Abstract
BACKGROUND
The role of nonmyeloablative allogeneic stem cell transplantation (NST) in the treatment of chronic lymphocytic leukemia (CLL) is not well established. The authors report on long-term experience with NST in relapsed/refractory CLL and define prognostic factors associated with outcome.
METHODS
The authors reviewed the outcome of 86 patients with relapsed/relapsed CLL enrolled in sequential NST protocols.
RESULTS
The median patient age was 58 years. Patients were heavily pretreated before transplantation, and 43 required immunomanipulation after NST for persistent or recurrent disease. Immunomanipulation included withdrawal of immunosuppression, rituximab, and step-wise donor lymphocyte infusions. Of 43 patients receiving immunomanipulation, 20 (47%) experienced a complete remission. Patients with human leukocyte antigen (HLA) genotype A1+/A2−/B44− were more likely to experience a complete remission (P ¼ .0009), with rates of 9%, 36%, 50%, and 91%, respectively, for 0, 1, 2, and 3 of these HLA factors. This resulted in significant improvement in progression-free-survival rates of 68.2% at 5 years for patients with all 3 HLA factors. Overall, the estimated 5-year survival rate was 51%. In a multivariate model, a CD4 count of <100/mm3 and a below normal serum immunoglobulin G level at study entry were associated with a short survival duration (P < .0001).
CONCLUSIONS
These results confirm the potential cure of relapsed/refractory CLL with NST and provide the first evidence that immunoglobulin G and CD4 levels are predictive of overall survival after NST in CLL and that human leukocyte antigen alleles predict response to immunomanipulation.
Keywords: chronic lymphocytic leukemia, human leukocyte histocompatibility antigen, nonmyeloablative allogeneic transplantation, stem cell transplantation
Allogeneic stem cell transplantation (SCT) after nonmyeloablative SCT (NST) or reduced-intensity regimens has resulted in long-term remissions in a subset of patients with advanced chronic lymphocytic leukemia (CLL) who develop resistance to conventional treatments.1-4 NST can overcome adverse prognostic factors such as the presence or absence of somatic mutations in the immunoglobulin heavy-chain variable region,5,6 Zeta chain-associated protein 70 kDa expression,7 and the presence of chromosomal aberrations, including 17p13.1 deletion.6,8 Recent studies have suggested that patients’ hematopoietic SCT comorbidity index,4 tumor bulk, age, and disease refractoriness were important determinants of survival.
Patients with CLL are typically severely immunosuppressed because of prior therapy with purine analogs9 and alemtuzumab.10 This pretransplantation immunodeficiency may be significant, but information about its effect on overall survival after NST has not been well studied.
The effectiveness of allogeneic NST in CLL has been attributed to a graft-versus-leukemia (GVL) effect because of elimination of tumor cells by alloimmune effector lymphocytes.5,11 In patients with aggressive disease, tumor growth may outpace the development of effector T cells. In a previous study, we found that when patients with leukemia develop relapse after allogeneic NST, immunomanipulation via withdrawal of immuno-suppression and donor lymphocyte infusion (DLI) with rituximab can induce sustained remissions in some patients.12 The underlying mechanism of this GVL effect is unknown. The simultaneous presence of the killer immunoglobulinlike receptor 3DL1 and its corresponding human leukocyte antigen (HLA) class I ligands bearing the Bw4 epitope has been described in CLL.13 Donor T cells that recognize a host-derived minor histocompatibility antigen on the cell surface of major histocompatibility complex class I and class II molecules have been implicated in both graft-versus-host disease (GVHD) and GVL reactions in patients with hematologic malignancies.14,15
This report updates our long-term experience with allogeneic NST in CLL, including the effect of pretrans-plantation immunodeficiency and hematopoietic SCT comorbidity index. Considering that the HLA class I molecules may act as restriction elements for GVL targets after NST in CLL, we also investigated the potential association of certain common class I HLA alleles and long-term outcome.
MATERIALS AND METHODS
Patient Population and Disease Assessment
All patients with CLL who had undergone NST in sequential phase 2 protocols at The University of Texas MD Anderson Cancer Center (Houston, Tex) from February 1996 to August 2007 were analyzed. The protocols had been approved by the institutional review board at our center, and all patients gave informed consent for enrollment on these studies.
Eligibility criteria for these protocols included age 19 to 70 years and relapsed CLL with demonstrated resistance to purine analog-based chemoimmunotherapy. Post-transplantation responses were scored according to recommendations from the National Cancer Institute-Sponsored Working Group.16,17 Disease extent was further assessed by computed tomography (CT) scans of the chest, abdomen, and pelvis and gallium scanning or positron emission tomography. Patients were evaluated at 1, 3, 6, and 12 months after transplantation or DLI, and thereafter every 6 months.
HLA Alleles Studied and Typing
In our analysis, we examined the most common alleles and serotypes expressed in the patients studied. These included HLA-A1, A2, A3, A24, B7, B8, B35, B44, B60, B62, BW4, BW6, and CW7. These allele groups are known to be common in most world populations. We chose to include only common HLA alleles and groups because if we examined other less common alleles, the statistical power would be significantly weaker.
Patients and donors were typed for alleles at HLA-A and HLA-B, HLA-C, DRB1, DRB3/4/5, and DQB1 by polymerase chain reaction (PCR) amplification and oligo-nucleotide hybridization using commercial kits from Invitrogen (Carlsbad, Calif), ELPHA (Hercules, Calif), or One Lambda (Canoga Park, Calif) that result in intermediate resolution. Patients were also typed for these loci using high-resolution methods with PCR amplification and nucleotide sequencing (Abbott, Abbott Park, Ill). HLA haplotypes were assigned by analyzing the segregation of HLA alleles as genetically transmitted units. The parental origin of haplotypes was then ascertained. HLA matching was evaluated from the intermediate-resolution and high-resolution results of patients and donors.
