluated using either gene expression profiling or simple Ki-67 immunohistochemical staining, are also associated with a poor outcome.9,10 Most recently, SOX11 (SRY [sex determining region Y)-box 11] expression in patients with MCL was reported to be a biological marker, with an absence of SOX11 expression found to be associated in some studies with an indolent form of the disease, not requiring the immediate initiation of aggressive chemotherapy.11,12
Conventional chemoimmunotherapy for patients with advanced MCL has led to improved outcomes but is not curative.3,13 Multiple research groups have attempted to improve the efficacy of chemotherapy by consolidating with early (during the first partial or complete remission) autologous stem cell transplantation (ASCT). In the pre-rituximab era, such strategies prolonged the first remission to 3 to 4 years, but no cured patient subgroups were evident on long-term follow-up. However, with the incorporation of the monoclonal anti-CD20 antibody, the results of ASCT appear to be superior.5 In 2009, we published our results with frontline ASCT, both with and without rituximab.5 After the initial posttransplantation period, it became apparent that the natural history of patients treated with rituximab differed from that of patients who were not treated with rituximab, with the progression-free survival (PFS) curves separating after 24 months. These data suggested that long-term disease-free survival is possible. The small number of patients, however, precluded firm conclusions or the analysis of predictors of outcome.
In this study, we combined a new group of patients with the group reported previously to analyze the effectiveness of frontline ASCT with rituximab in patients with MCL. We also assessed SOX11 expression and prognostic factors, including the Ki-67 index.
Materials and Methods
Study Group
The current study includes all patients with MCL who were treated in sequential phase 2 protocols at The University of Texas MD Anderson Cancer Center in Houston between May 1, 1999 and October 31, 2010, and who had received rituximab as part of their conditioning regimen before ASCT was administered during their first remission. Twenty-one of these patients have been reported previously.5 Eligibility criteria included patients aged ≤70 years a Zubrod performance status score of ≤2, and no uncontrolled active infection or symptomatic organ dysfunction; in addition, patients were required to have chemosensitive disease. All eligible patients had biopsy-proven MCL, supported by the results of ancillary studies,14 and all provided informed consent.
Historical Control Group
Thirty patients with newly diagnosed MCL who had been treated with hyper-CVAD (cyclophosphamide, vincristine, doxorubicin, and dexamethasone) and ASCT, but not rituximab, at the study institution between 1994 and 1996 formed the historical control group in the current study. These patients were reported previously5 and were retrospectively compared with the patients in the study group.
Transplantation Strategy
Transplantation strategy has evolved over the past several years. For patients in their first remission after chemotherapy, ASCT had been performed as consolidation therapy before 2001.5 After 2001, and because of the reported favorable clinical outcomes after chemotherapy with rituximab and hyper-CVAD (R-hyper-CVAD), ASCT was performed during the first remission in patients who had not experienced a complete response (CR) after R-hyper-CVAD and those who had undergone less intensive induction chemotherapy (eg, R-CHOP [rituximab and cyclophosphamide, doxorubicin, vincristine, and prednisone]).
Preparative Regimens, Stem Cell Collection, and Autologous Stem Cell Infusion
The details of the study patients' stem cell collection, preparative regimens, supportive care, and infections have been previously published.5 Patients received rituximab during stem cell collection, as previously described, with rituximab administered at a dose of 375 mg/m2 on the day before chemotherapy for stem cell mobilization and again at a dose of 1000 mg/m2 7 days later.5 Patients received an additional dose of 1000 mg/m2 of rituximab on days +1 and +8 after ASCT. The predominant ASCT preparative conditioning regimens in this study group were rituximab, carmustine, etoposide, cytarabine, and melphalan and rituximab, high-dose cyclophosphamide, and total body irradiation, which were used in 77% and 23% of patients, respectively.
Patients were evaluated 1 month, 3 months, 6 months, and 12 months after ASCT; every 6 months for up to 5 years; and yearly thereafter. Responses were scored using standard criteria for patients with lymphoma.15,16
SOX11 and Ki-67 Immunohistochemical Analysis
At the time the patients were treated, SOX11 and Ki-67 were not evaluated routinely for all cases of MCL. For the current study, we performed immunostaining for these markers retrospectively, collecting all initial diagnostic pretransplantation material when unstained slides were available. Formalin-fixed, paraffin-embedded tissue biopsy sections were assessed using heat-induced antigen retrieval and by immunohistochemical analysis using anti-SOX11 rabbit polyclonal (dilution of 1:1500) (Abcam, Cambridge, Mass) and anti-Ki-67 (dilution of 1:100) (Dako, Carpinteria, Calif) monoclonal antibodies. For SOX11 controls, we used a tissue microarray that included 13 cases of MCL as well as full tissue sections from 1 MCL case. Two cases of small lymphocytic lymphoma/chronic lymphocytic leukemia involving the lymph nodes served as negative controls for SOX11 immunostaining.
