Abstract
Radiographic hip osteoarthritis (RHOA) is associated with increased hip bone mineral density (aBMD). We examined whether femoral geometry was associated with RHOA independently of aBMD.
Participants from the Study for Osteoporotic Fractures (SOF) with pelvis radiographs at visits 1 and 5 (8.3yrs apartt) and hip DXA (2yrs after baseline) were included. Prevalent and incident RHOA phenotypes were defined as composite (osteophytes and joint space narrowing (JSN)), atrophic (JSN without osteophytes) and osteophytic (femoral osteophytes without JSN). Analogous definitions of progression were based on minimum joint space and total osteophyte score. Hip DXA scans were assessed using the hip structural analysis program to derive geometric measures including femoral neck length, width and centroid position. Relative risks (95% CI) for prevalent, incident and progressive RHOA per SD increase in geometric measure were estimated in a hip-based analysis using multinomial logistic regression and adjusting for age, body mass index, knee height and total hip aBMD.
In 5245 women (mean age 72.6 yrs), a wider femoral neck with more medial centroid position was associated with prevalent and incident osteophytic and composite RHOA phenotypes (p<0.05). Increased neck width and centroid position were significantly associated with osteophyte progression (both p<0.05). No significant geometric associations were found with atrophic RHOA. Differences in proximal femoral bone geometry and distribution occur early in hip OA and predict prevalent, incident and progressive osteophytic and composite, but not the atrophic, phenotypes. These bone differences may reflect responses to loading occuring early in the natural history of RHOA.
Introduction
Osteoarthritis (OA) of the hip is an important cause of pain and disability in the elderly and is the most common indication for total hip replacement surgery [1]. There is an urgent need to understand the mechanisms involved in both incident and progressive disease to enable targeting with interventional therapies to those at greatest risk as well as provide prognostic information for patients. While OA is considered to be a disease of cartilage with changes in bone structure occurring later in the disease, there is an increasing body of evidence highlighting the role of bone in the pathogenesis of OA [2, 3]. Studies have suggested an inverse relationship between osteoarthritis and osteoporosis at the hip [4] and we have previously demonstrated that radiographic hip OA (RHOA) is associated with higher areal bone mineral density (aBMD) at axial and appendicular sites suggesting a systemic bone phenotype in hip OA [5]. However measurements of aBMD are affected by bone size [6] and the distribution of bone mineral within the periosteal envelope may differ in hips with the same size and areal density.
In addition, abnormal loading of the joint is an important risk factor for OA of the hip and knee [7] and, once established, OA in turn alters the loading on the affected joint, often in ways that are not predictable. Since it is well known that bones adapt their mass and geometry to loading conditions [8], the altered loading conditions associated with OA should be expected to produce changes in peri-articular bone geometry. While RHOA is associated with higher BMD of the proximal femur, the geometric changes in the proximal femur associated with the development and progression of OA have not been studied using epidemiological methods.
In this study we used a technique that permits a geometric interpretation of dual energy x-ray absorptiometry (DXA) scans, acquired for measuring hip BMD, in order to evaluate the association of proximal femur geometry and mineral distribution with the prevalence, incidence and progression of RHOA.
Methods
Subjects were participants in the Study of Osteoporotic Fractures, a multicentre cohort study to determine risk factors for osteoporotic fractures in 9704 white women. Participants were all aged 65 years and older at the baseline examination and were recruited between September 1986 and October 1988 from four population-based listings of the United States: Baltimore, MD, Minneapolis, MN, Portland, OR and Monongahela Valley near Pittsburgh, PA [9]. Non-white women were excluded because of their low incidence of hip fracture, as were women who were non-ambulatory or had undergone bilateral hip replacement. The study was approved by the appropriate committees on human research and all women gave written informed consent.
Radiographic OA imaging and definitions
Participants had a supine antero-posterior pelvic radiograph with their legs in 15–30 degrees of internal rotation at baseline and at visit 5. The mean follow-up period was 8.3 years (range 7.4 to 10.4 yrs). Follow-up radiographs were obtained from 62% of the original cohort and 73% of the survivors at the time of follow-up [10]. 467 women received their follow-up radiographs at home using portable equipment.
Pelvis radiographs were read for individual radiographic features of hip OA using an atlas to standardize the readings [11] [12]. Each hip was graded for joint space narrowing (JSN) on a scale of 0 (normal) to 4 (bone on bone) at two locations: lateral (from a point perpendicular to the femoral head to the lateral margin of the acetabular roof, excluding osteophytes) and medial (from a point perpendicular to the femoral head to the point where the medial continuation of the acetabular roof becomes indistinct). Osteophytes were graded 0 (normal) to 3 (severe) at four locations: lateral femoral, lateral acetabular, inferior femoral and inferior acetabular. Minimum joint space (MJS), from the lateral margin of the acetabular roof to the point where the medial continuation of the acetabular roof becomes indistinct, was measured using a calliper and reticule.
Hip osteoarthritis is recognized as a heterogeneous disease and there is no single accepted definition of RHOA for use in epidemiological studies. Most current grading schema focus on severity [13] using a combination of osteophytes, JSN and other subchondral features. As we were interested in bone changes of the proximal femur, we wished to identify hips with a predominant bone (osteophytic) response vs. those hips without such a response (atrophic/ JSN only) as well as those hips with both osteophytes and JSN. Hence we used three validated definitions [14] encompassing the common phenotypes of RHOA: a composite definition that required the presence of both definite JSN and osteophytes (grade ≥ 2 in any location) and which is equivalent to a Kellgren-Lawrence grade of 2 or more [11]; a definition of osteophytic RHOA that required definite osteophytes and no definite JSN (inferior or superior femoral osteophytes grade ≥ 2 and JSN ≤ 1 in all locations) and a definition of atrophic RHOA that required definite JSN and no definite osteophytes (grades ≥2 lateral JSN or grades ≥3 medial JSN and osteophytes ≤ 1 in all locations). These three definitions are mutually exclusive in that a hip can match only one of them at a given time. A hip with incident OA by each definition met the criteria for that definition at follow-up but not any definition at baseline.
