Abstract
Objectives
To evaluate the impact of a genomic classifier (GC) test for predicting metastasis risk after radical prostatectomy (RP) on urologists' decision-making about adjuvant treatment of patients with high-risk prostate cancer.
Subjects and Methods
Patient case history was extracted from the medical records of each of the 145 patients with pT3 disease or positive surgical margins (PSMs) after RP treated by six high-volume urologists, from five community practices. GC results were available for 122 (84%) of these patients. US board-certified urologists (n = 107) were invited to provide adjuvant treatment recommendations for 10 cases randomly drawn from the pool of patient case histories. For each case, the study participants were asked to make an adjuvant therapy recommendation without (clinical variables only) and with knowledge of the GC test results. Recommendations were made without knowledge of other participants' responses and the presentation of case histories was randomised to minimise recall bias.
Results
A total of 110 patient case histories were available for review by the study participants. The median patient age was 62 years, 71% of patients had pT3 disease and 63% had PSMs. The median (range) 5-year predicted probability of metastasis by the GC test for the cohort was 3.9 (1–33)% and the GC test classified 72% of patients as having low risk for metastasis. A total of 51 urologists consented to the study and provided 530 adjuvant treatment recommendations without, and 530 with knowledge of the GC test results. Study participants performed a mean of 130 RPs/year and 55% were from community-based practices. Without GC test result knowledge, observation was recommended for 57% (n = 303), adjuvant radiation therapy (ART) for 36% (n = 193) and other treatments for 7% (n = 34) of patients. Overall, 31% (95% CI: 27–35%) of treatment recommendations changed with knowledge of the GC test results. Of the ART recommendations without GC test result knowledge, 40% (n = 77) changed to observation (95% CI: 33–47%) with this knowledge. Of patients recommended for observation, 13% (n = 38 [95% CI: 9–17%]) were changed to ART with knowledge of the GC test result. Patients with low risk disease according to the GC test were recommended for observation 81% of the time (n = 276), while of those with high risk, 65% were recommended for treatment (n = 118; P < 0.001). Treatment intensity was strongly correlated with the GC-predicted probability of metastasis (P < 0.001) and the GC test was the dominant risk factor driving decisions in multivariable analysis (odds ratio 8.6, 95% CI: 5.3–14.3%; P < 0.001).
Conclusions
Knowledge of GC test results had a direct effect on treatment strategies after surgery. Recommendations for observation increased by 20% for patients assessed by the GC test to be at low risk of metastasis, whereas recommendations for treatment increased by 16% for patients at high risk of metastasis. These results suggest that the implementation of genomic testing in clinical practice may lead to significant changes in adjuvant therapy decision-making for high-risk prostate cancer.
Keywords: prostate cancer, prognosis, metastasis, decision impact, patient management, clinical practice
Introduction
More than 230 000 men are diagnosed annually with prostate cancer in the USA, and nearly 30 000 will die from the disease [1]. Half of newly diagnosed men undergo radical prostatectomy (RP). Of these, approximately half will have one or more adverse pathological features, such as seminal vesicle invasion (SVI), extraprostatic extension (EPE), or positive surgical margins (PSMs) indicating an increased potential for disease recurrence [2,3].
Three randomised clinical studies on adjuvant radiation therapy (ART) vs observation among patients with adverse pathological features have been reported, showing significant reductions in biochemical recurrence [4–6], local recurrence [7,8], metastasis and increased overall survival for patients in the ART group [7]. Based on this evidence, the AUA updated its 2013 clinical practice guideline statement to recommend that ART should be offered as the ‘standard of care’ for all patients with adverse pathological features [9].
The decision for a patient to undergo ART after RP involves weighing the benefits of a ∼50% reduction in the risk of recurrence reported in randomised trials, with the risk of complications associated with radiation such as proctitis, rectal bleeding and urethral strictures [9]. Numerous predictive algorithms based primarily on pathological risk factors have been developed to assist in decision-making concerning the use of ART [10]; however, a well-recognised limitation of existing predictive algorithms is their low specificity in men with high-risk disease who all tend to have adverse pathology [11], such that many patients will be predicted to have a high risk of recurrence when the actual risk is low. In response, researchers have been seeking to develop new predictive algorithms that will increase the specificity of risk prediction.
Several previous studies have shown that genomic features in the primary tumour provide a quantitative and objective measure of biological potential for disease progression and metastasis [12]. The postoperative genomic classifier (GC) test (Decipher®; GenomeDx Biosciences, San Diego, CA, USA) was developed from an analysis of patients who had undergone RP at the Mayo Clinic, and was designed to predict early clinical metastatic after RP, with high specificity [13]. The GC test has subsequently been validated as an independent predictor of metastasis risk [14–16]. In these studies, the GC test was found to predict metastasis following RP more accurately than individual clinical variables, combinations of clinical variables, or other available predictive algorithms.
In comprehensive assessments of novel molecular tests, policy makers, third-party payers, and professional guidelines, and technology review groups request and review evidence on how such tests influence clinical management recommendations [17–19]. The present prospective, multicentre, decision-impact study (the ASSESS-D study) was intended to build on and add to evidence from a previously reported study, in which a 43% change in urologist adjuvant therapy recommendations after receiving the GC test results was observed [20]. The present ASSESS-D study was designed to evaluate the effect of knowledge of an individualised estimate of risk, provided by the GC test, on urologist treatment decision-making in a population of patients having undergone RP in a community-based practice; the intent was to reflect the distribution of test results and risk of metastasis among patients with high-risk disease seen in a broader range of clinical settings rather than just academic centres. The primary objective of the ASSESS-D study was to determine how urologists' knowledge of the GC test results influenced treatment planning for patients eligible for ART according to current AUA clinical guidelines. The secondary objective was to investigate the relative influence of the GC test results and clinical variables on urologist decision-making.
