Abstract
Heat stress provides a potent stimulus for activation of visceral sympathetic nerve discharge (SND) in young but not aged rats. Central mechanisms mediating attenuated SND responses to heating in aged rats have not been investigated. Because the GABAergic system in the rostral ventral lateral medulla (RVLM) is tonically inhibitory to SND, it is plausible to hypothesize that withdrawal of RVLM GABA tone as a strategy to activate renal SND to heating is not engaged to the same degree in aged compared with young rats. The effect of bilateral RVLM disinhibition produced by bicuculline (BIC, GABAA receptor antagonist, 100 pmol) microinjections on renal SND in anesthetized young (3-6 months old) and aged (22-24 months old) Fischer 344 rats was determined after core body temperature (Tc) had been increased to 41.5°C. Renal SND at 41.5°C was significantly increased from control levels in young but not aged rats, whereas RVLM BIC microinjections at 41.5°C produced marked renal sympathoexcitation in both groups. RVLM BIC microinjections at 38°C in young and aged rats increased renal SND to similar levels as produced by RVLM BIC microinjections at 41.5°C. The enhanced heating-induced renal sympathoactivation in young compared with aged rats; coupled with marked RVLM BIC-induced SND excitation under hyperthermic and normothermic conditions in both young and aged rats, suggests age-dependent changes in withdrawal of RVLM GABA tone as a strategy to activate renal SND in response to acute heating.
Keywords: RVLM, renal sympathetic nerve discharge, bicuculline, aged F344 rats, heat stress
INTRODUCTION
Elevated internal body temperature (Tc) produced by external heat stress induces activation of muscle sympathetic nerve discharge (SND) in humans (Crandall et al., 1999; Niimi et al., 1997) and visceral SND in young rats (Hosking et al., 2009, Kregel et al., 1994; Kenney et al., 1995, 1998, 2000, 2001, 2011; Kenney and Fels, 2002, 2003; Margiocco et al., 2010). Heating-induced activation of peripheral sympathetic nerve activity plays an important role in mediating cardiovascular responses to hyperthermia (Kenney et al., 1998; Kregel et al., 1988; Kregel and Gisolfi, 1989). The rostral ventral lateral medulla (RVLM) is critically involved in SND regulation (Dampney, 1994; Dampney et al., 2003; Guyenet, 2006; Horiuchi et al., 2004; Sun, 1995) and the functional balance between excitatory and inhibitory inputs, mediated primarily by excitatory amino acid and gamma-amino butyric acid (GABA) neural systems (Dampney et al., 2003; Guyenet, 2006; Horiuchi et al., 2004; Sun, 1995), plays a key role in regulating RVLM neuronal activity. Recent studies have investigated the role of this nucleus in SND regulation to external heat stress in anesthetized young rats. Inhibition of RVLM synaptic activity produced by muscimol (GABAA receptor agonist) microinjections at peak hyperthermia (Tc increased to 41.5°C) eliminates heating-induced activation of renal SND and splenic SND (Hosking et al., 2009), indicating the functional integrity of RVLM neural circuits is crucial for sustaining visceral SND activation in response to acute increases in Tc. Kenney et al. (2013) reported that disinhibition of RVLM sympathetic neural circuits contributes to heating-induced visceral sympathoexcitation in young Fischer 344 (F344) rats, suggesting that in these animals withdrawal of RVLM GABA tone is at least one strategy used to activate SND in response to acute heating.
Accompanying the persistent growth in the world’s population is a dramatic increase in the number of aged persons (United Nations, 2004). Physiological function is modified with advancing age, including alterations in sympathetic nervous system regulation (Kenney, 2010; Seals and Esler, 2000). During heat waves aged humans suffer excess mortality from hyperthermia and cardiovascular disease (Argaud et al., 2007; Dematte et al., 1998; Naughton et al., 2002). Visceral SND and blood flow redistribution responses to heating are attenuated in aged compared with young F344 rats (Kenney and Fels, 2002, 2003; Kenney and Musch, 2004; Margiocco et al., 2010), and the redistribution of blood flow from visceral circulations is attenuated during acute heating in aged compared with young human subjects (Minson et al., 1998). These findings demonstrate altered sympathetic neural and cardiovascular responses to heat stress in aged subjects, supporting the hypothesis that advancing age attenuates sympathetic nervous system responsivity to increased Tc, although central neural mechanisms have not been investigated.
