Abstract
Environmental factors, including exposure to stress, are known to contribute to the propensity to consume ethanol. However, stress produces inconsistent effects on ethanol drinking in rodent models. Therefore, the present study examined the impact of different stressors on limited access ethanol consumption and determined whether there were sex-dependent differences in response to stress. To this end, male and female C57BL/6J mice had 2-hr access to a water and 10% ethanol solution, beginning 30 minutes before onset of the dark cycle. Once ethanol intake was stable, the effect of restraint, tail suspension, predator odor, foot shock, and tail pinch on subsequent intake was explored. Both plasma corticosterone (CORT) and allopregnanolone (ALLO) were assessed as indices of hypothalamic-pituitary-adrenal (HPA) axis activity and of endogenous neurosteroid levels respectively, following restraint, tail suspension and predator odor. Ethanol intake was decreased following restraint, tail suspension, foot shock, and tail pinch in both sexes, with stressor-related differences in the duration of the suppression. The effect of predator odor on ethanol intake was biphasic in females; ethanol consumption was significantly reduced on the day of stress but significantly increased on the following two days. In males, predator odor produced a delayed significant increase in ethanol intake on the second day after stress. All three stressors increased plasma CORT, with higher CORT levels in females when compared with males. Notably, there was a significant positive correlation between CORT levels immediately after predator odor stress and ethanol intake on the following day as well as a significant positive linear relationship between CORT levels immediately after restraint stress and ethanol intake on the following day in females. Furthermore, the three stressors produced a greater increase in ALLO levels in female versus male mice, but ALLO levels following predator odor were not correlated with subsequent ethanol intake. In summary, the type of stressor administered had a profound impact on subsequent ethanol consumption, with subtle sex differences in the magnitude and persistence of the effect. These findings are the first to demonstrate that a single, acute exposure to restraint, tail suspension, and predator odor stress increased plasma CORT and ALLO levels in animals with a history of ethanol consumption and that female mice were more responsive than males to the ability of stress to increase CORT levels as well as to the ability of predator odor stress to produce a delayed increase in ethanol intake. Because predator odor stress is a model of posttraumatic stress disorder, the present sex differences have important implications for future preclinical studies modeling the comorbidity of posttraumatic stress disorder and alcohol use disorders.
Keywords: environmental stress, corticosterone, allopregnanolone, predator odor, foot shock, tail pinch
Introduction
In human studies, research has clearly demonstrated a link between alcohol consumption and stress with those individuals that experience higher levels of stress exhibiting higher levels of alcohol consumption (reviewed in Keyes et al., 2012). Stress, either an internal or environmental event, disrupts homeostasis via an activation of the hypothalamic-pituitary-adrenal (HPA) axis, which leads to the rapid secretion of corticotropin-releasing hormone (CRH) and ultimate release of glucocorticoids such as corticosterone (CORT) in the rodent (reviewed in Biggio et al., 2014; Gunn et al., 2011; Makino et al., 2002). Disruptions in HPA axis function are believed to influence alcohol self-administration (Koob and Kreek, 2007; Koob and Le Moal, 2001), and alcohol itself increases brain levels of CRH and plasma levels of CORT (Lee et al., 2004; Richardson et al., 2008; Rivier, 1999; Willey et al., 2012), thereby activating the HPA axis. Once the HPA axis is activated, metabolic processes are accelerated, with the magnitude of the stress response depending on a wide variety of variables including genetics, sex, previous stress experience, and neurosteroid responsivity (Anisman and Matheson, 2005; Gunn et al., 2011; Herman et al., 2004).
The CRH neurons of the paraventricular nucleus (PVN) of the hypothalamus receive projections that send an inhibitory gamma-aminobutyric acid (GABA) signal to these neurons (Cullinan et al., 2008); thus, GABA is capable of regulating the activity of the HPA axis under basal conditions and in response to stress. Additionally, the neurosteroid allopregnanolone (ALLO), a potent positive allosteric modulator of GABAA receptors, inhibits HPA axis activity and the stress-induced increase in plasma CORT (Patchev et al., 1996). Considering that exposure to various stressors as well as ethanol self-administration have been shown to increase concentrations of ALLO in a time- and sex-dependent manner (reviewed in Barbaccia et al., 2001; Finn et al., 2004), further exploration of the relationship between stress-induced alterations in ALLO and CORT during ethanol consumption is warranted.
It is generally accepted that stressful life events influence alcohol drinking in the human population (Keyes et al., 2012). However, animal models examining stress and ethanol interactions are plentiful but demonstrate conflicting evidence in terms of the change in ethanol consumption. In fact, the relationship between stress and alcohol consumption contains complexities related to alcohol consumption modeling (history of use, level of drinking, and timing of administration) and stress-related factors (type, duration, and timing of administration), which then relate to biological factors (genetics, age, and sex) (reviewed in Becker et al., 2011). In studies conducted using mice, most research indicates that administration of an acute stressor fails to alter or reduces ethanol consumption and/or self-administration (i.e. Chester et al., 2006, 2008; Matthews et al., 2008; Tambour et al., 2008; Yang et al., 2008; reviewed in Becker et al., 2011). However, these studies are in direct contrast to research indicating that an acute or chronic stressor increases ethanol consumption in mice (i.e. Chester et al., 2006; Farook et al., 2009; Lopez et al., 2011; Matthews et al., 2008; Pelloux et al., 2005; Racz et al., 2003; reviewed in Becker et al., 2011). With variations in stressors administered, genotype and sex of the mouse used, and ethanol access paradigm employed, interpretation of the impact of stress on ethanol consumption in mice is difficult. While some chronic stress models (e.g., maternal separation, adolescent social isolation) can produce a more consistent increase in subsequent ethanol intake (Becker et al., 2011), the purpose of the current study was to examine the impact of acute exposure to a a combination of physical stressors (restraint, foot shock, and tail pinch) as well as psychological stressors (tail suspension and predator odor) on limited access ethanol consumption (water vs. 10% v/v ethanol; 10E) in both male and female C57BL/6J mice in order to evaluate whether one form of acute stress exposure would have a greater impact on subsequent ethanol consumption than the other stress exposures.
Our choice of these particular stressors was based on evidence that acute exposure to each stressor increased ethanol intake in particular rodent genotypes (reviewed in Becker et al., 2011). However, only footshock and restraint stress were examined and reported to increase ethanol intake in male C57BL/6J mice (females not examined; Farook et al., 2009; Matthews et al., 2008). Thus, male and female C57BL/6J mice have not been systematically tested for their responsivity to the effects of various stressors on ethanol intake. We used a drinking procedure in which animals consume ethanol at levels that result in physiologically relevant blood ethanol concentrations (BECs; e.g., Finn et al., 2004) to test the hypothesis that the subsequent ethanol consumption following stress exposure would vary in a sex- and stressor-dependent way. In support of this general hypothesis, limited available data in other genotypes indicate that repeated restraint stress significantly increased ethanol intake in male and significantly decreased ethanol intake in female mice that were selectively bred for high alcohol preference (HAP; Chester et al., 2006), and that tail suspension stress significantly increased ethanol intake only in female but not in male CD-1 mice with high immobility scores (Pelloux et al., 2005). Finally, because perceived predation risk has a profound biological effect across species (Zanette et al., 2011), and predator odor stress significantly increased ethanol self-administration in male Wistar rats (Edwards et al., 2013; Roltsch et al., 2014), we predicted that exposure to predator odor stress would significantly increase ethanol intake and that the effect might be more pronounced in female mice.
Additionally, it has been suggested that one potential mechanism underlying sex differences in the impact of stress on ethanol intake could be alterations in HPA axis responsivity (Chester et al., 2006), as measured by plasma CORT or ALLO levels in response to stress. Therefore, the current study also examined whether a sex difference existed in the stress-induced alteration in CORT and ALLO levels following predator odor, tail suspension, and restraint stress. Finally, the relationship between stress-induced CORT and ALLO levels and subsequent ethanol consumption was evaluated in order to determine whether there was a positive correspondence between the stress-induced changes in CORT and/or ALLO levels and subsequent ethanol intake and whether this relationship differed in male and female mice.
Materials and Methods
Animals
A total of 22 female and 21 male adult (minimum age of 8 weeks) C57BL/6J mice were obtained from a breeding colony maintained by the Portland Alcohol Research Center in the Department of Veterans Affairs Veterinary Medical Unit (Portland, OR) in two separate cohorts. Mice were maintained on a 12-hr reverse light/dark cycle (lights off 1100) in a temperature (22 ± 2°C) and humidity controlled environment. Rodent chow (Labdiet 5001 rodent diet; PMI International, Richmond, IN) and water were available ad libitum. All rodent cages were changed once per week. Following a one week period of acclimation, animals were individually housed in clear polycarbonate cages (28 × 18 × 13 cm) on Ecofresh bedding and given two water sipper tubes. All procedures complied with the National Institute of Health Guidelines for the Care and Use of Laboratory Animals and were approved by the local Institutional Animal Care and Use Committee.
Limited access 2-bottle choice procedure
After animals were single-housed, a limited access 2-bottle choice procedure began (Sinnott et al., 2002). At 30-min before lights out, mice had access to 10E versus water for 2 hours. Volume before and after each access period was recorded in order to calculate total mLs consumed as well as g/kg consumed, based on the animal’s body weight, which was determined weekly. Animals were given 10E access on 5 days per week with only access to water over the weekend. After 14-15 days of ethanol intake (depending on cohort) to establish a stable baseline (less than 20% variability across 3 days), the initial blood collection or administration of environmental stressors began (Figure 1).
Figure 1.
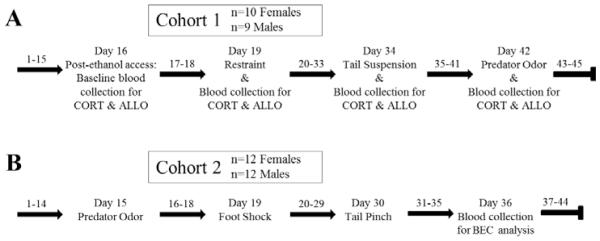
Experimental timeline for (A) cohort 1 and (B) cohort 2. Dark arrows refer to days of limited access 10% ethanol (10E) intake without any experimental manipulation. Days of drinking are numbered consecutively, with weekends excluded (since animals had no ethanol access on the weekends). The order and specific day of drinking that each stressor was administered are shown. In cohort 1 (panel A; n=10 Females and n=9 Males), a tail blood sample was taken immediately after the stress exposure on days 19, 34, and 42 for corticosterone (CORT) and allopregnanolone (ALLO) levels and prior to returning the animal to its home cage. A tail blood sample also was taken on day 16 after the 2 hr 10E intake, to assess a pre-stress “baseline”. In cohort 2 (panel B; n=12 Females and n=12 Males), an orbital blood sample was on day 36 to assess blood ethanol concentration (BEC) on a non-stress day.