For analysis of Ki-67 results, the entire slide was evaluated and representative areas with uniform staining were selected. The number of positive cells was assessed semiquantitatively in intervals of 10% as follows: < 10%, 10% to < 20%, 20% to < 30%, 30% to < 40%, etc. In addition, Ki-67 was analyzed in 17 cases as part of an earlier study by manually counting up to 1000 cells per case. There were no discrepancies found between the semi-quantitative estimate and the manual count in this subset of cases. The percentage of Ki-67-positive cells was the proliferation index.
Cytogenetic and Molecular Status
We tested patients' bone marrow for the presence of immunoglobulin H (IgH)-CCND1 fusion indication of t(11;14)(q13;q32) using fluorescence in situ hybridization or real-time polymerase chain reaction (PCR).17 Conventional cytogenetic analysis was also performed at the time of diagnosis in a subset of patients. Assessment was performed at the time of diagnosis and follow-up was performed before and after ASCT, which was encouraged but not mandatory. All available initial and follow-up molecular and cytogenetic data were reviewed and analyzed.
Statistical Analysis
Actuarial overall survival (OS) and PFS rate estimates were calculated using the Kaplan-Meier method.18 OS was estimated from the date of transplantation to the date of death or last follow-up. PFS was estimated from the date of transplantation to the date of disease recurrence or death in disease remission. The incidence of disease progression was estimated using the cumulative incidence method. Predictors of outcome were assessed using the Cox proportional hazards regression analysis. P values < .05 were considered to be statistically significant.19
Results
Patients
The study group comprised 39 patients. Patients' pre-transplantation characteristics and demographics are summarized in Table 1. Patient age ranged from 38 years to 73 years, with a median age of 54 years. Thirteen patients (33%) were aged > 60 years. All had advanced disease at the time of diagnosis: 34 patients (87%) had stage IV disease, 30 patients (77%) had bone marrow involvement, and 14 patients (36%) had involvement of the gastrointestinal tract. The MIPI score was 0 to 2 in 18 patients (46%), 3 or 4 in 12 patients (31%), and ≥ 5 in 6 patients (15%). Induction chemotherapy consisted of R-hyper-CVAD alternating with R-methotrexate and high-dose cytarabine in 27 patients (69%) and R-CHOP in 12 patients (31%). Ten patients (26%) had tumors with blastic morphologic features. Before transplantation, CR (or unconfirmed CR) was achieved in 25 patients (64%); 14 patients (36%) experienced a partial response, and 6 patients (15%) had undergone a positron emission tomography-positive scan. For patients treated with R-hyper-CVAD, 6 patients (22%) were treatment-resistant and did not experience a CR after 6 t0 8 cycles.
TABLE 1. Patient Characteristics.
| Characteristic | Study Group (Treated With Rituximab) | Control Group (Not Treated With Rituximab) | P |
|---|---|---|---|
| No. of patients | 39 | 30 | |
| Age (range), y | 54 (38-73) | 57 (42-66) | .7 |
| Male sex, % | 85 | 90 | .4 |
| Ann Arbor stage IV disease at diagnosis, % | 87 | 88 | .2 |
| B symptoms at diagnosis, % | 36 | 20 | .1 |
| Gastrointestinal involvement at diagnosis, % | 36 | 47 | .4 |
| Blood involvement at diagnosis, % | 21 | 7 | .09 |
| Lactate dehydrogenase >normal, % | 13 | 17 | .6 |
| Median β-2 microglobulin (range) | 3.1 (1.7-14.4) (in 22 patients) | 2.1 (1.2-4.6) | .002 |
| Blastic histology, % | 26 | 3 | .01 |
| Median MIPI at diagnosis (%) | |||
| 0-2 | 18 (46) | 8 (26) | .6 |
| 3-4 | 12 (31) | 17 (57) | |
| ≥5 | 6 (15) | 3 (10) | |
| Unknown | 3 (8) | 2 (7) | |
| Median IPI at diagnosis (range) | 2 (0-4) | 1 (0-2) | <.001 |
| Ki-67, no. (%) | |||
| >30% | 11 (28) | — | |
| ≤ 30% | 16 (41) | 5 (17) | |
| Unknown | 12 (31) | 25 (83) | |
| Induction with high-dose cytarabine, % | 69 | 93 | .01 |
| Resistant to HCVAD, % | 22 | 3 | .03 |
| CR/CRu at transplant, % | 64 | 37 | .02 |
Abbreviations: CR, complete remission; CRu, unconfirmed complete response; HCVAD, hyper-CVAD (cyclophosphamide, vincristine, doxorubicin, and dexamethasone); IPI, International Prognostic Index; MIPI, Mantle Cell Lymphoma International Prognostic Index.
