Abstract
Background
Reporting ototoxicity is frequently complicated by use of various ototoxicity criteria. The International Society of Pediatric Oncology (SIOP) ototoxicity grading scale was recently proposed for standardized use in reporting hearing loss outcomes across institutions. The aim of this study was to evaluate the concordance between the Chang and SIOP ototoxicity grading scales. Differences between the two scales were identified and the implications these differences may have in the clinical setting were discussed.
Procedure
Audiological evaluations were reviewed for 379 patients with newly diagnosed medulloblastoma (ages 3–21 years). Each patient was enrolled on one of two St. Jude clinical protocols that included craniospinal radiation therapy and four courses of 75 mg/m2 cisplatin chemotherapy. The latest audiogram conducted 5.5 – 24.5 months post-protocol treatment initiation was graded using the Chang and SIOP ototoxicity criteria. Clinically significant hearing loss was defined as Chang grade ≥ 2a and SIOP ≥2. Hearing loss was considered serious (requiring a hearing aid) at the level of Chang grade ≥ 2b and SIOP ≥ 3.
Results
A strong concordance was observed between the Chang and SIOP ototoxicity scales (Stuart’s tau-c statistic = 0.89, 95% CI: 0.86, 0.91). Among those patients diagnosed with serious hearing loss, the two scales were in good agreement. However, the scales deviated from one another in classifying patients with less serious or no hearing loss.
Conclusions
Although discrepancies between the Chang and SIOP ototoxicity scales exist primarily for patients with no or minimal hearing loss, the scales share a strong concordance overall.
Keywords: ototoxicity, hearing loss, cisplatin, late effects, medulloblastoma
Introduction
Cisplatin is frequently used in chemotherapy regimens to treat a variety of pediatric solid and central nervous system malignancies. Unfortunately, ototoxicity is a potential adverse effect of cisplatin use, typically resulting in permanent bilateral sensorineural hearing loss, first in the high frequency region then extending to the lower frequencies with continued exposure [1–3]. Younger children are at greater risk for developing permanent hearing loss from cisplatin exposure compared to older children [4], which can lead to significant delays in speech, language, and social development [5]. Loss of hearing in the high frequency range diminishes the ability to hear and recognize certain phonemes, primarily fricatives, which are important for the development and comprehension of speech, particularly for young children [6]. Even mild degrees of hearing impairment can negatively impact communication, academic performance, and psychosocial outcomes in young children [7, 8]. Because early detection of hearing loss and early intervention in infants and children are essential for optimizing speech, language, and social-emotional development [9–11], implementing a clinically relevant grading scale sensitive to identifying high frequency hearing loss in young patients receiving ototoxic drugs is an important component to successfully managing these patients [12].
The assessment of ototoxicity in patients has become more objective with the establishment of ototoxicity monitoring protocols [13–17] and hearing loss grading scales [1, 2, 18–22]. Several ototoxicity grading schemes have been proposed over the past two decades for various purposes, such as classifying hearing loss severity [2], identifying early changes in hearing sensitivity [18, 19], correlating hearing loss classifications to functional outcomes [19, 20], and reporting ototoxicity as an adverse event in oncologic clinical trials [1, 21, 22]. The purpose of a grading scale is to objectively define and report ototoxicity. However, the implementation of different ototoxicity grading scales across institutions has made analyzing ototoxicity studies challenging to interpret and has contributed to the variability in reporting the prevalence of platinum-associated hearing loss throughout the literature [23].
