Abstract
Rationale
Alcohol may be self-administered for its anxiolytic effects to alleviate symptoms of stress, but different types of stressors have varying effects on alcohol intake. Social stress is particularly relevant to alcohol drinking, and a primate model of stress-induced alcohol self-administration would be useful.
Objective
The objective of the study is to determine if social stresses of different lengths and intensities affect voluntary alcohol intake in monkeys.
Materials and methods
Subjects were adult male and female squirrel monkeys (Saimiri sciureus) housed in social colonies. Subjects were trained to drink a solution of ethanol and sucrose, alternated daily with a control solution of quinine and an equal concentration of sucrose in 15-min sessions. Drinking was tested during 20-min acute, social separations and 1-week, extended, social separations. Dominance status was quantified using observational records of social interactions within the colonies. Salivary cortisol was sampled in the home colony and during extended social separation.
Results
Dominance rank was inversely correlated with alcohol intake during social housing but was not correlated with control fluid intake. Acute social separation abolished drinking of both fluids, accompanied by increased anxiety-like behavior. Extended social separation increased salivary cortisol and alcohol drinking but not control fluid intake in males. In females, drinking was unchanged by extended separation.
Conclusions
The chronic stress of social subordination is correlated with increased alcohol drinking. Acute social separation stress suppresses drinking behavior, while extended separation preferentially increases alcohol intake in a subset of individuals. These findings suggest that social stressors of different time-courses and intensities have opposing effects on alcohol self-administration.
Keywords: Alcohol, Ethanol, Self-administration, Stress, Cortisol, Vocalization, Squirrel monkey, Dominance, Social behavior
One of the challenges of creating animal models of alcohol drinking is the difficulty of inducing animals to self-administer alcohol under conditions similar to those in which human drinking occurs. Among humans, most drinkers are not physiologically dependent on alcohol, and their consumption takes place intermittently, in social settings, and without food or water deprivation. An animal model encompassing these characteristics would facilitate inquiry into how social and environmental factors alter an individual’s propensity to self-administer alcohol and shed light on the circumstances that increase the vulnerability to heavy alcohol use.
Non-human primate models of alcohol self-administration are especially desirable because of the close phylogenetic link to humans. Several studies have shown that oral alcohol functions as a reinforcer in non-deprived Old World monkeys (Ator and Griffiths 1992; Kornet et al. 1990; Stewart et al. 1996). Voluntary alcohol drinking has been successfully established in socially housed rhesus macaques without food and water deprivation (Barr et al. 2004b; Fahlke et al. 2000; Higley et al. 1991). These protocols provide limited access to up to 8.5% alcohol in a sweetened vehicle along with an alternative sweetened fluid and lead to alcohol intake from 0.4 g/kg to over 1 g/kg. New World monkeys tend to be less accepting of alcohol than Old World monkeys, but socially housed squirrel monkeys can voluntarily drink pharmacologically relevant doses of alcohol in a sweetened solution (Mandillo et al. 1998).
The tension reduction hypothesis of alcohol self-administration suggests that alcohol may be self-administered to alleviate the unpleasant symptoms of anxiety (Cloninger 1987; Conger 1956). Studies in both humans and rats have found that anxiety-like behavior can be predictive of alcohol self-administration (Spanagel et al. 1995; Swendsen et al. 2000; Willinger et al. 2002). Experimental stressors such as electric shock and physical restraint have also had some success in increasing alcohol self-administration in laboratory rats, although in many cases, laboratory stressors suppress alcohol drinking (Anisman and Waller 1974; Lynch et al. 1999). Because stressors of different types, durations, and severities can have vastly different effects, when examining the effects of stress on alcohol intake in laboratory animals, it will be useful to focus on stressors that may more closely mimic the psychosocial stressors implicated in human alcohol use (Pacak and Palkovits 2001).
Groups of social animals tend to form social dominance hierarchies, an individual’s position in which can have wide ranging consequences for its behavior and physiology. It has been shown in both rodent and primate species that occupation of a subordinate position can be a significant stressor, resulting in anxiety-like behaviors and alterations of the hypothalamic–pituitary–adrenal (HPA) axis (Blanchard et al. 1993; Sapolsky et al. 1997; Shively 1998). Subordinate primates in both captive and wild populations have been found to show reduced suppression of cortisol in response to dexamethasone challenge, suggesting dysregulation of the HPA axis, a condition associated with chronic stress (Sapolsky et al. 1997; Shively 1998). The effect of dominance status on alcohol intake has been examined in rodents, and studies of rats housed in social groups have found that subordinate animals may drink more alcohol than dominants (Blanchard et al. 1987; Wolffgramm and Heyne 1991). In rhesus macaques, low social competency, which is associated with low dominance rank, has been found to correlate with high alcohol consumption (Higley et al. 1996a; Higley et al. 1996b).
While living in a social environment can be a significant stressor, social isolation is also very stressful for social animals. In the wild, male baboons with few social interactions were found to have higher basal cortisol levels than males that spent more time interacting with social partners (Sapolsky et al. 1997). Squirrel monkeys and rhesus macaques that are experimentally separated from their social group respond to isolation with an increase in cortisol (Higley et al. 1992; Lyons et al. 1999). Pretreatment with alcohol has anxiolytic-like effects in socially separated juvenile squirrel monkeys, reducing both explosive motor behavior and isolation calls (Weerts and Miczek 1996). Rhesus macaques have also been shown to increase their alcohol intake and anxiety-like behavior during periods of social isolation, again suggesting a link between increased stress and alcohol self-administration (Higley et al. 1991; Kraemer and McKinney 1985).
