Abstract
Serine palmitoyltransferase (SPT) is the key enzyme for the biosynthesis of sphingolipids. It has been reported that oral administration of myriocin (an SPT inhibitor) decreases plasma sphingomyelin (SM) and cholesterol levels, and reduces atherosclerosis in apoE knockout (KO) mice. We studied cholesterol absorption in myriocin-treated WT or apoE KO animals and found that, after myriocin treatment, the mice absorbed significantly less cholesterol than controls, with no observable pathological changes in the small intestine. More importantly, we found that heterozygous Sptlc1 (a subunit of SPT) KO mice also absorbed significantly less cholesterol than controls. To understand the mechanism, we measured protein levels of Niemann-Pick C1-like 1 (NPC1L1), ABCG5, and ABCA1, three key factors involved in intestinal cholesterol absorption. We found that NPC1L1 and ABCA1 were decreased, whereas ABCG5 was increased in the SPT deficient small intestine. SM levels on the apical membrane were also measured and they were significantly decreased in SPT deficient mice, compared with controls. In conclusion, SPT deficiency might reduce intestinal cholesterol absorption by altering NPC1L1 and ABCG5 protein levels in the apical membranes of enterocytes through lowering apical membrane SM levels. This may be also true for ABCA1 which locates on basal membrane of enterocytes. Manipulation of SPT activity could thus provide a novel alternative treatment for dyslipidemia.
Keywords: Myriocin, SPT gene knockout, Cholesterol absorption, Dyslipidemia, Atherosclerosis
1. Introduction
Lowering plasma cholesterol is important because its levels closely relate to the cardiovascular and metabolic disorders. About 30% of plasma cholesterol is derived by intestinal absorption [1]. It has been estimated that a 36–37% reduction in plasma cholesterol levels could be achieved by total inhibition of cholesterol absorption [1] Absorption is a multi-step process in which cholesterol is micellized by bile acids and phospholipids in the intestinal lumen, taken up by the enterocytes, assembled into lipoproteins, and transported to the lymph and the circulation.
Accumulating evidence indicates that Niemann-Pick C1-like 1 (NPC1L1) protein plays a key role in the influx of cholesterol into the enterocytes [2]. NPC1L1 deficiency significantly reduces cholesterol absorption and has been shown to be the target of ezetimibe, a well-known cholesterol absorption inhibitor, in the small intestine [3] and probably in the liver as well [4]. After uptake, some of the cholesterol is believed to be capable of secreting back into the intestinal lumen by means of the heterodimeric ATP-binding cassette transporters G5 and G8 (ABCG5/G8) [5]. A majority of the cholesterol taken up by the enterocytes are transported to the plasma. It has been shown that this process may involve at least two pathways: apolipoprotein (apo) B-dependent, and apoB-independent [6]. The apoB-dependent pathway requires apoB and microsomal triglyceride transfer protein (MTP) activity [6]. Apo AI and ABCA1 have been shown to play a role in the apoB-independent or the HDL pathway [7].
Located in the endoplasmic reticulum (ER) membranes, serine palmitoyltransferase (SPT) is the rate-limiting enzyme in the biosynthesis of sphingolipids, including sphingomyelin (SM) [8]. Mammalian SPT contains two subunits, Sptlc1 and Sptlc2, encoding 53- and 63 kDa proteins, respectively [9,10]. A recent report described a third subunit, Sptlc3, an isoform of Sptlc2. Both Sptlc2 and Sptlc3 can bind Sptlc1 independently, so that varying the amounts of Sptlc2 and -3 might be a cellular mechanism for adjusting SPT activity to meet tissue-specific requirements for sphingolipids [11].
The interaction of SM, cholesterol, and glycosphingolipids drives the formation of plasma membrane rafts [12]. These lipid rafts, formed in the Golgi apparatus, are targeted to the plasma membranes, where they are thought to exist as islands within the sea of bulk membranes. Despite disagreement as to their content, the rafts are considered in most reports to comprise approximately 3500 lipid molecules and 30 proteins [13]. As much as 65% of total cellular SM is located in these rafts [14].
The apical membrane of an intestinal cell is enriched in SM and cholesterol [15], indicative of the presence of lipid rafts [16]. It has been proposed that lipid rafts in the enterocyte apical membranes play an important role in cholesterol absorption and trafficking [17]. Both NPC1L1 and ABCG5/G8 are expressed in the small intestine and localized at the enterocyte apical surface [2,18]. It is conceivable that alteration of SM levels on this surface could change NPC1L1 and ABCG5/G8 levels, thus influencing cholesterol absorption. Indeed, Park et al. reported that oral administration of myriocin (a specific SPT inhibitor) in apoE KO mice reduced not only plasma SM levels but plasma cholesterol levels as well [19].
In this study, we utilized a pharmacological approach (myriocin treatment) and a genetic one (Sptlc1 gene knockout) to investigate the effect of SPT decreasing on cholesterol absorption. We hypothesized that intestine-specific SPT deficiency might alter NPC1L1 and ABCG5/G8 protein levels by directly influencing the apical surface structure of the enterocytes, thereby reducing cholesterol absorption.
2. Materials and methods
2.1. Materials
[9,10(N)-3H]triolein was from NEN Life Science Products. [4-14C] cholesterol and [9,10(N)-3H]oleic acid were from Amersham. [5,6-3H] sitostanol was from American Radiolabeled Chemicals. Oleic acid (OA) was from Sigma. Dulbecco's modified Eagle's medium (DMEM) was from Invitrogen.
2.2. Mice and diet
Male C57BL/6J and apoE KO mice with a C57BL/6J background were obtained from the Jackson Laboratory (Bar Harbor, ME). Sptlc1 heterozygous KO mice with a C57BL/6 background were created and bred in our laboratory. Myriocin was mixed with a chow diet. Eight- to 12-week-old WT or apoE KO (n = 6) mice received myriocin 0.3 mg/kg−1/d−1 for 12 weeks. The myriocin dose was chosen from a previous dose-dependent experiment with apoE KO mice. Controls consisted of WT or apoE KO mice fed a rodent chow diet (Purina Rodent Chow, # 5001, from Research Diets Inc., New Brunswick, New Jersey, USA) (n = 6). Male Sptlc1 heterozygous KO (Sptlc1+/−) (n = 6) and WT mice were also fed the chow diet. All experiment mice were under fast condition (removed food at 9:00am and perform experiment around 2:00pm). All procedures and protocols involving the use of animals were approved by the SUNY Downstate Medical Center Animal Care and Use Committee, and conformed with the “Guide for the Care and Use of Laboratory Animals” published by the US National Institutes of Health (NIH publication No. 85-23, revised 1996).
2.3. Cholesterol absorption studies
A classical fecal dual-isotope ratio method was used for the cholesterol absorption study [6,20,21]. Briefly, a mixture of [14C]-labeled (0.1 µCi) and unlabeled cholesterol (0.5 mg) and [3H]sitostanol (0.2 µCi) in 15 µl of olive oil was fed to mice (10–12 weeks old). Feces were collected for 24 h. The cholesterol absorption ratio was calculated as: % absorption = {1-[fecal(14C/3H)] / administered (14C/3H)}× 100. In some cases, mice were sacrificed, plasma collected, and radioactivity counted. Small intestines (from duodenum to ileum) were washed and cut into 2 cm segments. Each of these, as well as a part of the liver, was digested and radioactivity was counted individually.
2.4. Cholesterol uptake by primary enterocyte
The enterocyte cholesterol uptake study was carried out as we reported previously [6].
2.5. Enterocyte lipid measurement
The total cholesterol, triglyceride, sphingomylein, and phosphatidylcholine were quantified in lipid extracts of enterocytes using enzymatic reagents as described previously [22,23].
2.6. Tissue SPT activity assay
Mouse small intestine (0.2 g) was homogenized in 0.5 ml of 50 mM Tris–HCl, pH 7.4, 5 mM EDTA, and 250 mM sucrose. SPT activity in the homogenates was measured with [14C]-serine and palmitoyl-coenzyme A for substrates, as previously described [24].
2.7. In situ lysenin treatment and cell mortality measurement
Overnight-fasted mice were anesthetized and small intestines were isolated from WT animals with or without myriocin treatment, as well as Sptlc1 heterozygous and control mice. Contents of the intestinal lumen were removed and washed with buffer containing 100 mM NaCl, 5.4 mM KCl, 1 mM NaH2PO4, 26 mM NaHCO3 and 5.5 mM glucose (pH 7.4). Intestines were turned inside-out and cut into 0.5 cm segments from jejunum. These segments were then bathed in 0.5 ml of oxygenated DMEM containing 5% glutamine with lysenin (5 µg/ml), or without it as a control, at 37 °C for 30 min. WST-1 cell proliferation reagent (Roche) was added to monitor cell mortality. After continuous incubation at 37 °C for 15 min, the solution was transferred to an Eppendorf tube and spun (12,000 rpm) to pellet cell debris. Supernatant was then measured OD at 450 nm, a reading for viable cells (WST-1 reagent with no cell incubation being the background reading). Buffers and medium were gassed with 95% O2/5% CO2 for 20 min, and maintained at 37 °C prior to use.