Subjects and Methods
Study Design
This ASSESS-D study was a multicentre, prospective, decision-impact study assessing urologist management recommendations in randomly presented case histories of patients who had received RP and had undetectable PSA <0.2 ng/mL (Fig. 1). Case histories were presented to participants with and without knowledge of the GC test results to determine how these results influenced management plans.
Figure 1.
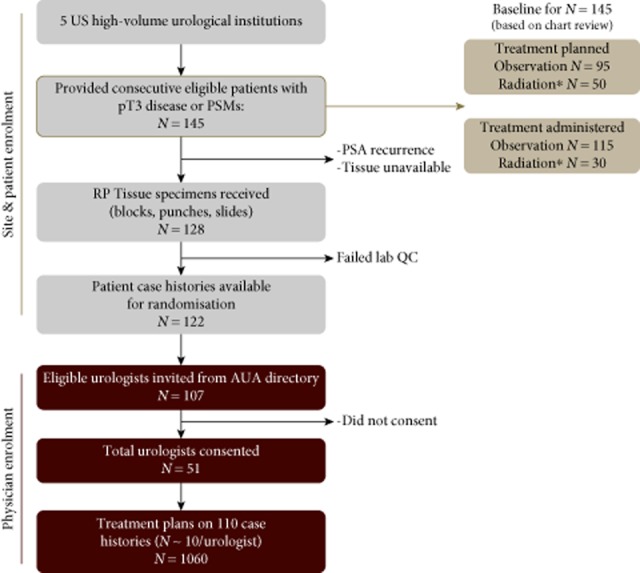
Flow diagram of the decision-impact, ASSESS-D, study. PSM, positive surgical margin. *Includes combination with hormone therapy or chemotherapy.
Case Histories
Patient case history was extracted from the medical records of patients seen by six high-volume urologists from five US community-based practices, who are currently using the GC test (Fig. 1). Records of consecutive patients presenting with pT3 disease or with positive surgical margins (PSMs) after surgery were eligible for data extraction. A total of 145 patient records that satisfied these criteria were extracted. The records of 17 patients who did not achieve PSA nadir after RP (PSA <0.2 ng/mL), or in whom adequate prostate tissue was unavailable, were excluded. Six samples failed laboratory quality control screens, leaving 122 patient records with complete clinical, pathological, treatment, and GC test results data available from which to develop case histories. Demographic and clinical information, as well as planned and administered treatment information after RP, was extracted from each patient's medical chart and collected using a case report form. All data were de-identified before the GC test analysis and presentation to urologist participants.
Genomic Classifier Test Analysis
Formalin-fixed paraffin-embedded tissue specimens from selected patient cases were submitted as sections, punches or blocks to a pathologist for re-review and preparation of tissue samples for processing in a Clinical Laboratory Improvement Amendments (CLIA)-certified medical laboratory. Tissue specimens were prepared for total RNA extraction, amplified, and hybridised to a high-density oligonucleotide microarray, and the 5-year probability of metastatic risk was computed for each of the patient cases based on the ‘locked’ 22-marker genomic classifier, as previously described [13].
Study Participants
Eligible study participants were US board-certified urologists who were recruited to the study through identification of key opinion leaders from the AUA membership directory, in addition to, high-volume surgeons referred by the urology co-authors of the study, who are among the early commercial users of the GC test. All participants provided written consent for study participation.
Data Collection
A study package on the GC test, a web link to the study's informed consent form, and electronic data collection instruments were provided to the urologists who consented to participate in the study. The study was conducted in accordance with the Declaration of Helsinki and the Belmont Report and was reviewed and approved by an independent institutional review board (Quorum Review Inc., Seattle, WA, USA). The study is registered on clinicaltrials.gov (NCT02034825).
Each study participant was presented with 10 randomly selected patient case histories based on clinical variables only (without GC test results). For the clinical only assessment, the following variables were presented to the urologists for each patient: age at surgery, ethnicity, preoperative PSA level (last measured PSA level before surgery), biopsy and pathological Gleason score, presence or absence of EPE, SVI, surgical margin status and lymph node involvement. The first measured PSA level after surgery was presented to verify all patients had achieved PSA nadir (Fig. S1A). The participants recorded their treatment recommendations based on the clinical information provided, with options including referral to a radiation oncologist and/or initiation of hormones, observation with regular clinical follow-up (i.e. monitoring until rise in PSA level), or any other plan not listed on the data collection instruments.
The GC test results were then provided for the same de-identified patient case histories (Fig. S1B). Case histories were randomly re-ordered to minimise recall bias. Urologists were then asked to record treatment recommendations with the knowledge of GC test results. All demographic information, clinical variables and the GC results were provided to urologists through a secure, Health Insurance Portability and Accountability Act-compliant, online platform.
Data Analysis
Statistical analyses were performed using R v3.0 software [21] and sas 9.2 (Cary, NC, USA). The chi-squared or Fisher's exact test were used to test for differences between groups for categorical variables. Exact binomial CIs were constructed to measure the changes in treatment from pre- to post-GC test results knowledge settings and all observations were considered independently, although a corroborative analysis adjusting for intra-urologist correlation was included. Generalised linear mixed-effect models were considered to account for urologist-specific behaviour in determining treatment plan changes. Univariable and multivariable regression models were used to assess the impact of GC test results and clinical variables in relation to treatment plan (logistic model) and change in intensity of the treatment plan (multinomial model) without and with GC test results. Age and preoperative PSA level were treated as continuous variables. Pathological Gleason score was dichotomised into ≤7 and 8, considering the small number of patient cases with Gleason score ≤6. EPE (present vs absent), SVI (present vs absent) and surgical margins (positive vs negative) were treated as binary variables. GC test results were categorised into low- and high-risk groups based on previously described thresholds [14].
