Abstract
We previously demonstrated induction and expression of CD62E and CD62P in the lungs of mice primed and then challenged with intratracheal (IT) SRBC. The current study examined accumulation of endogenous lymphocytes in the lungs of endothelial selectin-deficient (E-P-) mice after IT-SRBC challenge. Compared to syngeneic wild type (wt) mice, E-P- mice showed an 85-95% decrease in CD8+ T-cells and B cells in the lungs at both early and late time points. In contrast, CD4+ T-cell accumulation was reduced by ~60% early, but equivalent to wt levels later. Surprisingly, many γ/† T cells were found in lungs and blood of E-P- mice, but were undetectable in the lungs and blood of wt mice. Absolute numbers of peripheral blood CD4, CD8 and B-lymphocytes in E-P- mice equaled or exceeded the levels in wt mice, particularly after challenge. Trafficking studies using α/β T lymphoblasts confirmed that the recruitment of circulating cells after challenge was markedly reduced in E-P- mice. Furthermore, Ag-priming occurred normally in both the selectin-deficient and wt mice, because primed lymphocytes from both groups transferred Ag sensitivity into naive wt mice. Lung production of mRNA for six CC and two CXC chemokines after challenge was equivalent by RT-PCR analysis in wt and E-P- mice. Therefore, reduced lung accumulation of α/β T-cells and B-cells in E-P- mice did not result from reduced delivery of circulating lymphocytes to the lungs, unsuccessful antigen-priming or defective pulmonary chemokine production. Selectin-dependent lymphocyte recruitment into the lungs following IT-SRBC challenge is subset-specific and time-dependent.
Keywords: Adhesion Molecules, Cell Trafficking, Rodent, Transgenic/Knockout, T cell receptors
INTRODUCTION
Recruitment of lymphocytes into lung parenchyma is centrally involved both in host defense against a wide variety of pathogens and in the development of immunologic lung diseases. Lymphocyte recruitment is a multi-step process involving adhesive interactions between lymphocytes and a variety of cell adhesion molecules (CAMs) on vascular endothelial cells (1). Direct in vitro and intra-vital study of leukocyte-endothelial adhesive interactions established the sequential roles of individual adhesion receptors to recruitment under high shear flow conditions and identified the physiologically relevant adhesion receptors expressed during inflammatory responses in selected sites. However, many microvascular beds and disease processes are not amenable to in vitro modeling or intra-vital study. Consequently, the contributions of adhesion receptors must be evaluated in vivo using experimental models of inflammation and immunity.
Such in vivo analysis is particularly important for the lung, which possess a unique dual vascular supply that impacts directly on leukocyte recruitment. The pulmonary circulation carries the entire output of the right ventricle to the extensive capillary network investing the alveoli. The bronchial circulation branches off the aorta and supplies oxygenated blood directly to the peribronchial and submucosal tissues (2). Depending on the inciting agent and mode of entry, the pulmonary inflammatory response may be alveolar, bronchial and peribronchial or a combination of both. Recruitment into alveoli generally occurs across capillaries where changes in leukocyte distensibility can arrest cells directly (3-6), although the independence of this process from adhesion molecules has been contested recently (7, 8). In contrast, recruitment into the bronchial wall and peribronchial tissues most likely involves larger vessels, especially intact post-capillary venules (6, 9-12), and hence may utilize the tethering receptors that mediate immune reactions at other sites with a similar microvascular organization.
In order to test this hypothesis, we chose an established model of CD4-dependent alveolar and peribronchial lymphocyte recruitment in mice sensitized to SRBC and then challenged with intratracheal SRBC (IT-SRBC) (13-18). Initial studies from our group and others showed that CD62E, CD62P and VCAM are expressed on the lung microvasculature in sensitized mice after challenge with IT-SRBCs (19, 20). All three receptors are expressed throughout the period of initial lymphocyte influx (days 2-4). However, E-selectin expression falls to baseline more rapidly than either P-selectin or VCAM. In addition, IT-SRBC challenge transiently increased the percentage of circulating T-cell expressing selectin ligands and resulted in the accumulation of selectin-ligand positive T-cells in the lung. Finally, trafficking studies with cultured T-lymphoblasts derived from fucosyltransferase-VII deficient animals suggested that selectin-ligands and α4-integrins mediated independent pathways of T-cell recruitment into IT-SRBC challenged lungs (16, 21). The current study used the SRBC-model and mice with gene-targeted deletions in both P- and E-selectins to directly evaluate the contributions of the selectins to alveolar and peribronchial lymphocyte accumulation. Substantial differences were observed in the accumulation of CD4 and CD8 positive T-cells, B-cells and γ/† T-cells in the airways and interstitial tissue of the lung. The findings indicate that selectins influence the accumulation of both α/β T-cell and B-cells, but suggest that the contribution of selectins is subset and time dependent.
MATERIALS AND METHODS
mAbs
The following epitopes were assessed by three-color flow cytometry analyses using directly-conjugated mAbs (PharMingen; San Diego, CA) (clone, isotype and fluorochrome are indicated in parentheses): CD4 (RM4-4; rat IgG2a; cychrome), CD8 (53-6.72; rat IgG2a; FITC), CD19 (1D3; rat IgG2a; phycoerythrin), CD45 (30-F-11; rat IgG2b; cychrome), γ/†-TCR (GL3; hamster IgG2; cychrome), Gr-1 (RB6-8C5; rat IgG2b; phycoerythrin), pan-NK cell (DX-5; rat IgM; FITC), CD14 (rmC5-3; rat IgG1; FITC), and Mac-3 (M3/84; rat IgG1; FITC). The fluorochromes were chosen based on the fluorescence intensities for each epitope and the level of background observed with isotype-matched control reagents.
Mice
Mice containing null mutations for both E-selectin and P-selectin (E-P- mice) were generated by gene targeting in 129/Sv embryonic stem cells, as previously described (22). E−/−P- mice used in this study had been backcrossed to C57BL/6 mice for five generations. Pathogen-free female C57BL/6 mice for use as controls were purchased at 7-8 weeks from Charles River Laboratory Inc. (Wilmington, MA). Although early reports of E-P- mice highlighted their frequent ulcerative skin lesion (22, 23), such lesions have been uncommon in our colony; no mice with visible skin lesions were used in this study. Mice were housed in the Animal Care Facility at the Ann Arbor VA Medical Center, which is fully accredited by the American Association for Accreditation of Laboratory Animal Care. This study complied with the NIH “Guide for the Care and Use of Laboratory Animals” {DHEW Publication No. (NIH) 80-23} and followed a protocol approved by the Animal Care Committee of the local Institutional Review Board. Mice were fed standard animal chow (Rodent Lab chow 5001, Purina; St. Louis, MO) and chlorinated tap water ad libitum. Mice were used at 8-14 weeks of age.
Induction of pulmonary immune response
SRBC (sheep 4151; Colorado Serum Co.; Denver, CO) were washed three times in 7 ml normal saline before use. Mice were Ag primed by IP injection with 1 × 108 SRBC in 0.5 ml normal saline. Beginning 2-4 wks later, mice were intratracheally (IT) challenged with 5 × 108 SRBC in 50 μl normal saline as previously described (19).
Sample collection
At 4 or 7 d after IT Ag challenge, mice were deeply anesthetized with pentobarbital (80 mg/kg IP) and killed humanely by exsanguination and induction of bilateral pneumothoraces. Peripheral blood was collected by cardiac puncture. The peripheral blood specimens were centrifuged through Lympholyte-M (Accurate Chemical and Scientific Corporation; Westbury, NY), lysed in a UNOPETTE (Becton-Dickinson; Franklin Lakes, NJ), washed and resuspended in Dulbecco’s minimal essential medium containing 0.1% azide and 0.1% BSA (DMEM+). Lungs were then processed in one of three ways. For cellular analysis, lungs were initially perfused in situ using normal saline to clear the pulmonary vasculature, and then the inflammatory cells in the airways and alveolar spaces were collected by bronchoalveolar lavage (BAL) (3). Next, the lavaged lungs were dissected free of extra-pleural lymphatic tissue, and to release the interstitial inflammatory cells for further analysis, lungs were finely minced and enzymatically digested (collagenase plus DNAse) (24). Aliquots of both BAL and lung mince preparations were washed and resuspended in DMEM+ before further analysis. Second, for histologic analysis, some lungs were inflated (1 ml) and immersed in 10% neutral buffered formalin for 18 to 24 h. Parasagittal slices of the fixed lungs were embedded in paraffin, sectioned at 5 μm thickness and stained with hematoxylin and eosin or Masson’s trichrome stain. Finally, the lungs for mRNA and protein analysis were perfused with normal saline, separated from the mainstem bronchi at the medial pleural surface, taking care to exclude extra-pleural lymphatic tissue, snap frozen in liquid nitrogen and stored at −70° C until use.