2.8. In situ lysenin staining
Overnight-fasted mice were anesthetized and small intestines were isolated from control and Sptlc1 heterozygous KO mice. Contents from the intestinal lumen were removed, washed with buffer containing 100 mM NaCl, 5.4 mM KCl, 1 mM NaH2PO4, 26 mM NaHCO3 and 5.5 mM glucose (pH 7.4). A 3 cm long jejunum was cut out and sealed at both ends, lysenin-red fluorescent protein (RFP) (1 µg in 500 µl buffer) was put into the intestinal lumen. The intestinal segments were bathed in oxygenated DMEM containing 5% glutamine at 37 °C for 30 min, and then washed with the above buffer. Frozen sections were prepared and lysenin-RFP binding was examined under a microscope. The lysenin-RFP, a gift from Dr. Toshihide Kobayashi, has no toxicity but binds SM [25].
2.9. Mouse small intestine hematoxylin and eosin staining
The small intestine was dissected out and put into 4% paraformaldehyde for fixation overnight. The tissue was paraffin-embedded. The tissue was then sliced (10 µm thick). Each slice was deparaffinized and stained with hematoxylin and eosin.
2.10. Real-time PCR examined genes' expression
Mice were sacrificed by cervical dislocation. Jejunum was dissected, and total RNA extracted with Trizol (Invitrogen). cDNA was synthesized with an Invitrogen kit. Polymerase chain reaction (PCR) was performed on a total volume of 20 µl with the sybergreen kit from Applied Biosystems, 18 S being used as an internal control. The amplification program consisted of activation at 95 °C for 10 min, followed by 40 amplification cycles: 95 °C for 15 s, 60 °C for 1 min. Each sample was triplicated. The genes' relative expression was expressed as mean ± SD. Mouse NPC1L1 primers: forward, ATCCT-CATCCTGGGCTTTGC; reverse, GCAAGGTGATCAGGAGGTTGA. Mouse ABCG5 primers: forward, GCAGGGACCAGTTCCAAGACT; reverse, ACGTCTCGCGCACAGTGA. Mouse ABCG8 primers: forward, AAAGT-GAGGAGTGGACAGATGCT; reverse, TGCCTGTGATCACGTCGAGTAG. Mouse ABCA1 primers: forward, TTGGCGCTCAACTTTTACGAA; reverse, GAGCGAATGTCCTTCCCCA. 18S rRNA: forward, AGTCCCTGCC-CTTTGTACACA; reverse, GATCCGAGGGCCTCA CTAAAC.
2.11. Preparation and western blot analysis of small intestine homogenates
A total of 50–100 mg of small intestine sample was homogenized and lysed proteins were immunoblotted, as previously described, with a polyclonal rabbit antihuman NPC1L1 antiserum 69 B, a polyclonal rabbit antimouse ABCG5 antiserum, a polyclonal antimouse ABCA1 antibody, and a monoclonal mouse anti-β-actin antibody.
2.12. Statistical analysis
Data were expressed as mean ± SD. Differences between groups were evaluated by Mann–Whitney U test (non-parametric test). P values less than 0.05 were considered significant.
3. Results
3.1. Myriocin treatment or Sptlc1 deficiency significantly decreases SPT activity without altering the epithelial structure of the small intestine
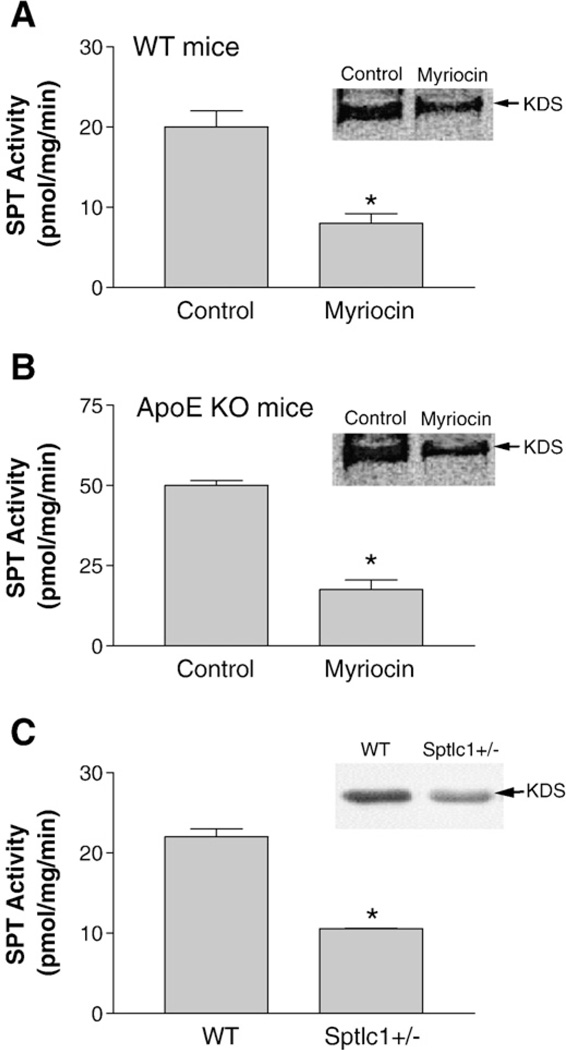
To investigate the relationship between small intestine SPT activity and cholesterol absorption, we utilized pharmacological and genetic approaches. For the first set of experiments: four groups (n = 6 per group) of 12-week-old male WT and apoE KO mice were used. Groups 1 and 2 were WT mice fed a chow diet with or without myriocin for 3 weeks; groups 3 and 4 were apoE KO mice fed a chow diet with or without myriocin for 3 weeks. Myriocin-treated mice had 60 to 65% less SPT activity respectively than controls in the small intestine (Fig. 1A and B). For the second set of experiments: male Sptlc1 heterozygous KO (Sptlc1+/−) mice [24] (n = 6) and WT controls (n = 6) were utilized. Sptlc1+/− mice had 52% less SPT activity than controls in the small intestine (Fig. 1C). These studies indicated that myriocin treatment and Sptlc1 deficiency reduced small intestinal SPT activity.
Fig. 1.
SPT deficiency decreased SPT activity in mouse small intestine. SPT activity of small intestine homogenate was measured with [3H]serine and palmitoyl-coenzyme A as substrates. TLC was performed to separate the product, 3-keto-dihydrosphingosine (KDS, inset). KDS band was scanned. (Panel A) WT mice fed chow diet or chow diet plus myriocin; (Panel B) apoE KO mice fed chow diet or chow diet plus myriocin; (Panel C) WT and Sptlc1+/− mice. Values are mean ± SD (n = 5, P<0.01).
As shown in Table 1, myriocin treatment significantly decreased plasma SM (48%, P<0.001) and cholesterol (37%, P<0.01) in apoE KO mice, compared with controls. On the other hand, myriocin treatment caused no significant effect on plasma lipids, including SM, PC, and cholesterol levels, in WT mice. As we have reported previously [24], Sptlc1+/− and WT mice had identical plasma lipid levels (Table 1). We also measured HMGCoA reductase (the key enzyme for cholesterol biosynthesis) mRNA levels in the liver from WT mice with or without myriocin treatment, as well as apoE KO mice with or without myriocin treatment. We did not find significant changes (Supplement Fig. 1).
Table 1.
Mouse plasma lipid measurement
| SM mg/dl |
PC | Chol | TG | |
|---|---|---|---|---|
| WT mice | ||||
| Control | 25 ± 5 | 139 ± 19 | 101 ± 17 | 79 ± 12 |
| Myriocin | 22 ± 2 | 155 ± 31 | 95 ± 12 | 71 ± 15 |
| ApoE KO mice | ||||
| Control | 75 ± 9 | 289 ± 25 | 556 ± 39 | 105 ± 19 |
| Myriocin | 39 ± 4* | 334 ± 49 | 350 ± 42* | 89 ± 13 |
| Mice | ||||
| WT | 22 ± 3 | 127 ± 24 | 91 ± 11 | 89 ± 16 |
| Sptlc1+/− | 19 ± 5 | 138 ± 33 | 99 ± 8 | 92 ± 10 |
Value, mean ± SD.
P < 0.01, N = 6.
SM, sphingomyelin; PC, Phosphatidylcholine; Chol, cholesterol; TG, triacylglycerols.
