Abstract
Purpose of review
To describe the roles of apolipoprotein C-III (apoC-III) and apoE in VLDL and LDL metabolism
Recent findings
ApoC-III can block clearance from the circulation of apolipoprotein B (apoB) lipoproteins, whereas apoE mediates their clearance. Normolipidemia is sustained by hepatic secretion of VLDL and IDL subspecies that contain both apoE and apoC-III (VLDL E+C-III+). Most of this VLDL E+C-III+ is speedily lipolyzed, reduced in apoC-III content, and cleared from the circulation as apoE containing dense VLDL, IDL, and light LDL. In contrast, in hypertriglyceridemia, most VLDL is secreted with apoC-III but without apoE, and so it is not cleared until it loses apoC-III during lipolysis to dense LDL. In normolipidemia, the liver also secretes IDL and large and medium-size LDL, whereas in hypertriglyceridemia, the liver secretes more dense LDL with and without apoC-III. These pathways establish the hypertriglyceridemic phenotype and link it metabolically to dense LDL. Dietary carbohydrate compared with unsaturated fat suppresses metabolic pathways mediated by apoE that are qualitatively similar to those suppressed in hypertriglyceridemia.
Summary
The opposing actions of apoC-III and apoE on subspecies of VLDL and LDL, and the direct secretion of LDL in several sizes, establish much of the basic structure of human apoB lipoprotein metabolism in normal and hypertriglyceridemic humans.
Keywords: apolipoprotein C-III, apolipoprotein E, lipoproteins, metabolism
Introduction
Because the apolipoprotein B (apoB) lipoproteins, VLDL and LDL, cause atherosclerosis, mechanisms that produce high levels are important to identify, especially mechanisms that sustain levels of potently atherogenic subspecies such as those that have apoC-III. Some diets and drugs may suppress mechanisms that form atherogenic lipoproteins and some may accelerate their removal from plasma. Knowing which treatments do this could be useful to anticipate beneficial clinical effects before results from clinical outcome trials become available.
VLDL and LDL Metabolism are Both Independent and Connected
A common view of apoB lipoprotein metabolism has the liver producing mostly VLDL, a large spherical particle that has a core rich in triglyceride and cholesterol. Exposure to lipoprotein lipase (LpL) first, and hepatic lipase later, hydrolyzes most of the triglyceride to unesterified fatty acids, which are delivered to muscle and fat, and to the liver. During this process, some VLDL is cleared from the circulation by interacting with hepatic receptors. Over a few hours in circulation, the remaining VLDL is metabolized in plasma to LDL, a much more slowly turning-over lipoprotein that circulates for days. LDL, cholesterol-rich and triglyceride-poor, is viewed mostly as a product of VLDL metabolism, and VLDL is considered mainly as a precursor of LDL.
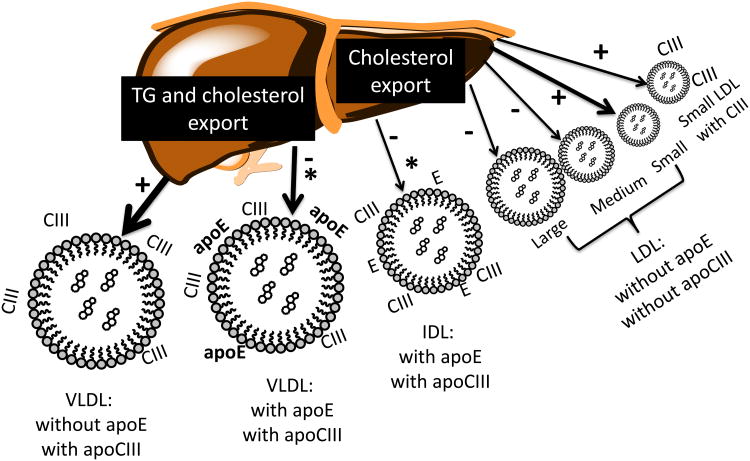
In reality, this standard framework for apoB lipoprotein metabolism oversimplies a fascinatingly intricate, finely tuned metabolic system that handles two very different lipids, triglyceride used for cellular energy production, and cholesterol for diverse physiological functions like bile acid synthesis, steroid hormone synthesis, lipoprotein synthesis, and many vital processes carried out by cell membranes. The body's need for metabolites of triglyceride and cholesterol differs over time. It would be efficient for VLDL and LDL secretion into plasma to be loosely coupled, so as to respond to time-varying nutritional status and needs of hepatic and extrahepatic tissues. Lipoprotein kinetic studies, using diverse techniques of labeling, lipoprotein preparation, and kinetic analysis, have identified secretion of LDL by the liver, at the same time the liver secretes VLDL [1–8]. Nonetheless, LDL is still most commonly thought of as a product of lipolysis of VLDL. A substantial percentage of plasma LDL is secreted directly, for example, 38% in normolipidemic individuals compared with 27% in hypertriglyceridemia [7]. The percentage of LDL that is directly secreted decreases as plasma triglyceride increases [8]. Fisher et al. [9] proposed that the relative amounts of VLDL and LDL secreted into plasma reflect the body's status of triglyceride and cholesterol pools that need to be mobilized during metabolism. Direct secretion of LDL presumably serves an important function in regulating hepatic and extrahepatic cholesterol content. It also plays a rolein the establishment of the dense LDL phenotype in hypertriglyceridemia by increasing the secretion of dense LDL as explained later (Fig. 1).