Immunohistochemical Detection of P53 and P21
It has been suggested that a p53+/p21− pattern of immunohistochemical expression is reliable as a surrogate for TP53 mutation in CLL.18 We retrospectively performed immunohistochemical studies of 200 nuclei using routinely fixed and processed bone marrow trephine biopsy samples and heat-induced epitope retrieval, as described previously.19 We chose bone marrow samples that were collected within 3 months pre-NST. All cases were scored independently by at least 2 researchers. Neoplasms (PAX 5+) with p53 staining in >20% of tumor cells were considered positive. Neoplasms with p21 staining in <5% of tumor cells were considered negative, as previously suggested.18
Preparative Regimens, Transplantation, and Post-Transplantation Immunomanipulation
Details of the preparative regimens, supportive care, and infection and GVHD prophylaxis and early results have been previously published.1,7,12 The predominant preparative regimen used was 30 mg/m2 of fludarabine, 750 mg/m2 of cyclophosphamide given intravenously on Days −5 to −3 before transplantation, and 375 mg/m2 of rituximab on Day −13 and 1000 mg/m2 on Days −6, + 1, and +8 (n = 78). Eight patients received other preparative regimens (fludarabine, melphalan, and rituximab [n = 6] and rituximab, carmustine, etoposide, cytarabine, and melphalan [n = 2]). Forty-three (50%) patients received transplants from histocompatible siblings, and 43 (50%) who had no matched sibling donors received transplants from unrelated donors. GVHD prophylaxis consisted of tacrolimus and methotrexate (5 mg/m2 on Days +1, +3, and +6, with an additional dose on Day +11 for nonsibling transplants) as previously described.1,7,12
Post-transplantation immunomanipulation (with-drawal of immunosuppression, infusion rituximab, and step-wise DLI) was performed in patients with progressive or residual disease using previously established methods.7,12 Rituximab was given at a dose of 375 mg/m2 intravenously, followed by 3 weekly doses of 1000 mg/m2. A DLI of 1 × 106 CD3-positive T cells/kg was administered after the first 2 doses of rituximab if no GVHD occurred. An escalated DLI dose was given at 6-week intervals if there was persistent active disease and no GVHD. DLIs were not routinely administered in patients with stable mixed chimerism if they remained in remission. Acute and chronic GVHD was graded according to consensus criteria.20 GVHD after DLI was graded according to National Institutes of Health consensus criteria.21
Immune Assays During Immunomanipulation
To determine the immune parameters related to response, we monitored natural killer (NK) activity in 13 patients who had undergone immunomanipulation. This prospective analysis was undertaken on heparinized blood samples collected at 5 time points: prestudy entry, before each of the first 2 doses of rituximab, before DLI, and then after the last dose of rituximab given with immunomanipulation. DLIs were provided between the second and third doses of rituximab. The blood samples were obtained according to an institutional review board-approved protocol at The University of Texas MD Anderson Cancer Center.
Statistical Analysis
Disease and transplantation characteristics were compared by using Fisher exact test for categorical variables and the Mann-Whitney U test for continuous variables. Actuarial estimates of overall survival (OS) rates were calculated using the Kaplan-Meier method.22 The incidences of disease progression and GVHD were estimated using the cumulative incidence method,23 considering death with disease and death without GVHD as competing risks. The current progression-free survival (PFS) rate, accounting for salvage therapy after DLI, was estimated using a linear combination of Kaplan-Meier estimates, as described by Klein et al.24 Risk factors for disease progression and death were evaluated using Cox proportional hazards. Logistic regression was used to assess predictors of response to immunomanipulation. Exact logistic regression was used in analyses involving a small number of events. For proportional hazards and logistic regression analyses, univariate models were fit initially, and all factors that were statistically significant were fit jointly in multivariate analyses. Model selection was performed by backward stepwise regression.25 The maximum statistically significant P value was .05.
RESULTS
Study Group
Eighty-six patients (70 men and 16 women) were analyzed. Before transplantation, patients had been exposed to the therapies listed in Table 1. Of the 86 patients studied, 71 (83%) had a history of purine-analog refractoriness according to the National Cancer Institute/International Workshop on Chronic Lymphocytic Leukemia definition.17 In addition, 64 (74%) patients were resistant to chemoimmunotherapy (>first salvage, nonresponders, or relapse within 1 year of therapy). However, because of successful use of salvage chemotherapy, only 15 (17%) patients had refractory (but low volume disease) at transplantation. Combinations such as fludarabine, cyclophosphamide, rituximab, and alemtuzumab; oxaliplatin, fludarabine, cytarabine, and rituximab; and rituximab, cyclophosphamide, vincristine, and prednisone with doxorubicin were used in CLL patients who did not experience a response to traditional treatments such as fludarabine and rituximab, with or without cyclophosphamide, patients with bulky disease of >5 cm in diameter, and patients with suspected Richter transformation.
Table 1.
