Abstract
Background
Injectable bulking treatment for fecal incontinence (FI) is intended to expand tissue in the anal canal and prevent fecal leakage. Use of injectable bulking agents is increasing because it can be performed in an outpatient setting and with low risk for morbidity. This study evaluated the long-term (36-month) clinical effectiveness and safety of injection of non-animal stabilized hyaluronic acid/dextranomer (NASHA Dx) on FI symptoms.
Methods
In a prospective multicenter trial, 136 patients with FI received the NASHA Dx bulking agent. Treatment success defined as a reduction in number of FI episodes by 50% or more compared with baseline (Responder50). Change from baseline in Cleveland Clinic Florida Fecal Incontinence Score (CCFIS) and Fecal Incontinence Quality of Life Scale (FIQL), and adverse events were also evaluated.
Key Results
Successful decrease in symptoms was achieved in 52% of patients at 6 months and this was sustained at 12 months (57%) and 36 months (52%). Mean CCFIS decreased from 14 at baseline to 11 at 36 months (p < 0.001). Quality-of-life scores for all four domains improved significantly between baseline and 36 months of follow-up. Severe adverse events were rare and most adverse events were transient and pertained to minor bleeding and pain or discomfort.
Conclusions & Inferences
Submucosal injection of NASHA Dx provided a significant improvement of FI symptoms in a majority of patients and this effect was stable during the course of the follow-up and maintained for 3 years.
Keywords: fecal incontinence, injectable bulking agents, NASHA Dx, non-animal stabilized hyaluronic acid/dextranomer
Introduction
Recently, several new treatment options for fecal incontinence (FI) have been introduced,1–5 and additional options are under development.6 The clinical utility of some techniques is limited because of their morbidity profile.7,8
Injectable bulking treatment for FI is intended to expand tissue in the anal canal and prevent fecal leakage. It is an increasingly used treatment option, as it can be performed in an outpatient setting and with low risk for morbidity. Previous studies have been limited in size or duration, and the optimal substance and injection location are yet not determined.2,9–14
We have previously reported that non-animal stabilized hyaluronic acid/dextranomer (NASHA Dx; Solesta®, Salix Pharmaceuticals, Inc. Raleigh, NC, USA), a biocompatible, injectable bulking agent, is effective in the treatment of FI at short-term follow-up.15 The aim of the present study was to evaluate the long-term clinical effectiveness of injection of NASHA Dx on FI symptoms and its safety.
Materials and Methods
Patients
Patients were enrolled and followed up prospectively at eight US and five European centers between September 2006 and October 2011 under a strict protocol approved by the US Food and Drug Administration (FDA). For the short-term analysis, patients were randomly assigned to receive either submucosal anal injection of NASHA Dx (active treatment) or sham treatment, and these results have been presented previously.15 The present study includes the 136 patients who were randomized to active treatment and evaluates the outcome up to 36 months follow-up.
Inclusion and exclusion criteria have been presented previously.15 Patients were 18–75 years of age, had a Cleveland Clinic Florida Fecal Incontinence Score (CCFIS) of 10 or higher, had at least four recorded incontinence episodes during 2 weeks, FI symptoms for at least 12 months, and had failed conservative treatment under medical supervision (tried dietary avoidance of beverages or food that cause diarrhea or urgency, fiber supplementation, or antidiarrheal medications such as loperamide hydrochloride or diphenoxylate/atropine). Exclusion criteria included incontinence to flatus only, complete external sphincter disruption, significant mucosal prolapse or full thickness rectal prolapse, active anorectal sepsis, anorectal tumor, current anal fissure, rectal anastomosis <12 cm from anal verge, active proctitis, idiopathic anorectal bleeding, bleeding diathesis or on anticoagulant therapy (such as warfarin, heparin or heparin-like substance), anorectal stenosis, significant chronic anorectal or pelvic pain, anorectal surgery (including sphincteroplasty and/or Secca procedure) within the last 12 months prior to the study, anorectal implants (including sacral nerve stimulation), previous stapled transanal rectal resection or stapled hemorrhoidectomy, anorectal malformation, active inflammatory bowel disease, severely compromised immune defense or on immunosuppressive therapy, malignancy in remission for less than 2 years prior to the study, chemotherapy within the last 12 months prior to the study, previous pelvic radiotherapy, pregnant or breast-feeding woman, less than 1 year post-partum, women of child-bearing potential and not practicing adequate contraception, participation in any other clinical study within 3 month prior to the prestudy visit. Patients with grade IV hemorrhoids and patients who had undergone rubber banding of hemorrhoids <3 months ago.
Procedures
The procedure has been described in detail previously.15 Patients were treated in an outpatient setting and injections were given without anesthesia. One milliliter of NASHA Dx was injected through an anoscope into each quadrant of the submucosa (total dose = 4 mL), roughly 5 mm above the dentate line. After injection, the needle was retained for 10 s to avoid backward leakage of the substance through the injection channel.
