Abstract
Scope
Xanthohumol (XN) is a bioactive prenylflavonoid from hops. A single-dose pharmacokinetic (PK) study was conducted in men (n=24) and women (n=24) to determine dose-concentration relationships.
Methods and results
Subjects received a single oral dose of 20, 60, or 180 mg XN. Blood was collected at 0, 0.25, 0.5, 1, 2, 4, 8, 12, 24, 48, 72, 96, and 120 h. Plasma levels of XN and its metabolites, isoxanthohumol (IX), 8-prenylnaringenin (8PN) and 6-prenylnaringenin (6PN) were measured by LC-MS/MS. Xanthohumol and IX conjugates were dominant circulating flavonoids among all subjects. Levels of 8PN and 6PN were undetectable in most subjects. The XN PK profile showed peak concentrations around 1h and between 4-5h after ingestion. The maximum XN concentrations (Cmax) were 45±7 μg/L, 67±11 μg/L, and 133±23 μg/L for the 20, 60, and180 mg dose, respectively. Using non-compartmental modeling, the area under the curves (AUC0→∞) for XN were 92±68 h×μg/L, 323±160 h×μg/L, and 863±388 h×μg/L for the 20, 60, and 180 mg dose, respectively. The mean half-life of XN was 20 h for the 60 and 18 h for the 180 mg dose.
Conclusion
Xanthohumol has a distinct biphasic absorption pattern with XN and IX conjugates being the major circulating metabolites.
Keywords: Flavonoids, Hops, Human Metabolism, Pharmacokinetics, Xanthohumol
1 Introduction
Type 2 diabetes has emerged as major public health issue because of its prevalence, impact on health care costs, and its role in development of other chronic diseases such as cardiovascular disease and obesity. In 2010, the United States (US) Centers for Disease Control (CDC) estimated that 8% of Americans (25 million) suffered from diabetes with 95% of diagnosed cases in adults (≥ 20 y of age) being type 2 diabetes. It has been estimated that diabetes resulted in direct health care costs of $176 billion in 2012 [1]. Type 2 diabetes patients are also at increased risk for cardiovascular disease, which is one of the leading causes of death in the US [2].
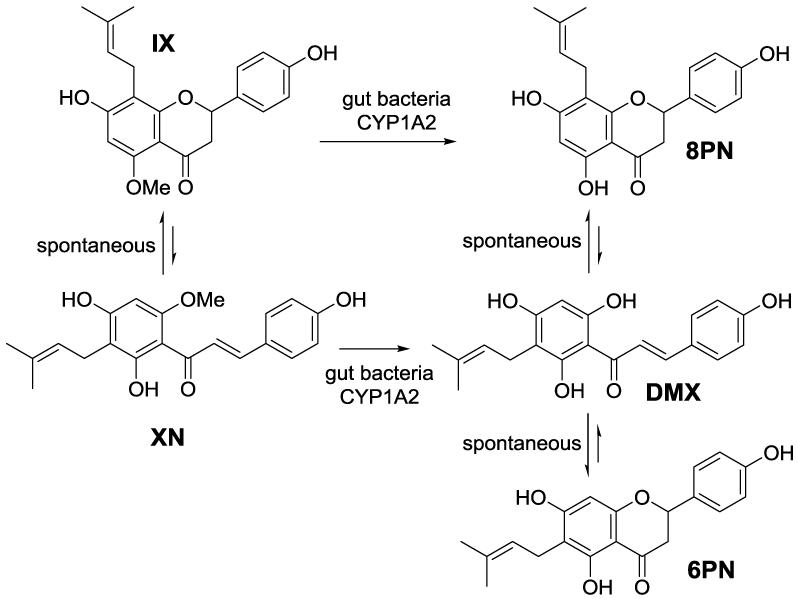
Current findings suggest flavonoids might aid in prevention and/or mitigation of type 2 diabetes [3, 4]. In particular, recent evidence suggests that xanthohumol (XN), a prenylflavonoid from hops (Humulus lupulus), may have therapeutic potential for metabolic diseases including type 2 diabetes [5, 6]. Investigations have shown that XN possesses a broad range of biological activities such as antioxidant [7], anti-inflammatory [8, 9], anti-microbial [10], and immune modulatory activity [11]. Some of the health benefits and potential adverse effects of XN may be related to the estrogenic activity of one of its metabolites, 8-prenylnaringenin (8PN), which possesses higher estrogenic activity than the soy isoflavonoid genistein [12-14]. The major pathways for XN metabolism have been reviewed in [15]. XN is subject to spontaneous conversion into isoxanthohumol (IX) via an intramolecular Michael addition. IX undergoes enzymatic O-demethylation to 8PN via hepatic CYP1A2 [16] or gut microbial enzymes [17-19]. Xanthohumol can also undergo direct metabolic conversion to desmethylxanthohumol (DMX) which is later converted into either 6-prenylnaringenin (6PN) or 8PN (Figure 1).
Figure 1.
Pathway for xanthohumol (XN) metabolism and production of its metabolites: isoxanthohumol (IX), 6-prenylnarigenin (6PN), 8-prenylnarigenin (8PN) and desmethylxanthohumol (DMX). Reproduced from [23].
Xanthohumol may impact lipid and glucose metabolism by acting as a ligand for the farnesoid X receptor (FXR), which regulates bile acid homeostasis as well as genes involved in lipid metabolism, lipid transport, and glucose homeostasis [6]. Nozawa has proposed that XN acts as a ligand for FXR and its supplementation in mice led to lower plasma lipid and glucose levels [6]. Studies conducted in our lab showed that XN lowers fasting plasma glucose levels[5] and ameliorates dysfunctional lipid metabolism [20] in obese male Zucker fa/fa rats. Despite immense work in vitro and recent in vivo animal studies, there is limited data on XN metabolism in humans. This study was conducted in healthy men and women to determine basic pharmacokinetics (PK) parameters for XN with the aim to establish dose-concentration relationships and to predict dose-effect relationships in humans diagnosed with metabolic syndrome.