Blood Ethanol Concentrations (BECs)
BECs were measured in the second cohort of animals following 2-hr access to 10E (Figure 1). A 20-μ1 blood sample was taken from the retro-orbital sinus immediately following the session, and the blood sample was analyzed for ethanol content via headspace gas chromatography (Finn et al., 2007).
Environmental Stressors
Each stressor was administered directly before presentation of the 10E bottle. Upon completion of the stressor, animals were returned to the home cage, and 10E bottles were presented. Two separate cohorts of animals were used, with each cohort receiving select stressors (see Figure 1 for details). Use of 2 cohorts enabled us to stagger the start time for each stressor to ensure that upon completion of the stress exposure, animals were returned to their home cages and drinking commenced immediately. It also allowed us to test predator odor twice (3rd in Cohort 1; 1st in Cohort 2) to examine potential order effects. Cohort 1 included 10 females and 9 males; tail blood collection occurred on day 16 (prior to any stress exposures) and on days 19, 34, and 42 (immediately after each stressor and prior to returning animals to the home cage for ethanol access). Cohort 2 included 12 females and 12 males; orbital blood collection occurred on day 36 after the 2 hr ethanol session.
Restraint Stress (Cohort 1)
Mice were removed from their home cage and placed in custom-made polycarbonate restrainers (1 ¼-inch diameter, 3 ¾-inch length; Flair Plastics, Portland, OR) for 30 minutes (Burrows et al., 1998).
Tail Suspension Stress (Cohort 1)
Mice were removed from their home cage and suspended by taping their tails with medical adhesive tape to a metal bar (approximately 3” tall × 2” wide × 36” long) that was approximately 24” off of the floor. All activity was video-recorded for 6 minutes. Behavior was measured as latency to first period of immobility (a 2 second period during which no movement occurred), time spent immobile, percent of time spent immobile, and total episodes of immobility. Behavior was scored by two technicians and then averaged (see Table 1; Barfield et al., 2010; Pelloux et al., 2005).
Table 1.
Sex differences in immobility scores during the 6-minute tail suspension stress.
| Sex | Latency to First Immobility (sec) |
Time Spent Immobile (sec) |
Percent of Time Immobile |
Episodes of Immobility |
|---|---|---|---|---|
| Male | 15.9±2.9* | 197.8±13.5+ | 55.0±3.7+ | 15.9±0.9 |
| Female | 42.7±11.8 | 160.0±16.3 | 44.4±4.5 | 15.9±1.1 |
Immobility scores are given in mean ± SEM for 9 male and 10 female mice. Immobility was determined as any period in which the animal stopped all movement for at least 2 seconds. Latency to first immobility is the amount of time (in sec) it took the animal to become immobile for the first time. Total time spent immobile was calculated and then divided by the total session time (360 sec) to yield percent of time immobile. Total episodes of immobility were also recorded. In general, males tended to spent more time immobile, when compared with females, and latency to first immobility was significantly shorter in the male versus female mice.
p<0.05
p<0.10 vs. respective female value (t-test).
Predator Odor Stress (Cohort 1 and 2)
Mice were removed from their home cage and placed in a polycarbonate cage containing dirty rat bedding for 30 minutes. Dirty rat bedding was attained from male and female adult rat cages after the rats had been housed in their cages for 1 week (Hebb et al., 2003). In a pilot study, we found that 30 min exposure to dirty rat bedding from male and female rats significantly increased CORT levels in ethanol naïve male and female C57BL/6J mice. Mean ± SEM CORT levels were 41.39 ± 2.83 μg/dL (males, n=5) and 53.43 ± 4.16 μg/dL (females, n=5), and values were significantly higher in female versus male mice [F(1,8) = 5.734, p<0.05].
Foot Shock Stress (Cohort 2)
Animals were removed from the home cage and placed into an operant box fitted with a metal floor. The Precision Animal Shocker (Coulbourn Instruments, Whitehall, PA) was used to deliver a 0.5 mA foot shock for 5 seconds every minute, for a total of 5 minutes (Sanford et al., 2010).
Tail Pinch Stress (Cohort 2)
A wooden clothespin was affixed near the base of the animal’s tail for 15 minutes, and the animal was monitored while in the home cage during the stress (Butts et al., 2011). If the animal was able to remove the clothespin, researchers immediately replaced it.
Tail Blood Collection
Mice in Cohort 1 were gently restrained using the custom-made mouse restrainers, and a tail blood sample was collected into a 20-μL micro-capillary pipet (Kimble Glass Inc., Vineland, NJ), centrifuged for 20-min at 914 × g to isolate plasma, and then stored at −80 °C until assayed. The baseline blood sample was taken on day 16, immediately after the 2 hr period of 10E intake. Blood samples also were taken immediately following exposure to each stressor (see Figure 1), before returning the animal to its home cage, in order to measure CORT levels at the time of peak increase following stress exposure. Blood collection took under 1 minute, which minimized the potential for added mild stress prior to the measurement of ethanol intake.
Corticosterone (CORT) Levels
Steroid concentrations were measured using a commercially available 125I radioimmunoassay kit (ImmuChem Double Antibody Corticosterone for rodents; MP Biomedicals, Orangeburg, NY). The manufacturer supplied protocol was implemented, with minor modifications. In brief, CORT concentration in plasma samples (5-μL) was single-determined via interpolation from a standard curve derived from six standards (ranging from 25 to 1000 ng/mL; i.e., 2.5 – 100 μg/dL).
Allopregnanolone (ALLO) Levels
Neurosteroid concentrations were measured using a commercially available enzyme immunoassay kit (DetectX Allopregnanolone Immunoassay kit; Arbor Assays, Ann Arbor, MI). The manufacturer supplied protocol for plasma was implemented without extraction. In brief, ALLO concentration in plasma samples (3-μL) was single-determined via interpolation from a standard curve derived from 8 standards (ranging from 0.391 to 50 ng/mL).
Statistics
Analysis of BEC was conducted using t-tests. Analysis of body weight was conducted using a 2 × 2 × 2 analysis of variance (ANOVA; Time × Sex × Cohort) with time as a within subjects factor, and average ethanol intake across time was analyzed with a 2 × 2 ANOVA (Sex × Cohort). For the analysis of the impact of select stressors on subsequent ethanol consumption, water consumption and preference ratio, pre-stressor baseline was calculated as the 2 day average intake prior to the day of stress. Alterations in intake (ethanol and water) and preference were tested using a repeated measures ANOVA with sex as a between subjects factor. Due to our a priori hypothesis that there would be sex-specific differences in the response to stress, deconstruction of the ANOVAs were conducted by grouping the data by sex and performing subsequent one-way repeated measures ANOVAs and post-hoc paired t-tests. Linear least squares regression was conducted to determine the relationship between ethanol intake (g/kg) and BEC (mg/ml), CORT (μg/dL) levels, ALLO (ng/ml) levels or percent time immobile. CORT and ALLO levels were analyzed using a 2-way ANOVA with day/stressor and sex as factors (Due to loss of data points, we did not conduct a repeated measures ANOVA). All analyses were performed by Systat 11 (San Jose, CA), with significance set at p<0.05. All figures were derived from Prism version 4.0 (La Jolla, CA).
Results
Overall ethanol consumption and body weight
At the beginning of the ethanol intake procedure, mice weighed 27.6±0.5 g (male) and 21.7±0.5 g (female) in Cohort 1 and 24.3±0.6 g (male) and 20.8±0.7 g (female) in Cohort 2. Analysis of body weight across time revealed significant effects of sex [F(1,39)=62.151, p<0.001], time [F(8,312)=30.819, p<0.001], and sex × time interaction [F(8,312)=2.788, p<0.01], with male mice from both cohorts weighing significantly more than female mice at baseline (Cohort 1: t17=7.95, p≤0.001; Cohort 2: t22=3.851, p≤0.001 and upon completion of the studies (Cohort 1- males: 26.9±0.4 g, females: 22.4±0.4 g, t17=7.290, p≤0.001; Cohort 2- males: 25.7±0.6 g, females 22.5±0.5 g, t22=4.510, p≤0.001). Additionally the significant effect of time indicated that animals continued to gain weight throughout the study. However, a significant effect of Cohort [F(1,39)=9.962, p<0.01], which lead to time × Cohort [F(8,312)=19.382, p<0.001] and time × sex × Cohort [F(8, 312)=2.636, p<0.01] interactions, was also present. Deconstruction of this ANOVA by sex revealed that body weight in female mice increased significantly over time [F(8,160)=29.558, p<0.001], but there was no effect of Cohort (p>0.05). In male mice, body weight was significantly influenced by time [F(8,152)=9.178, p<0.001] and Cohort [F(1,19)=10.424, p<0.001]. Closer evaluation of body weight in male mice revealed that male mice in the first Cohort weighed significantly more than male mice in the second Cohort at the start of the procedures (t19=4.133, p<0.01). However, body weight in the two Cohorts of male mice did not differ upon completion of the studies (t19=1.618, p>0.05).Average 10E intake (across all days of the study in g/kg) was 1.65±0.13 for females and 1.42±0.13 for males in Cohort 1 and 2.38±0.10 for females and 1.75±0.11 for males in Cohort 2, and 10E intake was significantly affected by sex [F(1,39)=11.784, p≤0.001] and Cohort [F(1,39)=20.572, p≤0.001]. The main effect of sex was due to the significantly higher 10E intake in females than in males when collapsed across Cohort (t41=2.858, p<0.01), while the main effect of Cohort was due to the significantly higher 10E intake in Cohort 2 versus Cohort 1 (t41=3.857, p≤0.001). Interestingly, when deconstructed by sex, male mice did not differ in ethanol intake across cohorts (p>0.05). Thus, the significant cohort effect was the result of female mice in the second cohort drinking on average more ethanol than those in the first cohort (t20=4.610, p≤0.001).