After 1 month, all patients with a CCFIS of 10 or more and with no persistent adverse effects at the time were offered a repeat procedure.15
At each follow-up, patients underwent clinical assessment, adverse events were recorded, patients completed a 14-day bowel diary, and assessments with CCFIS and Fecal Incontinence Quality of Life Scale (FIQL).16 Treatment success was defined as a reduction in number of FI episodes by 50% or more per week compared with baseline (Responder50).
Statistical analysis
For continuous (numerical) efficacy data, change from baseline was analyzed with the Wilcoxon signed-rank test.
Missing data were handled using the last-observation-carried-forward (LOCF) method, where missing data were imputed using data from the preceding visit. The therapeutic success rate (Responder50; the proportion of subjects with ≥50% reduction in incontinence episodes per week) at 36 months was also evaluated using two alternative methods: complete case analysis, which included only subjects that completed the assessments at 36 months, and the worst-case analysis, where subjects with missing 36-month data were considered treatment failures.
All statistical tests were performed at a two-sided significance level of 0.05. Statistical analyses were performed with SAS version 9.2 (SAS Institute Inc., Cary, NC, USA). This trial was approved by the local institutional review board at each institution. ClinicalTrials.gov registration: NCT00605826.
Results
Of 278 patients who were screened, 206 were deemed eligible to be included in the study. Of these, 136 patients were randomized to active treatment and 70 to sham treatment. The 136 patients who received active treatment (n = 136) constitute the patient population of the present study. One hundred twenty-two of 136 patients (90%) were female and mean age was 61 (range, 33–76) years (Table1).
Table 1.
Demographic and baseline characteristics
| Parameter | NASHA Dx (n = 136) |
|---|---|
| Age, y, mean (range) | 61.8 (55.5–68.3) |
| Sex, n (%) | |
| Male | 14 (10.3) |
| Female | 122 (89.7) |
| Race, n (%) | |
| White | 122 (89.7) |
| Black | 6 (4.4) |
| Other | 8 (5.9) |
| Body mass index, kg/m2, mean (range) | 25.8 (23.3–29.8) |
| Cause of incontinence, n (%) | |
| Obstetric | 56 (41.2) |
| Neurogenic* | 27 (19.9) |
| Iatrogenic | 30 (22.1) |
| Other | 23 (16.9) |
| Duration of symptoms, n (%) | |
| 1–5 years | 65 (47.8) |
| >5 years | 71 (52.2) |
| Previous treatment, n (%) | |
| Diet change | 84 (61.8) |
| Fiber supplementation | 110 (80.9) |
| Antidiarrheal drug treatment | 82 (60.3) |
| Biofeedback | 82 (60.3) |
| Surgery | 21 (15.4) |
Included injured or diseased nervous system. NASHA Dx, non-animal stabilized hyaluronic acid/dextranomer. Adapted from Ref. 15.
Of the 136 patients at baseline, 132 patients were available for follow-up after 6 months, 128 patients after 12 months, and 112 patients after 36 months (Fig.1). In total 112/136 patients (82%) were treated a second time 1 month after the initial treatment. Ninety-seven (87%) of these retreated patients were available for follow-up after 36 months. No patient received any further injections beyond the initial per protocol treatment and retreatment.
Figure 1.
