Abstract
Rationale
Escalated, binge-like patterns of cocaine self-administration are engendered by repeated, intermittent exposure to episodes of social defeat stress, as well as by extended drug access.
Objectives
The present study investigated if prior exposure to brief episodes of social defeat stress would intensify the escalation of cocaine self-administration associated with extended access conditions. The consequences of both stress sensitization and prolonged access were further assessed with progressive ratio (PR) break points and during a 24-h variable dose “binge”.
Methods
Male Long–Evans rats were exposed to four episodes of defeat stress (days 1–4–7–10), and their locomotor response to cocaine was assessed 10 days later. Rats were subsequently implanted with intravenous catheters. After acquisition, stressed and control rats were allowed daily short (1 h/day) or extended (6 h/day) sessions of cocaine self-administration for 14 days (0.75 mg/kg/infusion). In sequence, we determined break points for cocaine on PR tests and assessed drug intake patterns during a 24-h variable dose binge.
Results
Defeat stress induced cross-sensitization to a cocaine challenge, increased break points for cocaine, and produced persistent, escalated cocaine taking during a 24-h binge. Rats with extended access to cocaine—both stressed and controls—similarly escalated their drug intake throughout the 14 days. Extended access conditions accelerated the rate of cocaine self-administration in the first half of the binge, indicated by shorter post-infusion intervals, but failed to amplify the accumulated drug intake in non-stressed controls.
Conclusions
Both social defeat stress and drug access conditions may engender escalated cocaine intake via distinct mechanisms that regulate drug self-administration.
Keywords: Self-administration, Escalation, Sensitization, Stress, Long access, Intravenous
Introduction
Experience with specific environmental conditions may facilitate the transition from a more controlled and stable pattern of drug use towards an escalated, binge-like pattern (Koob and Le Moal 1997; Miczek et al. 2008; Roberts et al. 2007). In rodents, exposure to stressors (i.e., social defeat stress, social isolation, maternal separation, immobilization stress, footshock stress, etc.) and activation of neural and hormonal stress mechanisms can produce behavioral and neurochemical adaptations that render individuals more prone to drug-seeking and drug-taking behaviors (“behavioral and neurochemical sensitization”; see Goeders 2002; Marinelli and Piazza 2002; Miczek et al. 2008; Moffett et al. 2007). Similar to the repeated administration of psychostimulants and other drugs of abuse, repeated exposure to stress can induce augmented sensitivity to drug-induced psychomotor stimulation. In some cases, this sensitized behavioral response is correlated with enhanced drug-induced dopamine and glutamate responses in the nucleus accumbens and increased cellular activation of reward-associated brain regions (Deroche et al. 1995; Miczek et al. 2004; Nikulina et al. 2004; Pacchioni et al. 2002; Rouge-Pont et al. 1995). These results suggest that environmental stressors, similarly to drugs of abuse, can activate and cause long-term changes in the function of brain reward pathways.
Social defeat stress is one of such stressors that can sensitize an individual to psychomotor stimulation and can escalate psychostimulant self-administration (Covington and Miczek 2001, 2005; Kabbaj et al. 2001; Miczek and Mutschler 1996). During a typical, brief social defeat episode, an “intruder” animal is placed into the home cage of an experienced aggressive male resident, where it will be threatened and attacked by the resident until it shows clear signs of submission, usually within minutes of the confrontation. Although the confrontation is a stressful event for both resident and intruder, being associated with increased corticosterone secretion in both, only stressed intruders will engage in escalated patterns of cocaine taking (Covington and Miczek 2005). Repeated, intermittent exposure to brief social defeat stress episodes can produce persistent long-term consequences in rats and mice, that include faster acquisition of drug self-administration (Kabbaj et al. 2001; Tidey and Miczek 1997), increased motivation to obtain drugs on a progressive ratio (PR) schedule of reinforcement, and more accumulation of drug when given a 24-h unlimited access “binge” session (Covington et al. 2008; Covington and Miczek 2001; Covington and Miczek 2005).
Extended access is another condition that can escalate drug intake by allowing rats the opportunity to intravenously self-administer drugs (e.g., cocaine or heroin) for 6 h/day vs. 1 h/day for controls (Ahmed et al. 2000; Ahmed and Koob 1998). Typically, while short-access controls will show very controlled and stable levels of drug self-administration, rats with prolonged access conditions will progressively accelerate and escalate drug intake over the course of two or more weeks (Ahmed and Koob 1998, 1999; Koob and Kreek 2007; Mantsch et al. 2004; Roth and Carroll 2004). As a consequence of escalation, long-access rats are more vulnerable to drug- and footshock-induced reinstatement of drug-seeking behavior (Ahmed and Cador 2006; Ferrario et al. 2005; Knackstedt and Kalivas 2007; Mantsch et al. 2008). However, long-access rats do not consistently show increased breakpoints on a PR test (Liu et al. 2005 vs. Paterson and Markou 2003), and there seems to be a dissociation between the escalation of drug intake and the sensitization of psychomotor responses to the drug (Ahmed and Cador 2006; Knackstedt and Kalivas 2007; Lenoir and Ahmed 2007; but see Ferrario et al. 2005).
Thus, while both stress or drug-induced sensitization and extended drug access conditions can promote escalated drug intake, these two factors may do so via different mechanisms. Interactions between the two factors have been shown. After repeated i.p. administration of amphetamine, rats showing a sensitized response to amphetamine showed a more rapid escalation of cocaine intake when self-administering cocaine under prolonged access conditions (Ferrario and Robinson 2007). Furthermore, Mantsch and Katz (2007) were able to produce an escalation of cocaine-taking behavior by presenting rats with footshock stress prior to the self-administration sessions, even though these sessions were limited to 2 h (and not 6 h).
In the present study, we investigated whether prior exposure to intermittent, brief episodes of social defeat stress would amplify the escalation of cocaine self-administration associated with extended access conditions to the drug. We also determined the consequences of both stress sensitization and prolonged access on the motivation to obtain cocaine as assessed by PR break points, and on cocaine intake patterns during a 24-h variable dose “binge”.
Materials and methods
Subjects
Fifty male Long–Evans hooded rats from Charles River Laboratories (Wilmington, MA, USA), weighing 225–250 g at arrival, were the experimental subjects of this study. They were individually housed in customized acrylic chambers (30×30.5×24 cm) and were acclimated for 1 week before the beginning of the experiment. The chambers contained wire mesh panels as side walls, and the floor was covered with Cellu-Dri pellet bedding (Shepherd Specialty Papers, Kalamazoo, MI, USA), which was replaced every 5–7 days. They were housed in a vivarium with controlled temperature (21±1°C) and humidity (35–40%) in a reversed light cycle (lights on from 2000 to 0800 hours) and had free access to food and water during all the phases of the experiment. All rats were weighed and handled daily after arrival.
Additional experienced aggressive Long–Evans male rats (“residents”, n=10) were housed in pairs with females in an adjacent room, under similar controlled conditions and free access to food and water. They were housed in large custom-built stainless steel and acrylic chambers (45.7×71.1×45.7 cm), which were lined with sawdust bedding material. The residents served as the aggressive stimulus animals for the social defeat episodes. All experimental procedures were approved by the Tufts University Institutional Animal Care and Use Committee and followed the Guide for the Care and Use of Laboratory Animals (National Research Council 1996).