Figure 1.
Spectrum of apoB lipoprotein secretion by the liver across VLDL, IDL, and LDL subspecies. The figure illustrates VLDL, IDL, and LDL subspecies that shift in hypertriglyceridemia compared with normal, and on a high-carbohydrate compared with high-unsaturated fat diet. A need to export triglyceride stimulates secretion of VLDL, whereas cholesterol export occurs on both VLDL and LDL. In hypertriglyceridemia, secretion of the subspecies of VLDL that has apoC-III but not apoE is increased; and decreased of VLDL that has both apoC-III and apoE. In hypertriglyceridemia, secretion of dense LDL with or without apoC-III is increased. +: secretion increased in hypertriglyceridemia. −: secretion decreased in hypertriglyceridemia. *: secretory pathways decreased by dietary carbohydrate.
Apolipoprotein E and Apolipoprotein C-Iii Define Subspecies of Apolipoprotein B Lipoproteins
Besides the obligatory protein, apoB, some VLDL and LDL have small apolipoproteins on their surfaces that are not required for their synthesis or secretion but modulate the metabolism of these particles and their lipid contents. ApoE and apoC-III are the most extensively studied of these. ApoE and apoC-III are not uniformly distributed among every VLDL and LDL, even though there is enough apoC-III in apoB lipoproteins for every VLDL and LDL particle to have at least one molecule, and there is also enough apoE to exist on every VLDL particle and on most LDL [7,8,10]. Instead, apoE and apoC-III cluster on a portion of VLDL and LDL and thereby form distinct subspecies that contain many molecules of each (Fig.2). In normolipidemic people, 35–60% of VLDL has apoE and40–80%has apoC-III, varying with age and level of triglycerides [7,8,10–12]. On average, a VLDL with apoC-III has 60–100 molecules of apoC-III, and VLDL with apoE has 10–20 molecules of apoE. Some VLDL and LDL have both apoE and C-III. This concept of speciation was proposed and developed by the late Petar Alaupovic who named apoB lipoproteins according to the proteins they contained [13,14]. Alaupovic's idea was that the proteins on lipoproteins governed their function and their relation to coronary heart disease (CHD), which has been proven true. For example, apoE and apoC-III have opposing effects on the metabolism of VLDL and LDL [7,8,10] that align with their divergent associations with risk of CHD [15–17,18■■].
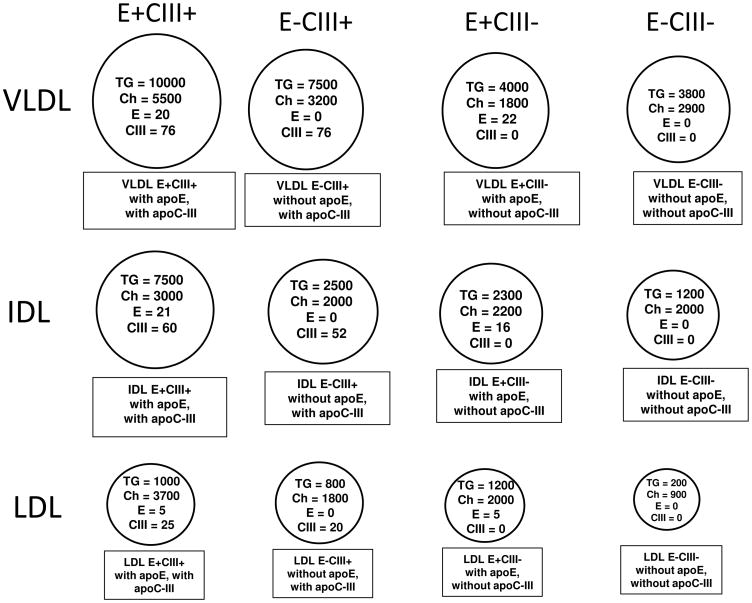
Figure 2.
Speciation of apoB lipoproteins by content of apoE and apoC-III [7]. Plasma was separated first by anti-apoE immunoaffinity chromatography. Second, the bound fraction containing apoE and the unbound fraction not having apoE were separated by anti-apoC-III immunoaffinity chromatography. In this way, four subfractions were prepared; E−C-III−, no apoE or apoC-III; E−C-III+, no apoE, apoC-III present; E+C-III−, apoE present, no apoC-III; and E+C-III+, apoE and apoC-III present. Third, VLDL, IDL, and LDL were separated from each subfraction by ultracentrifugation. Numbers are the numbers of molecules of TG, cholesterol, apoE and apoC-III per apoB in each subspecies.
Control of VLDL and LDL Metabolism by Apolipoprotein E and C-III
ApoE is a high-affinity ligand for the LDL receptor and other hepatic receptors and proteoglycan [19,20]. Chylomicrons and VLDL utilize apoE as their main ligand for clearance from the circulation. Experiments in mice, rats, and rabbits, which altered content or functionality of apoE, showed predicted effects on apoB lipoprotein levels and metabolism [21–24]. ApoE mimetic treatment reduced hypercholesterolemia and prevented atherosclerosis in apoE null mice and in LDL receptor-deficient mice [25■,26].