Hips with RHOA at baseline according to any of the above three definitions were categorized for progression outcome based on loss of minimum joint space of ≥0.5 mm and/or an increase of ≥2 in the aggregate osteophyte score (sum of osteophyte scores in all 4 locations in a hip) [10]. In those hips with < 0.5mm at baseline, a reduction of ≥0.2 mm was minimum joint space progression. We defined three radiographic phenotypes of progression analogous to the radiographic phenotypes of prevalent OA using the above definition of minimum joint space and osteophyte progression: atrophic by loss of minimum joint space only, osteophytic by an increase in the aggregate osteophyte score only or composite if hip had changes in both JSN and osteophyte scores. Those hips that underwent arthroplasty were excluded from analyses, as we were unable to identify the phenotype of radiographic progression,
DXA imaging for BMD and bone geometry
Participants had a hip DXA scan (Hologic QDR 1000, Hologic Inc. Waltham, MA, USA) at visit 2 (mean 2.2 yrs (range 1.0– 4.0) after baseline). Generally, the right hip was scanned unless there was a fracture, implant, hardware or other problem preventing the right hip from being scanned, and then the left hip was scanned (11% of women).
For each DXA image, the Hip Structure Analysis (HSA) program was used to measure BMD and geometry within 3 locations in the coronal plane [15]: at a line across the femoral neck at its narrowest point (FN), at a parallel line across the inter-trochanteric (IT) region and at a line across the shaft at a distance equal to 1.5 times the narrow neck width distal to the intersection of the neck and shaft axes (Figure 1). For each proximal femur location, 5 parallel profiles of bone mass of one 1 pixel spacing were derived from one bone margin to the other.
Figure 1.
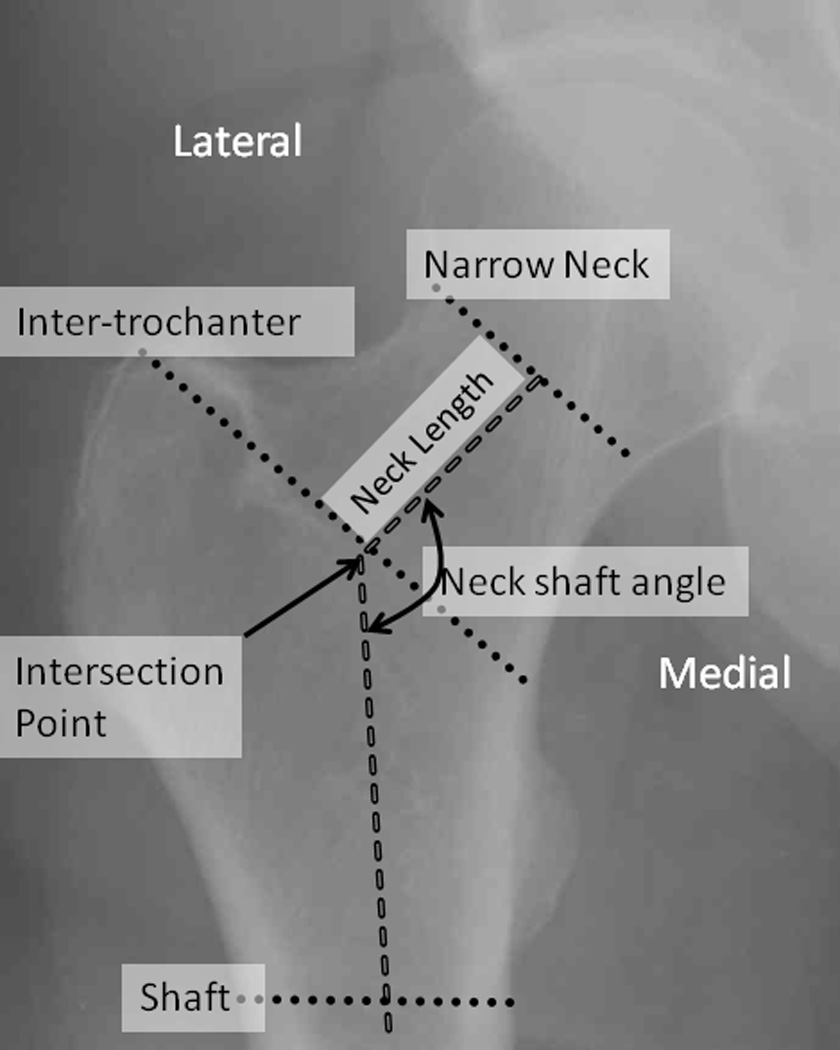
Measurement locations for hip structure analysis.