Recent attempts have been made to create a standardized ototoxicity scale that could be widely adapted in pediatric oncology settings. In 2010, Chang and Chinosornvatana proposed an ototoxicity grading scale based on absolute hearing threshold levels that better correlated hearing loss categories to clinical recommendations compared to the Common Terminology Criteria for Adverse Events (CTCAE) grading criteria currently used in many clinical trials [20]. The Chang scale was developed as a modification to the frequently-used Brock ototoxicity scale, a scale developed specifically for children receiving cisplatin chemotherapy [2]. The Chang scale detected milder degrees of hearing loss and better correlated with functional outcomes compared to the Brock scale [20]. The Chang scale represents typical grading scales often used by the medical community to report adverse events. Toxicity scales typically consist of 5 levels of severity, ranging from 0 (no complications) to 4 (severe complications). Chang divided grades 1 and 2 into grades 1a, 1b, 2a, and 2b to more precisely distinguish between different degrees of functional hearing loss; however this resulted in a scale which exceeded the typical 5 levels of severity customarily seen in toxicity grading. In 2012, a panel of experts published the SIOP ototoxicity grading scale for the purpose of comparing end-of-treatment hearing loss in oncologic clinical trials across institutions [1]. The SIOP scale, a modification of the Children’s Hospital of Boston functional hearing loss scale [19], is based on absolute hearing threshold measurements and is sensitive to high frequency hearing loss and mild degrees of impairment while retaining the customary 5 levels of severity [1]. We chose to compare the Chang and SIOP ototoxicity scales as they both represent improvements upon existing scales and reliability and validity measures have yet to be reported for the SIOP scale. The purpose of this study was to evaluate concordance between the Chang and the SIOP ototoxicity grading scales and identify areas of disparity between the two scales.
Methods
Patient Characteristics
The source population for this study was patients newly-diagnosed with average or high risk medulloblastoma treated on either the SJMB96 or SJMB03 treatment protocols at St. Jude Children’s Research Hospital (SJCRH) or one of the nine collaborative study sites. Informed consent was obtained from all parents or guardians of study participants and both clinical protocols were approved by the Human Subjects Institutional Review Boards at St. Jude Children’s Research Hospital and each of the participating institutions. Gajjar et al [24] and Fouladi et al [25] previously described eligibility criteria and course of therapy for the SJMB96 and SJMB03 protocols. Briefly, eligibility for these protocols included patients with newly diagnosed medulloblastoma aged 3–21 years having received no prior chemotherapy or radiotherapy. Protocol treatment included a surgical resection, six weeks of craniospinal radiation therapy, and chemotherapy. Average and high risk patients received 23.4 Gy and 36–39.6 Gy craniospinal radiotherapy, respectively with 55.8 Gy to the primary tumor bed. The clinical target volume of the boost dose to the tumor bed was reduced from 2.0 cm in the SJMB96 protocol to 1.0 cm in the SJMB03 protocol. The chemotherapy regimen for these patients consisted of four cycles of cyclophosphamide, vincristine, cisplatin, and stem cell or bone marrow rescue. Cisplatin dose included 75 mg/m2 each cycle totaling 300 mg/m2 cumulative dose. The SJMB96 protocol began enrolling patients in October 1996 and was amended in August 1999 to include amifostine [25], a thiophosphate cytoprotective agent given to reduce toxicities associated with radiotherapy and alkylating and platinum-containing agents [26]. Amifostine was administered immediately prior to and again three hours into each of the four cycles of cisplatin treatments to minimize ototoxic effects [25]. Audiometric evaluations were included as standard of care in both protocols. Eligibility for this analysis was limited to patients diagnosed with medulloblastoma from September 1996 to March 2012 who received at least one audiometric evaluation between 5.5–24.5 months from initiation of protocol-based treatment. Patients who did not receive cisplatin chemotherapy and those with permanent hearing loss in at least one ear at baseline were excluded from this analysis.
Audiological Methods
Various audiological testing methods were used to assess hearing dependent upon the patient’s age, cognitive and developmental abilities, and level of cooperation. Tympanometry was reviewed to determine the integrity of the conductive mechanism at the time of testing. Pure-tone air conduction thresholds were evaluated at frequencies 0.25, 0.5, 1, 2, 3, 4, 6, and 8 kHz in decibel (dB) hearing level (HL). Pure tone bone conduction thresholds were assessed at frequencies 0.25, 0.5, 1, 2, 3, and 4 kHz to determine the nature of the hearing impairment (i.e., conductive, sensorineural, or mixed). Click and tone-burst auditory brainstem response (ABR), auditory steady-state response, and/or distortion-product otoacoustic emissions (DPOAE) measurements were evaluated on patients who were unable to participate in conventional audiometric testing due to young age, cognitive or developmental delay, or lack of cooperation. The ototoxicity monitoring schedule consisted of an evaluation at the following time points: baseline (occurred within 2 weeks of initiation of radiation therapy), prior to each high dose cisplatin chemotherapy cycle, and at 9, 12, 15, and 24 months following diagnosis. Audiometric data from St. Jude and the nine collaborative sites were reviewed and assigned an ototoxicity grade by a single research audiologist at St. Jude (JKB). Each audiological evaluation was given an ototoxicity grade based on the Chang Ototoxicity Grading Scale [20] and the International Society of Pediatric Oncology Ototoxicity Scale (Table I) [1]. The latest audiometric evaluation that occurred between 5.5–24 months from on-treatment date was used for the analysis.