Results
Of the 122 available case histories, 110 were presented and reviewed by at least one of the study participants (Table 1). The median (range) patient age was 62 (44–75) years and 85.5% of patients were Caucasian. The median (range) preoperative PSA level was 4.6 (0.6–23.6) ng/mL and 90.0% of patients had PSA < 10 ng/mL. Preoperatively, most patients were assessed as having D'Amico low- (21.8%) or intermediate-risk (60.0%) disease. Postoperatively, 71% of patients were found to have pT3 disease (EPE, SVI, or both), 63% had PSMs and 35% had both. The median (interquartile range) value of the first postoperative PSA measurement for this cohort was 0.04 (0.0063–0.1) ng/mL.
Table 1.
Demographic and clinical characteristics of reviewed case histories (N = 110)
| No. | |
|---|---|
| Median (min., max.) age at RP, years | 62 (44, 75) |
| Ethnicity, n (%) | |
| Caucasian | 94 (85.5) |
| Black | 8 (7.3) |
| Hispanic | 5 (4.5) |
| Not Available | 3 (2.7) |
| Preoperative PSA, n (%) | |
| <10 ng/mL | 99 (90.0) |
| 10–20 ng/mL | 9 (8.2) |
| >20 ng/mL | 2 (1.8) |
| Preoperative D'Amico risk groups, n (%) | |
| Low | 24 (21.8) |
| Intermediate | 66 (60.0) |
| High | 20 (18.2) |
| Biopsy Gleason score, n (%) | |
| 6 | 25 (22.7) |
| 7 (3 + 4) | 52 (47.3) |
| 7 (4 + 3) | 17 (15.4) |
| 8 | 10 (9.1) |
| 9 | 6 (5.5) |
| Pathological Gleason score, n (%) | |
| 6 | 10 (9.1) |
| 7 (3 + 4) | 57 (51.8) |
| 7 (4 + 3) | 28 (25.4) |
| 8 | 8 (7.3) |
| 9 | 7 (6.4) |
| Extraprostatic extension, n (%) | |
| Present | 75 (68.2) |
| Not assessed | 2 (1.8) |
| Seminal vesicle invasion, n (%) | |
| Present | 8 (7.3) |
| Not assessed | 1 (0.9) |
| Positive surgical margins, n (%) | 69 (62.7) |
| Postoperative CAPRA-S groups, n (%) | |
| Low | 23 (20.9) |
| Intermediate | 68 (61.8) |
| High | 16 (14.6) |
| Unknown | 3 (2.7) |
| Risk probability at 5 years after RP according to GC test | |
| Median (min, max), % | 3.85 (1.2, 33.4) |
| Low risk according to GC, n (%)* | 79 (71.8) |
CAPRA-S, Cancer of the Prostate Risk Assessment Surgical; RP, radical prostatectomy; GC, genomic classifier.
Using a previously defined threshold of 6% [14].
Figure 2A shows the distribution of GC test results in the full cohort, reported as 5-year probability of metastases (median 3.9%; range 1.2–33.4%). GC-based risk groups using pre-specified thresholds [14] classified 71.8% of these patients as being at low risk for metastasis. The 5-year probability of metastases by GC result increases with increasing tumour stage without (Fig. 2B) and with PSMs (Fig. S2). Among the 145 patients for whom medical chart records were extracted, 50 (35%) were recommended to receive adjuvant therapy after RP, whereas 30 (21%) actually received ART (Table S1).
Figure 2.
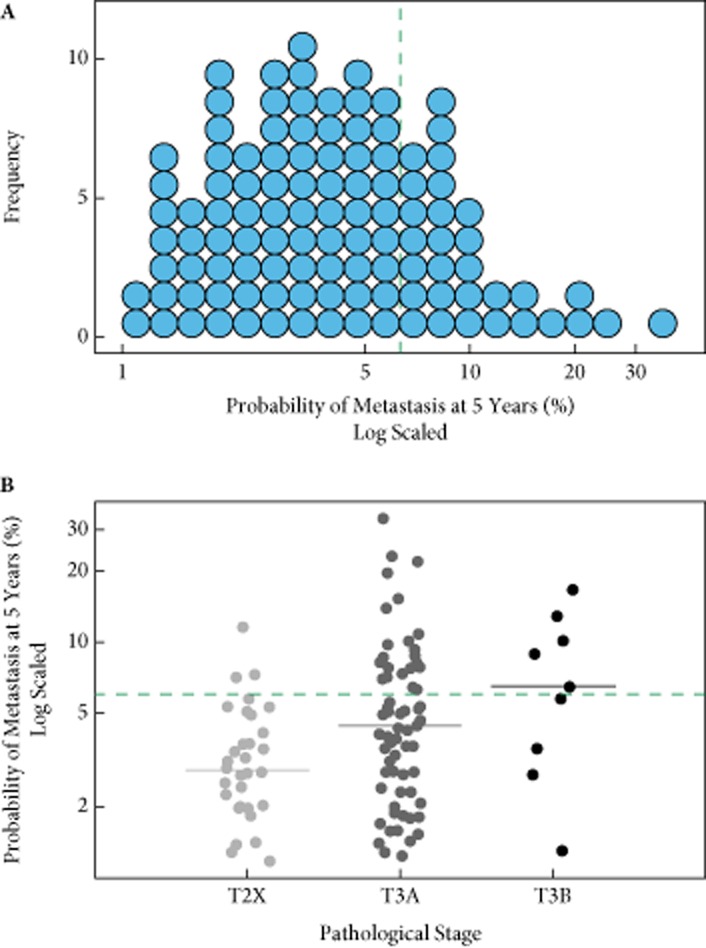
Distribution of genomic classifier test results A, in the overall cohort and B, stratified by pathological stage subsets. The green line indicates pre-specified threshold for low- or high-risk classification (<6% or ≥6% probability of metastasis 5 years after radical prostatectomy). T2X, pathological stage pT2A, pT2B or pT2C.