Patient and Donor Characteristics
| Characteristic | Patients, n = 86 |
|---|---|
| Median age, y [range] | 58 [36-70] |
| Sex, No. (%) | |
| Male | 70 (81) |
| Female | 16 (19) |
| Median time from initial diagnosis, mo [range] | 62 [6-307] |
| Richter transformation, No. (%) | 19 (22) |
| Binet stage B and C, No. (%) | 55/65 (85) |
| Median CD5/CD19+ in marrow, % [range] | 29 [0-94] |
| PET/gallium positive at SCT, No. (%) | 26/78 (33) |
| β2-microglobulin ≥3 mg/L, No. (%) | 43/84 (51) |
| High lactate dehydrogenase, No. (%) | 36 (42) |
| Hematopoietic SCT comorbidity index distribution, No. (%) | |
| 0 | 14 (16) |
| 1 | 18 (21) |
| 2 | 10 (12) |
| 3 | 19 (22) |
| 4 | 11 (13) |
| 5-8 | 14 (16) |
| B-symptoms present, No. (%) | 21/76 (28) |
| ZAP70+, No. (%) | 45/56 (80) |
| Unmutated immunoglobulin heavy chain variable region | 28/39 (72) |
| p53+/p21- on immunohistochemical analysis, No. (%) | 15/65 (23) |
| IgG below normal at SCT, No. (%) | 56/84 (67) |
| IgA below normal at SCT, No. (%) | 64/82 (78) |
| IgM below normal at SCT, No. (%) | 41/82 (50) |
| CD4 ≤100/mm3 at SCT, No. (%) | 18/68 (26) |
| CD4 ≤100/mm3 and IgG below normal, No. (%) | 15/68 (22) |
| Purine-analog refractory, No. (%) | 71 (83) |
| No. of patients exposed to the following therapies: | |
| FCR | 58 |
| FR/PCR | 16/5 |
| CFAR or OFAR | 20 |
| R-Hyper-CVAD | 26 |
| R-CHOP/ESHAP | 21/4 |
| F | 26 |
| FC | 34 |
| Chlorambucil | 19 |
| R+high-dose methylprednisolone | 9 |
| R+alemtuzumab | 19 |
| Alemtuzumab alone | 10 |
| Autologous SCT | 2 |
| Other | 28 |
Abbreviations: CFAR, fludarabine, cyclophosphamide, rituximab, alemtuzumab; ESHAP, etoposide, methylprednisolone, cytarabine, cisplatin; F, fludarabine; FC, fludarabine, cyclophosphamide; FCR, fludarabine, cyclophosphamide, rituximab; FR, fludarabine, rituximab; Ig, immunoglobulin; OFAR, oxaliplatin, fludarabine, cytarabine, rituximab; PCR, pentostatin, cyclophosphamide, rituximab; PET, positron emission tomography; R-CHOP, rituximab, cyclophosphamide, doxorubicin, vincristine, prednisone/prednisolone; R-Hyper-CVAD, rituximab, cyclophosphamide, vincristine, prednisone; SCT, stem cell transplantation.
Clinical Outcome
A median of 4.8 × 106/kg CD34-positive cells and 150 × 106/kg CD3-positive cells were infused. Eighty-three of the 86 patients experienced donor cell engraftment. The median values of donor T cells and myeloid cells by Day 90 to 100 after transplantation were 92% (range, 0%-100%) and 97% (range, 0%-100%), respectively. One patient who experienced a primary graft failure had auto-logous hematopoietic recovery. Two other patients were not tested for donor cell engraftment because of early death; 1 died of a flare-up of a pre-existing fungal infection, and the other died of a choking accident while eating.
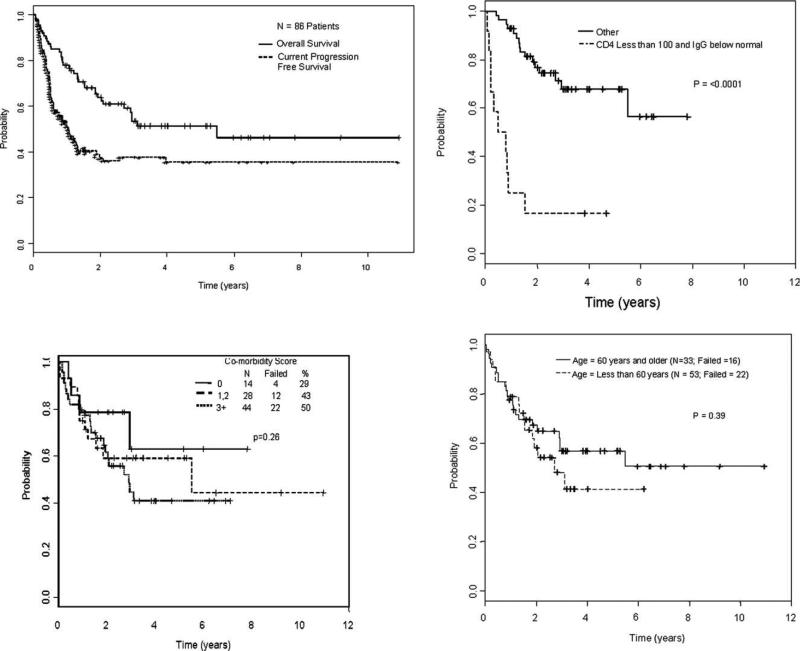
The median follow-up duration of surviving patients was 37.2 months (range, 11.4-131.1 months). The median follow-up time from diagnosis to transplantation was 62 months (range, 6-307 months). The median follow-up time from diagnosis to last follow-up for survivors was 106 months (range, 23-361 months). The 1-year, 3-year, and 5-year actuarial OS rates were 78% (95% confidence interval [CI], 0.68-0.85), 53% (95% CI, 0.41-0.64), and 51% (95% CI, 0.39-0.62), respectively. The 1-year, 3-year, and 5-year current PFS rates were 51% (95% CI, 0.40-0.60), 38% (95% CI, 0.27-0.48), and 36% (95% CI, 0.25-0.46), respectively (Fig. 1A). Clinical, molecular, and HLA factors were analyzed to determine their association with OS and PFS (Table 2). In univariate models, CD4 counts <100/mm3, below normal immunoglobulin G (IgG) levels, severe acute GVHD, and the presence of HLA-A24 expression were associated with a shorter survival duration (Table 3). Severe immunosuppression did not correlate with the number of prior chemotherapies the patients had received pretransplantation nor with their prior exposure to alemtuzumab.
Figure 1.
(A) Overall survival (OS)and current progression-free survival rates of 86 chronic lymphocytic leukemia patients who underwent nonmyeloablative stem cell transplantation are shown. OS is shown (B) according to CD4/mm3 and immunoglobulin G (IgG) levels at the time of study entry, (C) according to the hematopoietic stem cell transplantation comorbidity-index, and (D) according to age (< or 60 years).