The diminished SND responses to acute heating in aged rats may be a function of an age-dependent altered neural strategy that acts to actively suppress SND in response to increased Tc. Aging may shift the functional balance of medullary mechanisms mediating heating-associated SND responses to a state characterized by enhanced synaptic inhibition. Because the endogenous RVLM GABAergic system is inhibitory to presympathetic neurons, it is plausible to hypothesize that withdrawal of RVLM GABA tone as a strategy to activate renal SND to acute heating is not engaged to the same extent in aged compared with young rats. In the present study the effect of bilateral disinhibition of RVLM neural circuits produced by bicuculline (BIC, GABAA receptor antagonist) microinjections on the level of renal SND in anesthetized young (3-6 months old) and aged (22-24 months old) F344 rats was determined after Tc had been increased to 41.5°C. Additional experiments in young and aged F344 rats were completed by determining renal SND responses to RVLM BIC microinjections with Tc maintained at 38°C.
METHODS
The experimental procedures and protocols were completed in accordance with the American Physiological Society’s guiding principles for research involving animals and approved by the Institutional Animal Care and Use Committee at Kansas State University.
General Procedures
Experiments were completed in young (3-6 months old; 341±9 g) and aged (22-24 months old; 460±6 g) male F344 rats (Charles River Laboratories, contracted with the National Institute on Aging). Anesthesia was induced by isoflurane (3-5%; Butler Animal Science) and maintained during surgical procedures using isoflurane (1.5%-2.5%), α-chloralose (80 mg/kg, ip; Sigma), and urethane (800 mg/kg, ip; Sigma) (Kenney et al., 2013). A catheter was placed in the femoral vein for the intravenous administration of maintenance doses of α-chloralose (35-45 mg/kg/hr). Maintenance doses of urethane (200 mg/kg every 4 hours) were administered intraperitoneally. The trachea was cannulated and rats were paralyzed with gallamine triethiodide (5-10 mg/kg iv, initial dose; 10-15 mg/kg/hr, maintenance dose; Sigma) and artificially ventilated using a small animal ventilator (Harvard) (Kenney et al., 2011). Isoflurane anesthesia was discontinued following surgical procedures. Femoral arterial pressure was monitored using a pressure transducer connected to a blood pressure analyzer (Digi-Med BPA). Heart rate (HR) was derived from the pulsatile arterial pressure output of the blood pressure analyzer. Tc was measured with a thermistor probe inserted approximately 5-6 cm into the colon and was kept at 38.0°C during surgical interventions by a temperature-controlled table. The adequacy of anesthesia was indicated by the absence of a cardiovascular response to mechanical stimulation of the tail or hindlimb.
Sympathetic Nerve Recordings
Renal nerve activity was recorded biphasically with a platinum bipolar electrode after capacity-coupled preamplification (bandpass 30-3000 Hz) (Grass Instruments) from the central end of cut renal sympathetic nerves (Kenney et al., 2013). The left renal nerve was isolated retroperitoneally and nerve-electrode preparations were covered with silicone gel (World Precision Instruments) to prevent exposure to room air. Sympathetic nerve potentials were full wave rectified and integrated and total power in renal SND was quantified as µvolts × seconds (µV·s). SND recordings were corrected for background noise after administration of the ganglionic blocker, chlorisondamine (5 mg/kg iv; Sigma) (Margiocco et al., 2010).
Microinjections
RVLM microinjections were completed using standard stereotaxic procedures and target coordinates (Ito and Sved, 1997; Schreihofer et al., 2005). RVLM microinjections were completed using multibarrel glass micropipettes (3 barrels, outside tip diameter 40-70 μm) filled with appropriate drugs. Microinjection volumes were determined with the aid of a surgical microscope by measuring the displacement of the fluid meniscus in the micropipette barrel with respect to a horizontal grid. The RVLM was functionally identified by completing glutamate (10 mM, 40 nl; Sigma) microinjections across a range of coordinates to locate the RVLM site producing the largest increase in mean arterial pressure (MAP) (Moffitt et al., 2002).
Experimental Protocols
Before initiation of experimental protocols, anesthetized rats were allowed to stabilize for 60 min. Two protocols were completed, each involving RVLM microinjections. Microinjectate volume was 100 nl for each microinjection procedure. Renal SND, MAP and HR were recorded continuously in all experiments. The first protocol was designed to determine the effect of disinhibition of RVLM neural circuits on renal SND regulation in response to hyperthermia. Tc was increased from 38°C to 41.5°C at an approximate rate of 0.08-0.09°C/min using a heat lamp. Bilateral RVLM microinjections of BIC (100 pmol) were administered at a Tc of 41.5°C in young (n=11) and aged (n=9) F344 rats. Tc was maintained at 41.5°C for approximately 3-5 min before completion of RVLM BIC microinjections. End-tidal CO2 was measured using a micro-capnometer (Columbus Instruments) and was maintained near 4.5% during heating by adjusting the frequency of ventilation (control ventilatory rate, ~80-90 strokes/min; 41.5°C ventilatory rate, ~110-120 strokes/min). A second set of bilateral RVLM BIC microinjections (same dose as the initial microinjection) were completed at least 6 min after the initial microinjection with Tc sustained at 41.5°C in young (n=4) and aged (n=6) rats, followed by bilateral RVLM glutamate microinjections completed 3-5 min after the second RVLM BIC microinjections (Tc sustained at 41.5°C) (young, n=4; aged, n=6). Vehicle control experiments (young, n=4; aged, n=3) were completed by determining SND responses to bilateral microinjections of saline (100 nl) at a Tc of 41.5°C. In the second protocol the effect of bilateral RVLM BIC (100 pmol) (young, n=5; aged, n=5) or saline (young, n=4; aged, n=4) microinjections on renal SND were determined with Tc maintained at 38°C. At the conclusion of experiments rats were euthanized via an overdose of methohexital sodium (Brevital, 150 mg/kg, iv; JHP Pharmaceutical).