Patients in the control and study groups were similar with regard to age and baseline characteristics (Table 1), including sex distribution, serum lactate dehydrogenase levels, gastrointestinal tract and blood involvement, and MIPI score. However, more patients in the study group had blastic features (P = .01), an elevated β-2 microglobulin level (P = .002), and an IPI > 1 (P < .001). In addition, more patients in the control group received high-dose cytarabine as their induction chemotherapy compared with the study group (P = .01) (Table 1). Only 5 patients in the control group had been tested for Ki-67 and none was tested for SOX11.
SOX11
We performed immunohistochemical analysis for SOX11 retrospectively using formalin-fixed, paraffin-embedded tissue sections from 18 patients for whom diagnostic material was available. Seventeen of 18 tumors (94%) were positive for SOX11 expression. The biopsy specimens that were positive for SOX11 were obtained from the lymph nodes (n = 7), gastrointestinal tract (n = 5), bone marrow (n = 4), and testis (n = 1) (Fig. 1).
Figure 1.
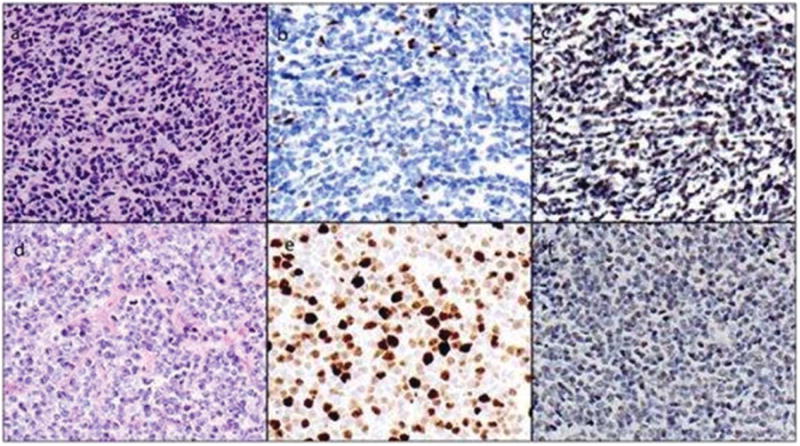
Composite picture from 2 cases is shown. (a) Case 1 was a mantle cell lymphoma (MCL) with classic morphological characteristics. (b) A few cells were positive for Ki-67, reflecting the low proliferation index, and (c) the majority were positive for SOX11. (d) The second case had blastoid morphological characteristics, with (e) a high proliferation index and (f) positive SOX11 expression.
The single SOX11-negative case also could be considered equivocal. This was a small gastrointestinal tract biopsy specimen with limited tumor involvement and scattered positive cells.
Survival and Effect of Ki-67 on the Risk of Disease Progression
All patients in the study group experienced a CR after ASCT with rituximab. The median follow-up interval for surviving patients was 38 months (range, 11 months-145 months). The estimated 4-year OS and PFS rates were 82% (95% confidence interval (95% CI), 0.61-0.92) and 59% (95% CI, 0.39-0.75), respectively. Twelve patients experienced disease recurrence; 11 occurred within 3 years of ASCT, with a clear plateau emerging after 3 years.
