Abstract
Background
Human skin aging is a multifactorial and complex biological process affecting the different skin constituents. Even if the skin aging mechanism is not yet fully unravelled is evident that epidermis loses the principal molecule responsible for binding and retaining water molecules, resulting in loss of skin moisture and accounting for some of the most striking alterations of the aged skin.
Objectives
This Study investigated the cosmetic filling efficacy of Fillerina® in decreasing the skin aging signs and in improving facial volume deficiencies.
Methods
A placebo-controlled, double-blind, randomized clinical trial was carried out on 40 healthy female subjects showing mild to moderate clinical signs of skin aging. The effect of the treatment on skin surface and on face volumes was assessed both in the short-term (3 h after a single product application) and in the long-term (7, 14, and 30 days after continuative daily use).
Results
Three hours after a single application and after 7, 14, and 30 days of treatment the lips volume was increased by 8.5%, 11.3%, 12.8%, and 14.2%. After 7, 14, and 30 days: (1) skin sagging of the face contours was decreased by −0.443 ± 0.286, −1.124 ± 0.511, and −1.326 ± 0.649 mm, respectively; (2) skin sagging of the cheekbones contours was decreased by −0.989 ± 0.585, −2.500 ± 0.929, and −2.517 ± 0.927 mm, respectively; (3) cheekbones volume was increased by 0.875 ± 0.519, 2.186 ± 0.781, and 2.275 ± 0.725 mm, respectively; (4) wrinkle volume was decreased by −11.3%, −18.4%, and −26.3%, respectively; and (5) wrinkle depth was decreased by −8.4%, −14.5%, and −21.8% respectively.
Conclusion
This study demonstrated the positive filling effect of Fillerina® in decreasing the clinical signs of skin aging and in improving the face volumes.
Keywords: anti-aging, face volumes, hyaluronic acid, skin aging, skin wrinkles
Introduction
Human skin aging is a multifactorial and complex biological process affecting the different constituents of the skin that is not yet fully understood. Skin aging is the result of two biologically independent processes. The first is intrinsic or innate aging, an unpreventable process, which affects the skin in the same way as it affects all others organs. The second is extrinsic aging, which is the result of exposure to environmental factors (i.e. ultraviolet irradiation, pollutants, etc.) and it is commonly referred to as photoaging.1
In elderly the skin turgor, resilience, and pliability are decreased, presumably due to altered patterns and levels of glycosaminoglycans (GAGs), especially hyaluronic acid (HA) and dermatan sulfate, which are the most common.2 HA is a nonsulfated GAG with an unique capacity to bind and retain water molecules.3 Chemically, HA is composed of repeating polymeric disaccharides of D-glucuronic acid and N-acetyl- D-glucosamine linked by a glucuronidic β (1→3) bond.4,5 Unlike other GAG, HA is not covalently linked to a protein core, but it may form aggregates with proteoglycans.6 HA polymers occur in a large number of configurations and shapes, depending on their size, salt concentration, pH, and associated cations.7
HA is widely distributed, from prokaryotic8,9 to eukaryotic cells.10 In humans, HA is most abundant in the skin,11–15 accounting for the 50% of the total body HA.16 HA is produced primarily by mesenchymal cells, even if other cell types are capable to produce HA.17–21 The use of biotinylated HA-binding peptide revealed that not only cells of mesenchymal origin are capable of synthesizing HA and permitted the histolocalization of HA in the dermal compartment of skin and the epidermis.22–24 This technique enabled the visualization of HA in the epidermis, mainly in the extracellular matrix molecules (ECM) of the upper spinous and granular layers, whereas in the basal layer HA is predominantly intracellular.12
HA has a dynamic turnover rate with a half-life less than a day in the skin and it is degraded into fragments of varying size by hyaluronidases (HYAL) by hydrolyzing the hexosaminidic β (1–4) linkages between N-acetyl-D-glucosamine and D-glucuronic acid residues in HA.25
The most dramatic histochemical change observed in senescent skin is the marked disappearance of epidermal HA. With increasing aging, a steady decline of HA occurs in the upper epidermal layer, with concomitant increases in the basal layer of the epidermis and the upper portions of the papillary dermis, while at senescence HA is entirely absent in the epidermis and present in the upper dermis.26 The reasons for this change in HA homeostasis with aging is unknown. Progressive reduction of the size of the HA polymers in skin as a result of aging has also been reported.22
In the skin photoaging were also reported an abnormal GAG content and distribution and a diminished HA concentration.27 Using photoexposed and photoprotected human skin tissue specimens, obtained from the same patient, a significant increase in the expression of HA of lower molecular mass in photoexposed skin, as compared with photoprotected skin was reported, suggesting that HA homeostasis exhibits a distinct profile in intrinsic skin aging, which is totally different from the characteristic profile in extrinsic skin aging.28
Even if the mechanism of skin aging is not yet fully unravelled is evident that during aging the epidermis loses the principal molecule responsible for binding and retaining water molecules, resulting in loss of skin moisture and accounting for some of the most striking alterations of the aged skin, including decreased turgidity, less support for microvessels, wrinkling, altered elasticity and loss of face volumes especially as regards to the cheekbones and lips.2
Currently, HA fillers are an established intervention for correcting facial volume deficiencies.29 However, they are costly, invasive, painful, and may have side effects. Thus, a topical, non-invasive, effective cosmetic treatment to replenish the skin with the lost HA could be more acceptable. The aim of the present study was to evaluate the filling efficacy of a commercially available HA based dermocosmetic treatment (Fillerina®) claimed to be effective in improving the facial volume deficiencies.