Table 2.
Factors Included in the Outcome Analysis
| Age | CD5, CD19 at study entry | CD3 infused |
|---|---|---|
| Sex | % marrow lymphocytes | Neutrophil recovery |
| B-symptoms at study entry | IgG level at study entry | Platelet recovery |
| Hematopoietic SCT comorbidity index | IgM level at study entry | Donor Tcells at Day 90 |
| Prior alemtuzumab | IgA level at study entry | Donor myeloid cells at Day 90 |
| Total prior treatments | CD8 levels at study entry | Acute GVHD risk |
| Refractory vs sensitive disease at study entry | CD4 level at study entry | Chronic GVHD risk |
| Stage at study entry | Mutational status of immunoglobulin heavy chain variable region | Donor age |
| PET/gallium at study entry | p53 by immunohistochemical analysis | ABO mismatch between donor and patient |
| White blood cell count at study entry | Conditioning regimen | Time from diagnosis to transplantation |
| Hb <11 at study entry | Cell source (blood vs marrow) | Cytomegalovirus status of donor and patient |
| Platelets < 100,000 at study entry | Sibling vs nonsibling donor | Sex mismatch between donor and patient |
| 132-microglobulin at study entry | CD34 infused | |
| Lactate dehydrogenase at study entry |
Abbreviations: GVHD, graft-versus-host disease; Hb, hemoglobin; Ig, immunoglobulin; PET, positron emission tomography.
Human leukocyte antigen subtypes: A1, A2, A3, A24, B7, B8, B35, B44, B60, B62, BW4, BW6, CW7, and KIR2DL1, 2DL2, or 2DL3 ligand.
Table 3.
Summary of Results From OS Cox Model
| Group | Univariate Analysis | Multivariate Analysis | ||
|---|---|---|---|---|
| Median, mo | P | Hazard Ratio | P | |
| CD4 ≤100 and IgG below normal (vs CD4 >100 and normal IgG) | a vs 8 | <.0001 | 7.1 | <.0001 |
| IgG below normal vs normal level | 25 vs a | .001 | ||
| CD4 >100 vs ≤100 | 66 vs 10 | .005 | ||
| 0-2 vs 3 or 4 acute GVHD | 66 vs 7 | .009 | ||
| Matched vs mismatched allogeneic type | 66 vs 6 | .001 | ||
| HLA-A24 absence (n = 68) | a vs 17 | .038 | ||
Abbreviations: GVHD, graft-versus-host disease; HLA, human leukocyte antigen; IgG, immunoglobulin G.
Indicates the median survival not reached.
In a multivariate model, the major determinant of survival was CD4 counts <100/mm3 and below normal IgG levels at the time of transplantation. The 5-year OS rates were 68% (95% CI, 0.51-0.80) versus 17% (95% CI, 0.03-0.41; P < .0001; Fig. 1B) for patients with CD4 counts >100/mm3 and normal IgG levels versus those with CD4 counts <100/mm3 and below normal IgG levels at the time of study entry, respectively (Fig. 1B).
Outcome According to Comorbidity Index and Age
In our study, 33 (38.4%) patients were ≥60 years of age. In addition, 44 (51%) patients had a hematopoietic SCT comorbidity index of ≥3, including 14 (16%) with an index of 5 to 8. We found that the hematopoietic SCT comorbidity index (Fig. 1C) and age (Fig. 1D) were not associated with survival (P = .25 and .39, respectively) or PFS (P = .73 and .95, respectively).
Outcome According to P53+/P21−
Sixty-five patients were tested for p53 and p21. Fifteen (23%) were p53+/p21− . We found that the expression of p53+/p21− did not predict OS rates, immunomanipulation response, or current PFS.
Immunomanipulation, Independent Prognostic Factors
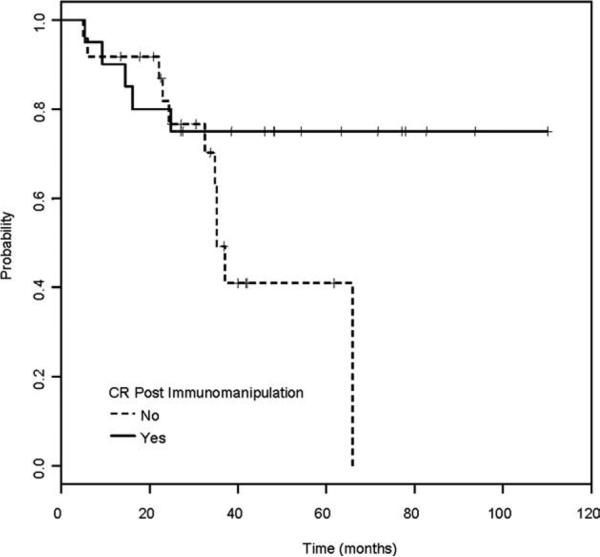
Patients had advanced and heavily pretreated disease at transplantation; therefore, immunomanipulation was considered necessary in some patients. In our study, 43 patients required immunomanipulation after NST, because of persistent disease (n = 4) or disease progression (n = 39). The median time for needing immunomanipulation was 183 days (range, 63-909 days [only 2 patients were beyond 18 months]) after NST. The median number of DLIs received was 2 (range, 1-6); the median maximal dose was 16.8 × 106 CD3+/kg (range, 1-200 × 106 CD3+/kg). Of the 43 patients who underwent immuno-manipulation, 20 (47%) experienced a complete response (CR). The median OS for these 20 CR patients has not yet been reached with a median follow-up time of 47 months (range, 5.4-110.2 months), whereas the median OS for the patients who did not achieve CR after immunomanipulation was 35 months (P = .08; Fig. 2). Taking into account the responses to immunomanipulation, a relapse rate of 39% (95% CI, 0.27-0.59) at 3 years was observed.
