Abstract
Purpose
Panitumumab, a fully human anti-epidermal growth factor receptor monoclonal antibody (mAb), has demonstrated efficacy in patients with wild-type KRAS metastatic colorectal cancer (mCRC). Rilotumumab and ganitumab are investigational, fully human mAbs against hepatocyte growth factor (HGF)/scatter factor and IGF1R, respectively. Here we evaluate combining rilotumumab or ganitumab with panitumumab in previously treated patients with wild-type KRAS mCRC.
Experimental Design
Part 1 was a phase Ib dose-finding study of panitumumab plus rilotumumab. The primary endpoint was the incidence of dose-limiting toxicities (DLT). Part 2 was a randomized phase II trial of panitumumab in combination with rilotumumab, ganitumab, or placebo. The primary endpoint was objective response rate (ORR); safety, progression-free survival (PFS), and overall survival (OS) were secondary endpoints. Archival tissue specimens were collected for exploratory correlative work.
Results
In part 1, no DLTs were reported. A recommended phase II dose of 10 mg/kg rilotumumab was selected. In part 2, for the panitumumab plus rilotumumab (n = 48), panitumumab plus ganitumab (n = 46), and panitumumab plus placebo arms (n = 48), the ORRs were 31%, 22%, and 21%, respectively. The median PFS was 5.2, 5.3, and 3.7 months and median OS 13.8,10.6, and 11.6 months, respectively. Adverse events were tolerable. Exploratory biomarker analyses, including MET and IGF-related protein expression, failed to indicate conclusive predictive evidence on efficacy endpoints.
Conclusions
Panitumumab plus rilotumumab met the prespecified criterion for improvement in ORR whereas ganitumab did not. This is the first study to suggest a benefit for combining an HGF inhibitor (rilotumumab) with panitumumab in previously treated patients with wild-type KRAS mCRC.
Introduction
Worldwide, colorectal cancer remains the third most common malignancy in men and the second most common malignancy in women, affecting a total of 1.2 million individuals and resulting in 608,000 deaths (1). With few treatment options for patients who have progressed on chemotherapy, patients with metastatic colorectal cancer (mCRC) continue to search for innovative therapies. Panitumumab, a fully human monoclonal antibody (mAb) against the epidermal growth factor receptor (EGFR), has demonstrated efficacy as a single agent and in combination with chemotherapy in wild-type (WT) KRAS mCRC tumors (2–5).
MET and the insulin-like growth factor 1 receptor (IGF1R) are tyrosine kinases that share multiple downstream pathways with EGFR, including the Ras–Raf–MAPK and PI3K/Akt pathways (6–9). Hepatocyte growth factor/ scatter factor (HGF/SF) is the only known ligand for MET. This pathway is required for normal physiologic development, including wound healing, tissue regeneration, and embryonic development (10). Dysregulation of MET has been associated with malignant development promoting tumor growth, migration, invasion, metastases, and drug resistance (11, 12). Rilotumumab (AMG 102), a fully human IgG2 mAb targeting HGF, neutralizes HGF-dependent MET signaling.
IGF1 and IGF2 bind IGF1R leading to the activation of survival and proliferative pathway signals. The IGF1R axis has been implicated in oncogenesis in several tumor types, including colorectal cancer where IGF2 is the most commonly overexpressed gene compared with normal mucosa (13). Ganitumab (AMG 479) is a fully human IgG1 mAb targeting human IGF1R and inhibiting binding by IGF1 and IGF2.
Experiments have suggested that the MET and IGF1R pathways interact in tumors with the EGFR pathway (14–17) and therefore, combined use of agents that block these pathways may generate additive anticancer effects.
A phase Ib/II clinical trial of these novel biologic agents was initiated to evaluate the safety and efficacy of rilotumumab or ganitumab in combination with panitumumab in previously treated patients with WT KRAS mCRC. Phase Ib data were available supporting the use of 12 mg/kg ganitumab with 6 mg/kg panitumumab (18).
Materials and Methods
Patients
Eligible patients were ≥ 18 years of age, had an Eastern Cooperative Oncology Group (ECOG) performance score of 0 or 1, and had histologically or cytologically confirmed metastatic adenocarcinoma of the colon or rectum. Radiographic evidence of disease progression during or following prior treatment with irinotecan- and/or oxaliplatin-based chemotherapy for mCRC was required. At least 1 unidi-mensionally measurable lesion per modified RECIST 1.0 was required. Archival tumor tissue was confirmed by a central laboratory to be WT KRAS using a validated test method.
Patients were excluded if they received prior treatment with an anti-EGFR inhibitor (e.g., panitumumab, cetuximab, erlotinib, and/or gefitinib) unless treatment was received in the adjuvant setting ≥6 months before enrollment. Prior treatment with MET or IGF1R inhibitors was not allowed. Additional exclusion criteria included the use of systemic chemotherapy or radiotherapy ≤21 days before enrollment and the use of any targeted therapies ≤30 days before enrollment (including bevacizumab).
The study protocol was approved by the institutional review boards or the ethics committees at all participating institutions. Written, informed consent was provided by all patients before any study-related treatment was conducted. Amgen Inc. maintained the database for this study. All authors had access to the complete data.
Study design and treatment
This is an exploratory, global, multicenter 3-part phase Ib/II trial designed to evaluate the efficacy and safety of rilotumumab or ganitumab in combination with panitumumab versus panitumumab plus placebo in previously treated patients with WT KRAS mCRC (NCT00788957; Supplementary Fig. S1). Exploratory correlative work was conducted only in the phase II portion of the study.
Part 1 (phase Ib) consisted of a dose de-escalation study to evaluate and select a tolerable dose of rilotumumab in combination with panitumumab to be used in part 2.
Part 2 (phase II) consisted of a 3-arm randomized trial to evaluate the safety and efficacy of panitumumab in combination with rilotumumab, ganitumab, or placebo in previously treated patients with WT KRAS mCRC.
In part 3 (phase II), patients in the panitumumab plus placebo arm of part 2 could be randomized to receive either rilotumumab or ganitumab monotherapy, if the patient ended their treatment because of disease progression or intolerability but remained eligible by protocol standards for additional therapy. Part 3 of the study is ongoing and results will be reported at a later date.
Phase Ib: Evaluation of DLT(s) in part 1
Patients received 6 mg/kg panitumumab in combination with 10 mg/kg rilotumumab intravenously once every 2 weeks (Q2W). The starting dose of rilotumumab was based on unpublished pharmacokinetic data. A human plasma concentrations of 10 mg/kg rilotumumab administered Q2W showed a median trough concentration of 158.6 µg/mL, which exceeds the IC90 values (approximately 75 (µg/mL) predicted from in vitro cell proliferation assays and therefore was likely to be an effective dose. Based on the incidence of dose-limiting toxicities (DLT), dose de-escalation to 5 mg/kg rilotumumab was allowed. No dose reduction in panitumumab was planned. A DLT evaluable patient was defined as a patient who had received at least 2 doses of panitumumab and rilotumumab as scheduled (i.e., week 1 and 3) and had a minimum 4 weeks (28 days) of follow-up for safety or had received at least 1 dose of panitumumab and rilotumumab and had a DLT within the first 4 weeks (28 days) on study.