BECs on a non-stress day
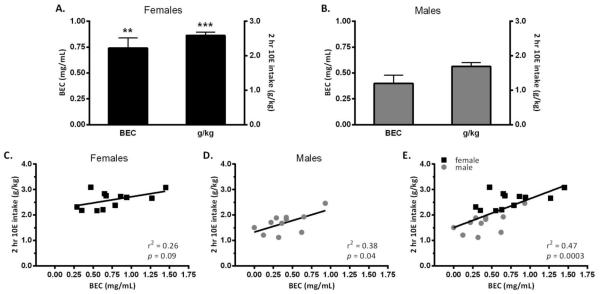
BECs were measured in Cohort 2. On the day of sampling, 10E intake (t22=5.213, p<0.001) and BECs (t22=2.954, p<0.01) were significantly higher in female versus male mice (Figure 2A and 2B, respectively). Examination of the relationship between 10E intake and BEC revealed that in females there was a trend toward a significant linear relationship (r2=0.25, p=0.09; Figure 2C) and in males this relationship was significant (r2=0.378, p<0.05; Figure 2D). However, when data for both sexes were combined, the linear relationship between 10E intake and the subsequent BEC reached greater significance (r2=0.47, p≤0.001) (Figure 2E).
Figure 2.
Blood ethanol concentrations (BECs) and ethanol intake during the 2-hr 2-bottle choice paradigm. Mean (±SEM) BEC (mg/mL) and ethanol intake (g/kg) in female (A) and male (B) mice following 2-hour access to both water and 10% ethanol (10E). Female mice not only consumed greater amounts of 10E during the access period but also had higher BECs when compared to male mice. ***p<0.001, **p<0.01 vs. male mice. (C-E) Regression analysis of 10E intake and BECs, confirming a significant positive relationship between amount of ethanol consumed and the respective BEC in this paradigm. Male mice (n=11) are presented in gray circles, females (n=12) are presented in black squares.
Restraint stress
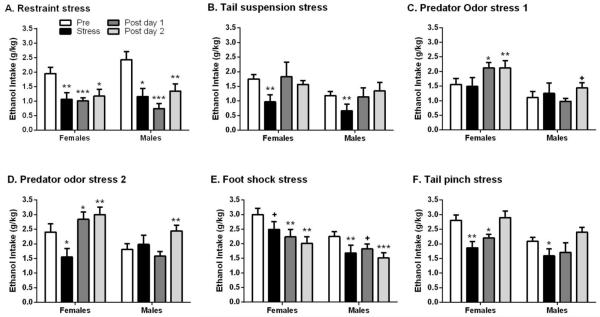
Restraint stress significantly decreased ethanol consumption without producing a sex-dependent effect [Figure 3A; Sex: F(1,17)=0.338, p>0.05, Day: F(3,51)=14.275, p≤0.001, Sex × Day: F(3,51)=1.203, p>0.05]. The ability of restraint stress to reduce 10E intake was significant in both males and females, with effects present at least two days following administration of the stressor (p<0.05). Analysis of water consumption revealed a transient increase as a result of restraint stress [Table 2; Sex: F(1,17)=2.708, p>0.05; Day: F(3,51)=3.592, p>0.05; Sex × Day: F(3,51)=0.540, p<0.05]. Water consumption was significantly increased in male mice on the day of restraint stress (p<0.05) and tended to be increased in females (p=0.08). To further elucidate the impact of restraint stress on ethanol consumption, analysis of ethanol preference (Table 3) was conducted. Again this analysis revealed a significant impact of day [F(3,39)=5.935, p<0.01] but no effect of sex [F(1,13)=0.898, p>0.05] nor an interaction between sex and day [F(3,39)=1.472, p>0.05]. The effect of day was driven by a significant decrease in ethanol preference in males on the day of restraint (p<0.05) as well as a trend on the first post-day (p=0.08). Females were more resistant to alteration in ethanol preference following restraint, with only a trend toward a decrease in preference on the day of the restraint (p=0.08). Altogether these data suggest that restraint stress produced a decrease in ethanol consumption in both sexes that lasted for at least 2 days following the stressor and that was accompanied by a slight increase in water consumption.
Figure 3.
Impact of environmental stressors on ethanol intake. Mean (±SEM) of 10% ethanol (10E) intake (g/kg/2hr) in female and male mice following (A) 30-min restraint (Cohort 1), (B) 6-min tail suspension (Cohort 1), (C) 30-min predator odor (Cohort 1), (D) 30-min predator odor (Cohort 2), (E) 5-min intermittent foot shock (Cohort 2), and (F) 15-min tail pinch stress (Cohort 2). Baseline is the average of the two drinking days immediately prior to the stress day. ***p<0.001, **p<0.01, *p<0.05 and +p≤0.09 vs. respective baseline intake (post hoc t-test). Cohort 1 group size was 9 (males) and 10 (females); Cohort 2 group size was 12 (males) and 12 (females).
Table 2.
Influence of environmental stressors on water intake during the limited-access 2-bottle choice paradigm.
| Restraint |
Tail
Suspension |
Predator
Odor 1 |
Predator
Odor 2 |
Foot Shock | Tail Pinch | |
|---|---|---|---|---|---|---|
| Females | ||||||
| Baseline | .07±.02 (10) | .02±.01(10) | .08±.04(10) | .11±.04(12) | .08±.03(12) | .06±.02(12) |
| Stress | .18±.06(10)+ | .06±.04(10) | .18±.12(10) | .43±.06 (12)*** | .15±.04(12) | .17±.06(12) |
| Post Day 1 | .06±.03 (10) | .04±.03 (10) | .04±.03 (10) | .12±.04(12) | .10±.03(12) | .08±.04(12) |
| Post Day 2 | .08±.03 (10) | .06±.03 (10) | .02±.02(10) | .08±.03 (12) | .03±.02 (12)+ | .15±.04(12)* |
|
| ||||||
| Males | ||||||
| Baseline | .12±.09(9) | .07±.05 (9) | .06±.02 (9) | .05±.02 (12) | .06±.02 (12) | .03±.01 (12) |
| Stress | .38±.12(9)* | .04±.03 (9) | .27±.06 (9)* | .25±.06 (12)** | .05±.04 (12) | .07±.04(12) |
| Post Day 1 | .18±.09(9) | .04±.04 (9) | .02±.02 (9) | .07±.04 (12) | .07±.03 (12) | .02±.02(12) |
| Post Day 2 | .24±.13(9) | .09±.07 (9) | .04±.03 (9) | .08±.03 (12) | .02±.02 (12) | .10±.05(12) |
Mean (±SEM) of water intake (in mLs) in female and male mice following 30-min restraint (Cohort 1), 6-min tail suspension (Cohort 1), 30-min predator odor (1= Cohort 1, 2= Cohort 2), 5-min intermittent foot shock (Cohort 2), and 15-min tail pinch stress (Cohort 2). Baseline is the average of the two drinking days immediately prior to stress day. Group size is given in parentheses.
p≤0.001
p<0.01
p<0.05
p<0.09 vs. respective baseline intake (post hoc paired t-test).
Table 3.
Influence of environmental stressors on ethanol preference during the limited-access 2-bottle choice paradigm.
| Restraint | Tail Suspension |
Predator Odor 1 |
Predator Odor 2 |
Foot Shock | Tail Pinch | |
|---|---|---|---|---|---|---|
| Females | ||||||
| Baseline | .87±.04 (9) | .95±.03 (8) | .90±.03 (10) | .83±.07 (12) | .92±.03(12) | .94±.02(12) |
| Stress | .63±.13(9)+ | .85±.10(8) | .73±.13(10) | .45±.07 (12)*** | .82±.06 (12) | .79±.07(12)+ |
| Post Day 1 | .87±.07 (9) | .94±.06 (8) | .95±.04(10) | .85±.06 (12) | .85±.05 (12)+ | .96±.10(12) |
| Post Day 2 | .80±.08 (9) | .92±.06 (8) | .98±.02(10) | .91±.04 (12) | .94±.05 (12) | .85±.04(12)* |
|
| ||||||
| Males | ||||||
| Baseline | .87±.08 (6) | .96±.04 (6) | .91±.04 (9) | .92±.03 (12) | .93±.03 (12) | .98±.02(10) |
| Stress | .43±.14(6)* | .86±.09 (6) | .57±.12(9)* | .72±.06 (12)* | .90±.08 (12) | .92±.06(10) |
| Post Day 1 | .64±.13(6) | 1.0±.01 (6) | .96±.04 (9) | .90±.06 (12) | .89±.05 (12) | .97±.03 (10) |
| Post Day 2 | .89±.ll (6) | .92±.08 (6) | .93±.05 (9) | .91±.04 (12) | .96±.04 (12) | .89±.05 (10) |
Mean (±SEM) of ethanol preference in female and male mice following 30-min restraint (Cohort 1), 6-min tail suspension (Cohort 1), 30-min predator odor (1= Cohort 1, 2= Cohort 2), 5-min intermittent foot shock (Cohort 2), and 15-min tail pinch stress (Cohort 2). Baseline is the average of the two drinking days immediately prior to stress day. Group size is given in parentheses; only those animals that consumed fluid during the limited access procedure across all days of interest were included (as no fluid consumed indicates that preference could not be calculated).
p<0.001
p<0.01
p<0.05
p<0.09 vs. respective baseline preference (post hoc t-test).
Tail suspension stress
In terms of behavior in the tail suspension test, latency to first immobility was significantly shorter in the male versus female mice (p<0.05), and males tended to spend more time immobile, when compared with females (p<0.10; Table 1). However, tail suspension stress produced a modest decrease in ethanol intake that was not influenced significantly by sex [Figure 3B; Sex: F(1,17)=2.326, p>0.05, Day: F(3,51)=3.334, p>0.05, Sex × Day: F(3,51)=0.305, p<0.05]. The reduction in 10E intake was transient, with deconstruction by sex revealing a significant decrease in ethanol intake only on the day of the stressor in both females (p<0.01) and males (p<0.01). Additionally, tail suspension stress did not alter water consumption (Table 2) or ethanol preference (Table 3) in either sex, suggesting that tail suspension stress induced a transient decrease in ethanol consumption. When the relationship between percent time immobile (Table 1) and 10E intake was examined, there was a trend for a negative relationship on the day of tail suspension stress in male and female mice (r2=0.15, p = 0.10; not shown). When the regressions were conducted for each sex, there was a significant negative relationship between percent time immobile and 10E intake only in the male mice (r2=0.47, p = 0.04) on the day of tail suspension stress (not shown). Similar results were found when the relationship between immobility time (Table 1) and 10E intake was examined (not shown). These results indicate that 47% of the variance in 10E intake could be accounted for by the variation in percent time immobile on the day of the tail suspension test in male mice.
