Abstract
P2Y receptors are G protein-coupled receptors stimulated by extracellular nucleotides. Both the P2Y1 and the P2Y6 receptors are preferentially activated by nucleoside 5′-diphosphates, but favor different base moieties. In the case of the P2Y1 receptor the preferred base is adenine, while the P2Y6 receptor is activated by uracil nucleotides. To identify potential amino acid domains that interact with the base moiety, we used a chimeric receptor approach, employing the human P2Y1 receptor as core structure to investigate the role in receptor activation of extracellular loops (ELs) and transmembrane domains (TMs) of the rat P2Y6 receptor. The chimeric receptors were expressed in COS-7 cells and measured for stimulation of phospholipase C (PLC) induced by the potent P2Y1 receptor agonist 2-MeSADP or the potent P2Y6 receptor agonist UDP. Replacement of the N-terminus or EL2 resulted in low (~50 μM) potency of the agonist 2-MeSADP, thus confirming the importance of EL2 in ligand recognition. Upon replacement of several regions, the potency of the P2Y1 agonist 2-MeSADP was either 1–2 μM (N-terminus and EL1, or EL1 and EL3) or 72 μM (N-terminus and EL3). Concurrent replacement of three regions (N-terminus, EL1, and EL3) completely precluded activation by 2-MeSADP. Our study identified domains of the P2Y6 receptor that contribute to receptor activation by UDP and hence seem to be involved in uracil recognition. Upon replacement with extracellular domains of the P2Y6 receptor sequence we observed a trend toward gain of receptor-induced PLC activation by UDP, for example, in the chimera containing replacements of both the N-terminus and EL1. Exchange of three receptor domains led to a construct with an EC50 value for UDP of 19 μM and a maximal inositol phosphate accumulation similar to the native P2Y6 receptor. Within receptor constructs of combined domain exchanges the additional substitution of Tyr110 by the corresponding Asn from the P2Y6 receptor showed a significant increase for activation by UDP, but only when combined with the N-terminal domain and TM1. The residue Tyr110 was identified to play an important role in the recognition of the nucleobase in the P2Y1 and P2Y6 receptors.
Keywords: P2Y1 nucleotide receptor, G protein-coupled receptor, Pyrimidines, Mutagenesis, Phospholipase C
1. Introduction
Extracellular nucleotides, such as ATP, act in cellular signaling through two families of membrane-bound P2 receptors: P2X subtypes, ligand-gated ion channels which are activated principally by adenine nucleotides; and P2Y subtypes, G protein-coupled receptors (GPCRs) which are activated by both adenine and uracil nucleotides [1]. Seven P2X and eight P2Y subtypes have recently been cloned. P2 receptor modulation has also been suggested for developing new treatments for pain, thrombosis, inflammation, immune disorders, septic shock, stroke, urinary incontinence, muscle disorders, psoriasis, erectile dysfunction, and cardiovascular diseases [2].
The P2Y1 receptor is a GPCR that stimulates PLC in response to adenine nucleotides (ADP > ATP) and is present in the heart, smooth muscles, vascular endothelium, prostate, ovary, brain, and platelets [3]. It has been cloned from the chicken, human, rat, cow, and mouse [4,5]. It is one of the three subtypes of P2 receptors found in platelets. Study of a P2Y1 receptor-null mouse [6] suggested that this receptor is associated with the initial shape change and subsequent steps of platelet aggregation. A selective P2Y1 receptor antagonist might be useful as an antithrombotic agent [7–9]. The P2Y6 receptor is found in the placenta, pancreas, spleen, thymus, bone, lung, intestines, smooth muscle, and epithelia, and has been cloned from the human, rat, mouse, chick, and turkey [10]. At P2Y6 receptors, UDP is the preferred ligand and is more potent than UTP. Recently the P2Y6 receptor was described to regulate interleukin-8 production and release in monocytes [11] and to antagonize TNF-α induced apoptosis in astrocytoma cells [12]. Furthermore the activation of P2Y6 receptors was found to be involved in growth regulation of vascular smooth muscle cells and was regulated by factors that are important in the development of vascular disease [13]. Selective ligands might be useful for treatment of cardiovascular or inflammatory disorders.
Molecular modeling of cloned P2Y1 receptor sequences, using a rhodopsin template, has focused on both the seven transmembrane domains (TMs) and the extracellular loops (ELs) [14,15]. The features of the putative binding site identified were consistent with mutagenesis results, as well as known ligand specificities. The modeling of the entire family of P2Y receptors has been based on a multiple sequence alignment of 68 related receptors [16]. This sequence analysis has delineated two separate subclasses among P2Y receptors. Within these subclasses P2Y1 and P2Y6 receptors belong to the same class. In order to ascertain which residues of the human P2Y1 receptor are involved in ligand recognition, we have mutated both the TMs (helices 3–7) and the ELs. A cluster of residues of positively charged amino acids (Lys and Arg) near the exofacial side of TMs 3, 6, and 7 putatively coordinates the phosphate moieties of nucleotide agonists and antagonists [17]. Two disulfide bridges in the extracellular domains, essential for structural integrity of the receptor, have been identified and several charged residues in the EL2 (E209) and three (R287) have been shown to be critical for receptor activation. This suggested that the role of the ELs in ligand recognition is as important as that of the TMs [18,19]. The present study investigates the role of both the ELs and TMs in the recognition of the base moiety, through the use of a chimeric receptor approach, employing the human P2Y1 receptor as the core structure with portions substituted from the rat P2Y6 receptor.
2. Experimental procedures
2.1. Materials
The expression construct coding for the human P2Y1 receptor (pCDP2Y1) was prepared as previously described [17]. The construct coding for the rat P2Y6 receptor was a gift from Dr. R.A. Nicholas at the University of North Carolina, Chapel Hill, NC. Vent DNA polymerase and all endonuclease restriction enzymes used in this study were obtained from New England Biolabs. The agonist 2-MeSADP, UDP, glucose, hexokinase, and o-phenylenediamine dihydrochloride were purchased from Sigma Chemical Co. [3H]-myo-inositol (15 Ci/mmol) was obtained from American Radiolabeled Chemicals. Fetal bovine serum (FBS) was from Gibco. The Sequenase Kit Version 2.0 was from Amersham. All oligonucleotides were synthesized by Bioserve Biotechnologies. A monoclonal antibody (12CA5) against a hemagglutinin epitope (HA) was purchased from Roche Biochemicals, and goat anti-mouse IgG antibody conjugated with horseradish peroxidase was purchased from Sigma. DEAE-dextran was obtained from Pharmacia-LKB.
2.2. Plasmid construction and site-directed mutagenesis
All chimeric constructs were introduced into pCDP2Y1 [17] using the following procedure. For each construct three PCR fragments were generated by standard techniques [20]. To generate overlapping fragments we used 42mer primers, of which 21 bases were from the corresponding sequence of hP2Y1 and the remaining 21 bases were from rP2Y6. Fragments 1 and 3 consisted mainly of P2Y1 sequence while fragment 2 was dominated by P2Y6. In a second PCR we combined fragments 1 and 2 by overlap extention PCR. Finally, in a third PCR reaction we fused this construct with fragment 3 for the desired construct. Those PCR fragments were then ligated into the pCDP2Y1 expression vector using the following restriction sites: N-terminal and TM1, as well as EL1 and TM3 chimeras, RsrII and AgeI; EL 2 and TM5 chimeras, AgeI and BglII; EL3, TM6 and TM7, BglII and XbaI. The accuracy of all PCR-derived sequences was confirmed by dideoxy sequencing of the mutant plasmids [21].