Conventional areal BMD (aBMD) was measured in the standard manner and used in the derivation of the hip geometric parameters. Sub-periosteal bone width was computed as the ‘blur corrected’ distance between the bone margins of the aBMD profile from medial to lateral cortices. The cross-sectional area (CSA) at each location was calculated by dividing the pixel values (g/cm2) at each point in the profile by the effective tissue density of bone mineral in fully mineralized adult bone and summing the values. The centroid position is a measure of the center of mass of bone across the profile for each of the three locations expressed in as the ratio of the distance from the medial bone margin to the center of mass divided by total sub-periosteal width. The higher the centroid position the more medial the center of mass. Two standard indices of bending strength, the cross sectional moment of inertia (CSMI) and the section modulus were calculated using the above directly measured parameters. Following Beck et al. [15], the CSMI was derived from the integral of bone mass times the square of its distance from the centroid position divided by the effective mineral density. Sectional modulus was computed as the ratio of CSMI to the maximum distance from the medial or lateral cortical margin to the centroid.
Data Analysis
For these analyses we included women with both a baseline pelvic radiograph read for OA features and a visit 2 hip DXA read for HSA measures. We excluded 206 women who had a previous hip fracture or hip surgery, rheumatoid arthritis, defined as a self-reported physician’s diagnosis with consistent hand radiographic findings, or Paget’s disease of bone.
As the geometric measures were only available on one side, we examined the relationship between hip geometric parameters and RHOA outcomes in hips ipsi-lateral to the side of hip DXA.
Differences in baseline characteristics of subjects by OA status were analyzed by ANOVA for continuous outcomes and chi-square statistic for dichotomous outcomes. We analyzed the association between hip geometric parameters and the three defined phenotypes of RHOA at baseline using multinomial logistic regression with robust standard errors to estimate the relative risk ratio (RR) using hips without RHOA as the referent group. The same approach was used to analyze incidence and progression of RHOA based on the three phenotypes using hips that did not have incident or progressive RHOA respectively as the referent group. Models were adjusted for age, baseline body mass index (BMI), knee height (because current height in elderly women may be affected by vertebral fractures) and aBMD at the total hip. All analyses were performed using Stata intercooled v10.0 (StataCorp LP, TX, USA).
Results
From the 9704 women seen at baseline, 5987 women had a pelvis radiograph at baseline read for radiographic features of OA and 5245 of these had a DXA hip scan at visit 2 with proximal hip geometry measurements available. Compared with the remainder of the cohort at baseline, the women included in this analysis differed significantly (p<0.05) on several characteristics but these differences were small. The women in this sample were slightly older (2.1 years), taller (0.73 cm) and heavier (0.70 kg) than the rest of the cohort but did not differ by BMI (26.4 vs. 26.4 kg/m2; p=0.24).
The number of women without RHOA in either hip, with prevalent RHOA and with incident RHOA is shown in Table 1. The number of hips with prevalent, progressive or incident RHOA by phenotypic definition is shown in Table 2. The measures of FN geometry were significantly (p<0.01) correlated with each other: neck axis length was positively associated with neck angle (r=+0.30) and negatively with width, centroid position, CSA, CSMI and section modulus (r=−0.05 to −0.26); neck width, CSA, CSMI and section modulus were positively associated with each other with the weakest correlation between width and CSA (r=+0.23) and the highest correlations between section modulus and CSMI (+0.93); centroid position was negatively associated with CSA and section modulus (r=−0.10) and positively with width (r=0.23) and CSMI (r=0.10).
TABLE 1.
Baseline characteristics of women with no RHOA at baseline or follow-up, prevalent RHOA at baseline and incident RHOA at follow-up in 5,245 white women
| No RHOA at BL or FU |
Prevalent RHOA in either hip |
Incident RHOA in one or more hips |
|
|---|---|---|---|
| N | 4,472 | 332 | 441 |
| Age (years)1 | 72.5 (4.5) | 73.9 (5.3) | 73.5 (4.8) |
| Knee Height (m)2 | 49.3 (2.3) | 49.6 (2.3) | 49.6 (2.4) |
| BMI (kg/m2)3 | 25.7 (17.0, 41.5) | 26.1 (17.9, 37.8) | 25.7 (17.9, 38.2) |
| Hip DXA measurements2 | |||
| aBMD (g/cm2) | 0.767 (0.13) | 0.765 (0.13) | 0.766 (0.13) |
| Neck length (cm) | 4.35 (0.62) | 4.28 (0.67) | 4.29 (0.64) |
| Neck shaft angle | 127.2 (6.1) | 126.8 (6.3) | 127.2 (6.2) |
| Width (cm) | 3.07 (0.28) | 3.20 (0.34) | 3.13 (0.33) |
| Centroid position4 | 0.535 (0.02) | 0.536 (0.02) | 0.539 (0.02) |
| CSA (cm2) | 2.06 (0.34) | 2.21 (0.47) | 2.14 (0.39) |
| CSMI (mm4) | 1.65 (0.43) | 1.96 (0.66) | 1.80 (0.53) |
| Section modulus | 0.99 (0.20) | 1.12 (0.29) | 1.05 (0.24) |
Mean (SD) shown, 1At visit 1;
At visit 2;
median (IQR).
Higher ratio implies more medial position of mass centre
Prevalent RHOA in either hips 5 Incident RHOA in either hips
Table 2.
Frequency of prevalent, incident and progressive RHOA phenotypes in 5,245 Caucasian women followed up for 8.3 years
Prevalent composite RHOA = definite joint space narrowing (JSN) & osteophytes (JSN grades = 2,3,4 & osteophyte grades ≥ 2);
Atrophic = definite JSN and no/minimal osteophytes (grades medial JSN= 3,4 or lateral 2,3,4 with osteophyte ≤ 1;
Trophic = definite femoral osteophytes and no/ minimal JSN (grades femoral osteophyte ≥ 2 with JSN grade ≤ 1.