Predator odor stress
The impact of predator odor stress on ethanol intake was assessed in both cohorts. In the first cohort (Figure 3C), 10E intake after exposure to predator odor was significantly influenced by sex [F(1,17)=5.723, p<0.05] and day [F(3,51)=3.013, p<0.04], with a trend toward a significant interaction [F(3,51)=2.667, p=0.057]. The effect of predator odor in the first cohort was driven by females, with a significant increase in alcohol consumption occurring on post days 1 and 2 (p<0.05). Males in the first cohort had a very modest increase in alcohol consumption on post day 2 that failed to reach statistical significance (p=0.09). Analysis of water consumption (Table 2) revealed a significant effect of day [F(3,51)=5.848, p<0.01] but not sex [F(1,17)=0.203, p>0.05] and no interaction [F(3,51)=0.457, p>0.05]. In this case, the effect of predator odor on water consumption was driven by males, with a significant increase in water consumption occurring on the day of the predator odor stress (p<0.05). Analysis of ethanol preference ratio (Table 3) also revealed an effect of day [F(3,51)=8.485, p≤0.001]. Similar to the results with water consumption, only males showed a significant decrease in preference occurring on the day of the predator odor stress (p<0.05).
As the first predator odor stress was preceded by both restraint and tail suspension stress and was followed by tail blood collection, we tested predator odor stress as the first stressor in the second cohort of animals and eliminated the tail blood collection to evaluate the possible impact of these factors on the subsequent drinking behavior. In the second cohort, the effect of sex on the response to predator odor stress was more robust as evident from the significant interaction between sex and day [Sex: F(1,22)=3.357, p=0.08, Day: F(3,66)=9.327, p≤0.001, Sex × Day: F(3,66)=7.291, p≤0.001]. As shown in Figure 3D, predator odor stress altered 10E intake in both females [F(3,33)=12.433, p≤0.001] and males [F(3,33)=4.064, p<0.05]. Subsequent analyses revealed that predator odor stress produced a transient increase in 10E intake in males that was only present on the second post day (p<0.01), while females showed a biphasic effect with predator odor first decreasing intake on the day of the stressor (p<0.05) and then increasing 10E intake on both days post stress (p<0.05 and p<0.01, respectively). Analysis of water intake revealed both an effect of sex and day [Table 2; Sex: F(1,22)=4.287, p<0.05; Day: F(3,66)=22.620, p≤0.001; Sex × Day: F(3,66)=2.080, p>0.05]. The increase in water intake was significant on the day of predator odor stress (females: p≤0.001; males: p<0.01). Analysis of ethanol preference (Table 3) revealed an effect of predator odor [Day: F(3,66)=22.922, p≤0.001] and an interaction [Sex × Day: F(3,66)=3.526, p<0.05], with a slight trend in the effect of sex [F(1,22)=3.137, p=0.09]. Subsequent deconstruction by sex revealed a significant decrease in ethanol preference that occurred only on the day of predator odor stress (females p≤0.001 vs. males p<0.01).
Foot shock stress
Foot shock stress significantly decreased ethanol intake without resulting in an interaction between intake and sex [Figure 3E; Sex: F(1,22)=6.557, p<0.05, Day: F(3,66)=10.234, p≤0.001, Sex × Day: F(3,66)=0.723, p>0.05]. Interestingly, there was a progressive decrease in 10E intake across days in females (day of stress: p=0.06; post day 1: p<0.01; post day 2: p<0.01), while 10E intake was more variable in males after foot shock stress (day of stress: p<0.01; post day 1: p=0.06; post day 2: p≤0.001). Analysis of water intake revealed an effect of foot shock stress [Table 2; Sex: F(1,22)=2.471, p>0.05; Day: F(3,66)=3.233, p>0.05; Sex × Day: F(3,66)=1.135, p<0.05]. Subsequent analyses revealed that foot shock stress caused a trend towards a decrease in intake on the second post day (p=0.08) in female mice, whereas water intake in male mice was unaffected (p>0.05). Analysis of ethanol preference (Table 3) did not initially reveal any significant effects, although female mice did have a trend toward a significant reduction in preference on the first post day (p=0.06). Altogether these data suggest that foot shock stress decreased ethanol intake in males and in females, with the effects lasting for at least two days following the stressor.
Tail pinch stress
Tail pinch stress significantly decreased ethanol consumption [Figure 3F; Sex: F(1,22)=6.400, p<0.05, Day: F(3,66)=10.542, p≤0.001, Sex × Day: F(3,66)=0.460, p>0.05]. The suppression in 10E intake persisted until the second day post stress in females (day of tail pinch: p<0.01; post day 1: p<0.05) while 10E intake was significantly decreased only the day of the stress in males (p<0.05). Additionally, analysis of water intake revealed a slight increase in water consumption after tail pinch stress [Sex: F(1,22)=5.434, p<0.05; Day: F(3,66)=2.923, p>0.05; Sex × Day: F(3,66)=0.312, p<0.05]. However, post-hoc t-tests only revealed a significant increase in water consumption in females on the second day post stress (p<0.05). Although initial analysis of ethanol preference failed to reveal an effect of tail pinch stress [Sex: F(1,20)=1.648, p>0.05; Day: F(3,60)=1.933, p>0.05; Sex × Day: F(3,60)=0.395, p>0.05; Table 3], post-hoc t-tests revealed that, in complement with the water intake data, tail pinch stress reduced ethanol preference only in females to very near significance on the day of the stress as well as significantly reduced preference on post day 2 (p=0.06 and p=0.04, respectively).
CORT levels following select stressors
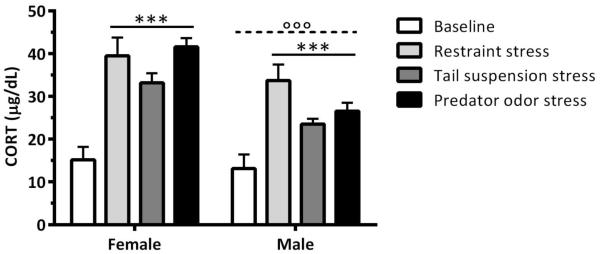
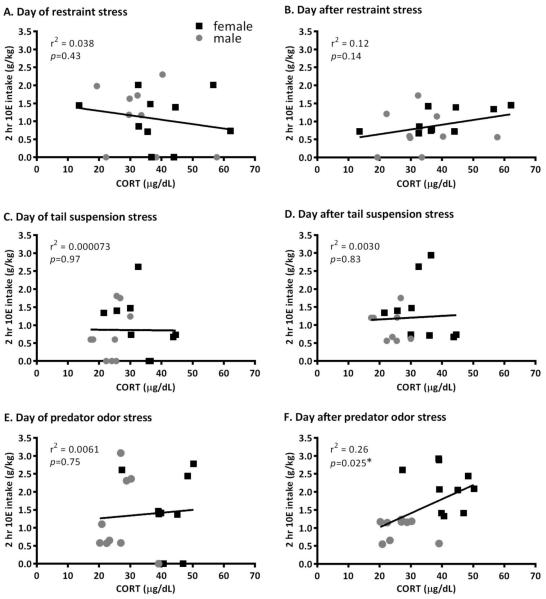
Plasma CORT levels were higher in females versus males and were increased significantly following restraint, tail suspension, and predator odor stress [Figure 4; Sex: F(1,67)=15.111, p≤0.001; Stressor: F(3,67)=22.948, p≤0.001; Sex × Stressor: F(3,67)=1.745, p>0.05]. When collapsed across sex, linear regression analysis indicated that there was no relationship between CORT levels and ethanol intake on either the day of the stressor or the first day post stressor for restraint and tail suspension stress (Figure 5A-D; p’s>0.05), and there was no relationship between CORT levels and ethanol intake on the day of the predator odor stress (Figure 5E, p>0.05). However, there was a significant positive relationship between CORT levels and ethanol intake on the first day post predator odor stress (r2=0.26, p<0.05) (Figure 5F). The goodness of fit of the regression line indicates that 26% of the variance in 10E intake (g/kg) on the day after predator odor stress could be accounted for by the variation in CORT levels on the day of the stress exposure. When the regressions were conducted for each sex, there was a significant positive relationship between CORT levels and ethanol intake on the first day post restraint stress in females (r2=0.42, p<0.05) (Figure 5B). This result indicates that 42% of the variance in 10E intake in female mice after restraint stress could be accounted for by the variation in CORT levels following the stressor. Altogether, these data indicate that the amount of HPA activation the animal experienced following the specific stressors (predator odor stress for both, restraint stress for females) impacted the amount of ethanol that the animal consumed on the next day.
Figure 4.
Plasma corticosterone (CORT) levels following select stressors. Mean (±SEM) CORT levels (μg/dL) in female and male mice from Cohort 1 at “baseline” and following exposure to 30-min restraint, 6-min tail suspension, and 30-min predator odor. ***p≤0.001 vs. “baseline” level (ANOVA, main effect of stressor), °°°p≤0.001 vs. females (ANOVA, main effect of sex); Group size for females was 10 except for baseline where one sample was lost; Group size for males was 9.
Figure 5.
Regression analysis of g/kg ethanol (10E) intake and μg/dL corticosterone (CORT) levels following select stressors in Cohort 1. There was no relationship between CORT levels in male and female mice following restraint or tail suspension stress and 10E intake on the day of (A and C, respectively) or the day after (B and D, respectively) administration of the stressor. However, in female mice, there was a significant positive linear relationship between CORT levels and ethanol intake on the first day post restraint stress (B; r2=0.42, p<0.05), indicating that 42% of the variance in 10E intake on the day after restraint stress could be explained by CORT levels immediately after restraint. Although levels of CORT were not related to 10E intake on the day of predator odor stress (E), there was a significant positive relationship (p<0.05) between CORT levels and ethanol intake on the day after exposure to the predator odor (F) in male and female mice. The r2 value indicates that 26% of the variance in 10E intake on the day after predator odor stress could be explained by CORT levels immediately after predator odor exposure.