The studies reported here use socially housed squirrel monkeys under neither food nor fluid restrictions to create a model of voluntary moderate alcohol self-administration. Naturalistic social stressors, dominance status, and acute and extended social separation are used to examine the relationship between alcohol self-administration and social context.
Materials and methods
Subjects
Subjects were eight adult male and five adult female squirrel monkeys (Saimiri sciureus), aged 4–23 years; because different individuals were drinking over different time periods, not all of the subjects were used in every experiment. Monkeys were housed in five multi-generational, mixed-sex social groups of between four and ten individuals each. The colony rooms were 1.8×2.2×2.1 m, with a stainless steel grid covering the ceiling. Each room contained a pole with wooden perches, two corner perches, a swing, ropes suspended from the ceiling, and pine shavings covering the floor. One wall of each colony room was a one-way mirrored glass window allowing for observation. The temperature (minimum 22°C), humidity (44–55%), and light cycle (lights on from 0800 to 2000 hours) were controlled. Heat lamps elevated the room temperature to 30°C between 1100 and 1500 hours. The monkeys had free access to tap water from lick spouts, and high-protein monkey chow (Purina, St. Louis, MO, USA) was continuously available. Their diet was supplemented with ZuPreem Primate Diet (Premium Nutritional Products, Inc., Mission, KS, USA), fresh fruit, chewable multivitamins (Bayer, Morristown, NJ, USA), peanuts, and sunflower seeds given in the evening after experiments ended. Squirrel monkeys have short 8–10-day estrous cycles and do not cycle outside of the breeding season (Dukelow 1978). There are no external indicators of estrous cycle phase in this species, and methods used to determine phase are sufficiently intrusive to constitute a significant stressor when preformed with the necessary frequency. Females in this study drank throughout the year with no seasonal change in drinking patterns. Experimental procedures were carried out in accordance with the guidelines of the “Guide for the Care and Use of Laboratory Animals” (1996) and were supervised by the Tufts Committee on Animal Care and Use.
Assessment of dominance
The dominance status of each monkey within the colony was ascertained by observational records. Trained observers recorded all instances of social behavior in 20-min samples taken between 800 and 1100 in the morning. Recorded behaviors classified as associative included proximity, huddling, playing, and grooming; behaviors classified as agonistic included aggressive behavior (fighting, biting, chasing, and grabbing), dominance displays (genital displays, branch shaking, and ear flapping), and displacement (Hopf et al. 1974; Miczek and Yoshimura 1982). Behaviors were recorded in sender–receiver sociometric matrix format (Altmann 1974). Dominance index was determined by calculating the percent of agonistic behaviors sent versus those received for each dyad and averaging the scores of each individual with all of that individual’s social partners (Zumpe and Michael 1986). Dominance index was calculated every 6 months (January–June and July–December) from 8–20 h of observation per colony.
Self-administration
Monkeys were induced to drink ethanol by a previously described procedure (Mandillo et al. 1998). Each monkey was trained to enter a plexiglass chamber (54×48×50 cm) located in the window of its home colony, from which it had full visual and auditory contact with the rest of the colony. A plastic graduated cylinder fitted with a rubber stopper and ball-tip sipper tube containing 60 ml of drinking solution was attached to the outside of the chamber, and each subject was allowed to drink for 15 min each day. A plexiglass door was closed between the subject and the rest of the colony during the drinking session so that only one subject at a time had access to the drinking solution. The drinking bottle was weighed before and after drinking to determine solution intake in grams of fluid. This method of assessing intake is accurate to 1 g. On the first 2 days of fluid presentation, the solution was 15% (w/v) sucrose; ethanol was then gradually added in increments of 0.5% (w/v) increasing every 2 days until a concentration of 2% ethanol 15% sucrose was reached. A control solution containing the same proportion of sucrose as the alcohol solution plus 0.08% quinine was then introduced on alternating days so that the monkeys had the opportunity to drink one solution per day. This quinine percentage was chosen because most of the subjects consumed it in a similar volume to the alcohol solution. All of the subjects drank the solutions of 15% sucrose with either 2% alcohol or 0.08% quinine in the intake/dominance correlations and the extended isolation experiments. Three of the females were added to the experiment after the 0.08% quinine concentration was chosen for the control fluid, and they showed a significant preference for alcohol over this quinine solution. The two of these females used in the colony context experiment were given a lower concentration of quinine 0.02% or 0.04% to better equalize their drinking of the two fluids.
Assessment of colony context effects
Subjects (n=9, five males, four females) entered the drinking chamber as usual, then one of a series of colony manipulations took place.
Baseline No change was made to the colony situation
Colony disruption
The experimenter entered the colony room and placed all of the other monkeys into a cage in the center of the room, then the experimenter left the room. The subject was able to see and hear the other monkeys from the drinking chamber.
Social separation
The experimenter entered the colony room and placed all of the other monkeys in to a cage then removed the cage from the colony room. The subject was able to see the empty colony room but could not see the other monkeys from the drinking chamber.
When the colony manipulation was in place, the subject was offered alcohol or control solution for 15 min then released back into the colony room. Five minutes after the end of the drinking session, the colony manipulation was terminated, and any monkeys that were caged or removed were released back into the colony room. During the 5 min following the drinking session, the subjects’ behavior was recorded by a single, unblinded trained observer using a computer running The Observer 5.0 behavioral scoring software (Noldus). Behavioral observations were taken on six of the nine subjects (four males and two females) because the recording equipment was not available at the time the other three were tested. Behaviors recorded were locomotion, alert/vigilant, foraging/eating/drinking, sitting in a relaxed posture, scratching, scent marking, calling, and stereotyped head rolling (Hopf et al. 1974). Social behaviors were also recorded but were not analyzed as they were not available during social separation. All behaviors were recorded as frequency and duration except for calls and head rolls, for which only frequency was tallied.