2.3. Epitope tagging
As previously described [17] a nine-amino acid sequence derived from the influenza virus hemagglutinin (HA) protein (TAC CCA TAC GAC GTG CCA GAC TAC GCG; peptide sequence: YPYDVPDYA) was inserted after the initiating Met residue in the extracellular N-terminus of the human P2Y1 receptor gene. A hexahistidine tag [22] was also included at the C-terminus, immediately after the ultimate Leu residue resulting in a construct suitable for potential affinity chromatography using a nickel column.
2.4. Transient expression of chimeric receptors in COS-7 cells
COS-7 (4 × 106) cells were seeded into 150 mm culture dishes containing 25 ml Dulbecco’s Modified Eagle’s Medium (DMEM) supplemented with 10% FBS, 100 units/ml penicillin, 100 μg/ml streptomycin and 2 μmol/ml glutamine. Cells were transfected approximately 24 h later with plasmid DNA (10 μg DNA/dish) using the DEAE-dextran method [23] for 40 min, followed by treatment with 100 μM chloroquine for 2.5 h, and grown for an additional 24 h at 37 °C with 5% CO2.
2.5. Inositol phosphate determination
Assays were carried out according to the general approach of Harden et al. [24]. About 24 h after transfection, the cells were split into 6-well plates (Costar, ~0.75 × 106 cells/well) in 2 ml DMEM culture medium supplemented with 1 μCi/ml [3H]-myo-inositol. After a 24 h labeling period of the cells, 10 μl of a LiCl, glucose, and hexokinase solution (see next paragraph) was added and cells were further incubated for 20 min at room temperature. The mixtures were gently swirled to insure uniformity. Following the addition of agonists (see next paragraph), cells were incubated for 30 min at 37 °C, 5% CO2. The supernatant was removed by aspiration, and 750 μl of cold 20 mM formic acid was added to each well. After 30 min incubation at 4 °C, cell extracts were neutralized with 250 μl of 60 mM NH4OH. The inositol monophosphate fraction was then isolated by anion exchange chromatography [25]. The contents of each well were applied to a small anion exchange column (Bio-Rad AG-1-X8) that had been pretreated with 15 ml 0.1 M formic acid/3 M ammonium formate, followed by 15 ml water. The columns were then washed with 10 ml water followed by 15 ml of a solution containing 5 mM sodium borate and 60 mM sodium formate. [3H]-Inositol phosphates were eluted with 4.5 ml of 0.1 M formic acid/0.2 M ammonium formate and quantified by liquid scintillation spectrometry (LKB Wallace 1215 Rackbeta scintillation counter).
Pharmacological parameters were analyzed using the KaleidaGraph program (Abelbeck Software, Version 3.01).
2.6. Pre-treatment of agonist stock solutions and background response in COS-7 cells
Control experiments of vector-transfected COS-7 cells indicated no response of these cells to 2-MeSADP, but indicated that an endogenous response to UTP was present (EC50 ≅ 1.5 μM, data not shown), confirming previous observations [26]. Nicholas et al. [27] had shown that commercial UDP contained small amounts of UTP. To prevent misleading results from possible impurities of UTP, we pre-incubated the UDP stock solution for 2 h at 37 °C with 22 mM glucose and 10 units/ml hexokinase. This procedure was described to effectively convert all UTP impurities into UDP, as was confirmed by HPLC [27]. A further potential complication was that trace amounts of ATP, released by cells into the culture medium, might serve as phosphate donors to the enzyme nucleoside diphosphate kinase, which might phosphorylate UDP to UTP [28]. To prevent formation of UTP at the stage of inositol phosphate accumulation, we supplemented a 2 M LiCl solution with glucose and hexokinase for a final assay concentration of 10 mM LiCl, 5 mM glucose, and 2 units hexokinase per well (see previous paragraph). Experiments under these conditions led to a response of vector-transfected COS-7 cells to UDP with an EC50 value slightly above 100 μM. The stimulation observed with UDP in vector-transfected COS-7 cells typically was 30–40% of the maximal effect in cells transfected with cDNA of multiply-substituted mutant receptors. These data were in agreement with published data [27] and accepted as background activity. Control COS-7 cells without transfection displayed nearly identical responses, thus this background response, which complicated interpretation of results, did not arise from the transfection.
2.7. Determination of cell-surface expression of mutant receptors
For cell surface ELISA (enzyme-linked immunosorbent assay) measurements, cells were transferred to 96 well dishes ((4–5) × 104 cells/well) one day after transfection. About 72 h after transfection, cells were fixed in 4% formaldehyde in phosphate buffered saline (PBS) for 30 min at room temperature. After washing three times with PBS and blocking with DMEM (containing 10% FBS), cells were incubated with the HA-specific mono-clonal antibody (12CA5), 20 μg/ml, for 3 h at 37 °C. Plates were washed and incubated with a 1:2000 dilution of a peroxidase-conjugated goat anti-mouse IgG antibody (Sigma) for 1 h at 37 °C. Hydrogen peroxide and o-phe-nylenediamine (each 2.5 mM in 0.1 M phosphate–citrate buffer, pH 5.0) served as substrate and chromogen, respectively. The enzymatic reaction was stopped after 30 min at room temperature with 1 M H2SO4 solution containing 0.05 M Na2SO3, and the color development was measured bichromatically in the BioKinetics reader (EL312, Bio Tek Instruments, Inc., Winooski, VT) at 490 and 630 nm (baseline). The reading for cells transfected with expression constructs coding for the human P2Y1 wild-type receptor was approximately 0.4 OD units, and approximately 0.15 OD units for cells transfected with vector DNA alone. The difference was normalized as 100% surface expression.
3. Results
3.1. Design and construction of mutant receptors
To take into account several definitions of the TM boundaries [19,29], we have generated overlapping chimeric receptors differing by only a few amino acids. Based on the loop/TM boundaries defined according to Moro et al. (Fig. 1) [19], we have designed additional receptor chimeras fused above or below the boundary point. The resulting constructs were numbered according to the sequence of the human P2Y1 receptor replaced with the corresponding amino acid sequence of the rat P2Y6 receptor (Fig. 1). All constructs were confirmed by dideoxy sequencing of the mutant plasmids [21] and transiently expressed in COS-7 cells.
Fig. 1.
Sequence alignment of human P2Y1 and rat P2Y6 nucleotide receptors. The numbers above indicate sequence positions of the human P2Y1 receptor. The transmembrane regions (TM), as predicted by Moro et al. [14], are marked with black bars above the sequence. The created constructs, listed in Table 1, were named according to number of the substituted amino acid sequences. As an example, construct 110–133 consists of the P2Y1 sequence, but from position 110 to position 133 the P2Y1 sequence was substituted with the corresponding amino acids from P2Y6. For reasons of consistent numbering in the alignment figure, two short regions within the intracellular loops of the rat P2Y6 receptor sequence are not shown. These regions were unimportant for this study and are indicated by small letters. The complete sequence would read hKr and pVAQEr, respectively.
3.2. Mutation of EL1
The first construct with an exchanged EL1 domain (111–123) showed a 330-fold potency decrease in stimulation of inositol phosphate production by 2-MeSADP (Table 1, Fig. 2). To further investigate this effect, we created a construct (111–118) in which only six amino acids in the N-terminal portion of the EL1 were substituted. This construct exhibited a slightly larger decrease in potency of 2-MeSADP (460-fold), but was not significantly different from construct 111–123. Thus, either the C-terminal part of EL1 did not play a critical role in receptor activation by 2-MeSADP or amino acids that might contribute to receptor activation were conserved among P2Y1 and P2Y6 receptors. Further substitution of portions within TM3 (construct 111–133, Table 1) did not further alter the potency of 2-MeSADP.
Table 1.