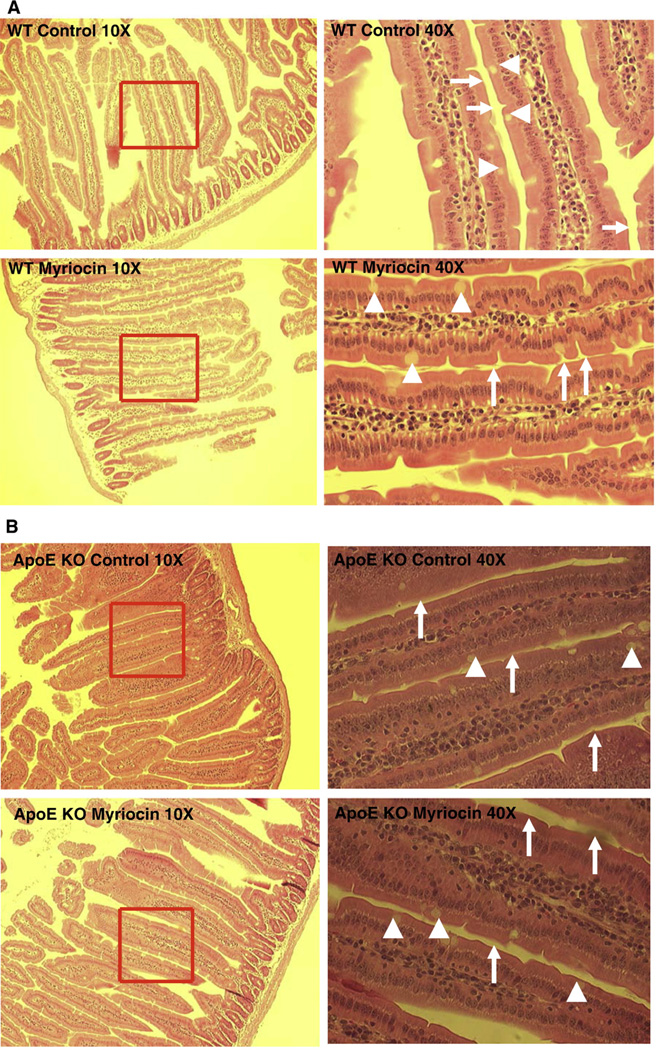
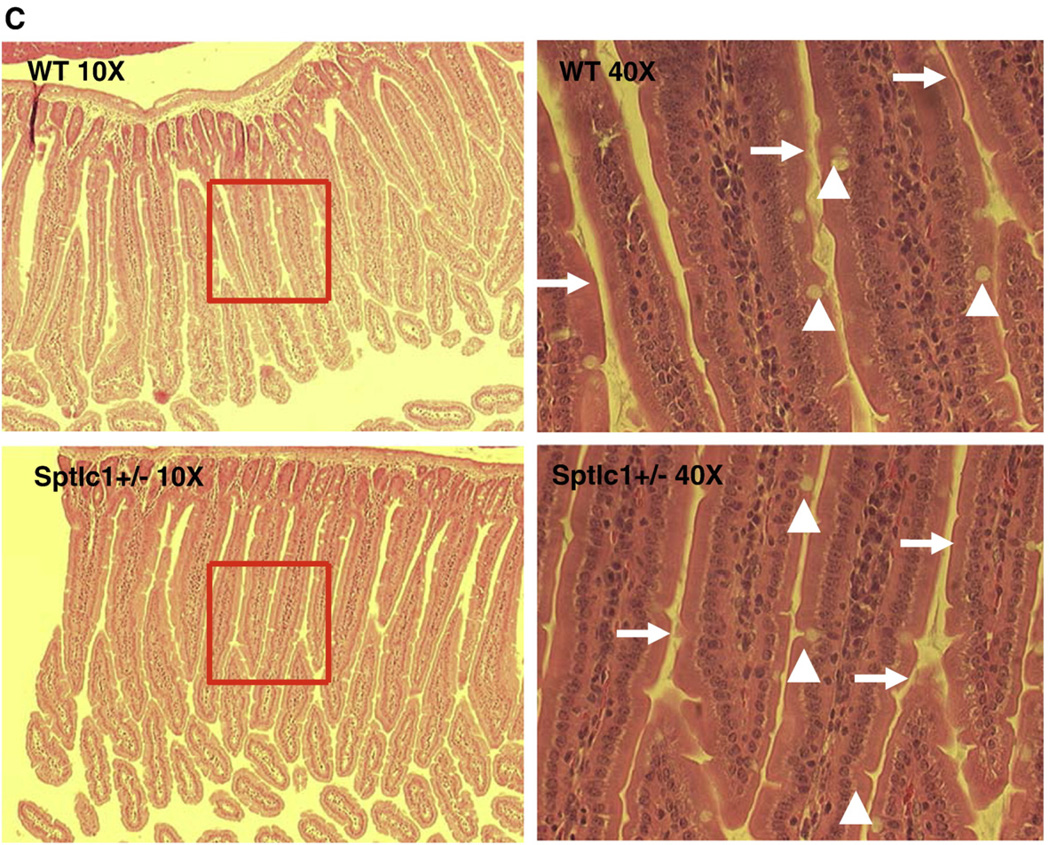
To investigate whether myriocin treatment or Sptlc1+/− had any impact on small intestine morphology, we stained intestinal sections with hematoxylin and eosin. As shown in Fig. 2A–C, neither myriocin treatment nor Sptlc1+/− influenced the morphology of the small intestine. All specimens had intact villi, epithelia, enterocytes, goblet cells, and brush borders. Therefore, SPT deficiency does not affect gross small intestinal morphology. Moreover, neither myriocin treatment nor Sptlc1+/− influenced body weight (data not shown).
Fig. 2.
SPT reduction did not change the morphology of the mouse small intestine. The small intestine was dissected out and put into 4% paraformaldehyde for fixation overnight. The tissue was then sliced (10 µm thick). Each slice was deparaffinized and stained with hematoxylin and eosin. The morphology was checked by microscope. (Panel A) WT mice fed chow diet or chow diet plusmyriocin; (Panel B) apoE KO mice fed chow diet or chow diet plusmyriocin; (Panel C) WT mice and Sptlc1+/− mice. The arrows point to bush border and arrow heads point to goblet cells.
3.2. Myriocin treatment or Sptlc1 deficiency significantly decreases cholesterol but not triacylglycerol absorption
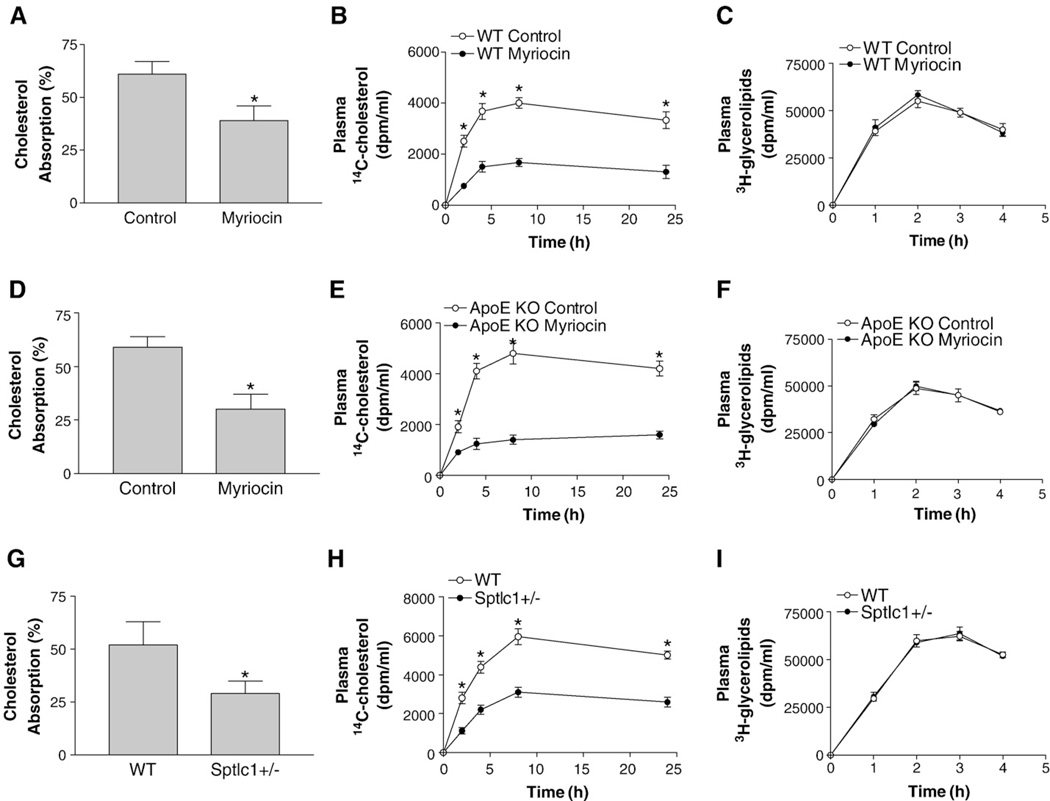
The observed decrease of plasma cholesterol levels in apoE KO mice after myriocin treatment (Table 1) could be due to a defect in cholesterol absorption. To explore the relationship between SPT deficiency and cholesterol absorption, we did studies after a single gavage, using the conventional fecal dual-isotope ([14C]-cholesterol/[3H]sitostanol) ratio method. As shown in Fig. 3, there was a significant reduction in cholesterol absorption in myriocin-treated WT (36%, P<0.01) (Fig. 3A), myriocin-treated apoE KO (50%, P<0.001) (Fig. 3D), and Sptlc1+/− mice (43%, P<0.001) (Fig. 3G), compared with controls.
Fig. 3.
SPT deficient mice absorbed less cholesterol. Mice (n = 6,10–12 weeks old) were fasted for 5 h and gavaged with 0.1 µCi [14C]cholesterol and 0.2 µCi [3H]sitostanol with 0.5 mg unlabeled cholesterol dissolved in 15 µl of olive oil. Feces were collected after 24 h and lipids were extracted for counting (Panels A, D, and G, cholesterol absorption, WT vs WT/myriocin; apoE KO vs apoE KO/myriocin; WT vs Sptlc1+/−, respectively). Blood was collected during the 24 h period (Panels B, E, and H, [14C]cholesterol in blood, WT vs WT/myriocin; apoE KO vs apoE KO/myriocin; WT vs Sptlc1+/−, respectively). To test specificity, we also gavaged the mice with 0.2 µCi [3H]triolein together with 0.5 mg/ml cold cholesterol. Blood was collected during the 24 h period (Panels C, F, and I, [3H]glycerolipids in blood, WT vs WT/myriocin; apoE KO vs apoE KO/myriocin; WT vs Sptlc1+/−, respectively). Value is mean ± SD. *P<0.01.