Progression defined as composite (MJS reduction ≥ 0.5mm & total osteophyte score increased ≥ 2); atrophic (MJS reduction ≥ 0.5mm & total osteophyte score increased < 2) and osteophytic (total osteophyte score increased ≥ 2 & MJS reduction < 0.5mm).
Incident RHOA defined as meeting definitions for RHOA at visit 5 but not at baseline.
Associations of hip geometry parameters with Prevalent RHOA
For the femoral neck measures, a wider bone width, a more medial centroid position, and greater CSMI, section modulus and cross sectional area were associated with an increased risk of prevalent RHOA defined by the composite and osteophytic phenotype, but not by the atrophic definition before and after adjusting for age, BMI, knee height and total hip aBMD (Figure 2). A longer femoral neck was associated with a decreased risk of prevalent RHOA defined by the composite phenotype. When the further distal IT and shaft locations were examined, most geometric measures at these sites were not significantly associated with RHOA types. However, there was a significantly increased risk of osteophytic RHOA with greater bone width at the IT (adjusted RR 1.6 [1.0–2.4]) and shaft (adjusted RR 1.4 [1.0–2.0]) subregions.
Figure 2.
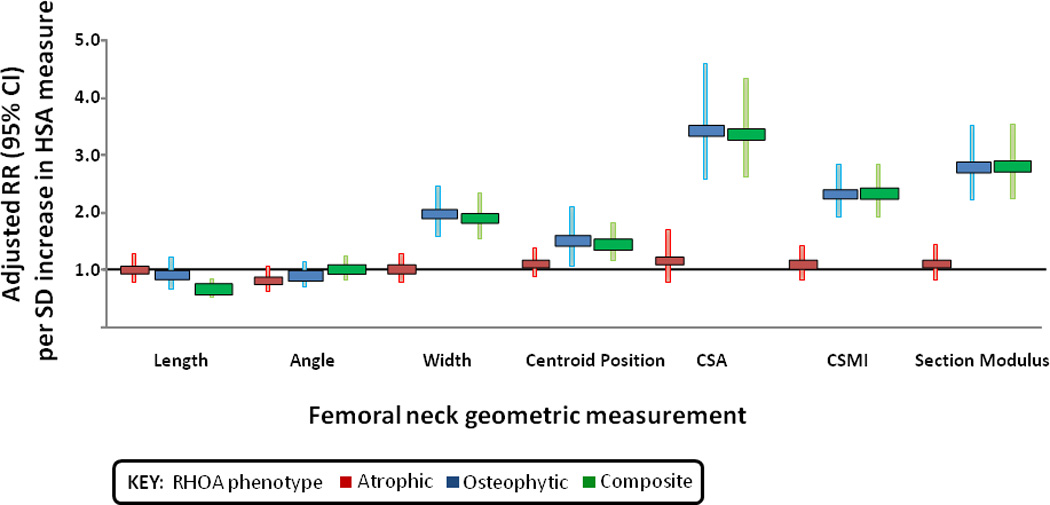
Association between baseline bone geometry at the femoral neck and prevalence of radiographic hip osteoarthritis by phenotype. Red, blue, and green bars represent the atrophic phenotype, the osteophytic phenotype, and the composite phenotype, respectively (see Patients and Methods for phenotype definitions). RR ϭ relative risk (adjusted for age, body mass index, knee height, and total hip areal bone mineral density); 95% CI ϭ 95% confidence interval; HSA ϭ hip structure analysis; CSA ϭ cross-sectional area; CSMI ϭ cross-sectional moment of inertia.
Geometric predictors of Incident RHOA
For the femoral neck measures, the risk of incident RHOA using both the osteophytic and composite definitions was significantly greater in hips with a wider neck, a more medial centroid position, and greater CSA, CSMI and section modulus (Table 3). At the further distal locations, there was also an increased risk of osteophytic RHOA in hips with a greater CSMI at the IT (adjusted RR 1.7 [1.2–2.3]) and shaft (adjusted RR 1.4 [1.0–1.9]) subregions and greater section modulus at the IT (adjusted RR 1.7 [1.2–2.3]) and shaft (adjusted RR 1.5 [1.0–2.1]) subregions. A longer femoral neck was associated with a decreased risk of incident RHOA RR 0.8 [0.7–1.0] using the composite definition, but was not associated with the other definitions of incident RHOA.
Table 3.
Adjusted Relative risk ratio of incident RHOA per SD increase in baseline bone geometry measured at the femoral neck sub-region
| Atrophic | Osteophytic | Composite | |
|---|---|---|---|
| Femoral neck: | |||
| Width | 0.92 (0.69,1.24) | 1.45 (1.04,2.02)* | 1.48 (1.20,1.81)** |
| Centroid position | 1.02 (0.81,1.28) | 1.35 (1.03,1.76)* | 1.29 (1.02,1.63)* |
| CSA | 1.23 (0.88,1.72) | 1.96 (1.32,2.89)*** | 2.10 (1.62,2.73)*** |
| CSMI | 1.04 (0.78,1.39) | 1.68 (1.26,2.23)*** | 1.72 (1.41,2.10)*** |
| Section modulus | 1.03 (0.77,1.39) | 1.72 (1.22,2.42)** | 1.85 (1.48,2.32)*** |
p≤ 0.05;
p≤0.01;
p≤0.001 Adjusted for age, baseline BMI, knee height and total hip aBMD
Geometric predictors of Progression of RHOA
A wider femoral neck (p=0.04) and medial centroid position (p<0.001) predicted an increase in osteophyte score and increase in both osteophyte and loss of joint space, with the association somewhat stronger for the latter. None of the geometric measures were associated with loss of joint space alone. (Table 4). Of the more distal subregions, a more medial centroid position of the shaft predicted osteophytic progression (adjusted RR 1.64 [1.0 – 2.7]). There were no other significant associations.