Owing to speciation of VLDL according to presence or absence of apoE on the lipoprotein, metabolism of the VLDL species that has apoE (VLDL apoE+) can be compared directly with the VLDL species that does not have apoE (VLDL apoE−) to determine how apoE functions on VLDL in vivo in humans. VLDL apoE+, prepared by anti-apoE immunoaffinity chromatography and ultracentrifugation, was cleared from the circulation much faster than VLDL apoE−, and was not readily converted to IDL [27]. In contrast, dense VLDL apoE− was the VLDL subspecies converted to IDL. These findings supported a role in humans for apoE as a ligand for receptor-mediated clearance of VLDL. However, apoC-III was present on most VLDL apoE+, and could have partially obscured the effect of apoE.
To evaluate separately the metabolism in plasma of apoE and apoC-III containing apoB lipoproteins, our next kinetic studies separated from plasma by sequential anti-apoE and anti-apoC-III immunoaffinity chromatography four subspecies: (1) E–C-III−, no apoE or apoC-III; (2) E-C-III+, no apoE, apoC-III present; (3) E+C-III−, apoE present, no apoC-III; and (4) E+C-III+, apoE and apoC-III present. Next, we prepared from each subspecies six apoB lipoprotein types using ultracentrifugation: light VLDL, dense VLDL, IDL, large LDL, medium LDL, and small LDL. This procedure resulted in four apolipoprotein-defined subspecies for each lipoprotein density classes shown in simplified form in Figure 2. These are distinct subspecies whose concentration is stable among individuals [28], that vary in metabolism [7,10], respond selectively to dietary macronutrients and statins [28,29], differ in hypertriglyceridemia [8], and have diverse associations with CHD [15–17].
We found that the dominant effect of apoC-III is to reduce clearance by the liver of triglyceride-rich VLDL particles [7,8,10], as found in animal models [21,30–32] (Fig. 3). Delayed clearance allows VLDL to circulate, while its triglyceride is transferred to peripheral tissues. VLDL and IDL that have apoC-III are speedily and nearly quantitatively metabolized to LDL [7,8,10]. The rate constants for lipolytic conversion of light VLDL to dense VLDL, which is LpL-mediated, were actually higher for apoC-III+ than apoC-III−. Similarly, the rate constants for metabolism of dense VLDL to IDL, effected by both lipoprotein and hepatic lipase, were also faster in C-III+ than C-III−. The metabolism of VLDL, IDL, and large LDL that have both apoE and apoC-III is divided between continued lipolysis to smaller subfractions and clearance from plasma, showing the actions of both apoE and apoC-III. During lipolytic conversion of larger to smaller apoB lipoproteins, apoC-III content per particle progressively decreases. This allows apoE and apoB100 access to hepatic receptors that clear its associated lipoprotein from the circulation. In summary, the presence of apoE and apoC-III appeared to markedly influence the metabolism of the apoB lipoproteins (Fig. 3). VLDL and IDL that have apoE but not apoC-III are cleared rapidly from the circulation before they can be metabolized to smaller lipoproteins. In fact, LDL E+C-III− is nearly undetectable in plasma, and LDL E+C-III+ is a quantitatively minor subspecies in contrast to its major presence in VLDL. Starkly contrasting, VLDL and IDL that do not have apoE or apoC-III are mostly converted by lipolysis to LDL, and have a lower fractional catabolic rate (FCR) than their counterparts with apoE, as summarized in Figure 3. This metabolic heterogeneity of apoE and apoC-III containing VLDL, IDL, and LDL is present in participants who are normolipidemic or hypertriglyceridemic [7,10]; on high-carbohydrate or high-fat diets [29]; or in the fasting or continuous postprandial states.
Figure 3.
VLDL, IDL, and LDL metabolism in plasma. Percentages indicate the percentage of flux of a lipoprotein species converted to another lipoprotein or cleared from the circulation. Width of arrows represents the amount of flux of apoB in a metabolic pathway. Arrows pointing up or down show flux pathways that are increased or decreased by dietary carbohydrate compared with unsaturated fat. +: flux pathways that are increased in hypertriglyceridemia −: flux pathways that are decreased in hypertriglyceridemia.
Isolating the Influence of Apolipoprotein C-III Itself on Metabolism of VLDL and IDL
ApoB lipoproteins that have apoC-III also may carry other apolipoproteins besides apoE that could affect their metabolism such as apoA-II, apoC-I, or apoC-II. Approximately 23% of plasma VLDL apoC-III+ and 76% of VLDL apoC-III− do not have these other apolipoproteins [8]. We prepared these subpopulations by immunoaffinity column chromatography of plasma first using antibodies against apoA-II, C-I, C-II, and E together on a gel filtration column, and second by antiapoC-III chromatography of the bound and unbound fractions. We compared the metabolism of these two subpopulations, which we called ‘OtherApos-C-III+’ and ‘OtherApos-C-III−’. They had similar conversion rates to IDL. Thus, again we did not find evidence for slow lipolysis of apoC-III containing VLDL and IDL [8]. We also studied the ‘OtherApos+ C-III+’ and ‘OtherApos+C-III−’ sub-populations. Light VLDL ‘Other apos+C-III+’ had faster conversion to dense VLDL compared with light VLDL ‘Other apos+C-III−’; and in turn, dense VLDL ‘Other apos+C-III+’ had faster conversion to IDL than dense VLDL ‘Other apos+C-III−’. VLDL and IDL ‘Other apos+C-III+’ had much slower clearance than ‘Other apos+C-III−’. These results support a specific and dominating role for apoC-III in decreasing the clearance of triglyceride-rich apoB lipoproteins, whereas they are efficiently lipolyzed to LDL, whether or not apoA-II, apoC-I, apoC-II, or apoE are also present.