We also monitored blood [14C]-cholesterol levels within 24 h after a single gavage. We found that myriocin-treated WT (Fig. 3B), myriocin-treated apoE KO (Fig. 3E), and Sptlc1+/− mice (Fig. 3H) had significantly less [14C]-cholesterol in the circulation than controls, confirming defective cholesterol absorption in these animals.
To investigate whether the effect of SPT deficiency was specific to cholesterol, we fed experimental mice with 0.1 µCi [3H]triolein instead of [14C]cholesterol, and collected blood at different time points within 4 h. No significant changes in the [3H]triolein-derived counts in the plasma between SPT deficiency (myriocin treated or Sptlc1+/−) animals and controls were observed (Fig. 3C, F, and I), indicating that SPT deficiency has no effect on triglyceride absorption.
We next sought to measure the amounts of [14C]cholesterol present in the intestine and those transported to plasma, liver, and bile in 24 h after a single bolus of radiolabeled cholesterol. Myriocin-treated WT and apoE KO, as well as Sptlc1+/− small intestines, plasma, livers, and bile contained significantly less [14C]cholesterol than controls (Table 2). Lower counts in plasma, liver, and bile indicated that SPT deficiency in mice caused less efficiency in cholesterol absorption.
Table 2.
Absorption of [14C]cholesterol after a single gavage
| Intestine (dpm/g) | Liver (dpm/g) | Bile (dpm/ml) | Plasma (dpm/ml) | |
|---|---|---|---|---|
| WT mice | ||||
| Control | 10103 ± 792 | 8311 ± 1038 | 5092 ± 661 | 3321 ± 674 |
| Myriocin | 5237 ± 478* | 4131 ± 575* | 2108 ± 394* | 1304 ± 390* |
| ApoE KO mice | ||||
| Control | 9714 ± 1184 | 7371 ± 781 | 4478 ± 742 | 4201 ± 615 |
| Myriocin | 4158 ± 499* | 3337 ± 649* | 2284 ± 521* | 1590 ± 142* |
| Mice | ||||
| WT | 11122 ± 1335 | 9927 ± 1249 | 5687 ± 577 | 5291 ± 711 |
| Sptlc1+/− | 6945 ± 816* | 4492 ± 398* | 2652 ± 662* | 2992 ± 606* |
Mice were fed with either 0.1 µCi of [14C]cholesterol and 1 µCi of [3H]sitostanol together with 0.5 mg of unlabeled cholesterol in 15 µl of olive oil. After 24 h, plasma, small intestine, liver, and bile were collected and used for radioactivity measurements. Values are mean ± SD, n = 5.
P < 0.01.
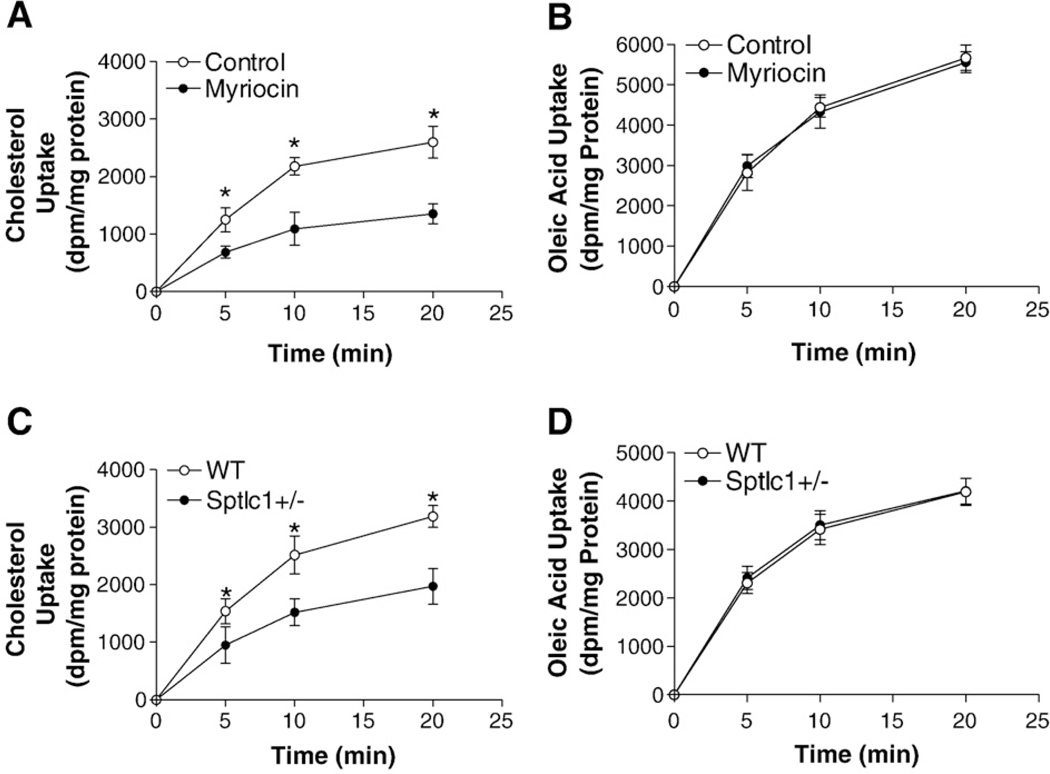
For further confirmation of the above in vivo observations, we isolated enterocytes from myriocin-treated or Sptlc1+/− mice, as well as controls, incubated them with radiolabeled cholesterol for varying times, and determined cellular radioactivity. We found that myriocin-treated or Sptlc1 deficient enterocytes took up significantly less radioactivity than controls, indicating a defect in cholesterol uptake (Fig. 4A and C). Experiments were performed to study the uptake of [3H]oleic acid. No significant changes were observed (Fig. 4B and D). These data indicate that myriocin treatment or Sptlc1 deficiency specifically decreases cholesterol uptake by the enterocytes.
Fig. 4.
SPT deficient enterocytes absorb less cholesterol than controls. Enterocytes were isolated from mice (n = 4, 12–14 weeks old) and resuspended in 4 ml of DMEM containing 0.05 µCi/ml of either [14C]cholesterol (Panels A and C) or [3H]oleic acid (Panels B and D). Enterocytes (100 µl) were collected at 5, 10, and 20 min, washed twice with DMEM, and cellular radioactivity was determined. The amounts of lipids were normalized to protein and plotted against time. (Panels A and B) WT vs WT/myriocin; (Panels C and D) WT vs Sptlc1+/−, Values are mean ± SD. *P<0.01.
3.3. Myriocin treatment or Sptlc1 deficiency significantly decreases small intestine apical membrane SM levels, decreases NPC1L1 and increases ABCG5/G8 protein levels
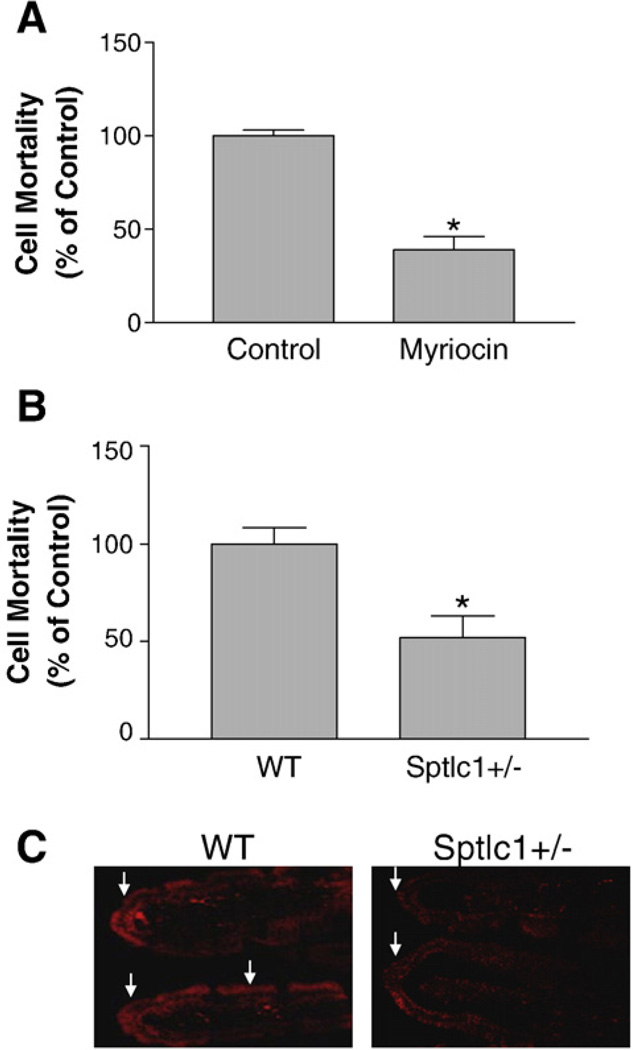
We explored the mechanisms responsible for decreased cholesterol absorption in myriocin-treated or Sptlc1+/− mice. First, we measured lipid levels in enterocytes isolated from myriocin-treated and Sptlc1+/− mice, as well as controls. We found that myriocin-treatment significantly decreases SM levels in enterocytes, but has no effect on cellular cholesterol, phosphatidylcholine, or triacylglycerol levels (Table 3). We also found that Sptlc1+/− and WT mice have same lipid levels (Table 3). These results suggest that cellular lipid levels may have little or no effect on the observed phenotype, i.e. decreasing cholesterol absorption. Second, we took advantage of lysenin, a recently discovered SM-specific cytotoxin to measure apical membrane SM levels, since lysenin can recognize SM only when it forms aggregates or microdomains in the plasma membranes [26]. We carried out an in situ lysenin assay: intestines were turned inside-out and cut into 0.5 cm segments from jejunum, and these were incubated with 5 µg/ml lysenin. Cell metabolic capacity in tissue segments was measured by adding WST-1 cell proliferation reagent. As indicated in Fig. 5A and B, intestinal segments from myriocin-treated or Sptlc1+/− mice showed significantly less sensitivity to lysenin-mediated the reduction of metabolic capacity than controls, indicating a decrease of SM levels in the apical membranes. To further investigate that Sptlc1 deficiency has impact on lysenin affinity, we cut out a 3 cm jejunum and sealed at both ends. We then injected lysenin-RFP into the lumen of the sealed intestine which underwent incubation at 37 °C for 30 min. We checked intestinal frozen sections and found that Sptlc1+/− small intestine has less lysenin-RFP binding compared with that of controls (Fig. 5C).