Flow cytometry analysis and determination of absolute lymphocyte counts
A minimum of three replicates was performed for each epitope. For each specimen, up to 1 × 105 events were collected without color compensation on a Beckman-Coulter EXCELL Flow Cytometer. Color compensation and analysis were conducted offline using the WinList analysis program (Verity Software House, Inc., Topsam, Maine). Light-scatter parameters were used to gate on the region containing the majority of lymphocytes in each specimen. The percentage of cells expressing each epitope was determined from one-parameter histograms. The threshold for “positive events” was based on isotype-matched control reagents. The percentage of “positive events” remaining in the control tube was subtracted from the percentage of “positive events” with each leukocyte specific antibody to determine the prevalence (percentage) for the various subsets.
Preliminary studies indicated that 95-100% of the CD45-positive cells in the light-scatter gate expressed the CD4, CD8, CD19, γ/† TCR or Gr-1 epitopes. Gr-1 (also known as Ly-6G) is expressed by murine neutrophils and eosinophils; consequently, the positive cells in the “lymphocyte” gate using this flow cytometer are most likely degranulated granulocytes. These cells accounted for a variable but significant percentage of the events in the gated region (up to 25%), particularly in the lung specimens. Therefore, the lymphocyte subset percentages used in the following calculations were corrected for the number of Gr-1 positive cells contaminating the analysis gate. It should be noted that this type of adjustment was not performed in previous studies using this model system (13, 14, 16, 18, 19, 24, 25), because the light scatter characteristics of the flow cytometers used previously did not result in such contamination of the lymphocyte gates (24). The means of the replicate determinations for each subset were then used to calculate the absolute number of cells as detailed below. The “absolute lymphocyte count” in each specimen was calculated from the “white blood cell count” (WBC) and “morphologic differential count”. The white blood cell counts were performed with a hemocytometer as previously described (26). Morphologic differential counts of the granulocytes, monocytes/macrophages and lymphocytes in each specimen were performed on stained filter preparations as previously described (24). The number of lymphocytes in each subset was then calculated from the absolute lymphocyte counts (WBC count × % lymphocytes from the morphologic differentials) and the lymphocyte subset percentages measured by flow cytometry. The results for each animal in a cohort were then used for the statistical analyses reported in the figures and tables.
Recruitment assay
Lymph node T cells from normal wt mice were activated using immobilized anti-CD3 mAb and their numbers expanded in IL-2 to produce T lymphoblasts, as previously described (16). After 5 days of expansion, T lymphoblasts were labeled with 5-chloromethylfluorescein diacetate (CMFDA) and transferred by tail vein injection into primed recipients, consisting either of wt or E-P- mice, which had been IT Ag challenged four or seven days earlier.
Adoptive transfer assay
To confirm the capacity of T-cells of E-P- mice to be Ag-primed in vivo, in one experiment splenocytes from E-P- donor mice or wt donor mice that had been primed with 1 × 108 SRBC six days previously by the IP route were transferred to unprimed wt recipient (2 × 107 splenocytes per recipient mouse in 0.2 ml PBS by the IV route). Subsequent Ag-driven accumulation of lung lymphocytes was assayed in BAL four days after IT SRBC challenge.
Isolation of RNA
Lungs were homogenized in 2 ml of TRIzol reagent (Life Technologies, Grand Island, NY) and RNA isolated as described in the TRIzol protocol. RNA was quantitated spectrophotometrically. To remove genomic DNA, all RNA samples was treated with DNase (DNA-free method, Ambion; Austin, Tx). The integrity of individual RNA samples was confirmed by electrophoresing aliquots on a 2% agarose gel containing 0.5 μg/ml ethidium bromide and observing 28- and 18-S rRNA bands. RNA samples was stored at −70° C.
RT-PCR detection of cytokine mRNA
Isolated RNA was reverse-transcribed to DNA and amplified by PCR as previously described in detail (17). The primer sequences used are defined in Table I. The amplification scheme was an initial 5 min at 95° C, repeated cycles of 15 sec at 95° C, 20 sec at 58° C, 30 sec at 72° C, and a final extension period of 6 min at 72° C. Positive and negative controls were included in each assay and the authenticity of reaction products was verified by Southern analysis using a chemiluminescent detection system (ECL; Amersham Life Science, Little Chalfont, Buckinghamshire, England). The amplified DNA was analyzed by electrophoresis on 1.5% agarose containing 0.5 μg/ml ethidium bromide. To quantitate cDNA bands, the ethidium-bromide stained agarose gels were photographed using Polaroid 667 film, scanned using a Scan Jet IIcx (Hewlett Packard) and analyzed on a Macintosh PowerPC G3 computer using the public domain NIH Image software (Version 1.6; available at http://rsb.info.nih.gov/nih-image/). Results are expressed as a ratio of OD signal for a given cytokine to that for cyclophilin in the same sample.
Table 1.
Primer sequences used for PCR amplification
| Mouse Gene | Synonym | Sequence (5’-3’) | Cycle # | |
|---|---|---|---|---|
| MCP-1 | CCL2 | sense | GCTGCCTCCTGTGCTAACT | 30 |
| antisense | CAGGATTGGAGGCAGTAG | |||
| MIP-1α | CCL3 | sense | CTACAGCCGGAAGATTCCAC | 32 |
| antisense | AGGAGATGGAGCTATGCAGG | |||
| MIP-1β | CCL4 | sense | TCTCTCTCCTCTTGCTCGTG | 29 |
| antisense | ACAGCAATGGTGGACCATCT | |||
| RANTES | CCL5 | sense | CACCTGCCTCACCA | 29 |
| antisense | CTTCACAGAGCAATGACTCC | |||
| MCP-3 | CCL7 | sense | GATCTCTGCCACGCTTCTGT | 33 |
| antisense | GGCTTCAGCGCAGACTTCCA | |||
| Eotaxin | CCL11 | sense | TTCTATTCCTGCTGCTCACGG | 30 |
| antisense | AGGGTGCATCTGTTGTTGGTG | |||
| MIP-2 | CXCL2 | sense | AAGGCAAGGCTAACTGAC | 30 |
| antisense | TGAACTCTCAGACAGCGA | |||
| IP10 | CXCL10 | sense | GCTGCAACTGCATCCATA | 32 |
| antisense | TCCGGATTCAGACATCTC | |||
| cyclophilin | sense | ACCTAAAGTCACAGTCAAGG | 25 | |
| antisense | TGGTGTCTTTGCCTGCATTG |
Statistical analysis
Data were expressed as mean ± SEM. Statistical calculations were performed using Statview and SuperANOVA programs (Abacus Concepts, Inc.; Berkeley, CA) on a Macintosh PowerPC G3 computer. Unpaired Student t test (for two samples) or ANOVA (for multiple comparisons) were used to evaluate continuous ratio scale data with post hoc analysis by the Tukey-Kramer test (27). Percentage data were arcsine transformed before analysis to convert them from a binomial to a normal distribution using tables in Zar (27). Results of RT-PCR experiments were analyzed by unpaired nonparametric Mann-Whitney test. Significant differences were defined as p<0.05.
RESULTS
Lung lymphocyte accumulation is altered after IT challenge of E-P- mice
Lung lymphocyte numbers did not differ significantly between wt control mice and E-P- mice before IT challenge, either in BAL (n = 6 mice per group, p = 0.08, unpaired t test) or minced lung preparations (p = 0.2) (Fig. 1). Within groups of either wt mice or E-P- mice, there was no statistical difference in total lymphocyte numbers between untreated mice and mice receiving IP priming only, and these two groups were pooled to derive day 0 numbers.
Figure 1.
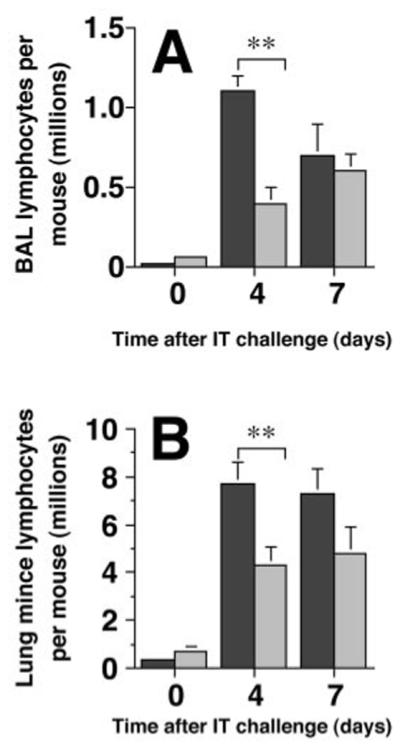
Lung lymphocyte numbers are reduced in E-P- mice after Ag challenge. Lung lymphocytes were collected by BAL (A) or from enzymatically-digested minced preparation of the perfused and lavaged lungs (B) of SRBC-primed mice four or seven days after IT SRBC challenge. Dark bars, wt C57BL/6 mice; light bars, E-P- mice. Absolute lymphocyte numbers were determined as the product of total BAL cell count (by hemocytometer) and lymphocyte percentage on differential cell count of hematoxylin and eosin stained preparations. Note differences in scales between A & B. Data represent mean ± SEM of six mice per group (days 0 and 4) or 11-12 mice per group (day 7) assayed individually in at least two experiments. **, p<0.01, unpaired Student t test.