Receptor-stimulated PLC activation and surface expression assays (ELISA) of wild-type and chimeric P2Y1/6 receptorsa
| Construct | 2-MeSADP EC50 (μM) | n | Surface expression | Max. effect of UDP (%)b | UDPc EC50 (μM) | n |
|---|---|---|---|---|---|---|
| Human P2Y1 | 0.0072 ± 0.0017 | 7 | 100 ± 4 | 32 | (98.6 ± 1.6) | 4 |
| Rat P2Y6 | No stimulation | 2 | n.d. | 100 | 0.069 ± 0.021 | 5 |
| N-terminus | ||||||
| 37–46 | 51.8 ± 4.2 | 5 | 6 ± 3 | 30 | (109 ± 5) | 3 |
| 37–51 | No stimulation | 3 | 127 ± 2 | 29 | (104 ± 17) | 3 |
| 37–61 | No stimulation | 2 | 29 ± 4 | 62 | 37.4 ± 6.4** | 4 |
| Extracellular loop 1 | ||||||
| 111–118 | 3.37 ± 0.76 | 3 | 97 ± 3 | 28 | (107 ± 10) | 3 |
| 110–118 | No stimulation | 2 | 106 ± 3 | 27 | (113 ± 18) | 3 |
| 111–123 | 2.40 ± 0.79 | 3 | 173 ± 2 | 29 | (129 ± 12) | 3 |
| 110–123 | No stimulation | 4 | 172 ± 2 | 31 | (104 ± 5) | 3 |
| 111–133 | 2.17 ± 1.22 | 3 | 83 ± 2 | 26 | (112 ± 9) | 3 |
| 110–133 | No stimulation | 4 | 119 ± 2 | 34 | (86 ± 9) | 3 |
| Extracellular loop 2 | ||||||
| 192–210 | 46.8 ± 7.8 | 4 | 190 ± 2 | 28 | (120 ± 6) | 3 |
| 192–213 | No stimulation | 3 | 36 ± 2 | 31 | (114 ± 18) | 3 |
| 192–215 | No stimulation | 2 | 15 ± 3 | 29 | (112 ± 15) | 3 |
| 192–225 | No stimulation | 2 | 16 ± 5 | 33 | (131 ± 10) | 3 |
| Extracellular loop 3 | ||||||
| 274–298 | No stimulation | 3 | 73 ± 2 | 31 | (109 ± 11) | 3 |
| 284–298 | 0.440 ± 0.190 | 4 | 112 ± 1 | 50 | 61.0 ± 6.0* | 6 |
| 284–303 | No stimulation | 3 | 47 ± 2 | 31 | (112 ± 12) | 3 |
| 284–307 | No stimulation | 3 | 6 ± 2 | 34 | (114 ± 18) | 3 |
| 284–313 | No stimulation | 3 | 34 ± 2 | 27 | (96.0 ± 7.5) | 3 |
| Combinational chimeras | ||||||
| 111–133/284–298 | 1.94 ± 0.93 | 3 | 88 ± 2 | 66 | 46.6 ± 2.4** | 4 |
| 110–133/284–298 | No stimulation | 2 | 62 ± 3 | 59 | 51.3 ±19.8* | 3 |
| 37–61/110–133 | No stimulation | 2 | 166 ± 3 | 90 | 19.3 ± 2.2** | 5 |
| 37–61/111–133 | 1.35 ± 0.43 | 3 | 37 ± 5 | 73 | 41.6 ± 4.9** | 3 |
| 37–61/284–298 | 72.1 ± 4.1 | 3 | 88 ± 2 | 56 | 62.9 ± 6.4* | 3 |
| 37–61/110–133/284–298 | No stimulation | 2 | 74 ± 3 | 92 | 18.7 ± 2.1** | 4 |
| 37–61/111–133/284–298 | No stimulation | 2 | 49 ± 3 | 86 | 28.6 ± 3.2** | 4 |
Determination of surface expression represents the average of six to eight experiments. Construct numbers indicate the sequence positions that were exchanged between receptors as defined in Fig. 1. EC50 values are mean values ± S.E. of two to seven independent experiments each done in duplicate. Maximal stimulation of ([3H]-inositol phosphate accumulation for each receptor was between two- and four-fold over basal.
Control COS-7 cells transfected with vector alone displayed a background response of less than maximal amplitude to UDP with an EC50 value slightly above 100 μM and no stimulation by 2-MeSADP (n = 4). Control COS-7 cells without transfection displayed nearly identical responses.
Maximal stimulation of PLC observed, by 1.0 mM UDP, relative to the rat P2Y6 response.
Values in parentheses are not statistically different from the background response present in COS-7 cells.
P < 0.01 compared to P2Y1 wt or vector transfected COS-7 cells.
P < 0.001 compared to P2Y1 wt or vector transfected COS-7 cells.
Fig. 2.
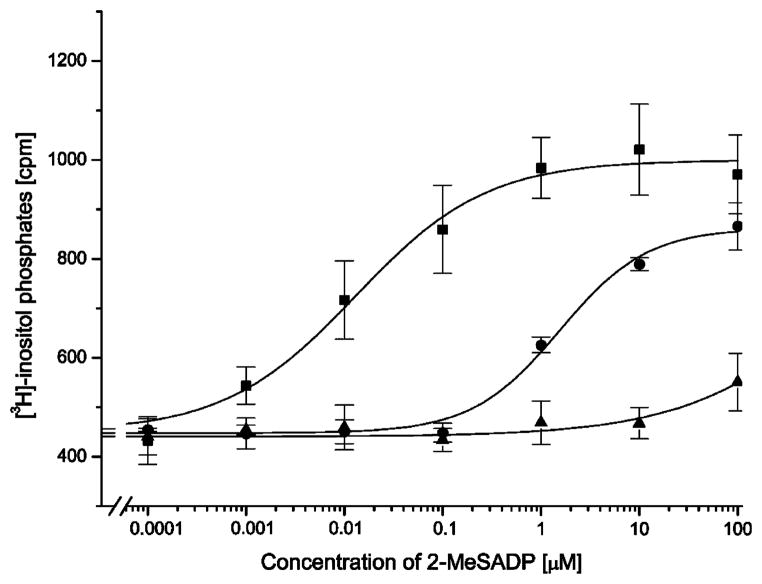
Concentration-response curves of P2Y1 receptor and P2Y1/6 chimeric receptors with exchanges in the EL1. Wild-type human P2Y1 receptors (squares) or chimeric receptors 111–123 (circles) or 110–123 (triangles) were transiently expressed in COS-7 cells. [3H]-Inositol phosphate accumulation was measured following a 30-min incubation with increasing concentrations of 2-MeSADP in the presence of 10 mM LiCl (see Section 2 for details). Maximal responses ranged from 2.0- to 3-fold increases in [3H]-inositol phosphate accumulation over basal. Concentration response curves represent the mean values ± S.E. of three to four replicate experiments.
We also created a construct starting at position 110, which is Asn in P2Y6 receptors and Tyr in other P2Y-family members. Surprisingly, the construct 110–123 was not activated by 2-MeSADP at <100 μM (Table 1 and Fig. 2), thus Tyr110 contributed a >50-fold shift in receptor activation by 2-MeSADP. The possibility that the lack of activity was due to failed expression or trafficking of the mutant receptors was eliminated. The receptor constructs all contained an HA-Tag at the N-terminus to enable determination of receptor cell surface expression by means of ELISA. Receptor expression was similar for both constructs, and lack of effect on surface expression upon position 110 replacement was confirmed using two other EL1 constructs (Table 1).