Data and Statistical Analysis
A computer-based ADInstruments Powerlab data acquisition system was used to collect all experimental data. Data included in the manuscript were obtained at the following experimental times: 1) during control prior to initiation of heating or prior to RVLM microinjections at 38°C, 2) following RVLM microinjections after increasing Tc to 41.5°C, and 3) following RVLM microinjections with Tc maintained at 38°C. Values are means ± SE. Renal SND responses are reported as percent change from baseline values (control). Control values of renal SND were considered as 100%. Statistical analyses included Student t-tests for pairwise comparisons and analysis of variance techniques with a repeated-measures design followed by Bonferroni post hoc corrections. Statistical analyses were completed on renal SND, MAP, and HR data. The overall level of statistical significance was p<0.05.
RESULTS
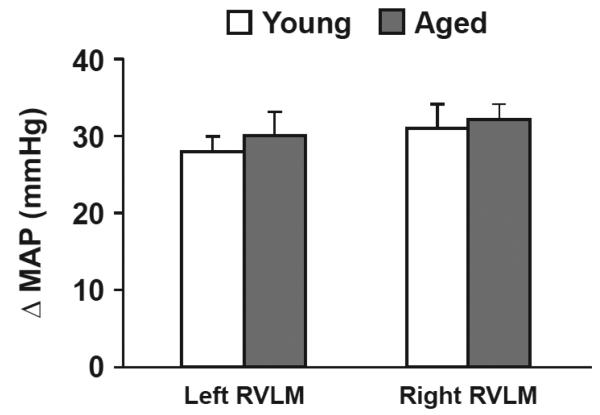
Increases in MAP produced by RVLM L-glutamate microinjections during RVLM mapping procedures did not differ between young and aged rats (Figure 1). Figure 2 shows original renal SND traces from representative young (top) and aged (bottom) F344 rats recorded at control (38°C), during acute heating that increased Tc to 41.5°C, and 4 min following bilateral RVLM BIC microinjections with Tc maintained at 41.5°C. Renal SND was increased from control levels during heating in the young but not in the aged rat, whereas RVLM BIC microinjections at 41.5°C markedly activated SND in both rats.
Figure 1.
Increases in mean arterial pressure (Δ MAP, mmHg) in response to bilateral microinjections of L-glutamate in left and right rostral ventral lateral medulla (RVLM) sites in young (open bars) and aged (closed bars) F344 rats.
Figure 2.
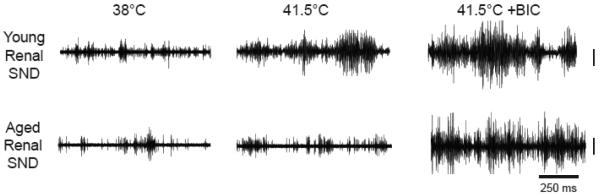
Renal sympathetic nerve discharge (SND) traces from representative young (top) and aged (bottom) F344 rats recorded at control (38°C), during acute heating that increased core body temperature (Tc) to 41.5°C, and 4 min following bilateral RVLM bicuculline (BIC, 100 pmol) microinjections with Tc maintained at 41.5°C. Vertical calibration is 50 µV. Horizontal calibration is 250 ms.