After IT SRBC challenge, lymphocyte accumulation in the airway and lung parenchyma was significantly lower in primed E-P- mice than in primed wt control mice (Fig. 1). Total lung lymphocyte numbers differed significantly between the two groups of mice at day 4 after IT challenge both in the BAL (63% reduction relative to wt mice) and in the minced lung preparation (44% reduction). Lung lymphocyte numbers were also lower in the minced lung preparation at day 7 (34% reduction), although this difference was not statistically significant (p = 0.11). By contrast, total numbers of BAL lymphocytes were essentially identical in the two groups of mice at day 7 after challenge (p = 0.8). Accumulation of mononuclear phagocytes did not differ significantly between the two groups of mice in either BAL or minced lung preparation at any time point (data not shown).
Detailed analysis of lung lymphocyte subset distribution revealed the full extent of the abnormal lymphocyte accumulation in the selectin-deficient mice. Total leukocyte counts, leukocyte differentials (200-cell count of stained filter preparation) and lymphocyte subset differentials (flow cytometry) were performed on leukocytes recovered from the BAL, interstitial mince and peripheral blood. The “absolute counts” for CD4, CD8, CD19 and γ/† T-cell subsets were then calculated as described in Materials & Methods. CD4+ T cells, CD8+ T cells and B-cells were reduced in both the BAL and the lung interstitium of E-P- mice throughout the period of maximal lymphocyte recruitment (Fig. 2). The magnitude of the decrease was subset dependent: accumulation of the CD4+ subset was reduced by 2- to 3-fold while the CD8+ subset and B-cells fell by 8- to greater than 10-fold at the 4-day time point. The CD8+ subset and B-cells remained low in both compartments at the 7-day time point. In contrast, the CD4+ subset remained low in the interstitial compartment but returned to wt levels in the BAL.
Figure 2.
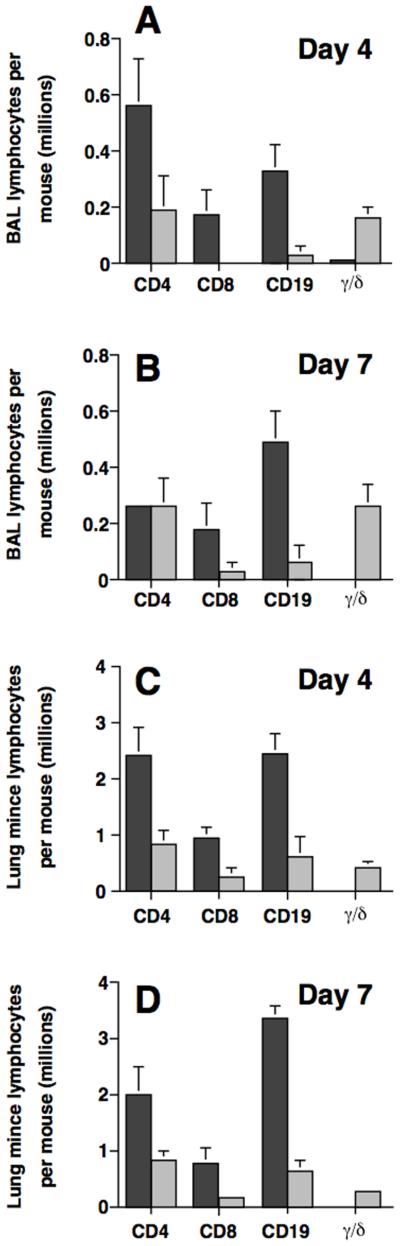
Endothelial selectin deficiency affects lung lymphocyte subsets differentially. Lymphocytes recovered by BAL (A, B) or enzymatic-digestion (C, D) of the lungs of mice following IT SRBC challenge were stained with mAbs and analyzed by flow cytometry. Dark bars, wt mice; light bars, E-P- mice. Day 4, A, C; day 7, B, D. Data represent mean ± SEM of six mice per group (days 0 and 4) or 11-12 mice per group (day 7) assayed individually in at least two experiments. **, p<0.01, unpaired Student t test.
An unexpected finding was a massive increase in the recovery of γ/† T-cells from both lung compartments in the E-P- mice after IT-SRBC challenge (Fig. 2). Indeed, γ/† T-cells accounted for ~40% of all lymphocytes recovered from the BAL of E-P- mice at both days 4 and 7. This subset constituted a much smaller percentage of the lymphocytes recovered from the interstitium of the E-P- mice but the level remained markedly higher than that obtained from wt animals. To further investigate the significance of pulmonary γ/† T-cell accumulation after challenge, we compared numbers of lymphocytes in BAL and lung mince in wt mice and E-P- mice, both in the untreated state and after IP priming only. There were no differences between the two groups of mice from either treatment state or in either lung compartment in numbers of CD4, CD8, or CD19 cells (data not shown). In comparing the untreated to the primed state within a given group of mice, slight (≤ 2-fold) differences, which were for the most part not statistically significant, were noted for each of these three lymphocyte subsets in both lung compartment. By contrast, γ/† T-cells were significantly increased in numbers in both lung compartments of untreated E-P- mice relative to wt mice (Table II). Similar disparity between the two groups of mice was seen after IP priming in BAL, but no significant difference was found between the two groups in numbers of γ/† T-cells recovered from lung minces after IP priming (Table II). Thus, E-P- mice had baseline increases in numbers of lung γ/† T-cells that further increased markedly after IT challenge (in E-P- mice, BAL γ† T-cells: 1.55 ± 0.15 × 105 on day 4 and 2.63 ± 0.32 × 105 on day 7; lung mince γ† T-cells: 4.13 ± 0.04 × 105 on day 4 and 2.86 ± 0.05 × 105).
Table II.
γδ T-cell numbers are increased in lungs of E-P- mice before IT challenge *
| BAL (cells per mouse) | Lung mince (cells per mouse) | |||
|---|---|---|---|---|
| Untreated |
IP only |
Untreated |
IP only |
|
| E-P- mice | 11,347 ± 509 † | 32,450 ± 5,344 † | 136,629 ± 37,436 † | 50,584 ± 19,241 N.S. |
| wt mice | 3,663 ± 178 | 5,662 ± 384 | 20,292 ± 4,600 | 45,659 ± 560 |
absolute numbers of γδ T-cells in BAL and lung mince were calculated as the product of the total leukocyte count, the differential lymphocyte counts (adjusted as described in Materials and Methods) and the percentage of γδ TCR + cells by flow cytometry. Mean ± SEM, n = 3 mice per group assayed individually.
significantly different, p<0.05, unpaired t-test compared to wt mice of same treatment; N.S., not significantly different.
Endothelial selectin deficiency does not impair lung granulocyte accumulation
Before IT SRBC challenge, granulocyte numbers in E-P- mice were elevated 3-fold (BAL) and over 7-fold (in LM) relative to wt control mice (Fig. 3). As with lymphocyte numbers, within groups of either wt mice or E-P- mice, there was no statistical difference in granulocyte numbers between untreated mice and mice receiving IP priming only, and these two groups were pooled to derive day 0 numbers.
Figure 3.
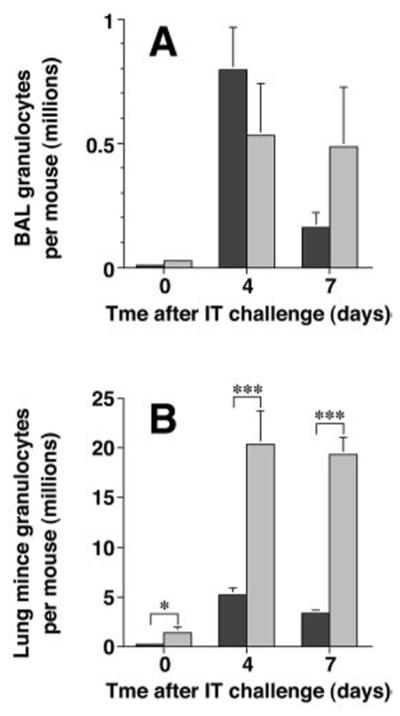
Endothelial selectin deficiency does not impair lung granulocyte accumulation. Granulocytes recovered by BAL (A) or enzymatic-digestion (B) of the lungs of mice following after IP-priming only (day 0) or after IT SRBC challenge were enumerated as described in the legend to Figure 1. Note the marked difference in scales between the two panels. Dark bars, wt mice; light bars, E-P- mice. Data represent mean ± SEM of six mice per group (days 0 and 4) or 11-12 mice per group (day 7) assayed individually in at least two experiments. *, p<0.05, ***, p<0.001, unpaired Student t test.
After IT SRBC challenge, granulocyte numbers in the BAL was similar in E-P- mice and wt mice (p = 0.23) (Fig. 3A). However, the number of granulocytes in the minced lung preparations of the E-P- mice was markedly greater than in wt mice at both the 4-day (p <0.001) and 7-day time points (p <0.001) (Fig. 3B). As in most previous studies in this model system, we did not separate neutrophils and eosinophils in this enumeration, as distinguishing the two types of granulocytes in the mouse is problematic. Caution should be exerted in interpreting these data. Although the pulmonary vasculature of both groups of mice was perfused in an identical fashion, E-P- mice have previously been reported to have a marked peripheral blood leukocytosis (28). Thus, we cannot exclude the possibility that granulocyte numbers recovered by mincing lung tissue of E-P- mice were spuriously increased by incomplete removal of cells trapped in pulmonary capillaries. Supporting this possibility, histologic analysis confirmed identical overall anatomic distribution and magnitude of interstitial inflammation in the two groups of mice (data not shown), with prominent perivenous and peribronchoarterial accumulation of mononuclear cells and granulocytes (9). Nevertheless, these data clearly indicate that lung granulocyte accumulation after Ag challenge is not impaired by absence of endothelial selectins.