If ≤1 of the first 6 evaluable patients experienced a DLT(s), the 6 mg/kg panitumumab plus 10 mg/kg rilotumumab dose was determined tolerable and used in part 2. If 2 of the first 6 evaluable patients experienced a DLT(s), then 3 additional patients were enrolled to receive the 6 mg/kg panitumumab plus 10 mg/kg rilotumumab dose. If ≥3 of the first 6 evaluable patients experienced a DLT (s), then the dose of panitumumab plus rilotumumab was determined not tolerable and a lower dose of 5 mg/kg rilotumumab was to be evaluated in combination with panitumumab.
DLTs were defined as any grade 3 or 4 therapy-related adverse event (AE) or laboratory abnormality that was deemed clinically significant by the investigator. Fatigue, nausea, vomiting, diarrhea, hypomagnesemia, rash, neutropenia, thrombocytopenia, and increased aspartate aminotransferase (AST)/alanine aminotransferase (ALT) were not considered to be DLTs unless the following criteria were met: grade 3 fatigue that persisted for >7 days, or grade 4 fatigue; grade 3 or 4 nausea, diarrhea, or vomiting despite maximum supportive care; grade 4 hypomagnesemia despite IV maximal magnesium replacement; grade 4 rash/desquamation; grade 3 or 4 neutropenia with persistent fever ≥38.5°C; grade 4 neutropenia or thrombocytopenia >7 days; elevated ALT or AST > 10 × upper limit of normal.
DLT-evaluable patients received ≥2 doses of panitumumab plus rilotumumab as scheduled and had a minimum 28 days of follow-up for safety or received ≥1 dose of panitumumab plus rilotumumab and had a DLT within the first 28 days on study.
Phase II: Randomization in part 2
In part 2, patients were randomized in a ratio of 1:1:1 to treatment arms: 6 mg/kg panitumumab plus 10 mg/kg rilotumumab; 6 mg/kg panitumumab plus 12 mg/kg ganitumab; and 6 mg/kg panitumumab plus placebo. Randomization was stratified by prior chemotherapy regimen (treated with irinotecan or oxaliplatin vs. both). Panitumumab was open-label; rilotumumab, ganitumab, and placebo were double-blinded. Treatments were administered Q2W until disease progression, intolerability, withdrawal, or death.
Tumor response was assessed by the investigator using RECIST 1.0 criteria at weeks 8, 12, 16, 24, 32, 40, 48, and every 12 weeks until disease progression. Responses were confirmed no earlier than 28 days after the criteria for response were first met.
Statistical analysis
In part 1, the primary objective was to identify a tolerable dose of panitumumab plus rilotumumab based on the incidence of DLTs. A secondary objective was to evaluate the safety of panitumumab plus rilotumumab.
In part 2, the primary objective was to evaluate the efficacy as measured by the objective response rate (ORR) of panitumumab plus rilotumumab and panitumumab plus ganitumab versus panitumumab plus placebo. Key secondary objectives included safety (AEs and laboratory values), duration of response, disease control [complete response plus partial response plus stable disease (SD); SD required patients to be on study for ≥49 days before SD], progression-free survival (PFS), and overall survival (OS).
Bayesian analysis was used for the statistical interpretation of ORR. This method calculates the posterior distribution of the odds ratio (OR) of ORRs for the experimental arms and the control arm using prior distributions of ORRs. It was prespecified that if there was ≥90% posterior probability that the combination therapy was better than panitumumab alone (i.e., OR > 1) as evaluated by objective tumor response, the combination was considered promising. If there was between 50% and 90% probability, the combination was considered indeterminate. If there was <50% probability, the combination was considered not promising. An ORR prior distribution for panitumumab monotherapy was derived from 4 prior studies in which patients had received both prior oxaliplatin and irinotecan (5, 19). The ORR prior distributions for the combination arms were assumed to have the same mean as the panitumumab alone arm and the ORR posterior distribution for each arm combines the prior distributions with observed ORRs from the study.
Results
Part 1: Phase Ib
Eleven patients from 3 sites (1 each in the USA, Belgium, and Spain) were enrolled to the starting dose of 10 mg/kg rilotumumab. Accrual was not halted until the dose decision for part 2 was made and therefore >6 patients were enrolled. No DLTs were reported for the first 6 DLT-evaluable patients, thus the Q2W dose of 6 mg/kg panitumumab plus 10 mg/kg rilotumumab was used in part 2. Capillary leak syndrome was reported in one of the first 6 DLT-evaluable patients (64 days after the first dose), but this occurred outside of the DLT reporting window. DLTs, defined as any grade 3 or 4 treatment-related AE or laboratory abnormality that was deemed clinically significant by the investigator, were not reported beyond the first 6 DLT-evaluable patients. However, AEs were assessed for all 11 patients in part 1 (described below).
Patient demographics and disease characteristics are summarized in Table 1. Treatment-related grade 3 or 4 AEs consisted of acneiform dermatitis/rash (55%), paronychia (18%), infection (9%), capillary leak syndrome (9%), erythema (9%), nail disorder (9%), and pruritus (9%). Treatment-emergent grade 3 or 4 AEs that occurred in ≥15% of patients were acneiform dermatitis/rash (55%), fatigue (18%), and paronychia (18%). Five patients had serious AEs. None were fatal and consisted of acneiform dermatitis (grade 3) and capillary leak syndrome (grade 3), both considered to be related to study treatment, intestinal obstruction (grade 4), anemia/ general health deterioration (grade 3), and cerebrovascular accident (grade 4). There was 1 death on study (within 30 days of the last dose of protocol-specified therapy) because of progressive disease.
Table 1.