When transfected COS-7 cells were treated with purified UDP (see Section 2) to increase inositol phosphate production, the constructs in which EL1 and part of TM3 were exchanged exhibited similar EC50 values for UDP as compared to P2Y1-transfected control cells. Thus, the EL1 did not seem to contribute significantly to receptor activation by UDP. However, a tendency for activation by UDP emerged upon additional exchange of regions including position 110. In activation of PLC by UDP, there was a trend towards slightly increased potency from construct 110–118 with an EC50 value of 114 μM to construct 110–133 with 86 μM. Thus, activation appeared to increase when longer parts of EL1 and TM3 were transferred. However, no statistical significance was achieved for these constructs when EC50 values for activation by UDP were compared to P2Y1-transfected cells.
3.3. Mutation of EL2
EL2 of the rat P2Y6 receptor is one residue shorter in length than the EL2 of the P2Y1 receptor. Based on an alignment of the two sequences (Fig. 1), the missing amino acid occurs at position 201 at the N-terminal site of the Cys that forms part of the conserved disulfide bond between EL2 and TM3 (Fig. 1). Thus, all constructs with altered EL2 were shorter by one amino acid. The first receptor construct (192–210) was already severely impaired in its ability to be activated by 2-MeSADP and exhibited a >6500-fold shift (Table 1). All additional constructs with further substitutions in EL2 and TM5 did not show receptor activation even at 100 μM 2-MeSADP. Upon receptor stimulation with treated UDP, no gain of activation was observed for any of the EL2 constructs.
However, there were differences in the receptor surface expression for EL2 receptor constructs. The surface expression decreased with the length of the exchanged amino acid sequence in the construct. The most pronounced difference was seen for constructs 192–210 and 192–213 (Table 1) in which case the surface expression dropped five-fold and remained low for the other EL2 constructs.
3.4. Mutation of EL3
EL3 of the rat P2Y6 receptor is two amino acids shorter than the EL3 of the P2Y1 receptor. The missing amino acids correspond to positions 294 and 295 at the N-terminal side of the Cys that is part of the confirmed disulfide bond between EL3 and the N-terminus [18] (Fig. 1). Thus, all constructs with altered EL3 were shorter by two amino acids. Despite this shortening, the first construct (284–298), in which only a short P2Y6 sequence was transferred, exhibited receptor activation, and the EC50 value for stimulation by 2-MeSADP was shifted only 60-fold (Table 1). Thus, the effect on potency of the loss of two amino acids in EL3 was not as dramatic as the substitution of the Cys residue by Ala in EL3 [18], therefore correct formation of disulfide bonds seems likely to occur in this mutant receptor. All additional constructs with further substitutions in EL3 and TM7 did not show receptor activation even at 100 μM 2-MeSADP (Table 1). Thus, the initial construct was further used to elucidate the role of the exofacial part of TM6. The resulting construct (274–298), in which EL3 and an additional 10 amino acids from TM6 were exchanged, was not activated by 2-MeSADP.
Within constructs having exchanged amino acid sequences in EL3, only construct 284–298 displayed a small but statistically significant increase in the response (both potency and maximal effect) to UDP in comparison to P2Y1 control (Table 1, Fig. 3). The potency shift was found to be statistically significant using the two population-independent t-test. No other constructs having single modifications in EL3 displayed this gain of function in response to UDP. EL3 was similar to the EL2 region, in that the surface expression decreased with the length of the exchanged amino acid sequence in the construct, reached a minimum, and increased again (Table 1).
Fig. 3.
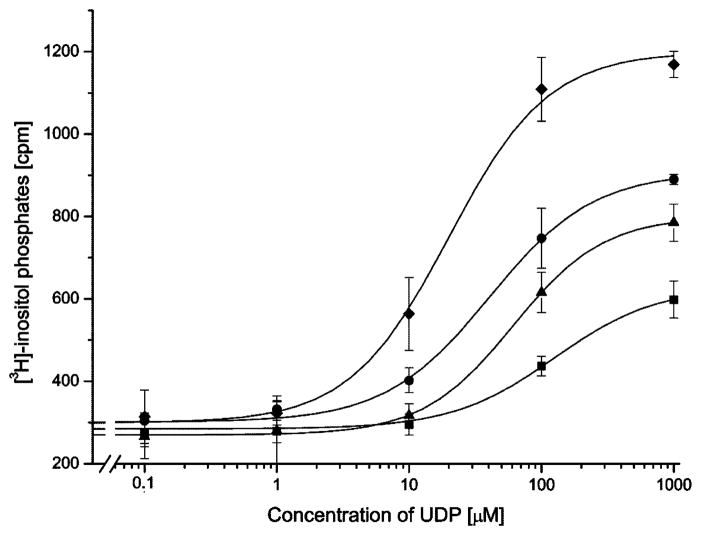
Concentration-response curves of P2Y1-transfected COS-7 cells and P2Y1/6 chimeric receptors with exchanges in multiple receptor domains. P2Y1 receptors (squares) or chimeric receptors 284–298 (triangles), 110–133/284–298 (circles), or 37–61/110–133/284–298 (diamonds) were transiently expressed in COS-7 cells. [3H]-Inositol phosphate accumulation was measured following a 30-min incubation with increasing concentrations of treated UDP in the presence of 10 mM LiCl (see Section 2 for details). Maximal responses in fold increase of [3H]-inositol phosphate accumulation compared to basal were 2-fold for P2Y1 receptors, 2.5-fold for construct 284–298, 2.8-fold for construct 110–133/284–298, and 3.8-fold for construct 37–61/110–133/284–298. The basal counts for the P2Y6 receptor were 320 cpm and stimulated counts were 1300 cpm. Concentration response curves show a single representative experiment of three independent experiments.
3.5. Mutation of the N-terminal domain
A comparison of the N-terminal sequences of the rat P2Y6 receptor and the human P2Y1 receptor showed low homology (Fig. 1). We decided to vary only a small portion of the N-terminal domain in the present constructs, as was included in the previous receptor model [19], i.e. including the Cys residue at position 42 and five preceding amino acids. Thus, the chimeric exchange began with position 37.
Stimulation of the N-terminal receptor constructs by 2-MeSADP was only observed for the construct 37–46. The EC50 value for activation by 2-MeSADP was increased more than 7000-fold for this construct (Table 1). Neither of the two other constructs was stimulated by ≤100 μM 2-MeSADP.
There was no gain of potency of UDP to activate constructs 37–46 and 37–51 (Table 1), while a strong and significant gain was detected for the construct 37–61. For this construct the activation curve by UDP was shifted three-fold to the left, which was significant at P < 0.01 when compared to the P2Y1 control (Table 1). All N-terminal constructs were detected on the cell surface using ELISA.
3.6. Combinational chimeras
By exchanging single domains we identified three potential amino acid clusters within the P2Y6 receptor that contributed to receptor activation by UDP: namely region 284–298 in EL3, region 37–61 in the N-terminus and TM 1, and to a lesser extent region 110–133 in EL1 and TM 3 (see Table 1).