2 Materials and Methods
2.1 Human Subjects
Study subjects were recruited for the PK study by the NIH Clinical Translational Research Center at Oregon Health Sciences University (OHSU) based on following entry criteria: a body mass index (BMI) between 18-32 kg/m2, normal metabolic blood panel, no current use of prescription drugs, and no acute medical conditions. Subjects were also required to abstain from beer consumption prior to (4 days before) and throughout the study. Subject characteristics are detailed in Table 1. All procedures and protocols involving human subjects were approved by and conducted in accordance with guidelines of the OHSU and Oregon State University Institutional Review Boards (IRB Protocol: IRB00005373). Written informed consent was obtained from all subjects. All subjects were allowed to withdraw from the study at any time. Healthy subjects (24 men and 24 women) were enrolled and completed the PK study. There were no treatment-related adverse events reported in the study.The study was registered with the ClinicalTrials.gov identifier NCT01367431.
Table 1.
Subject characteristics for human pharmacokinetics study of XN
| Subjects | ||
|---|---|---|
|
| ||
| Characteristics a | Males n=24 |
Females n=24 |
| Age (y) | 31 ± 2 | 35 ± 2 |
| Height (cm) | 178 ± 2 | 166 ± 2 |
| Weight (kg) | 78 ± 3 | 67 ± 3 |
Values are reported as mean ± SEM.
2.2 Treatment Groups
Subjects were randomized based on gender to one of the following oral treatments: 20, 60, or 180 mg XN. Blood was collected at 0, 0.25, 0.5, 1, 2, 4, 8, and 12 h through use of an intravenous catheter. Blood samples at 24, 48, 72, 96, and 120 h were collected by venous puncture. Subjects were advised to fast 8 h before the 0, 24, 48, 72, 96 and 120 h blood draws. Subjects resumed eating two hours after the 0 h time point and immediately following the 24, 48, 72, 96 and 120 h time points. Study participants and investigators were blinded to randomization of study treatment assignments.
2.3 Dose and sampling
Xanthohumol capsules were prepared using food-grade 99% pure XN powder (Hopsteiner, Inc., New York, NY). Capsules contained a self-emulsifying isotropic mixture of XN, oleic acid, Tween 80 and propylene glycol as previously described[5]. Capsules were formulated at Lloyd Center Pharmacy (Portland, OR) and had a non-transparent appearance to eliminate any potential visual distinction between treatments. Before beginning the study, a pilot batch of XN capsules was formulated and underwent chemical and physical stability tests after 3, 6 and 9 months of storage. All capsules were found to be within 10% of the nominal dose. Capsules were analyzed by dissolving in 10 mL water, followed by dilution with methanol to a final volume of 100 mL. Aliquots were chromatographed using a 4 × 250 mm Luna C18 column (Phenomenex, Torrance, CA) eluded with a gradient of 40 to 100% acetonitrile in 0.1% trifluoroacetic acid in 15 min. Xanthohumol was quantified using a UV detector set at 368 nm.
2.4 Sample preparation
Following each blood draw, samples were collected in sterile vacuum blood container tubes coated with lithium heparin (BD Vacutainer®, BD Diagnostics, Franklin Lakes, NJ, USA). Samples were centrifuged before removal and plasma was stored at −80°C until sample processing and analysis. Preliminary work on a subset of plasma samples was conducted to verify complete enzymatic hydrolysis. Representative plasma samples from all treatment groups were analyzed for glucuronide and sulfate conjugates before and after hydrolysis. XN glucuronides were detected via LC-MS/MS using selected reaction monitoring (SRM) of plasma samples before enzymatic hydrolysis. Following enzymatic hydrolysis, an increase in XN levels was observed and XN glucuronides could no longer be detected (data not shown). XN sulfate conjugates were not detected either before or after enzymatic hydrolysis of native plasma samples. To confirm that our LC-MS/MS method could detect XN sulfates, XN monosulfate was synthesized. Synthesis of a XN monosulfate standard was performed based on a modified procedure from [21, 22] and described in Supporting Information. XN monosulfate was characterized by LC-QToF MS analysis (Supporting Information Figures S1 and S2) and detected when spiked into blank plasma samples. Its level was reduced following enzymatic hydrolysis. The incubation mixture for the enzymatic hydrolysis consisted of the following components: a plasma aliquot (100 μL), 0.1 M sodium acetate buffer (pH 4.7, 380 μL), an internal standard, 4,2′-dihydroxychalcone (DHC, 10μL), methanol or various standards dissolved in methanol (XN, or a mixture of IX, 6PN and 8PN; 10 μL) and Helix pomatia sulfatase / glucuronidase (600U/incubation ~ 0.2 mg/mL, 100μL) for a total volume of 600 μL. Samples were incubated for 2 h at 37°C in 2 mL screw-cap tubes. Following enzymatic hydrolysis, samples were vortexed (10s) and centrifuged (13,000 rpm, 2 min). For sample extraction, a new method was developed using 8 × 45 mm pieces of Whatman #1 filter paper. All samples were extracted using a paper strip extraction method instead of the previously used liquid-liquid extraction method [23]. The paper strip extraction method offers several benefits over liquid-liquid extraction including less solvent use, lower labor costs, and less investigator exposure to organic solvents. Method validation studies in our lab showed that the paper strip extraction method met U.S. Food and Drug Administration guidelines in terms of accuracy (≤ 15% average error), precision (6-12% RSD), and reproducibility (≤ 15% RSD) across a large concentration range. A full description of the paper strip extraction method will be detailed elsewhere. In brief, a pointed paper strip was placed in each sample tube with the tip immersed in the incubation mixture. Samples were dried onto the paper strips overnight (vacuum dessicator over Drierite). Dried paper strips were extracted with acidified (0.1% formic acid) methanol (0.5 mL) by vortexing (30 s) and shaking (30 min). After removal of paper strips, extracts were centrifuged (13,000 rpm, 2 min) and analyzed by LC-MS/MS using the selected reaction monitoring (SRM) mode to determine total concentrations (free and conjugated) of XN and its metabolites (IX, 8PN and 6PN).