Figure 2.
Overall survival (OS) rate of 43 chronic lymphocytic leukemia patients after receiving immunomanipulation is shown according to response (solid line, complete response [CR]; dotted line, no CR). The median OS for CR patients (median follow-up time of 47 months [range, 5.4-110.2 months]) from the time of immunomanipulation has not been reached yet, whereas the median OS for the patients who did not achieve CR to immunomanipulation was 35 months (P = .08).
Patient characteristics at the time of study entry and at the time of initiation of immunomanipulation (Table 2), lymph node size (≥3 vs <3 cm by CT scans), DLI doses and numbers, chimerism, GVHD, and HLA alleles or groups of alleles were assessed for an association with CR. The major determinants of CR after immunomanipulation included a peripheral blood progenitor cell graft and good (≥90%) donor chimerism at Day 90 (P = .035) and being HLA-A1 positive/HLA-A2 negative/HLA-B44 negative (P = .0009, Table 4). The rates of CR to immunomanipulation were 9%, 36%, 50%, and 91% in patients with 0, 1, 2, and 3 of the HLA factors described, respectively. To determine whether responses observed for HLA subtypes were independent of or related to GVHD, we analyzed the incidence of acute and chronic GVHD for the HLA-A1, HLA-A2, and HLA-B44 subtypes, and found no statistically significant difference among the groups.
Table 4.
Predictors of Response to Immunomanipulation
| Group | Univariate Analysis | Multivariate Analysis | ||
|---|---|---|---|---|
| CR rate | P | Hazard Ratio | P | |
| HLA type | ||||
| HLA-A1 positive | 73 (vs 32) | .023 | ||
| HLA-A2 negative | 68 (vs 24) | .008 | ||
| HLA-B44 negative | 67 (vs 13) | .001 | ||
| HLA A1 positive, A2 negative, B44 negative | 91 (vs 9) | .017 | – | .0009 |
| Peripheral blood stem cell graft and best chimerism ≥90% (Day 90) | 100 (vs 36) | .035 | – | .035 |
Abbreviations: CR, complete response; HLA, human leukocyte antigen.
Current PFS
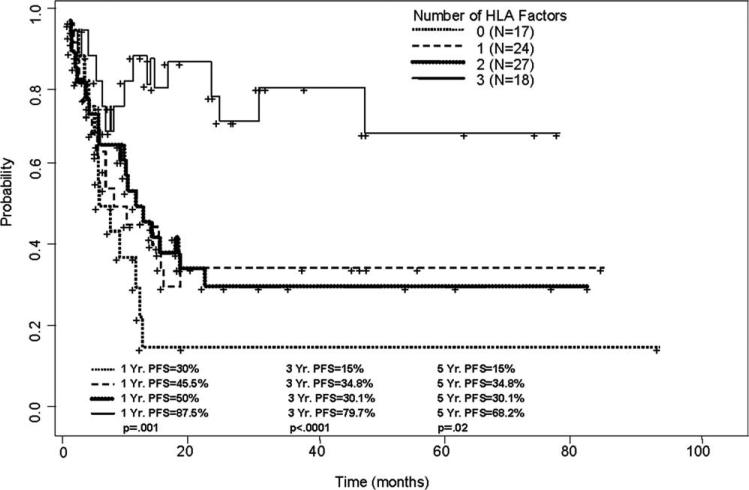
In univariate models, being HLA-A1 positive (P = .04), HLA-A2 negative (P = .014), HLA-B44 negative (P = .026), HLA-A1 positive/HLA-A2 negative/HLA-B44 negative (P < .001), or having peripheral blood graft and good chimerism (>90% donor T cells by Day 90) (P .007) were associated with improved current PFS. In multivariate analysis, patients with all 3 HLA factors (79.7% vs 15% [at 3 years] for patients with 0 factors, P < .0001; Fig. 3) and patients who had peripheral blood graft and good chimerism (61% versus 35.7% [at 3 years], P = .001, for patients who received marrow graft and had a suboptimal chimerism) were associated with improved current PFS.
Figure 3.
Current progression-free survival (PFS) rate of 86 chronic lymphocytic leukemia patients who underwent nonmyeloablative stem cell transplantation is shown according to human leukocyte antigen (HLA) subtype. Patients who were HLA-A1 positive, HLA-A2 negative, and HLA-B44 negative had higher current PFS rates than did other patients who had 0, 1, or only 2 of these HLA factors.
NK Cell Activity With Immunomanipulation
A linear mixed model was used to assess whether response had an effect on NK lysis. NK lysis was measured in 13 patients who had undergone immunomanipulation at 5 time points. We categorized patients as those who had experienced a response (n = 5) and those who had not (n = 8). There was evidence that response had an effect on NK lysis (P = .011). On average, responders’ NK lysis was 13 units higher than that of nonresponders. Four of the 5 responders were HLA-A1 positive/HLA-A2 negative/HLA-B44 negative.
Toxicity and GVHD
Overall, 36 patients died. Nonrelapse mortality rates at 100 days and at 1 year were 3% (95% CI, 0.9-0.39) and 17.4% (95% CI, 0.35-0.57), respectively. The causes of death varied according to IgG levels at study entry. Of the 28 patients with normal IgG levels, 4 (14%) died (disease progression [n = 1] and secondary malignancies [n = 3]). Of the 56 patients with below normal IgG levels at study entry, 32 (57%) died. The cause of death was related to infection in 15 (47%) cases, 5 of which occurred in the setting of GVHD. Five infections were atypical: 2 were related to encephalitis, 1 to viral meningitis, and 1 to Pneumocystis jiroveci pneumonia; 1 patient died of toxoplasmosis. Nine (28%) patients died of progressive disease, 3 (9%) patients died secondary to organ failure, 2 (6%) patients died of GVHD, and 2 (6%) patients had accidental deaths (1 patient choked while eating, 60 days after transplantation, and 1 had cerebrovascular bleeding after a fall that occurred in the setting of thrombocytopenia). One patient was lost to follow-up and died of unknown reasons 18 months after transplantation.