Patient demographics and disease characteristics at baseline
| Part 1: Phase Ib Panitumumab + rilotumumab (AMG 102) (n = 11) |
Part 2: Phase II Panitumumab + rilotumumab (AMG 102) (n = 48) |
Panitumumab + ganitumab (AMG 479) (n = 46) |
Panitumumab + placebo (n = 48) |
|
|---|---|---|---|---|
| Men, n (%) | 5(45) | 29 (60) | 25 (54) | 28 (58) |
| Age, mean years (range) | 56.5 (37–75) | 62.1 (45–78) | 62.0 (33–81) | 55.0(19–75) |
| Age ≥ 65 years, n (%) | 4(36) | 20 (42) | 18(39) | 9(19) |
| ECOG status, n (%) | ||||
| 0 | 6(55) | 24 (50) | 18(39) | 15(31) |
| 1 | 5(45) | 23 (48)a | 28 (61) | 33 (69) |
| Metastatic sites, n (%) | ||||
| Liver only | 3(27) | 5(10) | 4(9) | 5(10) |
| Liver + other sites | 5(45) | 32 (67) | 29 (63) | 27 (56) |
| Prior line of therapy for mCRC, n (%) | ||||
| First-line therapy | 11 (100) | 48 (100) | 46(100) | 46 (96)b |
| Second-line therapy | 8(73) | 33 (69) | 26 (57) | 31 (65) |
| Third-line therapy and later | 3(27) | 16 (33) | 12 (26) | 14(29) |
| Prior therapies for mCRC, n (%) | ||||
| Oxaliplatin | 8(73) | 42 (88) | 40 (87) | 39 (81) |
| Irinotecan | 11 (100) | 32 (67) | 26 (57) | 30 (63) |
| Oxaliplatin and irinotecan | 8(73) | 26 (54) | 20 (44) | 23 (48) |
| Bevacizumab | 5(45) | 17(35) | 12 (26) | 19(40) |
One patient with ECOG performance score of 2 was enrolled and was not counted as either ECOG performance score of 0 or 1; data from this patient were included in all efficacy and safety analyses.
Two patients had not received first-line therapy for mCRC; both patients had received oxaliplatin-based chemotherapy for non-mCRC in the adjuvant setting and progressed on therapy before entering the study.
Part 2: Phase II patient demographics
From June 9,2009 to February 5, 2010, 142 patients were randomly assigned from 37 sites in 11 countries (Table 1). The mean number of patients enrolled per site was 3.8 and the median was 3.0. All patients received at least one dose of study therapy. Median follow-up time (from the date of first dose to the last on-study or long-term follow-up visit or death date) was 7.2 months (range, 0.9–12.2), 6.8 months (range, 0.7–13.4), and 6.9 months (range, 0.5–12.0 months), in the panitumumab plus rilotumumab, panitumumab plus ganitumab, and panitumumab plus placebo arms, respectively.
Demographics and baseline characteristics were generally balanced across treatment arms (Table 1), with the exception of fewer patients with ECOG 1 performance status in the panitumumab plus rilotumumab arm and approximately twice as many patients ≥65 years old in the combination arms compared with the panitumumab plus placebo arm. One patient had an ECOG performance score of 2 and was in the panitumumab plus rilotumumab arm. Two patients in the control arm had not received first-line therapy for mCRC; both patients had received adjuvant oxaliplatin-based chemotherapy for non-mCRC and had progressed while on therapy less than a year before entering the study. All 3 patients received investigational therapy and are included in the efficacy and safety analysis.
Part 2: Efficacy
Objective response rate
ORR was 31% for patients receiving panitumumab plus rilotumumab versus 21% for patients receiving panitumumab plus placebo (Table 2). No complete responses were noted. For the panitumumab plus rilotumumab arm, the posterior probability that the OR is greater than 1 was 0.93 and therefore met the prespecified criteria that the combination regimen was considered promising.
Table 2.
Primary endpoint: ORR
| Panitumumab + rilotumumab (AMG 102) (n = 48) |
Panitumumab + ganitumab (AMG 479] (n = 46) |
Panitumumab + placebo (n = 48) |
|
|---|---|---|---|
| Patients with baseline measurable disease, n (%) | 48(100) | 46(100) | 48(100) |
| Objective response, n (%) | 15(31) | 10(22) | 10(21) |
| Complete response | 0(0) | 0(0) | 0(0) |
| Partial response | 15(31) | 10(22) | 10(21) |
| Stable disease | 19(40) | 18(39) | 17 (35) |
| Progressive disease | 11 (23) | 15(33) | 16(33) |
| Unevaluable/not done | 3(6) | 3(6) | 5(10) |
| Disease control ratea, % (95% CI) | 71 (56–83) | 61 (45–75) | 56 (41–71) |
| Duration of response, median months (95% CI) | 5.1 (3.7–5.6) | 3.7 (3.6–5.8) | 3.7 (3.6–NE) |
| Posterior probability of odds ratio > 1b | 0.93 | 0.63 |
Abbreviation: NE, not estimable.
Disease control rate = complete response + partial response + stable disease.
Odds ratio is calculated based on ORR; an odds ratio of >1 favors the combination arm over panitumumab alone.
The ORR was 22% for patients receiving panitumumab plus ganitumab versus 21% for patients receiving panitumumab plus placebo. For the panitumumab plus ganitumab arm, the posterior probability that the OR is greater than 1 was 0.63 and therefore the combination was indeterminate.
Nineteen patients in the panitumumab plus rilotumumab arm (15 of which had an objective response), 12 patients in the panitumumab plus ganitumab arm (10 of which had an objective response), and 15 patients in the panitumumab alone arm (10 of which had an objective response) had a sum of the longest diameter (SLD) reduction >30% on RECIST defined target lesions (Supplementary Fig. S2).
Median durations of response were 5.1 months [95% confidence interval (CI), 3.7–5.6 months], 3.7 months (95% CI, 3.6–5.8 months), and 3.7 months (95% CI, 3.6 to not estimable months) in the panitumumab plus rilotumumab, panitumumab plus ganitumab, and panitumumab plus placebo arms, respectively. The disease control rates were 71%, 61%, and 56% in the panitumumab plus rilotumumab, panitumumab plus ganitumab, and panitumumab plus placebo arms, respectively (Table 2).