2.5 Mass spectrometry
Liquid chromatography tandem mass spectrometry (LC-MS/MS) conditions for quantification of XN and its metabolites were similar to those detailed in our previous work [23]. In brief, LC-MS/MS was performed on an Applied Biosystems 4000QTRAP hybrid linear ion trap-triple quadrupole instrument (AB Sciex, Concord, Canada) operated at a source temperature of 600°C with a needle voltage of −4500 kV. Chromatographic separations of XN and its metabolites were achieved on a 2 × 50 mm Zorbax 300SB C8 column (Agilent, Santa Clara, CA) eluted with a gradient of 25 to 60 % solvent B (0.1 % formic acid in acetonitrile) in solvent A (aqueous 0.1 % formic acid) in 4 min at a flow rate of 0.5 mL/min. For detection of glucuronide conjugates, the following SRM transitions were used: m/z 529 → 353 for IX and XN. For detection of sulfate conjugates, the following SRM transition was used: m/z 433 → 119 for XN monosulfate. A quadrupole-time of flight mass spectrometer (ABSCIEX TripleTOF 5600, AB Sciex, Foster City, CA) was used to characterize XN conjugates. The 5600 TripleTOF was operated in positive ion mode with a source temperature of 600°C and needle voltage of 5500 kV. XN monoglucuronide (C21H31O11+) and diglucuronide (C33H39O17+) as well as XN monosulfate (C21H23O8S+) and disulfate (C21H23O11S2+) were analytes of interest in the TOF MS analysis; however, only one XN monoglucuronide with a mass peak at m/z 531.1873 [M+H]+ (calculated for C21H31O11: 531.1861) was identified in plasma samples. The chromatography conditions remained the same as those for XN analysis using the 4000QTRAP.
2.6 Pharmacokinetic model and parameters
Mean plasma concentration-time profiles for both XN and IX were generated using GraphPad Prism software (version 4.03, San Diego, CA, USA). Maximum plasma concentration (Cmax) and the time to reach Cmax (Tmax) were determined from the plasma concentration-time curves. Pharmacokinetic parameters were estimated by fitting a non-compartmental model to the experimental XN plasma concentration versus time data. Optimized PK parameter estimates from the model were obtained using regression analysis and WinNonlin software (WinNonlin 5.0.1; Pharsight, Sunnyvale, CA). Pharmacokinetic parameter estimates were used to calculate the following values: area under the plasma concentration-time curve (AUC0→∞), half-life (t1/2), and systemic total body clearance (CL/F). The fraction of dose absorbed (F) was not determined because an I.V. dose was not administered to the test subjects.
2.7 Statistics
Pharmacokinetic parameters of XN and IX are shown as means ± SEM. Using Statistical Analysis Software (SAS), Version 9.1 (SAS Institute, Cary, NC, USA), the effects of dose and gender on pharmacokinetic parameters of XN and IX were calculated in PROC GLM with dose (60 and 180 mg XN), gender (male and female), and their interaction as fixed effects. To achieve normality, the pharmacokinetic kinetic parameters were transformed to their natural logarithmic values. Statistical significance was declared at p ≤ 0.05 and a tendency at 0.05 < p ≤ 0.10.
3 Results and Discussion
3.1 Pharmacokinetic parameters of XN and its metabolites
Circulating levels of free XN were found to be very low compared to conjugate levels (< 4 %, see Table 2). In previous studies conducted with Zucker rats, we demonstrated a correlation between biological effect and total (free plus conjugated) XN levels, but not levels of free XN ()[5, 20]. There are a number of explanations for this observation: 1) plasma levels of free XN may not accurately reflect tissue levels of free XN or an active metabolite (the long half-life of XN may be indicative of tissue accumulation), 2) XN conjugates themselves may exert biological effects, and 3) gut microbiota may produce bioactive XN metabolites whose levels correlate with total XN plasma levels. Our earlier findings indicate that total XN levels reflect exposure of molecular targets to XN-derived active principles better than plasma levels of free XN. Therefore, we decided to focus on total plasma levels of XN in this study.
Table 2.
Extent of conjugation of xanthohumol and its metabolites in plasma samples at different time points (1 and 24 hr) following a single oral administration of 60 (n=2) or 180 (n=2) mg XN
| Metabolite | % Conjugation | |||
|---|---|---|---|---|
| 60 mg XN | 180 mg XN | |||
|
| ||||
| 1hr plasma | 24 hr plasma | 1hr plasma | 24 hr plasma | |
| Xanthohumol (XN) | 100 | 100 | 96 ± 3 | 100 |
| Isoxanthohumol (IX) | 100 | 100 | 100 | 100 |
| 8-prenylnaringenin (8PN) | 100 | 100 | 100 | 100 |
Values are reported as mean of biological replicates ± SEM. Samples were analyzed in triplicate.