Apolipoprotein C-III and Lipoprotein Lipase
ApoC-III is thought to slow the metabolism of chylomicrons and VLDL by inhibiting lipoprotein lipase and blocking the engagement of apoE and apoB-100 with their hepatic receptors. Slowing the rate of hydrolysis of lipoprotein triglyceride presumably makes unesterified fatty acids available more gradually to extrahepatic tissues. Plausible as it seems, this mechanism has not been elucidated in humans. The metabolic studies in humans described previously [7,8,10] showed normal conversion rates to LDL for VLDL apoC-III+ and IDL apoC-III+; in fact, they were faster than for VLDL and IDL apoC-III−. As these steps are performed partly by lipoprotein lipase and partly by hepatic lipase [33–35], the findings suggest that apoC-III does not inhibit lipolytic enzymes in vivo, at least enough to slow the conversion rates. In-vitro incubation studies show that apoC-III reduces triglyceride hydrolysis by LpL [36–38]. These experiments use ratios of apoC-III to apoC-II, a required cofactor of LpL, from 10:1 to 100:1. A recent study added further insight on the inhibitory effect of apoC-III by showing that apoC-III, in at least a 5:1 ratio with apoC-II, reduced triglyceride hydrolysis by displacing LpL from the surface of lipid droplets, allowing it to be inactivated by angptl2 [39■]. In marked contrast, the apoC-III to apoC-II ratio in human VLDL apoC-III+ is approximately 0.7 to 2.0 [8,10]. Thus, the apoC-III content in human plasma VLDL of normal or mildly hypertriglyceridemic individuals may not be high enough to inhibit LpL in vivo. Two laboratories produced human apoC-III-over-expressing mice and found that LpL had normal activity toward the apoC-III-enriched lipoproteins [30,32]. Tissue LpL activity was also normal. Reduced VLDL FCR was caused by slow VLDL clearance from plasma. Reduced VLDL FCR and the clearance rate occurred in both apoC-III low-expressing and high-expressing strains in a dose effect [30]. Thus, results of human metabolic studies are consistent with those of these apoC-III over-expression mouse models showing that apoC-III slows metabolism of VLDL by impairing the clearance mechanism.
Other experiments in mice and humans provided some support for LpL inhibition by apoC-III. Mice overexpressing human apoC-III at very high levels had slow clearance of VLDL-triglyceride, and their sera produced less activity of LpL than sera from control mice [40]. Clearance of VLDL-triglyceride was reduced, and thought to be secondary to impaired binding of apoC-III-enriched VLDL to glycosaminoglycan [40]. The amount of apoC-III on the VLDL was very high, judging from the severely elevated triglyceride levels, perhaps in the range in which apoC-III inhibits LpL in vitro, but more than in the previous apoC-III overexpression experiments [30,32] and in normal or mildly hypertriglyceridemic people [8,10]. Furthermore, since clearance of VLDL apoB was not studied, it cannot be assumed that lipolysis inhibition accounted for the full extent of the hyperlipidemia. Other research found that apoC-III increases the binding of apoB lipoproteins to a vascular proteoglycan, biglycan [41,42]. A different approach in mouse models utilized single or double knockout apoE and apoC-III [43]. The apoC-III knockout mice, whether singly or in combination with apoE knockout, cleared triglyceride from plasma more rapidly than control mice, suggesting that apoC-III inhibits LpL. ApoB metabolism was studied in two patients who had apoC-III deficiency in the context of apoA5/A4/C3/A1 gene cluster mutation [44]. The phenotype is remarkable for very low apoA-I and HDL-cholesterol levels. The patients' VLDL not only lacked apoC-III but also apoE. The VLDL FCR was very high attributed to the absent apoC-III, and the rapidly metabolized VLDL was converted to LDL. Sera from the apoC-III-deficient patients did not inhibit LpL, whereas control sera that had apoC-III did. The experiment suggests that apoC-III inhibits LpL, in vivo [44]. Still, the very low apoE content of VLDL could have reduced clearance from plasma and shifted the flux of VLDL to conversion to LDL. Multiple apolipoprotein abnormalities in apoAI-C-III deficiency lend caution to an interpretation that focuses only on apoC-III.
