Abstract
Background
Diet is the primary way cadmium (Cd) enters the body in those without occupational exposure and who do not inhabit Cd-polluted regions. Findings on the relationship between dietary Cd exposure and breast cancer (BC) risk have been inconsistent; a meta-analysis has supported this association but 2 recent cohort studies showed inconsistent results. Hence, we performed an updated meta-analysis to re-evaluate the association between dietary Cd exposure and BC risk.
Material/Methods
We searched PubMed, Medline, and EMBASE to identify relevant studies published through September 2014. Combined relative risks (RRs) and the corresponding 95% confidence intervals (CIs) were used to assess the association between dietary Cd exposure and BC risk.
Results
We identified 6 studies involving 321 315 participants and 11 978 cases. Our study suggested there was no statistically significant positive association between dietary Cd exposure and BC risk, the combined RR and corresponding 95% CI was 1.01 [0.88, 1.14]. The result was not modified by menopause status, geographic area, or study design.
Conclusions
Our study did not find a statistically significant positive association between dietary Cd exposure and BC risk. It is necessary to investigate this relationship among the high-risk groups and more cohort studies based on diverse populations are needed.
MeSH Keywords: Cadmium, Breast Cancer, Meta-analysis
Background
Cadmium (Cd) is often the result of environmental pollution caused by industrial and agricultural activities and it is widely dispersed into the environment [1]; hence, humans are exposed to it ubiquitously. The relationship between Cd and human health has been attracting increasing research attention [2]. An earlier study showed that Cd acts as a stressor and leads to metabolic alterations [3].
Humans always come into contact with Cd by occupational exposure, smoking, or diet. However, diet is the primary way Cd enters the bodies of those without occupational exposure and who do not live in Cd-polluted regions [2]. It has been reported that dietary Cd exposure is mainly from the relatively high Cd content of agricultural crops and their products, such as rice, bread, potatoes, and vegetables [4]. After entering the human body, Cd acts as a component of metallothionein and is deposited in organs and tissues [5].
The long-time biological effect of Cd is that it may be a carcinogen [6]. In 2008, a population-based cohort study suggested that chronic dietary Cd intake could increase risk of hormone-related cancers [7]. Similarly, another population-based prospective cohort study reported that dietary Cd intake played an important role in prostate cancer development [8]. Thus, dietary Cd intake may be associated with hormone-related cancers. For women, BC is an estrogen-related cancer, and a laboratory study indicated that Cd could malignantly transform breast cells, stimulate breast cell proliferation, and inhibit apoptosis [9]. The relationship between dietary Cd exposure and BC risk has been supported by some observational studies, but the results of these studies were inconsistent [10–13], hence, a meta-analysis was conducted by Cho et al. to clarify these inconsistent results, and this study found that there was a significant positive relationship between dietary Cd intake and BC risk [14]. Recently, 2 recent prospective cohort studies have observed a relationship between dietary Cd intake and BC risk; however, neither of these studies found that dietary Cd intake was a risk for BC [15,16].
Thus, we performed a meta-analysis to update and quantitatively re-assess the association between dietary Cd exposure and the risk of BC by summarizing the inconsistent results of all published studies. Thus, we intended to provide the best available evidence as to whether dietary Cd exposure is a risk of BC.
Material and Methods
Literature search strategy
We searched PubMed, Medline, and EMBASE to identify studies on the relationship between dietary Cd exposure and risk of BC, published through September 2014. The following search terms were used in the searching strategy: “cadmium” combined with “breast cancer” and “breast carcinoma”. In addition, references cited within relevant reviews were retrieved, and we contacted the authors for useful information.
Study selection
We included studies that met the following criteria: 1) study designed as case-control or cohort study; 2) the exposure of interest was dietary Cd exposure; 3) the outcome was BC incidence; and 4) RR and corresponding 95% CI for the highest compared with the lowest of dietary Cd exposure were reported.