Table 3.
Mouse enterocyte lipid measurement
| SM µg/mg protein |
PC | Chol | TG | |
|---|---|---|---|---|
| WT mice | ||||
| Control | 10.5 ± 0.5 | 30.6 ± 0.7 | 15.0 ± 1.1 | 11.3 ± 1.7 |
| Myriocin | 6.2 ± 0.9* | 27.9 ± 2.3 | 16.2 ± 1.2 | 11.1 ± 1.9 |
| Mice | ||||
| WT | 9.8 ± 1.1 | 32.1 ± 1.8 | 16.0 ± 0.4 | 12.0 ± 0.4 |
| Sptlc1+/− | 9.3 ± 1.4 | 30.4 ± 1.6 | 15.7 ± 0.2 | 11.7 ± 0.2 |
Value, mean ± SD.
P < 0.01, N = 5–7.
SM, sphingomyelin; PC, phosphatidylcholine; Chol, cholesterol; TG, triacylglycerols.
Fig. 5.
SPT deficiency decreased SM levels on the apical surface of mouse small intestine. In (Panels A and B) small intestines were turned inside-out and cut into 0.5 cm of segments from jejunum. The segments were then bathed in 0.5 ml of oxygenated DMEM containing 5% glutamine with 5 µg/ml lysenin or without it as control, at 37 °C for 30 min. Then 0.05 ml of WST-1 cell proliferation reagent was added and continuously incubated at 37 °C for 15 min. After incubation, solutions were transferred to an Eppendorf tube and quick-spun at maximum speed to pellet cell debris. Supernatants were read OD at 450 nm, a reading for viable cells. Background reading was the WST-1 reagent with no cell treatment. (Panel A) WT vs WT/myriocin; (Panel B) WT vs Sptlc1+/−. Value is mean ± SD (n = 4). *P<0.01. (Panel C) lysenin-RFP staining. A 3 cm long jejunum was cut out and sealed at both ends, lysenin-RFP was put into the intestinal lumen. The intestinal segments were bathed in oxygenated DMEM containing 5% glutamine at 37 °C for 30 min, and then washed with the above buffer. Frozen sections were prepared. The lysenin-RFP staining was examined under microscope (×40). The arrows point to bush borders.
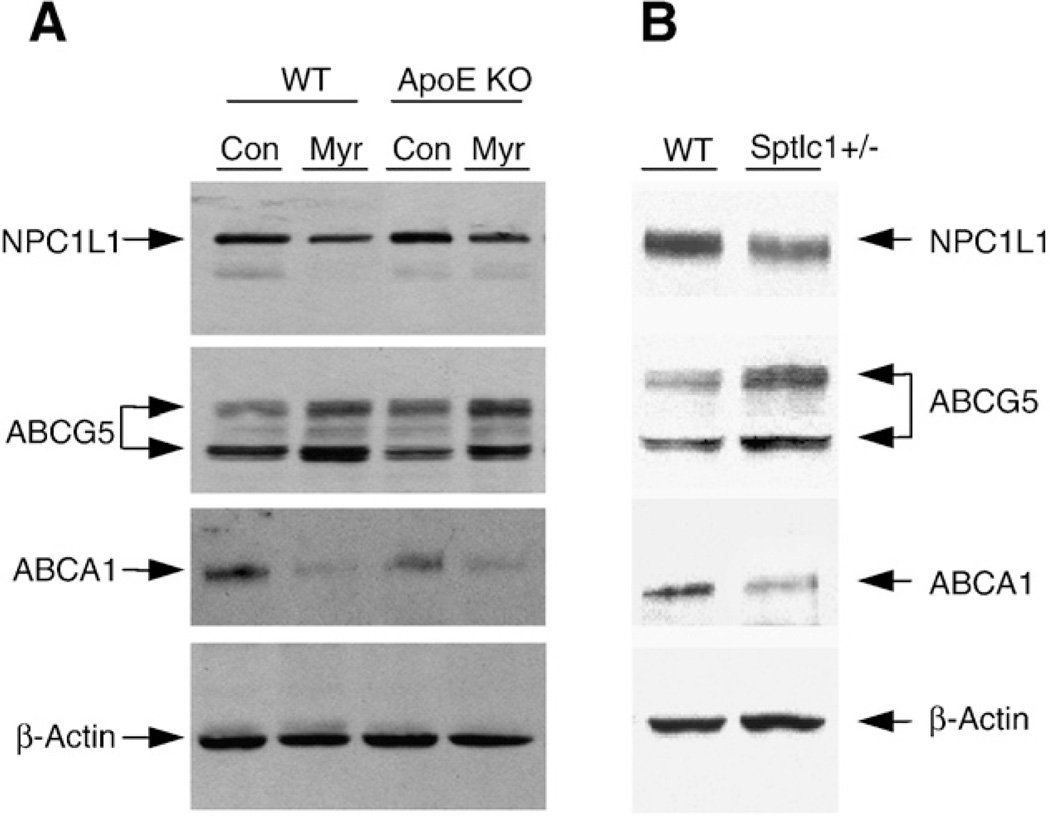
We next sought to do western blot measurement of NPC1L1 and ABCG5/G8, which are located in apical membranes of the enterocytes [2,18]. We found that myriocin treatment significantly decreased NPC1L1 (55 ± 7% and 50 ± 5%, P<0.01, n = 3, respectively) and increased ABCG5 protein mass (61 ± 8% and 59 ± 10%, P<0.01, n = 3, respectively) in WT and apoE KO mice, compared with non-treated animals (Fig. 6A). Moreover, small intestine from Sptlc1+/− mice contained significantly less NPC1L1 (66 ± 5%, P<0.01, n = 3) and more ABCG5 (70 ± 6%, P<0.01, n = 3) than that from WT mice (Fig. 6B). These results suggested that a decrease in apical membrane SM levels might decrease those of NPC1L1 and increase those of ABCG5/G8, thus diminishing cholesterol absorption. We also measured ABCA1, which is located in the basal membranes of the enterocytes and is involved in cholesterol secretion [6]. We found that ABCA1 was decreased in SPT-decreasing small intestines (60 ± 11%, 68 ± 4%, and 58 ± 9%, P<0.01, n = 3, respectively) compared with controls (Fig. 6A and B), suggesting that the decrease in ABCA1 might also contribute to lower cholesterol absorption in these mice.
Fig. 6.
SPT deficiency decreased NPC1L1 and ABCA1, and increased ABCG5 in mouse small intestine. Tissue homogenates were prepared as described in Methods, and equal amounts of homogenates from each mouse were pooled in each group (n = 4–5). A total of 50 µg pooled sample from each group was immunoblotted with antibodies against NPC1L1, ABCA1, and ABCG5. Immunoblots of β-actin were used as a loading control. These results are representative of three independent experiments. (A) WT and apoE KO mice with or without myriocin treatment; (B) WT and Sptlc1+/− mice.