Social defeat stress
Each brief social defeat episode consisted of three phases, which began by removing the female from the large “resident” chamber and placing the home cage of the experimental rat (“intruder”) in the large chamber, for 10 min. During this initial phase, the intruder is protected from attacks, but the wire mesh walls of the cage allow for social interactions and species-typical threats from the male aggressive resident, serving the function of instigation and provocation (Covington and Miczek 2001; Fish et al. 1999). Secondly, the intruder was then removed from its protective cage and placed directly into the resident’s chamber for the confrontation, which lasted no more than 5 min. The defeat was defined as the display of supine posture for five consecutive seconds by the intruder, a response that typically occurs after three to five biting attacks from the resident. Once the defeat was unambiguously observed, the confrontation was terminated, and in the third and final phase, the intruder was immediately returned to its home cage, which was again placed into the larger chamber for another 10 min to allow for social threats by the resident. Socially defeat-stressed animals (n=25) were exposed to four episodes of social defeat, on days 1, 4, 7, and 10 (Tornatzky and Miczek 1993), as shown in the timeline in Fig. 1, and were weighed and handled daily. Non-stressed controls (n=25) were only weighed and handled daily.
Fig. 1.
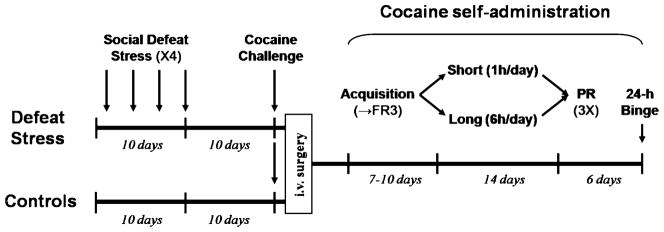
Experimental timeline
Assessment of locomotor sensitization to cocaine
Ten days after the last defeat episode (experimental day 20), defeat-stressed and non-stressed controls were challenged with an i.p. administration of cocaine (10 mg/kg) to test for the expression of behavioral cross-sensitization, as previously reported (Covington et al. 2008; Covington and Miczek 2001, 2005). Rats were weighed and moved to an adjacent room within their home cages and received an initial administration of saline (0.1 ml/100 g; i.p.), followed 15 min later by a cocaine administration (10 mg/kg; i.p.). Behavioral activity was monitored in the home cage by video recordings, in 5-min samples. Behavioral analyses of each video record were carried out by a trained observer using a customized keyboard and commercially available software (The Observer, Noldus Information Technology, Wageningen, The Netherlands). The frequency and duration of walking, rearing, grooming, sniffing, digging, and inactivity were recorded for 5–10 min after the saline administration, and 5–10 and 25–30 min after the cocaine administration.
Surgery for implantation of intravenous catheters
In the 4 days after the cocaine challenge, rats were permanently implanted with intravenous catheters into the right jugular vein under sterile procedures, using a combination of ketamine HCl (100 mg/kg, i.p.) and xylazine (6 mg/kg, i.p.) anesthesia. One end of the silastic catheter (Silastic® silicon tubing, ID 0.63 mm, OD 1.17 mm) terminated into the opening of the right atrium, and the other end was passed subcutaneously to the rat’s back where it exited through a small incision between the scapulae and was affixed to a plastic pedestal (Plastics One, Roanoke, VA, USA) mounted inside a harness system (Instech Laboratories, Plymouth Meeting, PA, USA). After surgery, each rat was allowed to recover for 5–6 days, and only body weight was monitored during this time.
Cocaine self-administration
In a separate room, each intravenous self-administration chamber was placed inside a larger enclosure, equipped with a ventilation fan and a house light. The chamber was identical to the home cage, except that the wire mesh panels on the walls were substituted with acrylic panels, one of which contained two retractable levers (Med Associates Inc.) and two cue lights. A red cue light above the active lever signaled drug delivery, and a green light in the middle of the panel signaled drug availability. Holes in the ceilings of the experimental chamber and the enclosure allowed for the connection between the rat’s catheter with an infusion line, single channel swivel, and drug syringe, as previously described (Covington and Miczek 2005; Miczek and Mutschler 1996). Each morning before the start of the self-administration session, each rat was weighed and flushed with sterile heparinized saline (20 IU/ml) before being tethered to the drug infusion system. Whenever cocaine was not available, sterile 0.17 ml saline pulses were delivered through the infusion line every 30 min, to help maintain catheter patency.
Acquisition of cocaine self-administration
For acquisition of self-administration behavior, rats were reinforced with a 0.75-mg/kg cocaine infusion after each lever press (fixed ratio schedule of reinforcement; FR), with a 30-s time-out period following the infusion. No drug priming was used in any phase of the experiment. After completing 15 cocaine infusions within 5 h for two consecutive days, the FR requirement was gradually increased to FR3. Rats were maintained on FR3 for 4 days, with each session being terminated when 15 cocaine infusions were obtained. When rats did not begin lever pressing during the first two daily sessions, female rat urine or palatable powdered food (e.g., Fruit Loops) was applied on top of the active lever to attract the animals (for approximately 30% of animals). Once animals started pressing the active lever, the lever was wiped clean at the end of the session, and no more urine or food was applied thereafter.
Escalation of cocaine self-administration
After acquisition, rats from both groups (defeat-stressed and controls) were randomly assigned to one of two conditions: short access (1 h/day) or prolonged access (6 h/day) to cocaine (0.75 mg/kg/infusion, according to a FR3 schedule of reinforcement), for 14 days (see Fig. 1). During these sessions, there was no limitation in the number of infusions, but only in the duration of the daily self-administration session (1 vs. 6 h), as initially described by Ahmed and Koob (1998). The extended access condition is used to induce progressive increases (escalation) of cocaine intake across 14 days of self-administration. One rat was lost during the escalation phase due to catheter patency failure, and its data were not included in the analysis.
Determination of break points on a progressive ratio schedule of reinforcement
After 14 days of the escalation phase, three test probes were scheduled in alternating days, in which rats self-administered 0.3 mg/kg cocaine/infusion according to a PR schedule of reinforcement. A lower dose of cocaine was used as an optimal dose to reveal subtle changes in the reinforcing effects of cocaine, as previously described (Covington et al. 2008; Covington and Miczek 2005). Under the PR schedule, the response requirement was progressively increased after each successive cocaine infusion, using an algorithm developed by Richardson and Roberts (1996): 1, 2, 4, 6, 9, 12, 15, 20, 25, 32, 40, 50, 62, 77, 95, 118, 145, 178…. The PR session was terminated when the rat did not meet the response requirement in order to be reinforced with an infusion within 60 min, and the break point was defined as the final infusion delivered in the session. For each rat, the average number of total infusions accumulated across the three PR tests was calculated as the dependent variable (Hodos 1961). The PR test sessions were alternated with FR3 sessions, in which the rats were allowed to self-administer cocaine (0.75 mg/kg/infusion, FR3) for the duration of their previous access condition (1 vs. 6 h/day for short vs. prolonged access, respectively).