Initially, we reviewed the titles and abstracts to ascertain reports of interest; if uncertain, a subsequent full-text assessment was conducted. The study retrieval was conducted by 2 independent authors. All disputes were resolved by discussion.
Data extraction and quality assessment
We extracted the following contents: the first author’s surname, publication year, geographic area, age of the participants, number of cases and participants or controls, study design, range of Cd exposure; RR (95% CI) for the highest vs. the lowest dietary Cd exposure and variables used in a multivariate model.
The key components of designs (e.g., selection of study populations, ascertainment of exposure and outcome, duration of follow-up) were used to estimate the quality of primary studies [17], rather than reporting the aggregate scores.
Data analysis
STATA version 12.0 was used to analyze data. RR and the 95% CI were used to measure the association of dietary Cd exposure and the risk of BC. A random-effects model was used to compute the combined RR. Homogeneity test was performed with the use of Q statistic and I2 statistic, and subgroups analysis was used to identify the source of heterogeneity. Sensitivity analysis was used to assess the influence of a single study on the overall risk estimate. Publication bias was detected by Begg’s and Egger’s test, if the P-value was less than 0.05, it was considered as statistically significant.
Results
Literature search and study characteristics
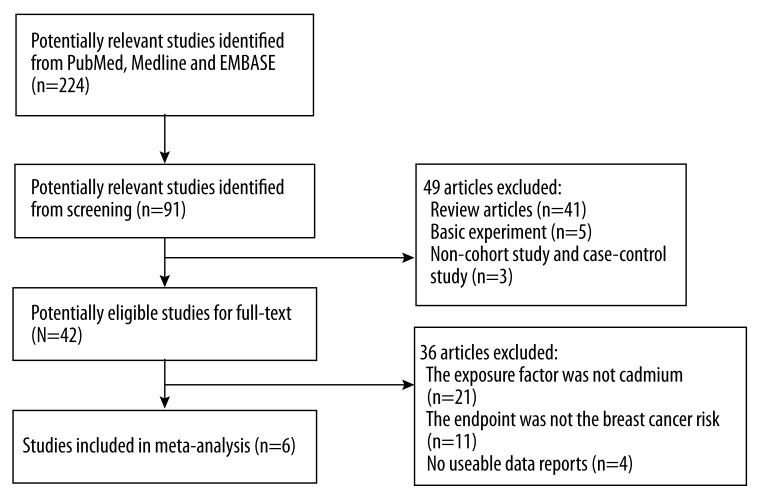
We initially identified 224 potential articles from the databases. After screening the abstracts and titles, most of these were excluded because they were reviews, the exposure or outcome did not relate to our analysis, or they were laboratory studies. Overall, we identified 6 studies involving 321 315 participants and 11 978 cases [10–13,15,16]. Figure 1 is a flow chart of study selection.
Figure 1.
Flow chart of study selection.
In the included studies, 2 were performed in Europe [11,15], 2 in the USA [10,16], and 2 in Japan [12,13]. In addition, 5 studies were cohort studies [10–12,15,16] and one was designed as case-control study [13]. Five results of these studies were reported by menopausal status in 4 studies [10,11,13,15], 4 results were conducted in postmenopausal women, and only 1 in premenopausal women. Table 1 summarizes the general data from the 6 included studies.
Table 1.
The characteristics of six included studies on the relationship between dietary cadmium exposure and breast cancer risk.
