Abstract
Weight regain after weight loss is a substantial challenge in obesity therapeutics. Dieting leads to significant adaptations in the homeostatic system that controls body weight, which promotes overeating and the relapse to obesity. In this review, we focus specifically on the adaptations in white adipose tissues that contribute to the biological drive to regain weight after weight loss. Weight loss leads to a reduction in size of adipocytes and this decline in size alters their metabolic and inflammatory characteristics in a manner that facilitates the clearance and storage of ingested energy. We present the hypothesis whereby the long-term signals reflecting stored energy and short-term signals reflecting nutrient availability are derived from the cellularity characteristics of adipose tissues. These signals are received and integrated in the hypothalamus and hindbrain and an energy gap between appetite and metabolic requirements emerges and promotes a positive energy imbalance and weight regain. In this paradigm, the cellularity and metabolic characteristics of adipose tissues after energy-restricted weight loss could explain the persistence of a biological drive to regain weight during both weight maintenance and the dynamic period of weight regain.
Keywords: Adipogenesis, dieting, obesity, weight regulation
Introduction
Over 60% of adults and close to 20% of children in the United States are overweight or obese (1,2). Weight loss strategies are only transiently effective for most people, as the vast majority of individuals who attempt to lose weight are not able to achieve and maintain a 10% reduction over a year (3). Over a third of lost weight tends to return within the first year and the majority is gained back within 3 to 5 years (4,5). A number of reasons have been proposed for the high recidivism rates (5,6), but there is substantial evidence for a biological drive to regain weight after weight loss (7,8). The objective of this review is to summarize the contribution of white adipose tissue to this biological drive and discuss how changes in its cellularity and metabolic characteristics may facilitate weight regain.
The biological drive to regain weight
The biological control of body weight involves a complex feedback loop between the brain and periphery. The brain receives signals from the periphery regarding long-term energy stores (i.e. adipose tissue triglyceride) and short-term nutrient availability (i.e. immediate availability of circulating nutrients) and based upon these integrated signals, adjusts energy balance to meet both the long-term and short-term objectives of energy homeostasis. This feedback system adapts when energy intake is cognitively (in humans) or forcefully (in animal models) restricted.
In a previous review (7), we summarized the adaptations to energy-restricted weight loss that are thought to promote weight regain (Fig. 1). This adaptive response involves coordinated changes in the brain, gut, muscle, liver, adipose tissue and neuroendocrine system, which culminate in a concerted effect on energy balance. Peripheral signals create an ‘anabolic’ neural profile in the hypothalamus and hindbrain, increasing appetite and sending neuroendocrine efferent signals to enhance metabolic efficiency in peripheral tissues. Metabolic requirements decline as a function of (i) lost mass, (ii) reduced consumption of food and (iii) increased metabolic efficiency of peripheral tissues. Peripheral tissues clear circulating nutrients more effectively and utilize fuels more efficiently to produce the energy they need. Signals from the periphery convey to the brain that energy stores are depleted and nutrient availability is low and these signals integrate in key circuits of the hypothalamus and hindbrain that serve as the primary control centres for energy balance regulation. The response to these integrated signals is that appetite increases and the expenditure of energy declines. We have referred to this quantitative difference between the caloric value reflecting appetite and expenditure requirements as the energy gap (9–11). To maintain the reduced weight, food intake must be cognitively (in humans) or forcefully (in animals) restricted to the level that expended energy is suppressed. During weight maintenance after weight loss, this energy gap reflects the magnitude of the daily burden that thwarts cognitive efforts to maintain the reduced weight. When efforts to restrict intake fail, overfeeding occurs, and the excess nutrients are rapidly cleared and stored, and the relapse to obesity begins. This pressure to continue to overfeed generally persists until the lost weight returns. In some cases, the biological pressures may lead to weight gain that surpasses the original weight.
Figure 1.
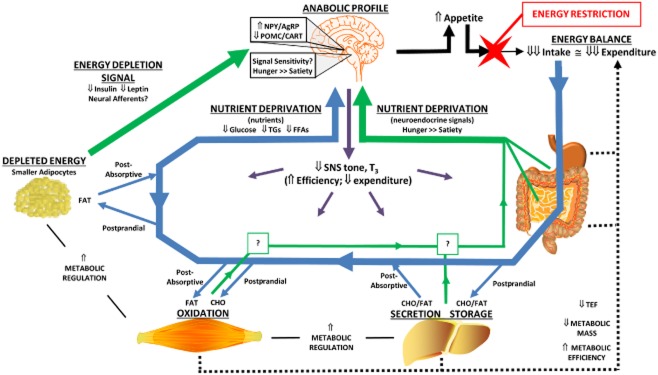
Homeostatic adaptations to weight loss that persist in weight maintenance.Neuroendocrine signals from the periphery (green arrows) convey a message of energy depletion (low leptin and insulin) and low nutrient availability (favouring signals of hunger over satiety/satiation) to the brain. Trafficking of absorbed nutrients (glucose, Glu; free fatty acids, FFA; triglycerides, TGs) to and from circulation is shown for both postprandial and post-absorptive metabolic states (blue arrows). Enhanced nutrient clearance reduces postprandial excursions in Glu and TGs and potentiates the postprandial suppression of FFAs, which may also convey a signal of nutrient deprivation to the brain. The signals of energy depletion and nutrient deprivation create an ‘anabolic’ neural profile in the hypothalamus and hindbrain, increasing appetite (solid black arrows) and sending efferent signals to enhance metabolic efficiency in peripheral tissues (purple arrows). The reduced metabolic mass, enhanced metabolic efficiency and lower thermic effect of food contribute to the suppression of energy expenditure (dotted black lines). A large energy gap is created between appetite and expenditure, and food intake must be cognitively (in humans) or forcefully (in animals) restricted to maintain the reduced weight. Adapted from fig. 1 of reference (7).
A fundamental understanding of this energy gap, dictated solely by biological pressures, has emerged from preclinical studies of weight regain in diet-induced obesity (DIO) models. The energy gap at the maintenance-relapse transition is influenced in predictable ways by diet composition (12), by the length of time in weight maintenance after weight loss (10) and by physical activity levels (13). Weight regain driven solely by this biological pressure reflects a first-order growth curve (4,11,13) such that the energy gap diminishes as the relapse to obesity progresses. As such, the magnitude of the energy gap is greatest at the nadir weight after weight loss (9,11,13). Furthermore, this energy gap does not dissipate with time in weight maintenance. Rather, studies indicate that the magnitude of the energy gap gradually increases the longer an animal maintains their reduced weight with an energy-restricted diet (10). The implications from these observations are that the biological pressures may strengthen with time during weight maintenance and with the amount of weight lost.
White adipose tissue is a critical node in the homeostatic system that controls body weight and it plays a particularly important role in the biological drive to regain lost weight. Over the past several decades, adipose tissue has been recognized as a dynamic, multifunctional organ with a number of different types of cells (14,15). It houses the majority of stored energy as triglyceride, which is thought to be the primary targeted parameter for regulation in long-term energy homeostasis. The adipocyte serves its primary purpose of long-term storage of energy and as weight is gained, lost and regained, adipocytes and their support cells must undergo a substantial amount of remodelling to accommodate the gain or loss of stored energy (16). As an integrated node in the feedback system, adipose tissues must send and receive important signals to and from the brain and other peripheral tissues to appropriately adjust the level of stored energy.