Combining Actions of Apolipoprotein E and C-III and Secretion of LDL to Form an Integrative Model of Apolipoprotein B Metabolism in Normolipidemic and Hypertriglyceridemic People
ApoC-III secretion is high in hypertriglyceridemia [45,46], which translates to more secretion of light and dense VLDL E-C-III+ and less of VLDL E+C-III+ (Fig. 1). ApoC-III itself may be involved in VLDL assembly in the liver, and may increase secretion of VLDL [47]. However, inhibition of apoC-III synthesis in the liver by apoC-III antisense treatment did not reduce VLDL secretion in mice [48■■], although it substantially lowered plasma apoC-III and triglyceride levels in mice, monkeys, and humans. It is not known whether apoC-III increases VLDL secretion in humans, or whether its influence is solely on metabolism of VLDL as it circulates in plasma. In humans, in hypertriglyceridemia, the shift in the secretion of VLDL away from apoE subspecies and toward apoC-III subspecies or subspecies having neither apoE or apoC-III (Fig. 1) reduces the flux in apoE-mediated clearance pathways, and increases the flux in the lipolysis pathways to smaller apoB lipoproteins all the way to dense LDL (Fig. 3). In hypertriglyceridemia, this apoC-III-dependent pathway from triglyceride-rich VLDL to dense LDL contributes to the excess production of dense LDL (Fig. 3). In addition, in hypertriglyceridemia, the liver secretes more dense LDL apoC-III+ and dense LDL apoC-III− than normal (Fig. 1). Normolipidemic compared with hypertriglyceridemic individuals secrete more large and medium-size LDL. Finally, for reasons that are unclear, dense LDL is cleared more slowly in hypertriglyceridemic than in normolipidemic people (10). Therefore, these pathways for apoB lipoproteins involving apoE and apoC-III establish the hypertriglyceridemic phenotype and link it metabolically to dense LDL.
Modulation of Apolipoprotein B Metabolism by Dietary Carbohydrate and Fat
Dietary carbohydrate when it replaces fat increases fasting blood triglyceride dose-dependently [49]. Carbohydrate increases the secretion into plasma of VLDL and IDL that does not have apoE, and decreases the secretion of VLDL and IDL that contains apoE [29] (Fig. 1). Carbohydrate also reduces the fractional catabolic rate of triglyceride-rich lipoproteins by decreasing apoE and increasing their conversion to LDL. These mechanisms are similar to those in hypertriglyceridemia. Diets high in carbohydrate compared with unsaturated fat, especially linoleic acid, are associated with increased risk of CHD [50,51■■]. The specific changes in metabolism of VLDL and LDL produced in hypertriglyceridemia and by dietary carbohydrate are thus likely to be a benchmark for atherogenic apoB lipoprotein metabolism.
Conclusion
The opposing actions of apoC-III and apoE present in subspecies of VLDL and LDL, and the direct secretion of LDL in several sizes, establish much of the basic structure of human apoB lipoprotein metabolism in normal and hypertriglyceridemic humans, and in response to changes in dietary carbohydrate and unsaturated fat.
Key Points.
Normal apoB lipoprotein metabolism is sustained by a balanced action of apoC-III and apoE; apoC-III delaying clearance of triglyceride-rich lipoproteins, giving time for their triglyceride to be hydrolyzed to fatty acids; and apoE binding to high affinity hepatic receptors to clear the lipoproteins after lipolysis removes some or all of the apoC-III.
In hypertriglyceridemia, excessive production of a VLDL subspecies that does not have apoE but has many molecules of apoC-III results in accumulation of triglyceride-rich VLDL that is metabolized by lipolytic enzymes to dense LDL before it is cleared.
In hypertriglyceridemia, the liver secretes dense LDL, which has a slow clearance rate from plasma. In contrast, in normolipidemia, the liver secretes large and medium-size LDL, which are converted rapidly to dense LDL. The dense LDL in normolipidemia has a faster clearance rate than in hypertriglyceridemia.
High concentrations of apoC-III, five or more times those in human VLDL, inhibit lipoprotein lipase, in vitro, by displacing the enzyme from triglyceride-rich droplets. However, kinetic studies in normal and mildly hypertriglyceridemic humans and in mice mildly overexpressing human apoC-III show that the principal action of apoC-III is to inhibit the removal of apoB lipoproteins from plasma and so direct them to active lipolytic pathways to become smaller lipoproteins.
Increasing intake of carbohydrate and decreasing unsaturated fat suppresses mechanisms in VLDL and LDL metabolism that involve apoE, increasing triglycerides, VLDL, and dense LDL, similar to what occurs in hypertriglyceridemia.
Acknowledgments
None.
Financial Support and sponsorship: The work described that was conducted in the author's laboratory was funded by grants from the National Heart, Lung and Blood Institute, Bethesda, Maryland, USA.
Abbreviations
- apo
apolipoprotein
- CIII+
containing apoC-III
- CIII-
not containing apoC-III
- E+
containing apoE
- E-
not containing apoE
- E+CIII+
containing apoE and apoC-III
- CHD
coronary heart disease
- IDL
intermediate density lipoproteins
- LDL
low density lipoproteins
- LpL
lipoprotein lipase
- VLDL
very low density lipoproteins
Footnotes
Conflicts of interest: The author has received consulting fees from ISIS Pharmaceuticals, AstraZeneca, Merck, and Omthera; research grants from Omthera and AstraZeneca; and is an inventor on a US patent awarded to Harvard University on use of apoC-III in CHD risk assessment.