| Study (year, country) | Age | Study design | Sample size (n) case/control (size) | Dietary cadmium exposure range | Adjusted RR (95% CI) (highest vs. lowest) | Variables used in multivariate model |
|---|---|---|---|---|---|---|
| Adams et al. (2012, USA) | 50–76 | Cohort | 1026/30543 | Q1 (<7.48) Q2 (7.48–10.05) Q3(10.06–13.30) Q4 (>13.30) |
Post.: 1.00 [0.72,1.41] | Age, total energy intake, race HRT use, smoking, vegetable consumption, BMI, physical activity, alcohol consumption, age at first childbirth, education, mammography multivitamin use |
| Julin et al. (2012, Sweden) | n | Cohort | 2112/55987 | T1 (<13) T2 (<13–16) T3 (>16) |
Post.: 1.21 [1.07,1.36] | Age, height, BMI, education, use of oral contraceptives, use of postmenopausal hormones, age at menarche, age at first birth, alcohol consumption, glycemic load, total energy intake, intake of whole grain and vegetables |
| Sawada et al. (2012, Japan) | 45–74 | Cohort | 402/48351 | T1: 19.2 T2: 24.9 T3: 32.3 |
0.87 [0.61,1.23] | Age, area, BMI, smoking, frequency of alcohol intake, leisure-time physical activity, intake of meat, soybean, vegetable, and fruit. Menopausal status and use of exogenous female hormones |
| Itoh et al. (2014, Japan) | 20–74 | Case-control | 390/390 | T1 (21.4) T2 (26.2) T3 (31.5) |
1.23 [0.76,2.00] Pre.: 0.95 [0.47,1.94] Post.: 1.49 [0.84,2.64] |
Age, area, menopausal status, physical activity, smoking, family, number of births, isoflavone, vegetable and total energy intake |
| Eriksen et al. (2014, Denmark) | 50–65 | Cohort | 1390/23815 | T1 (<11.9) T2 (11.9–15.3) T3 (>15.3) |
Post.: 0.97 [0.85,1.11] | Educational, smoking, number of births, age at first birth, HRT, HRT use, age at menarche, BMI, height, physical activity and alcohol intake |
| Adams et al. (2014, USA) | 50–79 | Cohort | 6658/150889 | Q1(<7.10) Q2 (7.10–9.24) Q3(9.24–11.35) Q4(11.35–14.21) Q5>(14.21) |
0.90 [0.81,1.00] | Total energy intake, age, race study component, BMI, alcohol consumption, education, physical activity, age at first birth and menarche, smoking age at menopause, unopposed estrogen use, estrogen and progesterone use, daily vegetable servings, daily grain servings, mammography 2 years before baseline |
Q – quartile; T – tertile; RR – rate ratio; BMI – body mass index; HRT – hormone replacement therapy; Pre – premenopausal woman; Post – postmenopausal woman.
Main analysis
Data of these 6 studies were analyzed in a random-effects model to estimate the relationship between dietary Ca exposure and BC risk. In our study, there was no statistically significant positive association between dietary Cd exposure and BC, and the combined RR and corresponding 95% CI was 1.01 [0.88, 1.14]. Strong evidence of heterogeneity was detected among these studies (I2=63.0%, P=0.019). Figure 2 presents the results of our meta-analysis.
Figure 2.
Forest plot for the relationship between dietary cadmium exposure and breast cancer risk.
Subgroup-analysis
According to menopause status, geographic area, and study design, we conducted subgroup-analysis to identify the source of heterogeneity. Evidence of heterogeneity was also found across the 2 European studies and the cohort design studies. However, among these 3 subgroups, no significant positive correlation between dietary Cd exposure and BC risk was found. The combined RR of BC was not modified by the different groups. The results of subgroup analyses are presented in Table 2.
Table 2.
Subgroup analysis of the association between cadmium exposure and breast cancer risk.