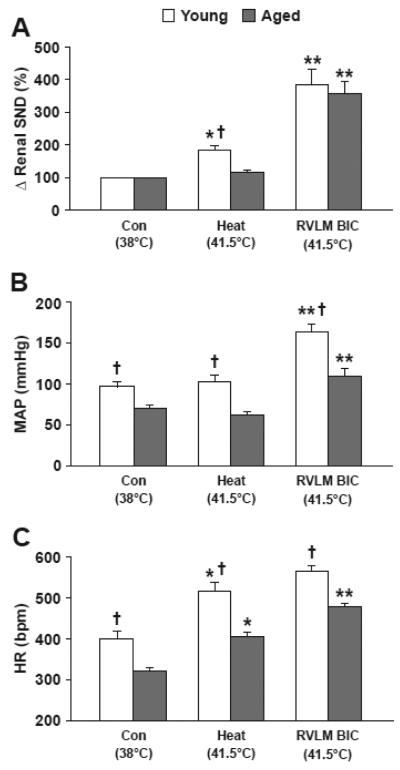
Figure 3 shows summarized renal SND (A), MAP (B), and HR (C) data during control (Con, 38°C), at peak hyperthermia after Tc had been increased to 41.5°C (Heat, 41.5°C), and 4-6 min following bilateral RVLM BIC microinjections with Tc maintained at 41.5°C in young (open bars, n=11) and aged (closed bars, n=9) F344 rats. Renal SND data during heating (41.5°C) and after RVLM BIC microinjections at 41.5°C are reported as percent change from control levels. Basal levels of MAP and HR were significantly lower in aged compared with young rats. Renal SND and HR were significantly increased from control levels after Tc was elevated to 41.5°C in young rats, whereas HR was increased from control levels in response to acute heating in aged rats. Age-related differences in each of the experimental variables were evident after increasing Tc to 41.5°C. Following RVLM BIC microinjections at 41.5°C, renal SND and MAP were significantly increased from peak hyperthermia levels in young rats whereas renal SND, MAP, and HR were significantly increased from peak hyperthermia levels in aged rats. Peak levels of renal sympathoexcitation recorded after completion of RVLM BIC microinjections at 41.5°C did not differ between young and aged rats, whereas age-related differences in MAP and HR persisted. RVLM vehicle microinjections at 41.5°C did not alter renal SND, MAP, or HR in young (n=4) or aged (n=3) rats (data not shown). A second set of bilateral RVLM BIC microinjections (same dose as the initial microinjection) completed 4-6 min after the initial microinjection with Tc maintained at 41.5°C did not alter the level of renal SND in young (n=4) or aged (n=6) rats, demonstrating the efficacy of the initial RVLM BIC microinjection. RVLM glutamate microinjections, completed 3-5 min after the second RVLM BIC microinjections with Tc maintained at 41.5°C, increased renal SND an additional 41±5% in young rats (n=4) and 36±6% in aged rats (n=6).
Figure 3.
Renal sympathetic nerve discharge (A; Δ renal SND, %), mean arterial pressure (B; MAP, mmHg), and heart rate (C; HR, beats per minute, bpm) recorded at control (Con, 38°C), during acute heating that increased Tc from 38°C to 41.5°C (Heat, 41.5°C), and 4-6 min following RVLM microinjections of bicuculline (BIC) with Tc maintained at 41.5°C (RVLM BIC, 41.5°C) in young (open bars, n=11) and aged (closed bars, n=9) F344 rats. *Peak hyperthermia (Heat 41.5°C) significantly different from control (38°C). **RVLM BIC significantly different from peak-heating levels. †Young F344 rats significantly different from aged F344 rats.
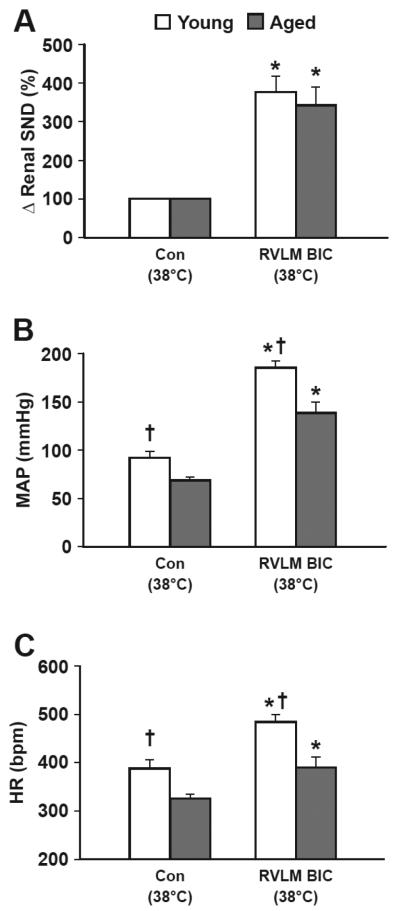
Figure 4 shows summarized renal SND (A), MAP (B), and HR (C) data recorded at a Tc of 38°C during control (Con, 38°C) and 4-6 min after bilateral RVLM BIC microinjections at 38°C in young (open bars, n=5) and aged (closed bars, n=5) F344 rats. Levels of renal SND after RVLM BIC microinjections are reported as percent change from control levels. Basal levels of MAP and HR were significantly lower in aged compared with young rats. With Tc maintained at 38°C, RVLM BIC microinjections significantly increased renal SND, MAP, and HR from control levels in both young and aged rats. RVLM BIC-induced renal sympathoactivation did not differ between young and aged rats whereas aged-related differences in MAP and HR persisted after RVLM BIC microinjections. RVLM vehicle microinjections at 38°C did not alter renal SND, MAP, or HR in young (n=4) or aged (n=4) rats (data not shown).
Figure 4.