Table I.
Ototoxicity Grading Scales*
| Chang | SIOP | ||
|---|---|---|---|
| Grade 0 | ≤ 20 dB at 1, 2, and 4 kHz | Grade 0 | ≤20 dB HL at all frequencies |
| Grade 1a Grade 1b |
≥ 40 dB at any freq 6 to 12 kHz > 20 and < 40 dB at 4kHz |
Grade 1 | >20 dB HL (i.e. 25 dB HL or greater) SNHL above 4000 Hz (i.e. 6 or 8 kHz) |
| Grade 2a Grade 2b |
≥ 40 dB at 4 kHz and above > 20 and < 40 dB at any freq below 4kHz |
Grade 2 | >20 dB HL SNHL at 4000 Hz and above |
| Grade 3 | ≥ 40 dB at 2 or 3 kHz & above | Grade 3 | >20 dB HL SNHL at 2000 Hz or 3000 Hz and above |
| Grade 4 | ≥ 40 dB at 1 kHz and above | Grade 4 | >40 dB HL (i.e. 45 dB HL or more) SNHL at 2000 Hz and above |
Sensorineural Hearing Threshold (dB HL) bone conduction or air conduction with normal tympanogram
Statistical approach
The objective of the statistical analysis was to evaluate the concordance in hearing levels between the Chang and SIOP scales. If hearing levels were asymmetrical, the level in the worst ear was used for the analysis. The Stuart’s tau-c statistic for testing association of two ordinal scales among non-square contingency tables was used as a measure of concordance [27].
Results
Patient enrollment
A total of 452 patients diagnosed with medulloblastoma were enrolled on either the SJMB96 (n=134) or SJMB03 (n=318) protocol as of March 2012. Of these, 418 patients diagnosed with medulloblastoma met eligibility criteria for having at least one audiology assessment between 5.5–24.5 months from treatment onset. Patients with non-transient hearing loss in at least one ear at baseline (n=35) or patients who did not receive cisplatin (n=4) were excluded from this analysis. Of the 379 evaluable patients, the median cumulative cisplatin dose was 300 mg/m2 (range 74–329 mg/m2). The median time from initiation of treatment to the latest audiological evaluation was 19.1 months. Table II provides an overview of the patient characteristics.
Table II.
Patient Characteristics
| Characteristic | n (%) (n = 379) | |
|---|---|---|
| Gender | Male | 243 (64%) |
| Female | 136 (36%) | |
| Race | White | 293 (77%) |
| Non-white | 86 (23%) | |
| Age at diagnosis (years) | Median | 8.2 |
| Range | 3.0–21.6 | |
| Interquartile Range | 6.2 – 10.9 | |
| Age at latest audiogram (years) | Median | 9.7 |
| Range | 3.7 – 22.5 | |
| Interquartile Range | 7.6 – 12.4 | |
| Time from treatment initiation to latest audiogram (months) | Median | 19.1 |
| Range | 5.6 – 24.5 | |
| Interquartile Range | 12.4 – 22.6 | |
| Disease Risk | Average | 263 (69%) |
| High | 116 (31%) | |
| Study | SJMB03 | 266 (70%) |
| SJMB96 | 113 (30%) | |
| Institution | St. Jude | 192 (51%) |
| Collaborative site | 187 (49%) | |
| Amifostine | No | 51(13%) |
| Yes | 328 (87%) | |
| Cisplatin cumulative dose (mg/m2) | Median | 300 |
| Range | 74 – 329 | |
| Interquartile Range | 295 – 302 |
Concordance between Chang and SIOP
Table III compares the number of patients in each hearing level of the Chang and SIOP scales. Based on these data, the Stuart’s tau-c statistic was estimated to be 0.89 (95% CI: 0.86, 0.91), indicating a very strong concordance.
Table III.