Of the 107 urologists notified of the study, 51 consented to participate. Study participants (Table 2) were all US board-certified urologists working in 33 institutions across 19 states (55% community-based). Participants had a median (range) of experience of 11 (2–30) years and performed a mean (range) of 130 (13–600) RPs/year. They provided 530 treatment recommendations with and 530 recommendations without knowledge of the GC test results.
Table 2.
Demographics of study participants (urologists; N = 51)
| No. | |
|---|---|
| Practice setting, n (%) | |
| Tertiary care | 23 (45) |
| Community (hospital, large urology group practice or private) | 28 (55) |
| No. years in practice, n | |
| Median (min, max) | 11 (2, 30) |
| No. RPs peformed per year | |
| Mean (min, max) | 130 (13, 600) |
| Geographic region (US Census Bureau), n (%) | |
| North-East | 11 (22) |
| Mid-West | 14 (27) |
| West | 5 (10) |
| South | 21 (41) |
RP, radical prostatectomy.
When the urologists did not have knowledge of GC test results (clinical information only), observation was recommended for 57% of patients (n = 303), ART for 36% (n = 193), and other treatments for 7% (n = 34; Table 3). Without GC test results, community-based providers planned treatment for 10% more cases than did academic/tertiary care providers (47 vs 37%; data not shown).
Table 3.
Effect of the genomic classifier (GC) test result on urologists' treatment recommendations after radical prostatectomy: change in urologists' treatment recommendations from without knowledge of GC tests result (clinical only) to with knowledge of GC test results (clinical + GC)
| Treatment planned |
Change with clinical + GC |
|||
|---|---|---|---|---|
| Without GC (clinical only) | With GC (clinical + GC) | Without GC (clinical only), n | Change, n (%) | 95% CI |
| Overall change* | 530 | 163 (31) | 27–35 | |
| Any treatment** | Observation | 223 | 85 (38) | 32–45 |
| ART | Observation | 193 | 77 (40) | 33–47 |
| ART+HT | Observation | 29 | 8 (28) | 13–47 |
| HT | Observation | 1 | 0 (0) | NA |
| Other | Observation | 4 | 4 (100) | 40–100 |
| ART | HT | 193 | 7 (4) | 1–7 |
| ART | ART+HT | 193 | 0 (0) | NA |
| ART | Other | 193 | 3 (2) | 0–4 |
| ART+HT | ART | 1 | 0 (0) | NA |
| ART+HT | ART+HT | 1 | 0 (0) | NA |
| ART+HT | Other | 1 | 1 (100) | 3–100 |
| HT | ART | 29 | 11 (38) | 21–58 |
| HT | HT | 29 | 0 (0) | NA |
| HT | Other | 29 | 1 (3) | 0–18 |
| Observation | Any treatment | 303 | 48 (16) | 12–20 |
| Observation | ART | 303 | 38 (13) | 9–17 |
| Observation | ART+HT | 303 | 9 (3) | 1–6 |
| Observation | HT | 303 | 1 (0) | 0–2 |
| Observation | Other | 303 | 3 (1) | 0–3 |
ART, adjuvant radiation therapy; HT, hormone therapy; NA, not applicable.
Results were virtually unchanged when intra-observer correlation was accounted for with overall change being 30% (95% CI: 25–36%).
Any treatment excludes case histories changed from ‘other’ to observation.
When the urologists did have knowledge of the GC test results, treatment recommendations changed by 31% from when they were without knowledge of GC (95% CI 27–35%; Table 3). A change in recommendation from treatment to observation represented 52% (n = 85) of the changed treatment recommendations. Of 193 recommendations for ART, 77 (40%; 95% CI: 33–47%) were changed with knowledge of GC test results to observation. For cases initially recommended for observation (n = 303), 38 (13%; 95% CI: 9–17%) changed to ART alone and nine (3%; 95% CI 1–6%) changed to ART+ hormone therapy (HT) with knowledge of the GC test results. Few treatment recommendations were changed from ART to HT (n = 7); of the 29 cases that were initially recommended for HT, 11 (38%) were changed to ART with knowledge of the GC test results.
With the knowledge of the GC test results, community-based providers and academic/tertiary care providers, recommended ART or ART+HT for 38% and 29% of cases, respectively. Although the number of ART recommendations was lower with GC test results than without, the difference between practice settings was maintained (9%; data not shown).
Treatment was recommended for 223 (42%) cases without GC test results. For the 342 (64.5%) case histories that were low risk according to the GC test results, observation was recommended for 276 (81%) with knowledge of these results (Fig. 3A). By contrast, observation was originally planned for 96 (51.1%) cases that were high risk according to the GC test results. With knowledge of the GC test results, observation was recommended in 65 (34.6%) cases (Fig. 3B); therefore, with knowledge of the GC test results, cases with a low risk according to the results were planned for treatment only 19% of the time, while those with a high risk were planned for treatment 65% of the time (P < 0.001). Results were similar when considering the subset of cases (n = 132) with an initial postoperative PSA level above the median value of 0.04 ng/mL (i.e. PSA 0.05–0.11 ng/mL; data not shown).