| Study group | No. of study | RR (95% CI) | P for heterogeneity | I2 |
|---|---|---|---|---|
| All | 6 | 1.01 [0.88, 1.14] | 0.019 | 63.0 |
| Menopause status | ||||
| Postmenopause | 4 | 1.09 [0.91, 1.26] | 0.077 | 56.1% |
| Premenopause | 1 | 0.95 [0.47, 1.94] | --- | --- |
| Geographic area | ||||
| USA | 2 | 0.91 [0.82, 1.00] | 0.584 | 0. 0% |
| Europe | 2 | 1.09 [0.85, 1.32] | 0.016 | 82.9% |
| Japan | 2 | 0.95 [0.66,1.23] | 0.309 | 3.5% |
| Study design | ||||
| Cohort | 5 | 1.00 [0.86, 1.13] | 0.012 | 69.0% |
| Case-control | 1 | 1.23 [0.76, 2.00] | --- | --- |
Sensitivity analysis and publication bias
Sensitivity analysis was conducted to estimate the influence of each individual study on combined RR by removing 1 study at a time. The combined RRs were similar with one another, and no single study significantly changed the combined result, which indicated that the result was statistically stable and reliable.
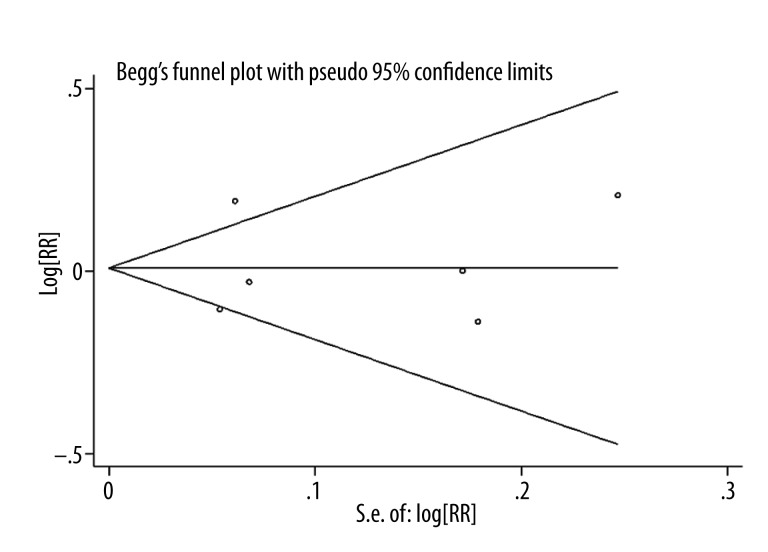
Publication bias was detected based in the shape of funnel plots and by Begg’s and Egger’s tests. Begg’s funnel plot and Egger’s regression test showed a low probability of publication bias (p=0.909). The result of Begg’s test is presented in Figure 3.
Figure 3.
Forest plot for publication bias.
Discussion
Our study did not show a statistically significant positive association between dietary Cd exposure and BC risk for the highest compared with the lowest category of dietary Cd exposure, and the combined RR(95% CI) of BC was 1.01 [0.88, 1.14].
Heterogeneity appears in a meta-analysis when the included studies have inconsistent conditions. In our study, evidence of heterogeneity was observed; thus, we conducted subgroup-analysis to identify the source of heterogeneity. Table 2 shows that the 2 European studies [11,15] were the main source of heterogeneity. Julin et al. adjusted for a wide range of potential confounders for BC: age, height, BMI, education, use of oral contraceptives and postmenopausal hormones, age at menarche, age at first birth, alcohol consumption, glycemic load, total energy intake, and dietary intake of whole vegetables and grains [11]; however, the Danish study of postmenopausal women adjusted for a relatively narrow range, not including age, total energy intake, or dietary intake of whole vegetables and grains [15].