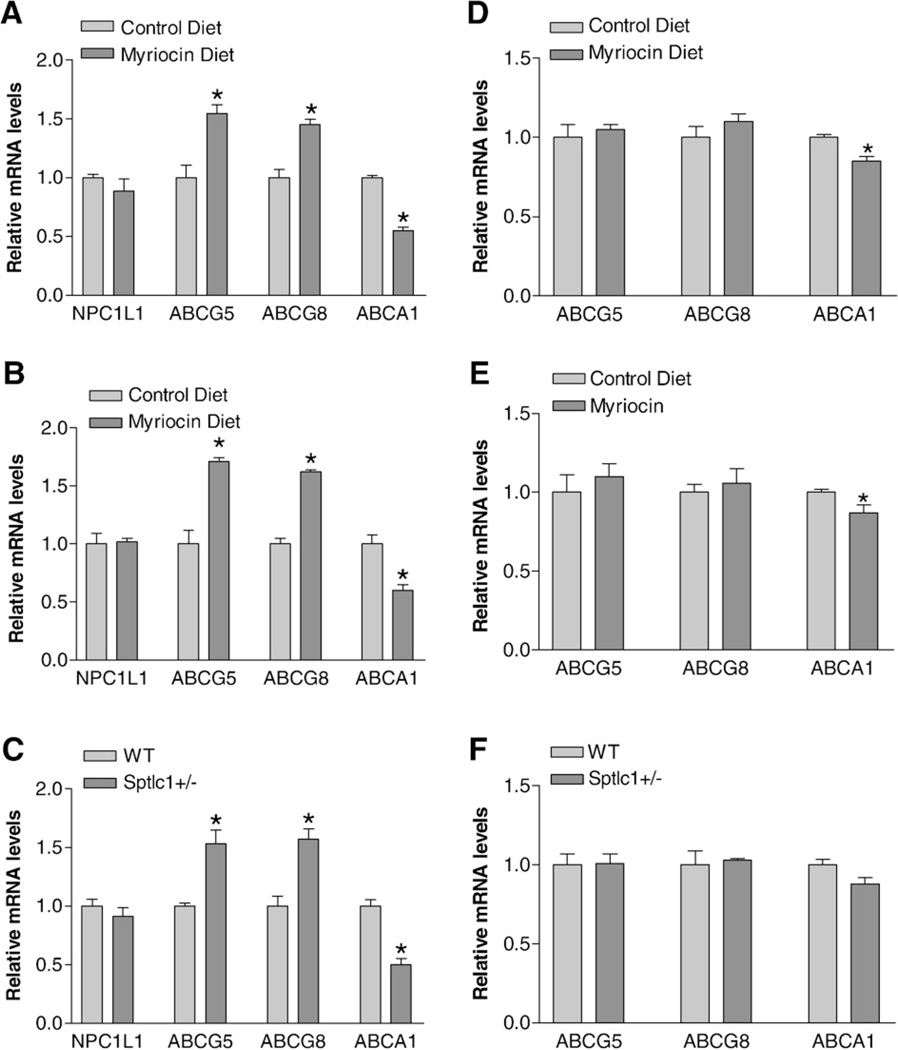
For further elucidation of the possible mechanisms for cholesterol absorption reduction in the SPT-deficient small intestine, we measured NPC1L1, ABCG5/G8, and ABCA1 mRNA levels in myriocin-treated WT, apoE KO, and Sptlc1+/− mice, as well as in controls. In the small intestine, we found that myriocin treatment or Sptlc1 gene decreasing significantly decreased ABCA1 mRNA levels (in WT mice, 48%, P<0.01; in apoE KO mice, 39%, P<0.01; and in Sptlc1+/− mice, 51%, P<0.01), compared with controls (Fig. 7A–C). It significantly increased ABCG5 and ABCG8 mRNA levels (in WT mice, 52 and 46%, P<0.01; in apoE KO mice, 69 and 63%, P<0.01; in Sptlc1+/− mice, 49 and 53%, P<0.01), respectively, compared with controls (Fig. 7A–C). No significant changes were observed in NPC1L1 mRNA levels (Fig. 7A–C). Moreover, intestinal MTP activity was not influenced by SPT deficiency (data not shown). We also measured liver mRNA levels, finding that myriocin treatment or Sptlc1 deficiency has no influence on ABCG5/G8 mRNA levels (Fig. 7D–F). However, myriocin treatment but not Sptlc1 decreasing significantly decreased ABCA1 mRNA levels (in WT mice, 20%, P<0.05; in apoE KO mice, 18%, P<0.05; and in Sptlc1+/− mice, 16%, P = 0.075) (Fig. 7D–F). We could not detect NPC1L1 mRNA in WT or Sptlc1+/− livers (data not shown).
Fig. 7.
Mouse small intestine and liver NPC1L1, ABCG5/G8, and ABCA1 mRNA measurements. NPC1L1, ABCG5/G8, and ABCA1 mRNA levels were measured by real-time PCR as described in “Materials and methods.” (Panel A) jejunum from WT mice with or without myriocin treatment; (Panel B) jejunum from apoE KO mice with or without myriocin treatment; (Panel C) jejunum from WT and Sptlc1+/− mice; (Panel D) liver from WT mice with or without myriocin treatment; (Panel E) liver from apoE KO mice with or without myriocin treatment; (Panel F) liver from WT and Sptlc1+/− mice. Values are mean + SD, n = 5. *P<0.01.
4. Discussion
The present study provides the first evidence that SPT plays an important role in intestinal cholesterol absorption. We also observed that SPT ablation decreased cholesterol but not triglyceride absorption. Decreased absorption was correlated with lower levels of NPC1L1 and ABCA1, and higher levels of ABCG5/G8, in the small intestine.
We have reported that myriocin treatment (intraperitoneal injection) decreases plasma SM levels and atherosclerosis in apoE KO mice [27] and this was confirmed by other groups of researchers [19,28–30]. Our results were identical with those obtained by Park et al., except for plasma cholesterol levels [19]. Their results indicated that myriocin treatment (oral administration) not only decreased SM but also cholesterol levels in apoE KO animals. We hypothesized that oral administration but not intraperitoneal injection of myriocin reduces cholesterol absorption in small intestine, thus decreasing plasma cholesterol levels in apoE KO mice. In fact, this hypothesis was the rationale of this study. We repeated Park et al.'s experiment (fed the apoE KO with myriocin-contained diet) and found that, indeed, myriocin treatment (oral) not only decreased SM but also cholesterol levels in apoE KO animals (Table 1). Many researches indicated that sphingolipid metabolism is closely related the development of atherosclerosis [19,27–31].
To investigate possible gastrointestinal toxicity, we specifically examined the morphology of the small intestine after myriocin treatment, finding no pathological changes in the tissue. As shown in Fig. 2, all specimens had intact villi, epithelia, enterocytes, goblet cells, and brush borders. This was likewise true for the small intestine specimens obtained from Sptlc1+/− mice. Thus, myriocin treatment and Sptlc1 deficiency cause physiological rather than morphological changes in the small intestine.
SPT deficiency reduced cholesterol absorption, which might explain why apoE KO mice had less cholesterol in the circulation. ApoE-mediated cholesterol clearance is a major pathway for removing chylomicron and remnant cholesterol from the circulation [32]. ApoE KO mice have a defect in removing apoB48-, cholesterol-, SM-rich particle pathway [33], those particles are accumulated in the circulation [33] (Table 1). On the other hand, WT and Sptlc1+/− animals have a normal apoE-mediated cholesterol clearance pathway and do not accumulate chylomicron remnants, so it is not surprising that the defect in cholesterol absorption is not reflected in plasma cholesterol levels (Table 1).
SPT activity makes an important contribution to the cell membrane structure. The interaction of SM and cholesterol drives the formation of plasma membrane rafts [12]. As much as 65% of all cellular SM is found in such rafts [14]. A general consensus has developed over the last few years that the rafts represent a platform for different cell functions [12,13]. Using in situ lysenin treatment, we observed for the first time that myriocin treatment or Sptlc1 deficiency decreases SM levels in the apical surface of the enterocytes (Fig. 5). Since lysenin recognizes SM only when it forms aggregates or domains [26], our data suggest that SPT activity is responsible for the level of apical membrane SM. This in turn modulates the formation or maintenance of microdomains in the membranes, influences the molecules (such as NPC1L1 and ABCG5/G8) that are located in such microdomains [2,18], and, thus, influences cholesterol absorption. We do not know the exact reason why small intestine SM levels in Spt1c1+/− mice did not decrease, while that of myriocin treated mice decreased significantly (Table 3). This is also true in the liver (Hojjati and Jiang unpublished observation). However, we speculate that myriocin treatment might create a more potent effect than Sptlc1 partial deficiency, in terms of tissue SM levels.
The present study indicates that SPT specifically regulates cholesterol uptake without affecting that of triacylglycerol. We observed that SPT deficiency decreased NPC1L1 (Fig. 6) protein but not mRNA levels (Fig. 7), suggesting that the regulation is translational or posttranslational. Moreover, since mouse liver has no detectable NPC1L1, the observed phenotype (decreased cholesterol in apoE KO mouse plasma) (Table 1), is small intestine-dependent. NPC1L1 is located on the epithelial layer bordering the luminal space [2]. NPC1L1 deficiency significantly reduces cholesterol absorption [3]. The cholesterol–NPC1L1 interaction and structural assembly of NPC1L1 may influence the kinetics of net cholesterol movement across the cell membranes of the enterocyte. It is therefore crucial to investigate how structural protein integrity or assembly at the cell membrane level is maintained during the intestinal absorption of cholesterol. It is possible that SPT deficiency affects the SM composition of cellular organelles, such as plasma membrane and the endoplasmic reticulum, and alters intracellular transport and localization of NPC1L1, resulting in reduced NPC1L1 proteins and assimilation of cholesterol by the enterocytes.
As shown in this study, SPT deficiency increases small intestine ABCG5 protein levels. Human sitosterolemia is caused by mutations in either ABCG5 or ABCG8 [34]. Intestinal absorption of cholesterol in patients with sitosterolemia is increased by about 30% and intestinal absorption of sitosterol is increased by about 800%, as measured by plasma dual-isotope labeling methods [35]. These increments in humans are consistent with mouse studies in ABCG5/G8 KO animals [36]. Overexpression of ABCG5 and ABCG8 reduces fractional absorption of dietary cholesterol [37]. Immunohistochemical analyses show that ABCG5 and ABCG8 proteins are apically localized in the small intestine [18]. It is possible that SPT deficiency affects SM composition on the apical surface of the enterocytes and increases ABCG5/G8 proteins or alters their localization, resulting in more cholesterol being pumped back into the lumen of the intestine, and less of it being absorbed. However, we can rule out the possibility that the regulation of ABCG5/G8 is transcriptional in the small intestine, since ABCG5/G8 mRNA levels are increased there but not in the liver (Fig. 7).