Materials
Participants and study design
In this monocentric randomized study, forty healthy Caucasian female subjects were included by a certified dermatologist. All the study procedures were carried out according to World Medical Association's (WMA) Helsinki Declaration and its amendments (Ethical Principles for Medical Research Involving Human Subjects, adopted by the 18th WMA General Assembly Helsinki, Finland, June 1964 and amendments). To participate in the study, each participant was fully informed on study risks and benefits, aims, and procedures. An informed consent form and a consent release for publication of photos were signed by each subject prior to participating in the study.
Eligible participants were all adult female subjects aged between 25 and 55 years old (mean age: active group 47.7 ± 5.7 years old, placebo group 46.3 ± 6.8 years old; mean ± SD) and showing mild-to-moderate clinical signs of skin aging on the face. The subjects were of general good health, had no obvious skin disease, known history of atopic dermatitis and/or skin elastosis on the face. Exclusion criteria were pregnancy or intention to become pregnant, lactation, allergy/sensitivity to cosmetics, participation in another similar study (at least 30 days prior to enrolling in the study), and unwillingness or unability to comply with the requirements of the study protocol. The study further excluded subjects using topical products containing moisturizing and/or anti-aging actives.
After the enrolment subjects were randomly assigned to one of the two study arms, in 1:1 ratio, to receive active or placebo products. For allocation a computer-generated, using PASS 11 statistical software (version 11.0.8 for Windows; PASS, LLC, Kaysville, UT, USA) restricted randomization list (biased coin using Efron's algorithm) was used. Subjects, investigator and her collaborators were kept blind to products assignment. Sequentially numbered, opaque, and sealed envelopes, reporting the unblinded treatment allocation, where prepared for each subject and stored in a safe place by the in site study Director.
The study took place at Farcoderm s.r.l. dermatological facilities in San Martino Siccomario (PV), Italy, from September to October 2012. Farcoderm s.r.l. is an independent testing laboratory, collaborating with the University of Pavia, for in vitro and in vivo safety and efficacy assessment of cosmetics, food supplements, and medical devices.
Interventions
The tested product was a commercially available dermocosmetic filling treatment named Fillerina® (Labo Cosprophar AG, Basel, Switzerland) formed by five specific products (Gel Filler, Nourishing Film, Day Cream, Night Cream and Eye/Lip Cream). Each product contained Sodium Hyaluronate crosspolymer and a mixture of HA of different molecular weight (hyaluronic acid 1 kDa, hydrolyzed sodium hyaluronate 5 kDa, hydrolyzed hyaluronic acid 50 kDa, sodium hyaluronate 200 kDa, and sodium hyaluronate 2000 kDa) as cosmetic active ingredients. The placebo formulations did not contain the above-mentioned actives. Active and placebo products were in the same physical form (gel or emulsion) and identical in appearance. Subjects applied by themselves the tested products according to the way of use reported in Figure1. Subjects were instructed to not share the test products with other household members. Subjects were allowed to continue the use of regular (without any claimed anti-aging effect) make-up products.
Figure 1.
Way of use.
Outcomes
The primary endpoints with respect to efficacy in decreasing the skin aging signs were the measurements of skin sagging/loss of volume of face contours, cheekbones, and lips by means of a morphometric image analysis technique (Fig.2). Wrinkle volume and depth were measured, in the periocular area (“crow's feet area”), as secondary endpoints by means of a 3D microtopography imaging system. All the measurement were carried out before study start (basal value) and after 7, 14, and 30 days of treatment, except for lips volume and wrinkles depth/volume, for which and additional measurement time was foreseen 3 h after the first product application at study start. The measurements were carried out on cleansed skin (except for the lips volume and the wrinkle depth/volume measurements 3 h after product application) under temperature (22 ± 2 °C) and relative humidity (50 ± 10%) controlled conditions. The treatment was stopped 8–12 h before any skin assessment were made.
Figure 2.
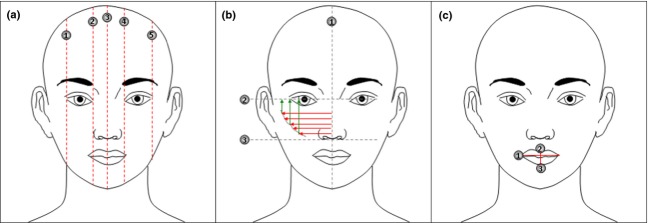
Morphometric evaluation of the face contours/volumes. (a) Face contour lifting effect is measured as the distance (red dotted lines) between the upper (forehead) and the lower (mandible) part of the face. The measurement is taken in the following points: (1) external side of the right eye, (2) internal side of the right eye, (3) nose apex, (4) internal side of the left eye, and (5) external side of the left eye. (b) Cheekbones contour pumpling effect is measured as the distance between the line passing from the nose apex (1) and the cheekbones contour upper (2) and lower (3) part. The measurement is taken in 5 points (red arrows). Cheekbones contour lifting effect is measured as the distance between the line passing perpendicularly to the eyes (2) and the cheekbones contour. The measurement is taken in 3 points (green arrows). (c) Lips volumizing effect is measured by means of mathematic interpolation of the following parameters: (1) length of the lip line, (2) height of the upper lip, and (3) height of the lower lip. The applied formula is the cone equation.