Variable dose binge
After completing one additional FR3 session subsequent to the last PR session, rats had access to cocaine for a final, 24-h variable dose binge protocol (Gerber and Wise 1989). The 24-h session started ca. 2 h into the dark phase (approximately at 10:00), and the dark/light cycle was regularly maintained throughout the session, with lights on at 20:00, and off again at 8:00. In the variable dose protocol, rats self-administered three different doses of cocaine (0.2, 0.4, and 0.8 mg/kg/infusion) that were randomly selected within each block of 15 infusions. Accordingly, each block of infusions offered five infusions at each dose, and each dose could be repeated no more than five times per block. As previously described (Covington et al. 2008), the different doses were achieved by changing the duration of the infusion, since the cocaine concentration in the syringe remained constant throughout the binge (5.0 mg/ml cocaine). As dependent measures for the variable dose binge, the amount of cocaine accumulated, number of infusions, post-infusion intervals for each unit dose, and patterns of responding were analyzed.
Forty-one rats completed the entire protocol. Two rats died as a consequence of anesthesia, resulting in 48 animals for the self-administration procedures. Forty-seven animals completed the 14-day escalation phase, 46 completed the PR tests, and 41 rats were assessed during a 24-h binge. Animals dropped out of the study due to death or severe health problems (four) or to catheters with no patency (three).
Statistical analyses
Cross-sensitization to cocaine
Behavioral sensitization was assessed by comparing the frequency of walking behavior after the initial saline injection and after the subsequent cocaine administration (averaged from min 5–10 to 25–30), between treatment groups (defeat stressed vs. controls) using a two-way repeated measures ANOVA. Walking behavior was chosen as the optimal parameter to detect sensitized psychomotor responses, as previously reported (e.g., Covington and Miczek 2001, 2005). Other behaviors (grooming, rearing, sniffing, digging, and inactivity) were also analyzed with two-way ANOVAs.
Cocaine self-administration
To determine whether or not social defeat stress influenced the escalation of cocaine self-administration in rats given extended drug access (6 h/day), the daily average number of infusions throughout the 14-day period was compared by a three-way ANOVA with days as a repeated measure, and treatment group (stress vs. control) and access conditions (short vs. extended) as main factors. Break points, or the maximum number of infusions obtained on each PR trial, were averaged for each rat throughout the three tests. Break points were compared using a two-way ANOVA with group and access condition as the main factors. Similarly, two-way ANOVAs were used to determine differences in the total amount of accumulated cocaine intake (milligram per kilogram) during the 24-h variable dose binge, as well as the duration of active self-administration throughout the binge (in hours). Typically, a post-infusion interval >60 min indicates that the animal has stopped cocaine-taking behavior, and the last infusion before that interval represents the end of the period of active self-administration. Rarely will rats resume drug taking after that point (no more than ten infusions). The patterns of cocaine self-administration during the 24-h binge were further examined in the number of infusions obtained in 2-h time bins. A three-way ANOVA with repeated measures was carried out with treatment group (stress vs. control), access condition (extended vs. short) and time bins (12 2-h bins). Finally, post-infusion intervals for the three doses of cocaine available during the variable dose binge (0.2, 0.4, and 0.8 mg/kg/infusion) were compared by a three-way ANOVA with group, access, and cocaine doses as the repeated measure. Post-infusion intervals >60 min were ignored for these measurements.
In all analyses, significant effects were detected when p< 0.05, and post hoc comparisons were carried out using more specific ANOVAs (e.g., when analyzing days or time bins during the binge or post-infusion intervals for a particular cocaine dose) and Tukey’s Honestly Significant Different tests for unequal group sizes.
Results
Cross-sensitization between social defeat stress and cocaine
When challenged with cocaine 10 days after the last defeat episode, stressed rats showed augmented cocaine-induced hyperactivity relative to non-stressed controls, as measured by increases in the rate of walking behavior (p<0.001). The cocaine challenge (10 mg/kg) induced locomotor stimulation in both stressed and control rats when compared to their behavioral response to saline (p<0.01), as depicted in Fig. 2a. A two-way ANOVA detected a main effect of social defeat stress (F(1, 48)=21.2, p<0.01) and a significant interaction between stress and drug administration (saline vs. cocaine; F(1, 48)=8.5, p<0.01). The stress effect was significant at both time points after the cocaine challenge (5–10 and 25–30 min after injection; time course not shown), but there were no significant group differences after the initial saline injection. There were no group differences or group × drug interactions in rearing, grooming, or digging behaviors (data not shown). Exposure to social defeat stress increased sniffing behavior (F(1, 48)=13.5, p<0.01), with no interaction with drug challenge. Inactivity was not further analyzed because these data failed normality and equal variance tests, probably due to very low counts and many animals with zero counts (all group averages were below 1 count/min in all time points).
Fig. 2.
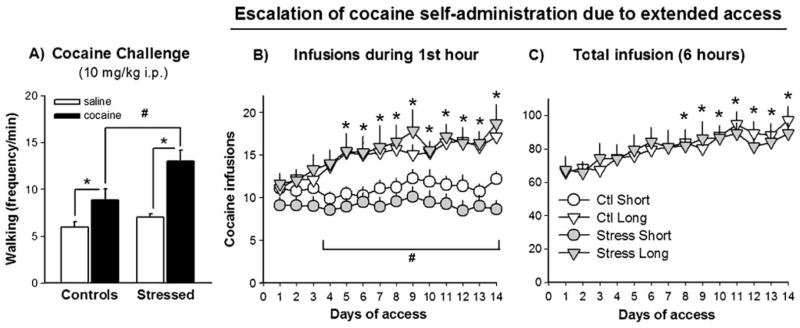
a Walking rate (in frequency per minute) in response to a cocaine challenge in stressed and control rats, as compared to the response to a saline challenge. Stressed animals showed augmented locomotor response to cocaine relative to control animals (#p<0.01), and to their own saline baseline (*p<0.05). Control rats showed psychomotor stimulation after the cocaine injection relative to their response to saline (*p<0.05). b Number of cocaine infusions (0.75 mg/kg/infusion) obtained in the first hour of each self-administration session by control and social defeat stress rats that were allowed 1 h (short) vs. 6 h (long) of daily cocaine access for 14 days. The bracket represents days in which there were significant effects of access conditions on cocaine self-administration (#p<0.05). Within-group escalation of cocaine intake was observed from days 5 to 14 relative to day 1 (*p<0.01). c Total number of cocaine infusions (0.75 mg/kg/infusion) obtained by rats with extended access to cocaine in daily 6-h sessions. Within-group escalation of cocaine intake was observed from days 8 to 14 relative to day 1 (*p<0.01)
Escalation of cocaine self-administration due to prolonged drug access
Rats acquired lever-pressing behavior within two to six daily sessions. There were no apparent differences in acquisition rate between control and stressed rats. Since lever-pressing responding was facilitated in animals that did not acquire after two daily sessions, further analyses of acquisition results are not appropriate in this study. Once rats were lever pressing for cocaine on a FR3 schedule of reinforcement for 4 days, they were assigned to short (1 h/day) or long (6 h/day) access conditions to cocaine. Between-group comparisons reveal that from days 3 to 14, rats with prolonged access to cocaine self-administered more infusions than short-access animals in the first hour of the session (Fig. 2b). Across the 14 days of 6-h long sessions of cocaine self-administration, there were significant effects of access condition (F(1, 43)=21.4, p< 0.001), days (F(13, 559)=6.7, p<0.001), and access × days interaction (F(13, 559)=4.7, p<0.001), but no group differences between stressed and control rats. Within-group comparisons show that starting from day 5, long-access rats escalated the number of cocaine infusions obtained in the first hour of the session when compared to day 1 (F(13, 273)=7.2, p<0.001), whereas short-access animals did not change their intake across 14 days (F(13, 312)=1.2). Considering the number of infusions during the 6-h sessions, rats with prolonged drug access showed increases in total cocaine intake from days 8 to 14 in relation to day 1 (F(13, 260)= 8.8, p<0.001), as shown in Fig. 2c. Inactive lever responding was recorded but there were no effects of group or access condition on inactive lever presses, which were minimal after acquisition of cocaine self-administration (data not shown).