Progression-free survival
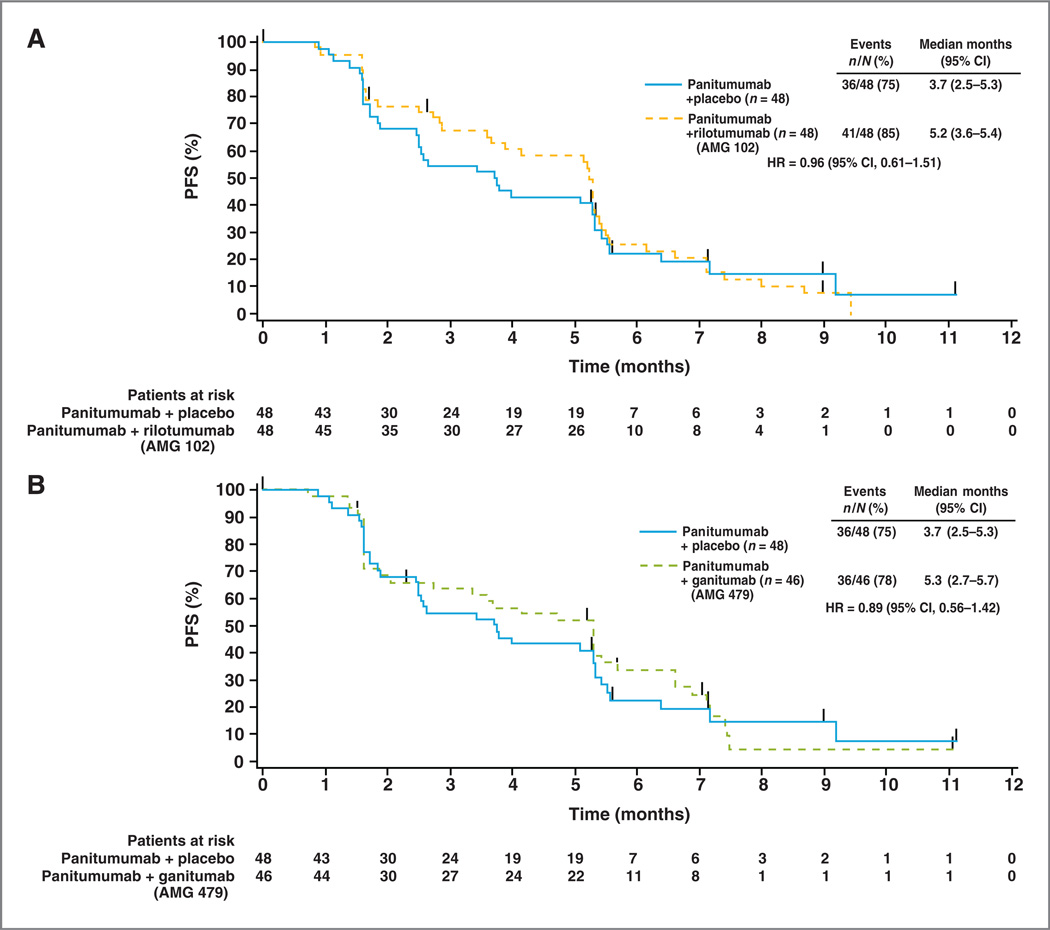
Median PFS for the 3 treatment groups was 5.2 months (95% CI, 3.6–5.4 months), 5.3 months (95% CI, 2.7–5.7 months), and 3.7 months (95% CI, 2.5–5.3 months) in the panitumumab plus rilotumumab, panitumumab plus ganitumab, and panitumumab plus placebo arms, respectively (Fig. 1). The PFS HR comparing the panitumumab plus rilotumumab arm with the panitumumab plus placebo arm was 0.96 (95% CI, 0.61–1.51) and that for the panitumumab plus ganitumab arm with the panitumumab plus placebo arm was 0.89 (95% CI, 0.56–1.42).
Figure 1.
Kaplan–Meier curves for PFS (central review) in patients treated with panitumumab plus rilotumumab (AMG 102;A) and panitumumab plus ganitumab (AMG 479; B) versus panitumumab plus placebo.
Overall survival
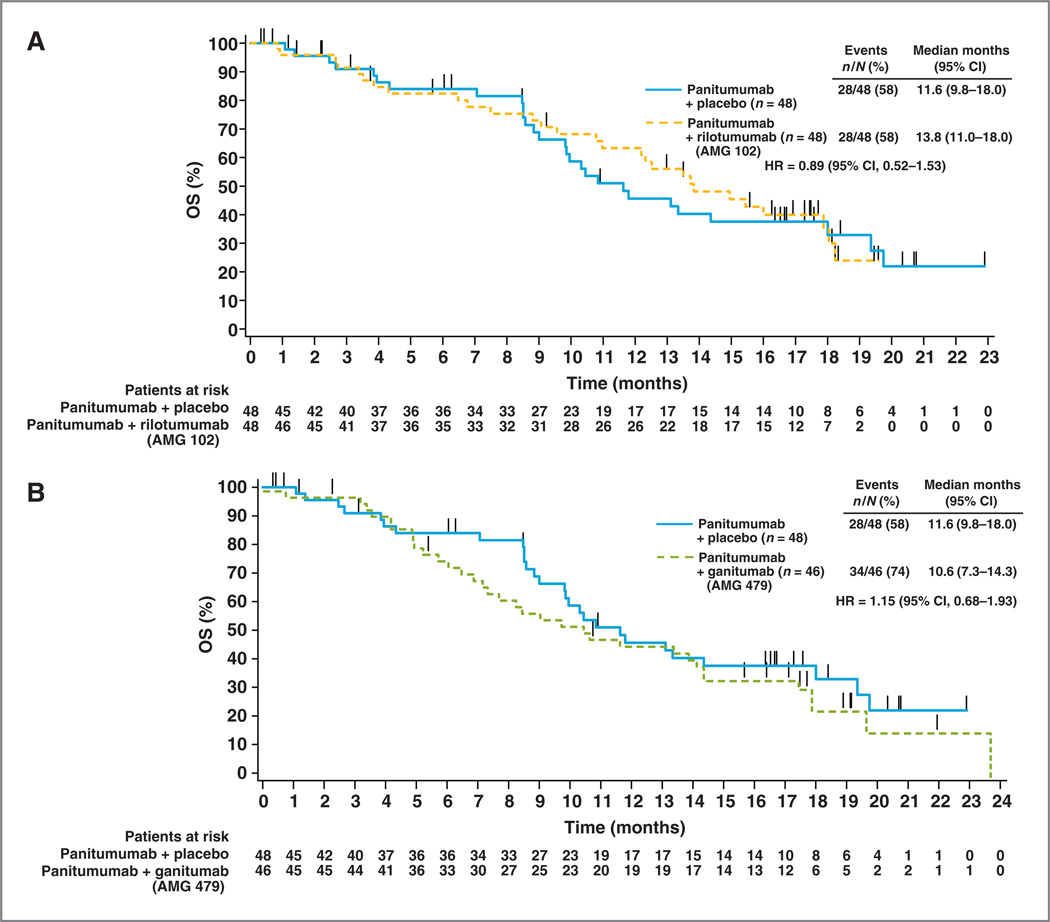
OS outcomes were immature at the time of the primary analysis with 40 (28%) deaths reported. An additional follow-up in June 2011 was conducted when the OS data were more mature, with 90 (63%) deaths reported. Median OS for the 3 treatment groups was 13.8 months (95% CI, 11.0–18.0 months), 10.6 months (95% CI, 7.3–14.3 months), and 11.6 months (95% CI, 9.8–18.0 months) in the panitumumab plus rilotumumab, panitumumab plus ganitumab, and panitumumab plus placebo arms, respectively (Fig. 2). The OS HR comparing the panitumumab plus rilotumumab arm with the panitumumab plus placebo arm was 0.89 (95% CI, 0.52–1.53), and that for the panitumumab plus ganitumab arm with the panitumumab plus placebo arm was 1.15 (95% CI, 0.68–1.93). Twenty-seven patients in the panitumumab plus placebo arm (56%) received subsequent therapy with 24 of these patients enrolling in part 3 of this phase II study where they received either subsequent rilotumumab or ganitumab monotherapy. Seven patients (15%) in the panitumumab plus rilotumumab arm and 12 (26%) patients in the panitumumab plus ganitumab arm received subsequent therapy.
Figure 2.