Peripheral blood analysis shows a leukocytosis in E-P- mice
To assess the delivery of circulating lymphocytes to the lung, peripheral blood lymphocyte counts in wt mice and E-P- mice were compared. In the absence of IP priming and of IT challenge, the numbers of CD4+, CD8+, CD19+ cells were significantly higher in E-P- mice than in wt mice (Fig. 4A), and this difference increased following SRBC sensitization and IT challenge. At the 7-day time-point, the absolute counts for these subsets were 2-4 fold higher in E-P- mice than in wt mice (Fig. 4B). In wt mice, γ/† T-cells were below the level of detection in both untreated wt mice and SRBC-challenged wt mice. In contrast, γ/† T-cells constituted ~ 7% and ~5% of the circulating pool in untreated and IT-SRBC challenged E-P- mice, respectively. Thus, the decreased accumulation of CD4, CD8, and CD19 subsets in the E-P- lungs did not result from reduced numbers of circulating cells. On the other hand, the increased accumulation of γ/† T cells reflected, in part, a systemic expansion of this subset in the E-P- mice.
Figure 4.
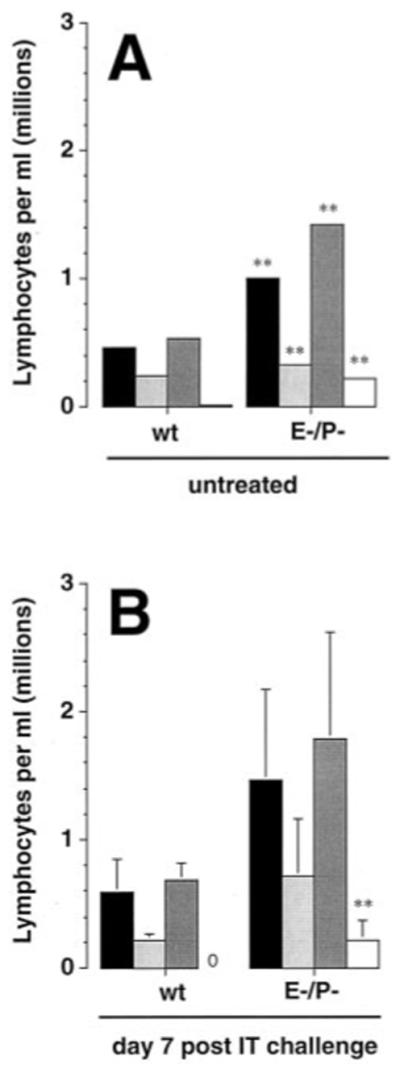
Peripheral blood analysis indicates a lymphocytosis in E-P- mice. Peripheral blood lymphocytes were analyzed by flow cytometry. Black bars, CD4 cells; light gray bars, CD8 cells; dark gray bars, CD19 cells; white bars, γ† cells. Data represent mean ± SEM of six mice per group (untreated) or 11-12 mice per group (day 7) assayed individually in at least two separate experiments. **, p<0.01, unpaired Student t test.
Recruitment of wild-type T-lymphoblasts into the lungs of E-P- mice is reduced
Steady-state lymphocyte counts in the lungs during a pulmonary immune response reflect the dynamic balance between lymphocyte recruitment and in situ proliferation on one hand, versus lymphocyte emigration and apoptosis on the other hand. To assess recruitment directly, we next conducted short-term trafficking assays using wt cultured T-lymphoblasts. T-lymphoblasts were prepared from splenic mononuclear cells under conditions that induced high levels of selectin-ligand synthesis (16). The T-lymphoblasts were labeled with the fluorophore CMFDA and administered iv to syngeneic wt mice and E-P- mice as previously described (16) on either day 3 or day 6 after IT SRBC. The overall level of trafficking into the BAL of wt mice was approximately 5-fold greater during the day 3-4 period than during the day 6-7 period, in agreement with our previous findings (16).However, at both time points, the number of fluorescent cells recovered from the E-P- mice was at least 10-fold lower than the number recovered from wt mice (Fig. 5). Consequently, recruitment of wild-type T-lymphoblasts is significantly reduced in the absence of endothelial selectins.
Figure 5.
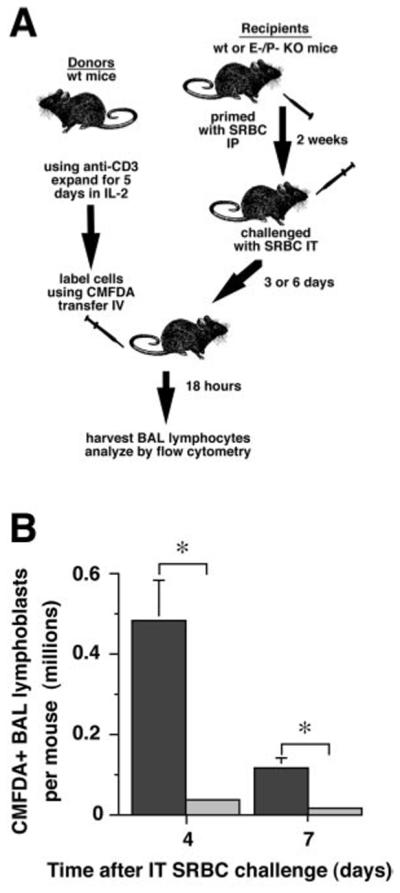
Recruitment of T lymphoblasts into lungs of E-P- mice is reduced. A. Experimental protocol. Lymph node T cells from normal wt mice were activated using immobilized anti-CD3 mAb and their numbers expanded in IL-2 to produce T lymphoblasts, as previously described (16). After 5 days of expansion, T lymphoblasts were labeled with CMFDA and transferred by tail vein injection into primed recipients consisting of either wt or E-P- mice which had been IT Ag challenged three or six days earlier. BAL lymphocytes were analyzed 18 hours later by flow cytometry. B. Results. Dark bars, wt recipients; light bars, E-P- recipients. Data are mean ± SD of individual recipient mice (wt, 3 mice, E-P-, 2 mice); *, p<0.05, unpaired t test.
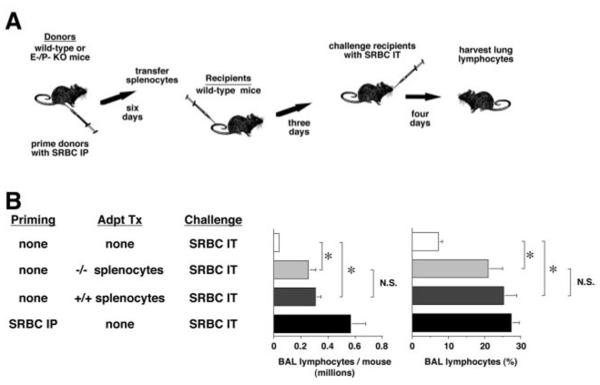
Adoptive transfer shows T cells of E-P- mice can be primed
Lung lymphocyte recruitment in the IT-SRBC model system is Ag-driven and requires that the same Ag be administered for both priming and IT challenge (24). Therefore, reduced or absent priming to SRBC Ags in the E-P- mice could also affect lymphocyte accumulation after IT challenge. To test this possibility, lymphocytes harvested from the spleens of primed mice (wt and E-P-) were adoptively transferred into two groups of unprimed wt mice 3-days before IT challenge with SRBCs. Subsequent lymphocyte recovery from the lungs of these two groups of mice four days after IT challenge was virtually identical, and both differed significantly from the modest levels observed in control mice receiving IT SRBC challenge without previous Ag priming (Fig. 6). Thus, wt and E-P- mice showed equal levels of priming after IP challenge with SRBC.
Figure 6.
T cells of E-P- mice can be Ag-primed by IP injection. A. Experimental protocol. Note that in this experiment, the transferred cell population was not CMFDA-labeled, and the experimental condition assayed was total Ag-driven lung lymphocyte accumulation. B. Results. Data are mean ± SEM of 3-5 mice per group analyzed individually. *, p<0.01, ANOVA, with post hoc analysis by the Tukey-Kramer test.
Chemokine elaboration is intact in E-P- mice after Ag challenge
Lymphocyte recruitment is dependent on both endothelial CAMs and chemokines. Therefore, CC and CXC chemokine production was analyzed using semi-quantitative RT-PCR on whole lung RNA extracts. The steady-state mRNA levels for the CC chemokines MCP-1 (CCL2 in the recently proposed nomenclature (29)), MIP-1α (CCL3), MIP-1β (CCL4), RANTES (CCL5), MCP-3 (CCL7), and eotaxin (CCL11) after IT-SRBC challenge were equivalent in the two groups of mice (Fig. 7). Additionally, lung mRNA production of the CXC chemokines MIP-2α (CXCL2) and IP10 (CXCL10) was equivalent except for a modest decrease in IP10 mRNA in the E-P- mice at the 7-day time point. Consequently, both groups developed virtually identical, robust and broad-spectrum chemokine responses to IP-SRBC challenge.