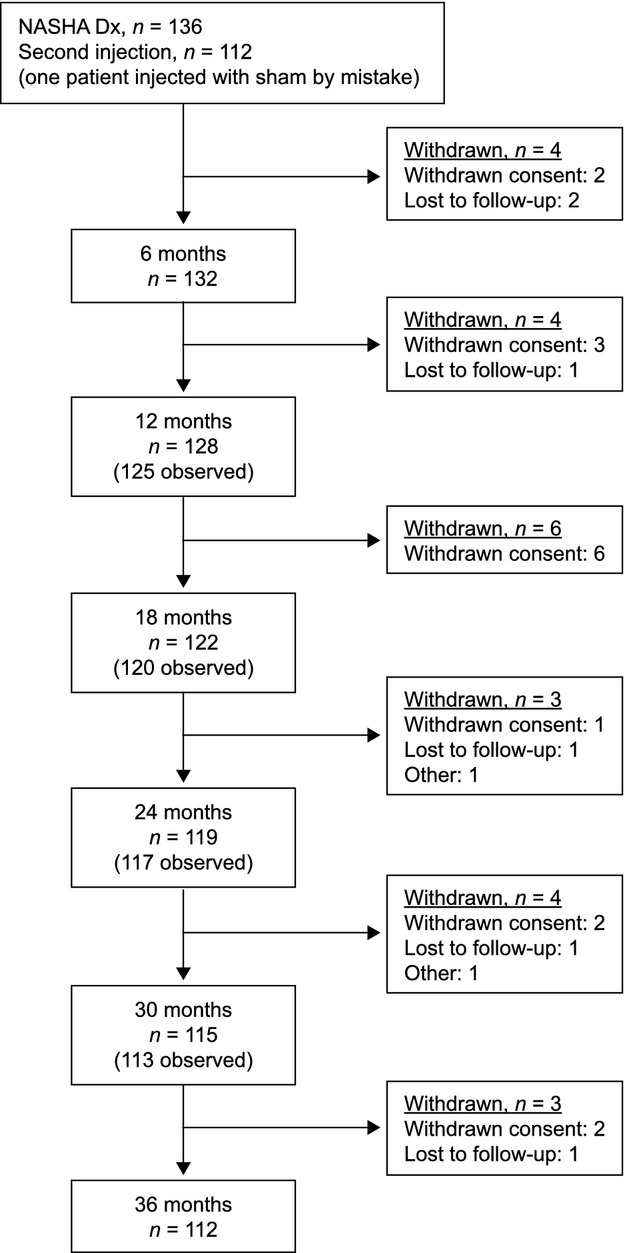
CONSORT diagram. NASHA Dx, non-animal stabilized hyaluronic acid/dextranomer.
During the 36-month study period, 3/136 patients (2%) underwent other treatments known to possibly influence continence function. One patient underwent a Delorme procedure for full thickness rectal prolapse 18 months before the study completion, one patient underwent a sphincteroplasty 1 week before study completion, and one patient underwent sacral nerve stimulation 1 month before study completion. All three patients were treatment failures, both before and after the additional treatments.
Treatment with NASHA Dx resulted in decreased symptoms (Responder50) in 52% of patients at 6 months and this was sustained at 36 months (52%; Fig.2). Six percent of patients experienced complete resolution of symptoms at 6 months and 13% at 36 months (Fig.2). A limited number of patients experienced worsening of symptoms (increase of FI episodes by ≥25%): 6% at 6 months, 12% at 12 months, and 15% at 36 months. Analyzing the success rate at 36 months in only the 112 patients who were followed up at this time point (complete case analysis) demonstrated a slightly higher success at 59% (Fig.3). The success rate was 44.9% when treating all missing patients as failures (worst-case analysis; Fig.3).
Figure 2.
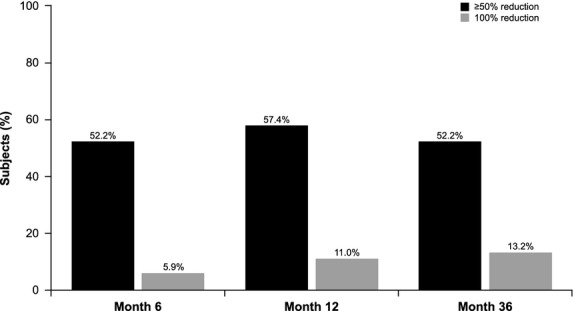
Percentage of subjects achieving a 50% (Responder50) and 100% reduction in fecal incontinence episodes at 6, 12, and 36 months.
Figure 3.
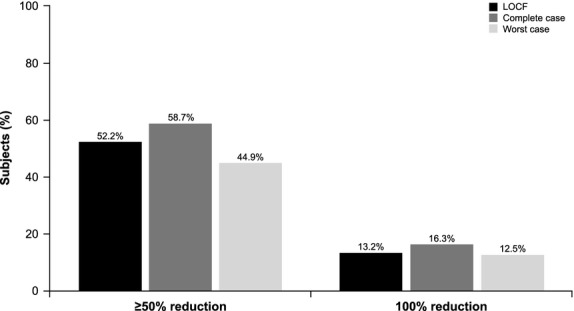
Percentage of subjects achieving a 50% (Responder50) and 100% reduction in fecal incontinence episodes at 36 months using three different imputation schemes for missing data: LOCF, Complete case analysis, and Worst-case analysis. LOCF, last observation carried forward.
Analyzing the effect of NASHA Dx, using a 14-day diary, the number of incontinence episodes decreased from median 15 at baseline to 7 at follow-up after 36 months (p < 0.001, Table2). Similarly, the number of incontinence-free days increased from 4.7 at baseline to 8.0 at 36 months (p < 0.001, Table2). Mean CCFIS decreased from 14 at baseline to 11 at 36 months (p < 0.001, Fig.4). The FIQL scores for all four domains improved between baseline and 36 months' follow-up (p < 0.001, Table3).
Table 2.