Total plasma concentrations of XN and its metabolites (IX, 8PN, 6PN) were determined using LC-MS/MS following enzymatic hydrolysis to remove conjugates Previous research has shown that both free and conjugated forms of flavonoids have biological activity [24]. In accordance with recommendations from the Food and Drug Administration [25], a total systemic exposure profile was determined with all potential active forms of XN and its metabolites for PK modeling. A subset of plasma samples was analyzed to determine extent of conjugation. Results showed that XN (96%) and its metabolites (100%) are primarily present in circulation as conjugates (Table 2). The great variability in XN plasma concentrations of subjects receiving the 20 mg dose as well as the presence of only trace amounts of IX made PK modeling for this dose level difficult and inconsistent. Therefore, PK parameters were only determined and compared for the 60 and 180 mg XN doses. Pharmacokinetic parameters for XN and IX are displayed in Tables 3 and 4. There was a tendency of a dose and gender interaction for XN on Tmax (p = 0.08) as Tmax was increased only at the 180 mg dose in males. Main effects of XN dosage were observed for AUC and Cmax; AUC (p < 0.0001 for XN and p = 0.0004 for IX) and Cmax, (p = 0.07 for XN and p < 0.0001 for IX) were higher at the 180 than the 60 mg XN dose level. Main effects for gender were observed in XN for Cmax and in IX for CL/F, as CL/F tended to be greater in males than females (p = 0.09) and Cmax tended to be lower in males than females (p = 0.10), which was significant at the 180 mg XN dose level (p = 0.05). Differences in Cmax could partially be attributed to the fact that our female subjects generally had a lower body mass than males (Table 1) and thus received a higher dose of XN per body weight (180 mg XN/66 kg vs. 180mg XN/78 kg in men). The Cmax and Tmax of XN agree with findings from human PK studies of other flavonoids [26-29], which show nanomolar plasma concentration ranges and peak levels between 1–3 h. Xanthohumol and IX conjugates were the dominant circulating metabolites among all subjects. Levels of 6PN were undetectable in all subjects, indicating that O-demethylation of XN is not a major metabolic pathway in humans (Figure 1). High levels of XN and IX as well as a lack of 6PN were also observed during our earlier XN rodent PK study [23]. However, levels of 8PN were very low in five subjects and not detectable in others. This observation contrasts with our previous PK study in rats [23], which showed high 8PN formation resulting in plasma concentrations of 39 nM. These findings may indicate that XN metabolism differs between humans and rodents, which should be considered for future work evaluating health actions of XN metabolites. Bolca et al. [14] found that 60% of post-menopausal women consuming a hops-based supplement for clinical therapy were poor 8PN producers based on urinary excretion. Thus, it could be that rodents may be strong producers of 8PN, whereas the ability to produce 8PN in humans varies greatly. This phenomenon has also been observed with other flavonoids, primarily with the soy isoflavone daidzein and its major metabolite, equol [25].
Table 3.
Pharmacokinetic (PK) parameters for total (free and conjugated) xanthohumol (XN) for human subjects given a single oral dose of 60 mg (n=13) or 180 mg (n=17) XNa,b
| Parameter | Units | XN Doses |
|||
|---|---|---|---|---|---|
| 60 mg | 180 mg | ||||
|
| |||||
| Males n=6 |
Females n=7 |
Males n=8 |
Females n=9 |
||
| AUC | h × μg L−1 | 287 ± 42 | 354 ± 75 | 712 ± 92 | 996 ± 149 |
| CL/F | L × h−1 | 160 ± 66 | 152 ± 47 | 268 ± 36 | 198 ± 32 |
| Cmax | μg × L−1 | 63 ± 16 | 70 ± 17 | 83± 22 | 178 ± 34§ |
| half-life, t1/2 | h | 20 ± 8 | 19 ± 6 | 16 ± 1 | 19 ± 2 |
| Tmax | h | 1.3 ± 0.5 | 1.1 ± 0.2 | 3.1 ± 0.9† | 1.1 ± 0.1 |
Values are reported as mean ± SEM.
§ denotes a statistical difference (p ≤ 0.05) and † represents statistical trend (0.05 ≤p ≤ 0.10) between males and females at the same dosage. Overall, AUC (p < 0.0001) and Cmax (p = 0.07) values were or tended to be greater at the 180 versus the 60 mg dose. Males tended to have lower Cmax values (p = 0.10) than females which was significant at 180 mg dose (p = 0.05). There also tended to be an interaction between dosage and gender for Tmax (p = 0.08), as Tmax was increased only at the 180 mg dosage in males.
Table 4.
Pharmacokinetic (PK) parameters for total (free and conjugated) isoxanthohumol (IX) for human subjects given a single oral dose of 60 mg (n=13) or 180 mg (n=17) xanthohumola,b
| Parameter | Units | XN Doses |
|||
|---|---|---|---|---|---|
| 60 mg | 180 mg | ||||
|
| |||||
| Males n=6 |
Females n=7 |
Males n=8 |
Females n=9 |
||
| AUC | h × μg L−1 | 216 ± 66 | 342 ± 95 | 511 ± 75 | 982 ± 141 |
| CL/F | L × h−1 | 318 ± 72 | 299 ± 136 | 357 ± 54 | 292 ± 143 |
| Cmax | μg × L−1 | 9.7 ± 2.0 | 9.3 ± 1.7 | 25 ± 6 | 37 ± 7 |
| half-life, t1/2 | h | 21 ± 6 | 30 ± 9 | 22 ± 3 | 24 ± 3 |
| Tmax | h | 6.3 ± 1.1 | 9.2 ± 2.7 | 6.5 ± 1.1 | 4.1 ± 0.6 |
Values are reported as mean ± SEM.