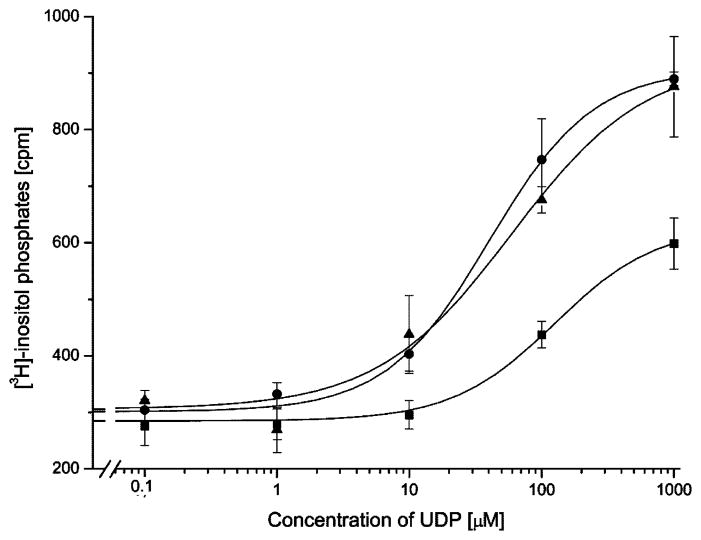
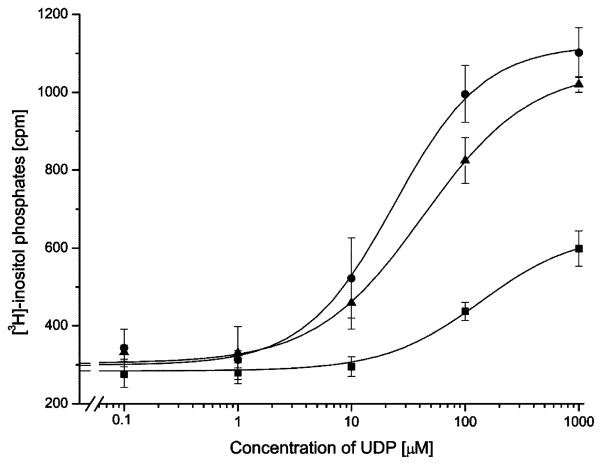
The combined exchange of domain 37–61 and 284–298 did not significantly improve the response of the receptor construct to UDP when it was compared to the single domain replacements (Table 1). Comparable results were obtained for the replacement of either domain 110–133 or 111–133 in combination with domain 284–298. When the resulting constructs were stimulated with UDP, the response was significantly improved over P2Y1-transfected control COS cells but only slightly different with respect to the single domain exchange constructs (Table 1 and Fig. 3). For these combined constructs the additional exchange at position 110 had no effect on the EC50 value for UDP stimulation (Fig. 4). This was different for the combination of domain 37–61 with domain 110–133 or 111–133. The receptor chimera 37–61/111–133 lowered the EC50 value for half maximal stimulation by UDP three-fold as compared to the control (Fig. 5). The additional exchange of amino acid 110 in construct 37–61/110–133 further increased the stimulation by UDP significantly from 3-fold to 6.5-fold over the control (Fig. 5). Two constructs were created in which all three domains were simultaneously substituted. The UDP stimulation was most enhanced for the construct 37–61/110–133/284–298. For this construct, the EC50 value for half-maximal stimulation was decreased by a factor of seven (Fig. 3), and the maximal level of [3H]-inositol phosphate production was increased four-fold over basal reaching the maximal level of [3H]-inositol phosphate production that was observed for the native P2Y6 receptor.
Fig. 4.
Concentration-response curves of P2Y1-transfected COS-7 cells and P2Y1/6 chimeric receptors with exchanges in multiple receptor domains. P2Y1 receptors (squares) or chimeric receptors 110–133/284–298 (circles), and 111–133/284–298 (triangles) were transiently expressed in COS-7 cells. [3H]-Inositol phosphate accumulation was measured following a 30-min incubation with increasing concentrations of purified UDP in the presence of 10 mM LiCl (see Section 2 for details). Maximal responses in fold increase of [3H]-inositol phosphate accumulation compared to basal were two-fold for P2Y1 receptors, threefold for construct for construct 110–133/284–298, and three-fold for construct 111–133/284–298. The basal counts for the P2Y6 receptor were 320 cpm and stimulated counts were 1300 cpm. Concentration response curves show a single representative experiment of three independent experiments. No significant difference was observed for the additional exchange of amino acid 110 in chimeric receptors.
Fig. 5.
Concentration–response curves of P2Y1-transfected COS-7 cells and P2Y1/6 chimeric receptors with exchanges in multiple receptor domains. P2Y1 receptors (squares) or chimeric receptors 37–61/110–133 (circles), and 37–61/111–133 (triangles) were transiently expressed in COS-7 cells. [3H]-Inositol phosphate accumulation was measured following a 30-min incubation with increasing concentrations of purified UDP in the presence of 10 mM LiCl (see Section 2 for details). Maximal responses in fold increase of [3H]-inositol phosphate accumulation compared to basal were 2-fold for P2Y1 receptors, 3.2-fold for construct for construct 37–61/111–133, and 3.8-fold for construct 37–61/110–133. Concentration response curves show a single representative experiment of three independent experiments. A significant difference was observed for the additional exchange of amino acid 110 in chimeric receptors.
4. Discussion
This study focuses on the role of the extracellular domains of the P2Y receptors in ligand recognition and receptor activation. Although the binding of nucleotides to P2Y receptors most closely resembles the binding of small molecules to other Type 1 GPCRs, such as biogenic amine receptors in which a small molecule ligand is coordinated within the transmembrane region surrounded by helical elements, the role of the ELs in the recognition process has been established in several recent studies [18,19,30]. There are at least two possible modes by which these loops may participate in the binding process: direct contact with the ligand in its TM binding site and contact regions in proposed “meta-binding” sites. According to the high resolution structure of rhodopsin [31], consistent with our prediction in a P2Y1 modeling study [19], the EL2 is closely associated with the ligand itself, in its putative, principal TM binding site. Thus, on one hand structural changes in EL2 might directly disrupt the coordination of the ligand in this site. On the other hand, EL2 and other exofacial regions of the P2Y receptors and other GPCRs may be involved in binding modes of agonists which are distinct from the principal TM site, and which may serve as transiently stabilized interactions that could guide the ligand in its approach to the TM region. Conceivably, there could be specific recognition elements present on extracellular domains required for the binding to meta-binding sites. Mutagenesis of single amino acids in conjunction with molecular modeling provided evidence for such sites on the P2Y1 receptor [18,19]. Thus, this study attempts to further explore the possibility of meta-binding by exchanging entire domains within these regions.
In a previous mutational study of the human P2Y1 receptor [18], the four Cys residues in the extracellular domains were found to form two disulfide bridges between the N-terminal domain and EL3, as well as between the exofacial end of TM3 and EL2, since these Cys residues are conserved within the family of P2Y receptors [29], it is likely that upon substitution of extracellular domains the same disulfide bond may form. However, the length of the EL domain or the position of the Cys varies by one or two amino acids (Fig. 1), which might have an effect on receptor activation. Proposed hypothetical boundaries of extracellular domains reported for the P2Y family vary slightly between two reports [19,29], and therefore we have included multiple constructs to take into account several boundary definitions. The Cys residue in the N-terminus of the P2Y6 receptor is offset by one amino acid in comparison to the P2Y1 receptor sequence, thus again this circumstance might contribute to changes in receptor activation.
In a recent study [32], regions of P2Y1 and P2Y2 receptors and the BLT1 leukotriene receptor predicted to interact with ligands were identified, and chimeric receptors were engineered to transpose the ligand binding site of one receptor onto another receptor. The ligand binding determinants of the BLT1 receptor are located in the upper regions of the helices and extracellular loops, and they had been successfully transferred to a receptor that normally binds unrelated ligands. Herold et al. [33] have analyzed chimeric receptors combining elements of two species homologues of the P2Y4 receptor. The second extracellular loop and the amino terminus form a functional motif that plays a key role in determining whether ATP functions as an agonist or antagonist at mammalian P2Y4 receptors.
The two receptors chosen for this study, P2Y1 and P2Y6, differ mainly in their base selectivity, although in ribose-modified, rigid (N)-methanocarba nucleotide analogues, there appears to be a fundamental difference between these two subtypes in the preference for ring twist conformation [34]. However, in the present study only ribosides were examined, thus the criterion for loss or gain of function was the recognition of either an adenine (P2Y1-like) or uracil moiety (P2Y6-like). Since radioligands for the P2Y receptors are still under development (e.g. [3H] MRS 2279 for the P2Y1 receptor), and no existing radioligands would be suitable in any case for mutant receptors with greatly reduced affinity for nucleotide ligands, it was necessary to study these receptor constructs in a functional assay. This did not permit the separation of binding and activation processes, nevertheless in this initial study of receptor chimera as well as in the previous P2Y1 mutagenesis studies, useful information about domains critical to the function of these P2Y subtypes could be obtained.