The lifting/reshaping effect for the face contours and the cheekbones, and the volumizing effect for the cheekbones and the lips were assessed using a morphometric image analysis technique as described in Figure2. Frontal images of the face were taken using a professional digital reflex camera NIKON D300 digital camera, Nikon Corporation Tokyo, Japan) equipped with a macro-objective (AF-S Micro NIKKOR 60 mm f/2.8G ED, Nikon Corporation Tokyo, Japan) and a flash system (Kit R1C1, Nikon Corporation). Subjects' position was regulated using a stereotactic device (Canfield Scientific, Inc., Fairfield, NJ, USA).
Wrinkles depth and volume were measured using a three-dimensional (3-D) microtopography imaging system (PRIMOS 3D lite, GFMesstechnik GmbH, Teltow, Germany). The imaging system projects structured light on a specific surface of the skin with a Digital Micro-mirror Device (DMD, Texas Instruments, Irving, TX, USA) and records the image with a CCD camera. Skin surface microtopography is reconstructed using temporal phase shift algorithms to generate 3-D images. The imaging system has an overlap feature which enables precise matching of photos taken at different visits. Depth and volume of the crow's feet area wrinkles were measured.
Sample size
Sample size was calculated with a two-sided 5% significance level and a power of 80% taking into account a 20% variation of the primary endpoints due to both inter-individual human variability and error in the measurement techniques. A sample size of 20 subjects per group was necessary given an anticipated dropout rate of 20%.
Statistical methods
Statistical analysis was carried out on the intention to treat (ITT) population using NCSS 8 (version 8.0.4 for Windows; NCCS, LLC) running on Windows Server® 2008 R2 64 Edition. Data normality (both for raw data and variations vs. the basal value) was verified using Shapiro–Wilk W normality test and data shape. Since data were normally distributed, the repeated measure analysis of variance (rm-anova) followed by Tukey–Kramer multiple comparison test was performed both for intra- and inter-group comparisons. The statistical significance probability value was set at P < 0.05. Values are reported as follows: (1) face contour lifting effect is reported as the mean variation of the upper to lower face contour measured in 5 point (Fig.2a), (2) cheekbones contour pumpling effect is reported as the mean variation of the nose to cheekbones contour measured in 5 points (Fig.2b), and (3) cheekbones contour lifting effect is reported as the mean variation of the eye to cheekbones contour measured in 3 points (Fig.2c). Values are expressed as arithmetic mean ± SD.
Results
Eligible subjects were recruited from August to September 2012. Only subjects who had not sun exposure during the summer period were enrolled. Subjects attended clinic visits at the time of randomization (baseline) and after 7, 14, 30 days of product use. During the baseline visit, subjects remained at the facility housing the trial for 3 h under temperature (22 ± 2 °C) and relative humidity (50 ± 10%) controlled conditions. All the randomized subjects (n = 20 per group) completed the trial (Fig.3). Subject's baseline demographics and clinical characteristic are reported in Table1. Before and after images are reported in Figure4.
Figure 3.
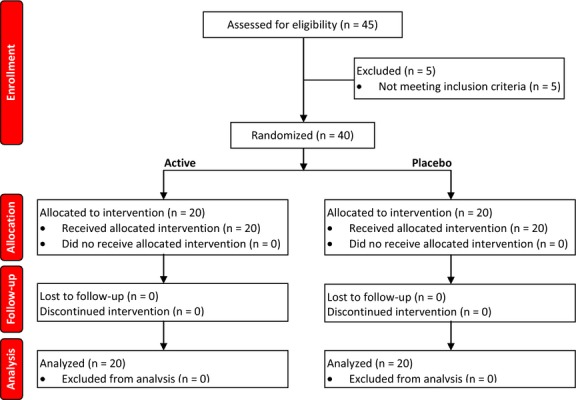
Flow diagram.
Table 1.
Subjects baseline demographics and clinical characteristic
| Active treatment | Placebo treatment | |
|---|---|---|
| Age (years) | 47.7 ± 5.7 | 46.3 ± 6.8 |
| Sex (female) | 20 | 20 |
| Ethnic origin | ||
| Caucasian | 20 | 20 |
| Face countour distances (cm) | ||
| Point 1 | 16.888 ± 0.991 | 16.967 ± 1.099 |
| Point 2 | 20.475 ± 0.898 | 20.415 ± 1.169 |
| Point 3 | 21,879 ± 1.106 | 22.071 ± 0.910 |
| Point 4 | 20.465 ± 0.876 | 20.629 ± 1.202 |
| Point 5 | 16.187 ± 0.889 | 16.894 ± 1.141 |
| Nose to cheekbones contour distances (cm) | ||
| Point 1 | 5.686 ± 0.358 | 5.857 ± 0.416 |
| Point 2 | 5.808 ± 0.354 | 5.993 ± 0.426 |
| Point 3 | 5.909 ± 0.370 | 6.094 ± 0.442 |
| Point 4 | 6.015 ± 0.380 | 6.215 ± 0.444 |
| Point 5 | 6.116 ± 0.387 | 6.309 ± 0.450 |
| Eye to cheekbones contour distances (cm) | ||
| Point 1 | 2.718 ± 0.473 | 2.374 ± 0.284 |
| Point 2 | 3.381 ± 0.324 | 3.119 ± 0.221 |
| Point 3 | 3.985 ± 0.244 | 3.831 ± 0.242 |
| Lip volume (cm3) | 2.697 ± 1.375 | 2.620 ± 1.118 |
| Wrinkle depth (μm) | 338.7 ± 70.2 | 311.2 ± 55.3 |
| Wrinkle volume (mm3) | 5.73 ± 1.97 | 5.64 ± 1.99 |
*Data are means ± SD or numbers.
Figure 4.