Extended social separation
Subjects (n=11, seven males, four females) were individually removed from their social colony and housed alone with no view of other monkeys in an 82×52×82-cm wire and plexiglass cage with two wooden perches and standard food and water continually available. After 2 days of individual housing, drinking sessions were resumed for 4 days beginning with an alcohol presentation. At the end of the week, the subject was returned to the social colony, and home-cage drinking was resumed as before.
Salivary cortisol sampling
Saliva samples were taken from four male monkeys just before drinking in the home colony (baseline) and in the extended social separation condition. These four males were used because they learned, and consistently preformed, the sampling procedure at the time of the isolations, and the other subjects had not. All samples were taken before drinking so that any differences in cortisol could be attributed to the social situation and would not be complicated by the fluid self-administration. Samples were taken in a 1-h window between 15:30 and 16:30 to control for diurnal rhythmicity of cortisol level. Salivary cortisol was collected by a noninvasive sampling method developed by Tiefenbacher et al. (2003). Briefly, subjects were trained to chew on a braided cotton dental rope (Richmond Dental, Charlotte, NC, USA) flavored with sucrose and fruit punch flavored drink mix then baked until dry. Saliva was extracted by placing the rope in Salivette® tubes and centrifuging at 1,000 rpm for 12 min. Saliva was stored at −80°C until cortisol assay. Squirrel monkeys have cortisol levels that are 100 times more than those of humans, so samples were diluted 1:50 in deionized water before being analyzed in duplicate by radioimmunoassay (Coat-A-Count® cortisol, DPC Diagnostics, Los Angeles, CA, USA; Klosterman et al. 1986).
Data analysis
The relationship between dominance index and home-cage self-administration of alcohol and control solution were determined using Pearson product-moment correlations. For each individual, the dominance index and solution intake used in the correlations was calculated by averaging values from each 6-month period in which both drinking and dominance values were available. One female was not used in this analysis because she never drank a measurable amount of the standard control solution. Because different animals were used at different times, the data is based upon between one and six 6-month periods. Due to the large age range in this group of subjects, a second set of correlations compared age to solution intake as well as dominance index.
Two-way repeated-measures ANOVAs were used to compare gram per kilogram solution intake and behavior across treatment conditions in the colony context tests. When a significant effect was revealed at the p<0.05 level, post hoc analysis was performed using the Holm–Sidak method (SigmaStat 3.1).
Drinking in the social versus the extended isolation conditions were compared by two-way repeated-measures ANOVAs and Holm–Sidak post hoc tests. The averaged intake values of each solution on first two presentation days immediately preceding and following isolation were used in the analysis for the before and after isolation drinking values. There were small but significant variations between the first and second exposure to both the alcohol and control fluid in isolation, so the less variable data from the second exposures were used for analysis. Change in cortisol level between social and isolated housing was analyzed by a one-way repeated-measures ANOVA and Holm–Sidak post hoc test.
Results
Dominance
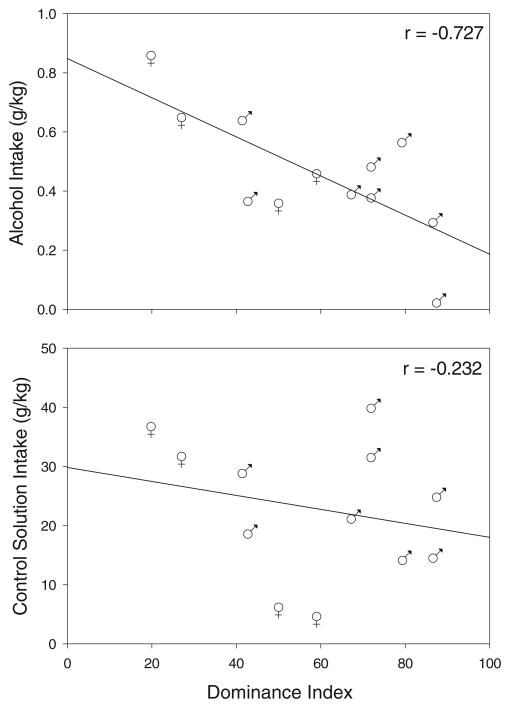
Individual dominance rank remained relatively stable over time, with no major shifts or rank reversals taking place during the study period. Average dominance index was compared to average fluid intake in individuals who drank through more than one 6-month period. Pearson product-moment correlation revealed a negative correlation between home-cage alcohol intake and dominance index (r[12]=−0.727; p= 0.007), with low ranking monkeys drinking more alcohol than high ranking animals (Fig. 1, top). There was no correlation between dominance and control fluid intake (Fig. 1, bottom). Age was positively correlated with home-cage alcohol intake (r[12] =0.610; p=0.035) but showed no relationship to control fluid intake or dominance index. On average, females drank more alcohol and less control fluid than males, with females drinking 0.57±0.11 g/kg alcohol and 19.37±8.39 g/kg control fluid, while males drank 0.40± 0.07 g/kg alcohol and 24.57±3.15 g/kg control fluid.
Fig. 1.