Upon replacement of various extracellular regions of the P2Y1 receptor, recognition of the adenine nucleotide 2-MeSADP was either weakened or abolished. Replacement of EL1, except for segments including the critical Tyr110, provided intermediate (μM) potency of the agonist, which normally activates the P2Y1 receptor at low nanonmolar concentrations. Replacement of EL3 also provided intermediate (μM) potency of the agonist, except when the region extended considerably into TM6 or TM7. Replacement of the N-terminus or EL2 resulted in even lower potency of the agonist. Thus, we have confirmed the importance of EL2 in ligand recognition. Upon replacement of several regions in the same chimeric receptor, the potency of the P2Y1 agonist 2-MeSADP was either intermediate (N-terminus and EL1, or EL1 and EL3) or weak (N-terminus and EL3). Concurrent replacement of three regions (N-terminus, EL1, and EL3) completely precluded activation by 2-MeSADP.
The shift of base preference to uracil was also studied in the chimeric receptors. Only the more pronounced shifts in the UDP response curves were indicative, due to a complicating, endogenous UDP response in the host COS-7 cells. Substitution with the important EL2 sequence of the P2Y6 receptor did not add the ability to recognize UDP. This reinforced the general observations that: (1) it is easier to subtract a receptor function through mutagenesis than to add a function, and (2) multiple recognition sites are required for the coordination of a given ligand in a receptor and single domain replacement is usually insufficient to add a function.
To further investigate the role of these domains we created combinational chimeras with all possible permutations of two domains and the corresponding chimera with all three domains. Since the effect on activation by UDP upon replacement of domain 110–133 alone was not statistically significant, we also made the corresponding chimeras in which the domain 111–133 was exchanged instead. The ability to be activated by UDP, albeit at μM concentrations, was obtained upon replacement of multiple exofacial domains of the P2Y1 receptor with the P2Y6 sequences (Fig. 6). Specifically, the chimera containing replacements of both the N-terminus and EL1 had this desired effect. The combination produced as much as a sixfold enhancement in the potency of UDP to stimulate PLC in transfected COS-7 cells. The potency of UDP at the native P2Y6 receptor was considerably greater than in these multiply substituted chimera, nevertheless the gain of function was statistically significant and occurred in several cases with consistent structure function pattern. Furthermore, the gain of stimulation by UDP was not only observed by a leftward shift of EC50 values, but also was visible in a larger increase in maximal counts of [3H]-inositol phosphates produced upon UDP stimulation (see Figs. 3–5). Thus, by combining different receptor domains an increase in potency and maximal stimulation of the chimeric receptors was achieved.
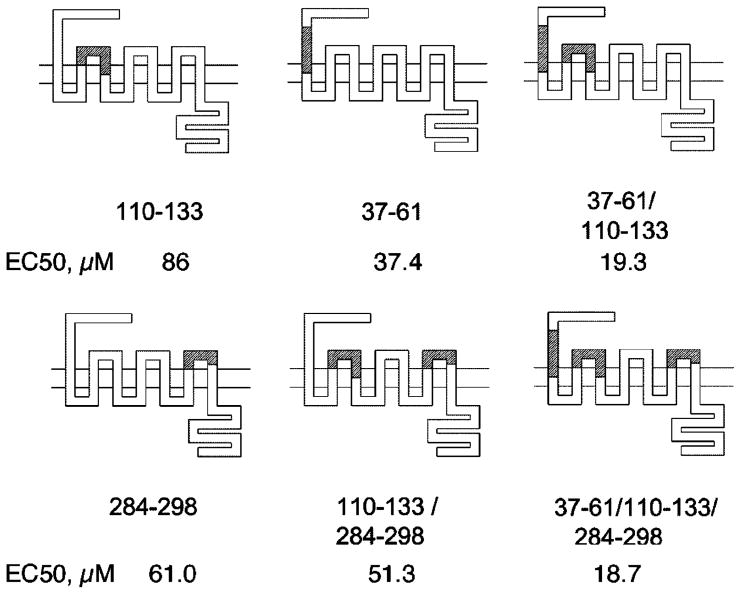
Fig. 6.
Diagrams of various P2Y1/6 receptor chimeras, showing the domains that were exchanged. White regions represent the human P2Y1 sequence and shaded regions represent the rat P2Y6 sequence. EC50 values for stimulation by UDP in the P2Y1/6 receptor chimeras are provided (values from Table 1). Constructs 37–61/110–133 and 37–61/110–133/284–298 exhibited a gain of receptor stimulation by UDP that was more than additive compared to single domain constructs. Using the notation that relates residues of different GPCRs [30], the following residues of the human P2Y1 sequence form the boundaries of these exchanged regions: Ser37 (N-terminal domain), Val61 (1.42), Tyr110 (2.63), Val133 (3.34), Leu284 (6.69), and Phe298 (EL3).
Specific ligand recognition elements in extracellular domains such as the N-terminus and EL1 are frequently found for peptide receptors and are not limited to P2 nucleotide receptors. For example, the N-terminal domain of the V1a vasopressin receptor close to the TM1 domain was found to be important for binding of agonists [35]. When substituting a similar domain, which in our case was achieved with construct 37–51, receptor activation by the agonist 2-MeSADP was lost. For small ligand receptors like the human A2A adenosine receptor, an amino acid in the exofacial region of TM1 was implicated in ligand binding [36]. A single amino acid (Glu13) was found to significantly contribute to agonist recognition, through a putative H-bond formed with TM7. In a similar study with the human A1 adenosine receptor a gain of ligand affinity was observed when Gly14 in the exofacial part of TM1 was replaced with the corresponding amino acid from human A2A adenosine receptor [37]. In the present study, a comparison of constructs 37–61 and 37–51 revealed a significant gain of potency of UDP, implying that features of the exofacial region of TM1 might be involved in agonist activation.
We have previously shown that the loss of mutant P2Y1 receptor surface expression was not due to reduced protein expression but rather due to an impaired trafficking of the receptor to the cell surface [18]. Thus, the exchanged amino acid sequences in EL2 that lead reduced surface expression may play a role in overall receptor stability, which affects trafficking.
The role in base recognition of amino acid 110 (residue 2.63, according to a standardized notation indicating TM and position relative to a conserved residue [38,39]) at the boundary of TM2 and EL1 was particularly striking. In all P2Y receptors this position corresponds to a Tyr residue, except for the P2Y6 receptor in which it is Asn. When Tyr110 was substituted with Asn, none of the corresponding receptor constructs responded to stimulation with 2-MeSADP (Table 1 and Fig. 2). However, when an EL1 mutation containing Asn110 was combined with domain exchanges at the N-terminus, a significant gain in UDP potency was observed (Fig. 6). For a similar combination of mutation of EL1 with a domain from EL3, the effect was less pronounced (Fig. 6). Furthermore, for constructs substituted in EL1 and TM3, an increased tendency for stimulation by UDP was evident for those constructs with additional exchange in position 110 (Table 1). This suggested that amino acids in TM2 and TM3 might interact directly in ligand recognition. An interaction of TM2 and TM3 in ligand recognition was found for the dopamine D2 receptor [40]. Additionally, in the human A2A adenosine receptor, selectivity for the base moiety of adenine was shown to involve amino acids of the exofacial part of TM3 [37]. Our present study further indicates a more complex interaction between these different domains in ligand recognition. When either domain 110–133 or 111–133 was combined with domain 284–298 we did not observe a significant difference in potency of UDP, neither when compared to the single domain constructs nor when both combined constructs were compared to each other (Fig. 4). However, when domain 110–133 or 111–133 was combined with domain 37–61, we did observe a significant difference in receptor activation by UDP, not only when compared to the single domain constructs, but more strikingly as well when both combined constructs were compared to each other (Fig. 5). This leads to the assumption that the amino acid in position 110 might interact with portions of TM1 and TM3 in receptor activation stimulated by UDP.