Renal sympathetic nerve discharge (A; Δ renal SND, %), mean arterial pressure (B; MAP, mmHg), and heart rate (C; HR, beats per min, bpm) recorded at control (Con, 38°C) and 4-6 min following RVLM bicuculline (BIC) microinjections with Tc maintained at 38°C (RVLM BIC, 38°C) in young (open bars, n=5) and aged (closed bars, n=5) F344 rats. *RVLM BIC significantly different from control (38°C). †Young F344 rats significantly different from aged F344 rats.
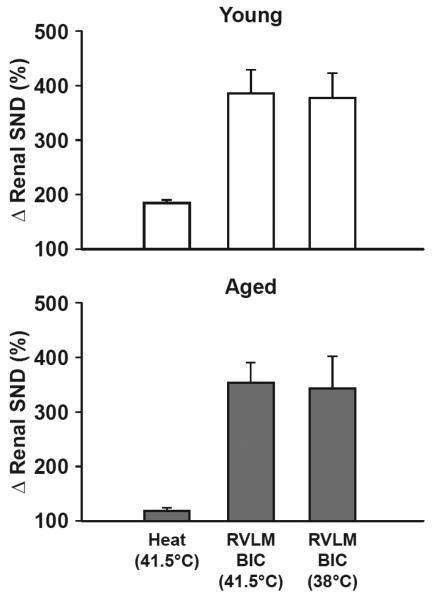
Renal SND data shown in Figures 3 and 4 are combined for presentation in Figure 5 as a means to compare renal SND responses to heating and RVLM BIC microinjections at 41.5°C (left two bars, data from Figure 3) with RVLM BIC microinjections at 38°C (right bar, data from Figure 4) in young (top) and aged (bottom) rats. Young rats demonstrated marked heating-induced activation of SND and peak RVLM BIC-induced renal sympathoexcitatory responses were similar under both hyperthermia and normothermia conditions. Aged F344 rats demonstrated modest increases in renal SND during heating and peak sympathetic activation responses to RVLM BIC microinjections were similar under each experimental condition (hyprthermia vs. normothermia).
Figure 5.
Renal sympathetic nerve discharge (Δ renal SND, %) responses to heating (Tc 41.5°C) and RVLM bicuculline (BIC) microinjections at 41.5°C (RVLM BIC, 41.5°C) (left two bars), and in response to RVLM BIC microinjections at 38°C (right bar) in young (top) and aged (bottom) rats.
DISCUSSION
Consistent with previous findings the renal sympathoexcitation to acute heat stress in the present study was attenuated in aged compared with young F344 rats. Disinhibition of RVLM neural circuits at 41.5°C resulted in immediate and significant increases in renal SND above heating-induced levels in both young and aged rats. In young F344 rats arterial blood pressure tended to be increased in parallel with renal SND at peak heating-induced elevations in Tc (41.5°C), consistent with previous findings supporting a role for sympathetic nervous system activation in mediating cardiovascular responses to acute heat stress (Kenney et al., 1998; Kregel et al., 1988; Kregel and Gisolfi, 1989). On the other hand, arterial blood pressure in aged rats tended to remain at or below control values at peak levels of Tc, consistent with the modest heating-induced renal SND excitation during acute heating in these animals. The persistence of a marked GABAergic inhibition to RVLM presympathetic neurons at peak hyperthermia appears to be a consistent physiological response profile in both young and aged rats, although it is a particularly interesting regulatory state in the aged rats as it would likely be advantageous for cardiovascular regulation if visceral sympathetic nerve outflow was more robustly activated at peak hyperthermia in the aged animals.
The level of renal sympathoexcitation at peak hyperthermia in young rats is not reduced in response to bilateral inhibition of RVLM ionotropic excitatory amino acid receptors (Kenney et al., 2011), indicating that activation of RVLM ionotropic glutamate receptors is not required for mediating visceral sympathoexcitation to acute heating, thereby suggesting a potential mediating role for GABAergic withdrawal. If endogenous disinhibition of RVLM presympathetic neurons via withdrawal of GABAergic tone does not contribute to visceral sympathoexcition to acute heating in young rats, then it would be predicted that peak levels of renal SND to RVLM BIC microinjections at 41.5°C would equal approximately the sum of heating-induced SND activation plus the level of SND excitation mediated by RVLM BIC microinjections at 38°C (Kenney et al., 2013). However, in the previous study and in the present experiments involving young rats, this was not the case as peak renal sympathoexcitatory responses to RVLM BIC microinjections at 38°C were similar to the sympathetic activation state recorded following RVLM BIC microinjections at peak hyperthermia (Kenney et al., 2013). Similarly, in the present study comparable levels of peak renal SND activation were observed in aged rats in response to RVLM BIC microinjections at 38°C and at peak hyperthermia (41.5°C). However, instead of the prominent heating-induced sympathoexcitation observed in young rats, renal SND was not significantly changed from control levels after increasing Tc to 41.5°C in the aged rats, indicating the presence of a sustained level of RVLM GABAergic tone at peak hyperthermia in these animals. Taken together these data support the hypothesis that withdrawal of RVLM GABA tone as a strategy to activate renal SND to acute heating is not engaged to the same extent in aged compared with young rats.