Top panel The correlation between subjects average gram per kilogram alcohol intake and dominance index. Bottom panel The correlation between dominance and subjects’ average gram per kilogram intake of control solution
Colony context
At baseline, monkeys in this experiment drank 28.58± 4.78 g/kg of control fluid and 24.36±3.26 g/kg of alcohol fluid, with an average alcohol intake of 0.49±0.07 g/kg. A two-way repeated-measures ANOVA indicated a significant effect of colony context on fluid intake (F[2, 16]=7.168; p=0.006) but no effect of fluid type or interaction between context and fluid type (Fig. 2). Post hoc comparison to control showed no effect of non-social colony disruption but a significant decrease in fluid intake after social separation (p=0.003).
Fig. 2.
Bars represent the average gram per kilogram intake of control solution (empty bars) and alcohol solution (filled bars) of nine subjects in three different colony contexts. Error bars represent SEM. Asterisks indicate a significant difference from respective baseline
There were significant effects of colony context on locomotion (F[2, 10]=10.64; p =0.003), foraging (F[2, 10]= 11.178; p=0.003), and calling (F[2, 10] =5.401; p=0.026) but not on the other behaviors (Table 1). Post hoc analysis revealed a significant increase in locomotion (p=0.001) and calling (p=0.016) after social separation and a significant decrease in foraging after both colony disruption and (p= 0.003) and social separation (p= 0.002). The only behavior that showed an effect of fluid type was calling (F[1, 5]= 7.630; p=0.04), with calling being more frequent after control fluid than after alcohol (p=0.04), but there was no interaction between colony context and fluid type.
Table 1.
Effects of colony context on frequency and duration of behaviors
| Behavior | After control fluid
|
After alcohol
|
||||
|---|---|---|---|---|---|---|
| Baseline | Colony disruption | Social disruption | Baseline | Colony disruption | Social disruption | |
| Head roll | 0 | 5.5±3.7 | 9.7±4.8 | 2.7±2.7 | 3.0±2.6 | 9.5±4.6 |
| Scratch | 5.7±2.2 | 7.0±1.8 | 1.7±0.8 | 3.8±1.2 | 4.0±1.8 | 1.8±1.5 |
| Scent mark | 0.7±0.3 | 1.8±1.1 | 0.7±0.4 | 0.8±0.5 | 0.5±0.3 | 0.2±0.2 |
| Call | 0 | 0.5±0.3 | 39.2±16.6 | 0 | 0.2±0.2 | 29.3±13.3 |
| Sit(s) | 49.3±28.4 | 59.4±37.6 | 0 | 38.5±26.2 | 69.1±44.2 | 0 |
| Locomotion(s) | 81.5±29.1 | 113.1±26.3 | 208.7±32.6 | 84.8±25.7 | 145.9±37.8 | 201.8±26.6 |
| Forage (s) | 110.5±47.1 | 7.2±6.7 | 0 | 89.3±29.2 | 13.0±10.9 | 0 |
| Vigilant (s) | 49.2±14.6 | 115.6±25.8 | 85.3±30.9 | 75.4±30.9 | 64.9±25.2 | 93.1±23.9 |
Behaviors are expressed as the mean ± SEM, n=6. Emboldened values are statistically different from baseline (p<0.05). Behaviors quantified in seconds rather than frequencies are denoted (s).
Extended social separation
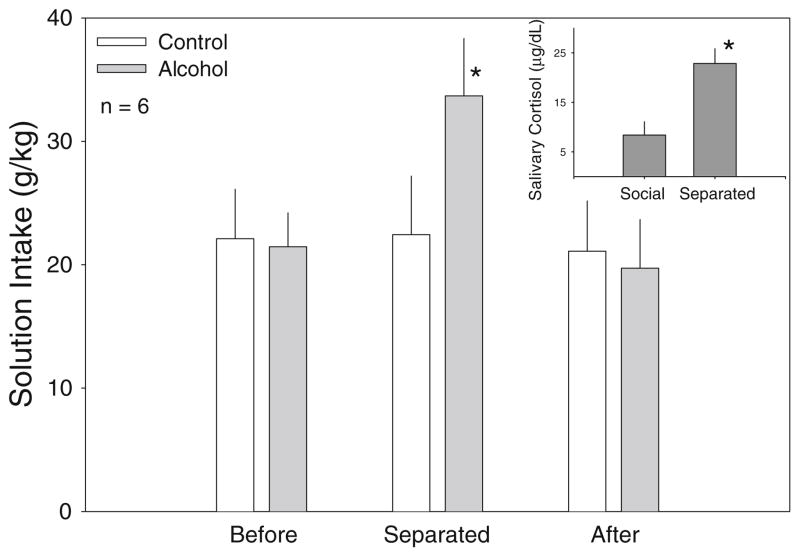
Extended social separation had substantially different effects on male and female drinking, so groups were divided by sex for analysis. One male did not drink either fluid while in the extended separation condition and so was not included in the analysis. At baseline, males drank 22.11± 4.00 g/kg of control fluid and 21.46±2.74 g/kg of alcohol fluid, with an average alcohol intake of 0.43±0.08 g/kg. A two-way repeated-measures ANOVA indicated a significant interaction between social condition and fluid type in male monkeys (F[2, 10] =8.078; p=0.008). Post hoc analysis showed that male subjects drank significantly more alcohol while separated than they did while housed socially both before (p<0.001) and after (p<0.001) extended separation, but control fluid intake was unaffected by social condition. During extended social separation, male monkeys drank significantly more alcohol than control fluid (p=0.03), but there was no difference in intake of the two fluids during social housing (Fig. 3). Female baseline fluid intake was 13.45±4.39 g/kg of control fluid and 28.92±6.67 g/kg of alcohol fluid, with an average alcohol intake of 0.58± 0.13 g/kg. In female monkeys, a two-way repeated-measures ANOVA revealed a significant effect of fluid type on solution intake (F[1,3]=71.424; p =0.003), but no effect of social condition or interaction between the two. Post hoc analysis showed that female monkeys had a distinct preference for alcohol solution over control solution (p=0.003) that did not depend upon social condition.