The relationship between Cd exposure and BC risk has been investigated to assess whether Cd has any modifying effects on the etiology and process of BC [18,19]. These studies investigated women who were exposed to Cd occupationally and who lived in Cd-polluted areas; a significant increase in frequencies of BC in Cd-exposed women was found by a meta-analysis and systematic review [20]. However, diet is the primary way Cd enters the human body among populations without occupational exposure and who do not live in Cd-polluted areas [2]. The relationship between dietary Cd exposure and BC risk also was considered in recent years. In our meta-analysis, 6 studies on this topic were identified, but the results were inconsistent. In 2012, Adams et al. conducted a cohort study to examine whether dietary Cd exposure increased risk of BC, and the result showed no significant association [10]. However, a Swedish population-based prospective cohort study including 55 987 participants and 2112 patients showed that dietary Cd intake increased risk of postmenopausal BC [11]. The 2 Japanese studies reported no significant positive relationships between dietary Cd exposure and BC risk [12,13], but the case-control study found a statistically significant association for estrogen receptor-positive tumors among postmenopausal women [13]. In 2013, a meta-analysis of the relationship between dietary Cd intake and cancer risk was conducted by Cho et al., who reported a significant association between dietary Cd exposure and BC risk, and found that dietary Cd intake increased risk of BC – the RR (95% CI) was 1.15 [1.04–1.28] [14]. However, in 2014, a prospective cohort study in Danish postmenopausal women did not support the above result and reported that dietary Cd intake was not linked to the risk of BC or other hormone-related cancers [15]. Similarly, another cohort study indicated dietary Cd intake had no statistical association with cancer risk [16]. Thus, we performed a meta-analysis to update and quantitatively re-assess this relationship and we did not find a statistically significant positive association between dietary Cd exposure and BC risk. The subgroup-analysis of our study was conducted at the same time and no significant positive association between dietary Cd exposure and BC risk was found in any subgroups.
Cd acts as a metallo-estrogen and may play an important role in the estrogenic signaling pathway [21]; it can malignantly transform breast cells, stimulate breast cell proliferation, and inhibit apoptosis [22]. In addition, Cd also can induce BC by a non-estrogen-related mechanism. It can change normal breast epithelial cells into a basal-like breast carcinoma characterized by negativity of the ER-a and the epidermal growth factor receptor 2 (HER2), leading to reduced expression of BC susceptibility gene 1, and increasing the expression of cytokeratin 5 and p63 [23].
Although our study did not find a statistically significant positive association between dietary Cd exposure and BC risk, many factors that may influence this association should be considered. First, some other trace elements may relate to the absorption of Cd. Flanagan et al. reported that iron deficiency led to increased absorption of Cd in animal and human studies [24]. In addition, zinc can limit absorption of Cd by decreasing in the intracellular Cd accumulation and by the sequestration of Cd by Cd-induced metallothionein [25]. Second, different types of diet may influence the bioavailability and/or kinetics of dietary Cd [26]. For example, the Swedish prospective cohort study showed that the low Cd and high whole grain and vegetables intake groups had the lowest risk, but they had the highest risk of BC in relation to diets high in Cd and low in whole grains and vegetables [11], which is why these food have a conflicting roles in the development of BC because whole grains and vegetables are relatively Cd-rich foods. Third, other lifestyle factors such as tobacco consumption may relate to Cd exposure [27]. For example, average urine Cd concentration in never-smokers was lower than that in smokers [12]. In our study, only 2 investigations reported a relationship between dietary Cd exposure and BC risk in smokers and never-smokers [10,13], but no study showed a positive association.
Our meta-analysis of 6 studies involving 321 315 participants and 11 978 cases improved the statistical power and found a more reliable result of the association between dietary Cd exposure and BC risk. Most investigations were cohort studies, which reduced the recall and selection bias. However, there are several limitations of our study. First, other factors may influence this association and the effect of dietary Cd exposure on the development of BC. Although most included studies adjusted for a wide range of factors, some studies did not exclude the potential factors. Second, in all included studies, Cd exposure was assessed with a food frequency questionnaire (FFQ), so measurement error in individual studies might have led to incorrect classification of Cd exposure. Third, none of the included studies reported whether the participants lived in a polluted area and, if so, for how long before the primary studies were started. Fourth, the inclusion criteria might induce selection bias and potential publication bias still existed. Fourth, all of our included studies were conducted in developed countries, and other studies in less-developed countries are warranted.