Overall, AUC (p = 0.0004) and Cmax (p < 0.0001) values were or tended to be greater at the 180 versus the 60 mg dose. Males tended to have greater CL/F values than females (p = 0.09).
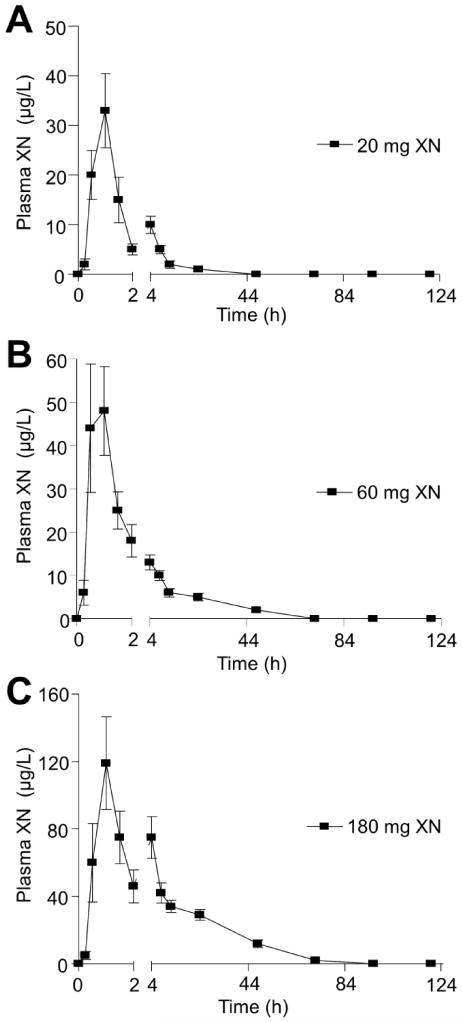
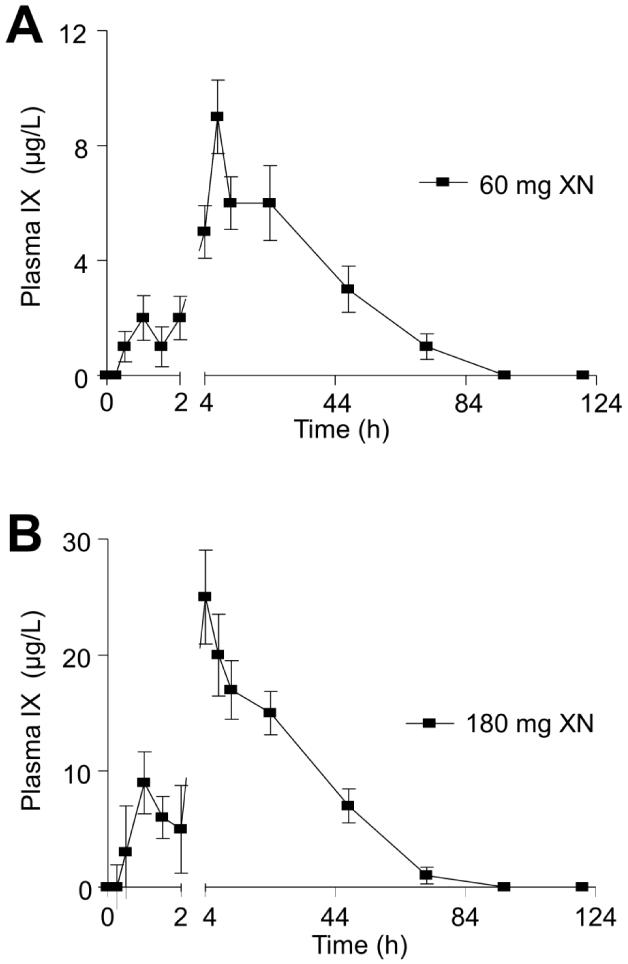
As seen previously with other flavonoids as well as in our earlier study of XN in rats [23], the average XN PK profiles show a second plasma concentration maximum following the initial absorption maximum for subjects who consumed a single dose of 20 or 180 mg XN (Figs. 2A, C). The initial absorption maximum was around 1 h and the second gradual increase in XN plasma levels occurred at about 4 h. The second gradual increase in XN plasma levels was not seen in the average PK profile for the 60 mg dose group (Fig. 2B). However, individual PK profiles of subjects consuming the 60 mg dose showed a second gradual increase in XN plasma levels and the timing of the increase differ greatly among subjects and thus was not apparent when evaluating the average PK profile. There are a variety of factors that could be contributing to the second increase in plasma XN levels including enterohepatic recirculation and/or additional absorption in the large intestine. The biphasic absorption pattern could also be due to an initial rapid absorption of XN from the vehicle solution followed by a delayed gradual absorption due to absorption of redissolved XN that may have precipitated in intestinal fluids. Dissolution studies are needed to determine the exact causes of the biphasic absorption pattern. In contrast to the XN PK profile, IX PK curves (Figs. 3A and B) show a small peak in plasma levels around 2 h and a larger plasma concentration maximum around 6 h for both the 60 and 180 mg dose groups, which is attributed to slow conversion of XN to IX over a period of hours.
Figure 2.
Plot of mean total (free and conjugated) xanthohumol (XN) plasma concentration vs. time data obtained after human subjects were given a single oral dose of A) 20 (n=18), B) 60 (n=13), or C) 180 mg (n=17) xanthohumol.
Figure 3.