Fig. 3.
Bars represent the average gram per kilogram intake of control solution (empty bars) and alcohol solution (filled bars) of six male subjects before, during, and after 1 week of social separation. Error bars represent SEM. Asterisks indicate a significant difference from respective level before social separation. Inset shows salivary cortisol level microgram per deciliter of four male subjects while socially housed and during 1 week of social separation. Asterisks indicate a significant change from socially housed level
Salivary cortisol
Salivary cortisol level during social housing was compared to cortisol level during extended social separation by one-way repeated-measures ANOVA, which revealed a significant effect of treatment (F[1, 3] =24.488; p=0.016). A Holm–Sidak post hoc analysis showed that salivary cortisol levels were increased during extended social separation (p= 0.016; Fig. 3, inset).
Discussion
The findings of the current study lend support to both the tension reduction hypothesis of alcohol drinking and the particular relevance of continuous low intensity social stress to drinking in primates (Cloninger 1987; Conger 1956). Both the extended social separation stress and the socially subordinant situation are associated with a preferential increase in alcohol intake. The inverse correlation between social dominance rank and alcohol intake reveals that animals chronically exposed to subordination stress show a concomitant increase in their alcohol intake. While arguments have been made that defense of a dominant position can sometimes be equally or more stressful than occupation of a subordinate rank, the social colonies studied here are very stable, such that dominant animals are rarely required to defend their position (Abbott et al. 2003; Barrett et al. 2002). Meanwhile, the captive situation necessitates that subordinates be in constant contact with higher ranking animals, and although there is rarely severe aggression, subordinates are characterized by continual vigilance and frequent avoidance behaviors.
Previous studies have found that the effects of alcohol on social behavior are different depending upon the dominance status of the alcohol-treated monkey (Winslow and Miczek 1985). Dominant monkeys showed increased aggression after low to moderate doses of alcohol, while the behavior of subordinate animals was relatively unaffected (Winslow and Miczek 1985). The current study reflects another aspect of this interaction that may similarly be moderated by endocrine and neurophysiological differences between dominant and subordinate animals (Shively 1998; Winslow and Miczek 1988). Subject age was found to be positively correlated with alcohol drinking, but not control fluid drinking. Age was not correlated with dominance status, so age represents a separate factor in alcohol drinking. One possible explanation for this finding is that older monkeys tend to have a longer history of alcohol exposure than younger animals, and although individual baseline intake is quite stable over time, monkeys with little alcohol experience tend to be less accepting of alcohol.
Further support for the hypothesis that monkeys may respond to social stress with increased alcohol drinking comes from the finding that extended social separation preferentially increases alcohol intake in male squirrel monkeys. The extended social separation stress may be similar in nature to the stress of social subordinance, as both are continuous psychological stressors that result in no direct tissue damage but have clear behavioral and endocrine effects (Lyons et al. 1999; Sapolsky et al. 1997; Shively 1998; Shively et al. 1997). This type of stress is likely to have very different consequences than more direct physical stressor such as shock or restraint, or even brief, intense, social defeats that have previously shown mostly suppressive effects on alcohol intake in rats (Funk et al. 2005; van Erp and Miczek 2001; van Erp et al. 2001). The study of social separation is complicated by the fact that the isolated condition encompasses several other factors that might contribute to increased drinking. The lack of normal social activity could cause a monkey to drink more simply because it has less to occupy its attention, but this should also cause an increase in control fluid intake, which did not take place in this case. Alternatively, the separation from the social group could potentially increase alcohol drinking by removing the motivation to maintain sobriety to avoid conflict with group members. The current data do not preclude this explanation, but the increase in cortisol during separation supports a stress hypothesis.
Unlike the male monkeys, the females used in this experiment showed no alteration in alcohol intake in response to extended social separation. One explanation for this sex difference is that in this group, two of the four females had an unusually high baseline intake of alcohol, so that on average, the females drank a third more alcohol-containing fluid than the males. In the short, 15-min, access period, this volume difference could point to a ceiling effect in which females could not reasonably consume more than they were already drinking at baseline. However, the two females that had lower baseline intake also showed no increase in their alcohol drinking during isolation. This suggests a possible sex difference in the way in which male and female squirrel monkeys react to extended social separation stress or in their propensity to drink alcohol in response to stress. Sex is also a potentially confounding factor in the drinking and dominance correlation because in mixed-sex squirrel monkey groups, females tend to be lower ranking than males. Female monkeys are frequently used in alcohol experiments, and some sex differences have previously been found in alcohol self-administration (Fahlke et al. 2000; Vivian et al. 2001). Male cynomolgus macaques self-administer more alcohol than females, although no sex difference was found in the discriminative stimulus effects of alcohol and other GABAA ligands in this species (Grant et al. 2000; Vivian et al. 2001). In rhesus macaques, males were also found to self-administer more alcohol than females, but sex did not affect the relationship between early stress and high alcohol intake in adulthood (Fahlke et al. 2000). Alternatively, a second study found that female rhesus exposed to early maternal separation show a higher ACTH response to alcohol than similarly reared males, suggesting an interaction between sex and stress in alcohol responsiveness (Barr et al. 2004a). A more detailed analysis of the behavioral and endocrine response of squirrel monkeys to the separation protocol will help elucidate whether the sex difference lies in the experience of the stress itself or in the alcohol self-administration.