In summary, replacement of the N-terminus or EL2 of the P2Y1 receptor resulted in low (~50 μM) potency of the agonist 2-MeSADP. This was consistent with previous studies indicating the importance of EL2 of GPCRs in recognition of small molecules. The loss of function resulting from the replacement of a small region of the N-terminal domain (e.g. 37–46) was substantial. Nevertheless, based on the rhodopsin structure and GPRC modeling it is unlikely that the relevant regions in the N-terminal domain and EL1 are involved in direct contact with the ligand while bound in the principal binding site [15,19], but rather affect the function of the receptor through overall structural perturbation or may possibly be involved in meta-binding sites for nucleotides. Upon replacement of several regions, the potency of the P2Y1 agonist 2-MeSADP was either 1–2 μM (N-terminus and EL1, or EL1 and EL3) or 72 μM (N-terminus and EL3). Concurrent replacement of three regions (N-terminus, EL1, and EL3) completely precluded activation by 2-MeSADP. The gain of function (i.e. ability of a mutant ADP receptor to recognize UDP) upon substitution of multiple exofacial regions of the receptor was only moderate although significant. These findings argue against recognition of uracil nucleotides in P2Y receptors occurring exclusively within the extracellular regions. Thus, nucleotide recognition must involve a combination of extracellular and transmembrane domains, possibly acting in concert but not necessarily simultaneously. The ligand may bind initially to the exofacial region and then move to a binding site in the TM regions. An extension of this work might be the replacement of all of the extracellular domains, as was done for P2Y1/2 receptor chimeras [32]. It is becoming clear that the extracellular domains of GPCRs have a role in recognition of small molecule ligands, which are thought to bind primarily within the TMs. For example, an ectopic activation site on the M1 muscarinic receptor was recently discovered [41]. The ability to bind a selective ligand for M1 muscarinic receptor could be transferred to the M5 muscarinic receptor when the M5 receptor contained portions of the N-terminus, TM1, and EL3 of the M1 receptor. Thus, the involvement of extracellular domains in GPCRs activated by endogenous small molecules might be a more common feature than previously anticipated.
Acknowledgments
We thank Dr. Ivar von Kügelgen and Dr. Jürgen Wess for helpful discussions and David F. Johnson for technical assistance. We thank Prof. R. Nicholas (University of North Carolina) for the construct coding for the rat P2Y6 receptor. We thank Brian A. Harris (NIDDK) for critically proofreading the manuscript.
Abbreviations
- 2-MeS-ADP
2-methylthioadenosine-5′-diphosphate
- DMEM
Dulbeccos modified Eagles medium
- EL
extracellular loop
- ELISA
enzyme-linked immunosorbent assay
- FBS
fetal bovine serum
- GPCR
G protein-coupled receptor
- HA
hemagglutinin
- PBS
phosphate buffered saline
- PCR
polymerase chain reaction
- PLC
phospholipase C
- TM
(helical) transmembrane domain
- UDP
uridine-5′-diphosphate
References
- 1.Fredholm BB, Abbracchio MP, Burnstock G, Dubyak GR, Harden TK, Jacobson KA, et al. Towards a revised nomenclature for P1 and P2 receptors. Trends Pharmacol Sci. 1997;18:79–82. doi: 10.1016/s0165-6147(96)01038-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jacobson KA, Jarvis MF, Williams M. Purine pyrimidine (P2) receptors as drug targets. J Med Chem. 2002;45:4057–93. doi: 10.1021/jm020046y. [DOI] [PubMed] [Google Scholar]
- 3.Palmer RK, Boyer JL, Schachter JB, Nicholas RA, Harden TK. Mol Pharmacol. 1998;54:1118–23. [PubMed] [Google Scholar]
- 4.Barnard EA, Webb TE, Simon J, Kunapuli SP. The diverse series of recombinant P2Y purinoceptors. Ciba Found Symp. 1996;198:166–80. doi: 10.1002/9780470514900.ch10. [DOI] [PubMed] [Google Scholar]
- 5.Schachter JB, Boyer JL, Li Q, Nicholas RA, Harden TK. Fidelity in functional coupling of the rat P2Y1 receptor to phospholipase C. Br J Pharmacol. 1997;122:1021–4. doi: 10.1038/sj.bjp.0701479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Leon C, Hechler B, Freund M, Eckly A, Vial C, Ohlmann P, et al. Defective platelet aggregation and increased resistance to thrombosis in purinergic P2Y1 receptor-null mice. J Clin Invest. 1999;104:1731–7. doi: 10.1172/JCI8399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jin J, Daniel JL, Kunapuli SP. Molecular basis for ADP-induced platelet activation. Part II. The P2Y1 receptor mediates ADP-induced intracellular calcium mobilization and shape change in platelets. J Biol Chem. 1998;273:2030–4. doi: 10.1074/jbc.273.4.2030. [DOI] [PubMed] [Google Scholar]
- 8.Hechler B, Leon C, Vial C, Vigne P, Frelin C, Cazenave JP, et al. The P2Y1 receptor is necessary for adenosine 5′-diphosphate-induced platelet aggregation. Blood. 1998;92:152–9. [PubMed] [Google Scholar]
- 9.Fagura MS, Dainty IA, McKay GD, Kirk IP, Humphries RG, Robertson MJ, et al. P2Y1-receptors in human platelets which are pharmacologically distinct from P2Y(ADP)-receptors. Br J Pharmacol. 1998;124:157–64. doi: 10.1038/sj.bjp.0701827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Li Q, Olesky M, Palmer RK, Harden TK, Nicholas RA. Evidence that the p2y3 receptor is the avian homologue of the mammalian P2Y6 receptor. Mol Pharmacol. 1998;54:541–6. doi: 10.1124/mol.54.3.541. [DOI] [PubMed] [Google Scholar]
- 11.Warny M, Aboudola S, Robson SC, Sevigny J, Communi D, Soltoff SP, et al. P2Y6 nucleotide receptor mediates monocyte interleukin-8 production in response to UDP or lipopolysaccharide. J Biol Chem. 2001;276:26051–6. doi: 10.1074/jbc.M102568200. [DOI] [PubMed] [Google Scholar]
- 12.Kim SG, Soltysiak KA, Gao ZG, Chang TS, Chung E, Jacobson KA. Tumor necrosis factor α-induced apoptosis in astrocytes is prevented by the activation of P2Y6, but not P2Y4 nucleotide receptors. Biochem Pharmacol. 2003;65:923–31. doi: 10.1016/s0006-2952(02)01614-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hou M, Harden TK, Kuhn CM, Baldetrop B, Lazarowski E, Pendergast W, et al. UDP acts as a growth factor for vascular smooth muscle cells by activation of P2Y6 receptors. Am J Physiol Heart Circ Physiol. 2002;282:784–92. doi: 10.1152/ajpheart.00997.2000. [DOI] [PubMed] [Google Scholar]