On univariate analysis, the only determinants that were found to be predictive of an increased risk of disease recurrence were blastoid morphologic features (P = .05) and a Ki-67 level of > 30% at diagnosis (P = .02) (Fig. 2A). Blastoid features were also found to be highly correlated with Ki-67 levels; 7 of 10 patients with blastoid features had a Ki-67 level > 30% and 13 of 16 patients without blastoid features had a Ki-67 level ≤ 30% (P = .01). Because of sample size limitations, the independent effects of blastoid features and Ki-67 level could not be assessed on multivariate analysis. Seven of 11 patients with a Ki-67 level > 30% experienced disease recurrence within the first 3 years versus only 3 of 16 patients with a Ki-67 level ≤ 30%. The only other single recurrence noted within the first 3 years occurred in patients with unknown Ki-67 levels.
Figure 2.
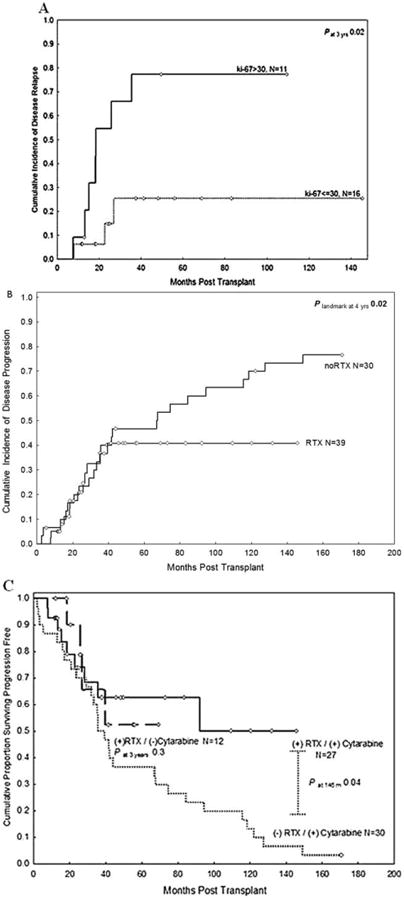
(A) The risk of disease recurrence after autologous stem cell transplantation (ASCT) with rituximab (RTX) according to the Ki-67 level at the time of diagnosis is shown. (B) The 39 patients who had undergone ASCT with RTX had lower rates of disease recurrence compared with the historical control group of 30 patients who had undergone induction therapy with hyper-CVAD (cyclophosphamide, vincristine, doxorubicin, and dexamethasone), high-dose cytarabine, and ASCT but not rituximab. (C) Progression-free survival rates are shown in the study group according to prior induction therapy, with or without high-dose cytarabine, versus those in the control group.
None of the factors listed in Table 1 was found to be significantly associated with an increased rate of disease recurrence, including the MIPI score (analyzed by low vs intermediate-to-high levels and stratified levels of 0-2 vs 3 or 4 vs ≥ 5), IPI, age > 60 years, disease status at the time of transplantation (CRvs partial response), or conditioning regimen.
Comparison With the Control Group
Thirty patients underwent ASCT without rituximab in the historical control group. All patients had undergone hyper-CVAD induction chemotherapy without rituximab. The median follow-up period in this group was 145 months (range, 62 months-187 months). The OS and PFS rates at 145 months (the last follow-up date in the study group) were inferior to those in the study group (OS rate of 14% [P = .06] and PFS of rate 7% [P = .03]).
The rate of disease recurrence for patients in the study group was comparable to that for those in the control group during the first 36 months after transplantation, despite the inclusion of 6 patients in the study group who did not experience a CR after 6 to 8 cycles of R-hyper-CVAD. However, differences in the recurrence rate became evident after 36 months (Fig. 2B). Only 1 of 16 patients in the study group who were alive and in CR at 36 months subsequently developed disease recurrence (3 months later); the remaining patients were alive and in remission at a median follow-up of 69 months (range, 38 months-145 months). This pattern was in stark contrast to the patients in the control group who did not receive rituximab, in which 11 of 15 patients experienced a disease recurrence after 36 months at a median of 74 months (range, 41 months-148 months) (hazard ratio, 4.9; P = .02 compared with patients treated with rituximab at the 3-year landmark).
The contribution of the Ki-67 percentage to disease progression in the group not treated with rituximab could not be fully assessed because adequate pathological material was available for only 5 patients; all had a Ki-67 level of ≤ 30%. One of these 5 patients experienced an early death secondary to gastrointestinal bleeding. Three of the remaining 4 patients experienced late disease recurrence at 40 months, 60 months, and 128 months, respectively. One patient remained alive and in disease remission at ≥ 187 months after transplantation.
