Abstract
In the second edition of this series, we described the use of cell tracking dyes in combination with tetramer reagents and traditional phenotyping protocols to monitor levels of proliferation and cytokine production in antigen-specific CD8+ T cells. In particular, we illustrated how tracking dye fluorescence profiles could be used to ascertain the precursor frequencies of different subsets in the T-cell pool that are able to bind tetramer, synthesize cytokines, undergo antigen-driven proliferation, and/or carry out various combinations of these functional responses.
Analysis of antigen-specific proliferative responses represents just one of many functions that can be monitored using cell tracking dyes and flow cytometry. In this third edition, we address issues to be considered when combining two different tracking dyes with other phenotypic and viability probes for the assessment of cytotoxic effector activity and regulatory T-cell functions. We summarize key characteristics of and differences between general protein- and membrane-labeling dyes, discuss determination of optimal staining concentrations, and provide detailed labeling protocols for both dye types. Examples of the advantages of two-color cell tracking are provided in the form of protocols for (a) independent enumeration of viable effector and target cells in a direct cytotoxicity assay and (b) simultaneous monitoring of proliferative responses in effector and regulatory T cells.
Keywords: Cell tracking, CellVue® dyes, CFSE, Cytotoxicity assay, Dye dilution proliferation assay, Flow cytometry, PKH dyes, Proliferation analysis, Regulatory T cells
1. Introduction
A multiplicity of fluorescent dyes is now commercially available for cell tracking and proliferation monitoring (reviewed in (1)). Although diverse in their chemistry and fluorescence characteristics, these reagents can be categorized into two main classes based upon their mechanism of cell labeling. Dyes of one class, here referred to as “protein dyes,” permanently combine with proteins by forming a covalent bond. Dyes of the other class, here referred to as “membrane dyes,” stably intercalate within cell membranes via strong hydrophobic associations. The term “proliferation dye” will be used here to refer to dyes of either class that (a) exhibit sufficiently good chemical and metabolic stability to partition approximately equally between daughter cells at mitosis, and (b) are sufficiently nontoxic that they can be used to label cells at initial intensities that are high enough to follow the resulting dye dilution through multiple rounds of cell division.
Due to their stability of cell association, cell tracking dyes of both classes have proven useful for in vivo assays of cell trafficking and recruitment in transplant and tissue repair studies (2–5) and for monitoring proliferation, differentiation, and effector functions in stem and immune cell biology (5, 6). In vivo cell tracking using fluorescent dyes also provides information complementary to that provided by MRI using paramagnetic (7) or superparamagnetic (8, 9) particles or polymers. For example, MR-active cell tracking agents offer superior in vivo 3D imaging resolution compared with whole body fluorescence imaging, but current agents either do not allow tracking of cell division history (e.g., MR-active micro- and nanoparticles do not necessarily partition equally between daughter cells) or exhibit greater toxicity than fluorescent cell tracking dyes (10). Similarly, bioluminescent reporter gene imaging is ideal for very long-term tracking studies where proliferation of the labeled population may exceed the detection limit of traditional fluorescent dyes (typically seven or eight generations), because all progeny of stably transfected parental cells will contain the reporter gene (11). Many investigators have found it advantageous to combine genetic labeling with fluorescent cell tracking dyes in order to quantify the number of cells in each generation or assess the frequency of precursors from whence they arose, something that is not possible using genetic markers alone (12, 13). Given the multitude of colors available to choose from (1), it seems likely that methods for combining fluorescent cell tracking dyes with bioluminescent markers will also be developed.
Cell tracking using fluorescent protein and membrane dyes has also proven beneficial for in vitro studies of cytotoxic effector mechanisms (see Subheading 3.3), cell membrane transfer (14, 15), and cell proliferation history (1, 4, 13, 16, 17). In vitro studies of stem/progenitor and immune cell proliferation by flow cytometry are among the most common applications of both classes of cell tracking dyes (reviewed in (1, 5)). This is true largely because of the limitations of alternate methods for proliferation monitoring. Tritiated thymidine (3H-thymidine) incorporation is reproducible and sensitive. However, it presents significant safety and regulatory issues, is ill-suited for analysis of mixed populations at the single cell level, detects only cells actively synthesizing DNA at the time of the pulse, and does not allow for the isolation of daughter cells for further analyses such as immunophenotyping, gene expression, proteomics, or functional studies. Click-iT® EdU technology (available commercially from Invitrogen) detects the incorporation of a modified thymidine analog into replicating DNA under much milder conditions than labeling with bromodeoxyuridine (BrdU), can be detected using a variety of fluorochromes, and is compatible with single-cell analysis by flow cytometry (18). However, it also detects only cells actively synthesizing DNA at the time of the ethynyl-deoxyuridine pulse and, because detection requires mild permeabilization and fixation, is unsuitable for the isolation of viable daughter cells for functional studies.