ALLO levels following select stressors
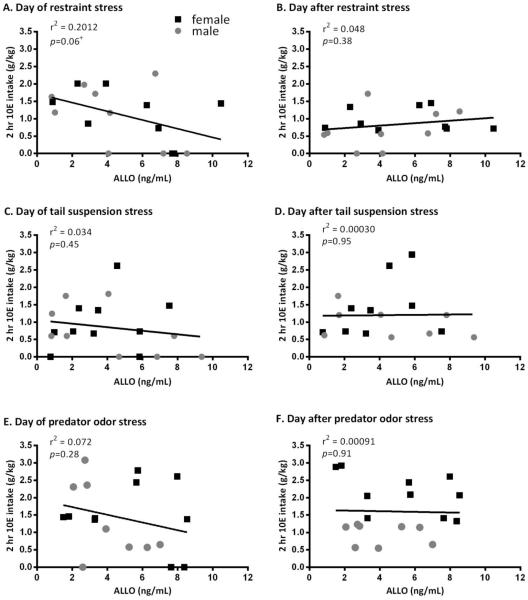
Plasma ALLO levels were also increased significantly following restraint, tail suspension, and predator odor stress [Figure 6; Sex: F(1,61)=0.795, p>0.05, Stressor: F(3,61)=3.002, p>0.05, Sex × Stressor: F(3,61)=0.476, p<0.05]. Linear regression analysis indicated that there was no relationship between ALLO levels and ethanol intake on either the day of the stressor or the first day post stressor for tail suspension and predator odor stress (Figure 7C-F; p’s>0.05). There was, however, a trend toward a significant negative relationship between ALLO levels and ethanol intake on the day of the restraint stress (r2=0.20, p=0.06) (Figure 7A), indicating that the higher ALLO levels circulating in the plasma following the stressor corresponded to lower ethanol intake immediately following the stress. This was only true on the day of the stressor, with no relationship between ALLO levels and ethanol intake on the day after the restraint stress (Figure 7B, p>0.05).
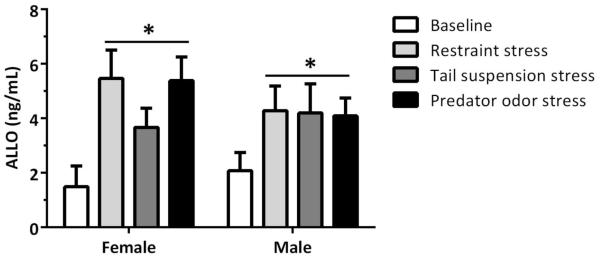
Figure 6.
Plasma allopregnanolone (ALLO) levels following select stressors. Mean (±SEM) ALLO levels (ng/mL) in female and male mice from Cohort 1 at “baseline” and following exposure to 30-min restraint, 6-min tail suspension, and 30-min predator odor. *p<0.05 vs. “baseline” level (ANOVA, main effect of stressor); Group size for females was 5 (baseline), 9 (restraint), and 10 (tail suspension and predator odor); Group size for males was 9 (baseline, restraint, and tail suspension) and 8 (predator odor). A change in group size is reflective of either loss of plasma or levels of ALLO falling below the sensitivity of the assay.
Figure 7.
Regression analysis of g/kg ethanol (10E) intake and ng/mL allopregnanolone (ALLO) levels following select stressors in Cohort 1. There was a trend toward a significant negative relationship between ALLO levels following restraint stress and 10E intake on the day of (A), but not the day after (B) administration of the stressor. The r2 value indicates that 20% of the variance in 10E intake on the day of restraint stress could be explained by ALLO levels immediately after 30 min of restraint. There was no relationship between ALLO levels following tail suspension or predator odor stress on the day of (C and E, respectively) or the day after (D and F, respectively) administration of the stressor.
Discussion
The present studies evaluated the impact of acute exposure to a variety of stressors with a strong physical or psychological component on limited access ethanol intake using adult male and female C57BL/6J mice. All five stressors significantly decreased 10E intake on the day of stress in males and females, with the exception of predator odor stress. In both sexes, the suppression in 10E intake was transient following tail suspension stress, but 10E intake remained suppressed for two days post restraint and footshock stress. However, female mice were more delayed in returning to baseline 10E intake than were males following tail pinch stress, and 10E intake was significantly increased on the two days following predator odor stress in female mice and increased on the second day following predator odor in males. Preference ratio was rarely altered by the stressors, but there was an increase in water intake and a decrease in preference ratio on the day of restraint stress and predator odor stress. The lack of parallel changes in preference ratio and 10E intake following all stress exposures is likely due to the low water intake during the 2 hr session and the high baseline preference ratio (range 0.833 – 0.975). Additionally, exposure to restraint, tail suspension, and predator odor stress significantly increased plasma CORT levels in male and female mice, with sex differences in the increase over baseline following predator odor stress (increase of 173.4% in females and 117.0% in males) and in the rank order of the stress-induced increase in CORT levels (predator odor > restraint > tail suspension in females; restraint > predator odor > tail suspension in males). It is notable that 26% of the variance in 10E intake in male and female mice on the day after predator odor stress as well as 42% of the variance in 10E intake in female mice could be explained by CORT levels immediately after predator odor or restraint stress exposure, respectively. Likewise, exposure to the three stressors produced a greater increase in ALLO levels in female versus male mice over baseline, especially for restraint stress and predator odor stress (increase of 267.3% & 261.5% for females; increase of 95.7% & 90.6% for males). Altogether, these data are the first to survey the impact of a variety of acute, environmental stressors on limited-access ethanol consumption in adult male and female C57BL/6J mice, and they reveal that the type of stressor administered has a profound impact on subsequent ethanol consumption, consistent with existing evidence (reviewed in Becker et al., 2011). Furthermore, the greater HPA activation following predator odor stress as well as in levels of a GABAergic neurosteroid following predator odor and restraint stress in female mice suggests that females may be more sensitive to these effects.
The main focus of this study was to evaluate the impact of various acute stressors on ethanol consumption using a limited access 2-bottle choice paradigm. In the present study, 10E intake fluctuated across time, reflecting the normal variation in 2 hr ethanol intake that we observe in male and female C57BL/6J mice, with the exception of the drop in 10E intake after restraint stress and prior to the tail suspension stress in male mice from Cohort 1. We always ensured that a stable baseline was established prior to administration of another stressor, and in most cases, at least one week elapsed between each stressor. Although we cannot rule out the potential for order effects in the present findings, we observed similar effects of predator odor stress on subsequent ethanol intake when it was the first (Cohort 2) or third (Cohort 1) stressor to be administered, suggesting that order effects did not contribute significantly to the present findings. Consistent with this idea, a recent study examined swim-, restraint-, and footshock-stress in male C57BL/6J mice for potential effects on depression-like behaviors and found no test interactions (Chourbaji et al., 2008), suggesting that each stressor did not affect performance in a subsequent behavioral test. Thus, we believe that order effects did not exert a significant confound to interpretation of the present results.
When evaluating baseline ethanol intake, female mice not only consumed greater levels of ethanol than male mice but they also had significantly higher BECs. This finding agrees with evidence that female mice consume higher doses of ethanol than male mice in a multitude of ethanol access conditions (e.g. Finn et al., 2004; Juárez and Barrios de Tomasi, 1999; Lancaster and Spiegel, 1992; Lopez et al., 2003, 2011; Middaugh and Kelley, 1999; Middaugh et al., 1999; Sinnott et al., 2002; Yoneyama et al., 2008). The greater ethanol intake in females extended across time in the present studies with one exception; when the impact of restraint stress was evaluated, baseline 10E consumption by females was slightly lower than that by males. Restraint stress was measured first in Cohort 1 on day 19 (Figure 1A), so it is unclear why a sex difference in 10E intake did not emerge until day 28 of the study. We did not monitor the females for stage of estrous cycle in the present studies, which can influence ALLO levels (e.g., Finn and Gee, 1994; Paul and Purdy, 1992) as well as ethanol consumption (Morin and Forger, 1982; Lancaster and Spiegel, 1992; Roberts et al., 1998). However, it is not known whether ethanol consumption or acute stress exposures would alter estrous cyclicity in the female mice in the present study. Limited data indicate that separate groups of female rats exposed to intermittent restraint stress, in the presence or absence of exposure to predator odor (female cat odor) stress, exhibited normal 4-5 day estrous cycles (Perrot-Sinal et al., 2004), suggesting that exposure of female rats to a female predator odor did not alter estrous cycling. In the present study, the dirty rat bedding was obtained from male and female rats; thus, it is unknown if the combination of male and female predator odor would alter estrous cyclicity. Likewise, the induction of physical dependence can disrupt normal estrous cycling in female mice (Veatch et al., 2007), but there is no evidence that female C57BL/6J mice with months of 2 hr limited access ethanol drinking exhibit signs of physical dependence. Thus, we are not aware of any data to show that the duration of limited access ethanol drinking in the present study (44-45 days) would alter estrous cycling in females. In the present study, baseline ALLO levels did not differ in male and female mice, and low levels in females at baseline suggested that the female mice were in diestrus-2 at that time point. However, our assessment of “baseline” ALLO levels was taken prior to any stressor exposure but after the 2 hr 10E intake session on day 16, and our earlier work indicated that brain ALLO levels did not differ in male and female C57BL/6J mice after 17 continuous days of 2 hr 10E intake (Finn et al., 2004). Thus, it is difficult to know if the lack of sex difference in “baseline” ALLO levels in the present study reflects an influence of 10E consumption or stage of the estrous cycle. Nonetheless, the range in variation in 10E intake across days did not differ in males or females, suggesting that estrous cycle phase did not contribute significant variability in the present studies.
As mentioned above, blood samples to measure “baseline” CORT and ALLO levels were collected following 16 days of limited access ethanol drinking. And while this does not reflect a true baseline measurement, we previously found that CORT levels did not differ in male and female C57BL/6J mice following 17 days of limited access 10E intake or 17 days of water intake (Finn et al., 2004). Thus, the “baseline” CORT measurement in the present study provided a good estimate of basal CORT levels in individually housed adult male and female mice from which to compare the increase in CORT levels following stress exposure. Importantly, exposure to restraint, tail suspension, and predator odor stress significantly increased CORT levels over “baseline” in male and female mice with a history of limited access ethanol intake, and the predator odor stress-induced increase in CORT levels was similar to the results from a pilot study in ethanol naïve male and female C57BL/6J mice (see Methods). In both the present and pilot study, CORT levels were significantly higher in the female versus male C57BL/6J mice. Thus, it is unlikely that a history of limited access ethanol intake in the present study, which would not be expected to produce physical dependence, altered the acute stress response, measured by plasma CORT levels.