Prior Exposure to High-Dose Cytarabine and Outcome
Patients who did not receive prior high-dose cytarabine with their induction chemotherapy were not found to be at risk for disease recurrence compared with other patients (P = .7). The 3-year PFS rate in patients who underwent induction with high-dose cytarabine before ASCT with rituximab was 62%, which was similar to that in patients treated with rituximab who did not receive high-dose cytarabine (66%; P = .7) (Fig. 2C). All patients in the control group received high-dose cytarabine; their PFS rate at 3 years was 50%, which was not statistically different from the other 2 groups at 3 years (P = .3). However, the curves later separated to reach statistical significance (P = .04) when comparing the patients treated with high-dose cytarabine in the study and control groups.
Molecular and Cytogenetic Status
Nine patients in whom t(11;14)/IgH-CCND1-positive cells were detectable by PCR at the time of ASCT were followed with subsequent testing. Seventy-one samples were collected at 1 month to 9 years after transplantation (Fig. 3). Two patients who were found to be positive on PCR at 1 month, 4 months, and 14 months, respectively, experienced disease recurrence. All other patients remained in continuous molecular remission.
Figure 3.
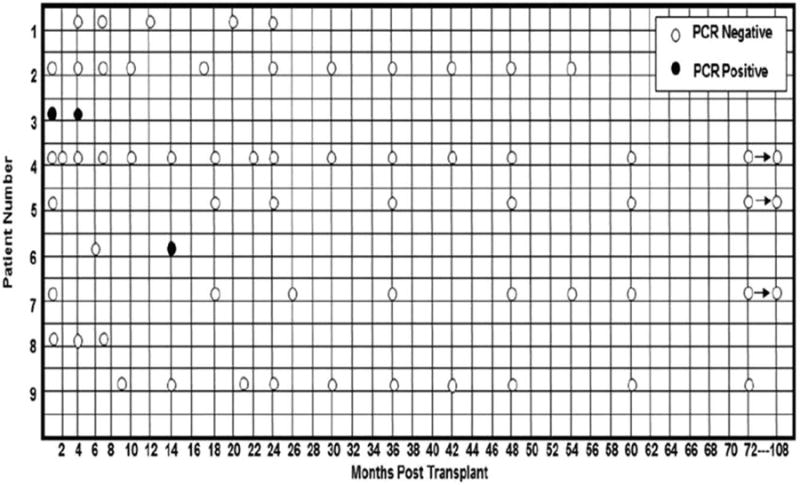
Molecular polymerase chain reaction status of 9 patients after autologous stem cell transplantation with rituximab is shown.
Discussion
We previously demonstrated that the addition of rituximab to conditioning regimens in patients treated with ASCT can induce a progression-free plateau in a subset of patients with MCL.5 The current study, which is supported by a larger number of patients and a longer follow-up period, provides confirmation of our findings from 2009.5 Most impressively, no cases of disease recurrence occurred after 39 months, despite the inclusion of patients who were resistant to the R-hyper-CVAD regimen. The quality of this remission is attested to by the persistent PCR negativity in 7 patients who were prospectively tested for up to 9 years after undergoing ASCT.
Disease recurrences continued to occur in patients treated with ASCT who had not received rituximab. This finding contrasts with our findings in such patients. This continuous pattern of disease recurrence was also observed in the updated results of the Nordic MCL2 trial,7 in which rituximab was used during stem cell collection but not immediately after transplantation, as we did for patients with minimal residual disease. In that trial, rituximab was used preemptively after ASCT in patients who had evidence of molecular disease recurrence from dormant clonal cells or malignant cells that had escaped in vivo purging during stem cell collection. The dose and schedule of rituximab in ASCT administered to patients with B-cell lymphoid malignancies appear to be important. Studies have suggested that higher doses20 or more doses21,22 of rituximab may increase the response rate in patients with lymphoma. CD20 is not expressed by hematopoietic stem cells, and we demonstrated that treatment with higher doses of rituximab at 1000 mg/m2 would not inhibit engraftment produced by these early progenitors. We previously reported improvements in ASCT for patients with diffuse large B-cell lymphoma,23 similar to the those reported in the current study. These observations warrant further evaluation in prospective, randomized trials.