Irrespective of age, the level of renal SND activation recorded after RVLM BIC microinjections at peak hyperthermia was enhanced by subsequent RVLM glutamate microinjections, indicating the level of SND activation after RVLM BIC microinjections does not represent an RVLM activation ceiling in either young or aged rats. It appears that a more complete withdrawal of GABAergic tone to RVLM sympathetic neural circuits and/or enhanced activation of RVLM ionotropic glutamate receptors could each theoretically be employed to enhance renal SND responses to heat stress, supporting the concept that central sympathetic neural circuits retain a substantial reserve capacity to further increase SND under physiologically-relevant hyperthermic conditions. It remains to be determined why such a prominent, endogenous GABAergic inhibitory tone to RVLM sympathetic neural circuits persists during acute heating, especially in the aged rats.
The RVLM was successfully targeted as demonstrated by significant increases in arterial blood pressure induced by bilateral glutamate microinjections at target sites. Peak increases in MAP induced by RVLM glutamate microinjections at a concentration of 10mM did not differ between young and aged rats, suggesting the lack of age-dependent effects on arterial blood pressure responses to activation of RVLM excitatory amino acid receptors. As expected, RVLM vehicle microinjections produced no substantial effect on renal SND, indicating that RVLM BIC-induced renal sympathoexcitatory responses were not mediated via vehicle or volume effects. The present experiments were completed in anesthetized rats because of methodological difficulties associated with implementing combined central microinjections and peripheral nerve recordings in conscious preparations. Although the anesthetic protocol employed is widely used in autonomic neurophysiological studies, it must be considered that the anesthetic state may contribute to age-related differences. For example, Erdos et al. (2010) reported that basal levels of MAP were significantly higher and basal levels of HR were significantly lower in conscious aged compared with conscious young F344 × Brown-Norway rats. In the present study basal levels of both MAP and HR were significantly lower in aged compared with young F344 rats. On the other hand, with regards to SND responses to increased Tc, acute heat stress increases sympathetic nerve activity in conscious humans (Crandall et al., 1999; Niimi et al., 1997), young, conscious rats (Kregel et al, 1994), and young, anesthetized rats (Hosking et al., 2009, Kenney et al., 1995, 1998, 2000, 2001, 2011, 2013), whereas SND has been reported to remain unchanged in response to acute heating in aged, conscious F344 rats (Stauss et al., 1997) and is significantly attenuated during acute heating in aged, anesthetized F344 rats (Kenney and Fels, 2002, 2003; Margiocco et al., 2010; present study). It is also worth noting that in the present study both young and aged rats demonstrated prominent SND activation to RVLM glutamate and BIC microinjections, suggesting the lack of a generalized anesthetic-associated, age-related reduction in SND responsivity.
The acute responsivity of visceral sympathetic nerve outflow is a prominent component of regulatory processes mediating acute physiological changes to increased internal body temperature in young but not aged rats (Kenney and Fels, 2002; Kenney and Fels, 2003; Margiocco et al., 2010). Despite the importance of sympathetic activation in mediating cardiovascular responses to acute heating, little to no information is known regarding central mechanisms that may contribute to the attenuated visceral sympathetic activation to heating in aged rats. The present study is the first to investigate the effects of advanced age on RVLM mechanisms mediating SND responses to acute heating. The current findings support the concept that central sympathetic neural circuits in both young and aged rats retain a substantial capacity to further increase SND in response to acute heat stress, and suggest that enhanced RVLM GABAergic tone contributes to mediating attenuated SND responses to heating with advanced age.
HIGHLIGHTS.
Sympathetic nerve discharge activation to heating is attenuated with advanced age.
RVLM role in mediating attenuated sympathetic responses investigated in aged rats.
RVLM disinhibition at peak heating was produced by bicuculline (GABAA antagonist).
Young and aged rats retained the capacity to further increase SND at peak heating.
Results suggest withdrawal of RVLM GABA tone to heating is attenuated in aged rats.
ACKNOWLEDGEMENTS
This work was supported by NIH grant AG-041948. The author thanks Richard Fels for technical and experimental assistance, and Taylor Richter and Mal Hoover for assistance with the preparation of the manuscript and figures.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
DISCLOSURES
No conflicts of interest are declared by the author.