References and Recommended Reading
Papers of particular interest, published within the annual period of review, have been highlighted as:
■ of special interest
■■ of outstanding interest
- 1.Beltz WF, Kesaniemi YA, Howard BV, Grundy SM. Development of an integrated model for analysis of the kinetics of apolipoprotein B in plasma very low density lipoproteins, intermediate density lipoproteins, and low density lipoproteins. J Clin Invest. 1985;76:575–585. doi: 10.1172/JCI112009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kesaniemi YA, Beltz WJ, Grundy SM. Comparisons of metabolism of apo-lipoprotein B in normal subjects, obese patients, and patients with coronary heart disease. J Clin Invest. 1985;76:586–595. doi: 10.1172/JCI112010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Teng B, Sniderman AD, Soutar AK, Thompson GR. Metabolic basis of hyperapobetalipoproteinemia. J Clin Invest. 1986;77:663–672. doi: 10.1172/JCI112360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Huff MW, Telford DE. Regulation of low density lipoprotein apoprotein B metabolism by lovastatin and cholestyramine in miniature pigs: effects on LDL composition and synthesis of LDL subfractions. Metabolism. 1989;38:256–264. doi: 10.1016/0026-0495(89)90084-x. [DOI] [PubMed] [Google Scholar]
- 5.Fisher WR, Zech LA, Kilgore LL, Stacpoole PW. Metabolic pathways of apolipoprotein B in heterozygous familial hypercholesterolemia: studies with a [3H] leucine tracer. J Lipid Res. 1991;32:1823–1836. [PubMed] [Google Scholar]
- 6.Gaw A, Packard CJ, Lindsay GM, et al. Overproduction of small very low density lipoproteins (Sf 20-60) in moderate hypercholesterolemia: relationships between apolipoprotein B kinetics and plasma lipoproteins. J Lipid Res. 1995;36:158–171. [PubMed] [Google Scholar]
- 7.Zheng CY, Khoo C, Ikewaki K, Sacks FM. Rapid turnover of apolipoprotein C-III-containing triglyceride-rich lipoproteins contributing to the formation of LDL subfractions. J Lipid Res. 2007;48:1190–1203. doi: 10.1194/jlr.P600011-JLR200. [DOI] [PubMed] [Google Scholar]
- 8.Mendivil C, Zheng C, Furtado J, et al. Metabolism of VLDL and LDL containing apolipoprotein C-III and not other small apolipoproteins. Arterioscler Thromb Vasc Biol. 2010;30:239–245. doi: 10.1161/ATVBAHA.109.197830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fisher WR, Zech LA, Stacpoole PW. ApoB metabolism in familial hypercholesterolemia. Arterioscler Thromb Vasc Biol. 1994;14:501–510. doi: 10.1161/01.atv.14.4.501. [DOI] [PubMed] [Google Scholar]
- 10.Zheng C, Furtado J, Khoo C, Sacks FM. Apolipoprotein C-III and the metabolic basis for hypertriglyceridemia and the dense LDL phenotype. Circulation. 2010;121:1722–1734. doi: 10.1161/CIRCULATIONAHA.109.875807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Campos H, Perlov D, Khoo C, Sacks FM. Distinct patterns of lipoproteins with apoB defined by presence of apoE or apoC-III in hypercholesterolemia and hypertriglyceridemia. J Lipid Res. 2001;42:1239–1249. [PubMed] [Google Scholar]
- 12.Khoo C, Campos H, Judge H, Sacks FM. Effects of estrogenic oral contraceptives on the lipoprotein B particle system defined by apolipoproteins E and CIII. J Lipid Res. 1999;40:202–212. [PubMed] [Google Scholar]
- 13.Alaupovic P. Significance of apolipoproteins for structure, function, and classification of plasma lipoproteins. Methods Enzymol. 1996;263:32–60. doi: 10.1016/s0076-6879(96)63004-3. [DOI] [PubMed] [Google Scholar]
- 14.Sacks FM, Brewer HB. Petar Alaupovic: the father of lipoprotein classification based on apolipoprotein composition. Arterioscler Thromb Vasc Biol. 2014;34:1111–1113. doi: 10.1161/atvbaha.114.303500. [DOI] [PubMed] [Google Scholar]
- 15.Alaupovic P, Mack WJ, Knight-Gibson C, Hodis HN. The role of triglyceriderich lipoprotein families in the progression of atherosclerotic lesions as determined by sequential coronary angiography from a controlled clinical trial. Arterioscler Thromb Vasc Biol. 1997;17:715–722. doi: 10.1161/01.atv.17.4.715. [DOI] [PubMed] [Google Scholar]
- 16.Lee SJ, Campos H, Moye LA, Sacks FM. LDL particles containing apolipoprotein CIII are independent risk factors for coronary events in diabetic patients. Arterioscl Thromb Vasc Biol. 2003;23:853–858. doi: 10.1161/01.ATV.0000066131.01313.EB. [DOI] [PubMed] [Google Scholar]
- 17.Mendivil CO, Rimm EB, Furtado J, et al. Low-density lipoproteins containing apolipoprotein C-III and the risk of coronary heart disease. Circulation. 2011;124:2065–2072. doi: 10.1161/CIRCULATIONAHA.111.056986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18■■.Mendivil CO, Rimm EB, Furtado J, Sacks FM. Apolipoprotein E in VLDL and LDL with apolipoprotein C-III is associated with a lower risk of coronary heart disease. J Am Heart Assoc. 2013;2:e000130. doi: 10.1161/JAHA.113.000130. This epidemiological study shows that the amount of apoE reduces the high risk of CHD associated with VLDL and LDL subspecies that contain apoC-III. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mahley RW. Apolipoprotein E: cholesterol transport protein with expanding role in cell biology. Science. 1988;240:622–630. doi: 10.1126/science.3283935. [DOI] [PubMed] [Google Scholar]