The incidences of acute grade II-IV and III-IV GVHD were 37% (95% CI, 0.27-0.47) and 7% (95% CI, 0.02-0.12), respectively. The cumulative incidence of extensive chronic GVHD at 60 months was 56% (95% CI, 0.45-0.68). One patient developed acute grade III GVHD, and 7 experienced extensive chronic GVHD after DLI.
DISCUSSION
In the past decade, the paradigm for allogeneic SCT for CLL has changed. Confidence in the power of the GVL effect has encouraged the use of transplantation after low-intensity, nonmyeloablative preparative regimens.26 Our study includes 86 patients with CLL treated with NST at a single institution and demonstrate the favorable results associated with allogeneic SCT in relapsed/refractory CLL. Among such patients, allogeneic SCT produced an estimated 5-year current PFS rate of 68.2% in patients who expressed HLA-A1+/A2− /B44− versus 15% for patients with none of the HLA factors described (P = .02).
Our study also describes the outcome of the largest series of patients who have received immunomanipulation after transplantation for persistent or recurrent disease. Among 43 such patients, the CR rate was 47% and was durable, confirming the results of our earlier reports.7 In our study, patients received rituximab concomitantly with DLI to enhance the GVL effect.27,28 Although responses of up to 54% have been observed with higher doses of rituximab in CLL patients,29 most of these have been partial responses of short duration. Our findings suggest that GVL effects of the engrafted donor immune system can induce long-term remissions, and possibly cures, in heavily pretreated patients with CLL.
Our preliminary data also provide the first evidence that besides several well-known clinical parameters, long-term outcome after rituximab and DLI may be influenced by genetic determinants such as the expression of certain class I HLA alleles. More specifically, we found that HLAA1 positive/A2 negative/Bw44 negative patients were most likely to experience a CR to immunomanipulation. The CR rate was 91% in patients who had this genotype versus 9% for those who lacked HLA-A1 and carried HLA-A2 and B44. These HLA molecules may act as GVL restriction elements by shaping the T-cell repertoire selection for GVL. In our study, we also found increased NK-mediated lysis in patients who experienced a response to immunomanipulation.
We also attempted to better define the risk factors for death in patients with CLL, using monoclonal antibodies and SCT. The Seattle research group4,30 suggested that comorbidities were more important than CLL-related variables for predicting OS and PFS; the researchers suggested that this was partly related to more deaths from acute GVHD among patients with comorbidity scores of >0 than among those with a score of 0. In our study, 25 (29%) patients had a comorbidity score of >3, whereas no Seattle patients were in that category.30 The incidence of GVHD in our study was not different between patients with different comorbidity scores or age groups (< vs ≥60 years).
It has been suggested that allogeneic transplantation in CLL should be reserved only for younger patients (<50 years) with no comorbidities.26 In our study, we found that neither of these factors had an impact on the transplant outcome, using the nonmyeloablative conditioning regimen and GVHD prophylaxis we proposed. Instead, we found that hypogammaglobulinemia and CD4 counts <100/mm3 had a major influence on survival. Patients with CLL are usually susceptible to infections because of hypogammaglobulinemia and defective T cells, which are both disease-related and therapy-related.31 In our study, 47% and 0% of deaths were related to infection in patients with below normal and normal IgG levels, respectively, at the time of transplantation. This relative risk of IgG and CD4 has not been previously described after NST for relapsed/refractory CLL, and should be corroborated by others.
Despite the discovery of new biologic prognostic factors in CLL, TP53 mutation or deletion remains the most important determinant of adverse clinical outcome.32,33 It is of paramount importance that novel therapeutic strategies be specifically assessed in CLL patients with p53 defects. It has been suggested that the p53+/p21− pattern of immunohistochemical expression can be used as a surrogate for TP53 mutations in CLL18 and other types of cancer.34,35 By using immunohistochemical analysis, we found that 23% of patients had tumor cells expressing p53+/p21− nuclear staining, a much higher incidence than is normally described in CLL,31 thus further suggesting advanced disease in the patients studied. We assessed the relationships between expression of p53+/p21− and OS, current PFS, immunomanipulation, and response to immunomanipulation. We could not demonstrate that patients with p53+/p21− had an inferior outcome. Recent studies have suggested that TP53 mutations in the absence of 17p deletions were found in 4.5% in patients studied (German CLL Study Group CLL trial 4) when TP53 mutations were assessed by denaturing high-performance liquid chromatography (exons 2-11).36 Prospective trials are needed to compare p53+/p21− results of immunohistochemistry, 17p deletions by fluorescent in situ hybridization, and modern molecular techniques to detect TP53 mutation.
In our study, we used combinations such as rituximab, cyclophosphamide, vincristine, and prednisone with doxorubicin to achieve disease control in patients who did not experience a response to traditional CLL treatments or in those with bulky disease. Therefore, in contrast to other reports,3,4,8 disease status at transplantation did not have a significant impact upon outcome, especially in that immunomodulation was incorporated in the treatment strategy.
In summary, our study demonstrates, in a large cohort of patients, the benefit of allogeneic SCT as therapy for relapsed/refractory CLL. This is also the first report demonstrating that certain HLA alleles may be predictive of response after immunomanipulation with DLI and rituximab in CLL. The described impact of pretrans-plantation immunodeficiency on survival may have important implications for the prevention of infections in patients with CLL undergoing NST, such as prophylactic immunoglobulin replacement, vaccination, and prophylactic antibiotic therapy.