Table 4.
Adjusted Relative risk ratio of progressive RHOA per SD increase in baseline proximal femoral geometry
| Atrophic1 | Osteophytic2 | Composite3 | |
|---|---|---|---|
| Femoral neck | |||
| Width | 1.17 (0.77, 1.78) | 1.85 (1.16, 2.98)* | 2.10 (1.34, 3.27)*** |
| Centroid position | 1.07 (0.73,1.57) | 1.52 (0.99,2.34) | 1.66 (1.11, 2.49)* |
| CSA | 1.145 (0.89, 2.36) | 1.38 (0.80,2.38) | 2.54 (1.58, 4.07)*** |
| CSMI | 1.17 (0.81, 1.68) | 1.44 (0.97, 2.13) | 1.94 (1.35, 2.81)*** |
| Section Modulus | 1.22 (0.82, 1.82) | 1.34 (0.86, 2.07) | 2.06 (1.38, 3.06)*** |
p≤ 0.05;
p≤0.01;
p≤0.001 Adjusted for age, baseline BMI, knee height and total hip aBMD
Progression defined as 1atrophic (MJS reduction ≥ 0.5mm & total osteophyte score increased < 2),
osteophytic (total osteophyte score increased ≥ 2 & MJS reduction < 0.5mm) and as
composite (MJS reduction ≥ 0.5mm & total osteophyte score increased ≥ 2).
DISCUSSION
In this large prospective cohort of women, we have found that a wider femoral neck and more medial centroid position of bone mineral in the femoral neck were associated with an increase risk of prevalent, incident and progressive RHOA, independent of aBMD of the hip. These associations were found for RHOA phenotypes defined by the presence of both JSN and osteophytes (composite phenotype) and by the presence of osteophytes without definite JSN (osteophytic phenotype) but not for RHOA defined by JSN without definite osteophytes (atrophic phenotype). We also found limited associations between RHOA and bone geometry at more distal sites of the proximal femur suggesting an underlying mechanism involving bone in more than the peri-articular regions of the femur.
Previous studies have found that persons with knee or hip osteoarthritis have a higher aBMD at axial sites [5, 16–19] [20, 21] and that these associations were strongest for osteophyte predominant disease [5, 17, 19, 20]. Higher aBMD of the hip and greater bone size are correlated [6]. Ours is the first study that has examined the association between proximal femur geometry and RHOA and demonstrated associations with prevalence, incidence and progression that are independent of aBMD and that vary by specific RHOA phenotype. A recent study found that statistical variation in the gross morphological profile of the proximal femur, derived using active shape modelling of AP pelvis radiographs, were predictive of RHOA incidence and progression [22]. Our study extends this work through the use of detailed measurements of bone geometry and mineral distribution at predetermined locations in the proximal femur, derived from DXA scans, which support inferences regarding biomechanical properties [23] and suggest mechanisms for the observed change in the proximal femur during the very early stages of hip osteoarthritis, for which there is little human published data. [24]
Our results could be interpreted in light of known variations in the morphology of the proximal femur associated with hip OA. Medial cortical buttressing is a common radiological finding of hip OA, found in approximately 50% of hips with end-stage disease [25]. Buttressing results from periosteal apposition in the femoral neck and may reflect increased joint forces transmitted through the neck [25]. Our findings of a greater neck width and a more medial mineral deposition in the femoral neck, of hips with prevalent and progressive OA is consistent with medial buttressing and anticipated lines of stress during weight-bearing. Importantly, we found both a wider femoral neck and medial shift in mineral distribution in radiographically normal hips that went on to develop RHOA during the study, suggesting for the first time that such changes may predispose to, or occur very early in, the development of hip OA. It is also noteworthy that we found that hips with RHOA at baseline, but not those that developed OA during the study, had greater bone widths at the more distal IT and shaft locations, suggesting that increased mineral apposition may also occur at these more distal sites, at least when OA is present.
In addition to possible changes in the medial cortical buttressing plate, we speculate that the more medial distribution of bone mass in hips with, and at risk for, RHOA, also reflects an increase in the amount of bone comprising the medial compressive strut [26]. This lies within the metabolically active trabecular compartment and originates from the superior femoral neck cortex, along the epiphyseal scar to the pressure buttress of the medial femoral neck the densest structure in the region [26]. This is supported by studies using macro-radiographs of the hip, describing increases in the thickness of the vertical trabeculae of the compressive strut in hips with OA [27]. In these studies there also appears to be a thinning of the remaining trabecula, suggesting a redistribution of mineral within the trabecular compartment in response to altered loads [28].
Femoroacetabular impingement is hypothesized to be an important cause of OA in non-dysplastic hips that arises from abnormal contact between the femoral head/neck junction and the acetabular rim during joint motion and is associated with morphological variants of the proximal femur (cam-type impingement) and/or the acetabulum (pincer-type impingement) [29]. Cam-type impingement is thought to result from non-spheroidal femoral head, as found in pistol grip deformity (PGD) [29]. Doherty and colleagues [30] have recently shown that hips with PGD have a reduced ratio of femoral head to femoral neck diameters (consistent with both a larger femoral neck diameter and/or a smaller femoral head) and that a smaller ratio is associated with the occurrence of RHOA in men. However, PGD is very uncommon in women (prevalence <0.4%), so this is unlikely to explain our finding that a larger femoral neck diameter is a risk factor for incident and progressive RHOA in women. Pincer-type impingement, which may be more common in women, involves anterior abutment of the head-neck junction against the acetabular rim in hips with acetabular over-coverage [29]. A larger neck diameter may increase the risk of this type of impingement, while an expansion of neck diameter and medial migration of the centroid position may both occur in response to mechanical stresses on the neck secondary to impingement.