Changes in adipocyte cellularity
Adipocyte size: highly modified
Weight loss is accompanied by a dramatic reduction in the size of adipocytes (Fig. 2), which is reversed when weight is regained (11,13,17–19). An individual adipose depot contains adipocytes that vary with respect to their size, and a size frequency distribution provides a clear picture of this variability within a depot. Because adipose depots exhibit differing cellularity profiles, a frequency distribution is often more informative than an average diameter. Studies in both humans and rodents suggest that adipocyte size is the most changeable aspect of cellularity characteristics in studies of weight loss and regain. During weight loss, energy stores are mobilized from adipocytes and adipocytes become smaller. During weight gain and weight regain, energy is accumulated and adipocytes become larger. The broad range for adipocyte size provides enormous flexibility for the amount of energy that can be stored at any one time. However, as adipocytes change size with the mobilization or accumulation of energy, the extracellular matrix must be remodelled to accommodate the change or a considerable mechanical strain will be imposed upon the adipocytes (16). Mariman has hypothesized that weight loss causes cellular stress in adipocytes, resulting in an altered metabolic profile that would relieve the stress via increased storage of lipid (8). From this perspective, one portion of the biological drive to regain weight could be based in the mechanical and molecular changes that are working to relieve the cellular stress and mechanical strain of the adipocyte.
Figure 2.
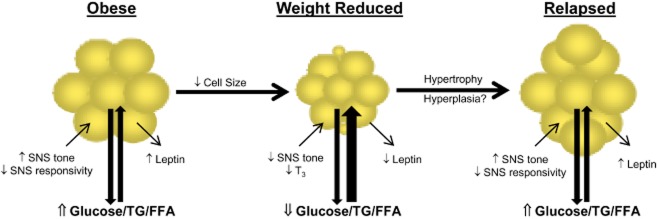
Adipocyte cellularity changes with weight loss and weight regain.Representative adipocytes are shown in the context of obesity, after weight loss and after weight regain. Weight loss would reduce the average size of resident adipocytes. Weight regain could involve both hypertrophy and hyperplasia. Changes in the neuroendocrine inputs (SNS tone and T3) that may be contributing to the adaptive response to weight loss are shown for each metabolic context. Likewise, changes in the secretion of the long-term adipose signal reflecting stored energy (leptin and insulin) are shown for each metabolic context. Finally, the systemic impact on nutrient availability is presented as the relative flux of glucose, triglycerides (TG) and free fatty acids (FFA). Both long-term (leptin) and short-term (nutrients and their surrogate signals) would be sensed by the hypothalamus and hindbrain to regulate appetite and metabolic requirements.
Adipocyte number: modified unidirectionally
Weight loss does not lead to any discernible change in the number of adipocytes in adipose tissue (11,13,17–19) (Fig. 2). The number of adipocytes in a normal, healthy individual remains relatively constant throughout adulthood (20), but there are conditions in which the number of adipocytes in particular adipose depots may increase. Our studies in a rodent paradigm of weight loss and regain suggest that the metabolic conditions during the relapse to obesity may provide the conditions that promote hyperplasia. Early in the relapse process, we observed the emergence of a population of very small (<20 μm) adipocytes, which was accompanied by an increase in total number of adipocytes in the depot (9). This increase in cell number persisted throughout the relapse process as all of the adipocytes became larger. We have speculated that this increased cell number partially explains animals in this model surpassing their pre-weight loss weight following relapse (11,13). While substantiating the temporal changes in cell size frequency distribution and total cell number in humans presents a logistical challenge, a hypercellularity phenomenon with similar characteristics has been reported in post-obese humans (21). Even so, this relapse-induced hyperplasia of adipose tissue, if it does occur, is likely limited to individuals who have a genetic predisposition for obesity. We have yet to observe an increase in cell number in diet-resistant rats or in DIO mice, which tend to relapse to their previous weight. Regardless, increasing the number of adipocytes in a depot in effect increases the overall capacity of that depot for triglyceride storage, and what flexibility exists for changing cell number appears to be unidirectional. There is very little evidence that the number of adipocytes is ever reduced under normal metabolic conditions associated with changes in weight. The implication is that increasing the number of adipocytes in a depot represents a permanent increase in the overall capacity of that depot to store triglyceride.
Adipocyte turnover: a tightly controlled balance
Because the number of adipocytes was observed to be relatively stable in normal, healthy adults, it was long thought that the adipocytes produced by puberty represented the population of cells that persisted throughout life. Tracer studies have discounted this notion by revealing that new adipocytes are being produced and mature adipocytes are being cleared with some regularity (22,23). A wide demographic study of Swedish adults observed that the turnover rate for adipocytes is approximately 8–10% per year. The generation of new adipocytes involves two distinct steps: (i) the proliferation of preadipocytes and (ii) the differentiation of preadipocytes into functioning adipocytes, capable of storing and releasing energy. The clearance of mature adipocytes is less understood, but is known to involve the recruitment of macrophages. The crown-like structures that are observed in adipose tissues represent adipocytes targeted for clearance, surrounded by the recruited macrophages (24). While the regulatory mechanisms for the generation and clearance of adipocytes are very different, they must be tightly linked to some global regulatory system that keeps them balanced, otherwise adipocyte number would be much less stable. The development of obesity is accompanied by a higher absolute amount of turnover, which is reflected in their greater fat mass and higher number of total adipocytes in their depots (16). The generation of new cells and clearance of mature cells remains, in general, balanced at a higher level in the obese. When adjusted for the difference in fat mass, the actual rate of cell turnover per unit fat mass is similar. At present, we do not know how adipocyte turnover is affected with weight loss or during the process of weight regain. However, if hyperplasia does occur, there must be some transient imbalance between new cell generation and mature cell clearance to account for the difference in cell number. Our ongoing studies will likely clarify how and when this balance is altered to elicit the hyperplasia we observed in our rodent paradigm of weight regain.
Metabolic capacity of the adipocyte
Changes in global gene expression
Adipose tissues experience a global down-regulation of gene expression in obese subjects in response to energy-restricted weight loss (25), which includes all of the key metabolic pathways. However, this effect is partly reversed at the transition to weight maintenance. With weight maintenance and during weight regain, an expression profile that would enhance energy conservation and the repletion of energy stores emerges (25–31). Markers of oxidative stress and inflammatory cytokines, which are also known to suppress appetite and increase expenditure, decline (29,32,33). The impaired induction of lipogenesis by insulin, glucose and feeding associated with obesity (34–37) resolves after energy-restricted weight loss (9,29,38–40). Finally, the enhanced metabolic response to ingested energy enhances nutrient clearance during weight maintenance and during sustained periods of overfeeding. These adaptive responses in the adipocyte prime the tissue to replete energy stores when nutrients once again become readily available.