SPT deficiency also decreases ABCA1 levels in the small intestine. We and others have shown that apoAI and ABCA1 play a role in intestinal cholesterol secretion, and this process also makes a contribution to cholesterol absorption [6,38]. ABCA1 resides in the basal membranes of the enterocytes [38]. ABCA1-dependent cholesterol export involves an initial interaction of apoA-I with lipid raft membrane domains [39]. It is conceivable that SPT reduction not only decreases SM levels on the apical surface of the enterocytes (Fig. 5), but also decreases SM levels in the basal membranes where ABCA1 is located. The downregulation of ABCA1 might also make a contribution to decreased cholesterol absorption in SPT deficient mice. On the other hand, ABCA1 could be decreased by the reduction in enterocyte cholesterol uptake, as reported in NPC1L1 KO mice [40]. Again, we cannot rule out the possibility that the regulation of ABCA1 is transcriptional in the small intestine, since ABCA1 mRNA levels are decreased there (Fig. 7A–C).
Although we favor SM-related mechanism, we cannot exclude the possible involvement of sphingolipids, other than SM, in the regulation of NPC1L1, ABCG5/G8, and ABCA1, since SPT is the key enzyme for biosynthesis of all the sphingolipids, including ceramide and sphingosine-1-phosphate [8]. Indeed, ceramide can regulate ABCA1 [41,42]. FTY720, an analogue of sphingosine-1-phosphate, increases plasma cholesterol levels in apoE KO mice [43]. It has also been reported that increase ceramide levels in lipid rafts by sphingomyelinase treatment regulates cell functions [44]. The detailed mechanism involved in the regulation of NPC1L1, ABCG5/G8, and ABCA1 in the SPT deficient small intestine requires further investigation.
In summary, we have shown that SPT deficiency leads to less cholesterol absorption. This is associated with reduced SM levels on the apical surface of the enterocytes, as well as decreased NPC1L1 and increased ABCG5 proteins in the small intestine. This may also relate to reduction of SM levels in the basal membrane of the enterocytes, and downregulation of ABCA1 in these cells. Decreased cholesterol absorption could be a mechanism contributing to the low cholesterol levels and decreased atherosclerosis found in SPT deficient apoE KO mice [19,27]. Thus, SPT might be an excellent candidate for therapeutic intervention, and its inhibition could be very useful in lowering plasma cholesterol levels and decreasing atherosclerosis.
Abbreviations
- SPT
serine palmitoylCoA transferase
- Sptlc1
serine palmitoylCoA transferase long chain base subunit 1
- ABC
ATP binding cassette protein
- NPC1L1
Niemann-Pick C1-like 1
- KO
knockout
- WT
wild type
Appendix A. Supplementary data
Supplementary data associated with this article can be found, in the online version, at doi:10.1016/j.bbalip.2009.01.010.
Footnotes
This work was supported by grants HL69817 and AHA Grant-in-Aid (0755922T). Liqing Yu and Weiqing Tang were supported by Scientist Development Grant 0635261N from the American Heart Association.
References
- 1.Gylling H, Miettinen TA. The effect of cholesterol absorption inhibition on low density lipoprotein cholesterol level. Atherosclerosis. 1995;117:305–308. doi: 10.1016/0021-9150(95)05566-f. [DOI] [PubMed] [Google Scholar]
- 2.Altmann SW, Davis HR, Jr, Zhu LJ, Yao X, Hoos LM, Tetzloff G, Iyer SP, Maguire M, Golovko A, Zeng M, Wang L, Murgolo N, Graziano MP. Niemann-Pick C1 like 1 protein is critical for intestinal cholesterol absorption. Science. 2004;303:1201–1204. doi: 10.1126/science.1093131. [DOI] [PubMed] [Google Scholar]
- 3.Garcia-Calvo M, Lisnock J, Bull HG, Hawes BE, Burnett DA, Braun MP, Crona JH, Davis HR, Jr, Dean DC, Detmers PA, Graziano MP, Hughes M, Macintyre DE, Ogawa A, O'neill KA, Iyer SP, Shevell DE, Smith MM, Tang YS, Makarewicz AM, Ujjainwalla F, Altmann SW, Chapman KT, Thornberry NA. The target of ezetimibe is Niemann-Pick C1-like 1 (NPC1L1) Proc. Natl. Acad. Sci. U. S. A. 2005;102:8132–8137. doi: 10.1073/pnas.0500269102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yu L, Bharadwaj S, Brown JM, Ma Y, Du W, Davis MA, Michaely P, Liu P, Willingham MC, Rudel LL. Cholesterol-regulated translocation of NPC1L1 to the cell surface facilitates free cholesterol uptake. J. Biol. Chem. 2006;281:6616–6624. doi: 10.1074/jbc.M511123200. [DOI] [PubMed] [Google Scholar]
- 5.Yu L, York J, von Bergmann K, Lutjohann D, Cohen JC, Hobbs HH. Stimulation of cholesterol excretion by the liver X receptor agonist requires ATP-binding cassette transporters G5 and G8. J. Biol. Chem. 2003;278:15565–15570. doi: 10.1074/jbc.M301311200. [DOI] [PubMed] [Google Scholar]
- 6.Iqbal J, Hussain MM. Evidence for multiple complementary pathways for efficient cholesterol absorption in mice. J. Lipid Res. 2005;46:1491–1501. doi: 10.1194/jlr.M500023-JLR200. [DOI] [PubMed] [Google Scholar]
- 7.Iqbal J, Anwar K, Hussain MM. Multiple, independently regulated pathways of cholesterol transport across the intestinal epithelial cells. J. Biol. Chem. 2003;278:31610–31620. doi: 10.1074/jbc.M301177200. [DOI] [PubMed] [Google Scholar]
- 8.Merrill AH, Jones DD. An update of the enzymology and regulation sphingomyelin metabolism. Biochim. Biophysi. Acta. 1990;1044:1–12. doi: 10.1016/0005-2760(90)90211-f. [DOI] [PubMed] [Google Scholar]
- 9.Weiss B, Stoffel W. Human and murine serine-palmitoyl-CoA transferase — cloning, expression and characterization of the key enzyme in sphingolipid synthesis. Eur. J. Biochem. 1997;249:239–247. doi: 10.1111/j.1432-1033.1997.00239.x. [DOI] [PubMed] [Google Scholar]
- 10.Hanada K, Hara T, Nishijima M, Kuge O, Dickson RC, Nagiec MM. A mammalian homolog of the yeast LCB1 encodes a component of serine palmitoyltransferase, the enzyme catalyzing the first step in sphingolipid synthesis. J. Biol. Chem. 1997;272:32108–32114. doi: 10.1074/jbc.272.51.32108. [DOI] [PubMed] [Google Scholar]
- 11.Hornemann T, Richard S, Rütti MF, Wei Y, von Eckardstein A. Mammalian serine-palmitoyltransferase — cloning and initial characterisation of a new subunit. J. Biol. Chem. 2006;281:37275–37281. doi: 10.1074/jbc.M608066200. [DOI] [PubMed] [Google Scholar]
- 12.Simons K, Ikonen E. Functional rafts in cell membranes. Nature. 1997;387:569–572. doi: 10.1038/42408. [DOI] [PubMed] [Google Scholar]
- 13.Simons K, Ikonen E. How cells handle cholesterol. Science. 2000;290:1721–1726. doi: 10.1126/science.290.5497.1721. [DOI] [PubMed] [Google Scholar]
- 14.Li Z, Hailemariam TK, Zhou H, Li Y, Duckworth DC, Peake DA, Zhang Y, Kuo MS, Cao G, Jiang XC. Inhibition of sphingomyelin synthase (SMS) affects intracellular sphingomyelin accumulation and plasma membrane lipid organization. Biochim. Biophys. Acta. 2007;1771:1186–1194. doi: 10.1016/j.bbalip.2007.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Christiansen K, Carlsen J. Microvillus membrane vesicles from pig small intestine. Purity and lipid composition. Biochim. Biophys. Acta. 1981;647:188–195. doi: 10.1016/0005-2736(81)90245-5. [DOI] [PubMed] [Google Scholar]
- 16.Brown DA, Rose JK. Sorting of GPI-anchored proteins to glycolipid-enriched membrane subdomains during transport to the apical cell surface. Cell. 1992;68:533–544. doi: 10.1016/0092-8674(92)90189-j. [DOI] [PubMed] [Google Scholar]
- 17.Field FJ, Born E, Murthy S, Mathur SN. Caveolin is present in intestinal cells: role in cholesterol trafficking? J. Lipid Res. 1998;39:1938–1950. [PubMed] [Google Scholar]
- 18.Graf GA, Yu L, Li WP, Gerard R, Tuma PL, Cohen JC, Hobbs HH. ABCG5 and ABCG8 are obligate heterodimers for protein trafficking and biliary cholesterol excretion. J. Biol. Chem. 2003;278:48275–48282. doi: 10.1074/jbc.M310223200. [DOI] [PubMed] [Google Scholar]