Break points on a progressive ratio schedule of cocaine reinforcement
When self-administering cocaine according to a PR schedule of reinforcement, socially defeated rats completed higher ratios of responding to obtain cocaine infusions when compared to controls (Fig. 3a). There were significant effects of social defeat stress (F(1, 42)=6.67, p<0.01), but no effects of access conditions (F(1, 42)=0.22) nor interactions between these two factors (F(1, 42)=1.11) on the break points. Unprotected Tukey post hoc tests were then used to further explore the stress effect in short- vs. long-access rats and revealed that this difference was only significant when break points achieved by the socially stressed group with prolonged drug access were compared with data from the control group with long (p<0.02), but not short access.
Fig. 3.
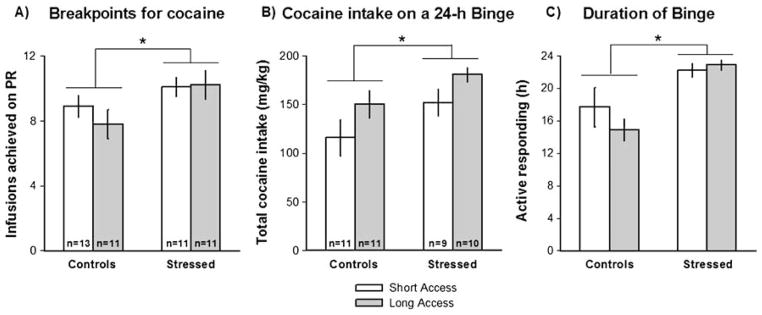
Control and social defeat stressed rats were allowed to self-administer cocaine for 14 days in short (1 h) or long (6 h) daily sessions and were subsequently assessed for cocaine self-administration patterns with progressive ratio (PR) tests (a) or a 24-h variable dose binge (b, c). a Cocaine infusions (0.3 mg/kg/infusion) obtained during a PR schedule of reinforcement by control and stressed rats. b Total cocaine accumulated during a 24-h variable dose binge (in milligram per kilogram), with three different cocaine doses available in randomized order (0.2, 0.4, and 0.8 mg/kg/infusion). c Duration of active cocaine self-administration during a 24-h variable dose binge (in hours). In both a, b, and c, stress significantly increased break points for cocaine and cocaine self-administration (*p<0.05)
Patterns of cocaine self-administration during a 24-h variable dose binge
Throughout the 24-h variable dose binge, socially defeated rats showed escalation of cocaine intake, as revealed by higher number of infusions and total amount of cocaine (in milligram per kilogram) accumulated relative to non-stressed controls (main effect of stress: F(1, 37)=4.2, p<0.05; Fig. 3b). A main effect of stress was also verified on the persistence of cocaine-taking behavior, as shown by a longer duration of active self-administration in the 24-h session (F(1, 37)=15.3, p<0.001). As shown in Fig. 3c, defeat-stressed rats were actively lever pressing for cocaine for ca. 22–23 h, while controls typically stopped self-administering after 15–17 h.
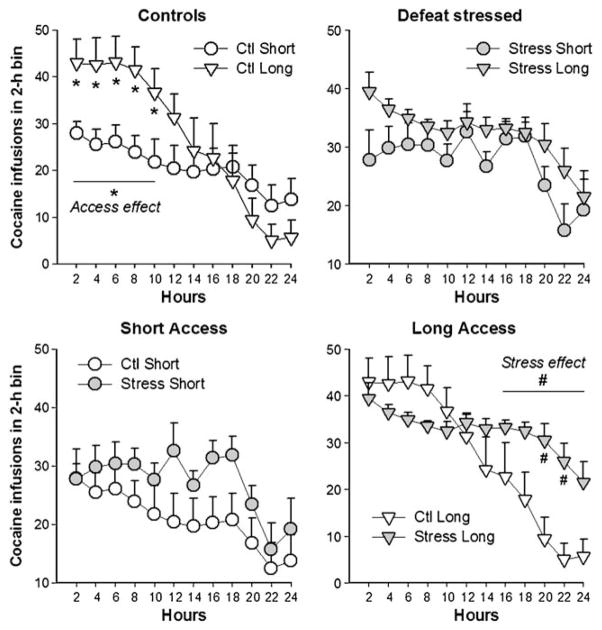
When the pattern of cocaine self-administration was further inspected using number of infusions obtained in 2-h bins, there were clear time-dependent changes induced by each different manipulation, as revealed by an interaction between stress, access condition, and time bins (F(11, 407)=3.1, p< 0.001). Figure 4 shows that the prolonged access condition was a major determinant in promoting escalated cocaine intake in the initial 10 h of the binge (main effects of access condition on each 2-h bin until hour 10, p<0.01). This effect was particularly important in control groups, in which rats with a history of escalation obtained more infusions than the non-escalated controls in hours 0–10 of the binge. In the groups with a history of social defeat stress, there was only a non-significant trend for escalated rats to self-administer more cocaine than for short-access rats in the initial 10 h of the binge. In the final 10 h of the binge, however, the effects of social defeat stress became the major determinant of cocaine self-administration. Defeated rats obtained more cocaine infusions than controls during each 2-h bin between hours 14 and 24, and this effect was more prominent when comparing long-access stressed rats to long-access controls in time bins 18–20 and 20–22 h (Fig. 4, p<0.001). When accumulated cocaine infusions were analyzed in the final 10 h of the binge, there was a main effect of stress (F(1, 37)=15.3, p<0.001), wherein both stressed short- and long-access rats took more cocaine relative to controls (p<0.05).
Fig. 4.
Patterns of cocaine self-administration during a 24-h variable dose binge, in which three doses of cocaine were randomly available (0.2, 0.4, and 0.8 mg/kg/infusion). Data are portrayed as average number of cocaine infusions in 2-h bins (+SEM) during the 24-h session. Top panels highlight comparisons of access conditions (short vs. long access) in controls (Ctl, top left) vs. defeat-stressed rats (top right). Bottom panels highlight differences promoted by social defeat stress in animals with short (1 h/day; bottom left) vs. long (6 h/day; bottom right) access conditions to cocaine. In the initial part of the 24-h binge, animals with a history of extended access (“long”) showed increased cocaine self-administration, particularly in control groups (*p<0.05). Defeat stress promoted overall escalated cocaine self-administration during the 24-h binge, particularly due to persistent active self-administration throughout the second half of the binge (#p<0.05). Stressed rats accumulated significantly more cocaine than controls, particularly in long-access groups (#p<0.05)
Post-infusion intervals for the three different doses of cocaine available were calculated to assess behavioral regulatory mechanisms of drug intake throughout the 24-h binge. Overall, both stressed and control rats that had developed escalated cocaine taking during prolonged access conditions showed reduced post-infusion intervals for the two higher cocaine doses, 0.4 and 0.8 mg/kg/infusion, when compared to short-access groups (Fig. 5). For the post-infusion intervals, the three-way ANOVA with repeated measures detected a main effect of access condition (F(1, 36)=7.58, p<0.01), cocaine doses (F(2, 72)=466.1, p<0.001), and a significant interaction between stress × access condition ×cocaine dose (F(2, 72)=3.2, p< 0.05). When post-infusion intervals were analyzed in the initial part of the binge (first 10 h), similar results were obtained as with the whole 24-h binge. In the final 10 h of the binge, a significant interaction between stress and cocaine doses was detected (F(2, 60)=4.01, p<0.05), but post hoc comparisons failed to identify these differences. Further analyses may have been compromised due to the fact that some control rats had already stopped lever pressing in the final period of the binge (two control short access; four control long access).