The treatment of MCL is nonstandardized. To the best of our knowledge, recent updates with 10 years of follow-up after intense conventional chemotherapy have not demonstrated a survival plateau.3 It was reported recently that a subset of patients with MCL requires observation only and that these patients may not require therapy until they become symptomatic.24 These observations highlight the potential for selection bias in single-center series. SOX11, a transcription factor that is normally involved in embryonic neurogenesis, has been found to be overexpressed in patients with MCL.11,12,25 The significance of a lack of SOX11 expression remains controversial. However, in at least 2 studies, a lack of SOX11 expression was linked to indolent forms of MCL.11,12 To address the potential selection bias in the current study, we retrospectively analyzed SOX11 expression in 18 patients with MCL for whom we had available tissue. In 17 patients, SOX11 was expressed by MCL with a nuclear pattern. In 1 patient, the SOX11 results were equivocal but were considered to be negative for the current study. In this patient, only a small gastrointestinal tract biopsy specimen with limited tumor was available for analysis. The biopsy specimen did have some scattered tumor cells with SOX11 overexpression. To our knowledge, the current study is the first to analyze SOX11 in patients with MCL who are undergoing ASCT.
Disease recurrence is a major cause of treatment failure after ASCT. As such, we analyzed the risks of recurrence and found that a proliferation index (Ki-67) > 30% was the only determining factor. Ki-67 expression has been found to be an important prognostic marker in patients with MCL.7,9,10 Using a quantitative image analysis, Schaffel et al26 suggested that a 30% proliferation index is an optimal cutoff. In the patient group in the current study, those patients whose tumors had a Ki-67 level > 30% also had a high risk of disease recurrence. Ki-67 distribution was found to be similar in patients who underwent pretransplantation induction chemotherapy with R-CHOP (cyclophosphamide, doxorubicin, vincristine, and prednisone) and R-hyper-CVAD, suggesting that high Ki-67 levels are an adverse prognostic marker, even in patients treated with aggressive chemotherapy before ASCT. We believe that Ki-67 levels should be routinely evaluated in all patients with MCL.
The results of recent studies suggest that ASCT is associated with a higher survival rate in patients with MCL if it is preceded by cytarabine-containing induction chemotherapy.27 In the current study, similar outcomes were observed in patients receiving R--CVAD/high-dose cytarabine or R-CHOP. We detected no difference in Ki-67 distribution between these 2 groups. This finding confirms the results that were recently published by Budde et al.6
We acknowledge the limitations of the current study. The small number of patients in this series and the absence of a prospective control group preclude firm conclusions regarding the prognostic significance of the MIPI score. In addition, it was difficult to determine whether the lower rate of disease recurrence in the study group compared with the control group was because of rituximab or a difference in Ki-67 distribution, because adequate pathological material for testing was only available for 5 patients in the control group. All 5 tested patients in the control group were found to have low Ki-67 levels, and the study group included patients who were resistant to R-hyper-CVAD; more patients in the study group had blastoid tumors, elevated β-2 microglobulin levels, and an IPI > 1, suggesting that the improvement was related to the use of rituximab.
In conclusion, ASCT with rituximab given during stem cell collection and immediately after transplantation may induce continuous long-term remissions in a substantial subset of patients with MCL who responded to induction chemotherapy, with improved results compared with historical controls not receiving rituximab. The dose and schedule of rituximab in patients treated with ASCT need to be studied in randomized trials. An elevated Ki-67 level appears to be the most significant determinant of disease recurrence. Innovative strategies are needed to treat these patients. At the study institution, we offer allogeneic stem cell transplantation to patients with MCL with a high Ki-67 index.
Acknowledgments
We thank Martin H. Nguyen for his technical assistance and Dr. Roberto N. Miranda for providing the tissue microarray that helped us optimize SOX11 immunohistochemical staining. We also thank all the pathologists and hematooncologists from outside our institution for providing paraffin blocks or unstained sections for SOX11 immunohistochemical analysis. We thank Ann Sutton for her editing of this article.
Funding Sources: No specific funding was disclosed.
Funding Statement: This article was supported by NIH. The grant number is P30 CA016672.
Footnotes
Presented in part as an abstract at the 53rd Annual Meeting of the American Society of Hematology; December 10-13 2011; San Diego, CA.
Conflict of Interest Disclosures: Dr. Bueso-Ramos has received payment from Novartis for the development of educational presentations.