Frequency comparison of SIOP vs. Chang hearing loss grades
| Table of SIOP by Chang | ||||||||
|---|---|---|---|---|---|---|---|---|
| SIOP | Chang | |||||||
| 0 | 1a | 1b | 2a | 2b | 3 | 4 | Total | |
| 0 | 98 | 0 | 0 | 0 | 0 | 0 | 0 | 98 |
| 1 | 30 | 68 | 0 | 0 | 0 | 0 | 0 | 98 |
| 2 | 0 | 0 | 27 | 24 | 0 | 0 | 0 | 51 |
| 3 | 0 | 0 | 0 | 0 | 25 | 74 | 1 | 100 |
| 4 | 0 | 0 | 0 | 0 | 0 | 21 | 11 | 32 |
| Total | 128 | 68 | 27 | 24 | 25 | 95 | 12 | 379 |
As shown in Figure 1a, the prevalence of any detectable hearing loss was 66% and 74% according to the Chang and SIOP scales, respectively. Among the 128 patients coded as having no hearing loss (grade 0) based on the Chang criteria, 30 (23%) were categorized as having SIOP grade 1, indicating non-concordance between the scales for some degree of high frequency hearing loss (Table III).
Figure 1.
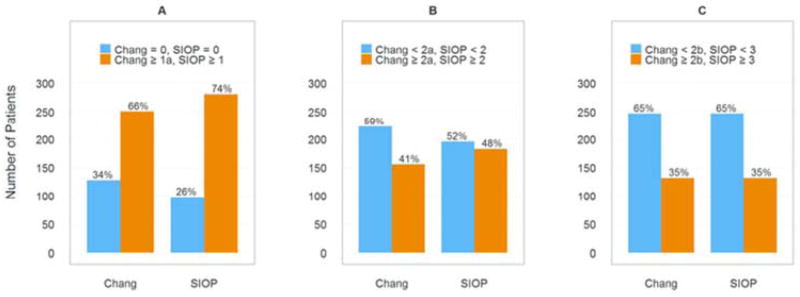
Prevalence of hearing loss by Chang and SIOP scales. A: Any detected hearing loss. B: Clinically significant hearing loss. C: Hearing loss requiring hearing aids.
Figure 1b compares the percentage of patients coded as having clinically significant hearing loss versus minimal or no hearing loss between the Chang and SIOP grading criteria. We considered “clinically significant hearing loss” as Chang grade ≥2a or SIOP grade≥ 2. Using the Chang scale, 156 (41%) patients were coded as having clinically significant hearing loss versus 183 (48%) coded with SIOP. For the 51 patients with a SIOP grade 2 hearing loss, 27 (53%) were coded as having a lesser grade by Chang grade 1b.
The scales were in better agreement in identifying more severe hearing impairment as evident in Figure 1c. For both scales, 132 (35%) patients were coded as having serious hearing loss requiring amplification. Minor differences were noted between the scales in defining grade 3 and 4 ototoxicity. Of the 95 patients assigned a Chang grade 3 hearing loss, 21 (22%) were classified by SIOP as grade 4. For grade 3 hearing loss, SIOP (n=100, 26%) and Chang (n=95, 25%) were similar in coding; however, for grade 4, SIOP coded 20 more patients (n=32, 8%) than Chang (n=12, 3%). As seen in Table III, one patient was classified as a SIOP grade 3 and Chang grade 4. This finding was unusual given that the SIOP criteria are more sensitive in defining grade 4 hearing loss compared to the Chang scale. This patient’s hearing threshold for 2 kHz was slightly better compared to 1 kHz, which is an atypical configuration for cisplatin-induced hearing loss and is considered a rare occurrence. However, this example represents a potential, albeit uncommon, disagreement between the Chang and SIOP scales for patients with serious hearing loss.
Discussion
We compared hearing loss levels in 379 pediatric patients with medulloblastoma who received high-dose cisplatin using two recently published ototoxicity grading scales, the SIOP and Chang scale. We observed an overall high degree of concordance between the two scales, particularly in classifying serious hearing loss requiring hearing aids. However, important differences were observed for mild and clinically significant degrees of hearing impairment.
The SIOP scale was more sensitive than the Chang scale in detecting mild levels of high frequency hearing loss. The definition for SIOP grade 1 hearing loss is 20 dB lower than Chang grade 1a, thus classifying milder forms of hearing loss between 25–35 dB HL as an ototoxic occurrence. Because the SIOP scale uses a lower hearing level threshold to define grade 1 hearing loss, it may be more sensitive than the Chang criteria for identifying any incidence of ototoxicity. However, if the goal is to identify serious ototoxicity as an adverse event, it appears there is no benefit of using the SIOP over the Chang scale.