Metabolic changes linked to adipocyte size
Insulin sensitivity is inversely related to size of the adipocyte (41). Compared with large adipocytes, small adipocytes exhibit higher rates of insulin-stimulated glucose uptake, higher levels of glucose oxidation and a lower sensitivity to antilipolytic action of insulin (42–44). In addition, smaller adipocytes exhibit a lower basal and catecholamine-induced lipolysis, have a lower rate of turnover of stored lipid and express genes favouring energy storage (28,45,46). The higher lipolytic capacity and triglyceride turnover in larger adipocytes is associated higher levels of Adipocyte triglyceride lipase (ATGL), Hormone sensitive lipase (HSL) and Lipoprotein lipase (LPL) (47–50). De novo lipogenesis is also down-regulated as adipocytes increase in size (51–54). Varlamov et al. (55) suggested that this relationship between cell size and metabolic function serves to protect against lipid overload and continual expansion, which could eventually have deleterious consequences for the health of the cell. It was suggested that when the adipocyte size approaches a critical threshold in an individual (∼100 μm), the capacity to take up and store circulating nutrients becomes diminished. If such a threshold exists, the implication is that an adipose tissue depot has a limited capacity to store energy, based upon the number of adipocytes it contains. Once that capacity is reached, the generation of new adipocytes (increasing the total capacity for storage) is the only avenue for storing more energy in the depot.
Functional changes of the adipocyte?
Beyond the cellularity characteristics, there is growing evidence to suggest that adipocytes have the capacity to alter their metabolic profiles and engage in wholesale changes in function, given the right metabolic context (14). White adipocytes have been observed in vivo to undergo transdifferentiation into brown adipocytes, which serve to dissipate, rather than store energy (56–58). Likewise, white adipocytes in the mammary gland have even been reported to transdifferentiate into glandular milk-producing epithelial cells during lactation, an effect that reverses after involution (57). These observations provide a novel perspective of the versatility of adipocytes that was once unappreciated. At present, few studies have considered such dramatic functional transformations in the context of weight loss, weight maintenance and weight regain studies. Given the metabolic extremes that can occur with weight loss and weight regain, it would be prudent for future studies to consider the extent to which adipocytes might be altered with energy restriction and gross overfeeding.
The versatility of metabolic profiles of adipocytes in changing environments may partly depend on the origins of the adipocytes. New adipocytes may primarily arise from resident preadipocytes and progenitors of the mesenchymal lineage, but recent findings demonstrate that bone marrow-derived progenitors (BMP) of the hematopoietic lineage can also migrate out of the skeleton and differentiate into adipocytes (59–62). Although this phenomenon needs to be demonstrated in humans, they may have an important role during weight regain if hyperplasia occurs. For instance, the observations of preferential homing and differentiation in visceral depots and lower leptin expression than white adipocytes suggest than BMP adipocytes could be a detriment to energy balance and metabolic health (60). The behaviour of these adipocytes during and after weight loss has not been determined, but would be essential for hypothesizing their relative role in energy balance and weight regain.
Neuroendocrine signals affecting adipose tissue
Energy-restricted weight loss from obesity is accompanied by a reduced sympathetic (SNS) tone (63–68) and reduced thyroid hormone levels (63,69–71). In contrast to the effects on SNS, the effect on thyroid hormones is observed less consistently and/or is more transiently tied to the early stages of weight loss (69,72,73). Collectively, these neuroendocrine changes can act upon adipose tissues to affect the size and number of resident adipocytes (Fig. 2). The SNS has established effects on the metabolic state and cellularity of adipose tissues (74,75) and a decline in SNS tone in this tissue could explain the shift in metabolic state favouring the uptake and deposition ingested energy, as well as the hyperplasia. Other studies indicate that both preadipocytes and adipocytes are responsive to Thyroid Stimulating Hormone (TSH) and thyroid hormones in a similar fashion (76–80). Both the SNS and thyroid hormones have inhibitory effects on preadipocyte proliferation and stimulatory effects on preadipocyte differentiation. As such, a decline in SNS tone and thyroid axis activity during weight maintenance may provide permissive conditions for preadipocyte proliferation, while the reversal of these neuroendocrine inputs during weight regain could underlie the hyperplasia. While these neuroendocrine inputs provide a plausible explanation for both metabolic and cellularity adaptations with weight loss and regain, their actual contribution to the adaptive response in adipose tissues requires further study.
Adipose signals for long-term energy stores
Leptin and insulin are often referred to as ‘adiposity signals’ because their levels generally reflect fat mass. Fasting levels of both hormones decrease with the decline in adiposity that occurs with weight loss (Fig. 2). The decline in leptin is more intuitive because it is secreted directly from adipocytes. The impact on insulin is indirect, reflecting the improvement in insulin sensitivity that occurs with weight loss (81–84). Interestingly, a number of studies have observed that leptin and insulin are actually reduced to a greater extent than would be expected for the amount of fat mass (11,21,65,85,86). We speculate that this may occur because leptin, and perhaps insulin, levels reflect both the amount of stored lipid and the size of the constituent adipocytes (87). Smaller adipocytes secrete less leptin and result in lower circulating levels for a given fat mass. Smaller adipocytes are also more insulin sensitive (45,46), which presumably means they require lower circulating levels of insulin to impart the same metabolic control. The reduction in cell size and the loss of total fat mass, therefore, may contribute independently to the decline in leptin and insulin. If new, very small adipocytes are generated early in the relapse process, the impact of cell size could be compounded. Regardless, the integrated adiposity signal conveyed to the brain is that the total energy reserves are low and that the adipocytes are far below their maximal capacity to store energy. The changes in these hormones directly contribute to enhanced hypothalamic expression of arcuate nucleus (ARC) neuropeptide Y (88–92) and agouti-related peptide (91,92), as well as decreased expression of proopiomelanocortin (89) (Fig. 1). These changes are the hypothalamic hallmark of an ‘anabolic’ state, leading to a positive energy imbalance and weight gain. Although the concept that the adiposity signals reflect both total stores and the fraction of maximal capacity filled is consistent with observations in weight loss studies, it needs to be tested more rigorously.
What complicates the role of leptin and insulin as ‘adipose signals’ is that their relationship to adiposity is maintained only during energy balance and the correlations only apply to fasted levels of the hormones. When an energy imbalance occurs, leptin and insulin reflect the metabolic state (anabolic or catabolic) of adipose tissue, as it deposits or mobilizes energy. Overfeeding increases circulating levels of leptin and insulin (93) and, with persistent overfeeding during weight regain, both leptin and insulin resolve long before the weight is fully regained (7). For this reason, leptin and insulin, by themselves, do not appear to sustain the signal of energy depletion as weight is being regained.
Nutrient availability as a reflection of the capacity to store excess energy
To complement the signal of energy depletion from these hormones, we have proposed that signals reflecting nutrient availability play a more critical role during the dynamic phases of weight regain (7). Signals could be either the nutrients or their surrogate neuroendocrine signals. The improvement in systemic metabolic regulation is often accompanied by lower fasting levels of glucose, free fatty acids (FFAs) and triglycerides (TGs), and more consistently yields reduced postprandial excursions of glucose and TGs with potentiated postprandial reductions in FFAs (7). This wholesale, consistent change in circulating nutrients undoubtedly imparts some homeostatic influence on the signals of nutrient status (Fig. 1). Levels of glucose are detected by nutrient-sensing systems in both the periphery (94–98) and brain (96,99), with consequences to energy balance and fuel utilization in the periphery. Triglycerides may even be sensed via their putative effects on leptin and insulin transport across the blood–brain barrier (9,100,101). FFAs are sensed, such as glucose, in the central and peripheral nutrient-sensing systems and can reduce subsequent food intake when infused into the gut (102,103), into the circulation (104) or directly into the brain (105,106). The cellular and metabolic adaptations in adipose tissues certainly contribute to the attenuated postprandial excursions of circulating nutrients following weight loss. The consequence to systemic metabolism is that postprandial glucose excursions would be attenuated and the postprandial suppression of circulating FFAs would be potentiated.