References
- 1.Swerdlow SH, Campo E, Seto M, et al. Mantle cell lymphoma. In: Swerdlow SH, Campo E, Harris NL, et al., editors. WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues. Lyon, France: IARC; 2008. pp. 229–232. [Google Scholar]
- 2.Majlis A, Pugh WC, Rodriguez MA, et al. Mantle cell lymphoma: correlation of clinical outcome and biologic features with 3 histologic variants. J Clin Oncol. 1997;15:1664–1671. doi: 10.1200/JCO.1997.15.4.1664. [DOI] [PubMed] [Google Scholar]
- 3.Romaguera JE, Fayad LE, Feng L, et al. Ten-year follow-up after intense chemoimmunotherapy with Rituximab-HyperCVAD alternating with Rituximab-high dose methotrexate/cytarabine (R-MA) and without stem cell transplantation in patients with untreated aggressive mantle cell lymphoma. Br J Haematol. 2010;150:200–208. doi: 10.1111/j.1365-2141.2010.08228.x. [DOI] [PubMed] [Google Scholar]
- 4.Hoster E, Dreyling M, Klapper W, et al. A new prognostic index (MIPI) for patients with advanced-stage mantle cell lymphoma. Blood. 2008;111:558–565. doi: 10.1182/blood-2007-06-095331. [DOI] [PubMed] [Google Scholar]
- 5.Tam CS, Bassett R, Ledesma C, et al. Mature results of the M. D. Anderson Cancer Center risk-adapted transplantation strategy in mantle cell lymphoma. Blood. 2009;113:4144–4152. doi: 10.1182/blood-2008-10-184200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Budde LE, Guthrie KA, Till BG, et al. Mantle cell lymphoma international prognostic index but not pretransplantation induction regimen predicts survival for patients with mantle-cell lymphoma receiving high-dose therapy and autologous stem-cell transplantation. J Clin Oncol. 2011;29:3023–3029. doi: 10.1200/JCO.2010.33.7055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Geisler CH, Kolstad A, Laurell A, et al. Nordic MCL2 trial update: six-year follow-up after intensive immunochemotherapy for untreated mantle cell lymphoma followed by BEAM or BEAC+ autologous stem-cell support: still very long survival but late relapse do occur. Br J Haematol. 2012;158:355–362. doi: 10.1111/j.1365-2141.2012.09174.x. [DOI] [PubMed] [Google Scholar]
- 8.Todorovic M, Balint B, Andjelic B, et al. Outcome prediction of advanced mantle cell lymphoma by international prognostic index versus different mantle cell lymphoma indexes: one institution study. Med Oncol. 2012;29:2212–2219. doi: 10.1007/s12032-011-0136-1. [DOI] [PubMed] [Google Scholar]
- 9.Rosenwald A, Wright G, Wiestner A, et al. The proliferation gene expression signature is a quantitative integrator of oncogenic events that predicts survival in mantle cell lymphoma. Cancer Cell. 2003;3:185–197. doi: 10.1016/s1535-6108(03)00028-x. [DOI] [PubMed] [Google Scholar]
- 10.Garcia M, Romaguera JE, Inamdar KV, et al. Proliferation predicts failure-free survival in mantle cell lymphoma patients treated with rituximab plus hyperfractionated cyclophosphamide, vincristine, doxorubicin, and dexamethasone alternating with rituximab plus high-dose methotrexate and cytarabine. Cancer. 2009;115:1041–1048. doi: 10.1002/cncr.24141. [DOI] [PubMed] [Google Scholar]
- 11.Fernandez V, Salamero O, Espinet B, et al. Genomic and gene expression profiling defines indolent forms of mantle cell lymphoma. Cancer Res. 2010;70:1408–1418. doi: 10.1158/0008-5472.CAN-09-3419. [DOI] [PubMed] [Google Scholar]
- 12.Ondrejka SL, Lai R, Smith SD, et al. Indolent mantle cell leukemia: a clinicopathological variant characterized by isolated lymphocytosis, interstitial bone marrow involvement, kappa light chain restriction, and good prognosis. Haematologica. 2011;96:1121–1127. doi: 10.3324/haematol.2010.036277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gressin R, Caulet-Maugendre S, Deconinck E, et al. French GOE-LAMS Group. Evaluation of the (R)VAD+C regimen for the treatment of newly diagnosed mantle cell lymphoma. Combined results of two prospective phase II trials from the French GOELAMS group. Haematologica. 2010;95:1350–1357. doi: 10.3324/haematol.2009.011759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Caraway NP, Gu J, Lin P, Romaguera JE, Glassman A, Katz R. The utility of interphase fluorescence in situ hybridization for the detection of the translocation t(11;14)(q13;q32) in the diagnosis of mantle cell lymphoma on fine-needle aspiration specimens. Cancer. 2005;105:110–118. doi: 10.1002/cncr.20923. [DOI] [PubMed] [Google Scholar]