Kaplan–Meier curves for OS in patients treated with panitumumab plus rilotumumab (AMG 102; A) and panitumumab plus ganitumab (AMG 479; B) versus panitumumab plus placebo.
Part 2: Safety
Twenty percent of patients receiving treatment had experienced at least one serious AE with 19% in the panitumumab plus rilotumumab arm, 20% in the panitumumab plus ganitumab arm, and 23% in the panitumumab plus placebo arm.
There were 9 deaths on study: 4 occurred in each of the combination arms and 1 occurred in the panitumumab plus placebo arm. Eight were because of disease progression and 1 was because of staphylococcal sepsis (panitumumab plus ganitumab arm). None were considered related to investigational treatment.
Grade 3 and 4 AEs, including events that occurred with ≥ 15% occurrence, are shown in Table 3. The safety profiles were similar for the 3 arms with the following exceptions: grade 3/4 hypomagnesemia (highest in the panitumumab alone arm) and grade 3/4 rash (highest in the panitumumab plus rilotumumab arm).
Table 3.
Adverse events (any grade in ≥15% of patients)
| Panitumumab + rilotumumab (AMG 102) (n = 48) |
Panitumumab + ganitumab (AMG 479) (n = 46) |
Panitumumab + placebo (n = 48) |
||||
|---|---|---|---|---|---|---|
| AE (preferred term), % | Any grade | Grade 3/4 | Any grade | Grade 3/4 | Any grade | Grade 3/4 |
| Any AE | 98 | 71 | 94 | 52 | 100 | 63 |
| Skin disorders | ||||||
| Rash | 58 | 29 | 52 | 8 | 48 | 13 |
| Acneiform dermatitis | 35 | 15 | 33 | 10 | 26 | 11 |
| Pruritus | 21 | 0 | 25 | 0 | 28 | 2 |
| Skin fissures | 15 | 2 | 17 | 0 | 26 | 0 |
| Paronychia | 31 | 4 | 15 | 2 | 20 | 2 |
| Dry skin | 23 | 2 | 15 | 0 | 22 | 0 |
| Gastrointestinal disorders | ||||||
| Constipation | 10 | 0 | 25 | 6 | 13 | 0 |
| Decreased appetite | 21 | 2 | 17 | 2 | 20 | 2 |
| Nausea | 8 | 0 | 17 | 0 | 7 | 0 |
| Vomiting | 6 | 0 | 15 | 0 | 11 | 0 |
| Abdominal pain | 10 | 4 | 15 | 6 | 9 | 7 |
| Diarrhea | 15 | 4 | 10 | 0 | 26 | 2 |
| Fatigue/asthenia | 18 | 4 | 36 | 2 | 30 | 6 |
| Hypomagnesaemia | 29 | 4 | 21 | 2 | 41 | 15 |
| Anemia | 4 | 0 | 17 | 8 | 2 | 0 |
| Peripheral edema | 19 | 2 | 13 | 0 | 2 | 0 |
| Epistaxis | 15 | 0 | 2 | 0 | 4 | 0 |
NOTE: There were nine grade5AEs. Four grade 5 AEs occurred in the panitumumab plus rilotumumab arm (all disease progression); 4 in the panitumumab plus ganitumab arm (3 disease progression, one staphylococcal sepsis); 1 in the panitumumab plus placebo arm (disease progression).
Twelve percent of the total number of rilotumumab doses were withheld and 4% of the ganitumab doses. Sixteen percent, 8%, and 8% of the total number of panitumumab doses were withheld in the rilotumumab plus panitumumab, ganitumab plus panitumumab, and the panitumumab alone arms, respectively. Of patients who had panitumumab withheld, skin- or nail-related toxicities was the reason for 62%, 38%, and 50% of patients in the rilotumumab, ganitumab, and the panitumumab alone arms, respectively.
Part 2: Phase II biomarker analysis
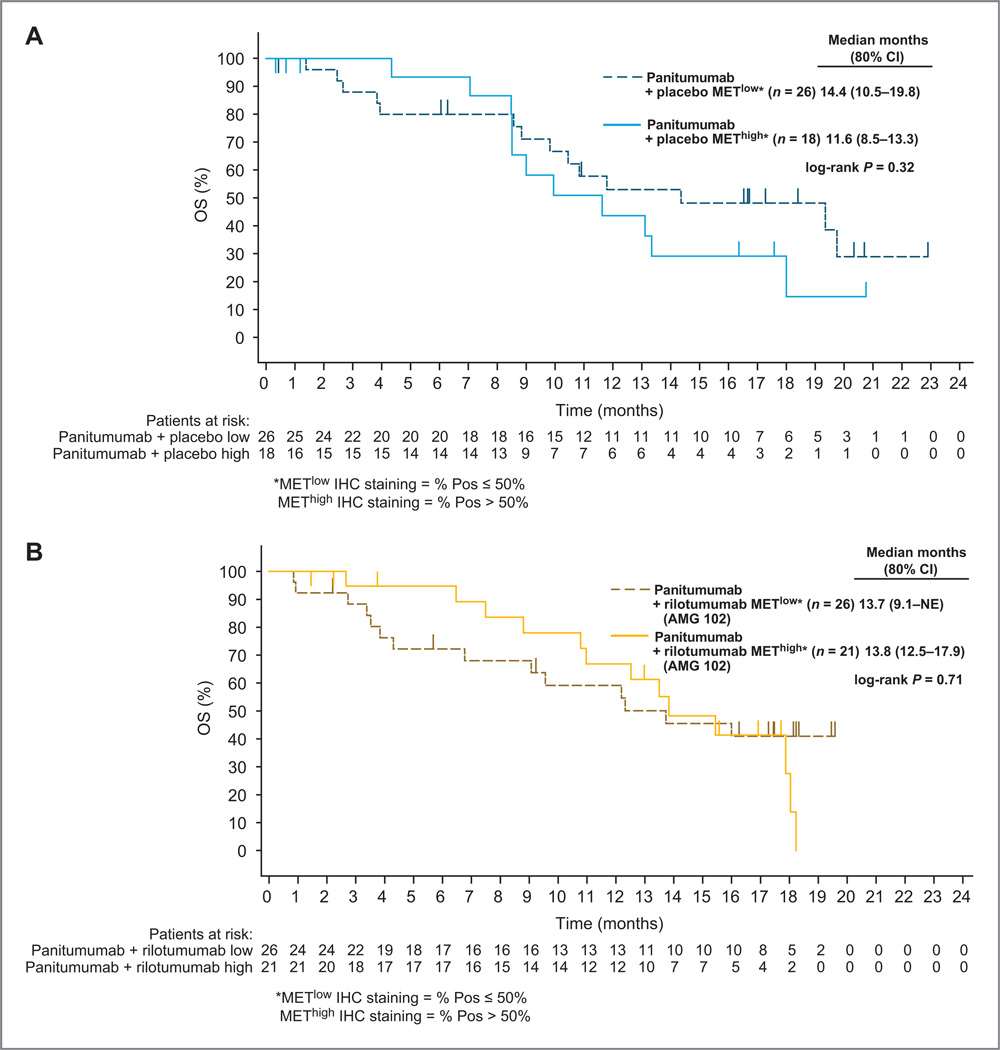
Cytoplasmic MET expression levels by IHC were obtained for 47 of 48 patients (98%) in the panitumumab plus rilotumumab arm and 44 of 48 patients (92%) in the panitumumab plus placebo arm (15% of samples were from metastatic lesions). No statistically significant differences were observed for the association of tumor MET cytoplasmic expression with PFS or OS in the panitumumab plus rilotumumab or panitumumab plus placebo arms (Fig. 3). Trends favoring high MET cytoplasmic levels were observed for ORR but the interaction P values indicated no statistically significant predictive treatment effects between the panitumumab plus rilotumumab and panitumumab plus placebo arms (Supplementary Fig. S3).