We also found that a shorter femoral neck length was associated with an increased risk of RHOA, although this was not independent of neck width. Several studies have found that in contrast to our findings for RHOA, a longer femoral neck length and lower values on indices of hip strength are associated with a greater risk of hip fractures [31] and may accuount for the observed inverse association between osteoporosis and hip osteoarthritis. Also, if true and not artefactual, a longer femoral neck must represent a true predictor rather than a consequence of early OA. Indicators of the strength of the femoral neck, including CSA, CSMI and section modulus showed the strongest associations with prevalent, incident and progressive RHOA in our study. Based on these indices, which take into account both the amount of bone mineral and its spatial distribution [15], our results suggest that the femoral necks of hips with, and at risk for, RHOA have substantially greater bending strength. How these factors contribute to the risk of hip OA requires further investigation.
Although the exact timing for the observed increase in neck width and more medial bone mineral distribution can not be established with certainty by this study, we found that at a minimum these changes occur early in the disease process and may be predisposing factors. In previous work we have demonstrated that, through programming of bone growth [32], such changes in bone shape may have their origins very early in life [33]. Alternatively, the differences in femoral neck width and mineral distribution could be an early response to altered loading conditions in the hip that increase the risk of developing OA or that result from effects of early OA on joint biomechanics. The bone changes may be bystanders that do not play a role in pathogenesis, or could influence pathogenesis through negative feedback loops involving increased bone stiffness and/or joint incongruence [24] leading to higher joint surfaces stress and cartilage damage. In addition osteoblasts in the loaded subchondral bone may stimulate cartilage breakdown through paracrine mechanisms [34].
The bone geometry associations very clearly differed between RHOA phenotypes defined by presence of osteophytes versus JSN alone. Several hip OA phenotypes were described by Solomon in 1976. They included a femoral tilt phenotype with increased sub-periosteal bone formation and medio/inferior thickening of the calcar (although this was a predominantly male condition (14:1)) and an atrophic type without osteophytes which was post-inflammatory with patchy cartilage loss not restricted to areas of maximal loading [35]. Our findings suggest adding broader morphological and bone mineral geometric paratemers to the described phenotypes. The potential mechanisms linking these bone parameters to the osteophytic phenotype are likely to include the wnt pathway, an integral regulator of bone remodelling [36] as well as ostephyte osteophyte growth [37]. Polymorphisms of wnt antagonists have been associated with hip OA in this cohort [38] and others [39] in addition to being associated with increases in axial aBMD [40, 41]. We speculate that abnormal loading or an increased responsiveness to increased loading, may activate bone formation through wnt signalling, stimulating osteoblast maturation and activity [42] with localized remodelling leading to the mineral distribution changes we observed in addition to osteophyte growth at the joint [37]. While in the atrophic this bone response may be dampened.
This study has certain strengths. It is the first to take advantage of DXA scans to investigate the relationship of proximal femur geometry with RHOA in a large community-based sample both cross-sectionally and longitudinally and to examine predictors of prevalent, incident and progressive disease. There are, however, also several limitations. Firstly, the measures of baseline RHOA and hip geometry were not contemporaneous, with a mean interval beween them of 2 years. This limits our interpretation of longitudinal associations as we cannot be certain the geometric characteristics precede radiographic features of hip OA at follow-up. However, hip OA is a slowly developing disease and so only a few hips would be expected to develop OA between the baseline and second visit. Radiographs are insensitive to the earliest manifestations of OA, which limits our ability to define the very earliest stage of disease. The subjects were generally healthy ambulatory volunteers and so, particularly at baseline, this would lead to fewer prevalent hip OA cases. In addition these findings may only apply to older white women as older black women and men were not included in the study. Femoral neck length dimensions are measured from PA DXA images and since the neck is typically angled relative to the implied coronal plane of the image these measurements may be biased; in particular neck length will be systematically decreased by limited internal rotation of the hip in those with hip pain and hip OA. However, the findings of this study were not significantly changed when we repeated the analysis excluding those with baseline pain on internal rotation. We could not measure femoral head diameters from DXA scans and so could not evaluate a ratio measure of head and neck diameter. Finally, hip OA is a clinical disease encompassing both symptoms and pathological changes and the focus of this analysis was on radiographic findings of disease only.
In summary, differences in the geometry of the femoral neck assessed from DXA scans, including femoral neck width and spatial distribution bone mass across the neck, are detectable early in the natural history of hip osteoarthritis as determined from radiographs. These geometric changes were associated with prevalent, incident and progressive RHOA defined by JSN and osteophytes and with an RHOA phenotype defined by osteophytes alone, but not with an atrophic phenotype defined by JSN without osteophytes. These data suggest that qualitative differences in bone remodelling may be critical to development of specific OA phenotypes at the hip and identify those hips at greatest risk of structural progression.