Plot of mean total (free and conjugated) isoxanthohumol (IX) plasma concentration vs. time data obtained after human subjects were given a single oral dose of A) 60 (n=13) or B) 180 mg (n=17) xanthohumol.
3.2 Steady-state plasma concentrations
Due to the complexity of XN pharmacokinetics, traditional PK equations for predicting steady state concentrations were not applicable for this study. Instead, projected steady state levels were calculated by utilizing representative mean plasma concentration data and projecting plasma concentration—time curves out to 120 h using the superposition principle [30]. Using this approach, steady-state plasma levels were calculated to be 1.5, 18, and 40 μg/L for the 20, 60, and 180 mg doses, respectively. In our previous study, chronic exposure of Zucker fa/fa rats to XN (16.9 mg/kg daily for 6 wks = human equivalent of 180 mg/d) resulted in a steady state plasma XN level of 138 μg/L and in significant reductions in body weight gain and fasting plasma glucose compared to control animals[5].
3.3 Projection of PK parameters using inter-species scaling
Xanthohumol PK showed a linear response with increasing dose as depicted in Figure 4. With linear kinetics, a modified allometric scaling approach [31] can be utilized to predict human PK parameters from animal PK data using the following equation:
| (Eq.1) |
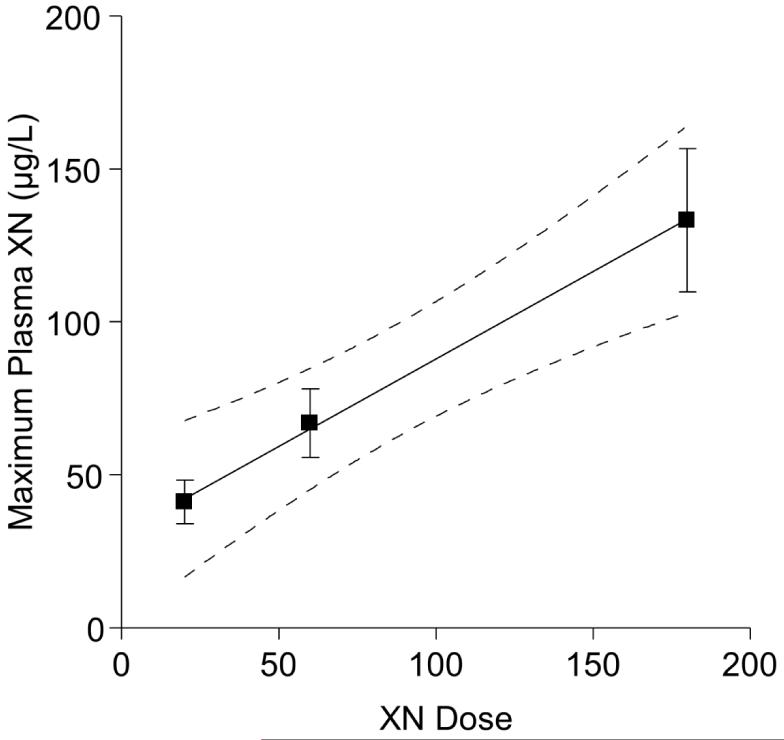
where CLhuman,pred and CLrat represents predicted XN clearance rate for humans and XN clearance rate for rats, respectively. The Wthuman and Wtrat represent the average body weight of human subjects and rats in our XN PK studies. Using previous findings from our 2010 rat XN PK study [23], the predicted human clearance rate for a single oral 180 mg XN dose (animal equivalent = 16.9 mg/kg BW) was 5.9 L/kg×h while the experimentally determined value was 3.2 L/kg×h (derived from data in Table 3). Other PK parameters such as Cmax and t1/2 were similarly in agreement between rat and human PK studies as detailed in Table 4 for an equivalent dose of XN. The AUC and Tmax are slightly higher in rats compared to humans for an equivalent dose. This difference is probably due to the different methods of oral XN administration in the studies. The rats received the XN doses via oral gavage with a proportionally greater volume of liquid (1 mL) compared to human subjects who received XN doses in the oral formulation capsule form (0.6 mL). Overall, the PK parameters in both rat and human PK studies agree for an equivalent dose of XN, thus confirming the validity of using the Food and Drug Administration (FDA) allometric interspecies scaling factor [25]. With PK parameters similar between rats and humans, it is reasonable to expect comparable biological effects of chronic exposure with equivalent doses. We have previously determined circulating levels in obese rodents that correlated with improvement of glucose and lipid metabolism [5, 20]This study provides new insight into XN metabolism and circulating levels in normal healthy subjects. Future work should confirm that XN metabolism is not altered in subjects suffering from metabolic syndrome.
Figure 4.
Linear relationship between total (free and conjugated) xanthohumol (XN) plasma concentration (Cmax) and dose. Human subjects were given a single oral dose of 20 (n=18), 60 (n=13), or 180 mg (n=17) XN.
4 Concluding Remarks
This is the first study that reports human PK parameters for XN. Xanthohumol PK shows a distinct biphasic absorption pattern with XN and IX conjugates being the major circulating metabolites following oral consumption of XN in humans. Results from this study as well as previous work demonstrate similarity of XN metabolism between animals and humans, thus allowing for translation of animal study findings to future clinical work. Findings from our earlier animal pharmacodynamic XN studies[5, 20] combined with data from this human PK study allows for selection of effective doses to be utilized in future clinical studies aimed at improving lipid and glucose metabolism in humans diagnosed with metabolic syndrome.
Supplementary Material
Table 5.