For this study, we considered Chang grade 2a and SIOP grade 2 as “clinically significant,” indicating intervention such as preferential classroom seating, additional educational accommodations, and/or use of assistive technology (i.e., FM system) but not requiring the use of hearing aids. Identifying children with clinically significant hearing loss is important as mild hearing impairment may result in significant language and academic deficits [7, 8]. A notable difference exists between the Chang and SIOP criteria in classifying patients with clinically significant hearing loss. Essentially half (53%) of SIOP grade 2 patients were coded with a milder Chang grade 1b. Again, the reason for this discrepancy is the difference in decibel level used to define each grade level between the two scales. SIOP grade 2 uses a lower decibel value of ≥25 dB compared to the Chang 2a decibel value of ≥40 dB. Thus, SIOP grade 2 is more sensitive in detecting patients with clinically significant hearing loss. Chang 2a was found to be the level of hearing loss that most corresponded to patients receiving FM systems [20]. The lower decibel threshold for SIOP grade 2 probably includes some patients who do not need an FM system.
Although Chang and SIOP ototoxicity outcomes were somewhat discrepant in patients experiencing milder degrees of high frequency hearing loss, the scales were congruent in identifying more severe hearing impairment. Patients diagnosed with a Chang grade≥2b or SIOP grade ≥3 hearing loss have serious hearing loss, typically requiring the use of hearing aids, other assistive listening technology, educational resources, and additional communicative strategies. While Chang grade ≥2b and SIOP grade ≥3 criteria identified the same number of patients (n=131, 34.6%) as having serious hearing loss, minor differences were noted between the scales in defining grade 4 ototoxicity. Overall, more patients were coded as having grade 4 hearing loss based on the SIOP scale (n=32, 8.4%) compared to the Chang scale (n=12, 3.2%). These two scales differ considerably in their definition for grade 4 hearing loss. As seen in Table I, grade 4 ototoxicity for SIOP indicates at least a moderate degree of hearing loss at ≥2 kHz while Chang grade 4 lowers the frequency range to include moderate hearing loss at ≥1 kHz. The difference between these two scales in defining grade 4 hearing loss may be inconsequential in most cases with the exception for reporting functional outcomes in oncologic clinical trials, particularly if a new drug or otoprotective agent were being investigated. In this case, differentiating between grade 3 and 4 may be valuable when evaluating adverse effects of a novel therapy or efficacy of an otoprotective drug.
Chang and Chinosornvatana [20] validated the Chang scale using the CTCAE v.3 criteria and demonstrated a strong correlation between grading and hearing aid recommendation using both scales; however, the Chang scale was more specific in predicting intervention (hearing aid or FM system recommendation) for the more severe grades compared to the CTCAE criteria making the Chang scale more clinically useful and relevant. The SIOP ototoxicity grading scale was created using the best attributes from all of the existing scales for the purpose of reporting end-of-treatment outcomes in clinical trials [1]. Although the SIOP grades have not been correlated with hearing loss recommendations, the strong concordance between Chang grades 2b-4 and SIOP grades 3–4 indicates that patients with SIOP grades 3 and 4 ototoxicity would likely need hearing aids at the end of therapy.
The authors proposed international consensus and use of the SIOP scale, given positive validation results. Our results indicate that the SIOP scale is reliable and clinically relevant in classifying patients with ototoxic hearing loss and predicting functional outcomes and clinical recommendations. The SIOP scale is easier to use and understand and is more sensitive in detecting mild hearing loss compared to the Chang scale, supporting its acceptance as the international ototoxicity grading scale.
Strengths of this study include a large sample size, a population homogenous for cisplatin exposure and high-quality, standardized treatment and ototoxicity monitoring protocols. However, study limitations should be considered when interpreting our results. Our population consisted of patients diagnosed with medulloblastoma with no other disease groups represented. Our patients also received cranial radiation therapy prior to chemotherapy, which has been shown to behave synergistically to exacerbate hearing loss when paired with cisplatin chemotherapy [28]. Finally, most of the patients (87%) in our cohort received amifostine to reduce or prevent hearing loss.