Number of FI episodes and incontinence-free days (14-day period)
| Time point | FI episodes |
Incontinence-free days |
||||
|---|---|---|---|---|---|---|
| Mean | Median | p-value* | Mean | Median | p-value* | |
| Baseline | 23.2 | 15.0 | 4.4 | 4.7 | ||
| 6 months | 14.6 | 7.2 | <0.001 | 7.5 | 8.3 | <0.001 |
| 12 months | 13.7 | 6.2 | <0.001 | 7.9 | 9.0 | <0.001 |
| 36 months | 13.2 | 7.0 | <0.001 | 8.1 | 8.0 | <0.001 |
For mean and median at each time point. Last observation carried forward; n = 136. 130 patients were evaluated at 6 months and 104 patients at 36 months. FI: fecal incontinence.
Figure 4.
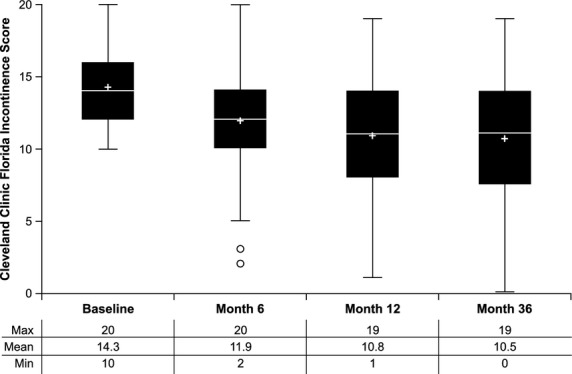
Box plots of Cleveland Clinic Florida Fecal Incontinence Scores at baseline, 6, 12, and 36 months. + symbols represent the mean and the line inside the box represents the median. The lower and upper edges of the box represent the first quartile (25th percentile) and the third quartile (75th percentile), and together bracket the interquartile range (IQR). Outliers at 6 months are 1.5 (IQR) below the 25th percentile.
Table 3.
Mean fecal incontinence quality-of-life (FIQL) scores
| Time point | Lifestyle | Coping | Depression/Self-perception | Embarrassment | p-value* |
|---|---|---|---|---|---|
| Baseline | 2.7 | 1.9 | 2.8 | 1.8 | |
| 6 months | 3.0 | 2.3 | 3.1 | 2.2 | <0.001 |
| 12 months | 3.2 | 2.5 | 3.3 | 2.5 | <0.001 |
| 36 months | 3.2 | 2.5 | 3.3 | 2.5 | <0.001 |
For each domain at each time point. Last observation carried forward; n = 136.
The use of antidiarrheal medications tended to decrease over time, in both responders and non-responders. The percentage of responders who used antidiarrheal medications was 46.5%, 24.7%, and 14.8% at 6, 12 and 36 months, respectively, whereas in non-responders, these percentages were 49.2%, 31.3%, and 25.6%. There was a trend that the percentage of responders who used antidiarrheal mediations was less compared with non-responders, although this difference did not reach statistical significance.
The most common treatment-related adverse events are found in Table4. During the first 6 months (blinded phase), 128 treatment-related adverse events were recorded.15 The most frequent adverse events were transient and pertained to minor bleeding and pain or discomfort. There were two serious adverse events. One patient developed a perianal abscess that required surgical intervention and one patient developed a prostatic abscess that resolved on antibiotic treatment. During the longer term follow-up, 6–36 months, an additional 20 treatment-related adverse events were recorded, the most common being proctalgia (n = 3) and injection site nodule (n = 3). Two patients rated proctalgia as mild and one as moderate. Treatments included Kenalog injection and xylocaine ointment. None of these patients underwent surgery and proctalgia resolved in all three cases.
Table 4.
Most common treatment-related adverse events (occurring in ≥2% of patients) and serious adverse events
| Adverse event | Timeframe postinjection n (%) |
|
|---|---|---|
| 0–6 months (n = 136) | >6 months–36 months (n = 133) | |
| Proctalgia | 19 (14.0) | 3 (2.3) |
| Pyrexia | 11 (8.1) | 0 |
| Rectal hemorrhage | 9 (6.6) | 2 (1.5) |
| Injection site bleeding | 7 (5.1) | 0 |
| Anorectal discomfort | 7 (5.1) | 0 |
| Injection site pain | 6 (4.4) | 0 |
| Diarrhea | 6 (4.4) | 2 (1.5) |
| Rectal discharge | 5 (3.7) | 0 |
| Anal hemorrhage | 5 (3.7) | 0 |
| Proctitis | 4 (2.9) | 0 |
| Chills | 4 (2.9) | 0 |
| Constipation | 3 (2.2) | 0 |
| Rectal abscess | 1 (0.7) | 0 |
| Prostate abscess* | 1 (0.7) | 0 |
| Injection site nodule | 0 | 3 (2.3) |
Serious adverse event.