Figure 7.
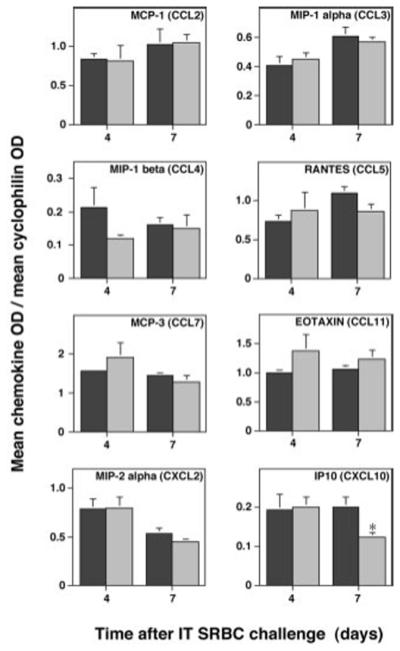
Whole lung CC chemokine production by E-P- mice is intact. Total lung RNA was extracted from SRBC-primed wt C57BL/6 mice (dark bars) or E-P- mice (light bars) at 4 days and 7 days after IT challenge. RNA from individual mice was reverse-transcribed and aliquots of cDNAs were amplified by PCR. Data are the ratio of the OD of the individual chemokine to the OD of cyclophilin as mean ± SEM of 3-5 mice. Note the difference in scale between individual cytokines. *, p<0.05, unpaired nonparametric Mann-Whitney test comparing groups at the same time point.
DISCUSSION
The principal finding of this study is that selectin-mediated lymphocyte accumulation in the lungs following IT SRBC-challenge in primed animals is subset dependent. In the E-P- mice, the accumulation of CD8+ T cells and B cells was reduced to a significantly greater degree than the accumulation of CD4+ T cells (>85-95% vs. <60%). In contrast, the recovery of γ/† T cells from the E-P- lungs was markedly (>> 10 fold) increased relative to wt controls reflecting, in part, a previously unrecognized systemic expansion of this subset in the selectin-deficient animals. The reduced T-cell trafficking from the circulation into IT-challenged E-P- lungs was confirmed using a polyclonal population of cultured T-lymphoblasts. Direct trafficking studies with γ/† T cells are problematic in the mouse; however, our data indicate that both constitutive and inflammation-induced trafficking of this subset into the lung is not invariably selectin-dependent in the mouse. The attenuated lymphocyte accumulation in the E-P- mice did not result from decreased numbers of circulating lymphocytes of any of the subsets, inadequate antigen priming, or a global deficiency in chemokine production.
These findings extend previous work from our group showing that IT-SRBC challenge of sensitized mice induced P- and E-selectin expression on the pulmonary vasculature, increased the number of circulating T-cells expressing selectin-ligands, and recruited selectin-ligand positive circulating T-cells into the lung (16, 19, 21). The current results are in agreement with our previous demonstration that lung lymphocyte recruitment is partially dependent on binding to endothelial cell selectins (16). That conclusion was based on analysis of in vitro activated T-lymphoblasts derived from gene-targeted mice lacking fucosyl-transferase VII (FucT VII −/−), the rate-limiting enzyme in the biosynthetic pathway necessary to produce functional leukocyte selectin ligands (30). Absence of ligands for L-selectin in the FucT VII −/− mice leads to defects in lymphocyte entry into lymph nodes and priming (30). Therefore, it is not feasible to compare the two genetic defects more directly by analyzing lung accumulation of individual lymphocyte subsets in response to IT-SRBC in sensitized FucT VII −/− mice themselves. The current results are also consistent with those of Pan and associates, who found that ganglioside analogues of the endothelial selectin ligand sialyl-Lewis × {SLe(x)} inhibited lung inflammation in a murine model of hypersensitivity pneumonitis in response to S. rectivirgula (31). Selectin-mediated Th1 lymphocyte recruitment has been documented in cutaneous and peritoneal immunologic lesions as well (32-34). However, selectins were not required for lymphocyte recruitment during immunologic reactions in the colon (35), liver (36) and brain (37). Indeed, Bartholdy and colleagues found that meningeal accumulation of CD8 + T-cells and CD8-mediated viral clearance from multiple organs was identical in E-P- and wt mice lethally infected with LCMV (38).
These seemingly disparate results may reflect the partial redundancy of adhesion receptors involved in lymphocyte recruitment and changes in receptor expression/utilization that occur as immunologic reactions progress. On T-cells, at least six receptors or receptor families mediate tethering interactions and at least two additional families can support the arrest/transmigration of tethered cells (39). Furthermore, several studies document changes in adhesion receptor expression and utilization during pulmonary immune responses. In SRBC-primed mice, IT-SRBC challenge was followed by transient expression of E-selectin and prolonged expression of P-selectin and VCAM on the microvasculature of the lung (19). In ovalbumin (OVA) sensitized mice, IT-OVA induced peribronchial inflammation was P-selectin dependent during the acute phase and CD49d-dependent during the late-phase response (40-42). In the current study, time-dependent changes were observed for the CD4 subset but not for the CD8 subset. In light of these complexities, it is not surprising that immune reactions employ a wide variety of adhesion receptors and that receptor utilization is not uniform across organs, disease processes or lymphocyte subsets.
This experimental model system examines the pulmonary response to the classic particulate Ag, SRBC (21). This system is relevant to human lung disease because many naturally-occurring inhaled or aspirated substances are complex particulates, the response to which may be poorly simulated by results from experimental systems using soluble antigens of lower complexity. SRBC do not proliferate or directly cause tissue damage, but do present a variety of glycopeptide and glycolipid moieties (notably the Forsmann antigen) in a three-dimensional context that may stimulate recognition by the innate immune system (43). The response to intratracheal (IT) challenge is dose-dependent and Ag-specific (24, 44). IT challenge induces intense inflammatory cell accumulation predominately in the bronchovascular bundles and around veins (9) that most closely resembles a type IV Gell and Coombs response. Although priming does not involve adjuvants, initial IT challenge induces a predominately type 2 cytokine response with prominent lung eosinophilia (17, 26). Repeated IT challenge evolves to a distinctly type 1 and waning pulmonary response (17), thus appearing to duplicate the spontaneous tolerance seen in most animal models of repeated IT Ag challenge (45-48). The SRBC model system has been used by a number of laboratories to analyze the anatomic and molecular mechanisms of lymphocyte recruitment to lung parenchyma (19, 20), the cytokine requirements for airways hyperresponsiveness (49), and the role of neuropeptides in development of lung inflammation (15).
In the SRBC model, lung lymphocyte accumulation is dependent on continuous recruitment from the periphery. This conclusion is based on the high rate of lymphocyte elimination by in situ apoptosis (50), the very low rate of in situ proliferation (26), and direct evidence that circulating T-lymphoblasts are trafficking into the lung throughout the immune response (16). Furthermore, previous experiments in this model indicated that systemic depletion of CD4+ T cells prior to IT-challenge with SRBC reduced the peribronchial accumulation of all leukocytes except CD8+ T cells (13). Conversely, CD8-depletion prior to IT-SRBC challenge did not alter the accumulation of either the CD4 subset or B-cells (25). Thus, the early recruitment of the CD4 and CD8 subsets in the SRBC model are mutually independent. These findings, coupled with the persistence of multiple T-cell directed chemokines in the lungs of E-P- animals, support the hypothesis that recruitment of CD8 and, to a lesser extent, the CD4 subset of T-cells is initially selectin-dependent following IT-SRBC challenge in primed mice.
B-cell accumulation in the lungs, on the other hand, is dependent on CD4+ T cells in the SRBC model (13). This dependence is distinct from the T-cell help required for antigen priming. Therefore, the profound reduction in B cell accumulation in selectin-deficient mice may reflect either direct involvement of selectins in B-cell recruitment or indirect changes in the lung microenvironment resulting from the altered trafficking of CD4+ T-cells. Ligands for both E- and P-selectin are synthesized by human B-cells and mediate tethering interactions in vitro (51-53); therefore, selectin mediated B-cell recruitment in vivo is a theoretical possibility. Alternatively, diminished accumulation of a critical T-cell subset might delay expression of endothelial adhesion receptors or chemokines essential for subsequent B-cell recruitment. For example, Gonzalo and colleagues proposed that CD4+ T-cells recruited early following IT-OVA administration in sensitized mice induced subsequent VCAM mediated eosinophil recruitment (40). B-cells express α4-integrins and interact with VCAM in vitro (54, 55). The α4-integrins clearly mediate memory B-cell trafficking in vivo, as shown for α4/β7:MadCAM-dependent localization of IgA-specific B-cells in the gastrointestinal tract (56, 57). Thus, the current results are compatible with either a direct or an indirect role for selectins in B-cell recruitment.