Comparison of animal (rat) [23] and clinical pharmacokinetic parameters for total xanthohumol (free and conjugated)a
| Parameter | Units | Rat | Human Subjects |
|---|---|---|---|
| Dose | 16.9 mg/kg BWb | 180 mg | |
| AUC | h × mg L−1 | 2.5 ± 0.1 | 1.0 ± 0.1 |
| Cmax | mg × L−1 | 0.15 ± 0.01 | 0.13 ± 0.02 |
| half-life, t1/2 | h | 18 ± 2 | 17 ± 1 |
| Tmax | h | 4.5 ± 0.6 | 2.0 ± 0.5 |
Values are reported as mean ± SEM.
Corresponds to an equivalent dose of 180 mg for an individual weighing 64 kg, calculated by allometric interspecies scaling.
Acknowledgements
Support from NIH (R21AT005294, S10 RR022589, P30 ES000210 and UL1TR000128), USANA Health Sciences, Inc., Salt Lake City, UT, and Hopsteiner Inc., New York, is gratefully acknowledged. We thank Mr. Jeffrey Morré for technical assistance.
Abbreviations
- 6PN
6-prenylnaringenin
- 8PN
8-prenylnaringenin
- AUC
area under the plasma concentration time curve
- Cmax
maximum plasma concentration
- CL/F
systemic total body clearance
- DHC
4,2′-dihydroxychalcone
- DMX
desmethylxanthohumol
- F
fraction of dose absorbed
- IV
intravenous
- IX
isoxanthohumol
- PK
pharmacokinetics
- SRM
selected reaction monitoring
- t1/2,
half-life
- Tmax
time required to reach maximum plasma concentration
- XN
xanthohumol
Footnotes
Conflict of Interest
The authors have declared no conflict of interest.
References
- [1].American Diabetes Association Economic costs of diabetes in the U.S. in 2012. Diabetes Care. 2013;36:1033–1046. doi: 10.2337/dc12-2625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Chiha M, Njeim M, Chedrawy EG. Diabetes and coronary heart disease: a risk factor for the global epidemic. Int J Hypertens. 2012;2012:697240. doi: 10.1155/2012/697240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Cumbie BC, Hermayer KL. Current concepts in targeted therapies for the pathophysiology of diabetic microvascular complications. Vasc Health Risk Manag. 2007;3:823–832. [PMC free article] [PubMed] [Google Scholar]
- [4].Nicolle E, Souard F, Faure P, Boumendjel A. Flavonoids as promising lead compounds in type 2 diabetes mellitus: molecules of interest and structure-activity relationship. Curr Med Chem. 2012;18:2661–2672. doi: 10.2174/092986711795933777. [DOI] [PubMed] [Google Scholar]
- [5].Legette LL, Moreno Luna AY, Reed RL, Miranda CL, et al. Xanthohumol lowers body weight and fasting plasma glucose in obese male Zucker fa/fa rats. Phytochemistry. 2013;91:236–241. doi: 10.1016/j.phytochem.2012.04.018. [DOI] [PubMed] [Google Scholar]
- [6].Nozawa H. Xanthohumol, the chalcone from beer hops (Humulus lupulus L.), is the ligand for farnesoid X receptor and ameliorates lipid and glucose metabolism in KK-A(y) mice. Biochem Biophys Res Commun. 2005;336:754–761. doi: 10.1016/j.bbrc.2005.08.159. [DOI] [PubMed] [Google Scholar]
- [7].Miranda CL, Stevens JF, Ivanov V, McCall M, et al. Antioxidant and prooxidant actions of prenylated and nonprenylated chalcones and flavanones in vitro. J Agric Food Chem. 2000;48:3876–3884. doi: 10.1021/jf0002995. [DOI] [PubMed] [Google Scholar]
- [8].Lupinacci E, Meijerink J, Vincken JP, Gabriele B, et al. Xanthohumol from hop (Humulus lupulus L.) is an efficient inhibitor of monocyte chemoattractant protein-1 and tumor necrosis factor-alpha release in LPS-stimulated RAW 264.7 mouse macrophages and U937 human monocytes. J Agric Food Chem. 2009;57:7274–7281. doi: 10.1021/jf901244k. [DOI] [PubMed] [Google Scholar]
- [9].Peluso MR, Miranda CL, Hobbs DJ, Proteau RR, Stevens JF. Xanthohumol and related prenylated flavonoids inhibit inflammatory cytokine production in LPS-activated THP-1 monocytes: structure-activity relationships and in silico binding to myeloid differentiation protein-2 (MD-2) Planta Med. 2010;76:1536–1543. doi: 10.1055/s-0029-1241013. [DOI] [PubMed] [Google Scholar]
- [10].Gerhauser C. Broad spectrum anti-infective potential of xanthohumol from hop (Humulus lupulus L.) in comparison with activities of other hop constituents and xanthohumol metabolites. Mol Nutr Food Res. 2005;49:827–831. doi: 10.1002/mnfr.200500091. [DOI] [PubMed] [Google Scholar]
- [11].Xuan NT, Shumilina E, Gulbins E, Gu S, et al. Triggering of dendritic cell apoptosis by xanthohumol. Mol Nutr Food Res. 2010;54(Suppl 2):S214–224. doi: 10.1002/mnfr.200900324. [DOI] [PubMed] [Google Scholar]
- [12].Milligan S, Kalita J, Pocock V, Heyerick A, et al. Oestrogenic activity of the hop phyto-oestrogen, 8-prenylnaringenin. Reproduction. 2002;123:235–242. [PubMed] [Google Scholar]