Discussion
The present study confirms that submucosal injection of NASHA Dx provides an improvement of FI symptoms in a majority of patients and that this effect is stable during the course of the follow-up and maintained long-term up to 3 years. Several previous, smaller, studies have demonstrated similar positive effects of injectable bulking agents in the treatment of FI.9,13,17–19
Slightly more than half of patients (52%) reported treatment success after injection of NASHA Dx. These patients had a clinically relevant improvement of symptoms and it is noteworthy that the improvement in FI symptoms was accompanied by a concomitant improvement in FI-related quality of life (QOL). At the same time, our study also demonstrated that there were a limited number of patients who experienced a complete resolution of symptoms, and a few patients deteriorated despite treatment. When counseling patients about treatment alternatives, it should therefore be mentioned that the success rate of NASHA Dx is moderate, only a limited number of patients will achieve complete resolution of symptoms, and that reinjection at a later stage or alternative treatments might be needed. Despite these limitations, injection therapy remains an alternative in the treatment of FI. The treatment is easily delivered and side effects are few. Moreover, this treatment offers an alternative in the office setting and does not require sick leave or hospitalization.
The efficacy and durability of NASHA Dx stand in contrast to findings observed for some other injectable bulking agents.20 The reasons for these discrepancies are not entirely clear. The composition of NASHA Dx differs from other injectable bulking agents and the size of the injected particles (80–120 μm) was engineered specifically to prevent migration of the substance.21 The study size may also have helped to demonstrate a significant difference. The durability of the product has previously been demonstrated with an identical biocompatible material (Deflux®, Salix Pharmaceuticals, Inc.) in the setting of vesico-uretal reflux.22
In the present study, 112 of 136 patients (82%) completed an assessment at the 36-month follow-up. Depending on the imputation method, the 24 patients who were not available at the 36-month follow-up impacted the efficacy results to varying degrees. With the LOCF method, which uses the most recent data collected as end point data when the 36-month data are missing, the Responder50 rate was 52%. On the one hand, this analysis takes into account patients who discontinued before the 36-month cut-off because of lack of efficacy. On the other hand, this analysis extrapolates that the function will remain stable over time in subjects without 36-month follow-up data, an assumption which may not be accurate in all patients. Limiting the analysis to the 112 patients who completed the 36-month assessment (complete case analysis) yields a higher Responder50 rate (59%). This method is commonly used, but does not take into account the efficacy achieved in patients who discontinued the study before 36 months because of lack of efficacy. At the other end of the spectrum, the worst-case analysis treats all subjects with missing 36-month data as treatment failures. Using this method, the success rate was lower at 44.9%. This method of analysis is conservative, considering that there are reasons other than lack of efficacy that account for study discontinuation.
Our previous NASHA Dx study15 is one of the few randomized, controlled trials in this field. Under ideal conditions, it would have been preferable to conduct a 36-month sham-controlled study. However, we believe that this would be quite difficult, if not impossible, with current treatment alternatives and patient expectations. In the present study, patients agreed to participate in the study with the knowledge that they would receive active treatment either immediately or with a 6-month delay. In addition, at the time of the study, there were limited treatment options available for FI. Today, enrollment in a 36-month, blinded, randomized trial with a sham arm would be more difficult with the FDA approvals of sacral nerve stimulation and NASHA Dx.
In our previous report,15 we found an unexpectedly high rate of success in the sham injection arm. This underlines that effects of any treatment modality should be interpreted with caution. It is noteworthy that the only other randomized study evaluating an injectable for treatment of FI23 did not find a difference between the active and sham treatment arms. This study used another type of injectable and was significantly smaller (22 patients in each arm). The present report, together with our previous report,15 indicates that injection of NASHA Dx provides a positive difference when compared with sham and that this treatment effect is maintained up to 36 months.
Fecal incontinence frequently impairs patients' QOL and patients frequently adapt their life style when they have FI symptoms.24 New treatment options for FI are therefore welcome. The present study demonstrates that treatment with NASHA Dx provides a beneficial effect for the majority of patients, with an increase in the number of incontinence-free days that is paralleled with an improvement of QoL for up to 36 months. This correlation between improvement in severity of symptoms and QoL is in accordance with other studies evaluating FI treatment options.25
Several patients with FI used fiber or loperamide to stabilize the stool consistency. In addition, loperamide has been shown to increase resting anal pressure tone by ∽20% in patients treated with restorative proctocolectomy.26 However, the effect of loperamide is unlikely to have influenced the outcome of the present study, as the use of antidiarrheal medications decreased in both responders and non-responders over time, and there was a trend (non-significant) that this decrease was more pronounced in the responder group.