Fig. 5.
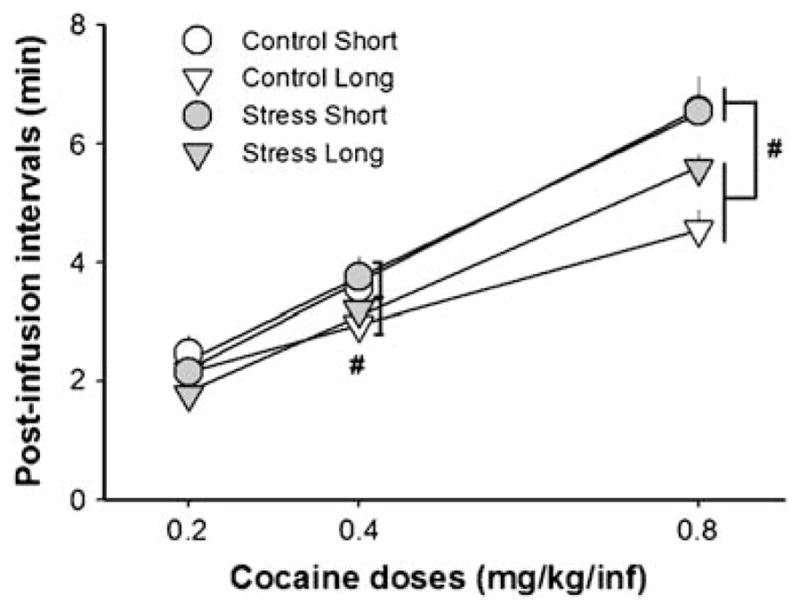
Post-infusion intervals (minute) for three different cocaine doses that were available during a 24-h variable dose binge. Rats with a history of extended access to cocaine (6-h sessions; “long”) showed shorter post-infusion intervals relative to those with a history of short access (1-h sessions), for the two higher cocaine doses, 0.4 and 0.8 mg/kg/infusion (#p<0.05). Results show average post-infusion intervals for each dose during the whole 24-h period (intervals longer than 60 min were excluded from analyses)
Discussion
The procedures used in the present study to induce escalated drug taking in rats can be associated with relevant features of addictive-like behaviors in humans. The escalation of drug taking due to extended access conditions (Ahmed and Koob 1998) can be associated with increases in frequency and/or amount of drug consumed over time, commonly observed in addicts (Gawin 1991; Gawin and Kleber 1988). Secondly, the association of stress with increased vulnerability to drug abuse and escalated drug intake has been of clinical concern. Post-traumatic stress disorders, physical and/or sexual abuse, harassment, and psychosocial adversity have all been associated with increased risk for drug abuse (for reviews, see Sinha 2001, 2008; Goeders 2003). Furthermore, the sensitizing effects of brief, intermittent social defeat episodes in rats, as in drug sensitization, may confer increased salience of drug effects and drug-predictive cues, thus promoting increased drug-seeking and drug-taking behaviors (Robinson and Berridge 1993, 2000).
Escalation of cocaine self-administration due to extended access
Typically, extended access conditions will produce acceleration of drug self-administration, particularly in the first hour of the session, and significant escalation effects are noticed within a few days of extended drug access (Ahmed and Cador 2006; Ahmed and Koob 1998; Liu et al. 2005; Mantsch et al. 2004). The increased rate of cocaine self-administration in rats with a history of escalation was further demonstrated in the present study by reduced post-infusion intervals for the two higher cocaine doses available during the 24-h binge. Rats with extended access to cocaine will wait shorter periods before self-administering the next drug infusion—both during the 6-h sessions (e.g., Liu et al. 2005), as well as during a 24-h binge (present study).
The escalation of drug intake due to extended access conditions promotes long-term behavioral and neurobiological changes in neural circuits which have been proposed to contribute to the transition to an addictive-like state (Ahmed et al. 2002; Ferrario et al. 2005; Mantsch et al. 2004; Oleson et al. 2009). Rats with a history of drug escalation are more sensitive to drug- and foot shock stress-induced reinstatement of cocaine-seeking behavior, suggesting an increased vulnerability to resume drug seeking in escalated animals (Ahmed and Cador 2006; Ferrario et al. 2005; Knackstedt and Kalivas 2007; Mantsch et al. 2008). In the present study, however, a history of escalation of cocaine intake failed to promote increases in the motivational effects of a low cocaine dose as measured by break points on a PR schedule of reinforcement (as in Liu et al. 2005; Oleson and Roberts 2009, but different from Paterson and Markou 2003; Wee et al. 2008). Furthermore, extended access conditions per se also failed to amplify accumulated cocaine intake during an unlimited, 24-h binge session. Despite the initial increased rate of cocaine self-administration during the first 10 h of the binge, most control rats with a history of escalation stopped lever pressing after 15–16 h, which prevented further drug accumulation, especially when compared to short-access controls (as reflected by increased number of infusions and shorter post-infusion intervals during the initial part of the binge).
Escalation of cocaine self-administration due to social defeat stress
The present study further confirmed the effects of brief social defeat stress in promoting cross-sensitization to the stimulant effects of a psychostimulant challenge, increasing break points for a cocaine infusion on a PR test, and in escalating cocaine intake during a 24-h variable dose binge (Covington et al. 2008; Covington and Miczek 2001, 2005; Kabbaj et al. 2001; Tornatzky and Miczek 2000). The consequences of defeat stress on cocaine intake during the 24-h binge seem to be due to an enduring persistence in drug self-administration relative to controls, as previously reported (Covington and Miczek 2001; Tornatzky and Miczek 2000). Socially defeated rats, in both short- and long-access subgroups, averaged 22 h of active cocaine taking (vs. 15–16 h in controls), permitting accumulation of very large amounts of cocaine. Due to the persistence in drug-taking behavior, the effects of stress on the time course of cocaine infusions obtained throughout the 24-h binge were particularly important during the second half of the session, when compared to control groups. From hours 14–24, rats with a history of social defeat stress accumulated significantly higher amounts of cocaine when compared to non-stressed control rats.
The effects of stress on cocaine self-administration have been typically attributed to stress-induced sensitization of brain reward mechanisms that control drug-related effects (see Marinelli and Piazza 2002; Miczek et al. 2008). As a consequence of drug- or stress-induced sensitization, an augmented psychomotor response after experimenter-administered drug injection is observed (Covington and Miczek 2001). Furthermore, increased break points on a PR schedule of reinforcement, as well as persistent, escalated patterns of cocaine self-administration during a 24-h cocaine binge, have been attributed to a sensitization of drug-reward mechanisms promoted by repeated defeat stress or drug administration (e.g., Covington and Miczek 2001, 2005; Covington et al. 2008; Vezina 2004).