- 15.Cheson BD, Horning SJ, Coiffier B, et al. Report of an international workshop to standardize response criteria for non-Hodgkin's lymphomas. NCI Sponsored International Working Group. J Clin Oncol. 1999;17:1244–1253. doi: 10.1200/JCO.1999.17.4.1244. [DOI] [PubMed] [Google Scholar]
- 16.Cheson BD, Pfistner B, Juweid ME, et al. International harmonization project on lymphoma. Revised response criteria for malignant lymphoma. J Clin Oncol. 2007;25:579–586. doi: 10.1200/JCO.2006.09.2403. [DOI] [PubMed] [Google Scholar]
- 17.Luthra R, Sarris AH, Hai S, et al. Real-time 5′→3′ exonuclease-based PCR assay for detection of the t(11;14)(q13;q32) Am J Clin Pathol. 1999;112:524–530. doi: 10.1093/ajcp/112.4.524. [DOI] [PubMed] [Google Scholar]
- 18.Kaplan EL, Meier P. Non-parametric estimation from incomplete observations. J Am Stat Assoc. 1958;53:457–481. [Google Scholar]
- 19.Kalbfleish JD, Prentice RL. The Statistical Analysis of Failure Time Data. Hoboken, NJ: John Wiley & Sons Inc; 2002. Relative Risk (Cox) Regression Models; pp. 95–142. [Google Scholar]
- 20.O'Brien SM, Kantarjian H, Thomas DA, et al. Rituximab dose-escalation trial in chronic lymphocytic leukemia. J Clin Oncol. 2001;19:2165–2170. doi: 10.1200/JCO.2001.19.8.2165. [DOI] [PubMed] [Google Scholar]
- 21.Byrd JC, Murphy T, Howard RS, et al. Rituximab using a thrice weekly dosing schedule in B-cell chronic lymphocytic leukemia and small lymphocytic lymphoma demonstrates clinical activity and acceptable toxicity. J Clin Oncol. 2001;19:2153–2164. doi: 10.1200/JCO.2001.19.8.2153. [DOI] [PubMed] [Google Scholar]
- 22.Piro LT, White CA, Grillo-Lopez AJ, et al. Extended rituximab (anti-CD20 monoclonal antibody) therapy for relapsed or refractory low grade or follicular non-Hodgkin's lymphoma. Ann Oncol. 1999;10:655–661. doi: 10.1023/a:1008389119525. [DOI] [PubMed] [Google Scholar]
- 23.Zipp L, Saliba RM, Valverde R, et al. Mature results of BEAM/high-dose rituximab vs BEAM/yttrium-90 ibritumomab tiuxetan (Zevalin) and autologous stem cell transplantation (ASCT) for relapsed CD20+ follicular and diffuse large b-cell lymphoma: survival outcomes and risk of secondary malignancies. Blood. 2011;118:2005. abstract. [Google Scholar]
- 24.Martin P, Chadburn A, Christos P, et al. Outcome of deferred initial therapy in mantle-cell lymphoma. J Clin Oncol. 2009;27:1209–1213. doi: 10.1200/JCO.2008.19.6121. [DOI] [PubMed] [Google Scholar]
- 25.Nygren L, Baumgartner Wennerholm S, Klimkowska M, Christensson B, Kimby E, Sander B. Prognostic role of SOX11 in a population-based cohort of mantle cell lymphoma. Blood. 2012;119:4215–4213. doi: 10.1182/blood-2011-12-400580. [DOI] [PubMed] [Google Scholar]
- 26.Schaffel R, Hedvat CV, Teruya-Feldstein J, et al. Prognostic impact of proliferative index determined by quantitative image analysis and the International Prognostic Index in patients with mantle cell lymphoma. Ann Oncol. 2010;21:133–139. doi: 10.1093/annonc/mdp495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Delarue R, Haioun C, Ribrag V, et al. Groupe d'Etude des Lymphomes de l'Adulte (GELA) CHOP and DHAP plus rituximab followed by autologous stem cell transplantation in mantle cell lymphoma: a phase 2 study from the Groupe d'Etude des Lymphomes de l'Adulte. Blood. 2013;121:48–53. doi: 10.1182/blood-2011-09-370320. [DOI] [PubMed] [Google Scholar]