In summary, we found strong concordance between the SIOP and Chang ototoxicity scales, particularly for patients with serious hearing loss. Meaningful discrepancies exist between the scales; mainly the SIOP scale is more sensitive in coding patients with milder degrees of hearing loss. Recent advancements in cancer treatment, otoprotection, and genetic-related ototoxicity studies support the need for a standardized, widely-accepted ototoxicity grading scale, such as the SIOP scale, that would allow for easier grading and more consistent outcome measures across institutions.
Acknowledgments
Supported in part by grants from the National Cancer Institute Cancer Center CORE grant CA 21765, the Noyes Brain Tumor Foundation, Musicians Against Childhood Cancer (MACC), and the America Lebanese Syrian Associated Charities (ALSAC).
Footnotes
Financial disclosures or potential conflicts of interest: None
References
- 1.Brock PR, Knight KR, Freyer DR, et al. Platinum-based ototoxicity in children: a consensus review on mechanisms, predisposition and protection including a new SIOP Boston Ototoxicity Scale. J Clin Oncol. 2012;30:2408–2417. doi: 10.1200/JCO.2011.39.1110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brock PR, Bellman SC, Yeomans EC, et al. Cisplatin ototoxicity in children: a practical grading system. Med Pediatr Oncol. 1991;19:295–300. doi: 10.1002/mpo.2950190415. [DOI] [PubMed] [Google Scholar]
- 3.Blakely BW, Myers SF. Patterns of hearing loss resulting from cis-platinum therapy. Otolaryngol Head Neck Surg. 1993;109:385–391. doi: 10.1177/019459989310900302. [DOI] [PubMed] [Google Scholar]
- 4.Li Y, Womer RB, Silber JH. Predicting cisplatin ototoxicity in children: the influence of age and the cumulative dose. Eur J Cancer. 2004;40:2445–2451. doi: 10.1016/j.ejca.2003.08.009. [DOI] [PubMed] [Google Scholar]
- 5.Knight KR, Kraemer DF, Neuwelt EA. Ototoxicity in children receiving platinum chemotherapy: underestimating a commonly occurring toxicity that may influence academic and social development. J Clin Oncol. 2005;23:8588–8596. doi: 10.1200/JCO.2004.00.5355. [DOI] [PubMed] [Google Scholar]
- 6.Stelmachowicz PG, Pittman AL, Hoover BM, et al. The importance of high-frequency audibility in the speech and language development of children with hearing loss. Arch Otolaryngol Head Neck Surg. 2004;130:556–562. doi: 10.1001/archotol.130.5.556. [DOI] [PubMed] [Google Scholar]
- 7.Bess FH, Dodd-Murphy J, Parker RA. Children with minimal sensorineural hearing loss: prevalence, educational performance, and functional status. Ear Hear. 1998;19:339–354. doi: 10.1097/00003446-199810000-00001. [DOI] [PubMed] [Google Scholar]
- 8.Davis JM, Elfenbein J, Schum R, Bentler RA. Effects of mild and moderate hearing impairments on language, educational, and psychosocial behavior of children. J Speech Hear Disord. 1986;51:53–62. doi: 10.1044/jshd.5101.53. [DOI] [PubMed] [Google Scholar]
- 9.Yoshinaga-Itano C, Sedey AL, Coulter DK, Mehl AL. Language of early- and later- identified children with hearing loss. Pediatrics. 1998;102:1161–1171. doi: 10.1542/peds.102.5.1161. [DOI] [PubMed] [Google Scholar]
- 10.Yoshinaga-Itano C. Benefits of early intervention for children with hearing loss. Otolaryngol Clin North Am. 1999;32:1089–1102. doi: 10.1016/s0030-6665(05)70196-1. [DOI] [PubMed] [Google Scholar]
- 11.Downs MP, Yoshinaga-Itano C. The efficacy of early identification and intervention for children with hearing impairment. Pediatr Clin North Am. 1999;46:79–87. doi: 10.1016/s0031-3955(05)70082-1. [DOI] [PubMed] [Google Scholar]
- 12.Chang KW. Clinically accurate assessment and grading of ototoxicity. Laryngoscope. 2011;121:2649–2657. doi: 10.1002/lary.22376. [DOI] [PubMed] [Google Scholar]