The ‘nutrient clearance’ hypothesis for the dynamic phase of weight regain
This hypothesis suggests that the energy gap between appetite and expended energy persists during weight regain as a function of the capacity of adipose tissue to clear and store excess energy (7) (Fig. 2). Early in relapse, the adipose tissue's capacity to clear excess energy is pitted against the rate at which nutrients are ingested and absorbed. As weight regain progresses, the adipocytes gradually increase in size and their capacity to clear excess energy diminishes. Excursions of glucose and TGs become larger and the suppression of FFA under dynamic (postprandial) states of metabolism would gradually become attenuated. Once the adipocytes near a critical threshold of size and the maximal capacity for stored energy is approached, the rate of weight regain would diminish. As the pre-weight loss weight is once again achieved, or surpassed if adipocyte hyperplasia has occurred, the fasting and postprandial levels of circulating nutrients would once again reflect the high levels observed with the insulin resistant state.
This simplistic hypothesis integrates the long-term adipose signals, reflecting the level of ‘stored energy’, with short-term signals of nutrient availability, which essentially reflect the ‘capacity to store energy’. Both signals are fundamentally rooted in the cellular and metabolic profiles of adipose tissues. Conceptually, the long-term signals provided by leptin and insulin would establish the global ‘anabolic’ tone in the hypothalamus, hindbrain and peripheral tissues. In this anabolic context, circulating nutrients and their surrogate neuroendocrine signals would become more important under postprandial conditions and during extended bouts of overfeeding while the weight is being regained. The convergence of these long-term and short-term signals in the energy homeostatic circuits of the brain would then dictate the magnitude and persistence of the energy gap.
The fundamental ideas behind this hypothesis are not entirely novel and they certainly present a simplified picture of the feedback system. Decades of research and numerous publications have provided a basic understanding of the key nodes of the homeostatic system controlling body weight and of adipocyte biology. Practically, the picture becomes much more complex as the integrated feedback signal from adipose tissues includes feedback from multiple adipose depots that have different metabolic and cellularity characteristics (15). Dieting and weight regain tend to alter visceral adipose depots more than subcutaneous depots (107–109), but less is understood about the interplay between depots, how they collectively establish a capacity-related ‘threshold’ for adipocyte size and about their relative contribution to the signals of energy depletion and nutrient availability during weight maintenance and weight regain. Furthermore, there is a large variability between individuals with respect to the metabolic and cellularity characteristics of their adipose depots (22). This variation may translate into different ‘thresholds’ for adipocyte size and, consequently, different maximal capacities for a given adipocyte number. Even so, the value of this hypothesis is that it provides a basic explanation for the persistence of the energy gap driving weight regain in both static (during weight maintenance) and dynamic (during weight regain) phases of the relapse to obesity. In addition, it frames the integration of long-term signals for stored energy and short-term signals of nutrient availability in a manner that links both to the cellular and metabolic characteristic of adipose tissues.
Conclusions
Adipose tissues represent a key node in the homeostatic system that regulates body weight. Weight loss from caloric restriction results in substantial changes that prime adipose tissues to take up and store ingested energy. In combination with the other adaptations in this homeostatic system, these changes in adipose tissues present a significant challenge for successful weight loss maintenance. Weight loss awakens the body's defence system in a manner that is persistent, saturated with redundancies and well-focused on the objective of restoring the body's depleted energy reserves. Successful, long-term weight loss requires recognition of the strength and persistence of these biological pressures and a better understanding of how they may be countered with environmental, behavioural and pharmaceutical interventions. Adipose tissues and, more specifically, the adipocytes may provide an important target for developing interventions, given their critical role in the adaptive response. To be effective, interventions aimed at preventing weight regain will likely need to be as comprehensive, persistent and redundant as the biological adaptations they are attempting to counter.
Conflict of interest statement
The authors declare no conflict of interest.
References
- 1.Ogden CL, Carroll MD, Curtin LR, McDowell MA, Tabak CJ, Flegal KM. Prevalence of overweight and obesity in the United States, 1999–2004. JAMA. 2006;295:1549–1555. doi: 10.1001/jama.295.13.1549. [DOI] [PubMed] [Google Scholar]
- 2.Catenacci VA, Hill JO, Wyatt HR. The obesity epidemic. Clin Chest Med. 2009;30:415–444. doi: 10.1016/j.ccm.2009.05.001. [DOI] [PubMed] [Google Scholar]
- 3.Kraschnewski JL, Boan J, Esposito J, et al. Long-term weight loss maintenance in the United States. Int J Obes (Lond) 2010;34:1644–1654. doi: 10.1038/ijo.2010.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Anderson JW, Konz EC, Frederich RC, Wood CL. Long-term weight-loss maintenance: a meta-analysis of US studies. Am J Clin Nutr. 2001;74:579–584. doi: 10.1093/ajcn/74.5.579. [DOI] [PubMed] [Google Scholar]
- 5.Weiss EC, Galuska DA, Kettel Khan L, Gillespie C, Serdula MK. Weight regain in U.S. adults who experienced substantial weight loss, 1999–2002. Am J Prev Med. 2007;33:34–40. doi: 10.1016/j.amepre.2007.02.040. [DOI] [PubMed] [Google Scholar]
- 6.Elfhag K, Rossner S. Who succeeds in maintaining weight loss? A conceptual review of factors associated with weight loss maintenance and weight regain. Obes Rev. 2005;6:67–85. doi: 10.1111/j.1467-789X.2005.00170.x. [DOI] [PubMed] [Google Scholar]