Acknowledgments
FUNDING SOURCES
No specific funding was disclosed.
FUNDING STATEMENT:
This article was supported by NIH. The grant number is P30 CA016672.
Footnotes
CONFLICT OF INTEREST DISCLOSURES
The authors made no disclosures.
REFERENCES
- 1.Khouri IF, Keating M, Korbling M, et al. Transplant-lite: induction of graft-versus-malignancy using fludarabine-based nonablative chemotherapy and allogeneic blood progenitor-cell transplantation as treatment for lymphoid malignancies. J Clin Oncol. 1998;16:2817–2824. doi: 10.1200/JCO.1998.16.8.2817. [DOI] [PubMed] [Google Scholar]
- 2.Schetelig J, Thiede C, Bornhauser M, et al. Evidence of a graft-versus-leukemia effect in chronic lymphocytic leukemia after reduced-intensity conditioning and allogeneic stem-cell transplantation: the Cooperative German Transplant Study Group. J Clin Oncol. 2003;21:2747–2753. doi: 10.1200/JCO.2003.12.011. [DOI] [PubMed] [Google Scholar]
- 3.Delgado J, Thomson K, Russell N, et al. Results of alemtuzumab-based reduced-intensity allogeneic transplantation for chronic lymphocytic leukemia: a British Society of Blood and Marrow Transplantation study. Blood. 2006;107:1724–1730. doi: 10.1182/blood-2005-08-3372. [DOI] [PubMed] [Google Scholar]
- 4.Sorror ML, Storer BE, Sandmaier BM, et al. Five year follow-up of patients with advanced chronic lymphocytic leukemia treated with allogeneic hematopoietic cell transplantation after nonmyeloablative conditioning. J Clin Oncol. 2008;26:4912–4920. doi: 10.1200/JCO.2007.15.4757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ritgen M, Stilgenbauer S, von Neuhoff N, et al. Graft-versus-leukemia activity may overcome therapeutic resistance of chronic lymphocytic leukemia with unmutated immunoglobulin variable heavy-chain gene status: implications of minimal residual disease measurement with quantitative PCR. Blood. 2004;104:2600–2602. doi: 10.1182/blood-2003-12-4321. [DOI] [PubMed] [Google Scholar]
- 6.Caballero D, Garcia-Marco JA, Martino R, et al. Allogeneic transplant with reduced intensity conditioning regimens may overcome the poor prognosis of B-cell chronic lymphocytic leukemia with unmutated immunoglobulin variable heavy-chain gene and chromosomal abnormalities (11q- and 17p-). Clin Cancer Res. 2005;11:7757–7763. doi: 10.1158/1078-0432.CCR-05-0941. [DOI] [PubMed] [Google Scholar]
- 7.Khouri IF, Saliba RM, Admirand J, et al. Graft-versus-leukaemia effect after non-myeloablative haematopoietic transplantation can overcome the unfavourable expression of ZAP-70 in refractory chronic lymphocytic leukaemia. Br J Haematol. 2007;137:355–363. doi: 10.1111/j.1365-2141.2007.06591.x. [DOI] [PubMed] [Google Scholar]
- 8.Dregher P, Dohner H, Ritgen M, et al. Allergenic stem cell transplantation provides durable disease control in poor-risk chronic lymphocytic leukemia: long-term clinical and MRD results of the German Study Group CLL3X trial. Blood. 2010;116:2438–2447. doi: 10.1182/blood-2010-03-275420. [DOI] [PubMed] [Google Scholar]
- 9.Wierda W, O'Brien S, Wen S, et al. Chemoimmunotherapy with fludarabine, cyclophosphamide, and rituximab for relapsed and refractory chronic lymphocytic leukemia. J Clin Oncol. 2005;23:4009–4012. doi: 10.1200/JCO.2005.12.516. [DOI] [PubMed] [Google Scholar]
- 10.Keating MJ, Flinn I, Jain V, et al. Therapeutic role of alemtuzumab (Campath-1H) in patients who have failed fludarabine: results of a large international study. Blood. 2002;99:3554–3561. doi: 10.1182/blood.v99.10.3554. [DOI] [PubMed] [Google Scholar]
- 11.Rondon G, Giralt S, Huh Y, et al. Graft-versus-leukemia effect after allogeneic bone marrow transplantation for chronic lymphocytic leukemia. Bone Marrow Transplant. 1996;18:669–672. [PubMed] [Google Scholar]
- 12.Khouri IF, Lee MS, Saliba RM, et al. Nonablative allogeneic stem cell transplantation for chronic lymphocytic leukemia: impact of rituximab on immunomodulation and survival. Exp Hematol. 2004;32:28–35. doi: 10.1016/j.exphem.2003.09.021. [DOI] [PubMed] [Google Scholar]
- 13.Verheyden S, Demanet C. Susceptibility to myeloid and lymphoid leukemia is mediated by distinct inhibitory KIRHLA ligand interactions. Leukemia. 2006;20:1437–1438. doi: 10.1038/sj.leu.2404279. [DOI] [PubMed] [Google Scholar]
- 14.Bleakley M, Riddle SR. Molecules and mechanisms of the graft-versus-leukaemia effect. Nat Rev Cancer. 2004;4:371–380. doi: 10.1038/nrc1365. [DOI] [PubMed] [Google Scholar]
- 15.Goulmy E. Minor histocompatibility antigens: from transplantation problems to therapy of cancer. Hum Immunol. 2006;67:433–438. doi: 10.1016/j.humimm.2006.03.012. [DOI] [PubMed] [Google Scholar]
- 16.Cheson BD, Bennett JM, Grever M, et al. National Cancer Institute-sponsored Working Group guidelines for chronic lymphocytic leukemia: revised guidelines for diagnosis and treatment. Blood. 1996;87:4990–4997. [PubMed] [Google Scholar]