Acknowledgements
MKJ was supported by an Arthritis Research Campaign travelling fellowship. The Study of Osteoporotic Fractures (SOF) is supported by National Institutes of Health funding. The following institutes provide support: the National Institute of Arthritis and Musculoskeletal and Skin Diseases (NIAMS) and the National Institute on Aging (NIA) under the following grant numbers: AG05407, AR35582, AG05394, AR35584, AR35583, R01 AG005407, R01 AG027576-22, 2 R01 AG005394-22A1, and 2 R01 AG027574-22A1. We would like to thank the research assistants and participants in SOF.
References
- 1.Felson DT, et al. Osteoarthritis: new insights. Part 1: the disease and its risk factors. Ann Intern Med. 2000;133(8):635–646. doi: 10.7326/0003-4819-133-8-200010170-00016. [DOI] [PubMed] [Google Scholar]
- 2.Radin EL, Rose RM. Role of subchondral bone in the initiation and progression of cartilage damage. Clin Orthop Relat Res. 1986;(213):34–40. [PubMed] [Google Scholar]
- 3.Hunter DJ, et al. Change in joint space width: hyaline articular cartilage loss or alteration in meniscus? Arthritis Rheum. 2006;54(8):2488–2495. doi: 10.1002/art.22016. [DOI] [PubMed] [Google Scholar]
- 4.Cooper C, et al. Osteoarthritis of the hip and osteoporosis of the proximal femur. Ann Rheum Dis. 1991;50(8):540–542. doi: 10.1136/ard.50.8.540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nevitt MC, et al. Radiographic osteoarthritis of the hip and bone mineral density. The Study of Osteoporotic Fractures Research Group. Arthritis Rheum. 1995;38(7):907–916. doi: 10.1002/art.1780380706. [DOI] [PubMed] [Google Scholar]
- 6.Looker AC, Beck TJ, Orwoll ES. Does body size account for gender differences in femur bone density and geometry? J Bone Miner Res. 2001;16(7):1291–1299. doi: 10.1359/jbmr.2001.16.7.1291. [DOI] [PubMed] [Google Scholar]
- 7.Cooper C, et al. Individual risk factors for hip osteoarthritis: obesity, hip injury, and physical activity. Am J Epidemiol. 1998;147(6):516–522. doi: 10.1093/oxfordjournals.aje.a009482. [DOI] [PubMed] [Google Scholar]
- 8.Frost HM. Why should many skeletal scientists and clinicians learn the Utah paradigm of skeletal physiology? J Musculoskelet Neuronal Interact. 2001;2(2):121–130. [PubMed] [Google Scholar]
- 9.Cummings SR, et al. Appendicular bone density and age predict hip fracture in women. The Study of Osteoporotic Fractures Research Group. JAMA. 1990;263(5):665–668. [PubMed] [Google Scholar]
- 10.Lane NE, et al. Progression of radiographic hip osteoarthritis over eight years in a community sample of elderly white women. Arthritis Rheum. 2004;50(5):1477–1486. doi: 10.1002/art.20213. [DOI] [PubMed] [Google Scholar]
- 11.Altman RD, et al. Atlas of individual radiographic features in osteoarthritis. Osteoarthritis Cartilage. 1995;3(Suppl A):3–70. [PubMed] [Google Scholar]
- 12.Lane NE, Kremer LB. Radiographic indices for osteoarthritis. Rheum Dis Clin North Am. 1995;21(2):379–394. [PubMed] [Google Scholar]
- 13.Croft P, Cooper C, Coggon D. Case definition of hip osteoarthritis in epidemiologic studies. J Rheumatol. 1994;21(4):591–592. [PubMed] [Google Scholar]
- 14.Arden NK, L N, Parimi N, Liu L, Hochberg M, Nevit MC. Defining Incident Radiographic Hip OA For Epidemiological Studies In Women - submitted. Arthritis Rheum. 2008 doi: 10.1002/art.24382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Beck TJ, et al. Predicting femoral neck strength from bone mineral data. A structural approach. Invest Radiol. 1990;25(1):6–18. doi: 10.1097/00004424-199001000-00004. [DOI] [PubMed] [Google Scholar]
- 16.Hordon LD, Wright V, Smith MA. Bone mass in osteoarthritis. Ann Rheum Dis. 1992;51(6):823–825. doi: 10.1136/ard.51.6.823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hannan MT, et al. Bone mineral density and knee osteoarthritis in elderly men and women. The Framingham Study. Arthritis Rheum. 1993;36(12):1671–1680. doi: 10.1002/art.1780361205. [DOI] [PubMed] [Google Scholar]
- 18.Burger H, et al. Association of radiographically evident osteoarthritis with higher bone mineral density and increased bone loss with age. The Rotterdam Study. Arthritis Rheum. 1996;39(1):81–86. doi: 10.1002/art.1780390111. [DOI] [PubMed] [Google Scholar]
- 19.Antoniades L, et al. A cotwin control study of the relationship between hip osteoarthritis and bone mineral density. Arthritis Rheum. 2000;43(7):1450–1455. doi: 10.1002/1529-0131(200007)43:7<1450::AID-ANR6>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 20.Hart DJ, et al. The relationship of bone density and fracture to incident and progressive radiographic osteoarthritis of the knee: the Chingford Study. Arthritis Rheum. 2002;46(1):92–99. doi: 10.1002/1529-0131(200201)46:1<92::AID-ART10057>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- 21.Hochberg MC, Lethbridge-Cejku M, Tobin JD. Bone mineral density and osteoarthritis: data from the Baltimore Longitudinal Study of Aging. Osteoarthritis Cartilage. 2004;12(Suppl A):S45–S48. doi: 10.1016/j.joca.2003.09.008. [DOI] [PubMed] [Google Scholar]