(a) Before and after digital pictures. (b) Before and after digital pictures. T0, basal picture, T3, 3 h after product application, T7/14/30, 7, 14, and 30 days after products use.
The results of the face contours lifting effect evaluation are reported in Table2 and Figure5 The baseline (Table1) mean upper to lower distance of face contours (measured in 5 points, see Fig.1a) was similar between active (19.305 ± 0.875 cm) and placebo (19.395 ± 1.044 cm) groups and not statistically significant (P > 0.05). At the 7, 14, and 30 days follow-up visit this distance is decreased in the active group by −0.443 ± 0.286, −1.124 ± 0.511, and −1.326 ± 0.649 mm, respectively. A slight worsening was seen in the placebo group by 0.151 ± 0.125, 0.157 ± 0.153, and 0.112 ± 0.145 mm at the 7, 14, and 30 days follow up visit, respectively. The decrease of the upper to lower distance of face contours is indicative of a decrease of the skin sagging.
Table 2.
Results of face contour lifting effect evaluation
| Active treatment |
Placebo treatment |
|||
|---|---|---|---|---|
| N | Mean (±SD) | n | Mean (±SD) | |
| T = 7 days | 20 | −0.443 ± 0.286 | 20 | 0.151 ± 0.125 |
| T = 14 days | 20 | −1.124 ± 0.511 | 20 | 0.157 ± 0.153 |
| T = 30 days | 20 | −1.326 ± 0.649 | 20 | 0.112 ± 0.145 |
The table report the mean (n = 5) variation of the upper to lower face contour distance measured as described in the Figure1a.
Figure 5.
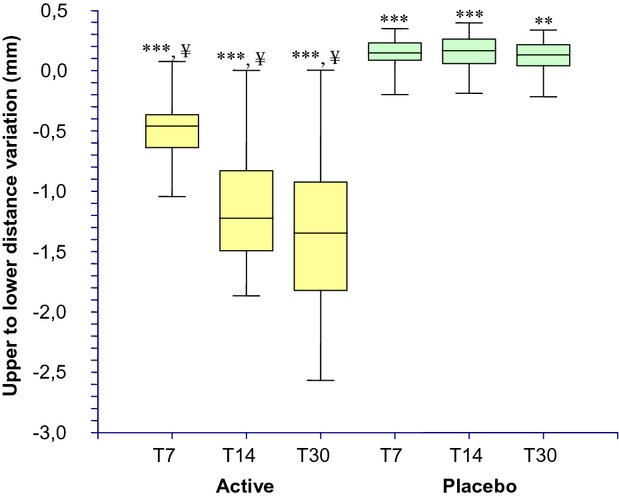
Upper to lower distance (face contour lifting effect) variation during the treatment. ***P < 0.001 vs. T0, **P < 0.01 vs. T0, ¥P < 0.001 active treatment vs. placebo. T0, baseline; T7, 7 days; T14, 14 days; T30, 30 days.
The results of the cheekbones volumizing/plumping effect evaluation are reported in Table3 and Figure6. The baseline (Table1) mean nose to cheekbones contours distance (measured in 5 points, see Fig.1b) was similar between active (5.907 ± 0.366 cm) and placebo (6.094 ± 0.433 cm) groups and not statistically significant (P > 0.05). At the 7, 14, and 30 days follow up visit this distance is increased in the active group by 0.875 ± 0.519, 2.186 ± 0.781, and 2.275 ± 0.725 mm, respectively. A slight worsening was seen in the placebo group by −0.221 ± 0.190, −0.217 ± 0.227, and −0.230 ± 0.268 mm at the 7, 14, and 30 days follow-up visit, respectively. The increase of the nose to cheekbones contours distance is indicative of more protruding cheekbones contours and increased volume.
Table 3.
Results of cheekbones volumizing/pumpling effect evaluation
| Active treatment |
Placebo treatment |
|||
|---|---|---|---|---|
| N | Mean (±SD) | n | Mean (±SD) | |
| T = 7 days | 20 | 0.875 ± 0.519 | 20 | −0.221 ± 0.190 |
| T = 14 days | 20 | 2.186 ± 0.781 | 20 | −0.217 ± 0.227 |
| T = 30 days | 20 | 2.275 ± 0.725 | 20 | −0.230 ± 0.268 |
The table report the mean (n = 5) variation of the nose to cheekbones contour distance measured as described in the Figure1b.
Figure 6.
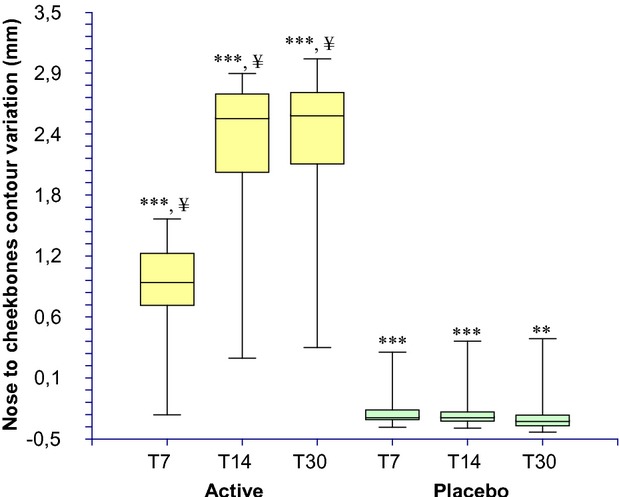
Nose to cheekbones contour distance (cheekbones pumpling effect) variation during the treatment. ***P < 0.001 vs. T0, **P < 0.01 vs. T0, ¥P < 0.001 active treatment vs. placebo. T0, baseline; T7, 7 days; T14, 14 days; T30, 30 days.