Conclusions
Our study did not find a statistically significant positive association between dietary Cd exposure and BC risk. It is necessary to investigate this potential relationship among high-risk groups and more cohort studies based on diverse populations are needed.
Footnotes
Conflict of Interest
None.
Source of support: Departmental sources
References
- 1.Hellstrom L, Persson B, Brudin L, et al. Cadmium exposure pathways in a population living near a battery plant. Sci Total Environ. 2007;373:447–55. doi: 10.1016/j.scitotenv.2006.11.028. [DOI] [PubMed] [Google Scholar]
- 2.Järup L, Akesson A. Current status of cadmium as an environmental health problem. Toxicol Appl Pharmacol. 2009;238:201–8. doi: 10.1016/j.taap.2009.04.020. [DOI] [PubMed] [Google Scholar]
- 3.Almeida JA, Novelli EL, Dal Pai Silva M, et al. Environmental cadmium exposure and metabolic responses of the Nile tilapia, Oreochromis niloticus. Environ Pollut. 2001;114:169–75. doi: 10.1016/s0269-7491(00)00221-9. [DOI] [PubMed] [Google Scholar]
- 4.Amzal B, Julin B, Vahter M, et al. Population toxicokinetic modeling of cadmium for health risk assessment. Environ Health Perspect. 2009;117:1293–301. doi: 10.1289/ehp.0800317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fujishiro H, Yano Y, Takada Y, et al. Roles of ZIP8, ZIP14, and DMT1 in transport of cadmium and manganese in mouse kidney proximal tubule cells. Metallomics. 2012;4:700–8. doi: 10.1039/c2mt20024d. [DOI] [PubMed] [Google Scholar]
- 6.Joseph P. Mechanisms of cadmium carcinogenesis. Toxicol Appl Pharmacol. 2009;238:272–79. doi: 10.1016/j.taap.2009.01.011. [DOI] [PubMed] [Google Scholar]
- 7.Akesson A, Julin B, Wolk A. Long-term dietary cadmium intake and postmenopausal endometrial cancer incidence: a population-based prospective cohort study. Cancer Res. 2008;68:6435–41. doi: 10.1158/0008-5472.CAN-08-0329. [DOI] [PubMed] [Google Scholar]
- 8.Julin B, Wolk A, Johansson JE, et al. Dietary cadmium exposure and prostate cancer incidence: A population-based prospective cohort study. Br J Cancer. 2012;107:895–900. doi: 10.1038/bjc.2012.311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Garcia-Morales P, Saceda M, Kenney N, et al. Effect of cadmium on estrogen receptor levels and estrogen-induced responses in human breast cancer cells. J Biol Chem. 1994;269:16896–901. [PubMed] [Google Scholar]
- 10.Adams Scott V, Newcomb Polly A, White Emily. Dietary cadmium and risk of invasive postmenopausal breast cancer in the VITAL cohort. Cancer Causes Control. 2012;23:845–54. doi: 10.1007/s10552-012-9953-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Julin B, Wolk A, Bergkvist L. Dietary Cadmium Exposure and Risk of Postmenopausal Breast Cancer: A Population-Based Prospective Cohort Study. Cancer Res. 2012;72:1459–66. doi: 10.1158/0008-5472.CAN-11-0735. [DOI] [PubMed] [Google Scholar]
- 12.Sawada N, Iwasaki M, Inoue M, et al. Long-term Dietary Cadmium Intake and Cancer Incidence. Epidemiology. 2012;23(3):368–76. doi: 10.1097/EDE.0b013e31824d063c. [DOI] [PubMed] [Google Scholar]
- 13.Itoh H, Iwasaki M, Sawada N, et al. Dietary cadmium intake and breast cancer risk in Japanese women: A case – control study. Int J Hyg Environ Health. 2014;217:70–77. doi: 10.1016/j.ijheh.2013.03.010. [DOI] [PubMed] [Google Scholar]