In selecting fluorescent cell tracking dye(s) for a given study, it is essential to understand the advantages and limitations of different probes in order to match the probe(s) to the needs of the application. In our experience, key considerations include (1) the ability to achieve bright initial staining intensities without altering the expression or function of cellular machinery, or otherwise affecting the functional or proliferative capabilities of labeled cells relative to unlabeled controls; (2) stability of dye–cell association sufficient to ensure that probe is not lost from labeled cells due to degradation and does not transfer to unlabeled cells over the time frame of the assay; and (3) spectral compatibility with available instrument configuration(s) and other fluorochromes to be used. Ideally, the cell-labeling protocol should also be simple, rapid, and robust (i.e., readily reproducible both intra-experimentally and intra-institutionally). In this chapter, we illustrate how these considerations are addressed in the context of two immune function assays, as well as the advantages and limitations associated with combining multiple tracking dyes to increase the information available from a given assay. In particular, we discuss protocols for a direct LAK cytotoxicity assay using PKH67 and CellVue Claret, and an in vitro suppression assay that simultaneously monitors the proliferative capacities of regulatory and effector T cells using CFSE and CellVue Claret.
2. Materials
2.1. Cell Isolation and Cell Culture
Complete medium (CM). RPMI 1640 supplemented with 10% heat-inactivated fetal bovine serum (FBS) (Atlanta Biologicals, Lawrenceville, GA), 25 mM HEPES, 0.1 mM nonessential amino acids, 1 mM sodium pyruvate, 2 mM fresh glutamine, and 50 µg/mL gentamicin sulfate and 5 × 10−5 M β-mercaptoethanol.
Phosphate-buffered saline (PBS). Prepare 10× stock with 1.37 M NaCl, 27 mM KCl, 100 mM Na2HPO4, and 18 mM KH2PO4. Adjust to pH 7.4 with HCl if necessary. Sterilize by 0.2-µm filtration and store at room temperature. Prepare working solution by diluting one part with nine parts of tissue culture grade water.
10% Acid citrate dextrose (ACD, Sigma–Aldrich, St. Louis, MO) in PBS.
Formaldehyde, 10%, methanol free, ultra pure (Polysciences, Inc., Warrington, PA). Dilute to 2% in PBS (pH 7.4) and store at 4–8°C.
Hanks’ balanced salt solution (HBSS) without phenol red, magnesium- and calcium-free (Invitrogen). Store at room temperature until opened, then at 4–8°C.
Histopaque®-1077 (Sigma–Aldrich). Store at 4–8°C and use at room temperature.
IL-2 (Aldesleukin, Proleukin for injection, NDC 53905-991-01; Novartis, New York, NY). Dilute stock (2.2 × 106 IU/mL) in sterile HBSS to 1 × 105 IU/mL and store at −80°C. Do not refreeze after thawing; store at 4–8°C and discard thawed product after 7 days.
Phytohemagglutinin-HA (PHA) (Remel, Lenexa, KS). Prepare stock of 10 mg/mL in CM from powder, sterilize by 0.2-µm filtration, and store frozen at −80°C in single-use aliquots. To induce polyclonal T-cell proliferation, incubate human peripheral blood mononuclear cells (hPBMC) at a final PHA concentration of 5 µg/mL.
TRIMA filters (Trima Accel Collection System; CaridianBCT, Inc., Lakewood, CO). WBC-retaining filters were obtained from the pheresis facility at Roswell Park Cancer Institute, after informed consent from donors, and used to isolate hPBMC in quantity for some of the studies described here.
Versene, 0.48 mM EDTA·4Na in PBS (Mediatech, Manassas, VA). Adjust to pH 7.4 with HCl if necessary.
K562 cell line. Kind gift of Dr. Myron S. Czuczman, Roswell Park Cancer Institute, Buffalo, New York. Also available for purchase, #CCL-243; American Type Culture Collection, Manassas, VA.
2.2. Antibodies
Anti-CD3 (clone OKT3) and anti-CD28 (clone 28.2). Azide-free, unconjugated preparations, each at a concentration of 1.0 mg/mL (eBioscience, San Diego, CA).
Fluorochrome-conjugated monoclonal antibodies (mAbs). CD4 phycoerythrin cyanine 7 (PECy7, clone SK3), CD25 allophycocyanin (APC, clone 2A3), and CD127 phycoerythrin (PE, clone hIL-7R-M21) (all from BD Biosciences, San Jose, CA); CD45 Pacific Blue (PacBlue, clone HI30) (BioLegend, San Diego, CA).