The present study used a limited amount of substance for injection. The amount was chosen from a pilot study9 in 34 patients where a similar degree of improvement was seen and it is unclear if an increased volume would have resulted in an improved effect. There are no comparative studies evaluating the optimal volume to inject. There is a vast experience from treatment of hemorrhoids using similar volumes of injectable substances.27 At the same time, 112/136 patients (82%) qualified and chose to be treated with repeat injections after 1 month. Future studies and clinical experience will determine the extent to which the current volume is satisfactory. Another unknown factor is the optimal injection site. Based on previous studies with NASHA Dx, the submucosal route was chosen, as it is safe, almost painless, can be performed without anesthesia, and is associated with a low risk of infection. An alternative location for injection is the intersphincteric space,19,20,28 which was not evaluated in the present study.
There were a significant number of adverse events reported in the present study. The study was conducted under a strict FDA-approved protocol, which mandates that all health problems be reported as adverse events. This reporting structure is consistent with previous studies in this field.4 There were two serious adverse events during the original 6-month trial, one of which required surgical intervention (drainage of abscess), and there were no additional treatment-related serious adverse events reported during the follow-up period through 36 months. Three patients reported proctalgia after 6 months. These symptoms resolved in all three patients after non-surgical management. This confirms that injection therapy is an attractive option from a safety standpoint when compared with other treatment alternatives.29
Patients suffering from FI have an increasing number of treatment options. The role of injectable agents in this treatment algorithm is not yet fully established. In addition, with the introduction of other new modalities for the treatment of FI, a combination of two or three modalities in the same patient will be increasingly common. In the future, it will be important to evaluate the outcomes after treatment with injectables and other modalities, such as sacral nerve stimulation and pelvic floor repair.
Key Messages
General statement: This study suggests that the efficacy of NASHA/Dx for reducing fecal incontinence episodes is stable for up to 3 years and that the incidence of adverse events remains low.
Aims/goals of the research: To evaluate the long-term clinical effectiveness and safety of anorectal submucosal injection of non-animal stabilized hyaluronic acid/dextranomer (NASHA Dx) on FI symptoms.
Basic methodology: Patients with FI were randomized to receive either the NASHA/Dx bulking agent or a sham treatment. Patients receiving the active treatment were evaluated for clinical effectiveness and safety for up to 36 months.
Results summary: The reduction of fecal incontinence episodes at 6 months was sustained at continued follow-up for 36 months. Additionally, mean Cleveland Clinic Florida Fecal Incontinence Score decreased significantly and Fecal Incontinence Quality of Life Scale scores improved significantly between baseline and 36 months of follow-up.
Acknowledgments
Additional members in the NASHA Dx Study Group were Duthie G (Castle Hill Hospital, East Yorkshire, UK); Eyring E (Center for Colon Rectal Disease, Murray, UT, USA); Haas E (Colorectal Surgical Associates, Houston, TX, USA); Zutshi M (Cleveland Clinic Foundation, Cleveland, OH, USA); Johansson C (Danderyd Hospital, Stockholm, Sweden); Marcet J (University South Florida, College of Medicine, Tampa, FL, USA); Roberts P (Lahey Clinic, Burlington, MA, USA); Starck M (Academic Hospital, Malmo, Sweden); and Varma M (UCSF Center for Pelvic Physiology, San Francisco, CA, USA).
Glossary
- CCFIS
Cleveland Clinic Florida Fecal Incontinence Score
- FDA
US Food and Drug Administration
- FI
fecal incontinence
- FIQL
Fecal Incontinence Quality of Life Scale
- IQR
interquartile range
- LOCF
last observation carried forward
- NASHA Dx
non-animal stabilized hyaluronic acid/dextranomer
- QoL
quality of life
Funding
This research was funded by Q-Med AB. Oceana Therapeutics acquired a license for worldwide commercialization rights for NASHA/Dx in 2009. Oceana Therapeutics was acquired by Salix Pharmaceuticals, Inc., in 2011. The authors affirm that they had complete access to the data that support the publication.
Disclosure
Dr. Mellgren's previous institution has received a grant from Q-Med for research. Dr. Mellgren also has served as a consultant for Salix Pharmaceuticals, Inc. Dr. Matzel has received consulting fees, support for travel to meetings, and fees for participation in trial design and analysis meetings from Q-Med. Dr. Pollack is deceased. Dr. Hull's institution has received a grant from Q-Med to perform FDA monitored research. Dr. Bernstein's institution has received a grant from Q-Med for research. Dr. Bernstein also has served as a consultant for Salix Pharmaceuticals, Inc. Dr. Graf has received travel accommodations from Oceana Therapeutics.
Author Contribution
AM, KEM, JP, TH, MB, and WG were involved in study conception and design, acquisition of data, and analysis and interpretation of data; provided critical revisions; and approved the final version of the manuscript submitted. AM drafted the article.