The results of the cheekbones contour lifting evaluation are reported in Table4 and Figure7. The baseline eye to cheekbones contours distance (measured in 3 points, see Fig.1b) was similar between active (3.361 ± 0.325 cm) and placebo (3.108 ± 0.226 cm) groups and not statistically significant (P > 0.05). At the 7, 14, and 30 days follow-up visit this distance is decreased in the active group by −0.989 ± 0.585, −2.500 ± 0.929, and −2.517 ± 0.927 mm, respectively. A slight worsening was seen in the placebo group by 0.199 ± 0.122 0.241 ± 0.153, and 0.265 ± 0.169 mm at the 7, 14, and 30 days follow-up visit, respectively. The decrease of the eye to cheekbones contours distance is indicative of a decrease of the skin sagging and an increase of cheekbones volume.
Table 4.
Results of cheekbones contour lifting effect evaluation
| Active treatment |
Placebo treatment |
|||
|---|---|---|---|---|
| N | Mean (±SD) | n | Mean (±SD) | |
| T = 7 days | 20 | −0.989 ± 0.585 | 20 | 0.199 ± 0.122 |
| T = 14 days | 20 | −2.500 ± 0.929 | 20 | 0.241 ± 0.153 |
| T = 30 days | 20 | −2.517 ± 0.927 | 20 | 0.265 ± 0.169 |
The table report the mean (n = 3) variation of the eye cheekbones contour distance measured as described in the Figure1b.
Figure 7.
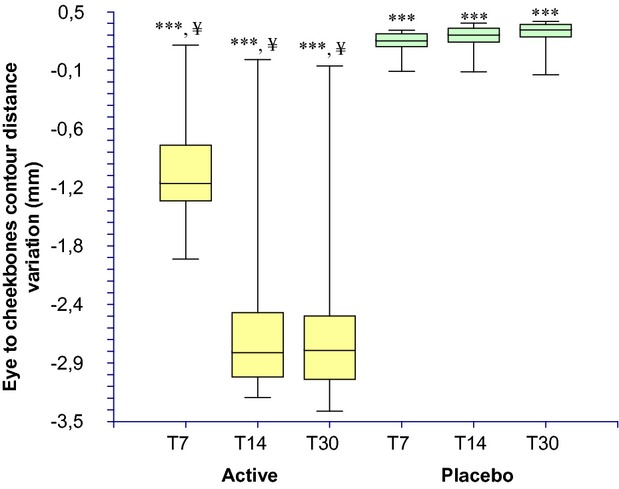
Eye to cheekbones contours distance (cheekbones contour lifting effect) variation during the treatment. ***P < 0.001 vs. T0, ¥P < 0.001 active treatment vs. placebo. T0, baseline; T7, 7 days; T14, 14 days; T30, 30 days.
The results of the treatment effect on lips volume are reported in Table5 and Figure8. The baseline mean value was similar between active (2.697 ± 0.307 cm3) and placebo (2.620 ± 0.250 cm3) groups and not statistically significant (P > 0.05). At the 3 h (2.917 ± 0.331 cm3) and 7 (2.886 ± 0.297 cm3), 14 (2.950 ± 0.314 cm3) and 30 (3.019 ± 0.339 cm3) days follow-up visit this distance was increased in the active group corresponding to a 8.5%, 11.3%, 12.8%, and 14.2% variation vs. the basal value, respectively. In the placebo group, no changes were observed at the 3 h (2.563 ± 0.235 cm3) and 7 (2.597 ± 0.250 cm3), 14 (2.581 ± 0.246 cm3), and 30 (2.556 ± 0.243 cm3) days follow-up visit, respectively.
Table 5.
Results of the lips volumizing effect evaluation
| Active treatment |
Placebo treatment |
|||
|---|---|---|---|---|
| N | Mean (±SD) | n | Mean (±SD) | |
| T = 0 h | 20 | 2.697 ± 0.307 | 20 | 2.620 ± 0.250 |
| T = 3 h | 20 | 2.917 ± 0.331 | 20 | 2.563 ± 0.235 |
| T = 7 days | 20 | 2.886 ± 0.297 | 20 | 2.597 ± 0.250 |
| T = 14 days | 20 | 2.950 ± 0.314 | 20 | 2.581 ± 0.246 |
| T = 30 days | 20 | 3.019 ± 0.339 | 20 | 2.556 ± 0.243 |
The table report the mean lips volume measured as described in the Figure1c.
Figure 8.
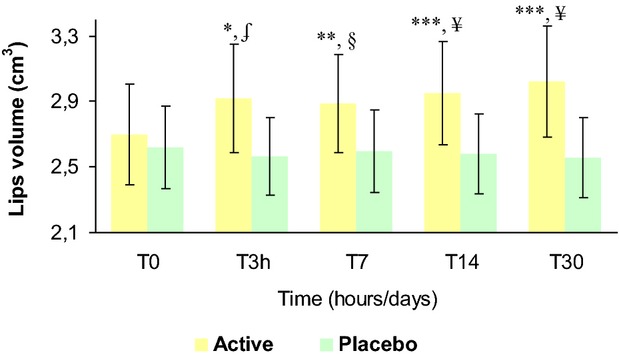
Lips volume variation during the treatment. *P < 0.05 vs. T0, **P < 0.01 vs. T0, ***P < 0.001 vs. T0, §P < 0.05 active treatment vs. placebo, §P < 0.01 active treatment vs. placebo, ¥P < 0.001 active treatment vs. placebo. T0, baseline; T3h, 3 hours; T7, 7 days; T14, 14 days; T30, 30 days.