Previous studies utilizing repeated or single episodes of foot shock stress found either no effect on subsequent ethanol intake in adult mice (male and female HAP mice in Chester et al., 2008; male DBA/2J and A/J mice in Matthews et al., 2008) or a modest and non-significant increase in ethanol intake in male C57BL/6J mice that was due to a decrease in ethanol intake in the control animals. In contrast to these results, the current studies determined that foot shock stress caused a persistent decrease in ethanol intake that became more pronounced across the three days of measurement in male and female C57BL/6J mice. While procedural differences, such as intensity and duration of the footshock, likely contribute to the different results across studies, the mechanism contributing to the decrease in ethanol intake in the present study is unknown. Acute and chronic footshock did not alter the acquisition of ethanol-induced conditioned place preference (CPP) in adult male and female Kunming mice to a 1 g/kg dose of ethanol (Song et al., 2007), suggesting that footshock stress does not alter ethanol’s rewarding properties to a low ethanol dose in adult mice. Additionally, footshock stress increased CORT levels by approximately 100% in male C57BL/6J mice (Chourbaji et al., 2008; Matthews et al., 2008), consistent with a stress-induced activation of the HPA axis, but it is not known whether footshock stress would produce a similar increase in CORT levels in female C57BL/6J mice. However, female versus male HAP mice show greater anxiety-related behavior following footshock stress, as evident by a greater startle response (Chester et al., 2008), which reflects well with human studies indicating that females are more susceptible and/or vulnerable to depression and anxiety disorders (Bruce et al., 2005; Cryan et al., 2005).
Repeated restraint stress also has produced varying results in adult mice. Ten days of 2-hr restraint, followed by 20 days of intermittent restraint stress, increased ethanol intake in male HAP mice, but decreased ethanol intake in female HAP mice (Chester et al., 2006). However, acoustic startle reactivity did not differ in the male and female HAP mice following a final 2-hr restraint stress session, when compared with control (no-stress) groups. So, it is not known whether HAP mice habituated to the ability of repeated restraint to increase anxiety-related behavior or whether HAP mice are resistant to an anxiogenic effect of restraint stress. Additionally, subtle changes in ethanol intake were detected following repeated restraint stress in male and female genetically heterogeneous mice (i.e., Withdrawal Seizure Control; Tambour et al., 2008) or in male C57BL/6J mice (Farook et al., 2009). In the study by Farook et al. (2009), daily 1-hr restraint stress increased continuous access ethanol consumption in C57BL/6J male mice, but this effect was only present after administration of the fifth stressor. In our current study, acute 30-min restraint stress produced a decrease in ethanol intake that lasted at least two days post-restraint and that impacted both sexes equally. Taken in conjunction with the results of Farook et al. (2009), we do not know whether there would be sex differences in the ability of repeated restraint stress to produce a delayed increase in ethanol intake. Nonetheless, we also found that restraint stress produced a similar activation of the HPA axis in male and female mice, measured by plasma CORT levels, consistent with earlier work in male and female inbred long-sleep and short-sleep selected lines (Parker et al., 2008). The restraint stress-induced increase in CORT levels in the present study was positively correlated with 10E intake on the following day only in female C57BL/6J mice. In fact, regression analysis indicated that 42% of the variance in 10E intake in female mice on the day after restraint stress in the present study could be explained by the plasma CORT concentrations immediately after restraint stress. In contrast to the similar increase in CORT levels, there was a much greater increase in levels of the GABAergic neurosteroid ALLO following restraint stress in female versus male C57BL/6J mice. Conversely, plasma ALLO levels in both males and females were negatively correlated with 10E intake on the day of restraint stress, with regression analysis indicating that 20% of the variance in 10E intake on the day of restraint stress could be explained by ALLO levels immediately after the restraint stress. Collectively, these findings suggest that there are sex differences in the relationship between restraint stress-induced changes in ALLO or CORT on subsequent ethanol intake.
Traditionally, tail suspension stress (more commonly referred to as tail suspension test or TST) is used to assess depression-like symptoms (see Cryan et al., 2005 for details), and one study investigated the relationship between immobility time in the TST and ethanol consumption in male and female CD-1 mice (Pelloux et al., 2005). Placing mice into subgroups for high immobility (HI) versus low immobility (LI) time did not influence ethanol intake in male mice. However, intake of 20% ethanol (but not 3%, 6%, or 10E) was significantly higher in HI (174 sec) versus LI (69 sec) female mice (Pelloux et al., 2005). Ethanol-induced CPP also differed in the HI versus LI female mice; HI females exhibited CPP to a 3 g/kg ethanol dose, whereas LI females exhibited CPP to a 1.5 g/kg ethanol dose. These findings suggest that immobility time can influence ethanol consumption and ethanol’s rewarding properties in female CD-1 mice. In the current study, tail suspension stress caused a transient reduction in ethanol consumption in both male and female C57BL/6J mice, with 10E intake returning to baseline levels on the day following the stressor. Tail suspension stress also produced a similar activation of the HPA axis in male and female mice, measured by the percent increase in CORT levels over baseline values, as well as a fairly similar increase in ALLO levels. Immobility times in the current study were equivalent to those associated with HI times in the study by Pelloux et al. (2005), but we found that there was a negative relationship between percent time immobile and 10E intake on the day of tail suspension stress in both male and female C57BL/6J mice, which was influenced primarily by the data in male mice. In other words, high percent time immobile was associated with low 10E intake, and in male mice, 47% of the variation in 10E intake could be explained by the percent time immobile. This negative relationship between immobility time and ethanol intake is opposite to that reported by Pelloux et al. (2005) for intake of 20% ethanol in female CD-1 mice, but intake of 10E did not differ in male and female CD-1 mice with HI versus LI times. Collectively, tail suspension stress and assessment of immobility as a measure of behavioral despair or depressive-like response may be useful in studies investigating the relationship between stress and alcohol consumption.
The current work is the first to examine the effects of predator odor and tail pinch stress on ethanol consumption in mice. Tail pinch is traditionally used either as a model of stress-induced eating, where the pressure applied to the tail induces a coping strategy involving consumption of food (e.g., Heinrichs et al., 1992), or as a model of nociception, where the pressure and subsequent behavior is used to assess the analgesia of a given compound (e.g., Bodnar et al., 1978). In Maudsley Reactive inbred rats, tail pinch was found to transiently increase alcohol consumption in both males and females, but this study involved 35 days of stress, which occurred prior to establishing alcohol consumption, as well as an inbred strain of rats created to be highly reactive to stress (Adams, 1995). The increase in ethanol consumption was marginal in male versus female Maudsley Reactive rats, but the increase in female rats after tail pinch stress was greater after re-exposure to ethanol following a 6 week period of abstinence (Adams, 1995). The delayed effect of tail pinch stress may explain why the above results contrast with the current findings, where tail pinch stress produced a transient decrease in ethanol consumption in male and female C57BL/6J mice. Intake of 10E returned to baseline by the 2nd day post tail pinch stress, and it did not vary by more than the normal day-to-day variations for the remaining 14 days of the study. Thus, it is unclear whether tail pinch can be considered as a stressor of biological significance that produces a delayed increase in ethanol intake, or whether it is an arousing stimulus that can alter ethanol consumption in a highly reactive species.
Similarly, predator odor stress has also been assessed in rats, and has begun to be used as a model of posttraumatic stress (e.g., Cohen & Zohar, 2004). In agreement with the current results that predator odor stress using dirty rat bedding increased subsequent ethanol consumption in male and female C57BL/6J mice, male Wistar rats increased operant responding for ethanol after exposure to predator odor stress using bobcat urine (at least in those animals which avoided the odor-paired chamber; Edwards et al., 2013; Roltsch et al., 2014). Exposure to bobcat urine also increased thermal nociception 5 days later and increased startle reactivity 2 days later (Roltsch et al., 2014), suggesting that a high arousal and high nociception state after predator odor stress may contribute to the increase in ethanol self-administration. Mice also exhibit increased anxiety after exposure to predator odor stress. When male mice from 9 mouse strains were examined for their anxiogenic-like behavior after unavoidable cat exposure or exposure to cat odor, C57BL/6J and DBA/2J were the 2 high reactive strains that displayed the greatest anxiogenic-like responses, using the free-exploration test (Belzung et al., 2001). Exposure to dirty rat bedding increased anxiety-like behavior in male CD-1 mice, measured by acoustic startle reactivity, that persisted for one week after the stress exposure (Hebb et al., 2003), but ethanol intake was not measured. Thus, we presume that exposure to dirty rat bedding will increase anxiety in male and female C57BL/6J mice, and future studies will test this assumption. Importantly, predator odor stress significantly increased CORT and ALLO levels in the present study, and also produced a sex-dependent effect, with female mice showing greater stress-induced increases in both steroids than male mice. Because ethanol intake was significantly increased following predator odor stress in females on both post-stress day measurements, the higher CORT levels in female mice support the possibility that HPA axis responsivity could be one potential mechanism underlying sex differences in the impact of stress on ethanol intake (Chester et al., 2006). Consistent with this idea, 26% of the variance in 10E intake on the day after predator odor stress could be explained by CORT levels immediately after predator odor exposure. Together these data support the notion that predator odor stress may be an excellent model of posttraumatic stress and compliment literature from human studies indicating that posttraumatic stress disorder and alcohol-use disorders are comorbid (e.g., Engdahl et al., 1998).
It is interesting that the acute stress-induced increase in plasma CORT and ALLO levels was not consistently correlated with subsequent 10E intake, implying differences in the effects of these steroids on HPA axis responsivity and in the potential impact on ethanol consumption. Although the mechanism is not known, a few possibilities will be considered. It is well documented that CORT exerts negative feedback at the level of the PVN to inhibit the synthesis and release of CRH (e.g., Makino et al., 2002), and recent findings indicate that physiological concentrations of ALLO (10 – 100 nM) inhibit the output of PVN neurons (i.e., CRH release) via a potentiation of GABAA receptors (see Gunn et al., 2011); both actions could contribute to a termination of the stress response, but the time course for the two steroids across various stressors likely differs. In mice that are drinking ethanol, it is possible that diminished negative feedback and/or enhanced positive feedback at the level of the amygdala (e.g., Makino et al., 2002) would offset an ALLO-induced constraint of the HPA axis. Alternately, GABAA receptor transmission can shift from hyperpolarization to depolarization, depending on the expression of cation-chloride co-transporters (CCC) that maintain the transmembrane chloride (Cl-) gradient (Blaesse et al., 2009). Recent work found that restraint stress down-regulated one of the CCCs, which produced a depolarizing action of GABA in the PVN and weakened the inhibitory constraint of the HPA axis (Hewitt et al., 2009). It is not known whether the various stressors employed would exert similar changes in the Cl- gradient and the impact on GABAA receptor-mediated transmission in the PVN. A final consideration is that a stress-induced increase in CRH also activates the locus coeruleus (LC) – norepinephrine (NE) stress/arousal system, and that there are sex differences in LC neuronal responses to CRH as well as in the trafficking of CRH-R1 receptors in LC dendrites and in the potential engagement of distinct cell signaling pathways (reviewed by Valentino et al., 2012). Under this scenario, the response to CRH could be either prolonged in females due to compromised receptor internalization or qualitatively distinct in males and females as a result of engaging different cell signaling pathways. Regardless, there are numerous potential mechanisms that may contribute to the present findings.