References
- 1.Norton C, Whitehead WE, Bliss DZ, Bliss DZ, Harari D, Lang J Conservative Management of Fecal Incontinence in Adults Committee of the International Consultation on Incontinence. Management of fecal incontinence in adults. Neurourol Urodyn. 2010;29:199–206. doi: 10.1002/nau.20803. [DOI] [PubMed] [Google Scholar]
- 2.Tan JJY, Chan M, Tjandra JJ. Evolving therapy for fecal incontinence. Dis Colon Rectum. 2007;50:1950–67. doi: 10.1007/s10350-007-9009-2. [DOI] [PubMed] [Google Scholar]
- 3.Tjandra JJ, Chan MKY, Yeh HCH. Injectable silicone biomaterial (PTQ) is more effective than carbon-coated beads (Durasphere) in treating passive faecal incontinence–a randomized trial. Colorectal Dis. 2009;11:382–9. doi: 10.1111/j.1463-1318.2008.01634.x. [DOI] [PubMed] [Google Scholar]
- 4.Wexner SD, Coller JA, Devroede G, Hull T, McCallum R, Chan M, Ayscue JM, Shobeiri AS, et al. Sacral nerve stimulation for fecal incontinence. Ann Surg. 2010;251:441–9. doi: 10.1097/SLA.0b013e3181cf8ed0. [DOI] [PubMed] [Google Scholar]
- 5.Wong MTC, Meurette G, Wyart V, Glemain P, Lehur PA. The artificial bowel sphincter: a single institution experience over a decade. Ann Surg. 2011;254:951–6. doi: 10.1097/SLA.0b013e31823ac2bc. [DOI] [PubMed] [Google Scholar]
- 6.Lehur P-A, McNevin S, Buntzen S, Mellgren AF, Laurberg S, Madoff RD. Magnetic anal sphincter augmentation for the treatment of fecal incontinence: a preliminary report from a feasibility study. Dis Colon Rectum. 2010;53:1604–10. doi: 10.1007/DCR.0b013e3181f5d5f7. [DOI] [PubMed] [Google Scholar]
- 7.Melenhorst J, Koch SM, van Gemert WG, Baeten CG. The artificial bowel sphincter for faecal incontinence: a single centre study. Int J Colorectal Dis. 2008;23:107–11. doi: 10.1007/s00384-007-0357-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Thornton MJ, Kennedy ML, Lubowski DZ, King DW. Long-term follow-up of dynamic graciloplasty for faecal incontinence. Colorectal Dis. 2004;6:470–6. doi: 10.1111/j.1463-1318.2004.00714.x. [DOI] [PubMed] [Google Scholar]
- 9.Danielson J, Karlbom U, Sonesson A-C, Wester T, Graf W. Submucosal injection of stabilized nonanimal hyaluronic acid with dextranomer: a new treatment option for fecal incontinence. Dis Colon Rectum. 2009;52:1101–6. doi: 10.1007/DCR.0b013e31819f5cbf. [DOI] [PubMed] [Google Scholar]
- 10.Maeda Y, Vaizey CJ, Kamm MA. Long-term results of perianal silicone injection for faecal incontinence. Colorectal Dis. 2007;9:357–61. doi: 10.1111/j.1463-1318.2006.01164.x. [DOI] [PubMed] [Google Scholar]
- 11.Schwandner O, Brunner M, Dietl O. Quality of life and functional results of submucosal injection therapy using dextranomer hyaluronic acid for fecal incontinence. Surg Innov. 2011;18:130–5. doi: 10.1177/1553350610392243. [DOI] [PubMed] [Google Scholar]
- 12.Smith S, Calleary J. Intra-anal collagen injection for the treatment of faecal incontinence [author reply in Br J Surg 2007;94:643–4] Br J Surg. 2006;93:1514–8. doi: 10.1002/bjs.5869. [DOI] [PubMed] [Google Scholar]
- 13.Aigner F, Conrad F, Margreiter R, Oberwalder M Coloproctology Working Group. Anal submucosal carbon bead injection for treatment of idiopathic fecal incontinence: a preliminary report. Dis Colon Rectum. 2009;52:293–8. doi: 10.1007/DCR.0b013e318197d755. [DOI] [PubMed] [Google Scholar]
- 14.Hussain ZI, Lim M, Stojkovic SG. Systematic review of perianal implants in the treatment of faecal incontinence. Br J Surg. 2011;98:1526–36. doi: 10.1002/bjs.7645. [DOI] [PubMed] [Google Scholar]
- 15.Graf W, Mellgren A, Matzel KE, Hull T, Johansson C NASHA Dx Study Group. Efficacy of dextranomer in stabilised hyaluronic acid for treatment of faecal incontinence: a randomised, sham-controlled trial. Lancet. 2011;377:997–1003. doi: 10.1016/S0140-6736(10)62297-0. [DOI] [PubMed] [Google Scholar]