The results of the treatment effect on wrinkle volume are reported in Table6 and Figure9. The baseline mean value was similar between active (5.72 ± 0.44 mm3) and placebo (5.64 ± 0.44 mm3) groups and not statistically significant (P > 0.05). At 7 (5.13 ± 0.44 mm3), 14 (4.75 ± 0.43 mm3), and 30 (4.30 ± 0.41 mm3) days follow-up visit wrinkle volume was decreased in the active group corresponding to a −11.3%, −18.4%, and −26.3% variation vs. the basal value, respectively. In the placebo group no changes were observed at 7 (5.59 ± 0.45 mm3), 14 (5.61 ± 0.45 mm3), and 30 (5.77 ± 0.48 mm3) days follow-up visit, respectively.
Table 6.
Results of the wrinkle volume evaluation
| Active treatment |
Placebo treatment |
|||
|---|---|---|---|---|
| N | Mean (±SD) | n | Mean (±SD) | |
| T = 0 h | 20 | 5.72 ± 0.44 | 20 | 5.64 ± 0.44 |
| T = 7 days | 20 | 5.13 ± 0.44 | 20 | 5.59 ± 0.45 |
| T = 14 days | 20 | 4.75 ± 0.43 | 20 | 5.61 ± 0.45 |
| T = 30 days | 20 | 4.30 ± 0.41 | 20 | 5.77 ± 0.48 |
Figure 9.
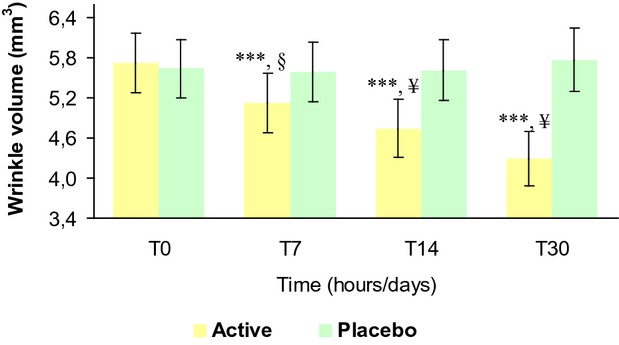
Wrinkle volume variation during the treatment. ***P < 0.001 vs. T0, §P < 0.01 active treatment vs. placebo, ¥P < 0.001 active treatment vs. placebo. T0, baseline; T3h, 3 hours; T7, 7 days; T14, 14 days; T30, 30 days.
The results of the treatment effect on wrinkle depth are reported in Table7 and Figure10. The baseline mean value was similar between active (338.7 ± 15.7 μm) and placebo (311.2 ± 12.4 μm) groups and not statistically significant (P > 0.05). At 7 (311.5 ± 18.2 μm), 14 (290.2 ± 16.5 μm), and 30 (265.1 ± 16.6 μm) days follow-up wrinkle depth was decreased in the active group corresponding to a −8.4%, −14.5%, and −21.8% variation vs. the basal value, respectively. In the placebo group no changes were observed at 7 (310.2 ± 12.0 μm), 14 (312.0 ± 12.3 μm), and 30 (315.6 ± 13.4 μm) days follow-up visit respectively.
Table 7.
Results of the wrinkle depth evaluation
| Active treatment |
Placebo treatment |
|||
|---|---|---|---|---|
| N | Mean (±SD) | n | Mean (±SD) | |
| T = 0 h | 20 | 338.7 ± 15.7 | 20 | 311.2 ± 12.4 |
| T = 7 days | 20 | 311.5 ± 18.2 | 20 | 310.2 ± 12.0 |
| T = 14 days | 20 | 290.2 ± 16.5 | 20 | 312.0 ± 12.3 |
| T = 30 days | 20 | 265.1 ± 16.6 | 20 | 315.6 ± 13.4 |
Figure 10.
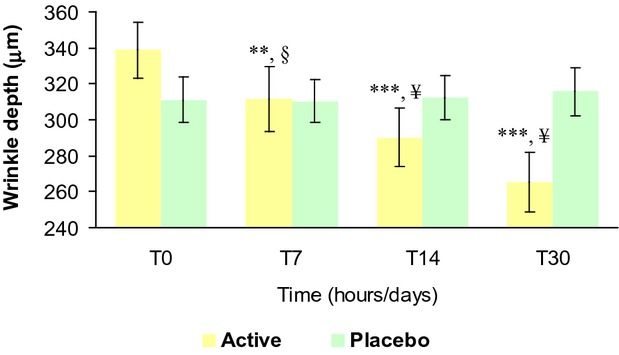
Wrinkle depth variation during the treatment. ***P < 0.001 vs. T0, **P < 0.01 vs. T0, §P < 0.01 active treatment vs. placebo, ¥P < 0.001 active treatment vs. placebo. T0, baseline; T3h, 3 hours; T7, 7 days; T14, 14 days; T30, 30 days.
Discussion
To our knowledge this is the first study demonstrating the filling effect of a cosmetic treatment based on six types hyaluronic at different molecular weight in improving facial contours and volumes. Compared to the placebo group, and to the baseline, the active treatment improved the skin sagging of both the face and the cheekbones contours, the lips volume, and decreased the wrinkle depth and volume. The efficacy of the product appeared to be greater in diminishing the cheekbones skin sagging compared to face contour skin sagging. This relative responsiveness on different site could be related to the skin sagging severity, and to the extent of the two skin sites.