- 20.Mahley RW, Huang Y. Atherogenic remnant lipoproteins: Role for proteoglycans in trapping, transferring, and internalising. J Clin Invest. 2007;117:94–98. doi: 10.1172/JCI30889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Windler E, Chao Y, Havel RJ. Regulation of the hepatic uptake of triglyceriderich lipoproteins in the rat: opposing effects of homologous apolipoprotein E and individual C apoproteins. J Biol Chem. 1980;255:8303–8307. [PubMed] [Google Scholar]
- 22.Yamada N, Shames DM, Stoudemire JB, Havel RJ. Metabolism of lipoproteins containing apolipoprotein B-100 in blood plasma of rabbits: Heterogeneity related to the presence of apolipoprotein E. Proc Natl Acad Sci. 1986;83:3479–3483. doi: 10.1073/pnas.83.10.3479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kashyap VS, Santamarina-Fojo S, Brown DR, et al. Apolipoprotein E deficiency in mice: gene replacement and prevention of atherosclerosis using adenovirus vectors. J Clin Invest. 1995;96:1612–1620. doi: 10.1172/JCI118200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pendse AA, Arbones-Mainar JM, Johnson LA, et al. Apolipoprotein E knockout and knock-in mice: atherosclerosis, metabolic syndrome, and beyond. J Lipid Res. 2009;50:S178–S182. doi: 10.1194/jlr.R800070-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25■.White CR, Garber DW, Anantharamaiah GM. Anti-inflammatory and cholesterol-reducing properties of apolipoprotein mimetics: a review. J Lipid Res. 2014;55:2007–2021. doi: 10.1194/jlr.R051367. This is a review on development of apoE mimetics in therapy of hyperlipidemia. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hadattu SP, Nayyar G, Garber DW, et al. Two apolipoprotein E mimetic peptides with similar cholesterol reducing properties exhibit differential atheroprotective effects in LDL-R null mice. Atherosclerosis. 2013;227:58–64. doi: 10.1016/j.atherosclerosis.2012.10.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tomiyasu K, Walsh BW, Ikewaki K, et al. Differential metabolism of human VLDL according to content of apolipoprotein E and CIII. Arterioscler Thromb Vasc Biol. 2001;21:1494–1500. doi: 10.1161/hq0901.094489. [DOI] [PubMed] [Google Scholar]
- 28.Lee SJ, Sacks FM. Effect of pravastatin on intermediate-density and low-density lipoproteins containing apolipoprotein CIII in patients with diabetes mellitus. Am J Cardiol. 2003;92:121–124. doi: 10.1016/s0002-9149(03)00524-1. [DOI] [PubMed] [Google Scholar]
- 29.Zheng C, Khoo C, Furtado J, et al. Dietary monounsaturated fat activates pathways for triglyceride-rich lipoproteins that involve apolipoproteins E and C-III. Am J Clin Nutr. 2008;88:272–281. doi: 10.1093/ajcn/88.2.272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Aalto-Setala K, Fisher EA, Chen X, et al. Mechanism of hypertriglyceridemia in human apo C-III transgenic mice: diminished VLDL fractional catabolic rate associated with increased apo C-III and reduced apo E on the particles. J Clin Invest. 1992;90:1889–1900. doi: 10.1172/JCI116066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Maeda N, Li H, Lee D, et al. Targeted disruption of the apolipoprotein C-III gene in mice results in hypotriglyceridemia and protection from postprandial hypertriglyceridemia. J Biol Chem. 1994;269:23610–23616. [PubMed] [Google Scholar]
- 32.de Silva HV, Lauer SJ, Wang J, et al. Overexpression of human apolipoprotein C-III in transgenic mice results in an accumulation of apolipoprotein B48 remnants that is corrected by excess apolipoprotein E. J Biol Chem. 1994;269:2324–2335. [PubMed] [Google Scholar]
- 33.Demant T, Carlson LA, Holmquist L, et al. Lipoprotein metabolism in hepatic lipase deficiency: studies on the turnover of apolipoprotein B and on the effect of hepatic lipase on high density lipoprotein. J Lipid Res. 1988;29:1603–1611. [PubMed] [Google Scholar]
- 34.Nicoll A, Lewis B. Evaluation of the roles of lipoprotein lipase and hepatic lipase in lipoprotein metabolism: in vivo and in vitro studies in man. Eur J Clin Invest. 1980;10:487–495. doi: 10.1111/j.1365-2362.1980.tb02090.x. [DOI] [PubMed] [Google Scholar]
- 35.Goldberg IJ, Le NA, Paterniti JR, et al. Lipoprotein metabolism during acute inhibition of hepatic triglyceride lipase on the cynomolgus monkey. J Clin Invest. 1982;70:1184–1192. doi: 10.1172/JCI110717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Brown WV, Baginsky ML. Inhibition of lipoprotein lipase by an apoprotein of human very low density lipoprotein. Biochem Biophys Res Commun. 1972;46:375–382. doi: 10.1016/s0006-291x(72)80149-9. [DOI] [PubMed] [Google Scholar]