- 19.Park TS, Panek RL, Mueller SB, Hanselman JC, Rosebury WS, Robertson AW, Kindt EK, Homan R, Karathanasis SK, Rekhter MD. Inhibition of sphingomyelin synthesis reduces atherogenesis in apolipoprotein E-knockout mice. Circulation. 2004;110:3465–3471. doi: 10.1161/01.CIR.0000148370.60535.22. [DOI] [PubMed] [Google Scholar]
- 20.Sehayek E, Ono JG, Shefer S, Nguyen LB, Wang N, Batta AK, Salen G, Smith JD, Tall AR, Breslow JL. Biliary cholesterol excretion: a novel mechanism that regulates dietary cholesterol absorption. Proc. Natl. Acad. Sci. U. S. A. 1998;95:10194–10199. doi: 10.1073/pnas.95.17.10194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang DQ, Carey MC. Measurement of intestinal cholesterol absorption by plasma and fecal dual-isotope ratio, mass balance, and lymph fistula methods in the mouse: an analysis of direct versus indirect methodologies. J. Lipid Res. 2003;44:1042–1059. doi: 10.1194/jlr.D200041-JLR200. [DOI] [PubMed] [Google Scholar]
- 22.Carr TP, Andresen CJ, Rudel LL. Enzymatic determination of triglyceride, free cholesterol, and total cholesterol in tissue lipid extracts. Clin. Biochem. 1993;26:39–42. doi: 10.1016/0009-9120(93)90015-x. [DOI] [PubMed] [Google Scholar]
- 23.Hojjati MR, Jiang XC. Rapid, specific, and sensitive measurements of plasma sphingomyelin and phosphatidylcholine. J. Lipid Res. 2006;47:673–676. doi: 10.1194/jlr.D500040-JLR200. [DOI] [PubMed] [Google Scholar]
- 24.Hojjati MR, Li Z, Jiang XC. Serine palmitoyl-CoA transferase (SPT) deficiency and sphingolipid levels in mice. Biochim. Biophys. Acta. 2005;1737:44–51. doi: 10.1016/j.bbalip.2005.08.006. [DOI] [PubMed] [Google Scholar]
- 25.Kiyokawa E, Baba T, Otsuka N, Makino A, Ohno S, Kobayashi T. Spatial and functional heterogeneity of sphingolipid-rich membrane domains. J. Biol. Chem. 2005;280:24072–24084. doi: 10.1074/jbc.M502244200. [DOI] [PubMed] [Google Scholar]
- 26.Ishitsuka R, Kobayashi T. Cholesterol and lipid/protein ratio control the oligomerization of a sphingomyelin-specific toxin, lysenin. Biochemistry. 2007;46:1495–1502. doi: 10.1021/bi061290k. [DOI] [PubMed] [Google Scholar]
- 27.Hojjati MR, Li Z, Zhou H, Tang S, Huan C, Ooi E, Lu S, Jiang XC. Effect of myriocin on plasma sphingolipid metabolism and atherosclerosis in apoE-deficient mice. J. Biol. Chem. 2005;280:10284–10289. doi: 10.1074/jbc.M412348200. [DOI] [PubMed] [Google Scholar]
- 28.Glaros EN, Kim WS, Wu BJ, Suarna C, Quinn CM, Rye KA, Stocker R, Jessup W, Garner B. Inhibition of atherosclerosis by the serine palmitoyl transferase inhibitor myriocin is associated with reduced plasma glycosphingolipid concentration. Biochem. Pharmacol. 2007;73:1340–1346. doi: 10.1016/j.bcp.2006.12.023. [DOI] [PubMed] [Google Scholar]
- 29.Park TS, Panek RL, Rekhter MD, Mueller SB, Rosebury WS, Robertson A, Hanselman JC, Kindt E, Homan R, Karathanasis SK. Modulation of lipoprotein metabolism by inhibition of sphingomyelin synthesis in ApoE knockout mice. Atherosclerosis. 2006;189:264–272. doi: 10.1016/j.atherosclerosis.2005.12.029. [DOI] [PubMed] [Google Scholar]
- 30.Park TS, Rosebury W, Kindt EK, Kowala MC, Panek RL. Serine palmitoyltransferase inhibitor myriocin induces the regression of atherosclerotic plaques in hyperlipidemic ApoE-deficient mice. Pharmacol. Res. 2008;58:45–51. doi: 10.1016/j.phrs.2008.06.005. [DOI] [PubMed] [Google Scholar]
- 31.Bodary PF, Shen Y, Vargas FB, Bi X, Ostenso KA, Gu S, Shayman JA, Eitzman DT. Alpha-galactosidase A deficiency accelerates atherosclerosis in mice with apolipoprotein E deficiency. Circulation. 2005;111:629–632. doi: 10.1161/01.CIR.0000154550.15963.80. [DOI] [PubMed] [Google Scholar]
- 32.Weisgraber KH, Innerarity TL, Rall SC, Jr, Mahley RW. Receptor interactions controlling lipoprotein metabolism. Can. J. Biochem. Cell Biol. 1985;63:898–905. doi: 10.1139/o85-111. [DOI] [PubMed] [Google Scholar]
- 33.Jeong TS, Schissel SL, Tabas I, Pownall HJ, Tall AR, Jiang XC. Increased sphingomyelin content of plasma lipoproteins in apolipoprotein E knockout mice reflects combined production and catabolic defects and enhances reactivity with mammalian sphingomyelinase. J. Clin. Invest. 1998;101:905–912. doi: 10.1172/JCI870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Berge KE, Tian H, Graf GA, Yu L, Grishin NV, Schultz J, Kwiterovich P, Shan B, Barnes R, Hobbs HH. Accumulation of dietary cholesterol in sitosterolemia caused by mutations in adjacent ABC transporters. Science. 2000;290:1771–1775. doi: 10.1126/science.290.5497.1771. [DOI] [PubMed] [Google Scholar]
- 35.Salen G, Tint GS, Shefer S, Shore V, Nguyen L. Increased sitosterol absorption is offset by rapid elimination to prevent accumulation in heterozygotes with sitosterolemia. Arterioscler. Thromb. 1992;12:563–568. doi: 10.1161/01.atv.12.5.563. [DOI] [PubMed] [Google Scholar]
- 36.Wang HH, Patel SB, Carey MC, Wang DQ. Quantifying anomalous intestinal sterol uptake, lymphatic transport, and biliary secretion in Abcg8(−/−) mice. Hepatology. 2007;45:998–1006. doi: 10.1002/hep.21579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yu L, Li-Hawkins J, Hammer RE, Berge KE, Horton JD, Cohen JC, Hobbs HH. Overexpression of ABCG5 and ABCG8 promotes biliary cholesterol secretion and reduces fractional absorption of dietary cholesterol. J. Clin. Invest. 2002;110:671–680. doi: 10.1172/JCI16001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Brunham LR, Kruit JK, Iqbal J, Fievet C, Timmins JM, Pape TD, Coburn BA, Bissada N, Staels B, Groen AK, Hussain MM, Parks JS, Kuipers F, Hayden MR. Intestinal ABCA1 directly contributes to HDL biogenesis in vivo. J. Clin. Invest. 2006;116:1052–1062. doi: 10.1172/JCI27352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gaus K, Kritharides L, Schmitz G, Boettcher A, Drobnik W, Langmann T, Quinn CM, Death A, Dean RT, Jessup W. Apolipoprotein A-1 interaction with plasma membrane lipid rafts controls cholesterol export from macrophages. FASEB J. 2004;18:574–576. doi: 10.1096/fj.03-0486fje. [DOI] [PubMed] [Google Scholar]
- 40.Davis HR, Jr, Zhu LJ, Hoos LM, Tetzloff G, Maguire M, Liu J, Yao X, Iyer SP, Lam MH, Lund EG, Detmers PA, Graziano MP, Altmann SW. Niemann-Pick C1 like 1 (NPC1L1) is the intestinal phytosterol and cholesterol transporter and a key modulator of whole-body cholesterol homeostasis. J. Biol. Chem. 2004;279:33586–33592. doi: 10.1074/jbc.M405817200. [DOI] [PubMed] [Google Scholar]
- 41.Witting SR, Maiorano JN, Davidson WS. Ceramide enhances cholesterol efflux to apolipoprotein A-I by increasing the cell surface presence of ATP-binding cassette transporter A1. J. Biol. Chem. 2003;278:40121–40127. doi: 10.1074/jbc.M305193200. [DOI] [PubMed] [Google Scholar]
- 42.Ghering AB, Davidson WS. Ceramide structural features required to stimulate ABCA1-mediated cholesterol efflux to apolipoprotein A-I. J. Lipid Res. 2006;47:2781–2788. doi: 10.1194/jlr.M600380-JLR200. [DOI] [PubMed] [Google Scholar]
- 43.Klingenberg R, Nofer JR, Rudling M, Bea F, Blessing E, Preusch M, Grone HJ, Katus HA, Hansson GK, Dengler TJ. Sphingosine-1-phosphate analogue FTY720 causes lymphocyte redistribution and hypercholesterolemia in ApoE-deficient mice. Arterioscler. Thromb. Vasc. Biol. 2007;27:2392–2399. doi: 10.1161/ATVBAHA.107.149476. [DOI] [PubMed] [Google Scholar]
- 44.Diaz O, Mébarek-Azzam S, Benzaria A, Dubois M, Lagarde M, Némoz G, Prigent AF. Disruption of lipid rafts stimulates phospholipase d activity in human lymphocytes: implication in the regulation of immune function. J. Immunol. 2005;175:8077–8086. doi: 10.4049/jimmunol.175.12.8077. [DOI] [PubMed] [Google Scholar]