REFERENCES
- Argaud L, Ferry T, Le QH, Marfisi A, Ciorba D, Achache P, Ducluzeau R, Robert D. Short-and long-term outcomes of heatstroke following the 2003 heat wave in Lyon, France. Arch Intern Med. 2007;167:2177–2183. doi: 10.1001/archinte.167.20.ioi70147. [DOI] [PubMed] [Google Scholar]
- Crandall CG, Etzel RA, Farr DB. Cardiopulmonary baroreceptor control of muscle sympathetic nerve activity in heat-stressed humans. Am J Physiol. 1999;277:H2348–2352. doi: 10.1152/ajpheart.1999.277.6.h2348. [DOI] [PubMed] [Google Scholar]
- Dampney RA. Functional organization of central pathways regulating the cardiovascular system. Physiol Rev. 1994;74:323–364. doi: 10.1152/physrev.1994.74.2.323. [DOI] [PubMed] [Google Scholar]
- Dampney RA, Horiuchi J, Tagawa T, Fontes MA, Potts PD, Polson JW. Medullary and supramedullary mechanisms regulating sympathetic vasomotor tone. Acta Physiol Scand. 2003;177:209–218. doi: 10.1046/j.1365-201X.2003.01070.x. [DOI] [PubMed] [Google Scholar]
- Dematte JE, O’Mara K, Buescher J, Whitney CG, Forsythe S, McNamee T, Adiga RB, Ndukwu IM. Near-fatal heat stroke during the 1995 heat wave in Chicago. Ann Intern Med. 1998;129:173–181. doi: 10.7326/0003-4819-129-3-199808010-00001. [DOI] [PubMed] [Google Scholar]
- Erdos E, Cudykier I, Woods M, Basgut B, Whidden M, Tawil R, Cardounel AJ, Tumer N. Hypertensive effects of central angiotensin II infusion and restraint stress are reduced with age. J Hyperten. 2010;28:1298–1306. doi: 10.1097/HJH.0b013e328338a075. [DOI] [PubMed] [Google Scholar]
- Guyenet PG. The sympathetic control of blood pressure. Nat Rev Neurosci. 2006;7:335–346. doi: 10.1038/nrn1902. [DOI] [PubMed] [Google Scholar]
- Horiuchi J, Killinger S, Dampney RA. Contribution to sympathetic vasomotor tone of tonic glutamatergic inputs to neurons in the RVLM. Am J Physiol Regul Integr Comp Physiol. 2004;287:R1335–1343. doi: 10.1152/ajpregu.00255.2004. [DOI] [PubMed] [Google Scholar]
- Hosking KG, Fels RJ, Kenney MJ. Inhibition of RVLM synaptic activation at peak hyperthermia reduces visceral sympathetic nerve discharge. Auton Neurosci. 2009;150:104–110. doi: 10.1016/j.autneu.2009.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito S, Sved AF. Tonic glutamate-mediated control of rostral ventrolateral medulla and sympathetic vasomotor tone. Am J Physiol. 1997;273:R487–494. doi: 10.1152/ajpregu.1997.273.2.R487. [DOI] [PubMed] [Google Scholar]
- Kenney MJ. Animal aging and regulation of sympathetic nerve discharge. J Appl Physiol. 2010;109:951–958. doi: 10.1152/japplphysiol.00506.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenney MJ, Barney CC, Hirai T, Gisolfi CV. Sympathetic nerve responses to hyperthermia in the anesthetized rat. J Appl Physiol. 1995;78:881–889. doi: 10.1152/jappl.1995.78.3.881. [DOI] [PubMed] [Google Scholar]
- Kenney MJ, Claassen DE, Bishop MR, Fels RJ. Regulation of the sympathetic nerve discharge bursting pattern during heat stress. Am J Physiol. 1998;275:R1992–2001. doi: 10.1152/ajpregu.1998.275.6.R1992. [DOI] [PubMed] [Google Scholar]
- Kenney MJ, Fels RJ. Forebrain and brain stem neural circuits contribute to altered sympathetic responses to heating in senescent rats. J Appl Physiol. 2003;95:1986–1993. doi: 10.1152/japplphysiol.00438.2003. [DOI] [PubMed] [Google Scholar]
- Kenney MJ, Fels RJ. Sympathetic nerve regulation to heating is altered in senescent rats. Am J Physiol Regul Integr Comp Physiol. 2002;283:R513–520. doi: 10.1152/ajpregu.00683.2001. [DOI] [PubMed] [Google Scholar]
- Kenney MJ, Ganta CK, Fels RJ. Disinhibition of RVLM neural circuits and regulation of sympathetic nerve discharge at peak hyperthermia. J Appl Physiol. 2013;115:1297–1303. doi: 10.1152/japplphysiol.00494.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenney MJ, Meyer CN, Hosking KG, Fels RJ. Is visceral sympathoexcitation to heat stress dependent on activation of ionotropic excitatory amino acid receptors in the rostral ventrolateral medulla? Am J Physiol Regul Integr Comp Physiol. 2011;301:R548–557. doi: 10.1152/ajpregu.00113.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenney MJ, Musch TI. Senescence alters blood flow responses to acute heat stress. Am J Physiol Heart Circ Physiol. 2004;286:H1480–H1485. doi: 10.1152/ajpheart.00857.2003. [DOI] [PubMed] [Google Scholar]