- [13].Milligan SR, Kalita JC, Heyerick A, Rong H, et al. Identification of a potent phytoestrogen in hops (Humulus lupulus L.) and beer. J Clin Endocrinol Metab. 1999;84:2249–2252. doi: 10.1210/jcem.84.6.5887. [DOI] [PubMed] [Google Scholar]
- [14].Milligan SR, Kalita JC, Pocock V, Van De Kauter V, et al. The endocrine activities of 8-prenylnaringenin and related hop (Humulus lupulus L.) flavonoids. J Clin Endocrinol Metab. 2000;85:4912–4915. doi: 10.1210/jcem.85.12.7168. [DOI] [PubMed] [Google Scholar]
- [15].Stevens JF, Page JE. Xanthohumol and related prenylflavonoids from hops and beer: to your good health! Phytochemistry. 2004;65:1317–1330. doi: 10.1016/j.phytochem.2004.04.025. [DOI] [PubMed] [Google Scholar]
- [16].Guo J, Nikolic D, Chadwick LR, Pauli GF, van Breemen RB. Identification of human hepatic cytochrome P450 enzymes involved in the metabolism of 8-prenylnaringenin and isoxanthohumol from hops (Humulus lupulus L.) Drug Metab Dispos. 2006;34:1152–1159. doi: 10.1124/dmd.105.008250. [DOI] [PubMed] [Google Scholar]
- [17].Bolca S, Possemiers S, Maervoet V, Huybrechts I, et al. Microbial and dietary factors associated with the 8-prenylnaringenin producer phenotype: a dietary intervention trial with fifty healthy post-menopausal Caucasian women. Br J Nutr. 2007;98:950–959. doi: 10.1017/S0007114507749243. [DOI] [PubMed] [Google Scholar]
- [18].Possemiers S, Bolca S, Grootaert C, Heyerick A, et al. The prenylflavonoid isoxanthohumol from hops (Humulus lupulus L.) is activated into the potent phytoestrogen 8-prenylnaringenin in vitro and in the human intestine. J Nutr. 2006;136:1862–1867. doi: 10.1093/jn/136.7.1862. [DOI] [PubMed] [Google Scholar]
- [19].Possemiers S, Heyerick A, Robbens V, De Keukeleire D, Verstraete W. Activation of proestrogens from hops (Humulus lupulus L.) by intestinal microbiota; conversion of isoxanthohumol into 8-prenylnaringenin. J Agric Food Chem. 2005;53:6281–6288. doi: 10.1021/jf0509714. [DOI] [PubMed] [Google Scholar]
- [20].Kirkwood JS, Legette LL, Miranda CL, Jiang Y, Stevens JF. A metabolomics driven elucidation of the anti-obesity mechanisms of xanthohumol. J Biol Chem. 2013;288:19000–19013. doi: 10.1074/jbc.M112.445452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Fairley B, Botting NP, Cassidy A. The synthesis of daidzein sulfates. Tetrahedron. 2003;59:5407–5410. [Google Scholar]
- [22].Soidinsalo O, Wahala K. Synthesis of phytoestrogenic isoflavonoid disulfates. Steroids. 2004;69:613–616. doi: 10.1016/j.steroids.2004.03.015. [DOI] [PubMed] [Google Scholar]
- [23].Legette L, Ma L, Reed RL, Miranda CL, et al. Pharmacokinetics of xanthohumol and metabolites in rats after oral and intravenous administration. Mol Nutr Food Res. 2012;56:466–474. doi: 10.1002/mnfr.201100554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Beekmann K, Actis-Goretta L, van Bladeren PJ, Dionisi F, et al. A state-of-the-art overview of the effect of metabolic conjugation on the biological activity of flavonoids. Food Funct. 3:1008–1018. doi: 10.1039/c2fo30065f. [DOI] [PubMed] [Google Scholar]
- [25]. [Accessed on February 27, 2013];Guidance for Industry Bioavailability and Bioequivalence Studies for Orally Administered Drug Products — General Considerations. 2003 Available at: http://www.fda.gov/downloads/Drugs/.../Guidances/ucm070124.pdf.
- [26].Egert S, Wolffram S, Bosy-Westphal A, Boesch-Saadatmandi C, et al. Daily quercetin supplementation dose-dependently increases plasma quercetin concentrations in healthy humans. J Nutr. 2008;138:1615–1621. doi: 10.1093/jn/138.9.1615. [DOI] [PubMed] [Google Scholar]
- [27].Hosoda K, Furuta T, Ishii K. Metabolism and disposition of isoflavone conjugated metabolites in humans after ingestion of kinako. Drug Metab Dispos. 39:1762–1767. doi: 10.1124/dmd.111.038281. [DOI] [PubMed] [Google Scholar]
- [28].Rufer CE, Bub A, Moseneder J, Winterhalter P, et al. Pharmacokinetics of the soybean isoflavone daidzein in its aglycone and glucoside form: a randomized, double-blind, crossover study. Am J Clin Nutr. 2008;87:1314–1323. doi: 10.1093/ajcn/87.5.1314. [DOI] [PubMed] [Google Scholar]
- [29].Setchell KD, Clerici C. Equol: pharmacokinetics and biological actions. J Nutr. 140:1363S–1368S. doi: 10.3945/jn.109.119784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Shargel L, Wu-Pong S, Yu A. Applied Biopharmaceutics and Pharmacokinetics. McGraw-Hill Companies Inc.; New York: 2012. pp. 153–157. [Google Scholar]
- [31].Tang H, Hussain A, Leal M, Mayersohn M, Fluhler E. Interspecies prediction of human drug clearance based on scaling data from one or two animal species. Drug Metab Dispos. 2007;35:1886–1893. doi: 10.1124/dmd.107.016188. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.