- 7.Maclean PS, Bergouignan A, Cornier MA, Jackman MR. Biology's response to dieting: the impetus for weight regain. Am J Physiol. 2011;301:R581–R600. doi: 10.1152/ajpregu.00755.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mariman EC. Human biology of weight maintenance after weight loss. J Nutrigenet Nutrigenomics. 2012;5:13–25. doi: 10.1159/000337081. [DOI] [PubMed] [Google Scholar]
- 9.Jackman MR, Steig A, Higgins JA, et al. Weight regain after sustained weight reduction is accompanied by suppressed oxidation of dietary fat and adipocyte hyperplasia. Am J Physiol. 2008;294:R1117–R1129. doi: 10.1152/ajpregu.00808.2007. [DOI] [PubMed] [Google Scholar]
- 10.MacLean PS, Higgins JA, Johnson GC, et al. Enhanced metabolic efficiency contributes to weight regain after weight loss in obesity-prone rats. Am J Physiol. 2004;287:R1306–R1315. doi: 10.1152/ajpregu.00463.2004. [DOI] [PubMed] [Google Scholar]
- 11.MacLean PS, Higgins JA, Jackman MR, et al. Peripheral metabolic responses to prolonged weight reduction that promote rapid, efficient regain in obesity-prone rats. Am J Physiol. 2006;290:R1577–R1588. doi: 10.1152/ajpregu.00810.2005. [DOI] [PubMed] [Google Scholar]
- 12.Higgins JA, Jackman MR, Brown IL, et al. Resistant starch and exercise independently attenuate weight regain on a high fat diet in a rat model of obesity. Nutr Metab (Lond) 2011;8:49. doi: 10.1186/1743-7075-8-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.MacLean PS, Higgins JA, Wyatt HR, et al. Regular exercise attenuates the metabolic drive to regain weight after long-term weight loss. Am J Physiol. 2009;297:R793–R802. doi: 10.1152/ajpregu.00192.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Giordano A, Smorlesi A, Frontini A, Barbatelli G, Cinti S. White, brown and pink adipocytes: the extraordinary plasticity of the adipose organ. Eur J Endocrinol. 2014;170:R159–R171. doi: 10.1530/EJE-13-0945. [DOI] [PubMed] [Google Scholar]
- 15.Lee MJ, Wu Y, Fried SK. Adipose tissue heterogeneity: implication of depot differences in adipose tissue for obesity complications. Mol Aspects Med. 2013;34:1–11. doi: 10.1016/j.mam.2012.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lee MJ, Wu Y, Fried SK. Adipose tissue remodeling in pathophysiology of obesity. Curr Opin Clin Nutr Metab Care. 2010;13:371–376. doi: 10.1097/MCO.0b013e32833aabef. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gurr MI, Jung RT, Robinson MP, James WP. Adipose tissue cellularity in man: the relationship between fat cell size and number, the mass and distribution of body fat and the history of weight gain and loss. Int J Obes (Lond) 1982;6:419–436. [PubMed] [Google Scholar]
- 18.Miller WH, Jr, Faust IM, Goldberger AC, Hirsch J. Effects of severe long-term food deprivation and refeeding on adipose tissue cells in the rat. Am J Physiol. 1983;245:E74–E80. doi: 10.1152/ajpendo.1983.245.1.E74. [DOI] [PubMed] [Google Scholar]
- 19.Portillo MP, Cantoral R, Macarulla MT. Effects of dietary fat content on adiposity during energy restriction in genetically obese rats. Reprod Nutr Dev. 1999;39:189–199. doi: 10.1051/rnd:19990204. [DOI] [PubMed] [Google Scholar]
- 20.Spalding KL, Arner E, Westermark PO, et al. Dynamics of fat cell turnover in humans. Nature. 2008;453:783–787. doi: 10.1038/nature06902. [DOI] [PubMed] [Google Scholar]
- 21.Lofgren P, Andersson I, Adolfsson B, et al. Long-term prospective and controlled studies demonstrate adipose tissue hypercellularity and relative leptin deficiency in the postobese state. J Clin Endocrinol Metab. 2005;90:6207–6213. doi: 10.1210/jc.2005-0596. [DOI] [PubMed] [Google Scholar]
- 22.Arner E, Westermark PO, Spalding KL, et al. Adipocyte turnover: relevance to human adipose tissue morphology. Diabetes. 2010;59:105–109. doi: 10.2337/db09-0942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Arner P, Bernard S, Salehpour M, et al. Dynamics of human adipose lipid turnover in health and metabolic disease. Nature. 2011;478:110–113. doi: 10.1038/nature10426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cinti S, Mitchell G, Barbatelli G, et al. Adipocyte death defines macrophage localization and function in adipose tissue of obese mice and humans. J Lipid Res. 2005;46:2347–2355. doi: 10.1194/jlr.M500294-JLR200. [DOI] [PubMed] [Google Scholar]
- 25.Capel F, Klimcakova E, Viguerie N, et al. Macrophages and adipocytes in human obesity: adipose tissue gene expression and insulin sensitivity during calorie restriction and weight stabilization. Diabetes. 2009;58:1558–1567. doi: 10.2337/db09-0033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Viguerie N, Vidal H, Arner P, et al. Adipose tissue gene expression in obese subjects during low-fat and high-fat hypocaloric diets. Diabetologia. 2005;48:123–131. doi: 10.1007/s00125-004-1618-x. [DOI] [PubMed] [Google Scholar]
- 27.Gummesson A, Jernas M, Svensson PA, et al. Relations of adipose tissue CIDEA gene expression to basal metabolic rate, energy restriction, and obesity: population-based and dietary intervention studies. J Clin Endocrinol Metab. 2007;92:4759–4765. doi: 10.1210/jc.2007-1136. [DOI] [PubMed] [Google Scholar]
- 28.Svensson PA, Gabrielsson BG, Jernas M, Gummesson A, Sjoholm K. Regulation of human aldoketoreductase 1C3 (AKR1C3) gene expression in the adipose tissue. Cell Mol Biol Lett. 2008;13:599–613. doi: 10.2478/s11658-008-0025-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kern PA, Saghizadeh M, Ong JM, Bosch RJ, Deem R, Simsolo RB. The expression of tumor necrosis factor in human adipose tissue. Regulation by obesity, weight loss, and relationship to lipoprotein lipase. J Clin Invest. 1995;95:2111–2119. doi: 10.1172/JCI117899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kolehmainen M, Vidal H, Ohisalo JJ, Pirinen E, Alhava E, Uusitupa MI. Hormone sensitive lipase expression and adipose tissue metabolism show gender difference in obese subjects after weight loss. Int J Obes (Lond) 2002;26:6–16. doi: 10.1038/sj.ijo.0801858. [DOI] [PubMed] [Google Scholar]
- 31.Berman DM, Nicklas BJ, Ryan AS, Rogus EM, Dennis KE, Goldberg AP. Regulation of lipolysis and lipoprotein lipase after weight loss in obese, postmenopausal women. Obes Res. 2004;12:32–39. doi: 10.1038/oby.2004.6. [DOI] [PubMed] [Google Scholar]