Altogether, the current study indicates that during baseline conditions female C57BL/6J mice consume greater amounts of ethanol during a 2-bottle choice limited-access paradigm as reflected by higher g/kg and BECs, when compared with male mice. Both physical and psychological stressors were examined, and the impact of acute stress on ethanol intake was dependent on type of stressor administered as well as the sex of the animal. We are the first to demonstrate that a single, acute exposure to restraint, tail suspension, and predator odor stress increased plasma CORT and ALLO levels in animals with a history of ethanol consumption and that female mice were more responsive than males to the ability of stress to increase CORT and ALLO levels as well as to increase10E intake following predator odor stress. The positive linear relationships between CORT levels following restraint stress or predator odor stress and 10E intake provide insight regarding HPA axis following an acute stressor and subsequent ethanol intake. Acute stress-related changes in immobility times and in anxiety-related behaviors also may be informative to studies modeling the relationship between anxiety- and/or depression-related symptoms and alcohol use disorders. Finally, perceived predation risk has a profound biological effect across species (Zanette et al., 2011). Because predator odor stress is a model of posttraumatic stress disorder, the present sex differences have important implications for future preclinical studies modeling the comorbidity of posttraumatic stress disorder and alcohol use disorders.
Acknowledgement
Funding for these studies was provided by a grant from the Department of Veterans Affairs (BX001070, DAF). Additionally, this material is the result of work supported with resources and the use of facilities at the Portland VA Medical Center (DAF). DKC was supported by a training grant (NIAAA, T32 AA007468) as well as RO1 AA106981 (NIAAA, awarded to DAF). CBH was supported by the VA College Summer Student Fellowship Program. The C57BL/6J mice were supplied from a breeding colony that was maintained by the Portland Alcohol Research Center (NIAAA, P60 AA10760 awarded to Dr. John C. Crabbe). We also thank two anonymous reviewers for their careful evaluation of this manuscript and their helpful suggestions.
References
- Adams N. Sex differences and the effects of tail pinch on ethanol drinking in Maudsley rats. Alcohol. 1995;12:463–468. doi: 10.1016/0741-8329(95)00032-m. [DOI] [PubMed] [Google Scholar]
- Anisman H, Matheson K. Stress, depression, and anhedonia: caveats concerning animal models. Neurosci. Biobehav. Rev. 2005;29:525–546. doi: 10.1016/j.neubiorev.2005.03.007. [DOI] [PubMed] [Google Scholar]
- Barbaccia ML, Serra M, Purdy RH, Biggio G. Stress and neuroactive steroids. Int. Rev. Neurobiol. 2001;46:243–272. doi: 10.1016/s0074-7742(01)46065-x. [DOI] [PubMed] [Google Scholar]
- Barfield ET, Barry SM, Hodgin HB, Thompson BM, Allen SS, Grisel JE. β-Endorphin mediates behavioral despair and the effect of ethanol on the tail suspension test in mice. Alcohol. Clin. Exp. Res. 2010;34:1066–1072. doi: 10.1111/j.1530-0277.2010.01182.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker HC, Lopez MF, Doremus-Fitzwater TL. Effects of stress on alcohol drinking: a review of animal studies. Psychopharmacology. 2011;218:131–156. doi: 10.1007/s00213-011-2443-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belzung C, El Hage W, Moindrot N, Griebel G. Behavioral and neurochemical changes following predatory stress in mice. Neuropharmacology. 2001;41:400–408. doi: 10.1016/s0028-3908(01)00072-7. [DOI] [PubMed] [Google Scholar]
- Biggio G, Pisu MG, Biggio F, Serra M. Allopregnanolone modulation of HPA axis function in the adult rat. Psychopharmacology. 2014 Mar 22; doi: 10.1007/s00213-014-3521-6. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- Blaesse P, Airaksinen MS, Rivera C, Kaila K. Cation-chloride cotransporters and neuronal function. Neuron. 2009;61:820–838. doi: 10.1016/j.neuron.2009.03.003. [DOI] [PubMed] [Google Scholar]
- Bodnar RJ, Kelly DD, Glusman M. Stress-induced analgesia: Time course of pain reflex alterations following cold water swims. Bulletin of the Psychonomic Society. 1978;11:333–336. [Google Scholar]
- Bruce SE, Yonkers KA, Otto MW, Eisen JL, Weisberg RB, Pagano M, Shea MT, Keller MB. Influence of psychiatric comorbidity on recovery and recurrence in generalized anxiety disorder, social phobia, and panic disorder: a 12-year prospective study. Am. J. Psychiatry. 2005;162:1179–1187. doi: 10.1176/appi.ajp.162.6.1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burrows HL, Nakajima M, Lesh JS, Goosens KA, Samuelson LC, Inui A, Camper SA, Seasholtz AF. Excess corticotropin releasing hormone-binding protein in the hypothalamic-pituitary-adrenal axis in transgenic mice. J. Clin. Invest. 1998;101:1439–1447. doi: 10.1172/JCI1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butts KA, Weinberg J, Young AH, Phillips AG. Glucocorticoid receptors in the prefrontal cortex regulate stress-evoked dopamine efflux and aspects of executive function. Proc. Natl. Acad. Sci. U.S.A. 2011;108:18459–18464. doi: 10.1073/pnas.1111746108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chester JA, Berrenha GD, Hughes ML, Kueneke KJ. Age- and sex-dependent effects of footshock stress on subsequent alcohol drinking and acoustic startle behavior in mice selectively bred for high-alcohol preference. Alcohol. Clin. Exp. Res. 2008;32:1782–1794. doi: 10.1111/j.1530-0277.2008.00763.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chester JA, de Paula Barrenha G, DeMaria A, Finnegan A. Different effects of stress on alcohol drinking behavior in male and female mice selectively bred for high alcohol preference. Alcohol Alcohol. 2006;41:44–53. doi: 10.1093/alcalc/agh242. [DOI] [PubMed] [Google Scholar]
- Chourbaji S, Brandwein C, Vogt MA, Dormann C, Gass P. Evaluation of effects of previous exposure to an acute stressor before testing for depression-like behaviours in mice. Stress. 2008;11:170–175. doi: 10.1080/10253890701560119. [DOI] [PubMed] [Google Scholar]
- Cohen H, Zohar J. An animal model of posttraumatic stress disorder: the use of cut-off behavioral criteria. Ann. N.Y. Acad. Sci. 2004;1032:167–178. doi: 10.1196/annals.1314.014. [DOI] [PubMed] [Google Scholar]
- Cryan JF, Mombereau C, Vassout A. The tail suspension test as a model for assessing antidepressant activity: review of pharmacological and genetic studies in mice. Neurosci. Biobehav. Rev. 2005;29:571–625. doi: 10.1016/j.neubiorev.2005.03.009. [DOI] [PubMed] [Google Scholar]
- Cullinan WE, Ziegler DR, Herman JP. Functional role of local GABAergic influences on the HPA axis. Brain Struct. Funct. 2008;213:63–72. doi: 10.1007/s00429-008-0192-2. [DOI] [PubMed] [Google Scholar]
- Edwards S, Baynes BB, Carmichael CY, Zamora-Martinez ER, Barrus M, Koob GF, Gilpin NW. Traumatic stress reactivity promotes excessive alcohol drinking and alters the balance of prefrontal cortex-amygdala activity. Transl. Psychiatry. 2013;3:e296. doi: 10.1038/tp.2013.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engdahl B, Dikel TN, Eberly R, Blank A., Jr. Comorbidity and course of psychiatric disorders in a community sample of former prisoners of war. Am. J. Psychiatry. 1998;155:1740–1745. doi: 10.1176/ajp.155.12.1740. [DOI] [PubMed] [Google Scholar]
- Farook JM, Lewis B, Littleton JM, Barron S. Topiramate attenuates the stress-induced increase in alcohol consumption and preference in male C57BL/6J mice. Physiol. Behav. 2009;96:189–193. doi: 10.1016/j.physbeh.2008.08.011. [DOI] [PubMed] [Google Scholar]
- Finn DA, Gee KW. The estrus cycle, sensitivity to convulsants and the anticonvulsant effect of a neuroactive steroid. J. Pharmacol. Exp. Ther. 1994;271:164–170. [PubMed] [Google Scholar]
- Finn DA, Sinnott RS, Ford MM, Long SL, Tanchuck M, Phillips TJ. Sex differences in the effect of ethanol injection and consumption on brain allopregnanolone levels in C57BL/6 mice. Neuroscience. 2004;123:813–819. doi: 10.1016/j.neuroscience.2003.11.017. [DOI] [PubMed] [Google Scholar]
- Finn DA, Snelling C, Fretwell AM, Tanchuck MA, Underwood L, Cole M, Crabbe JC, Roberts AJ. Increased drinking during withdrawal from intermittent ethanol exposure is blocked by the CRF receptor antagonist D-Phe-CRF(12-41) Alcohol. Clin. Exp. Res. 2007;31:939–949. doi: 10.1111/j.1530-0277.2007.00379.x. [DOI] [PubMed] [Google Scholar]
- Gunn BG, Brown AR, Lambert JJ, Belelli D. Neurosteroids and GABAA receptor interactions: a focus on stress. Front. Neurosci. 2011;5:131. doi: 10.3389/fnins.2011.00131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hebb ALO, Zacharko RM, Domingues H, Laforest S, Gauthier M, Levac C, Drolet G. Changes in brain cholecystokinin and anxiety-like behavior following exposure of mice to predator odor. Neuroscience. 2003;116:539–551. doi: 10.1016/s0306-4522(02)00710-8. [DOI] [PubMed] [Google Scholar]