- Kenney MJ, Musch TI, Weiss ML. Renal sympathetic nerve regulation to heating is altered in rats with heart failure. Am J Physiol Heart Circ Physiol. 2001;280:H2868–2875. doi: 10.1152/ajpheart.2001.280.6.H2868. [DOI] [PubMed] [Google Scholar]
- Kenney MJ, Pickar JG, Weiss ML, Saindon CS, Fels RJ. Effects of midbrain and spinal cord transections on sympathetic nerve responses to heating. Am J Physiol Regul Integr Comp Physiol. 2000;278:R1329–1338. doi: 10.1152/ajpregu.2000.278.5.R1329. [DOI] [PubMed] [Google Scholar]
- Kregel KC, Gisolfi CV. Circulatory responses to heat after celiac ganglionectomy or adrenal demedullation. J Appl Physiol. 1989;66:1359–1363. doi: 10.1152/jappl.1989.66.3.1359. [DOI] [PubMed] [Google Scholar]
- Kregel KC, Stauss H, Unger T. Modulation of autonomic nervous system adjustments to heat stress by central ANG II receptor antagonism. Am J Physiol. 1994;266:R1985–1991. doi: 10.1152/ajpregu.1994.266.6.R1985. [DOI] [PubMed] [Google Scholar]
- Kregel KC, Wall PT, Gisolfi CV. Peripheral vascular responses to hyperthermia in the rat. J Appl Physiol. 1988;64:2582–2588. doi: 10.1152/jappl.1988.64.6.2582. [DOI] [PubMed] [Google Scholar]
- Margiocco ML, Borgarelli M, Musch TI, Hirai DM, Hageman KS, Fels RJ, Garcia AA, Kenney MJ. Effects of combined aging and heart failure on visceral sympathetic nerve and cardiovascular responses to progressive hyperthermia in F344 rats. Am J Physiol Regul Integr Comp Physiol. 2010;299:R1555–R1563. doi: 10.1152/ajpregu.00434.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minson CT, Wladkowski SL, Cardell AF, Pawelczyk JA, Kenney WL. Age alters the cardiovascular response to direct passive heating. J Appl Physiol. 1998;84:1323–1332. doi: 10.1152/jappl.1998.84.4.1323. [DOI] [PubMed] [Google Scholar]
- Moffitt JA, Heesch CM, Hasser EM. Increased GABA(A) inhibition of the RVLM after hindlimb unloading in rats. Am J Physiol Regul Integr Comp Physiol. 2002;283:R604–614. doi: 10.1152/ajpregu.00341.2001. [DOI] [PubMed] [Google Scholar]
- Naughton MP, Henderson A, Mirabelli MC, Kaiser R, Wilhelm JL, Kieszak SM, Rubin CH, McGeehin MA. Heat-related mortality during a 1999 heat wave in Chicago. Am J Prev Med. 2002;22:221–227. doi: 10.1016/s0749-3797(02)00421-x. [DOI] [PubMed] [Google Scholar]
- Niimi Y, Matsukawa T, Sugiyama Y, Shamsuzzaman AS, Ito H, Sobue G, Mano T. Effect of heat stress on muscle sympathetic nerve activity in humans. J Auton Nerv Syst. 1997;63:61–67. doi: 10.1016/s0165-1838(96)00134-8. [DOI] [PubMed] [Google Scholar]
- Schreihofer AM, Ito S, Sved AF. Brain stem control of arterial pressure in chronic arterial baroreceptor-denervated rats. Am J Physiol Regul Integr Comp Physiol. 2005;289:R1746–1755. doi: 10.1152/ajpregu.00307.2005. [DOI] [PubMed] [Google Scholar]
- Seals DR, Esler MD. Human ageing and the sympathoadrenal system. J Physiol. 2000;528:407–417. doi: 10.1111/j.1469-7793.2000.00407.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stauss HM, Morgan DA, Anderson KE, Massett MP, Kregel KC. Modulation of baroreflex sensitivity and spectral power of blood pressure by heat stress and aging. Am J Physiol Heart Circ Physiol. 1997;272:H776–H784. doi: 10.1152/ajpheart.1997.272.2.H776. [DOI] [PubMed] [Google Scholar]
- Sun MK. Central neural organization and control of sympathetic nervous system in mammals. Prog Neurobiol. 1995;47:157–233. doi: 10.1016/0301-0082(95)00026-8. [DOI] [PubMed] [Google Scholar]
- United Nations . United Nations World Population Prospects: the 2003 Revision. In: World Population Prospects: United Nations Population Division. United Nations; New York: 2004. [Google Scholar]