- 32.Huang P, Li S, Shao M, et al. Calorie restriction and endurance exercise share potent anti-inflammatory function in adipose tissues in ameliorating diet-induced obesity and insulin resistance in mice. Nutr Metab (Lond) 2010;7:59. doi: 10.1186/1743-7075-7-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Palming J, Sjoholm K, Jernas M, et al. The expression of NAD(P)H:quinone oxidoreductase 1 is high in human adipose tissue, reduced by weight loss, and correlates with adiposity, insulin sensitivity, and markers of liver dysfunction. J Clin Endocrinol Metab. 2007;92:2346–2352. doi: 10.1210/jc.2006-2476. [DOI] [PubMed] [Google Scholar]
- 34.Minehira K, Vega N, Vidal H, Acheson K, Tappy L. Effect of carbohydrate overfeeding on whole body macronutrient metabolism and expression of lipogenic enzymes in adipose tissue of lean and overweight humans. Int J Obes (Lond) 2004;28:1291–1298. doi: 10.1038/sj.ijo.0802760. [DOI] [PubMed] [Google Scholar]
- 35.Delzenne N, Ferre P, Beylot M, et al. Study of the regulation by nutrients of the expression of genes involved in lipogenesis and obesity in humans and animals. Nutr Metab Cardiovasc Dis. 2001;11:118–121. [PubMed] [Google Scholar]
- 36.Diraison F, Dusserre E, Vidal H, Sothier M, Beylot M. Increased hepatic lipogenesis but decreased expression of lipogenic gene in adipose tissue in human obesity. Am J Physiol. 2002;282:E46–E51. doi: 10.1152/ajpendo.2002.282.1.E46. [DOI] [PubMed] [Google Scholar]
- 37.Ducluzeau PH, Perretti N, Laville M, et al. Regulation by insulin of gene expression in human skeletal muscle and adipose tissue. Evidence for specific defects in type 2 diabetes. Diabetes. 2001;50:1134–1142. doi: 10.2337/diabetes.50.5.1134. [DOI] [PubMed] [Google Scholar]
- 38.Oberkofler H, Fukushima N, Esterbauer H, Krempler F, Patsch W. Sterol regulatory element binding proteins: relationship of adipose tissue gene expression with obesity in humans. Biochim Biophys Acta. 2002;1575:75–81. doi: 10.1016/s0167-4781(02)00279-8. [DOI] [PubMed] [Google Scholar]
- 39.Santosa S, Hensrud DD, Votruba SB, Jensen MD. The influence of sex and obesity phenotype on meal fatty acid metabolism before and after weight loss. Am J Clin Nutr. 2008;88:1134–1141. doi: 10.1093/ajcn/88.4.1134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Faraj M, Jones P, Sniderman AD, Cianflone K. Enhanced dietary fat clearance in postobese women. J Lipid Res. 2001;42:571–580. [PubMed] [Google Scholar]
- 41.Foley JE, Laursen AL, Sonne O, Gliemann J. Insulin binding and hexose transport in rat adipocytes. Relation to cell size. Diabetologia. 1980;19:234–241. doi: 10.1007/BF00275275. [DOI] [PubMed] [Google Scholar]
- 42.Jacobsson B, Smith U. Effect of cell size on lipolysis and antilipolytic action of insulin in human fat cells. J Lipid Res. 1972;13:651–656. [PubMed] [Google Scholar]
- 43.Olefsky JM. Effects of fasting on insulin binding, glucose transport, and glucose oxidation in isolated rat adipocytes: relationships between insulin receptors and insulin action. J Clin Invest. 1976;58:1450–1460. doi: 10.1172/JCI108601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Smith U. Effect of cell size on lipid synthesis by human adipose tissue in vitro. J Lipid Res. 1971;12:65–70. [PubMed] [Google Scholar]
- 45.Lofgren P, Hoffstedt J, Naslund E, Wiren M, Arner P. Prospective and controlled studies of the actions of insulin and catecholamine in fat cells of obese women following weight reduction. Diabetologia. 2005;48:2334–2342. doi: 10.1007/s00125-005-1961-6. [DOI] [PubMed] [Google Scholar]
- 46.Bjorntorp P, Carlgren G, Isaksson B, Krotkiewski M, Larsson B, Sjostrom L. Effect of an energy-reduced dietary regimen in relation to adipose tissue cellularity in obese women. Am J Clin Nutr. 1975;28:445–452. doi: 10.1093/ajcn/28.5.445. [DOI] [PubMed] [Google Scholar]
- 47.Bjorntorp P, Sjostrom L. The composition and metabolism in vitro of adipose tissue fat cells of different sizes. Eur J Clin Invest. 1972;2:78–84. doi: 10.1111/j.1365-2362.1972.tb00573.x. [DOI] [PubMed] [Google Scholar]
- 48.Bezaire V, Mairal A, Ribet C, et al. Contribution of adipose triglyceride lipase and hormone-sensitive lipase to lipolysis in hMADS adipocytes. J Biol Chem. 2009;284:18282–18291. doi: 10.1074/jbc.M109.008631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Farnier C, Krief S, Blache M, et al. Adipocyte functions are modulated by cell size change: potential involvement of an integrin/ERK signalling pathway. Int J Obes (Lond) 2003;27:1178–1186. doi: 10.1038/sj.ijo.0802399. [DOI] [PubMed] [Google Scholar]
- 50.Farnier C, Krief S, Blache M, et al. The signaling pathway for beta1-integrin/ERKs is involved in the adaptation of adipocyte functions to cell size. Ann N Y Acad Sci. 2002;973:594–597. doi: 10.1111/j.1749-6632.2002.tb04706.x. [DOI] [PubMed] [Google Scholar]
- 51.Hoffstedt J, Forster D, Lofgren P. Impaired subcutaneous adipocyte lipogenesis is associated with systemic insulin resistance and increased apolipoprotein B/AI ratio in men and women. J Intern Med. 2007;262:131–139. doi: 10.1111/j.1365-2796.2007.01811.x. [DOI] [PubMed] [Google Scholar]
- 52.Roberts R, Hodson L, Dennis AL, et al. Markers of de novo lipogenesis in adipose tissue: associations with small adipocytes and insulin sensitivity in humans. Diabetologia. 2009;52:882–890. doi: 10.1007/s00125-009-1300-4. [DOI] [PubMed] [Google Scholar]
- 53.Hudgins LC, Baday A, Hellerstein MK, et al. The effect of dietary carbohydrate on genes for fatty acid synthase and inflammatory cytokines in adipose tissues from lean and obese subjects. J Nutr Biochem. 2008;19:237–245. doi: 10.1016/j.jnutbio.2007.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Etherton TD, Aberle ED, Thompson EH, Allen CE. Effects of cell size and animal age on glucose metabolism in pig adipose tissue. J Lipid Res. 1981;22:72–80. [PubMed] [Google Scholar]
- 55.Varlamov O, Somwar R, Cornea A, Kievit P, Grove KL, Roberts CT., Jr Single-cell analysis of insulin-regulated fatty acid uptake in adipocytes. Am J Physiol. 2010;299:E486–E496. doi: 10.1152/ajpendo.00330.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Cinti S. Adipocyte differentiation and transdifferentiation: plasticity of the adipose organ. J Endocrinol Invest. 2002;25:823–835. doi: 10.1007/BF03344046. [DOI] [PubMed] [Google Scholar]
- 57.Cinti S. Transdifferentiation properties of adipocytes in the adipose organ. Am J Physiol. 2009;297:E977–E986. doi: 10.1152/ajpendo.00183.2009. [DOI] [PubMed] [Google Scholar]