Figure 3.
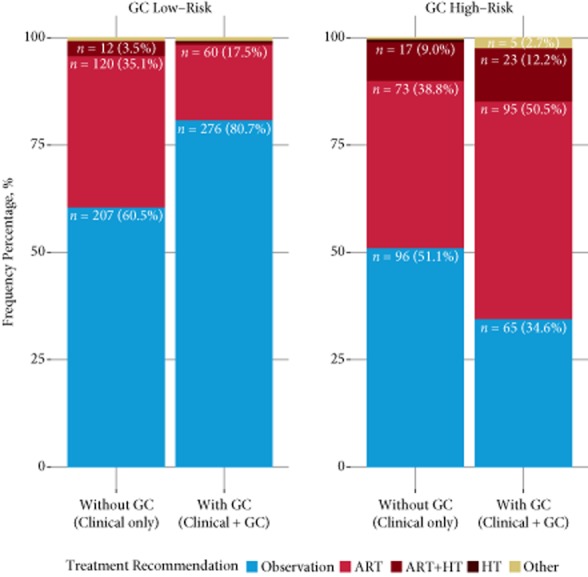
Treatment recommendation changes within genomic classifier (GC) risk groups (i.e. GC low risk and high risk according to the GC test result). ART, adjuvant radiation therapy; HT, hormone therapy; ART+HT, combined ART and HT. The y-axis indicates the frequency of treatment recommendations in the respective GC risk groups.
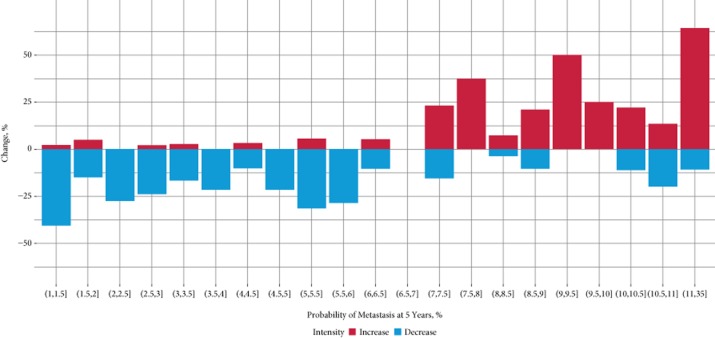
In addition, we examined how knowledge of GC test results affected treatment intensity (Fig. 4). Treatment intensity was defined as decreasing if recommendations were changed from ART to observation or from ART+HT to ART only. Alternatively, treatment intensity was defined as increasing if recommendation changed: from observation to ART; from ART only to ART+HT; or from any treatment to HT. A greater proportion of recommendations were to decrease treatment intensity for GC test results showing a <6.5% probability of metastasis. Conversely, a greater proportion of recommendations were to increase treatment intensity when the GC test results showed a ≥7.0% probability of metastasis (there were no GC test results in the range 6.5–7%). The correlation of recommended treatment intensity with probability of metastasis at 5 years according to the GC test was significant (P < 0.001). This pattern of recommendation for increasing treatment intensity with a higher probability of metastasis at 5 years was observed in both community and academic settings (Table S2).
Figure 4.
Treatment plan intensity waterfall plot by risk of metastasis at 5 years after radical prostatectomy, according to the genomic classifier (GC) test, across all reviewed patient case histories. Treatment intensity levels were ranked from lowest to highest as follows: observation, adjuvant radiation therapy (ART), ART+ hormone therapy (HT), and HT. The x-axis indicates the 5-year probabilities of metastasis (%) according to the GC test results and the y-axis indicates the percentage of change in treatment intensity with knowledge of the GC test results (%).
Without knowledge of GC test results, the factors that most influenced urologist recommendations were the presence of EPE, SVI or PSM and a high Gleason score, whereas age had little influence on treatment recommendations in the study cohort (Table 4). In multivariable analysis, knowledge of the GC test result was fourfold more influential than PSMs, the only other significant clinical variable.
Table 4.
Univariable and multivariable analyses to estimate the influence of demographic, clinical and genomic variables on treatment decision-making
| Without GC (clinical only) |
With GC (clinical + GC) |
|||||||
|---|---|---|---|---|---|---|---|---|
| Univariable |
Multivariable |
Univariable |
Multivariable |
|||||
| OR |
P | OR |
P | OR |
P | OR |
P | |
| (95% CI) | (95% CI) | (95% CI) | (95% CI) | |||||
| GC | – | – | – | – | 8.16 | <0.001 | 8.57 | <0.001 |
| (5.37–12.56) | (5.27, 14.26) | |||||||
| Margins | 2.68 | <0.001 | 4.23 | <0.001 | 1.42 | 0.075 | 2.22 | 0.002 |
| (1.84–3.94) | (2.77, 6.54) | (0.97–2.10) | (1.39–3.61) | |||||
| Age | 1 | 0.873 | 0.99 | 0.420 | 1.02 | 0.257 | 0.97 | 0.105 |
| (0.97, 1.03) | (0.96, 1.02) | (0.99, 1.05) | (0.94, 1.01) | |||||
| EPE | 2.53 | 0.001 | 4.07 | <0.001 | 2.8 | 0.001 | 1.66 | 0.109 |
| (1.60, 4.08) | (2.46, 6.91) | (1.70–4.79) | (0.90, 3.14) | |||||
| SVI | 3.22 | 0.002 | 2.08 | 0.085 | 3.37 | 0.001 | 1.7 | 0.23 |
| (1.57, 7.02) | (0.92, 4.95) | (1.65, 7.12) | (0.72, 4.11) | |||||
| Pathological Gleason score (>7) | 2.37 | <0.001 | 1.95 | 0.015 | 1.67 | 0.039 | 1.34 | 0.349 |
| (1.48, 3.86) | (1.14, 3.35) | (1.02, 2.70) | (0.72, 2.45) | |||||
| Preoperative PSA* | 1.06 | 0.02 | 1.06 | 0.092 | 0.97 | 0.25 | 1.01 | 0.855 |
| (1.01, 1.12) | (0.99, 1.13) | (0.91, 1.02) | (0.94, 1.07) | |||||
OR, odds ratio; GC, genomic classifier; EPE, extraprostatic extension; SVI, seminal vesical invasion. Models were adjusted for practice setting (private, community hospital or tertiary care).