Figure 3.
Effect of low (≤50%) and high (≥50%) cytoplasmic MET IHC staining (≥1 +) on OS in patients treated with panitumumab plus placebo (A) and panitumumab plus rilotumumab (AMG 102; B).
To identify possible predictive markers for ganitumab treatment, analysis of baseline circulating IGF and IGF-related proteins was performed. An association between lower values for IGFBP1 and IGFBP2 and improved OS was observed for all 3 treatment arms suggesting a prognostic effect. None of the analytes demonstrated a significant interaction with treatment (Supplementary Fig. S4). There were no strong associations between the tested analytes and ORR in ganitumab-treated patients (Supplementary Fig. S5).
There was an association between tumor EGFR staining and improved OS for the panitumumab plus ganitumab arm, and a similar trend was seen in the panitumumab plus placebo arm but the effect was not statistically significant. Several studies have indicated an association of PTEN with anti-EGFR therapy (20, 21). An analysis of PTEN IHC levels suggested a correlation between lower PTEN expression and improved OS but only in the panitumumab plus placebo arm. Taken together, the predictive ability of these biomarkers for the efficacy of the combination of panitumumab plus rilotumumab or ganitumab was not evident in this study.
Discussion
This is the first phase Ib/II study evaluating the safety and efficacy of the fully human mAbs against HGF/SF, rilotumumab, and IGF1R, ganitumab, in combination with panitumumab in previously treated patients with WT KRAS mCRC. The rationale for this unique trial design was that inhibition of the EGFR pathway, combined with MET or IGF1R pathway inhibition, could provide additive anticancer effects. To our knowledge, the data reported here are the first to suggest promising evidence of efficacy by an HGF (MET pathway) inhibitor (rilotumumab) when combined with an EGFR pathway inhibitor (panitumumab) in patients with WT KRAS mCRC who have progressed on standard chemotherapy.
The primary endpoint of ORR increased by 10 percentage points with the addition of rilotumumab to panitumumab compared with panitumumab alone (31% vs. 21%) and these results were considered statistically significant (per prospectively specified Bayesian criterion). The ORR in the panitumumab alone arm was 21% and similar to that previously reported in the panitumumab monotherapy setting (17% in the WT KRAS subset in trial 20020408; 2). The disease control rate and the duration of response also favored the rilotumumab combination arm. However, the improvement in ORR did not translate into significant PFS and OS benefits.
There was an imbalance of rash in the rilotumumab arm for all grade rash (58%, 52%, and 42% for the rilotumumab, ganitumab, and panitumumab alone arms, respectively) and for grade 3 and 4 rash (29%, 8%, 13% for the rilotumumab, ganitumab, and panitumumab alone arms, respectively). It is possible this may have led to unblinding of the treatment arms. However, all arms received panitumumab for which rash is a known toxicity, no prior or subsequent association of increased rash has been reported for rilotumumab, and it is not clear that the presence of rash alone would provide any strong indication of the treatment arm.
In part 1 (phase Ib) of this study, no DLTs were reported for the first 6 DLT-evaluable patients receiving panitumumab plus rilotumumab. Inpart2 (phaseII), the safety profile of the drug combination was similarto that of panitumumab plus placebo with the exception of a higher incidence of grade 3/4 rash in the panitumumab plus rilotumumab arm.
The median PFS in the panitumumab plus ganitumab arm was similar to what was observed in the rilotumumab-containing arm. However, there was no improvement in OS. Treatment-related grade 3/4 AEs were similar between the arms with the exception of an increase in hypomagne-semia in the panitumumab plus ganitumab arm.
Overall, these results are consistent to those reported elsewhere (22), that the combination of an anti-EGFR and an anti-IGF1R agent do not provide strong additive anticancer effects in patients with WT KRAS mCRC. One explanation for these results is that there is compensatory signaling via IGF2 through the insulin receptor (23,24) which is not downregulated by ganitumab. Additional patient selection markers beyond KRAS status may be required to determine which tumors are most dependent on combined signaling through EGFR and IGF1R as IGF1R expression levels alone do not seem to be sufficient to predict response (22).
Exploratory biomarker analysis was conducted to investigate the correlation of efficacy endpoints with tumor MET, EGFR, and PTEN and baseline circulating IGF1, IGF2, and IGFBP1–3 and −6 protein levels. For the analyzed biomarkers, there was no strong evidence of predictive potential on efficacy endpoints for rilotumumab or ganitumab in combination with panitumumab. Reasons for the lack of conclusive evidence may be attributed to the small number of patients per arm as well as potential imbalances in the arms for RAS mutations beyond KRAS exon 2, which are known negative predictive factors for anti-EGFR mAB therapies, and BRAF mutations, which are known prognostic factors in mCRC. Identification of patients in this study with additional RAS mutations is on-going.
To date, review of the literature has not identified a uniform approach to evaluation of the HGF/MET pathway in mCRC. MET is believed to be a late event in carcinogenesis and expression levels may be higher at sites of metastatic disease (11, 12, 25). MET amplification is uncommon and was not measured (26). In this analysis, 85% of the archival tissue samples originated from primary tumors.
This study was not directly planned to examine MET biomarkers, however, results suggest that the MET and EGFR pathways may be related in their impact on outcome. Recent literature in patient-derived colorectal cancer xenograft models, as well as a limited set of patient plasma, demonstrated that MET amplification is associated with EGFR resistance. In vitro this resistance may be overcome by MET kinase inhibitors (27). In an updated analysis of a phase II study in esophagogastric junction cancer (28), OS was 11.5 months in the rilotumumab plus chemotherapy arm and 5.7 months in the chemotherapy alone arm in patients with tumors expressing high MET (HR, 0.34; 95% CI, 0.15–0.78). Hence, for future clinical trial development in the HGF/MET pathway, we encourage the use of MET-positive patient populations. These studies would require baseline tissue from all patients to provide the power needed to address biomarker hypotheses. A specific definition of MET positivity would be one of the objectives as the definition of MET positivity would determine the specific biomarker hypothesis that would be tested.