- 13.Campbell KC, Durrant J. Audiologic monitoring for ototoxicity. Otolaryngol Clin North Am. 1993;26:903–914. [PubMed] [Google Scholar]
- 14.Fausti SA, Henry JA, Schaffer HI, et al. High-frequency monitoring for early detection of cisplatin ototoxicity. Arch Otolaryngol Head Neck Surg. 1993;119:661–666. doi: 10.1001/archotol.1993.01880180081015. [DOI] [PubMed] [Google Scholar]
- 15.Fausti SA, Larson VD, Noffsinger D, et al. High-frequency audiometric monitoring strategies for early detection of ototoxicity. Ear Hear. 1994;15:232–239. doi: 10.1097/00003446-199406000-00004. [DOI] [PubMed] [Google Scholar]
- 16.Vaughan NE, Fausti SA, Chelius S, et al. An efficient test protocol for identification of a limited, sensitive frequency test range for early detection of ototoxicity. J Rehabil Res Dev. 2002;39:567–574. [PubMed] [Google Scholar]
- 17.Vasquez R, Mattucci KF. A proposed protocol for monitoring ototoxicity in patients who take cochleo- or vestibulotoxic drugs. Ear Nose Throat J. 2003;82:181–184. [PubMed] [Google Scholar]
- 18.American Speech-Language-Hearing Association. Audiologic management of individuals receiving cochleotoxic drug therapy guidelines. [Accessed August 7, 2013];ASHA practice policy. http://www.asha.org/policy. Published 1994.
- 19.Lewis MJ, DuBois SG, Fligor B, et al. Ototoxicity in children treated for osteosarcoma. Pediatr Blood Cancer. 2009;52:387–391. doi: 10.1002/pbc.21875. [DOI] [PubMed] [Google Scholar]
- 20.Chang KW, Chinosornvatana N. Practical grading system for evaluating cisplatin ototoxicity in children. J Clin Oncol. 2010;28:1788–1795. doi: 10.1200/JCO.2009.24.4228. [DOI] [PubMed] [Google Scholar]
- 21.National Cancer Institute. [Accessed August 7, 2013];Common Terminology Criteria for Adverse Events, Version 3.0. http://ctep.cancer.gov/protocoldevelopment/electronic_applications/docs/ctcae_glossary.pdf. Published 10/22/2003.
- 22.National Cancer Institute. [Accessed August 7, 2013];Common Terminology Criteria for Adverse Events, Version 4.03. http://evs.nci.nih.gov/ftp1/CTCAE/CTCAE_4.03_2010-06-14_QuickReference_8.5x11.pdf. Published May 28, 2009.
- 23.Neuwelt EA, Brock P. Critical need for international consensus on ototoxicity assessment criteria. J Clin Oncol. 2010;28:1630–1632. doi: 10.1200/JCO.2009.26.7872. [DOI] [PubMed] [Google Scholar]
- 24.Gajjar A, Chintagumpala M, Ashley D, et al. Risk-adapted craniospinal radiotherapy followed by high-dose chemotherapy and stem-cell rescue in children with newly diagnosed medulloblastoma (St Jude Medulloblastoma-96): long-term results from a prospective, multicentre trial. Lancet Oncol. 2006;7:813–820. doi: 10.1016/S1470-2045(06)70867-1. [DOI] [PubMed] [Google Scholar]
- 25.Fouladi M, Chintagumpala M, Ashley D, et al. Amifostine protects against cisplatin- induced ototoxicity in children with average-risk medulloblastoma. J Clin Oncol. 2008;26:3749–3755. doi: 10.1200/JCO.2007.14.3974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.McKibbin T, Panetta JC, Fouladi M, et al. Clinical pharmacokinetics of amifostine and WR1065 in pediatric patients with medulloblastoma. Clin Cancer Res. 2010;16:1049–1057. doi: 10.1158/1078-0432.CCR-09-1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.De Muth JE. Basic statistics and pharmaceutical statistical applications. Baton Raton FL: Chapman & Hall/CRC; 2006. [Google Scholar]
- 28.Low WK, Toh ST, Wee J, et al. Sensorineural hearing loss after radiotherapy and chemoradiotherapy: a single, blinded, randomized study. J Clin Oncol. 2006;24:1904–1909. doi: 10.1200/JCO.2005.05.0096. [DOI] [PubMed] [Google Scholar]