- Heinrichs SC, Cole BJ, Pich EM, Menzaghi F, Koob GF, Hauger RL. Endogenous corticotropin-releasing factor modulates feeding induced by neuropeptide Y or a tail-pinch stressor. Peptides. 1992;13:874–884. doi: 10.1016/0196-9781(92)90044-4. [DOI] [PubMed] [Google Scholar]
- Herman JP, Mueller NK, Figueiredo H. Role of GABA and glutamate circuitry in hypothalamo-pituitary-adrenocortical stress integration. Ann. N.Y. Acad. Sci. 2004;1018:35–45. doi: 10.1196/annals.1296.004. [DOI] [PubMed] [Google Scholar]
- Hewitt SA, Wamsteeker JI, Kurz EU, Bains JS. Altered chloride homeostasis removes synaptic inhibitory constraint of the stress axis. Nature Neurosci. 2009;12:438–443. doi: 10.1038/nn.2274. [DOI] [PubMed] [Google Scholar]
- Juárez J, Barrios de Tomasi E. Sex differences in alcohol drinking patterns during forced and voluntary consumption in rats. Alcohol. 1999;19:15–22. doi: 10.1016/s0741-8329(99)00010-5. [DOI] [PubMed] [Google Scholar]
- Keyes KM, Hatzenbuehler ML, Grant BF, Hasin DS. Stress and alcohol: epidemiologic evidence. Alcohol Res. 2012;34:391–400. doi: 10.35946/arcr.v34.4.03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob G, Kreek MJ. Stress, dysregulation of drug reward pathways, and the transition to drug dependence. Am. J. Psychiatry. 2007;164:1149–1159. doi: 10.1176/appi.ajp.2007.05030503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob G, Le Moal M. Drug addiction, dysregulation of reward, and allostasis. Neuropsychopharmacology. 2001;24:97–129. doi: 10.1016/S0893-133X(00)00195-0. [DOI] [PubMed] [Google Scholar]
- Lancaster FE, Spiegel KS. Sex differences in pattern of drinking. Alcohol. 1992;9:415–420. doi: 10.1016/0741-8329(92)90041-8. [DOI] [PubMed] [Google Scholar]
- Lee S, Selvage D, Hansen K, Rivier C. Site of action of acute alcohol administration in stimulating the rat hypothalamic-pituitary-adrenal axis: comparison between the effect of systemic and intracerebroventricular injection of this drug on pituitary and hypothalamic responses. Endocrinology. 2004;145:4470–4479. doi: 10.1210/en.2004-0110. [DOI] [PubMed] [Google Scholar]
- Lopez MF, Becker HC. Gender differences in ethanol intake prior to and following repeated chronic ethanol exposure and withdrawal in C57BL/6J mice. Alcohol. Clin. Exp. Res. 2003;27:15A. doi: 10.1097/01.alc.0000149977.95306.3a. [DOI] [PubMed] [Google Scholar]
- Lopez MF, Doremus-Fitzwater TL, Becker HC. Chronic social isolation and chronic variable stress during early development induce later elevated ethanol intake in adult C57BL/6J mice. Alcohol. 2011;45:355–364. doi: 10.1016/j.alcohol.2010.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makino S, Hashimoto K, Gold PW. Multiple feedback mechanisms activating corticotropin-releasing hormone system in the brain during stress. Pharmacol. Biochem. Behav. 2002;73:147–158. doi: 10.1016/s0091-3057(02)00791-8. [DOI] [PubMed] [Google Scholar]
- Matthews DB, Morrow AL, O’Buckley T, Flanigan TJ, Berry RB, Cook MN, Mittleman G, Goldwitz D, Tokunaga S, Silvers JM. Acute mild footshock alters ethanol drinking and plasma corticosterone levels in C57BL/6J male mice, but not DBA/2J or A/J male mice. Alcohol. 2008;42:469–476. doi: 10.1016/j.alcohol.2008.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Middaugh LD, Kelley BM. Operant ethanol reward in C57BL/6 mice: influence of gender and procedural variables. Alcohol. 1999;17:185–194. doi: 10.1016/s0741-8329(98)00056-1. [DOI] [PubMed] [Google Scholar]
- Middaugh LD, Kelley BM, Brandy AL, McGroarty KK. Ethanol consumption by C57BL/6 mice: influence of gender and procedural variables. Alcohol. 1999;17:175–183. doi: 10.1016/s0741-8329(98)00055-x. [DOI] [PubMed] [Google Scholar]
- Morin LP, Forger NG. Endocrine control of ethanol intake by rats or hamsters: Relative contributions of the ovaries, adrenals and steroids. Pharmacol. Biochem. Behav. 1982;17:529–537. doi: 10.1016/0091-3057(82)90315-x. [DOI] [PubMed] [Google Scholar]
- Parker CC, Ponicsan H, Spencer RL, Holmes A, Johnson TE. Restraint stress and exogenous corticosterone differentially alter sensitivity to the sedative-hypnotic effects of ethanol in inbred long-sleep and inbred short-sleep mice. Alcohol. 2008;42:477–485. doi: 10.1016/j.alcohol.2008.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patchev VK, Hassan AH, Holsboer DF, Almeida OF. The neurosteroid tetrahydroprogesterone attenuates the endocrine response to stress and exerts glucocorticoid-like effects on vasopressin gene transcription in the rat hypothalamus. Neuropsychopharmacology. 1996;15:533–540. doi: 10.1016/S0893-133X(96)00096-6. [DOI] [PubMed] [Google Scholar]
- Paul SM, Purdy RH. Neuroactive steroids. FASEB J. 1992;6:2311–2322. [PubMed] [Google Scholar]
- Pelloux Y, Hagues G, Costentin J, Duterte-Boucher D. Helplessness in the tail suspension test is associated with an increase in ethanol intake and its rewarding effect in female mice. Alcohol. Clin. Exp. Res. 2005;29:378–388. doi: 10.1097/01.alc.0000156123.10298.fa. [DOI] [PubMed] [Google Scholar]
- Perrot-Sinal TS, Gregus A, Boudreau D, Kalynchuck LE. Sex and repeated restraint stress interact to affect cat odor-induced defensive behavior in adult rats. Brain Res. 2004;1027:161–172. doi: 10.1016/j.brainres.2004.08.056. [DOI] [PubMed] [Google Scholar]
- Racz I, Bilkei-Gorzo A, Toth ZE, Michel K, Palkovits M, Zimmer A. A critical role for the cannabinoid CB1 receptors in alcohol dependence and stress-stimulated ethanol drinking. J. Neurosci. 2003;23:2453–2458. doi: 10.1523/JNEUROSCI.23-06-02453.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson HN, Lee SY, O’Dell LE, Koob GF, Rivier CL. Alcohol self-administration acutely stimulates the hypothalamic-pituitary-adrenal axis, but alcohol dependence leads to a dampened neuroendocrine state. Eur. J. Neurosci. 2008;28:1641–1653. doi: 10.1111/j.1460-9568.2008.06455.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivier C. Gender, sex steroids, corticotropin-releasing factor, nitric oxide, and the HPA response to stress. Pharmacol. Biochem. Behav. 1999;64:739–751. doi: 10.1016/s0091-3057(99)00148-3. [DOI] [PubMed] [Google Scholar]
- Roberts AJ, Smith AD, Weiss F, Rivier C, Koob GF. Estrous cycle effects on operant responding for ethanol in female rats. Alcohol. Clin. Exp. Res. 1998;22:1564–1569. [PubMed] [Google Scholar]
- Roltsch EA, Baynes BB, Mayeux JP, Whitaker AM, Baiamonte BA, Gilpin NW. Predator odor stress alters corticotropin-releasing factor-1 receptor (CRF1R)-dependent behaviors in rats. Neuropharmacology. 2014;79:83–89. doi: 10.1016/j.neuropharm.2013.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanford LD, Yang L, Wellman LL, Liu X, Tang X. Differential effects of controllable and uncontrollable footshock stress on sleep in mice. Sleep. 2010;33:621–630. doi: 10.1093/sleep/33.5.621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinnott RS, Phillips TJ, Finn DA. Alteration of voluntary ethanol and saccharin consumption by the neurosteroid allopregnanolone in mice. Psychopharmacology. 2002;162:438–447. doi: 10.1007/s00213-002-1123-1. [DOI] [PubMed] [Google Scholar]
- Song M, Wang X-Y, Zhao, M., Wang X-Y, Zhai H-F, Lu L. Role of stress in acquisition of alcohol-conditioned place preference in adolescent and adult mice. Alcohol. Clin. Exp. Res. 2007;31:2001–2005. doi: 10.1111/j.1530-0277.2007.00522.x. [DOI] [PubMed] [Google Scholar]
- Tambour S, Brown LL, Crabbe JC. Gender and age at drinking onset affect voluntary alcohol consumption but neither the alcohol deprivation effect nor the response to stress in mice. Alcohol. Clin. Exp. Res. 2008;32:2100–2106. doi: 10.1111/j.1530-0277.2008.00798.x. [DOI] [PubMed] [Google Scholar]
- Valentino RJ, Reyes B, Van Bockstaele E, Bangasser D. Molecular and cellular sex differences at the intersection of stress and arousal. Neuropharmacology. 2012;62:13–20. doi: 10.1016/j.neuropharm.2011.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veatch LM, Wright TM, Randall CL. Only male mice show sensitization of handling-induced convulsions across repeated ethanol withdrawal cycles. Alcohol. Clin. Exp. Res. 2007;31:477–485. doi: 10.1111/j.1530-0277.2006.00328.x. [DOI] [PubMed] [Google Scholar]
- Willey AR, Anderson RI, Morales M, Ramirez RL, Spear LP. Effects of ethanol administration on corticosterone levels in adolescent and adult rats. Alcohol. 2012;46:29–36. doi: 10.1016/j.alcohol.2011.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang X, Wang S, Rice KC, Munro CA, Wand GS. Restraint stress and ethanol consumption in two mouse strains. Alcohol. Clin. Exp. Res. 2008;32:840–852. doi: 10.1111/j.1530-0277.2008.00632.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoneyama N, Crabbe JC, Ford MM, Murillo A, Finn DA. Voluntary ethanol consumption in 22 inbred mouse strains. Alcohol. 2008;42:149–160. doi: 10.1016/j.alcohol.2007.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zanette LY, White AF, Allen MC, Clinchy M. Perceived predation risk reduces the number of offspring songbirds produce per year. Science. 2011;334:1398–1401. doi: 10.1126/science.1210908. [DOI] [PubMed] [Google Scholar]