The challenge of inducing squirrel monkeys to voluntarily consume pharmacologically relevant doses of alcohol without food or water deprivation necessitated using a sweetened solution to increase palatability and a non-alcoholic solution to control for sucrose-specific responses (Mandillo et al. 1998). Although both fluids were equally sweetened, the monkeys clearly differentiate between the two with certain animals showing a baseline preference for one fluid or the other, and the extended isolation condition resulting in an increase only in alcohol but not the control fluid intake. These results show that using a sweetened solution enables testing of voluntary alcohol drinking in subjects disinclined to self-administer less palatable alcohol-only solutions.
The findings of the acute colony context test show that social separation causes a much greater reaction in squirrel monkeys than an environmental manipulation that does not alter the social situation. The only effect of colony disruption was a reduction in foraging, while social separation reduced foraging and drinking and increased locomotion and calling, suggesting that for squirrel monkeys, social separation is particularly stressful. Interestingly, the behavioral measures suggest a modest anxiolytic effect of alcohol in which calling behavior was dramatically increased during social disruption, but significantly less so after alcohol than after control fluid. It is not surprising that drinking should be reduced in acute social separation, as the monkeys seem to focus on activities that could reestablish contact with the social group. It has also been previously noted that alcohol drinking tends to take place after exposure to an acute stressor rather than during the stressor (Pohorecky 1990). Humans will sometimes abstain from alcohol, while a stressor is present to avoid impairment under conditions in which a behavioral response may be required (Pohorecky 1991). The monkeys in the acute social separation condition reduced intake of both the alcohol containing and the control solution, suggesting that the reduction in drinking was not only motivated by impairment avoidance. It is possible that if the subjects had access to drinking solutions after, rather than during, the acute separation there may have been a preferential increase in alcohol drinking.
The contrasting effects of extended versus acute separation on drinking take place after several days of isolation housing, suggesting that separation stress evolves over time beginning with a protest reaction that is eventually replaced with an adaptation phase during which alcohol may be used as a coping mechanism. Further data on the time-dependent effects of extended social separation on drinking, behavior, and salivary cortisol will help to clarify the details of this phenomenon (McKenzie-Quirk in prep.).
Acknowledgments
This research was supported by USPHS grant (AA013983) from NIAAA (KAM).
Contributor Information
Sara D. McKenzie-Quirk, Department of Psychology, Tufts University, 530 Boston Ave. (Bacon Hall), Medford, MA 02155, USA
Klaus A. Miczek, Email: klaus.miczek@tufts.edu, Department of Psychology, Tufts University, 530 Boston Ave. (Bacon Hall), Medford, MA 02155, USA, URL: http://www.tufts.edu/sackler/facultyIntros/miczekK.html. Departments of Psychiatry, Pharmacology, and Neuroscience, Tufts University, Medford, MA, USA
References
- Abbott DH, Keverne EB, Bercovitch FB, Shively CA, Mendoza SP, Saltzman W, Snowdon CT, Ziegler TE, Banjevic M, Garland T, Jr, Sapolsky RM. Are subordinates always stressed? A comparative analysis of rank differences in cortisol levels among primates. Horm Behav. 2003;43:67–82. doi: 10.1016/s0018-506x(02)00037-5. [DOI] [PubMed] [Google Scholar]
- Altmann J. Observational study of behavior: sampling methods. Behaviour. 1974;49:227–267. doi: 10.1163/156853974x00534. [DOI] [PubMed] [Google Scholar]
- Anisman H, Waller TG. Effects of inescapable shock and shock-produced conflict on self selection of alcohol in rats. Pharmacol Biochem Behav. 1974;2:27–33. doi: 10.1016/0091-3057(74)90131-2. [DOI] [PubMed] [Google Scholar]
- Ator NA, Griffiths RR. Oral self-administration of triazolam, diazepam and ethanol in the baboon: drug reinforcement and benzodiazepine physical dependence. Psychopharmacology (Berl) 1992;108:301–312. doi: 10.1007/BF02245116. [DOI] [PubMed] [Google Scholar]
- Barr CS, Newman TK, Lindell S, Becker ML, Shannon C, Champoux M, Suomi SJ, Higley JD. Early experience and sex interact to influence limbic–hypothalamic–pituitary–adrenal–axis function after acute alcohol administration in rhesus macaques (Macaca mulatta) Alcohol Clin Exp Res. 2004a;28:1114–1119. doi: 10.1097/01.alc.0000130973.94350.8c. [DOI] [PubMed] [Google Scholar]