Preoperative PSA values were log2-transformed.
Discussion
This multicentre, decision-impact ASSESS-D study, was designed to assess the effect of a GC that provides a quantitative measure of metastatic risk based on a biological readout (i.e. gene expression), on urologist treatment planning in the adjuvant setting. Unlike the previously reported DECIDE study, where patient cases were selected to ensure diversity of clinical and genomic-based risk levels, consecutive patient cases were prospectively accrued for ASSESS-D based on eligibility for adjuvant treatment as indicated by the current AUA guidelines. The 110 patients whose case histories were reviewed in the present study, therefore, reflect the metastatic risk profiles of patients with high-risk prostate cancer as found in urology practice settings, where, in the USA, a large proportion of patients with prostate cancer are treated [22].With knowledge of the GC test results, 31% of the treatment recommendations made by urologists based on patients' case histories changed. Notably, we measured this effect independently of practice setting (i.e. community or tertiary care provider), despite the fact that adjuvant treatment was recommended in 10% more cases for patients managed in the community.
According to AUA guidelines, all patients enrolled in the study should at least be offered ART because of their increased risk of metastasis (i.e. PSM or pT3) and because of the efficacy of ART (supported by data from three randomised clinical trials) [4–8]. Not all patients meeting these criteria, however, will experience recurrence after RP without adjuvant treatment, therefore, as stated in the guidelines, ‘prognostic biomarkers are greatly needed’ to assist in differentiating those at greater risk of disease progression among this population [9]. The GC test has been clinically validated as a predictor of metastasis [14–16]. Most (72%) of the patients included in the present study were predicted to have a low risk of metastasis according to their GC test results, with a median risk probability of just 2.8% at 5 years after RP compared with 8.8% for high risk results. Quantification of risk with the use of GC may therefore enhance a clinician's ability to discriminate among those who are less likely to experience disease progression, and avoid exposing these patients to unnecessary risks with adjuvant therapy; to identify those who are most at risk and who would derive the most to benefit from treatment intensification.
Knowledge of GC test results had a direct effect on treatment strategies after RP. In treatment plans made with knowledge of the GC test results, patients whose GC results showed a low risk of metastasis were planned for observation 81% of the time, while those whose results showed high risk were planned for treatment 65% of the time. Furthermore, treatment plans for patients with low risk according to the GC test had 50% less ART recommended in comparison with treatment plans based on clinical information alone. Multivariable analysis showed patients with high risk, according to the GC test, were nearly eight times more likely to be recommended for treatment than those with a low risk. Overall, intensification of treatment was highly correlated with higher risk according to the GC test. With knowledge of GC test results, urologists were nearly 1.4 times more likely to manage the patient by observation with PSA monitoring than to treat with ART or other secondary treatments (P < 0.01).
Extrapolated to a population of a 1000 patients with high-risk disease treated with RP (those with pT3 disease or with PSMs), implementation of the GC test could potentially increase the number of patients recommended to undergo observation by 72. Multiple validation studies have shown the superior accuracy of GC in predicting patient survival outcomes over clinical variables (such as Gleason score, PSA and tumour stage) that are currently used to make adjuvant treatment decisions. Recently, Den et al. [15] evaluated GC for predicting biochemical failure and metastasis in a cohort of 139 men treated after RP with radiation therapy (RT) over a 20-year period. Significant differences in biochemical and metastasis-free survival were observed only for patients who received ART (i.e. when PSA <0.2 ng/mL) in comparison with those who received salvage RT (i.e. when PSA ≥0.2 ng/mL) for the strata with high GC scores. No such differences were observed for patients with low GC scores. Accordingly, the authors concluded, patients with lower GC risk may benefit from delayed RT whereas those with higher GC risk from earlier RT. It is likely, therefore, that the effect of the GC test, if implemented into routine clinical practice, would be to direct adjuvant treatment more appropriately to patients who are most at risk of metastasis and who have the most to gain from this intervention. This would probably spare the majority of these patients from potentially unnecessary treatment and reduce the morbidity and costs associated with secondary therapy after RP overall. Despite strong evidence and guideline recommendations for ART for treatment of patients with high-risk disease, only 11.5–18.2% of such patients receive ART [23–25]. Several ongoing clinical trials are testing the hypothesis that early salvage RT is non-inferior to ART (e.g. the RADICALS and RAVES trials) although, in current clinical practice, with the availability of ultrasensitive PSA testing, most urologists prefer to wait for an initial rise in PSA level. Recently, Ross et al. [26] showed the predictive value of the GC test for patients with biochemical recurrence, and an ongoing trial by this group is underway to evaluate whether knowledge of the GC test may change treatment recommendations in the early salvage RT setting.