- 14.Cho YA, Kim J, Woo HD, et al. Dietary Cadmium Intake and the Risk of Cancer: A Meta-Analysis. PLoS One. 2013;8:e75087. doi: 10.1371/journal.pone.0075087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Eriksen KT, Halkjær J, Sørensen M, et al. Dietary Cadmium Intake and Risk of Breast, Endometrial and Ovarian Cancer in Danish Postmenopausal Women: A Prospective Cohort Study. PLoS One. 2014;9:e100815. doi: 10.1371/journal.pone.0100815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Adams SV, Quraishi SM, Shafer MM, et al. Dietary Cadmium Exposure and Risk of Breast, Endometrial, and Ovarian Cancer in the Women’s Health Initiative. Environ Health Perspect. 2014;122(6):594–600. doi: 10.1289/ehp.1307054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stroup DF, Berlin JA, Morton SC, et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Metaanalysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA. 2000;283:2008–12. doi: 10.1001/jama.283.15.2008. [DOI] [PubMed] [Google Scholar]
- 18.McElroy JA, Shafer MM, Trentham-Dietz A, et al. Cadmium Exposure and Breast Cancer Risk. J Natl Cancer Inst. 2006;98:869–73. doi: 10.1093/jnci/djj233. [DOI] [PubMed] [Google Scholar]
- 19.Nagata C, Nagao Y, Nakamura K, et al. Cadmium exposure and the risk of breast cancer in Japanese women. Breast Cancer Res Treat. 2013;138:235–39. doi: 10.1007/s10549-013-2414-4. [DOI] [PubMed] [Google Scholar]
- 20.Rahim F, Jalali A, Tangestani R. Breast Cancer Frequency and Exposure to Cadmium: A Meta-Analysis and Systematic Review. Asian Pac J Cancer Prev. 2013;14:4283–87. doi: 10.7314/apjcp.2013.14.7.4283. [DOI] [PubMed] [Google Scholar]
- 21.Liu Z, Yu X, Shaikh ZA. Rapid activation of ERK1/2 and AKT in human breast cancer cells by cadmium. Toxicol Appl Pharmacol. 2008;228:286–94. doi: 10.1016/j.taap.2007.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Garcia-Morales P, Saceda M, Kenney N, et al. Effect of cadmium on estrogen receptor levels and estrogen-induced responses in human breast cancer cells. J Biol Chem. 1994;269:16896–901. [PubMed] [Google Scholar]
- 23.Benbrahim-Tallaa L, Tokar EJ, Diwan BA, et al. Cadmium malignantly transforms normal human breast epithelial cells into a basal-like phenotype. Environ Health Perspect. 2009;117:1847–52. doi: 10.1289/ehp.0900999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Flanagan PR, McLellan JS, Haist J, et al. Increased dietary cadmium absorption in mice and human subjects with iron deficiency. Gastroenterology. 1978;74:841–46. [PubMed] [Google Scholar]
- 25.Kaji T, Mishima A, Koyanagi E, et al. Possible mechanism for zinc protection against cadmium cytotoxicity in cultured vascular endothelial cells. Toxicology. 1992;76:257–70. doi: 10.1016/0300-483x(92)90194-j. [DOI] [PubMed] [Google Scholar]
- 26.Vahter M, Berglund M, Nermell B, et al. Bioavailability of cadmium from shellfish and mixed diet in women. Toxicol Appl Pharmacol. 1996;136:332–41. doi: 10.1006/taap.1996.0040. [DOI] [PubMed] [Google Scholar]
- 27.Richter PA, Bishop EE, Wang J, et al. Tobacco smoke exposure and levels of urinary metals in the U.S. youth and adult population: the National Health and Nutrition Examination Survey (NHANES) 1999–2004. Int J Environ Res Public Health. 2009;6:1930–46. doi: 10.3390/ijerph6071930. [DOI] [PMC free article] [PubMed] [Google Scholar]