- 16.Rockwood TH, Church JM, Fleshman JW, Kane RL, Mavrantonis C, Thorson AG, Wexner SD, Bliss D, et al. Fecal Incontinence Quality of Life Scale: quality of life instrument for patients with fecal incontinence. Dis Colon Rectum. 2000;43:9–16. doi: 10.1007/BF02237236. discussion 17. [DOI] [PubMed] [Google Scholar]
- 17.Chan MKY, Tjandra JJ. Injectable silicone biomaterial (PTQ) to treat fecal incontinence after hemorrhoidectomy. Dis Colon Rectum. 2006;49:433–9. doi: 10.1007/s10350-005-0307-2. [DOI] [PubMed] [Google Scholar]
- 18.Ganio E, Marino F, Giani I, Luc AR, Clerico G, Novelli E, Trompetto M. Injectable synthetic calcium hydroxylapatite ceramic microspheres (Coaptite) for passive fecal incontinence. Tech Coloproctol. 2008;12:99–102. doi: 10.1007/s10151-008-0406-x. [DOI] [PubMed] [Google Scholar]
- 19.Soerensen MM, Lundby L, Buntzen S, Laurberg S. Intersphincteric injected silicone biomaterial implants: a treatment for faecal incontinence. Colorectal Dis. 2009;11:73–6. doi: 10.1111/j.1463-1318.2008.01544.x. [DOI] [PubMed] [Google Scholar]
- 20.Maeda Y, Laurberg S, Norton C. Perianal injectable bulking agents as treatment for faecal incontinence in adults. Cochrane Database Syst Rev. 2013;2:CD007959. doi: 10.1002/14651858.CD007959.pub3. [DOI] [PubMed] [Google Scholar]
- 21.Stenberg AM, Sundin A, Larsson BS, Lackgren G, Stenberg A. Lack of distant migration after injection of a 125iodine labeled dextranomer based implant into the rabbit bladder. J Urol. 1997;158:1937–41. doi: 10.1016/s0022-5347(01)64185-5. [DOI] [PubMed] [Google Scholar]
- 22.Stenberg A, Larsson E, Lackgren G. Endoscopic treatment with dextranomer-hyaluronic acid for vesicoureteral reflux: histological findings. J Urol. 2003;169:1109–13. doi: 10.1097/01.ju.0000053013.49676.89. [DOI] [PubMed] [Google Scholar]
- 23.Siproudhis L, Morcet J, Laine F. Elastomer implants in faecal incontinence: a blind, randomized placebo-controlled study. Aliment Pharmacol Ther. 2007;25:1125–32. doi: 10.1111/j.1365-2036.2007.03293.x. [DOI] [PubMed] [Google Scholar]
- 24.Bordeianou L, Rockwood T, Baxter N, Lowry A, Mellgren A, Parker S. Does incontinence severity correlate with quality of life? Prospective analysis of 502 consecutive patients. Colorectal Dis. 2008;10:273–9. doi: 10.1111/j.1463-1318.2007.01288.x. [DOI] [PubMed] [Google Scholar]
- 25.Mellgren A, Wexner SD, Coller JA, Devroede G, Lerew DR, Madoff RD, Hull T SNS Study Group. Long-term efficacy and safety of sacral nerve stimulation for fecal incontinence. Dis Colon Rectum. 2011;54:1065–75. doi: 10.1097/DCR.0b013e31822155e9. [DOI] [PubMed] [Google Scholar]
- 26.Hallgren T, Fasth S, Delbro DS, Nordgren S, Oresland T, Hultén L. Loperamide improves anal sphincter function and continence after restorative proctocolectomy. Dig Dis Sci. 1994;39:2612–8. doi: 10.1007/BF02087698. [DOI] [PubMed] [Google Scholar]
- 27.Al-Ghnaniem R, Leather AJ, Rennie JA. Survey of methods of treatment of haemorrhoids and complications of injection sclerotherapy. Ann Royal Coll Surg Engl. 2001;83:325–8. [PMC free article] [PubMed] [Google Scholar]
- 28.Tjandra JJ, Lim JF, Hiscock R, Rajendra P. Injectable silicone biomaterial for fecal incontinence caused by internal anal sphincter dysfunction is effective. Dis Colon Rectum. 2004;47:2138–46. doi: 10.1007/s10350-004-0760-3. [DOI] [PubMed] [Google Scholar]
- 29.Luo C, Samaranayake CB, Plank LD, Bissett IP. Systematic review on the efficacy and safety of injectable bulking agents for passive faecal incontinence. Colorectal Dis. 2010;12:296–303. doi: 10.1111/j.1463-1318.2009.01828.x. [DOI] [PubMed] [Google Scholar]