Although combined inhibition of the HGF/MET pathway in combination with EGFR inhibition appeared promising, there are no current plans to pursue further development of panitumumab plus rilotumumab in mCRC. However, rilotumumab continues to undergo further development in multiple malignancies, including in combination with bevacizumab in recurrent malignant glioma (NCT01113398), in combination with erlotinib in recurrent or progressive non-small cell lung carcinoma (NCT01233687), and in combination with chemotherapy in naïve gastroesophageal patients (NCTO1791374, NCT01443065, NCT01697072).
These data support further study of combined biologic blockade of cancer-related pathways without the requirement for cytotoxic agents and emphasize the need to identify patients that may benefit from combined EGFR and MET pathway inhibition.
Supplementary Material
Translational Relevance.
Upregulation of the hepatocyte growth factor (HGF)/MET and the IGF1R pathways have been suggested as potential mechanisms of signal escape in colorectal tumors upon treatment with epidermal growth factor receptor (EGFR) inhibitors. In this clinical study, it is suggested for the first time that the dual inhibition of the EGFR by panitumumab and HGF by rilotumumab translates in incremental antitumor activity over panitumumab as a single agent in patients with refractory wild-type KRAS metastatic colorectal cancer.
Acknowledgments
The authors thank the following individuals from Amgen Inc.: S. Lee, a medical writer, who provided writing assistance, and R. Loberg, who provided circulating IGF and IGF-related protein data. The authors also thank the patients and caregivers for their participation in the study. Amgen Inc. reviewed this article for data accuracy.
Grant Support
This was supported by Amgen Inc., Thousand Oaks, California.
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked advertisement in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
Footnotes
Disclosure of Potential Conflicts of Interest
E. Van Cutsem reports receiving a commercial research grant from Amgen Inc. C. Eng and N. Tebbutt report receiving commercial research grants from and are consultants/advisory board members for Amgen Inc. I. McCaffery and D. Smethurst were employees of and have ownership interest (including patents) in Amgen Inc. K. Oliner, L. Chen, J.L. Gansert are employees of Amgen Inc. E. Loh is an employee of and has ownership interest (including patents) in Amgen Inc. J. Tabernero is a consultant/advisory board member for Amgen Inc. No potential conflicts of interest were disclosed by the other authors.
Authors’ Contributions
Conception and design: E. Van Cutsem, C. Eng, E. Mitchell, R. Tang I. McCaffery, L. Chen, J. Gansert, E. Loh, D. Smethurst, J. Tabernero
Development of methodology: E. Van Cutsem, C. Eng, E. Mitchell, E. Elez, R. Tang, I. McCaffery, K.S. Oliner, L. Chen, J. Tabernero
Acquisition of data (provided animals, acquired and managed patients, provided facilities, etc.): E. Van Cutsem, C. Eng, E. Nowara, A. Świeboda-Sadlej, N.C. Tebbutt, E. Mitchell, J. Stephenson, E. Elez, H. Prenen, K.S. Oliner, J. Gansert, D. Smethurst, J. Tabernero
Analysis and interpretation of data (e.g., statistical analysis, biostatistics, computational analysis): E. Van Cutsem, C. Eng, E. Nowara, N.C. Tebbutt, E. Mitchell, E. Elez, H. Deng, R. Tang, I. McCaffery, K.S. Oliner, L. Chen, J. Gansert, E. Loh, D. Smethurst, J. Tabernero
Writing, review, and/or revision of the manuscript: E. Van Cutsem, C. Eng, E. Nowara, A. Świeboda-Sadlej, N.C. Tebbutt, E. Mitchell, I. Davidenko, J. Stephenson, E. Elez, H. Prenen, H. Deng, R. Tang I. McCaffery, K.S. Oliner, L. Chen, J. Gansert, E. Loh, J. Tabernero
Administrative, technical, or material support (i.e., reporting or organizing data, constructing databases): C. Eng, E. Nowara, H. Prenen, J. Gansert
Study supervision: C. Eng, E. Nowara, J. Stephenson, H. Prenen, J. Gansert, E. Loh, J. Tabernero
Note: Supplementary data for this article are available at Clinical Cancer Research Online (http://clincancerres.aacrjournals.org/).
References
- 1.Ferlay J, Shin HR, Bray F, Forman D, Mathers C, Parkin DM. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer. 2010;127:2893–2917. doi: 10.1002/ijc.25516. [DOI] [PubMed] [Google Scholar]
- 2.Amado RG, Wolf M, Peeters M, Van Cutsem E, Siena S, Freeman DJ, et al. Wild-type KRAS is required for panitumumab efficacy in patients with metastatic colorectal cancer. J Clin Oncol. 2008;26:1626–1634. doi: 10.1200/JCO.2007.14.7116. [DOI] [PubMed] [Google Scholar]
- 3.Douillard JY, Siena S, Cassidy J, Tabernero J, Burkes R, Barugel M, et al. Randomized, phase III trial of panitumumab with infusional fluorouracil, leucovorin, and oxaliplatin (FOLFOX4) versus FOLFOX4 alone as first-line treatment in patients with previously untreated metastatic colorectal cancer: the PRIME study. J Clin Oncol. 2010;28:4697–4705. doi: 10.1200/JCO.2009.27.4860. [DOI] [PubMed] [Google Scholar]
- 4.Peeters M, Price TJ, Cervantes A, Sobrero AF, Ducreux M, Hotko Y, et al. Randomized phase III study of panitumumab with fluorouracil, leucovorin, and irinotecan (FOLFIRI) compared with FOLFIRI alone as second-line treatment in patients with metastatic colorectal cancer. J Clin Oncol. 2010;28:4706–4713. doi: 10.1200/JCO.2009.27.6055. [DOI] [PubMed] [Google Scholar]
- 5.Van Cutsem E, Peeters M, Siena S, Humblet Y, Hendlisz A, Neyns B, et al. Open-label phase III trial of panitumumab plus best supportive care compared with best supportive care alone in patients with chemotherapy-refractory metastatic colorectal cancer. J Clin Oncol. 2007;25:1658–1664. doi: 10.1200/JCO.2006.08.1620. [DOI] [PubMed] [Google Scholar]
- 6.Hynes NE, Lane HA. ERBB receptors and cancer: the complexity of targeted inhibitors. Nat Rev Cancer. 2005;5:341–354. doi: 10.1038/nrc1609. [DOI] [PubMed] [Google Scholar]
- 7.Jo M, Stolz DB, Esplen JE, Dorko K, Michalopoulos GK, Strom SC. Cross-talk between epidermal growth factor receptor and c-Met signal pathways in transformed cells. J Biol Chem. 2000;275:8806–8811. doi: 10.1074/jbc.275.12.8806. [DOI] [PubMed] [Google Scholar]