The existence of large number of γ/† T cells in the peripheral blood and alveoli of E-P- mice has not previously been reported. The generalized increased in myelopoiesis observed in selectin-deficient animals has been attributed, in part, to increased production of key growth factors including IL-3 and GM-CSF (23). This dysregulation may account for the expansion of γ/† T cells as well; however, a compensatory increase due to deficiencies in either innate or acquired immunity cannot be ruled out. Although uncommon in rodents and humans, γ/† T cells comprise the major circulating lymphocyte in newborn ruminants (58). Interestingly, Jutila and associates have shown that P- and E-selectin support shear-dependent rolling of bovine γ/† T cells in vitro (59) and mediate recruitment into some immune reactions (58, 60). Nevertheless, the current findings indicate that γ/† T cells can enter the lungs without the endothelial selectins in the mouse, both in the unchallenged state and during lung inflammation.
The finding of abundant γ† T cells in E-P- mice is noteworthy because this cell type may contribute to host responses as both an effector and regulatory cell (61). γ/† T cells react directly with unprocessed Ag (62), and mediate effector functions including cytokine production, cytotoxicity, and presentation of Ag to αβ T cells (63-65). Pulmonary γ/† T cells comprise a heterogenous population, which are found both in normal mice and in a variety of infectious models (66-68). Unlike the case in the skin, where γ/† T cells lack TCR diversity, pulmonary γ/† T cells show considerable clonal diversity (66, 69). γ/† T cells may also play important immunoregulatory roles in autoimmunity and allergic inflammation (70). In both murine and rat model systems, small numbers of γ/† cells from Ag-exposed donors transferred Ag-specific tolerance to naive recipients via immune deviation (71, 72). In addition, γ/† T cells augmented IL-4 dependent, type 2-mediated airway inflammation to peptide antigens in some murine models (73). Therefore, the elevated numbers of γ/† T cells in E-P- mice and their capacity for recruitment to the lungs in the absence of endothelial selectins must be considered when using these mice for in vivo experimental model systems.
The current study found that granulocyte recovery from the lungs of E-P- mice was either unaltered (BAL) or significantly increased (minced tissues) compared to wt animals. This finding is consistent with previous reports documenting selectin-independent pathways for neutrophil recruitment into the lungs (3-6, 74). However, one cannot completely rule out a role for selectins in neutrophil recruitment. As previously reported (28), neutrophil counts were constitutively elevated in the E-P- mice. After IT-SRBC challenge, the absolute number of circulating neutrophils reached levels as high as 10-50 fold above the measurements in wt control animals (data not shown). Since neutrophil delivery to the lungs is markedly increased in E-P- mice, the relatively low numbers of neutrophils recovered from the BAL in particular may indicate that the “extraction efficiency” is actually decreased in absence of endothelial selectins.
In summary, the absence of endothelial selectins significantly reduces lymphocyte accumulation in the lungs following IT antigen challenge of sensitized mice. The impact is greatest on CD8+ cells and B-cells, less marked on CD4+ cells and without apparent effect on γ/† T-cells. Thus, agents designed to block the endothelial selectins may both diminish and skew pulmonary immune responses.
ACKNOWLEDGMENTS
We thank all members of the Ann Arbor VA REAP for helpful suggestions; Joyce O’Brien for secretarial support; and Dr. Antonello Punturieri for critiquing the manuscript.
Footnotes
2. Portions of these data were presented at the Midwest Autumn Immunology Meeting (Chicago, IL; November 20, 1999) and at the annual meeting of the American Association of Immunologists (Seattle, WA; May 15, 2000).
3. Supported by RO-1 HL56309 and RO-1 HL6157 (JLC), RO-1 CA73059 and P01 AI33189 (LMS) from the USPHS; and by Merit Review funding and a Research Enhancement Award Program (REAP) grant from the Department of Veterans Affairs. The Curtis and Stoolman laboratories contributed equally to this study.
- BAL
- bronchoalveolar lavage
- CAMs
- cell adhesion molecules
- DMEM+
- DMEM containing 0.1% azide and 0.1% BSA
- E-P- mice
- gene-targeted mice lacking both E-selectin and P-selectin
- FucT VII −/− mice
- gene-targeted mice lacking fucosyltransferase VII
- IT
- intratracheal
- IT-SRBC
- intratracheal SRBC
- OVA
- ovalbumin
- Pnad
- peripheral node addressins
- SLe(x)
- sialyl-Lewis X
References
- 1.Lowe JB, Ward PA. Therapeutic inhibition of carbohydrate-protein interactions in vivo. J Clin Invest. 1997;99:822. doi: 10.1172/JCI119244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Deffebach ME, Charan NB, Lakshminarayan S, Butler J. The bronchial circulation. Small, but a vital attribute of the lung. Am Rev Respir Dis. 1987;135:463. doi: 10.1164/arrd.1987.135.2.463. [DOI] [PubMed] [Google Scholar]
- 3.Worthen GS, Schwab B, III, Elson EL, Downey GP. Mechanics of stimulated neutrophils: cell stiffening induces retention in capillaries. Science. 1989;245:183. doi: 10.1126/science.2749255. [DOI] [PubMed] [Google Scholar]
- 4.Doherty DE, Downey GP, Schwab B, 3rd, Elson E, Worthen GS. Lipolysaccharide-induced monocyte retention in the lung. Role of monocyte stiffness, actin assembly, and CD18-dependent adherence. J Immunol. 1994;153:241. [PubMed] [Google Scholar]
- 5.Erzurum SC, Downey GP, Doherty DE, Schwab B, 3rd, Elson EL, Worthen GS. Mechanisms of lipopolysaccharide-induced neutrophil retention. Relative contributions of adhesive and cellular mechanical properties. J Immunol. 1992;149:154. [PubMed] [Google Scholar]
- 6.Doerschuk CM. Mechanisms of leukocyte sequestration in inflamed lungs. Microcirculation. 2001;8:71. [PubMed] [Google Scholar]
- 7.Sheikh S, Gratzer WB, Pinder JC, Nash GB. Actin polymerisation regulates integrin-mediated adhesion as well as rigidity of neutrophils. Biochem Biophys Res Commun. 1997;238:910. doi: 10.1006/bbrc.1997.7407. [DOI] [PubMed] [Google Scholar]
- 8.Burns JA, Issekutz TB, Yagita H, Issekutz AC. The beta2, alpha4, alpha5 integrins and selectins mediate chemotactic factor and endotoxin-enhanced neutrophil sequestration in the lung. Am J Pathol. 2001;158:1809. doi: 10.1016/s0002-9440(10)64137-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Curtis JL, Warnock ML, Arraj SM, Kaltreider HB. Histologic analysis of an immune response in the lung parenchyma of mice: angiopathy accompanies inflammatory cell influx. Am J Pathol. 1990;137:689. [PMC free article] [PubMed] [Google Scholar]
- 10.Ichikawa S, Shiozawa Y, Uchino S. Immune cell migration through the arterial wall in the murine lung during a pulmonary inflammatory response. Arch Histol Cytol. 1996;59:87. doi: 10.1679/aohc.59.87. [DOI] [PubMed] [Google Scholar]
- 11.Widdicombe J. The tracheobronchial vasculature: a possible role in asthma. Microcirculation. 1996;3:129. doi: 10.3109/10739689609148283. [DOI] [PubMed] [Google Scholar]
- 12.Persson CG, Erjefalt JS, Andersson M, Erjefalt I, Greiff L, Korsgren M, Linden M, Sundler F, Svensson C. Epithelium, microcirculation, and eosinophils--new aspects of the allergic airway in vivo. Allergy. 1997;52:241. doi: 10.1111/j.1398-9995.1997.tb00989.x. [DOI] [PubMed] [Google Scholar]
- 13.Curtis JL, Byrd PK, Warnock ML, Kaltreider HB. Requirement of CD4+ T cells for cellular recruitment to the lungs of mice in response to intratracheal antigen. J Clin Invest. 1991;88:1244. doi: 10.1172/JCI115428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Curtis JL, Kim S, Scott PJ, Buechner-Maxwell VA. Adhesion receptor phenotypes of murine lung CD4+ T cells during a pulmonary immune response to sheep erythrocytes. Am J Respir Cell Mol Biol. 1995;12:520. doi: 10.1165/ajrcmb.12.5.7537969. [DOI] [PubMed] [Google Scholar]