- 14.Moro S, Guo D, Camaioni E, Boyer JL, Harden TK, Jacobson KA. Human P2Y1 receptor: molecular modeling and site-directed mutagenesis as tools to identify agonist and antagonist recognition sites. J Med Chem. 1998;41:1456–66. doi: 10.1021/jm970684u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jacobson KA, Costanzi S, Ohno M, Joshi BV, Besada P, Xu B, et al. Molecular recognition at purine and pyrimidine nucleotide (P2) receptors. Curr Trends Med Chem. 2004;4:805–19. doi: 10.2174/1568026043450961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Costanzi S, Mamedova L, Gao ZG, Jacobson KA. Architecture of P2Y nucleotide receptors: structural comparison based on sequence analysis, mutagenesis, and homology modeling. J Med Chem. doi: 10.1021/jm049914c. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jiang Q, Guo D, Lee BX, van Rhee M, Kim YC, Nicholas RA, et al. A mutational analysis of residues essential for ligand recognition at the human P2Y1 receptor. Mol Pharmacol. 1997;52:499–507. doi: 10.1124/mol.52.3.499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hoffmann C, Moro S, Nicholas RA, Harden TK, Jacobson KA. The role of amino acids in extracellular loops of the human P2Y1 receptor in surface expression and activation processes. J Biol Chem. 1999;274:14639–47. doi: 10.1074/jbc.274.21.14639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Moro S, Hoffmann C, Jacobson KA. Role of the extracellular loops of G protein-coupled receptors in ligand recognition: a molecular modeling study of the human P2Y1 receptor. Biochemistry. 1999;38:3498–507. doi: 10.1021/bi982369v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Higuchi R. Using PCR to engineer DNA. In: Ehrlich HA, editor. PCR technology. New York: Stockton Press; 1989. pp. 61–70. [Google Scholar]
- 21.Sanger R, Nicklen S, Coulson AR. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci USA. 1977;74:5463–7. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Robeva AS, Woodard R, Luthin DR, Taylor HE, Linden J. Double tagging recombinant A1- and A2A-adenosine receptors with hexahistidine and the FLAG epitope. Development of an efficient generic protein purification procedure. Biochem Pharmacol. 1996;51:545–55. doi: 10.1016/0006-2952(95)02235-x. [DOI] [PubMed] [Google Scholar]
- 23.Cullen BR. Use of eukaryotic expression technology in the functional analysis of cloned genes. Methods Enzymol. 1987;152:684–704. doi: 10.1016/0076-6879(87)52074-2. [DOI] [PubMed] [Google Scholar]
- 24.Harden TK, Hawkins PT, Stephens L, Boyer JL, Downes P. Phosphoinositide hydrolysis by guanosine-(γ-thio)triphosphate activated phospholipase C of turkey erythrocyte membranes. Biochem J. 1988;252:583–93. doi: 10.1042/bj2520583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Berridge MJ, Dawson RM, Downes CP, Heslop JP, Irvine RF. Changes in the levels of inositol phosphates after agonist-dependent hydrolysis of membrane phosphoinositides. Biochem J. 1983;212:473–82. doi: 10.1042/bj2120473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Herold CL, Li Q, Schachter JB, Harden TK, Nicholas RA. Lack of nucleotide-promoted second messenger signaling responses in 1321N1 cells expressing the proposed P2Y receptor, p2y7. Biochem Biophys Res Commun. 1997;235:717–21. doi: 10.1006/bbrc.1997.6884. [DOI] [PubMed] [Google Scholar]
- 27.Nicholas RA, Watt WC, Lazarowski ER, Li Q, Harden TK. Uridine nucleotide selectivity of three phospholipase C-activating P2 receptors: identification of a UDP-selective, a UTP-selective, and an ATP-and UTP-specific receptor. Mol Pharmacol. 1996;50:224–9. [PubMed] [Google Scholar]
- 28.Lazarowski ER, Homolya L, Boucher RC, Harden TK. Identification of an ectonucleoside diphosphokinase and its contribution to inter-conversion of P2 receptor agonists. J Biol Chem. 1997;272:20402–7. doi: 10.1074/jbc.272.33.20402. [DOI] [PubMed] [Google Scholar]
- 29.Filtz TM, Harden KT, Nicholas RA. Structure, pharmacological selectivity, and second messenger signaling properties of G protein-coupled P2-purinergic receptors. In: Jacobson KA, Jarvis MF, editors. Purinergic approaches in experimental therapeutics. Wiley; 1997. pp. 39–53. [Google Scholar]
- 30.Stillman BA, Breyer MD, Breyer RM. Importance of the extracellular domain for prostaglandin EP2 receptor function. Mol Pharmacol. 1999;56:545–51. doi: 10.1124/mol.56.3.545. [DOI] [PubMed] [Google Scholar]
- 31.Palczewski K, Kumasaka T, Hori T, Behnke CA, Motoshima H, Fox BA, et al. Crystal structure of rhodopsin: a G protein-coupled receptor. Science. 2000;289:739–45. doi: 10.1126/science.289.5480.739. [DOI] [PubMed] [Google Scholar]
- 32.Gearing KL, Barnes A, Barnett J, Brown A, Cousens D, Dowell S, et al. Complex chimeras to map ligand binding sites of GPCRs. Protein Eng. 2003;16:365–72. doi: 10.1093/protein/gzg045. [DOI] [PubMed] [Google Scholar]
- 33.Herold CL, Qi AD, Harden TK, Nicholas RA. Agonist versus antagonist action of ATP at the P2Y4 receptor is determined by the second extracellular loop. J Biol Chem. 2004;279:11456–64. doi: 10.1074/jbc.M301734200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kim HS, Ravi RG, Marquez VE, Maddileti S, Wihlborg A-KK, Erling D, et al. Methanocarba modification of uracil and adenine nucleotides: High potency of Northern ring conformation at P2Y1, P2Y2, or P2Y4 and P2Y11, but not P2Y6 receptors. J Med Chem. 2002;45:208–18. doi: 10.1021/jm010369e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hawtin SR, Wesley VJ, Parslow RA, Patel S, Wheatley M. Critical role of a subdomain of the N-terminus of the V1a vasopressin receptor for binding agonists but not antagonists; functional rescue by the oxytocin receptor N-terminus. Biochemistry. 2000;39:13524–33. doi: 10.1021/bi0013400. [DOI] [PubMed] [Google Scholar]
- 36.Gao Z-GG, Jiang Q, Jacobson KA, IJzerman AP. Site-directed mutagenesis studies of human A2A adenosine receptors. Involvement of Glu13 and His278 in ligand binding and sodium modulation. Biochem Pharmacol. 2000;60:661–8. doi: 10.1016/s0006-2952(00)00357-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rivkees SA, Barbhaiya H, IJzerman AP. Identification of the adenine binding site of the human A1 adenosine receptor. J Biol Chem. 1999;274:3617–21. doi: 10.1074/jbc.274.6.3617. [DOI] [PubMed] [Google Scholar]
- 38.Ballesteros JA, Weinstein H. Integrated methods for the construction of three dimensional models and computational probing of structure-function relations in G-protein coupled receptors. Methods Neurosci. 1995;25:366–428. [Google Scholar]
- 39.van Rhee AM, Jacobson KA. Molecular architecture of G protein-coupled receptors. Drug Devel Res. 1996;37:1–38. doi: 10.1002/(SICI)1098-2299(199601)37:1<1::AID-DDR1>3.0.CO;2-S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Javitch JA, Ballesteros JA, Chen J, Chiappa V, Simpson MM. Electrostatic and aromatic microdomains within the binding-site crevice of the D2 receptor: contributions of the second membrane-spanning segment. Biochemistry. 1999;38:7961–98. doi: 10.1021/bi9905314. [DOI] [PubMed] [Google Scholar]
- 41.Spalding TA, Trotter C, Skjaerbaek N, Messier TL, Currier EA, Burstein ES, et al. Discovery of an ectopic activation site on the M(1) muscarinic receptor. Mol Pharmacol. 2002;61:1297–302. doi: 10.1124/mol.61.6.1297. [DOI] [PubMed] [Google Scholar]