- 58.Cinti S. Reversible transdifferentiation in the adipose organ. Int J Pediatr Obes. 2008;3(Suppl. 2):21–26. doi: 10.1080/17477160802404665. [DOI] [PubMed] [Google Scholar]
- 59.Crossno JT, Jr, Majka SM, Grazia T, Gill RG, Klemm DJ. Rosiglitazone promotes development of a novel adipocyte population from bone marrow-derived circulating progenitor cells. J Clin Invest. 2006;116:3220–3228. doi: 10.1172/JCI28510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Majka SM, Fox KE, Psilas JC, et al. De novo generation of white adipocytes from the myeloid lineage via mesenchymal intermediates is age, adipose depot, and gender specific. Proc Natl Acad Sci U S A. 2010;107:14781–14786. doi: 10.1073/pnas.1003512107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sera Y, LaRue AC, Moussa O, et al. Hematopoietic stem cell origin of adipocytes. Exp Hematol. 2009;37:1108–1120. doi: 10.1016/j.exphem.2009.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Tomiyama K, Murase N, Stolz DB, et al. Characterization of transplanted green fluorescent protein+ bone marrow cells into adipose tissue. Stem Cells. 2008;26:330–338. doi: 10.1634/stemcells.2007-0567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Rosenbaum M, Hirsch J, Murphy E, Leibel RL. Effects of changes in body weight on carbohydrate metabolism, catecholamine excretion, and thyroid function. Am J Clin Nutr. 2000;71:1421–1432. doi: 10.1093/ajcn/71.6.1421. [DOI] [PubMed] [Google Scholar]
- 64.Rosenbaum M, Goldsmith R, Bloomfield D, et al. Low-dose leptin reverses skeletal muscle, autonomic, and neuroendocrine adaptations to maintenance of reduced weight. J Clin Invest. 2005;115:3579–3586. doi: 10.1172/JCI25977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Jequier E. Leptin signaling, adiposity, and energy balance. Ann N Y Acad Sci. 2002;967:379–388. doi: 10.1111/j.1749-6632.2002.tb04293.x. [DOI] [PubMed] [Google Scholar]
- 66.Arone LJ, Mackintosh R, Rosenbaum M, Leibel RL, Hirsch J. Autonomic nervous system activity in weight gain and weight loss. Am J Physiol. 1995;269:R222–R225. doi: 10.1152/ajpregu.1995.269.1.R222. [DOI] [PubMed] [Google Scholar]
- 67.Levin BE, Dunn-Meynell AA. Defense of body weight against chronic caloric restriction in obesity-prone and -resistant rats. Am J Physiol. 2000;278:R231–R237. doi: 10.1152/ajpregu.2000.278.1.R231. [DOI] [PubMed] [Google Scholar]
- 68.Straznicky NE, Grima MT, Eikelis N, et al. The effects of weight loss versus weight loss maintenance on sympathetic nervous system activity and metabolic syndrome components. J Clin Endocrinol Metab. 2011;96:E503–E508. doi: 10.1210/jc.2010-2204. [DOI] [PubMed] [Google Scholar]
- 69.Torgerson JS, Carlsson B, Stenlof K, Carlsson LM, Bringman E, Sjostrom L. A low serum leptin level at baseline and a large early decline in leptin predict a large 1-year weight reduction in energy-restricted obese humans. J Clin Endocrinol Metab. 1999;84:4197–4203. doi: 10.1210/jcem.84.11.6089. [DOI] [PubMed] [Google Scholar]
- 70.Sari R, Balci MK, Altunbas H, Karayalcin U. The effect of body weight and weight loss on thyroid volume and function in obese women. Clin Endocrinol. 2003;59:258–262. doi: 10.1046/j.1365-2265.2003.01836.x. [DOI] [PubMed] [Google Scholar]
- 71.Kozlowska L, Rosolowska-Huszcz D. Leptin, thyrotropin, and thyroid hormones in obese/overweight women before and after two levels of energy deficit. Endocrine. 2004;24:147–153. doi: 10.1385/ENDO:24:2:147. [DOI] [PubMed] [Google Scholar]
- 72.Ho JT, Keogh JB, Bornstein SR, et al. Moderate weight loss reduces renin and aldosterone but does not influence basal or stimulated pituitary-adrenal axis function. Horm Metab Res. 2007;39:694–699. doi: 10.1055/s-2007-985354. [DOI] [PubMed] [Google Scholar]
- 73.Weinsier RL, Nagy TR, Hunter GR, Darnell BE, Hensrud DD, Weiss HL. Do adaptive changes in metabolic rate favor weight regain in weight-reduced individuals? An examination of the set-point theory. Am J Clin Nutr. 2000;72:1088–1094. doi: 10.1093/ajcn/72.5.1088. [DOI] [PubMed] [Google Scholar]
- 74.Bartness TJ, Song CK. Thematic review series: adipocyte biology. Sympathetic and sensory innervation of white adipose tissue. J Lipid Res. 2007;48:1655–1672. doi: 10.1194/jlr.R700006-JLR200. [DOI] [PubMed] [Google Scholar]
- 75.Bowers RR, Festuccia WT, Song CK, Shi H, Migliorini RH, Bartness TJ. Sympathetic innervation of white adipose tissue and its regulation of fat cell number. Am J Physiol. 2004;286:R1167–R1175. doi: 10.1152/ajpregu.00558.2003. [DOI] [PubMed] [Google Scholar]
- 76.Sorisky A, Bell A, Gagnon A. TSH receptor in adipose cells. Horm Metab Res. 2000;32:468–474. doi: 10.1055/s-2007-978672. [DOI] [PubMed] [Google Scholar]
- 77.Bell A, Gagnon A, Grunder L, Parikh SJ, Smith TJ, Sorisky A. Functional TSH receptor in human abdominal preadipocytes and orbital fibroblasts. Am J Physiol. 2000;279:C335–C340. doi: 10.1152/ajpcell.2000.279.2.C335. [DOI] [PubMed] [Google Scholar]
- 78.Schaffler A, Binart N, Scholmerich J, Buchler C. Hypothesis paper Brain talks with fat–evidence for a hypothalamic-pituitary-adipose axis? Neuropeptides. 2005;39:363–367. doi: 10.1016/j.npep.2005.06.003. [DOI] [PubMed] [Google Scholar]
- 79.Darimont C, Gaillard D, Ailhaud G, Negrel R. Terminal differentiation of mouse preadipocyte cells: adipogenic and antimitogenic role of triiodothyronine. Mol Cell Endocrinol. 1993;98:67–73. doi: 10.1016/0303-7207(93)90238-f. [DOI] [PubMed] [Google Scholar]
- 80.Viguerie N, Millet L, Avizou S, Vidal H, Larrouy D, Langin D. Regulation of human adipocyte gene expression by thyroid hormone. J Clin Endocrinol Metab. 2002;87:630–634. doi: 10.1210/jcem.87.2.8200. [DOI] [PubMed] [Google Scholar]
- 81.Lien LF, Haqq AM, Arlotto M, et al. The STEDMAN project: biophysical, biochemical and metabolic effects of a behavioral weight loss intervention during weight loss, maintenance, and regain. OMICS. 2009;13:21–35. doi: 10.1089/omi.2008.0035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.James AP, Watts GF, Barrett PH, et al. Effect of weight loss on postprandial lipemia and low-density lipoprotein receptor binding in overweight men. Metabolism. 2003;52:136–141. doi: 10.1053/meta.2003.50032. [DOI] [PubMed] [Google Scholar]