- 15.Kaltreider HB, Ichikawa S, Byrd PK, Ingram DA, Kishiyama JL, Sreedharan SP, Warnock ML, Beck JM, Goetzl EJ. Upregulation of neuropeptides and neuropeptide receptors in a murine model of immune inflammation in lung parenchyma. Am J Respir Cell Mol Biol. 1997;16:133. doi: 10.1165/ajrcmb.16.2.9032120. [DOI] [PubMed] [Google Scholar]
- 16.Wolber FM, Curtis JL, Lowe J, Mály P, Smith P, Yednock TA, Kelly RJ, Stoolman LM. Endothelial selectins and α4 integrin regulate independent pathways of T lymphocyte recruitment in pulmonary inflammation. J Immunol. 1998;161:4396. [PubMed] [Google Scholar]
- 17.Todt J, Sonstein J, Polak T, Seitzman GD, Hu B, Curtis JL. Repeated intratracheal challenge with particulate antigen modulates murine lung cytokines. J Immunol. 2000;164:4037. doi: 10.4049/jimmunol.164.8.4037. [DOI] [PubMed] [Google Scholar]
- 18.Gyetko MR, Sud S, Sonstein J, Polak T, Sud A, Curtis JL. Cutting Edge: Antigen-driven lymphocyte recruitment to the lung is diminished in the absence of urokinase-type plasminogen activator (uPA) receptor, but Is independent of uPA. J Immunol. 2001;167:5539. doi: 10.4049/jimmunol.167.10.5539. [DOI] [PubMed] [Google Scholar]
- 19.Wolber FM, Curtis JL, Milik AM, Seitzman GD, Fields KL, Kim K-M, Kim S, Sonstein J, Stoolman LM. Lymphocyte recruitment and the kinetics of adhesion receptor expression during the pulmonary immune response to particulate antigen. Am J Pathol. 1997;151:1715. [PMC free article] [PubMed] [Google Scholar]
- 20.Ichikawa S, Goto Y, Uchino S, Kaltreider HB, Goetzl EJ, Sreedharan SP. Changes in adhesion molecule expression during distinct patterns of immune cell migration in the inflamed lung. Arch Histol Cytol. 1996;59:443. doi: 10.1679/aohc.59.443. [DOI] [PubMed] [Google Scholar]
- 21.Curtis JL, Wolber FM, Sonstein J, Craig RA, Polak T, Knibbs RN, Todt J, Seitzman GD, Stoolman LM. Lymphocyte-endothelial cell adhesive interactions in lung immunity: lessons from the murine response to particulate antigen. Immunopharmacology. 2000;48:223. doi: 10.1016/s0162-3109(00)00221-6. [DOI] [PubMed] [Google Scholar]
- 22.Bullard DC, Kunkel EJ, Kubo H, Hicks MJ, Lorenzo I, Doyle NA, Doerschuk CM, Ley K, Beaudet AL. Infectious susceptibility and severe deficiency of leukocyte rolling and recruitment in E-selectin and P-selectin double mutant mice. J Exp Med. 1996;183:2329. doi: 10.1084/jem.183.5.2329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Frenette PS, Mayadas TN, Rayburn H, Hynes RO, Wagner DD. Susceptibility to infection and altered hematopoiesis in mice deficient in both P- and E-selectins. Cell. 1996;84:563. doi: 10.1016/s0092-8674(00)81032-6. [DOI] [PubMed] [Google Scholar]
- 24.Curtis JL, Kaltreider HB. Characterization of bronchoalveolar lymphocytes during a specific antibody-forming cell response in the lungs of mice. Am Rev Respir Dis. 1989;139:393. doi: 10.1164/ajrccm/139.2.393. [DOI] [PubMed] [Google Scholar]
- 25.Curtis JL, Byrd PK, Warnock ML, Beck JM, Kaltreider HB. Pulmonary lymphocyte recruitment: Depletion of CD8+ T cells does not impair the pulmonary immune response to intratracheal antigen. Am J Respir Cell Mol Biol. 1993;9:90. doi: 10.1165/ajrcmb/9.1.90. [DOI] [PubMed] [Google Scholar]
- 26.Seitzman GD, Sonstein J, Choy W, Kim S, Curtis JL. Lung lymphocytes proliferate minimally in the murine pulmonary immune response to intratracheal sheep erythrocytes. Am J Respir Cell Mol Biol. 1998;18:800. doi: 10.1165/ajrcmb.18.6.3063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zar JH. Biostatistical analysis. Prentice-Hall, Englewood Cliffs. 1974 [Google Scholar]
- 28.Robinson SD, Frenette PS, Rayburn H, Cummiskey M, Ullman-Culler M, Wagner DD, Hynes RO. Multiple, targeted deficiencies in selectins reveal a predominant role for P-selectin in leukocyte recruitment. Proc Natl Acad Sci U S A. 1999;96:11452. doi: 10.1073/pnas.96.20.11452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zlotnik A, Yoshie O. Chemokines: a new classification system and their role in immunity. Immunity. 2000;12:121. doi: 10.1016/s1074-7613(00)80165-x. [DOI] [PubMed] [Google Scholar]
- 30.Mály P, Thall A, Petryniak B, Rogers CE, Smith PL, Marks RM, Kelly RJ, Gersten KM, Cheng G, Saunders TL, Camper SA, Camphausen RT, Sullivan FX, Isogai Y, Hindsgaul O, von Andrian UH, Lowe JB. The α(1,3)fucosyltransferase Fuc-TVII controls leukocyte trafficking through an essential role in L-, E-, and P-selectin ligand biosynthesis. Cell. 1996;86:643. doi: 10.1016/s0092-8674(00)80137-3. [DOI] [PubMed] [Google Scholar]
- 31.Pan LH, Yamauchi K, Sawai T, Nakadate T, Kojima Y, Takahashi N, Adachi K, Kameyama A, Inoue H. Inhibition of binding of E- and P-selectin to sialyl-Lewis × molecule suppresses the inflammatory response in hypersensitivity pneumonitis in mice. Am J Respir Crit Care Med. 2000;161:1689. doi: 10.1164/ajrccm.161.5.9812016. [DOI] [PubMed] [Google Scholar]
- 32.Austrup F, Vestweber D, Borges E, Löhning M, Braüer R, Herz U, Renz H, Hallman R, Scheffold A, Radbruch A, Hamann A. P- and E-selectin mediate recruitment of T-helper-1 but not T-helper-2 cells into inflammed tissues. Nature. 1997;385:81. doi: 10.1038/385081a0. [DOI] [PubMed] [Google Scholar]
- 33.Borges E, Tietz W, Steegmaier M, Moll T, Hallmann R, Hamann A, Vestweber D. P-selectin glycoprotein ligand-1 (PSGL-1) on T helper 1 but not on T helper 2 cells binds to P-selectin and supports migration into inflamed skin. J Exp Med. 1997;185:573. doi: 10.1084/jem.185.3.573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tietz W, Allemand Y, Borges E, von Laer D, Hallmann R, Vestweber D, Hamann A. CD4+ T cells migrate into inflamed skin only if they express ligands for E- and P-selectin. J Immunol. 1998;161:963. [PubMed] [Google Scholar]
- 35.Chu A, Hong K, Berg EL, Ehrhardt RO. Tissue specificity of E- and P-selectin ligands in Th1-mediated chronic inflammation. J Immunol. 1999;163:5086. [PubMed] [Google Scholar]
- 36.Wong J, Johnston B, Lee SS, Bullard DC, Smith CW, Beaudet AL, Kubes P. A minimal role for selectins in the recruitment of leukocytes into the inflamed liver microvasculature. J Clin Invest. 1997;99:2782. doi: 10.1172/JCI119468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Engelhardt B, Vestweber D, Hallmann R, Schulz M. E- and P-selectin are not involved in the recruitment of inflammatory cells across the blood-brain barrier in experimental autoimmune encephalomyelitis. Blood. 1997;90:4459. [PubMed] [Google Scholar]
- 38.Bartholdy C, Marker O, Thomsen AR. Migration of activated CD8(+) T lymphocytes to sites of viral infection does not require endothelial selectins. Blood. 2000;95:1362. [PubMed] [Google Scholar]
- 39.von Andrian UH, Mackay CR. T-cell function and migration. Two sides of the same coin. N Engl J Med. 2000;343:1020. doi: 10.1056/NEJM200010053431407. [DOI] [PubMed] [Google Scholar]
- 40.Gonzalo JA, Lloyd CM, Kremer L, Finger E, Martinez AC, Siegelman MH, Cybulsky M, Gutierrez-Ramos JC. Eosinophil recruitment to the lung in a murine model of allergic inflammation. The role of T cells, chemokines, and adhesion receptors. J Clin Invest. 1996;98:2332. doi: 10.1172/JCI119045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Broide DH, Sullivan S, Gifford T, Sriramarao P. Inhibition of pulmonary eosinophilia in P-selectin- and ICAM-1-deficient mice. Am J Respir Cell Mol Biol. 1998;18:218. doi: 10.1165/ajrcmb.18.2.2829. [DOI] [PubMed] [Google Scholar]
- 42.Henderson WR, Jr., Chi EY, Albert RK, Chu SJ, Lamm WJ, Rochon Y, Jonas M, Christie PE, Harlan JM. Blockade of CD49d (alpha4 integrin) on intrapulmonary but not circulating leukocytes inhibits airway inflammation and hyperresponsiveness in a mouse model of asthma. J Clin Invest. 1997;100:3083. doi: 10.1172/JCI119863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bice DE, Shopp GM. Antibody responses after lung immunization. Exp Lung Res. 1988;14:133. doi: 10.3109/01902148809115121. [DOI] [PubMed] [Google Scholar]