A slight worsening of the skin sagging was seen in the placebo group. This slight variation could be due to normal variation of skin sagging.
Chronoaged skin contains significantly fewer levels of hyaluronic acid26 and hormone replacement treatment has demonstrated to increase HA concentration.30 The stratum corneum (SC) dryness has been proposed to play an important role in wrinkle formation.31 Fine wrinkle appearance has been linked to the dryness of the SC and increased appearance of fine wrinkles are observed in low humidity compared to high humidity environments.32 Moreover, SC water holding capacity and elasticity decrease during chronological aging.33 Simple moisturization by occlusion, due to the higher molecular weight HAs contained in the treatment used in this study, is likely to dampen these responses. Occlusion could have also a beneficial effect in decreasing the profibrotic signaling within the dermis.34
Nevertheless, improvements in skin aging biology are also expected by the low molecular weight HAs contained in the treatment used in the study. Topical application of low molecular weight HA was demonstrated to improve skin moisturization and elasticity associated with significant reduction of wrinkle depth.35
Irrespective of the potential mechanism of action, this study provide the first evidence that the use of six types hyaluronic acid at different molecular weight (Fillerina®) is able to provide an improvement in the appearance of chronoaged skin in subjects showing mild-to-moderate clinical signs of skin aging on the face.
Acknowledgments
This study was founded by Labo Cosprophar AG, Basel, Switzerland. The sponsor had no influence in the performance, analysis, and interpretation of the study. The authors thank the staff of Farcoderm for their professionalism and support during study development. Dr. Fulvio Marzatico is the guarantor for this article, and takes responsibility for the integrity of the work as a whole.
References
- 1.Gilchrest BA. A review of skin ageing and its medical therapy. Br J Dermatol. 1996;135:867–75. doi: 10.1046/j.1365-2133.1996.d01-1088.x. [DOI] [PubMed] [Google Scholar]
- 2.Ghersetich I, Lotti T, Campanile G, et al. Hyaluronic acid in cutaneous intrinsic aging. Int J Dermatol. 1994;33:119–22. doi: 10.1111/j.1365-4362.1994.tb01540.x. [DOI] [PubMed] [Google Scholar]
- 3.Baumann L. Skin ageing and its treatment. J Pathol. 2007;211:241–51. doi: 10.1002/path.2098. [DOI] [PubMed] [Google Scholar]
- 4.Weissmann B, Meyer K. The structure of hyalobiuronic acid and of hyaluronic acid from umbilical cord. J Am Chem Soc. 1954;76:1753–7. [Google Scholar]
- 5.Weissmann B, Meyer K, Sampson P, et al. Isolation of oligosaccharides enzymatically produced from hyaluronic acid. J Biol Chem. 1954;208:417–29. [PubMed] [Google Scholar]
- 6.Bates EJ, Harper GS, Lowther DA, Preston BN. Effect of oxygen-derived reactive species on cartilage proteoglycan-hyaluronate aggregates. Biochem Int. 1984;8:629–37. [PubMed] [Google Scholar]
- 7.Laurent TC. Structure of hyaluronic acid. In: EA Balazs., editor. Chemistry and Molecular Biology of the Intercellular Matrix. New York: Academic Press; 1970. p. 703. [Google Scholar]
- 8.Lowther DA, Rogers HJ. Biosynthesis of hyaluronate. Nature. 1955;175:435. doi: 10.1038/175435a0. [DOI] [PubMed] [Google Scholar]
- 9.MacLennan AP. The production of capsules, hyaluronic acid and hyaluronidase by 25 strains of group C streptococci. J Gen Microbiol. 1956;15:485–91. doi: 10.1099/00221287-15-3-485. [DOI] [PubMed] [Google Scholar]
- 10.Prehm P. Release of hyaluronate from eukaryotic cells. Biochem J. 1990;267:185–9. doi: 10.1042/bj2670185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Juhlin L. Hyaluronan in skin. J Intern Med. 1997;242:61–6. doi: 10.1046/j.1365-2796.1997.00175.x. [DOI] [PubMed] [Google Scholar]
- 12.Tammi R, Ripellino JA, Margolis RU, et al. Localization of epidermal hyaluronic acid using the hyaluronate binding region of cartilage proteoglycan as a specific probe. J Invest Dermatol. 1988;90:412–4. doi: 10.1111/1523-1747.ep12456530. [DOI] [PubMed] [Google Scholar]
- 13.Armstrong SE, Bell DR. Relationship between lymph and tissue hyaluronan in skin and skeletal muscle. Am J Physiol Heart Circ Physiol. 2002;283:H2485–94. doi: 10.1152/ajpheart.00385.2002. [DOI] [PubMed] [Google Scholar]
- 14.Tzellos TG, Sinopidis X, Kyrgidis A, et al. Differential hyaluronan homeostasis and expression of proteoglycans in juvenile and adult human skin. J Dermatol Sci. 2011;61:69–72. doi: 10.1016/j.jdermsci.2010.10.010. [DOI] [PubMed] [Google Scholar]
- 15.Tzellos TG, Klagas I, Vahtsevanos K, et al. Extrinsic ageing in the human skin is associated with alterations in the expression of hyaluronic acid and its metabolizing enzymes. Exp Dermatol. 2009;18:1028–35. doi: 10.1111/j.1600-0625.2009.00889.x. [DOI] [PubMed] [Google Scholar]