- 17.Hallek M, Cheson BD, Catovsky D, et al. Guidelines for the diagnosis and treatment of chronic lymphocytic leukemia: a report from the International Workshop on Chronic Lymphocytic Leukemia updating the National Cancer Institute-Working Group 1996 guidelines. Blood. 2008;111:5446–5456. doi: 10.1182/blood-2007-06-093906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cobo F, Martinez A, Hernandez L, et al. Multiple cycle regulator alterations in Richter’s transformation of chronic lymphocytic leukemia. Leukemia. 2002;16:1028–1034. doi: 10.1038/sj.leu.2402529. [DOI] [PubMed] [Google Scholar]
- 19.Giles F, Bekele BN, O'Brien S, et al. A prognostic model for survival in chronic lymphocytic leukemia based on p53 expression. Br J Haematol. 2003;121:578–585. doi: 10.1046/j.1365-2141.2003.04306.x. [DOI] [PubMed] [Google Scholar]
- 20.Przepiorka D, Weisdorf D, Martin P, et al. 1994 consensus conference on acute GVHD grading. Bone Marrow Transplant. 1995;15:825–828. [PubMed] [Google Scholar]
- 21.Filipovich AH, Weisdorf D, Pavletic S, et al. National Institutes of Health consensus development project on criteria for clinical trials in chronic graft-versus-host disease: 1. Diagnosis and staging working group report. Biol Blood Marrow Transplant. 2005;11:945–955. doi: 10.1016/j.bbmt.2005.09.004. [DOI] [PubMed] [Google Scholar]
- 22.Kaplan EL, Meier P. Non-parametric estimation from incomplete observations. J Am Stat Assoc. 1958;53:457–481. [Google Scholar]
- 23.Prentice RL, Kalbfleish JD, Peterson AV, Jr, et al. The analysis of failure times in the presence of competing risks. Bio-metrics. 1978;34:541–554. [PubMed] [Google Scholar]
- 24.Klein JP, Keiding N, Shu Y, Szydlo RM, Goldman JM. Summary curves for patients transplanted for chronic myeloid leukemia salvaged by a donor lymphocyte infusion: the current leukemia-free survival curve. Br J Haematol. 2000;109:148–152. doi: 10.1046/j.1365-2141.2000.01982.x. [DOI] [PubMed] [Google Scholar]
- 25.Wilikinson L, Dallal GE. Tests of significance in forward selection regression with an F-to enter stopping rule. Technometrics. 1921;23:377–380. [Google Scholar]
- 26.Delgado J, Milligan DW, Dreger P. Allogeneic hematopoietic cell transplantation for chronic lymphocytic leukemia: ready for primetime? Blood. 2009;114:2581–2588. doi: 10.1182/blood-2009-05-206821. [DOI] [PubMed] [Google Scholar]
- 27.Hsu FJ, Komarovskaya M. CTLA4 blockade maximizes antitumor T-cell activation by dendritic cells presenting idiotype protein or opsonized anti-CD20 antibody-coated lymphoma cells. J Immunother. 2002;25:455–468. doi: 10.1097/00002371-200211000-00002. [DOI] [PubMed] [Google Scholar]
- 28.Clynes RA, Towers TL, Presta LG, Ravetch JV. Inhibitory Fc receptors modulate in vivo cytotoxicity against tumor targets. Nat Med. 2000;6:443–446. doi: 10.1038/74704. [DOI] [PubMed] [Google Scholar]
- 29.O'Brien SM, Kantarjian H, Thomas D, et al. Rituximab dose-escalation trial in chronic lymphocytic leukemia. J Clin Oncol. 2001;19:2165–2170. doi: 10.1200/JCO.2001.19.8.2165. [DOI] [PubMed] [Google Scholar]
- 30.Sorror ML, Maris MB, Sandmaier BM, et al. Hematopoietic cell transplantation after nonmyeloablative conditioning for advanced chronic lymphocytic leukemia. J Clin Oncol. 2005;23:3819–3829. doi: 10.1200/JCO.2005.04.569. [DOI] [PubMed] [Google Scholar]
- 31.Tam CS, O'Brien S, Lerner S, et al. The natural history of fludarabine-refractory chronic lymphocytic leukemia patients who fail alemtuzumab or have bulky lymphadenopathy. Leuk Lymphoma. 2007;48:1931–1939. doi: 10.1080/10428190701573257. [DOI] [PubMed] [Google Scholar]
- 32.Dohner H, Fischer K, Bentz M, et al. p53 gene deletion predicts for poor survival and non-response to therapy with purine analogs in chronic B-cell leukemias. Blood. 1995;85:1580–1589. [PubMed] [Google Scholar]
- 33.Dohner H, Stilgenbauer S, Benner A, et al. Genomic aberrations and survival in chronic lymphocytic leukemia. N Engl J Med. 2000;343:1910–1916. doi: 10.1056/NEJM200012283432602. [DOI] [PubMed] [Google Scholar]
- 34.Bukholm IK, Nesland JM, Karesen R, Jacobsen U, Borresen AL. Relationship between abnormal p53 protein and failure to express p21 protein in human breast carcinomas. J Pathol. 1997;181:140–145. doi: 10.1002/(SICI)1096-9896(199702)181:2<140::AID-PATH745>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- 35.Zlotta AR, Noel JC, Fayt I, et al. Correlation and prognostic significance of p53,p21WAF1/CIP1 and Ki-67 expression in patients with superficial bladder tumours treated with bacillus Calmette-Guerin intravesical therapy. J Urol. 1999;161:792–798. [PubMed] [Google Scholar]
- 36.Zenz T, Eichhorst B, Busch R, et al. TP53 mutation and survival in chronic lymphocytic leukemia. J Clin Oncol. 2010;28:4473–4479. doi: 10.1200/JCO.2009.27.8762. [DOI] [PubMed] [Google Scholar]