The results of the present study provide insights into the role of the GC test in clinical practice despite the following study limitations. The association between GC test result and treatment recommendations were determined based on case histories extracted from chart review of patients treated by five of the authors, ‘early adopters’ of the GC test, who may not be representative of all urologists that treat prostate cancer. The study participants reflect a selected group of high-volume prostate cancer surgeons with extensive experience in managing high-risk prostate cancer that may not be representative of the wider urology community. Furthermore, the study participants did not have access to the complete clinical evaluation with respect to patient health status and detailed pathological information, such as the location and extent of PSMs or tumour volume. In addition, data on treatment recommendations were collected rather than details of the actual treatment received after knowledge of the GC test; however, the rate of ART recommendations for cases when the participants were without knowledge of the GC test was similar to the proportion of ART recommended to these patients by their treating urologists (35%; Table S1). Moreover, the participants who consented to the study may reflect a subset of urologists more willing to use novel risk tools, such as the GC test and may not be generalizable to the broader urology community. Although, the above limitations are typical for early-stage evaluations of new biomarker technologies, by sampling over 100 patient cases treated in the community and 51 urologists representing diverse practices to provide treatment recommendations, our results are very likely to reflect the impact of the GC test in real-world settings.
In conclusion, findings from the present study confirm that knowledge of individualised risk estimates from genomic profiling, rather than population-based average risks of cancer progression and recurrence, significantly change urologists' postoperative adjuvant treatment decisions in an at-risk population. Studies are ongoing to prospectively evaluate patient–urologist shared decision-making, impact on quality of life, outcomes and healthcare economics for the postoperative therapy of high-risk prostate cancer.
Acknowledgments
The authors would like to thank Dr Vladimir Mouraviev, Charity Cowley, Jael Johnson, Johnn Beuscher, Gillian Devlin, Marguerite du Plessis and Aleksey Vorona for their assistance in the conducting of the study. Kasra Yousefi is acknowledged for support in the analysis and Dr Heesun Shin for useful comments and discussion.
Glossary
- RP
radical prostatectomy
- SVI
seminal vesicle invasion
- EPE
extraprostatic extension
- PSM
positive surgical margin
- RT
radiation therapy
- ART
adjuvant radiation therapy
- GC
genomic classifier
- HT
hormone therapy
Conflict of Interest
This study was supported by GenomeDx Biosciences and the National Research Council of Canada Industrial Research Assistance Program. C.B and E.D. are employees of GenomeDx Biosciences. K.K.B reports grants from GenomeDx Biosciences, during the conduct of the study; grants from GenomeDx Biosciences, outside the submitted work. J.H. reports grants from GenomeDx during the conduct of the study; other from ASCO, outside the submitted work. D.J.T. reports other from GenomeDx, during the conduct of the study. A.S. reports other from GenomeDx, during the conduct of the study; other from GenomeDx, outside the submitted work. D.A., G.B., N.K. and D.H. have nothing to disclose.
Supporting Information
Additional Supporting Information may be found in the online version of this article at the publisher*s web-site:
Fig. S1 Example of case history without (clinical only) and with genomic classfier (GC) result information (clinical + GC) as presented to study participants.
Fig. S2 Genomic classfier (GC) test distribution in subsets of pathological stage and surgical margin status. The green line indicates pre-specified thresholds for low- or high-risk classification (<6% or ≥6% probability of metastasis 5 years after radical prostatectomy). PSM, positive surgical margins; SM-, negative surgical margins.
Recommended and administered secondary therapy for patient cases across five study sites (N = 145).
Treatment intensity changes by genomic classfier (GC) risk category overall and by practice setting (community and tertiary care).
References
- 1.Siegel R, Ma J, Zou Z, Jemal A. Cancer Statistics, 2014. CA Cancer J Clin. 2014;64:9–29. doi: 10.3322/caac.21208. [DOI] [PubMed] [Google Scholar]
- 2.Swanson GP, Riggs M, Hermans M. Pathologic findings at radical prostatectomy: risk factors for failure and death. Urol Oncol. 2007;25:110–114. doi: 10.1016/j.urolonc.2006.06.003. [DOI] [PubMed] [Google Scholar]
- 3.NCCN Clinical Guidelines in Oncology (NCCN Guideline) 2014. Prostate Cancer. Version 1 [Internet]. NCCN; 2014. Available at: http://www.nccn.org/professionals/physician_gls/pdf/prostate.pdf. Accessed January 2014.