- 16.Reed RK, Lilja K, Laurent TC. Hyaluronan in the rat with special reference to the skin. Acta Physiol Scand. 1988;134:405–11. doi: 10.1111/j.1748-1716.1988.tb08508.x. [DOI] [PubMed] [Google Scholar]
- 17.Toole BP. Hyaluronan: from extracellular glue to pericellular cue. Nat Rev Cancer. 2004;4:528–39. doi: 10.1038/nrc1391. [DOI] [PubMed] [Google Scholar]
- 18.Papakonstantinou E, Karakiulakis G, Roth M, et al. Platelet-derived growth factor stimulates the secretion of hyaluronic acid by proliferating human vascular smooth muscle cells. Proc Natl Acad Sci USA. 1995;92:9881–5. doi: 10.1073/pnas.92.21.9881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Papakonstantinou E, Roth M, Tamm M, et al. Hypoxia differentially enhances the effects of transforming growth factor-beta isoforms on the synthesis and secretion of glycosaminoglycans by human lung fibroblasts. J Pharmacol Exp Ther. 2002;301:830–7. doi: 10.1124/jpet.301.3.830. [DOI] [PubMed] [Google Scholar]
- 20.Papakonstantinou E, Kouri FM, Karakiulakis G, et al. Increased hyaluronic acid content in idiopathic pulmonary arterial hypertension. Eur Respir J. 2008;32:1504–12. doi: 10.1183/09031936.00159507. [DOI] [PubMed] [Google Scholar]
- 21.Lee JY, Spicer AP. Hyaluronan: a multifunctional, megaDalton, stealth molecule. Curr Opin Cell Biol. 2000;12:581–6. doi: 10.1016/s0955-0674(00)00135-6. [DOI] [PubMed] [Google Scholar]
- 22.Meyer LJ, Stern R. Age-dependent changes of hyaluronan in human skin. J Invest Dermatol. 1994;102:385–9. doi: 10.1111/1523-1747.ep12371800. [DOI] [PubMed] [Google Scholar]
- 23.Wang C, Tammi M, Tammi R. Distribution of hyaluronan and its CD44 receptor in the epithelia of human skin appendages. Histochemistry. 1992;98:105–12. doi: 10.1007/BF00717001. [DOI] [PubMed] [Google Scholar]
- 24.Bertheim U, Hellström S. The distribution of hyaluronan in human skin and mature, hypertrophic and keloid scars. Br J Plast Surg. 1994;47:483–9. doi: 10.1016/0007-1226(94)90031-0. [DOI] [PubMed] [Google Scholar]
- 25.Laurent UB, Dahl LB, Reed RK. Catabolism of hyaluronan in rabbit skin takes place locally, in lymph nodes and liver. Exp Physiol. 1991;76:695–703. doi: 10.1113/expphysiol.1991.sp003536. [DOI] [PubMed] [Google Scholar]
- 26.Stern R, Jedrzejas MJ. Hyaluronidases: their genomics, structures, and mechanisms of action. Chem Rev. 2006;106:818–39. doi: 10.1021/cr050247k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Longas MO, Russell CS, He XY. Evidence for structural changes in dermatan sulfate and hyaluronic acid with aging. Carbohydr Res. 1987;159:127–36. doi: 10.1016/s0008-6215(00)90010-7. [DOI] [PubMed] [Google Scholar]
- 28.Bernstein EF, Underhill CB, Hahn PJ, et al. Chronic sun exposure alters both the content and distribution of dermal glycosaminoglycans. Br J Dermatol. 1996;135:255–62. doi: 10.1111/j.1365-2133.1996.tb01156.x. [DOI] [PubMed] [Google Scholar]
- 29.Papakonstantinou E, Roth M, Karakiulakis G. Hyaluronic acid: a key molecule in skin aging. Dermatoendocrinol. 2012;4:253–8. doi: 10.4161/derm.21923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Patriarca MT, Barbosa de Moraes AR, Nader HB, et al. Hyaluronic acid concentration in postmenopausal facial skin after topical estradiol and genistein treatment: a double-blind, randomized clinical trial of efficacy. Menopause. 2013;20:336–41. doi: 10.1097/GME.0b013e318269898c. [DOI] [PubMed] [Google Scholar]
- 31.Tsukahara K, Hotta M, Fujimura T, et al. Effect of room humidity on the formation of fine wrinkles in the facial skin of Japanese. Skin Res Technol. 2007;13:184–8. doi: 10.1111/j.1600-0846.2007.00209.x. [DOI] [PubMed] [Google Scholar]
- 32.Hillebrand GG, Liang Z, Yan X, et al. New wrinkles on wrinkling: an 8-year longitudinal study on the progression of expression lines into persistent wrinkles. Br J Dermatol. 2010;162:1233–41. doi: 10.1111/j.1365-2133.2010.09709.x. [DOI] [PubMed] [Google Scholar]
- 33.Durai PC, Thappa DM, Kumari R, et al. Aging in elderly: chronological versus photoaging. Indian J Dermatol. 2012;57:343–52. doi: 10.4103/0019-5154.100473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mustoe TA, Gurjala A. The role of the epidermis and the mechanism of action of occlusive dressings in scarring. Wound Repair Regen. 2011;19(Suppl 1):s16–21. doi: 10.1111/j.1524-475X.2011.00709.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pavicic T, Gauglitz GG, Lersch P, et al. Efficacy of cream-based novel formulations of hyaluronic acid of different molecular weights in anti-wrinkle treatment. J Drugs Dermatol. 2011;10:990–1000. [PubMed] [Google Scholar]