Interactions between social defeat stress and extended access to cocaine
A previous history of repeated amphetamine exposure—which induced sensitization to the locomotor stimulant effects of amphetamine—facilitated the escalation of cocaine self-administration due to extended access conditions in rats (Ferrario and Robinson 2007). Amphetamine-sensitized rats rapidly escalated cocaine-taking behavior, which was observed as early as during the second day of extended access. In the present study, defeat stress also induced locomotor cross-sensitization to a cocaine challenge. However, we found no indication that stress sensitization facilitated or accelerated the escalation of cocaine self-administration due to extended access to the drug. While this observation could be due to different consequences of drug vs. stress sensitization on escalation, it is very likely that other methodological differences could have contributed. For example, the schedule of food restriction (to 85% of body weight) used by Ferrario and Robinson (2007) throughout all drug self-administration procedures may have served as a stressor, which in combination with a history of amphetamine exposure may have further facilitated the escalation of cocaine taking (see Cabib et al. 2000; Goeders 2002; Marinelli and Piazza 2002).
Exposure to foot shock stress immediately prior to each cocaine self-administration session promoted escalation of drug intake even in the absence of extended access conditions (Mantsch and Katz 2007). In that study, exposure to stress in close temporal proximity to drug access (5 min before the self-administration sessions) amplified drug taking of rats with access to cocaine in 2-h daily sessions—a condition that usually produces stable, non-escalated patterns of drug self-administration (Mantsch and Katz 2007). Exposure of rats to social stress immediately prior to a self-administration session also increased the rate of responding that is reinforced by cocaine (Miczek and Mutschler 1996). In the present study, the last exposure to social defeat stress occurred at least 2 weeks before acquisition of cocaine self-administration. While the pattern of cocaine escalation was similar in stressed and control rats with extended access conditions, long-term effects of social defeat stress were detected using different parameters of drug-taking behavior, as with increased break points in PR tests, and with more persistent and increased cocaine taking during a 24-h variable dose binge (Covington et al. 2008; present study). Thus, both temporal relations as well as stressor specificity may be critical factors in determining which particular aspects of drug self-administration are affected as a consequence of stress (Miczek et al. 2008).
In a recent study using behavioral economic analyses, Oleson and Roberts (2009) suggest a dissociation between the maximum “price” expended to obtain cocaine on PR or FR schedules from the actual amount of cocaine taken across a range of cocaine doses. While rats that escalated their final ratios after repeated PR sessions exert themselves more (i.e., are willing to “pay” a higher unit-price) for a drug infusion, rats with escalated consumption promoted by extended access on FR1 schedule do not (Oleson and Roberts 2009). Our study also suggests a somewhat similar dissociation, in which social defeat stress—but not extended access conditions—increased the reinforcing effects of cocaine as measured by the PR tests (e.g., Hodos 1961; Richardson and Roberts 1996), as well as increased the time spent taking cocaine during the 24-h binge session. One could speculate that stress sensitization and sensitization of break points (as in Morgan and Roberts 2004) may affect common regulatory mechanisms of drug self-administration to promote increased drug-seeking and drug-taking behaviors (Oleson and Roberts 2009; present study).
It is also remarkable that during the 24-h binge session, the initially accelerated rate of drug self-administration that seems to be associated with a history of long-access conditions disappears as differences emerge between long-access controls and stressed groups in the second half the of binge (see Fig. 4). Despite showing an increased rate of cocaine self-administration throughout the initial 10 h of the binge relative to short-access controls (as reflected by increased number of infusions and shorter post-infusion intervals), most control rats with a history of escalation stopped lever pressing within 15–16 h. Only stressed long-access rats continued to self-administer throughout most of the 24-h binge session, showing a different pattern of cocaine responding and accumulation relative to long-access controls.
Periods of drug withdrawal have been reported to affect motivational and consummatory aspects of drug seeking and drug taking in several conditions (see reviews by Lu et al. 2004 and Morgan and Roberts 2004; Grimm et al. 2001; Sorge and Stewart 2005). Morgan and Roberts (2004) suggest that a reduction in break points can be obtained when rats are tested 1 day after a 24-h cocaine binge, an effect that vanished after three to seven drug-free days (withdrawal). However, after escalation of cocaine self-administration, Liu et al. (2005) reported no changes in break points for cocaine when animals were assessed 1 day or 7 days after the last extended access session. Based on that study, it seems unlikely that our break point results would be critically modified by using a longer drug-free period. Concerning the 24-h binge session, it is possible that a longer drug-free period would affect the patterns and amount of cocaine accumulated. However, it was not within the scope of the current study to determine such effects, which are amenable for future investigation.
Overall, the present study suggests that social defeat stress and extended access conditions both promote escalated cocaine self-administration, by affecting distinctively different regulatory mechanisms of cocaine-taking behavior. The escalation due to extended access seems to be due to an accelerated rate of drug self-administration, apparent throughout the 6-h sessions as well as in the first half of the 24-h binge. The most prominent effects of social stress sensitization, on the other hand, are evident as the enduring and persistent responding for cocaine, which is particularly striking when animals are assessed for PR break points or are allowed to self-administer for prolonged periods as during the 24-h binge. Due to the increased persistence in drug-taking behavior, defeat-stressed animals accumulated more cocaine than controls during the binge—even when compared to controls with a history of extended access. Further characterization of these regulatory mechanisms of drug self-administration, as well as their underlying neurobiological substrates, may provide pharmacotherapeutic targets that may be useful for different types of addictive behaviors in humans.
Acknowledgments
This research was supported by NIDA research grant DA02632 (KAM, PI). We thank Mr. J. Thomas Sopko for outstanding technical assistance, and Jonaton Jang and Fabio C Cruz who assisted with experimental procedures.