- Barr CS, Newman TK, Lindell S, Shannon C, Champoux M, Lesch KP, Suomi SJ, Goldman D, Higley JD. Interaction between serotonin transporter gene variation and rearing condition in alcohol preference and consumption in female primates. Arch Gen Psychiatry. 2004b;61:1146–1152. doi: 10.1001/archpsyc.61.11.1146. [DOI] [PubMed] [Google Scholar]
- Barrett GM, Shimizu K, Bardi M, Asaba S, Mori A. Endocrine correlates of rank, reproduction, and female-directed aggression in male Japanese macaques (Macaca fuscata) Horm Behav. 2002;42:85–96. doi: 10.1006/hbeh.2002.1804. [DOI] [PubMed] [Google Scholar]
- Blanchard RJ, Hori K, Tom P, Blanchard DC. Social structure and ethanol consumption in the laboratory rat. Pharmacol Biochem Behav. 1987;28:437–442. doi: 10.1016/0091-3057(87)90502-8. [DOI] [PubMed] [Google Scholar]
- Blanchard DC, Sakai RR, McEwen B, Weiss SM, Blanchard RJ. Subordination stress: Behavioral, brain, and neuroendocrine correlates. Behav Brain Res. 1993;58:113–121. doi: 10.1016/0166-4328(93)90096-9. [DOI] [PubMed] [Google Scholar]
- Cloninger CR. Neurogenetic adaptive mechanisms in alcoholism. Science. 1987;236:410–416. doi: 10.1126/science.2882604. [DOI] [PubMed] [Google Scholar]
- Conger JJ. Alcoholism: theory, problem and challenge. II. Reinforcement theory and the dynamics of alcoholism. Q J Stud Alcohol. 1956;17:296–305. [PubMed] [Google Scholar]
- Dukelow WR. Reproduction in the squirrel monkeys (Saimiri sciureus) Rec Adv Primatol. 1978;2:195–200. [Google Scholar]
- Fahlke C, Lorenz JG, Long J, Champoux M, Suomi SJ, Higley JD. Rearing experiences and stress-induced plasma cortisol as early risk factors for excessive alcohol consumption in nonhuman primates. Alcohol Clin Exp Res. 2000;24:644–650. [PubMed] [Google Scholar]
- Funk D, Harding S, Juzytsch W, Le AD. Effects of unconditioned and conditioned social defeat on alcohol self-administration and reinstatement of alcohol seeking in rats. Psychopharmacology (Berl) 2005;183:341–349. doi: 10.1007/s00213-005-0194-1. [DOI] [PubMed] [Google Scholar]
- Grant KA, Waters CA, Green-Jordan K, Azarov A, Szeliga KT. Characterization of the discriminative stimulus effects of GABA (A) receptor ligands in macaca fascicularis monkeys under different ethanol training conditions. Psychopharmacology (Berl) 2000;152:181–188. doi: 10.1007/s002130000510. [DOI] [PubMed] [Google Scholar]
- Higley JD, Hasert MF, Suomi SJ, Linnoila M. Nonhuman primate model of alcohol abuse: effects of early experience, personality, and stress on alcohol consumption. Proc Natl Acad Sci USA. 1991;88:7261–7265. doi: 10.1073/pnas.88.16.7261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higley JD, Suomi SJ, Linnoila M. A longitudinal assessment of CSF monoamine metabolite and plasma cortisol concentrations in young rhesus monkeys. Biol Psychiatry. 1992;32:127–145. doi: 10.1016/0006-3223(92)90016-s. [DOI] [PubMed] [Google Scholar]
- Higley JD, Suomi SJ, Linnoila M. A nonhuman primate model of type II excessive alcohol consumption? Part 1. Low cerebrospinal fluid 5-hydroxyindoleacetic acid concentrations and diminished social competence correlate with excessive alcohol consumption. Alcohol Clin Exp Res. 1996a;20:629–642. doi: 10.1111/j.1530-0277.1996.tb01665.x. [DOI] [PubMed] [Google Scholar]
- Higley JD, Suomi SJ, Linnoila M. A nonhuman primate model of type II alcoholism? Part 2. Diminished social competence and excessive aggression correlates with low cerebrospinal fluid 5-hydroxyindoleacetic acid concentrations. Alcohol Clin Exp Res. 1996b;20:643–650. doi: 10.1111/j.1530-0277.1996.tb01666.x. [DOI] [PubMed] [Google Scholar]
- Hopf S, Hartmann-Wiesner E, Kuhlmorgen B, Mayer S. The behavioral repertoire of the squirrel monkey (saimiri) Folia Primatol (Basel) 1974;21:225–249. doi: 10.1159/000155602. [DOI] [PubMed] [Google Scholar]
- Klosterman LL, Murai JT, Siiteri PK. Cortisol levels, binding, and properties of corticosteroid-binding globulin in the serum of primates. Endocrinology. 1986;118:424–434. doi: 10.1210/endo-118-1-424. [DOI] [PubMed] [Google Scholar]
- Kornet M, Goosen C, Ribbens LG, van Ree JM. Analysis of spontaneous alcohol drinking in rhesus monkeys. Physiol Behav. 1990;47:679–684. doi: 10.1016/0031-9384(90)90077-h. [DOI] [PubMed] [Google Scholar]
- Kraemer GW, McKinney WT. Social separation increases alcohol consumption in rhesus monkeys. Psychopharmacology (Berl) 1985;86:182–189. doi: 10.1007/BF00431706. [DOI] [PubMed] [Google Scholar]
- Lynch WJ, Kushner MG, Rawleigh JM, Fiszdon J, Carroll ME. The effects of restraint stress on voluntary ethanol consumption in rats. Exp Clin Psychopharmacol. 1999;7:318–323. doi: 10.1037//1064-1297.7.4.318. [DOI] [PubMed] [Google Scholar]
- Lyons DM, Wang OJ, Lindley SE, Levine S, Kalin NH, Schatzberg AF. Separation induced changes in squirrel monkey hypothalamic–pituitary–adrenal physiology resemble aspects of hypercortisolism in humans. Psychoneuroendocrinology. 1999;24:131–142. doi: 10.1016/s0306-4530(98)00065-1. [DOI] [PubMed] [Google Scholar]