- 44.Kaltreider HB, Curtis JL, Arraj SM. The mechanism of appearance of specific antibody-forming cells in lungs of inbred mice after intratracheal immunization with sheep erythrocytes. II. Dose-dependence & kinetics. Am Rev Respir Dis. 1987;135:87. doi: 10.1164/arrd.1987.135.1.87. [DOI] [PubMed] [Google Scholar]
- 45.Richerson HB, Seidenfeld JJ, Ratjczak HV, Richards DW. Chronic experimental interstitial pneumonitis in the rabbit. Am Rev Respir Dis. 1978;117:5. doi: 10.1164/arrd.1978.117.1.5. [DOI] [PubMed] [Google Scholar]
- 46.Ratajczak HV, Richards DW, Richerson HB. Systemic and local lymphocyte responses in experimental hypersensitivity pneumonitis. Am Rev Respir Dis. 1980;122:761. doi: 10.1164/arrd.1980.122.5.761. [DOI] [PubMed] [Google Scholar]
- 47.Schuyler MR, Kleinerman J, Pensky JR, Brandt C, Schmitt D. Pulmonary response to repeated exposure to Micropolyspora faeni. Am Rev Respir Dis. 1983;128:1071. doi: 10.1164/arrd.1983.128.6.1071. [DOI] [PubMed] [Google Scholar]
- 48.Yiamouyiannis CA, Schramm CM, Puddington L, Stengel P, Baradaran-Hosseini E, Wolyniec WW, Whiteley HE, Thrall RS. Shifts in lung lymphocyte profiles correlate with the sequential development of acute allergic and chronic tolerant stages in a murine asthma model. Am J Pathol. 1999;154:1911. doi: 10.1016/S0002-9440(10)65449-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gavett SH, O'Hearn DJ, Li X, Huang SK, Finkelman FD, Wills-Karp M. Interleukin 12 inhibits antigen-induced airway hyperresponsiveness, inflammation, and Th2 cytokine expression in mice. J Exp Med. 1995;182:1527. doi: 10.1084/jem.182.5.1527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Milik AM, Beuchner-Maxwell VA, Kim S, Sonstein J, Seitzman GD, Beals TF, Curtis JL. Lung lymphocyte elimination by apoptosis in the murine response to intratracheal particulate antigen. J Clin Invest. 1997;99:1082. doi: 10.1172/JCI119236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Postigo AA, Marazuela M, Sanchez-Madrid F, de Landazuri MO. B lymphocyte binding to E- and P-selectins is mediated through the de novo expression of carbohydrates on in vitro and in vivo activated human B cells. J Clin Invest. 1994;94:1585. doi: 10.1172/JCI117500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wagers AJ, Lowe JB, Kansas GS. An important role for the alpha 1,3 fucosyltransferase, FucT-VII, in leukocyte adhesion to E-selectin. Blood. 1996;88:2125. [PubMed] [Google Scholar]
- 53.Montoya MC, Holtmann K, Snapp KR, Borges E, Sanchez-Madrid F, Luscinskas FW, Kansas G, Vestweber D, de Landazuri MO. Memory B lymphocytes from secondary lymphoid organs interact with E-selectin through a novel glycoprotein ligand. J Clin Invest. 1999;103:1317. doi: 10.1172/JCI4705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Postigo AA, Sanchez-Mateos P, Lazarovits AI, Sanchez-Madrid F, de Landazuri MO. Alpha 4 beta 7 integrin mediates B cell binding to fibronectin and vascular cell adhesion molecule-1. Expression and function of alpha 4 integrins on human B lymphocytes. J Immunol. 1993;151:2471. [PubMed] [Google Scholar]
- 55.Yago T, Tsukuda M, Tajima H, Nishi T, Kurata-Miura K, Ohkubo J, Minami M. Analysis of initial attachment of B cells to endothelial cells under flow conditions. J Immunol. 1997;158:707. [PubMed] [Google Scholar]
- 56.Quiding-Jabrink M, Nordstrom I, Granstrom G, Kilander A, Jertborn M, Butcher EC, Lazarovits AI, Holmgren J, Czerkinsky C. Differential expression of tissue-specific adhesion molecules on human circulating antibody-forming cells after systemic, enteric, and nasal immunizations. A molecular basis for the compartmentalization of effector B cell responses. J Clin Invest. 1997;99:1281. doi: 10.1172/JCI119286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Williams MB, Rose JR, Rott LS, Franco MA, Greenberg HB, Butcher EC. The memory B cell subset responsible for the secretory IgA response and protective humoral immunity to rotavirus expresses the intestinal homing receptor, alpha4beta7. J Immunol. 1998;161:4227. [PubMed] [Google Scholar]
- 58.Wilson E, Walcheck B, Davis WC, Jutila MA. Preferential tissue localization of bovine gamma delta T cell subsets defined by anti-T cell receptor for antigen antibodies. Immunol Lett. 1998;64:39. doi: 10.1016/s0165-2478(98)00077-7. [DOI] [PubMed] [Google Scholar]
- 59.Jutila MA, Bargatze RF, Kurk S, Warnock RA, Ehsani N, Watson SR, Walcheck B. Cell surface P- and E-selectin support shear-dependent rolling of bovine gamma/delta T cells. J Immunol. 1994;153:3917. [PubMed] [Google Scholar]
- 60.Wilson E, Aydintug MK, Jutila MA. A circulating bovine gamma delta T cell subset, which is found in large numbers in the spleen, accumulates inefficiently in an artificial site of inflammation: correlation with lack of expression of E-selectin ligands and L-selectin. J Immunol. 1999;162:4914. [PubMed] [Google Scholar]
- 61.Hayday AC. γ/† cells: a right time & a right place for a conserved third way of protection. Annu Rev Immunol. 2000;18:975. doi: 10.1146/annurev.immunol.18.1.975. [DOI] [PubMed] [Google Scholar]
- 62.Chien YH, Jores R, Crowley MP. Recognition by gamma/delta T cells. Annu Rev Immunol. 1996;14:511. doi: 10.1146/annurev.immunol.14.1.511. [DOI] [PubMed] [Google Scholar]
- 63.Wen L, Hayday AC. Gamma delta T-cell help in responses to pathogens and in the development of systemic autoimmunity. Immunol Res. 1997;16:229. doi: 10.1007/BF02786392. [DOI] [PubMed] [Google Scholar]
- 64.Carding SR. Role of gamma delta T cells in immunity to infectious diseases and the regulation of hematolymphoid cell development. Immunol Res. 1998;17:13. doi: 10.1007/BF02786426. [DOI] [PubMed] [Google Scholar]
- 65.Collins RA, Werling D, Duggan SE, Bland AP, Parsons KR, Howard CJ. γ† T cells present antigen to CD4+ αβ T cells. J Leukoc Biol. 1998;63:707. doi: 10.1002/jlb.63.6.707. [DOI] [PubMed] [Google Scholar]
- 66.Augustin A, Kubo RT, Sim G-K. Resident pulmonary lymphocytes expressing the γ/† T-cell receptor. Nature. 1989;340:239. doi: 10.1038/340239a0. [DOI] [PubMed] [Google Scholar]
- 67.D'Souza CD, Cooper AM, Frank AA, Mazzaccaro RJ, Bloom BR, Orme IM. An anti-inflammatory role for gamma delta T lymphocytes in acquired immunity to Mycobacterium tuberculosis. J Immunol. 1997;158:1217. [PubMed] [Google Scholar]
- 68.Saunders BM, Frank AA, Cooper AM, Orme IM. Role of gamma delta T cells in immunopathology of pulmonary Mycobacterium avium infection in mice. Infect Immun. 1998;66:5508. doi: 10.1128/iai.66.11.5508-5514.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Sim GK, Augustin A. Dominant expression of the T cell receptor BALB invariant delta (BID) chain in resident pulmonary lymphocytes is due to selection. Eur J Immunol. 1991;21:859. doi: 10.1002/eji.1830210352. [DOI] [PubMed] [Google Scholar]
- 70.Salerno A, Dieli F. Role of gamma delta T lymphocytes in immune response in humans and mice. Crit Rev Immunol. 1998;18:327. doi: 10.1615/critrevimmunol.v18.i4.30. [DOI] [PubMed] [Google Scholar]
- 71.McMenamin C, Pimm C, McKersey M, Holt PG. Regulation of IgE responses to inhaled antigen in mice by antigen- specific gamma delta T cells. Science. 1994;265:1869. doi: 10.1126/science.7916481. [DOI] [PubMed] [Google Scholar]
- 72.McMenamin C, McKersey M, Kuhnlein P, Hunig T, Holt PG. Gamma delta T cells down-regulate primary IgE responses in rats to inhaled soluble protein antigens. J Immunol. 1995;154:4390. [PubMed] [Google Scholar]
- 73.Zuany-Amorim C, Ruffie C, Haile S, Vargaftig BB, Pereira P, Pretolani M. Requirement for γ† T cells in allergic airway inflammation. Science. 1998;280:1265. doi: 10.1126/science.280.5367.1265. [DOI] [PubMed] [Google Scholar]
- 74.Mizgerd JP, Meek BB, Kutkoski GJ, Bullard DC, Beaudet AL, Doerschuk CM. Selectins and neutrophil traffic: margination and Streptococcus pneumoniae-induced emigration in murine lungs. J Exp Med. 1996;184:639. doi: 10.1084/jem.184.2.639. [DOI] [PMC free article] [PubMed] [Google Scholar]