Contributor Information
Isabel M. H. Quadros, Email: isabel.quadros@tufts.edu, Department of Psychology, Tufts University, 530 Boston Ave (Bacon Hall), Medford, MA 02155, USA. Center for Neuroscience Research, Department of Neuroscience, Tufts University, 136 Harrison Ave (ST331), Boston, MA 02111, USA
Klaus A. Miczek, Department of Psychology, Tufts University, 530 Boston Ave (Bacon Hall), Medford, MA 02155, USA. Department of Neuroscience, Tufts University, Boston, MA, USA. Department of Psychiatry, Tufts University, Boston, MA, USA. Department of Pharmacology and Experimental Therapeutics, Tufts University, Boston, MA, USA
References
- Ahmed SH, Cador M. Dissociation of psychomotor sensitization from compulsive cocaine consumption. Neuropsychopharmacology. 2006;31:563–571. doi: 10.1038/sj.npp.1300834. [DOI] [PubMed] [Google Scholar]
- Ahmed SH, Koob GF. Transition from moderate to excessive drug intake: change in hedonic set point. Science. 1998;282:298–300. doi: 10.1126/science.282.5387.298. [DOI] [PubMed] [Google Scholar]
- Ahmed SH, Koob GF. Long-lasting increase in the set point for cocaine self-administration after escalation in rats. Psychopharmacology. 1999;146:303–312. doi: 10.1007/s002130051121. [DOI] [PubMed] [Google Scholar]
- Ahmed SH, Walker JR, Koob GF. Persistent increase in the motivation to take heroin in rats with a history of drug escalation. Neuropsychopharmacology. 2000;22:413–421. doi: 10.1016/S0893-133X(99)00133-5. [DOI] [PubMed] [Google Scholar]
- Ahmed SH, Kenny PJ, Koob GF, Markou A. Neurobiological evidence for hedonic allostasis associated with escalating cocaine use. Nat Neurosci. 2002;5:625–626. doi: 10.1038/nn872. [DOI] [PubMed] [Google Scholar]
- Cabib S, Orsini C, Le Moal M, Piazza PV. Abolition and reversal of strain differences in behavioral responses to drugs of abuse after a brief experience. Science. 2000;289:463–465. doi: 10.1126/science.289.5478.463. [DOI] [PubMed] [Google Scholar]
- Covington HE, III, Miczek KA. Repeated social-defeat stress, cocaine or morphine. Effects on behavioral sensitization and intravenous cocaine self-administration “binges”. Psychopharmacology (Berl) 2001;158:388–398. doi: 10.1007/s002130100858. [DOI] [PubMed] [Google Scholar]
- Covington HE, III, Miczek KA. Intense cocaine self-administration after episodic social defeat stress, but not after aggressive behavior: dissociation from corticosterone activation. Psychopharmacology (Berl) 2005;183:331–340. doi: 10.1007/s00213-005-0190-5. [DOI] [PubMed] [Google Scholar]
- Covington HE, III, Tropea TF, Rajadhyaksha AM, Kosofsky BE, Miczek KA. NMDA receptors in the rat VTA: a critical site for social stress to intensify cocaine taking. Psychopharmacology (Berl) 2008;197:203–216. doi: 10.1007/s00213-007-1024-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deroche V, Marinelli M, Maccari S, Lemoal M, Simon H, Piazza PV. Stress-induced sensitization and glucocorticoids. 1. Sensitization of dopamine-dependent locomotor effects of amphetamine and morphine depends on stress-induced corticosterone secretion. J Neurosci. 1995;15:7181–7188. doi: 10.1523/JNEUROSCI.15-11-07181.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrario CR, Robinson TE. Amphetamine pretreatment accelerates the subsequent escalation of cocaine self-administration behavior. Eur Neuropsychopharmacol. 2007;17:352–357. doi: 10.1016/j.euroneuro.2006.08.005. [DOI] [PubMed] [Google Scholar]
- Ferrario CR, Gorny G, Crombag HS, Li Y, Kolb B, Robinson TE. Neural and behavioral plasticity associated with the transition from controlled to escalated cocaine use. Biol Psychiatry. 2005;58:751–759. doi: 10.1016/j.biopsych.2005.04.046. [DOI] [PubMed] [Google Scholar]
- Fish EW, Faccidomo S, Miczek KA. Aggression heightened by alcohol or social instigation in mice: reduction by the 5-HT1B receptor agonist CP-94, 253. Psychopharmacology. 1999;146:391–399. doi: 10.1007/pl00005484. [DOI] [PubMed] [Google Scholar]
- Gawin FH. Cocaine addiction: psychology and neurophysiology. Science. 1991;251(5001):1580–6. doi: 10.1126/science.2011738. [DOI] [PubMed] [Google Scholar]
- Gawin FH, Kleber HD. Evolving conceptualizations of cocaine dependence. Yale J Biol Med. 1988;61(2):123–136. [PMC free article] [PubMed] [Google Scholar]
- Gerber GJ, Wise RA. Pharmacological regulation of intravenous cocaine and heroin self-administration in rats: a variable dose paradigm. Pharmacol Biochem Behav. 1989;32:527–531. doi: 10.1016/0091-3057(89)90192-5. [DOI] [PubMed] [Google Scholar]
- Goeders NE. The HPA axis and cocaine reinforcement. Psychoneuroendocrinology. 2002;27:13–33. doi: 10.1016/s0306-4530(01)00034-8. [DOI] [PubMed] [Google Scholar]
- Goeders NE. The impact of stress on addiction. Eur Neuropsychopharmacol. 2003;13(6):435–41. doi: 10.1016/j.euroneuro.2003.08.004. [DOI] [PubMed] [Google Scholar]
- Grimm JW, Hope BT, Wise RA, Shaham Y. Neuroadaptation. Incubation of cocaine craving after withdrawal. Nature. 2001;412 (6843):141–142. doi: 10.1038/35084134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodos W. Progressive ratio as a measure of reward strength. Science. 1961;134:943–944. doi: 10.1126/science.134.3483.943. [DOI] [PubMed] [Google Scholar]
- Kabbaj M, Norton CS, Kollack-Walker S, Watson SJ, Robinson TE, Akil H. Social defeat alters the acquisition of cocaine self-administration in rats: role of individual differences in cocaine-taking behavior. Psychopharmacology (Berl) 2001;158:382–387. doi: 10.1007/s002130100918. [DOI] [PubMed] [Google Scholar]
- Knackstedt LA, Kalivas PW. Extended access to cocaine self-administration enhances drug-primed reinstatement but not behavioral sensitization. J Pharmacol Exp Ther. 2007;322:1103–1109. doi: 10.1124/jpet.107.122861. [DOI] [PubMed] [Google Scholar]
- Koob G, Kreek MJ. Stress, dysregulation of drug reward pathways, and the transition to drug dependence. Am J Psychiatry. 2007;164:1149–1159. doi: 10.1176/appi.ajp.2007.05030503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF, Le Moal M. Drug abuse: hedonic homeostatic dysregulation. Science. 1997;278:52–59. doi: 10.1126/science.278.5335.52. [DOI] [PubMed] [Google Scholar]
- Lenoir M, Ahmed SH. Heroin-induced reinstatement is specific to compulsive heroin use and dissociable from heroin reward and sensitization. Neuropsychopharmacology. 2007;32:616–624. doi: 10.1038/sj.npp.1301083. [DOI] [PubMed] [Google Scholar]
- Liu Y, Roberts DC, Morgan D. Effects of extended-access self-administration and deprivation on breakpoints maintained by cocaine in rats. Psychopharmacology (Berl) 2005;179:644–651. doi: 10.1007/s00213-004-2089-y. [DOI] [PubMed] [Google Scholar]
- Lu L, Grimm JW, Hope BT, Shaham Y. Incubation of cocaine craving after withdrawal: a review of preclinical data. Neuropharmacology. 2004;47(S1):214–226. doi: 10.1016/j.neuropharm.2004.06.027. [DOI] [PubMed] [Google Scholar]
- Mantsch JR, Katz ES. Elevation of glucocorticoids is necessary but not sufficient for the escalation of cocaine self-administration by chronic electric footshock stress in rats. Neuropsychopharmacology. 2007;32:367–376. doi: 10.1038/sj.npp.1301077. [DOI] [PubMed] [Google Scholar]