- Mandillo S, Titchen K, Miczek KA. Ethanol drinking in socially housed squirrel monkeys. Behav Pharmacol. 1998;9:363–367. [PubMed] [Google Scholar]
- Miczek KA, Yoshimura H. Disruption of primate social behavior by d-amphetamine and cocaine: Differential antagonism by antipsychotics. Psychopharmacology (Berl) 1982;76:163–171. doi: 10.1007/BF00435272. [DOI] [PubMed] [Google Scholar]
- Pacak K, Palkovits M. Stressor specificity of central neuroendocrine responses: implications for stress-related disorders. Endocr Rev. 2001;22:502–548. doi: 10.1210/edrv.22.4.0436. [DOI] [PubMed] [Google Scholar]
- Pohorecky LA. Interaction of ethanol and stress: research with experimental animals—an update. Alcohol Alcohol. 1990;25:263–276. doi: 10.1093/oxfordjournals.alcalc.a045000. [DOI] [PubMed] [Google Scholar]
- Pohorecky LA. Stress and alcohol interaction: an update of human research. Alcohol Clin Exp Res. 1991;15:438–459. doi: 10.1111/j.1530-0277.1991.tb00543.x. [DOI] [PubMed] [Google Scholar]
- Sapolsky RM, Alberts SC, Altmann J. Hypercortisolism associated with social subordinance or social isolation among wild baboons. Arch Gen Psychiatry. 1997;54:1137–1143. doi: 10.1001/archpsyc.1997.01830240097014. [DOI] [PubMed] [Google Scholar]
- Shively CA. Social subordination stress, behavior, and central monoaminergic function in female cynomolgus monkeys. Biol Psychiatry. 1998;44:882–891. doi: 10.1016/s0006-3223(97)00437-x. [DOI] [PubMed] [Google Scholar]
- Shively CA, Laber-Laird K, Anton RF. Behavior and physiology of social stress and depression in female cynomolgus monkeys. Biol Psychiatry. 1997;41:871–882. doi: 10.1016/S0006-3223(96)00185-0. [DOI] [PubMed] [Google Scholar]
- Spanagel R, Montkowski A, Allingham K, Stohr T, Shoaib M, Holsboer F, Landgraf R. Anxiety: a potential predictor of vulnerability to the initiation of ethanol self-administration in rats. Psychopharmacology (Berl) 1995;122:369–373. doi: 10.1007/BF02246268. [DOI] [PubMed] [Google Scholar]
- Stewart RB, Bass AA, Wang NS, Meisch RA. Ethanol as an oral reinforcer in normal weight rhesus monkeys: dose–response functions. Alcohol. 1996;13:341–346. doi: 10.1016/0741-8329(96)00004-3. [DOI] [PubMed] [Google Scholar]
- Swendsen JD, Tennen H, Carney MA, Affleck G, Willard A, Hromi A. Mood and alcohol consumption: an experience sampling test of the self-medication hypothesis. J Abnorm Psychol. 2000;109:198–204. [PubMed] [Google Scholar]
- Tiefenbacher S, Lee B, Meyer JS, Spealman RD. Noninvasive technique for the repeated sampling of salivary free cortisol in awake, unrestrained squirrel monkeys. Am J Primatol. 2003;60:69–75. doi: 10.1002/ajp.10080. [DOI] [PubMed] [Google Scholar]
- van Erp AM, Miczek KA. Persistent suppression of ethanol self-administration by brief social stress in rats and increased startle response as index of withdrawal. Physiol Behav. 2001;73:301–311. doi: 10.1016/s0031-9384(01)00458-9. [DOI] [PubMed] [Google Scholar]
- van Erp AM, Tachi N, Miczek KA. Short or continuous social stress: suppression of continuously available ethanol intake in subordinate rats. Behav Pharmacol. 2001;12:335–342. doi: 10.1097/00008877-200109000-00004. [DOI] [PubMed] [Google Scholar]
- Vivian JA, Green HL, Young JE, Majerksy LS, Thomas BW, Shively CA, Tobin JR, Nader MA, Grant KA. Induction and maintenance of ethanol self-administration in cynomolgus monkeys (Macaca fascicularis): long-term characterization of sex and individual differences. Alcohol Clin Exp Res. 2001;25:1087–1097. [PubMed] [Google Scholar]
- Weerts EM, Miczek KA. Primate vocalizations during social separation and aggression: effects of alcohol and benzodiazepines. Psychopharmacology (Berl) 1996;127:255–264. [PubMed] [Google Scholar]
- Willinger U, Lenzinger E, Hornik K, Fischer G, Schonbeck G, Aschauer HN, Meszaros K European fluvoxamine in alcoholism study group . Anxiety as a predictor of relapse in detoxified alcohol-dependent patients. Alcohol. 2002;37:609–612. doi: 10.1093/alcalc/37.6.609. [DOI] [PubMed] [Google Scholar]
- Winslow JT, Miczek KA. Social status as determinant of alcohol effects on aggressive behavior in squirrel monkeys (Saimiri sciureus) Psychopharmacology (Berl) 1985;85:167–172. doi: 10.1007/BF00428408. [DOI] [PubMed] [Google Scholar]
- Winslow JT, Miczek KA. Androgen dependency of alcohol effects on aggressive behavior: a seasonal rhythm in high-ranking squirrel monkeys. Psychopharmacology (Berl) 1988;95:92–98. doi: 10.1007/BF00212774. [DOI] [PubMed] [Google Scholar]
- Wolffgramm J, Heyne A. Social behavior, dominance, and social deprivation of rats determine drug choice. Pharmacol Biochem Behav. 1991;38:389–399. doi: 10.1016/0091-3057(91)90297-f. [DOI] [PubMed] [Google Scholar]
- Zumpe D, Michael RP. Dominance index: a simple measure of relative dominance status in primates. Am J Primatol. 1986;10:291–300. doi: 10.1002/ajp.1350100402. [DOI] [PubMed] [Google Scholar]