- 37.Wang CS, McConathy WJ, Kloer HU, Alaupovic P. Modulation of lipoprotein lipase activity by apolipoproteins. Effect of apoliprotein C-III. J Clin Invest. 1985;75:384–390. doi: 10.1172/JCI111711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Liu H, Talmud PJ, Lins L, et al. Recombinant wild type and site-directed mutations of apolipoprotein C-III: lipid binding, displacement of ApoE, and inhibition of lipoprotein lipase. Biochemistry. 2000;39:9201–9212. doi: 10.1021/bi0009441. [DOI] [PubMed] [Google Scholar]
- 39■.Larsson M, Vorrsjo E, Talmud P, et al. Apolipoproteins C-I and C-III inhibit lipoprotein lipase activity by displacement of the enzyme from lipid droplets. J Biol Chem. 2013;288:33997–34008. doi: 10.1074/jbc.M113.495366. The findings extend mechanistic understanding of how apoC-I and apoC-III slow lipolysis of triglyceride. ApoC-I and apoC-III in high amounts displace LpL from triglyceride-rich droplets and the LpL that is released is inactivated by angptl4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ebara T, Ramakrishnan R, Steiner G, Shachter NS. Chylomicronemia due to apolipoprotein CIII overexpression in apolipoprotein E-null nice. J Clin Invest. 1997;99:2672–2681. doi: 10.1172/JCI119456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Olin-Lewis K, Krauss RM, La Belle M, et al. ApoC-III content of apoB-containing lipoproteins is associated with binding to the vascular proteoglycan biglycan. J Lipid Res. 2002;43:1969–1977. doi: 10.1194/jlr.m200322-jlr200. [DOI] [PubMed] [Google Scholar]
- 42.Hiukka A, Stahlman M, Pettersson C, et al. ApoCIII-enriched LDL in type two diabetes displays altered lipid composition, increased susceptibility for sphingomyelinase, and increased binding to biglycan. Diabetes. 2009;58:2018–2026. doi: 10.2337/db09-0206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jong MC, Rensen PC, Dahlmans VE, et al. Apolipoprotein C-III deficiency accelerates triglyceride hydrolysis by lipoprotein lipase in wild-type and apoE knockout mice. J Lipid Res. 2001;42:1578–1585. [PubMed] [Google Scholar]
- 44.Ginsberg HN, Le NA, Goldberg IJ, et al. Apolipoprotein B metabolism in subjects with deficiency of apolipoproteins CIII and AI. Evidence that apolipoprotein CIII inhibits catabolism of triglyceride-rich lipoproteins by lipoprotein lipase in vivo. J Clin Invest. 1986;78:1287–1295. doi: 10.1172/JCI112713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cohn JS, Patterson BW, Uffelman KD, et al. Rate of production of plasma and very-low-density lipoprotein (VLDL) apolipoprotein C-III is strongly related to the concentration and level of production of VLDL triglyceride in male subjects with different body weights and levels of insulin sensitivity. J Clin Endocrinol Metab. 2004;89:3949–3955. doi: 10.1210/jc.2003-032056. [DOI] [PubMed] [Google Scholar]
- 46.Chan DC, Watts GF, Nguyen MN, Barrett PH. Apolipoproteins C-III and A-V as predictors of very-low-density lipoprotein triglyceride and apolipoprotein B-100 kinetics. Arterioscler Thromb Vasc Biol. 2006;26:590–596. doi: 10.1161/01.ATV.0000203519.25116.54. [DOI] [PubMed] [Google Scholar]
- 47.Sundaram M, Zhong S, Khalil MB, et al. Expression of apolipoprotein C-III in McA-RH7777 cells enhances VLDL assembly and secretion under lipid-rich conditions. J Lipid Res. 2010;51:150–161. doi: 10.1194/jlr.M900346-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48■■.Graham MJ, Lee RG, Bell TA, et al. Antisense oligonucleotide inhibition of apolipoprotein C-III reduces plasma triglycerides in rodents, nonhuman primates, and humans. Novelty and significance. Circ Res. 2013;112:1479–1490. doi: 10.1161/CIRCRESAHA.111.300367. This is the first report on inhibition of apoC-III by an antisense oligonucleotide. It comprehensively reports preclinical experiments and early clinical testing. ApoC-III inhibition greatly lowers plasma apoC-III and VLDL-triglyceride. [DOI] [PubMed] [Google Scholar]
- 49.Mensink RP, Zock PL, Kester AD, Katan MB. Effects of dietary fatty acids and carbohydrates on the ratio of serum total to HDL cholesterol and on serum lipids and apolipoproteins: a meta-analysis of 60 controlled trials. Am J Clin Nutr. 2003;77:1146–1155. doi: 10.1093/ajcn/77.5.1146. [DOI] [PubMed] [Google Scholar]
- 50.Hu FB, Stampfer MJ, Manson JE, et al. Dietary fat intake and the risk of coronary heart disease in women. N Engl J Med. 1997;337:1491–1499. doi: 10.1056/NEJM199711203372102. [DOI] [PubMed] [Google Scholar]
- 51■■.Farvid JS, Ding M, Pan A, et al. Dietary linoleic acid and risk of coronary heart disease: a systematic review and meta-analysis of prospective cohort studies. Circulation. 2014;130:1568–1578. doi: 10.1161/CIRCULATIONAHA.114.010236. This is a very important meta-analysis updating previous findings that dietary intake of linoleic acid, replacing either saturated fat or carbohydrate, is strongly associated with protection against CHD. This meta-analysis now includes over 12,000 cases of CHD. [DOI] [PMC free article] [PubMed] [Google Scholar]