- Mantsch JR, Yuferov V, Mathieu-Kia AM, Ho A, Kreek MJ. Effects of extended access to high versus low cocaine doses on self-administration, cocaine-induced reinstatement and brain mRNA levels in rats. Psychopharmacology (Berl) 2004;175:26–36. doi: 10.1007/s00213-004-1778-x. [DOI] [PubMed] [Google Scholar]
- Mantsch JR, Baker DA, Serge JP, Hoks MA, Francis DM, Katz ES. Surgical adrenalectomy with diurnal corticosterone replacement slows escalation and prevents the augmentation of cocaine-induced reinstatement in rats self-administering cocaine under long-access conditions. Neuropsychopharmacology. 2008;33:814–826. doi: 10.1038/sj.npp.1301464. [DOI] [PubMed] [Google Scholar]
- Marinelli M, Piazza PV. Interaction between glucocorticoid hormones, stress and psychostimulant drugs. Eur J NeuroSci. 2002;16:387–394. doi: 10.1046/j.1460-9568.2002.02089.x. [DOI] [PubMed] [Google Scholar]
- Miczek KA, Mutschler NH. Activational effects of social stress on IV cocaine self-administration in rats. Psychopharmacology. 1996;128:256–264. doi: 10.1007/s002130050133. [DOI] [PubMed] [Google Scholar]
- Miczek KA, Covington HE, Nikulina EA, Hammer RP. Aggression and defeat: persistent effects on cocaine self-administration and gene expression in peptidergic and aminergic mesocorticolimbic circuits. Neurosci Biobehav Rev. 2004;27:787–802. doi: 10.1016/j.neubiorev.2003.11.005. [DOI] [PubMed] [Google Scholar]
- Miczek KA, Yap JJ, Covington HE., III Social stress, therapeutics and drug abuse: preclinical models of escalated and depressed intake. Pharmacol Ther. 2008;120:102–128. doi: 10.1016/j.pharmthera.2008.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moffett MC, Vicentic A, Kozel M, Plotsky P, Francis DD, Kuhar MJ. Maternal separation alters drug intake patterns in adulthood in rats. Biochem Pharmacol. 2007;73:321–330. doi: 10.1016/j.bcp.2006.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan D, Roberts DCS. Sensitization to the reinforcing effects of cocaine following binge-abstinent self-administration. Neurosci Biobehav Rev. 2004;27:803–812. doi: 10.1016/j.neubiorev.2003.11.004. [DOI] [PubMed] [Google Scholar]
- National Research Council. Guide for the care and use of laboratory animals. National Academy Press; Washington DC: 1996. [Google Scholar]
- Nikulina EM, Covington HE, III, Ganschow L, Hammer RP, Jr, Miczek KA. Long-term behavioral and neuronal cross-sensitization to amphetamine induced by repeated brief social defeat stress: Fos in the ventral tegmental area and amygdala. Neuroscience. 2004;123:857–865. doi: 10.1016/j.neuroscience.2003.10.029. [DOI] [PubMed] [Google Scholar]
- Oleson EB, Roberts DC. Behavioral economic assessment of price and cocaine consumption following self-administration histories that produce escalation of either final ratios or intake. Neuropsychopharmacology. 2009;34(3):796–804. doi: 10.1038/npp.2008.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oleson EB, Talluri S, Childers SR, Smith JE, Roberts DC, Bonin KD, Budygin EA. Dopamine uptake changes associated with cocaine self-administration. Neuropsychopharmacology. 2009;34(5):1174–84. doi: 10.1038/npp.2008.186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pacchioni AM, Gioino G, Assis A, Cancela LM. A single exposure to restraint stress induces behavioral and neurochemical sensitization to stimulating effects of amphetamine: involvement of NMDA receptors. Ann N Y Acad Sci. 2002;965:233–246. doi: 10.1111/j.1749-6632.2002.tb04165.x. [DOI] [PubMed] [Google Scholar]
- Paterson NE, Markou A. Increased motivation for self-administered cocaine after escalated cocaine intake. NeuroReport. 2003;14:2229–2232. doi: 10.1097/00001756-200312020-00019. [DOI] [PubMed] [Google Scholar]
- Richardson NR, Roberts DCS. Progressive ratio schedules in drug self-administration studies in rats: a method to evaluate reinforcing efficacy. J Neurosci Methods. 1996;66:1–11. doi: 10.1016/0165-0270(95)00153-0. [DOI] [PubMed] [Google Scholar]
- Roberts DC, Morgan D, Liu Y. How to make a rat addicted to cocaine. Prog Neuropsychopharmacol Biol Psychiatry. 2007;31:1614–1624. doi: 10.1016/j.pnpbp.2007.08.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson TE, Berridge KC. The neural basis of drug craving: an incentive-sensitization theory of addiction. Brain Res Brain Res Rev. 1993;18:247–291. doi: 10.1016/0165-0173(93)90013-p. [DOI] [PubMed] [Google Scholar]
- Robinson TE, Berridge KC. The psychology and neurobiology of addiction: an incentive-sensitization view. Addiction. 2000;95:S91–S117. doi: 10.1080/09652140050111681. [DOI] [PubMed] [Google Scholar]
- Roth ME, Carroll ME. Sex differences in the escalation of intravenous cocaine intake following long- or short-access to cocaine self-administration. Pharmacol Biochem Behav. 2004;78:199–207. doi: 10.1016/j.pbb.2004.03.018. [DOI] [PubMed] [Google Scholar]
- Rouge-Pont F, Marinelli M, Le Moal M, Simon H, Piazza PV. Stress-induced sensitization and glucocorticoids. 2. Sensitization of the increase in extracellular dopamine induced by cocaine depends on stress-induced corticosterone secretion. J Neurosci. 1995;15:7189–7195. doi: 10.1523/JNEUROSCI.15-11-07189.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinha R. How does stress increase risk of drug abuse and relapse? Psychopharmacology (Berl) 2001;158(4):343–359. doi: 10.1007/s002130100917. [DOI] [PubMed] [Google Scholar]
- Sinha R. Chronic stress, drug use, and vulnerability to addiction. Ann N Y Acad Sci. 2008;1141:105–130. doi: 10.1196/annals.1441.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorge RE, Stewart J. The contribution of drug history and time since termination of drug taking to footshock stress-induced cocaine seeking in rats. Psychopharmacology (Berl) 2005;183(2):210–217. doi: 10.1007/s00213-005-0160-y. [DOI] [PubMed] [Google Scholar]
- Tidey JW, Miczek KA. Acquisition of cocaine self-administration after social stress: role of accumbens dopamine. Psychopharmacology (Berl) 1997;130:203–212. doi: 10.1007/s002130050230. [DOI] [PubMed] [Google Scholar]
- Tornatzky W, Miczek KA. Long-term impairment of autonomic circadian rhythms after brief intermittent social stress. Physiol Behav. 1993;53:983–993. doi: 10.1016/0031-9384(93)90278-n. [DOI] [PubMed] [Google Scholar]
- Tornatzky W, Miczek KA. Cocaine self-administration “binges”: transition from behavioral and autonomic regulation toward homeostatic dysregulation in rats. Psychopharmacology. 2000;148:289–298. doi: 10.1007/s002130050053. [DOI] [PubMed] [Google Scholar]
- Vezina P. Sensitization of midbrain dopamine neuron reactivity and the self-administration of psychomotor stimulant drugs. Neurosci Biobehav Rev. 2004;27:827–839. doi: 10.1016/j.neubiorev.2003.11.001. [DOI] [PubMed] [Google Scholar]
- Wee S, Mandyam CD, Lekic DM, Koob GF. Alpha 1-noradrenergic system role in increased motivation for cocaine intake in rats with prolonged access. Eur Neuropsychopharmacol. 2008;18:303–311. doi: 10.1016/j.euroneuro.2007.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]