- 8.Engelman JA, Zejnullahu K, Mitsudomi T, Song Y, Hyland C, Park JO, et al. MET amplification leads to gefitinib resistance in lung cancer by activating ERBB3 signaling. Science. 2007;316:1039–1043. doi: 10.1126/science.1141478. [DOI] [PubMed] [Google Scholar]
- 9.Lesko E, Majka M. The biological role of HGF-MET axis in tumor growth and development of metastasis. Front Biosci. 2008;13:1271–1280. doi: 10.2741/2760. [DOI] [PubMed] [Google Scholar]
- 10.Trusolino L, Bertotti A, Comoglio PM. MET signalling: principles and functions in development, organ regeneration and cancer. Nat Rev Mol Cell Biol. 2010;11:834–848. doi: 10.1038/nrm3012. [DOI] [PubMed] [Google Scholar]
- 11.Birchmeier C, Birchmeier W, Gherardi E, Vande Woude GF. Met, metastasis, motility and more. Nat Rev Mol Cell Biol. 2003;4:915–925. doi: 10.1038/nrm1261. [DOI] [PubMed] [Google Scholar]
- 12.Kammula US, Kuntz EJ, Francone TD, Zeng Z, Shia J, Landmann RG, et al. Molecular co-expression of the c-Met oncogene and hepatocyte growth factor in primary colon cancer predicts tumor stage and clinical outcome. Cancer Lett. 2007;248:219–228. doi: 10.1016/j.canlet.2006.07.007. [DOI] [PubMed] [Google Scholar]
- 13.Zhang L, Zhou W, Velculescu VE, Kern SE, Hruban RH, Hamilton SR, et al. Gene expression profiles in normal and cancer cells. Science. 1997;276:1268–1272. doi: 10.1126/science.276.5316.1268. [DOI] [PubMed] [Google Scholar]
- 14.Liska D, Chen CT, Bachleitner-Hofmann T, Christensen JG, Weiser MR. HGF rescues colorectal cancer cells from EGFR inhibition via MET activation. Clin Cancer Res. 2011;17:472–482. doi: 10.1158/1078-0432.CCR-10-0568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ahmad T, Farnie G, Bundred NJ, Anderson NG. The mitogenic action of insulin-like growth factor I in normal human mammary epithelial cells requires the epidermal growth factor receptor tyrosine kinase. J Biol Chem. 2004;279:1713–1719. doi: 10.1074/jbc.M306156200. [DOI] [PubMed] [Google Scholar]
- 16.Roudabush FL, Pierce KL, Maudsley S, Khan KD, Luttrell LM. Trans-activation of the EGF receptor mediates IGF-1-stimulated shc phosphorylation and ERK1/2 activation in COS-7 cells. J Biol Chem. 2000;275:22583–22589. doi: 10.1074/jbc.M002915200. [DOI] [PubMed] [Google Scholar]
- 17.Swantek JL, Baserga R. Prolonged activation of ERK2 by epidermal growth factor and other growth factors requires afunctional insulin-like growth factor 1 receptor. Endocrinology. 1999;140:3163–3169. doi: 10.1210/endo.140.7.6766. [DOI] [PubMed] [Google Scholar]
- 18.Sarantopoulos J, Mita AC, Mulay M, Romero O, Lu J, Capilla F, et al. A phase IB study of AMG 479, a type 1 insulin-like growth factor receptor (IGF1R) antibody, in combination with panitumumab (P)or gemcitabine (G) J Clin Oncol. 2008;(suppl 15):26. abstr 3583. [Google Scholar]
- 19.Hecht JR, Mitchell E, Neubauer MA, Burris HA3rd, Swanson P, Lopez T, et al. Lack of correlation between epidermal growth factor receptor status and response to Panitumumab monotherapy in metastatic colorectal cancer. Clin Cancer Res. 2010;16:2205–2213. doi: 10.1158/1078-0432.CCR-09-2017. [DOI] [PubMed] [Google Scholar]
- 20.Mao C, Liao RY, Chen Q. Loss of PTEN expression predicts resistance to EGFR-targeted monoclonal antibodies in patients with metastatic colorectal cancer. Br J Cancer. 102:2010. 940. doi: 10.1038/sj.bjc.6605575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sood A, McClain D, Maitra R, Basu-Mallick A, Seetharam R, Kaubisch A, et al. PTEN gene expression and mutations in the PIK3CA gene as predictors of clinical benefit to anti-epidermal growth factor receptor antibody therapy in patients with KRAS wild-type metastatic colorectal cancer. Clin Colorectal Cancer. 2012;11:143–150. doi: 10.1016/j.clcc.2011.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Reidy DL, Vakiani E, Fakih MG, Saif MW, Hecht JR, Goodman-Davis N, et al. Randomized, phase II study of the insulin-like growth factor-1 receptor inhibitor IMC-A12, with or without cetuximab, in patients with cetuximab- or panitumumab-refractory metastatic colorectal cancer. J Clin Oncol. 2010;28:4240–4246. doi: 10.1200/JCO.2010.30.4154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Garofalo C, Manara MC, Nicoletti G, Marino MT, Lollini PL, Astolfi A, et al. Efficacy of and resistance to anti-IGF-1R therapies in Ewing’s sarcoma is dependent on insulin receptor signaling. Oncogene. 2011;30:2730–2740. doi: 10.1038/onc.2010.640. [DOI] [PubMed] [Google Scholar]
- 24.Beltran PJ, Chung YA, Moody G, Mitchell P, Cajulis E, Vonderfecht S, et al. Efficacy of ganitumab (AMG 479), alone and in combination with rapamycin, in Ewing’s and osteogenic sarcoma models. J Pharmacol Exp Ther. 2011;337:644–654. doi: 10.1124/jpet.110.178400. [DOI] [PubMed] [Google Scholar]
- 25.Zeng ZS, Weiser MR, Kuntz E, Chen CT, Khan SA, Forslund A, et al. c-Met gene amplification is associated with advanced stage colorectal cancer and liver metastases. Cancer Lett. 2008;265:258–269. doi: 10.1016/j.canlet.2008.02.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Di Renzo MF, Olivero M, Giacomini A, Porte H, Chastre E, Mirossay L, et al. Overexpression and amplification of the met/HGF receptor gene during the progression of colorectal cancer. Clin Cancer Res. 1995;1:147–154. [PubMed] [Google Scholar]
- 27.Bardelli A, Corso S, Bertotti A, Hobor S, Valtorta E, Siravegna G, et al. Amplification of the MET receptor drives resistance to anti-EGFR therapies in colorectal cancer. Cancer Discov. 2013;3:658–673. doi: 10.1158/2159-8290.CD-12-0558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Davidenko I, Iveson T, Donehower RC, Tjulandin S, Deptala A, Jiang Y, et al. Updated efficacy, biomarker, and exposure-response data from a phase 2 study of rilotumumab (R) plus epirubicin, cisplatin, and capecitabine (ECX) in gastric (G) or esophagogastric junction (EGJ) cancer. Annals of Oncology. 2012;(susuppl 9):ix230. abstr P687. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.