- 4.Bolla M, van Poppel H, Collette L. Postoperative radiotherapy after radical prostatectomy: a randomised controlled trial (EORTC trial 22911) Lancet. 2005;366:572–578. doi: 10.1016/S0140-6736(05)67101-2. [DOI] [PubMed] [Google Scholar]
- 5.Thompson IM, Jr, Tangen CM, Paradelo J. Adjuvant radiotherapy for pathologically advanced prostate cancer: a randomized clinical trial. JAMA. 2006;296:2329–2335. doi: 10.1001/jama.296.19.2329. [DOI] [PubMed] [Google Scholar]
- 6.Wiegel T, Bottke D, Steiner U. Phase III postoperative adjuvant radiotherapy after radical prostatectomy compared with radical prostatectomy alone in pT3 prostate cancer with postoperative undetectable prostate-specific antigen: ARO 96-02/AUO AP 09/95. J Clin Oncol. 2009;27:2924–2930. doi: 10.1200/JCO.2008.18.9563. [DOI] [PubMed] [Google Scholar]
- 7.Thompson IM, Tangen CM, Paradelo J. Adjuvant radiotherapy for pathological T3N0M0 prostate cancer significantly reduces risk of metastases and improves survival: long-term followup of a randomized clinical trial. J Urol. 2009;181:956–962. doi: 10.1016/j.juro.2008.11.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bolla M, van Poppel H, Tombal B. Postoperative radiotherapy after radical prostatectomy for high-risk prostate cancer: long-term results of a randomised controlled trial (EORTC trial 22911) Lancet. 2012;380:2018–2027. doi: 10.1016/S0140-6736(12)61253-7. [DOI] [PubMed] [Google Scholar]
- 9.Thompson IM, Valicenti RK, Albertsen P. Adjuvant and Salvage Radiotherapy After Prostatectomy: AUA/ASTRO Guideline. J Urol. 2013;190:441–449. doi: 10.1016/j.juro.2013.05.032. [DOI] [PubMed] [Google Scholar]
- 10.Lughezzani G, Briganti A, Karakiewicz PI. Predictive and prognostic models in radical prostatectomy candidates: a critical analysis of the literature. Eur Urol. 2010;58:687–700. doi: 10.1016/j.eururo.2010.07.034. et al. European Association of Urology; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stephenson AJ, Kattan MW, Eastham J. Prostate cancer-specific mortality after radical prostatectomy for patients treated in the prostate-specific antigen era. J Clin Oncol. 2009;27:4300–4305. doi: 10.1200/JCO.2008.18.2501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sorensen KD, Orntoft TF. Discovery of prostate cancer biomarkers by microarray gene expression profiling. Expert Rev Mol Diagn. 2010;10:49–64. doi: 10.1586/erm.09.74. [DOI] [PubMed] [Google Scholar]
- 13.Erho N, Crisan A, Vergara IA. Discovery and validation of a prostate cancer genomic classifier that predicts early metastasis following radical prostatectomy. PLoS ONE. 2013;8:e66855. doi: 10.1371/journal.pone.0066855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Karnes RJ, Bergstralh EJ, Davicioni E. Validation of a genomic classifier that predicts metastasis following radical prostatectomy in an at risk patient population. J Urol. 2013;190:2047–2053. doi: 10.1016/j.juro.2013.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Den RD, Feng FY, Showalter TN. Genomic prostate cancer classifier predicts biochemical failure and metastases in patients after postoperative radiation therapy. IJROBP. 2014;89:1036–1044. doi: 10.1016/j.ijrobp.2014.04.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Klein EA, Li J, Stephenson AJ, Yousefi K, Kattan MW, Magi-Galluzzi C. Independent validation of a genomic classifier in an at risk population of men conservatively managed after radical prostatectomy. J Clin Oncol. 2014;32(4):16. (Suppl. [Google Scholar]
- 17.Hornberger J, Doberne J, Chien R. Laboratory-developed test–SynFRAME: an approach for assessing laboratory-developed tests synthesized from prior appraisal frameworks. Genet Test Mol Biomarkers. 2012;16:605–614. doi: 10.1089/gtmb.2011.0177. [DOI] [PubMed] [Google Scholar]
- 18.Teutsch SM, Bradley LA, Palomaki GE. The Evaluation of Genomic Applications in Practice and Prevention (EGAPP) Initiative: methods of the EGAPP Working Group. Genet Med. 2009;11:3–14. doi: 10.1097/GIM.0b013e318184137c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hornberger J. Assigning value to medical algorithms: implications for personalized medicine. Per Med. 2013;10:577–588. doi: 10.2217/pme.13.55. [DOI] [PubMed] [Google Scholar]
- 20.Badani K, Thompson DJ, Buerki C. Impact of a genomic classifier of metastatic risk on postoperative treatment recommendations for prostate cancer patients: a report from the DECIDE study group. Oncotarget. 2013;4:600–609. doi: 10.18632/oncotarget.918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.R Development Core Team. R: A Language and Environment for Statistical Computing. Vienna, Austria: R Foundation for Statistical Computing; 2008. [Internet].. Available at: http://www.r-project.org. Accessed January 2014. [Google Scholar]
- 22.Association of Community Cancer Centers. 2014. [Internet].. [cited ]. Available at: http://www.accc-cancer.org/association/. Accessed January 2014.
- 23.Ghia AJ, Shrieve DC, Tward JD. Adjuvant radiotherapy use and patterns of care analysis for margin-positive prostate adenocarcinoma with extracapsular extension: postprostatectomy adjuvant radiotherapy: a SEER analysis. Urology. 2010;76:1169–1174. doi: 10.1016/j.urology.2010.04.047. [DOI] [PubMed] [Google Scholar]
- 24.Hoffman KE, Nguyen PL, Chen MH. Recommendations for post-prostatectomy radiation therapy in the United States before and after the presentation of randomized trials. J Urol. 2011;185:116–120. doi: 10.1016/j.juro.2010.08.086. [DOI] [PubMed] [Google Scholar]
- 25.Sheets NC, Hendrix LH, Allen IM, Chen RC. Trends in the use of postprostatectomy therapies for patients with prostate cancer: a Surveillance, Epidemiology, and End Results Medicare analysis. Cancer. 2013;119:3295–3301. doi: 10.1002/cncr.28222. [DOI] [PubMed] [Google Scholar]
- 26.Ross AE, Feng FY, Ghadessi M. A genomic classifier predicting metastatic disease progression in men with biochemical recurrence after prostatectomy. Prostate Cancer Prostatic Dis. 2014;17:64–69. doi: 10.1038/pcan.2013.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1 Example of case history without (clinical only) and with genomic classfier (GC) result information (clinical + GC) as presented to study participants.
Fig. S2 Genomic classfier (GC) test distribution in subsets of pathological stage and surgical margin status. The green line indicates pre-specified thresholds for low- or high-risk classification (<6% or ≥6% probability of metastasis 5 years after radical prostatectomy). PSM, positive surgical margins; SM-, negative surgical margins.
Recommended and administered secondary therapy for patient cases across five study sites (N = 145).
Treatment intensity changes by genomic classfier (GC) risk category overall and by practice setting (community and tertiary care).