- 83.Kirk E, Reeds DN, Finck BN, Mayurranjan SM, Patterson BW, Klein S. Dietary fat and carbohydrates differentially alter insulin sensitivity during caloric restriction. Gastroenterology. 2009;136:1552–1560. doi: 10.1053/j.gastro.2009.01.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.McLaughlin T, Schweitzer P, Carter S, et al. Persistence of improvement in insulin sensitivity following a dietary weight loss programme. Diabetes Obes Metab. 2008;10:1186–1194. doi: 10.1111/j.1463-1326.2008.00877.x. [DOI] [PubMed] [Google Scholar]
- 85.Rosenbaum M, Nicolson M, Hirsch J, Murphy E, Chu F, Leibel RL. Effects of weight change on plasma leptin concentrations and energy expenditure. J Clin Endocrinol Metab. 1997;82:3647–3654. doi: 10.1210/jcem.82.11.4390. [DOI] [PubMed] [Google Scholar]
- 86.Levin BE, Keesey RE. Defense of differing body weight set points in diet-induced obese and resistant rats. Am J Physiol. 1998;274:R412–R419. doi: 10.1152/ajpregu.1998.274.2.R412. [DOI] [PubMed] [Google Scholar]
- 87.Arner P, Spalding KL. Fat cell turnover in humans. Biochem Biophys Res Commun. 2010;396:101–104. doi: 10.1016/j.bbrc.2010.02.165. [DOI] [PubMed] [Google Scholar]
- 88.Williams G, Shellard L, Lewis DE, et al. Hypothalamic neuropeptide Y disturbances in the obese (cp/cp) JCR:LA corpulent rat. Peptides. 1992;13:537–540. doi: 10.1016/0196-9781(92)90085-h. [DOI] [PubMed] [Google Scholar]
- 89.Levin BE, Dunn-Meynell AA. Chronic exercise lowers the defended body weight gain and adiposity in diet-induced obese rats. Am J Physiol. 2004;286:R771–R778. doi: 10.1152/ajpregu.00650.2003. [DOI] [PubMed] [Google Scholar]
- 90.Bi S, Robinson BM, Moran TH. Acute food deprivation and chronic food restriction differentially affect hypothalamic NPY mRNA expression. Am J Physiol. 2003;285:R1030–R1036. doi: 10.1152/ajpregu.00734.2002. [DOI] [PubMed] [Google Scholar]
- 91.Yu Y, Deng C, Huang XF. Obese reversal by a chronic energy restricted diet leaves an increased Arc NPY/AgRP, but no alteration in POMC/CART, mRNA expression in diet-induced obese mice. Behav Brain Res. 2009;205:50–56. doi: 10.1016/j.bbr.2009.07.003. [DOI] [PubMed] [Google Scholar]
- 92.Morton GJ, Mystkowski P, Matsumoto AM, Schwartz MW. Increased hypothalamic melanin concentrating hormone gene expression during energy restriction involves a melanocortin-independent, estrogen-sensitive mechanism. Peptides. 2004;25:667–674. doi: 10.1016/j.peptides.2004.02.007. [DOI] [PubMed] [Google Scholar]
- 93.Gloy VL, Lutz TA, Langhans W, Geary N, Hillebrand JJ. Basal plasma levels of insulin, leptin, ghrelin, and amylin do not signal adiposity in rats recovering from forced overweight. Endocrinology. 2010;151:4280–4288. doi: 10.1210/en.2010-0439. [DOI] [PubMed] [Google Scholar]
- 94.Seeley RJ, York DA. Fuel sensing and the central nervous system (CNS): implications for the regulation of energy balance and the treatment for obesity. Obes Rev. 2005;6:259–265. doi: 10.1111/j.1467-789X.2005.00193.x. [DOI] [PubMed] [Google Scholar]
- 95.Delaere F, Magnan C, Mithieux G. Hypothalamic integration of portal glucose signals and control of food intake and insulin sensitivity. Diabetes Metab. 2010;36:257–262. doi: 10.1016/j.diabet.2010.05.001. [DOI] [PubMed] [Google Scholar]
- 96.Lam TK. Neuronal regulation of homeostasis by nutrient sensing. Nat Med. 2010;16:392–395. doi: 10.1038/nm0410-392. [DOI] [PubMed] [Google Scholar]
- 97.Bartness TJ, Shrestha YB, Vaughan CH, Schwartz GJ, Song CK. Sensory and sympathetic nervous system control of white adipose tissue lipolysis. Mol Cell Endocrinol. 2010;318:34–43. doi: 10.1016/j.mce.2009.08.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Chaput JP, Tremblay A. The glucostatic theory of appetite control and the risk of obesity and diabetes. Int J Obes (Lond) 2009;33:46–53. doi: 10.1038/ijo.2008.221. [DOI] [PubMed] [Google Scholar]
- 99.Jordan SD, Konner AC, Bruning JC. Sensing the fuels: glucose and lipid signaling in the CNS controlling energy homeostasis. Cell Mol Life Sci. 2010;67:3255–3273. doi: 10.1007/s00018-010-0414-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Urayama A, Banks WA. Starvation and triglycerides reverse the obesity-induced impairment of insulin transport at the blood-brain barrier. Endocrinology. 2008;149:3592–3597. doi: 10.1210/en.2008-0008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Banks WA, Coon AB, Robinson SM, et al. Triglycerides induce leptin resistance at the blood-brain barrier. Diabetes. 2004;53:1253–1260. doi: 10.2337/diabetes.53.5.1253. [DOI] [PubMed] [Google Scholar]
- 102.Litherland NB, Thire S, Beaulieu AD, Reynolds CK, Benson JA, Drackley JK. Dry matter intake is decreased more by abomasal infusion of unsaturated free fatty acids than by unsaturated triglycerides. J Dairy Sci. 2005;88:632–643. doi: 10.3168/jds.S0022-0302(05)72727-2. [DOI] [PubMed] [Google Scholar]
- 103.French SJ, Conlon CA, Mutuma ST, et al. The effects of intestinal infusion of long-chain fatty acids on food intake in humans. Gastroenterology. 2000;119:943–948. doi: 10.1053/gast.2000.18139. [DOI] [PubMed] [Google Scholar]
- 104.Vandermeerschen-Doize F, Paquay R. Effects of continuous long-term intravenous infusion of long-chain fatty acids on feeding behaviour and blood components of adult sheep. Appetite. 1984;5:137–146. doi: 10.1016/s0195-6663(84)80034-3. [DOI] [PubMed] [Google Scholar]
- 105.Coccurello R, Caprioli A, Bellantuono S, et al. Effects of the increase in neuronal fatty acids availability on food intake and satiety in mice. Psychopharmacology (Berl) 2010;210:85–95. doi: 10.1007/s00213-010-1820-0. [DOI] [PubMed] [Google Scholar]
- 106.Obici S, Feng Z, Morgan K, Stein D, Karkanias G, Rossetti L. Central administration of oleic acid inhibits glucose production and food intake. Diabetes. 2002;51:271–275. doi: 10.2337/diabetes.51.2.271. [DOI] [PubMed] [Google Scholar]
- 107.Goodpaster BH, Kelley DE, Wing RR, Meier A, Thaete FL. Effects of weight loss on regional fat distribution and insulin sensitivity in obesity. Diabetes. 1999;48:839–847. doi: 10.2337/diabetes.48.4.839. [DOI] [PubMed] [Google Scholar]
- 108.Chaston TB, Dixon JB. Factors associated with percent change in visceral versus subcutaneous abdominal fat during weight loss: findings from a systematic review. Int J Obes (Lond) 2008;32:619–628. doi: 10.1038/sj.ijo.0803761. [DOI] [PubMed] [Google Scholar]
- 109.Chowdhury B, Kvist H, Andersson B, Bjorntorp P, Sjostrom L. CT-determined changes in adipose tissue distribution during a small weight reduction in obese males. Int J Obes (Lond) 1993;17:685–691. [PubMed] [Google Scholar]