- 22.Gregory JS, et al. Early identification of radiographic osteoarthritis of the hip using an active shape model to quantify changes in bone morphometric features: can hip shape tell us anything about the progression of osteoarthritis? Arthritis Rheum. 2007;56(11):3634–3643. doi: 10.1002/art.22982. [DOI] [PubMed] [Google Scholar]
- 23.Beck TJ. Extending DXA beyond bone mineral density: understanding hip structure analysis. Curr Osteoporos Rep. 2007;5(2):49–55. doi: 10.1007/s11914-007-0002-4. [DOI] [PubMed] [Google Scholar]
- 24.Herzog W, Federico S. Considerations on joint and articular cartilage mechanics. Biomech Model Mechanobiol. 2006;5(2–3):64–81. doi: 10.1007/s10237-006-0029-y. [DOI] [PubMed] [Google Scholar]
- 25.Dixon T, et al. Femoral neck buttressing: a radiographic and histologic analysis. Skeletal Radiol. 2000;29(10):587–592. doi: 10.1007/s002560000260. [DOI] [PubMed] [Google Scholar]
- 26.Stiehl JB, Jacobson D, Carrera G. Morphological analysis of the proximal femur using quantitative computed tomography. Int Orthop. 2007;31(3):287–292. doi: 10.1007/s00264-006-0182-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Papaloucas CD, et al. Cancellous bone changes in hip osteoarthritis: a short-term longitudinal study using fractal signature analysis. Osteoarthritis Cartilage. 2005;13(11):998–1003. doi: 10.1016/j.joca.2005.06.009. [DOI] [PubMed] [Google Scholar]
- 28.Frost HM. Joint anatomy, design, and arthroses: insights of the Utah paradigm. Anat Rec. 1999;255(2):162–174. doi: 10.1002/(SICI)1097-0185(19990601)255:2<162::AID-AR6>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- 29.Ganz R, et al. Femoroacetabular impingement: a cause for osteoarthritis of the hip. Clin Orthop Relat Res. 2003;(417):112–120. doi: 10.1097/01.blo.0000096804.78689.c2. [DOI] [PubMed] [Google Scholar]
- 30.Doherty M, et al. Nonspherical femoral head shape (pistol grip deformity), neck shaft angle, and risk of hip osteoarthritis: a case-control study. Arthritis Rheum. 2008;58(10):3172–3182. doi: 10.1002/art.23939. [DOI] [PubMed] [Google Scholar]
- 31.Faulkner KG, et al. Hip axis length and osteoporotic fractures. Study of Osteoporotic Fractures Research Group. J Bone Miner Res. 1995;10(3):506–508. doi: 10.1002/jbmr.5650100323. [DOI] [PubMed] [Google Scholar]
- 32.Cooper C, et al. Growth and bone development. Nestle Nutr Workshop Ser Pediatr Program. 2008;61:53–68. doi: 10.1159/000113170. [DOI] [PubMed] [Google Scholar]
- 33.Javaid MK, et al. Infant growth influences proximal femoral geometry in adulthood. J Bone Miner Res. 2006;21(4):508–512. doi: 10.1359/jbmr.051214. [DOI] [PubMed] [Google Scholar]
- 34.Sanchez C, et al. Subchondral bone osteoblasts induce phenotypic changes in human osteoarthritic chondrocytes. Osteoarthritis Cartilage. 2005;13(11):988–997. doi: 10.1016/j.joca.2005.07.012. [DOI] [PubMed] [Google Scholar]
- 35.Solomon L. Patterns of osteoarthritis of the hip. J Bone Joint Surg Br. 1976;58(2):176–183. doi: 10.1302/0301-620X.58B2.932079. [DOI] [PubMed] [Google Scholar]
- 36.Johnson ML, Kamel MA. The Wnt signaling pathway and bone metabolism. Curr Opin Rheumatol. 2007;19(4):376–382. doi: 10.1097/BOR.0b013e32816e06f9. [DOI] [PubMed] [Google Scholar]
- 37.Diarra D, et al. Dickkopf-1 is a master regulator of joint remodeling. Nat Med. 2007;13(2):156–163. doi: 10.1038/nm1538. [DOI] [PubMed] [Google Scholar]
- 38.Lane NE, et al. Wnt signaling antagonists are potential prognostic biomarkers for the progression of radiographic hip osteoarthritis in elderly Caucasian women. Arthritis Rheum. 2007;56(10):3319–3325. doi: 10.1002/art.22867. [DOI] [PubMed] [Google Scholar]
- 39.Loughlin J, et al. Functional variants within the secreted frizzled-related protein 3 gene are associated with hip osteoarthritis in females. Proc Natl Acad Sci U S A. 2004;101(26):9757–9762. doi: 10.1073/pnas.0403456101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gong Y, et al. LDL receptor-related protein 5 (LRP5) affects bone accrual and eye development. Cell. 2001;107(4):513–523. doi: 10.1016/s0092-8674(01)00571-2. [DOI] [PubMed] [Google Scholar]
- 41.Boyden LM, et al. High bone density due to a mutation in LDL-receptor-related protein 5. N Engl J Med. 2002;346(20):1513–1521. doi: 10.1056/NEJMoa013444. [DOI] [PubMed] [Google Scholar]
- 42.Robinson JA, et al. Wnt/beta-catenin signaling is a normal physiological response to mechanical loading in bone. J Biol Chem. 2006;281(42):31720–31728. doi: 10.1074/jbc.M602308200. [DOI] [PubMed] [Google Scholar]