2.3. Flow Cytometry Reagents
7-Aminoactinomycin D (7-AAD) (Invitrogen, Carlsbad, CA). Reconstitute powdered solid to 1 mg/mL in PBS and store at −20°C. Make a weekly working stock by diluting thawed 1-mg/mL stock to 100 µg/mL in PBS and store at 4–8°C. Add 4 µL of working stock to each 100 µL of cells (4 µg/mL final) and allow the cells to stand on ice for 30 min prior to data acquisition.
FCM buffer. PBS (pH 7.2) supplemented with 1% BSA, 0.1% sodium azide, and 40 µg/mL tetrasodium ethylenediaminetetraacetic acid.
Human IgG block. Reconstitute human IgG Cohn fraction II and III globulins (Sigma–Aldrich) to 12 mg/mL in RPMI 1640 supplemented with 25 mM HEPES, 20 µg/mL gentamicin sulfate, and 2 mg/mL BSA (Sigma–Aldrich). Store frozen at −20°C until use. Once thawed, store at 4–8°C for no longer than 1 month.
LIVE/DEAD® Fixable Violet (Invitrogen). Reconstitute with DMSO according to the manufacturer’s instructions. Store frozen at −20°C for no longer than 6 months. Thaw a fresh aliquot daily and dilute 1:50 in PBS. Add 5 µL per test and incubate for 30 min in a buffer free of exogenous protein before washing and fixing in 2% formaldehyde for assessment of viability by flow cytometry.
Spherotech AccuCount Ultra-Rainbow Fluorescent Particles (Spherotech, Lake Forest, IL). These are used for single platform enumeration of absolute cell numbers by flow cytometry as described in Subheading 3.3.
2.4. Cell Tracking Dyes
CellVue® Claret, PKH26, and PKH67 fluorescent cell linker kits (Sigma–Aldrich). The kits contain 1 mM of stock solutions in ethanol and cell-labeling diluent for general cell membrane labeling (Diluent C). CellVue Claret is also available from Molecular Targeting Technologies, Inc. (West Chester, PA). Store tightly capped at room temperature to avoid evaporation of ethanol and associated increases in dye concentration. If any dye solids are visible, sonicate the dye stocks to resolubilize before use and verify that dye absorbance remains within the range specified in the Certificate of Analysis available for each kit.
5-(and-6)-carboxyfluorescein diacetate, succinimidyl ester (CFDA-SE; Invitrogen). Reconstitute for cell labeling as described in Subheading 3.1. As the nonfluorescent CFDA precursor diffuses across the plasma membrane into the cytoplasm, its acetate substituents are cleaved by nonspecific esterases, forming the fluorescent amino-reactive product, carboxyfluorescein succinimidyl ester (CFSE).
2.5. Flow Cytometer and Other Equipment
For routine data acquisition, any flow cytometer capable of acquiring forward and side scatter, PacBlue, FITC, PE, PerCP/PECy5, PECy7, and APC would be appropriate. All of the data except for those presented in Figs. 2b, 4b, and 7 were acquired using an LSRII flow cytometer (BD Biosciences) fitted with a 25-mW 407-nm violet diode laser, a 20-mW 488-nm blue optically pumped semiconductor laser, and a 20-mW 635-nm HeNe laser. From the 407-nm laser, PacBlue or LIVE/DEAD Fixable Violet was detected using a 450/50-nm bandpass filter; from the 488-nm laser, CFSE or PKH67, PKH26, 7-AAD, and CD4 PECy7 fluorescence were detected using 530/30-, 575/26-, 685/35-, and 780/60-nm bandpass filters, respectively. CellVue Claret fluorescence was excited using the 633-nm line and collected using a 660/20-nm bandpass filter.
For sorting experiments, any flow cytometer capable of collecting peak area, width, and height and sorting based on forward and side scatter, PE, PECy7, and APC would be appropriate. The data shown in Fig. 7 were acquired on a FACSAria II flow cytometer (BD Biosciences) fitted with a 100-mW 355-nm solid state UV laser, a 100-mW 405-nm violet diode laser, a 50-mW 488-nm blue optically pumped semiconductor laser, and a 40-mW 639-nm red diode laser. From the 488-nm laser, CD127 PE and CD4 PECy7 fluorescence were detected using 575/26-nm and 780/60-nm bandpass filters, respectively. CD25 APC was excited using the 639-nm line and collected using a 660/20-nm bandpass filter.
A tube rotator (#13916-822; VWR, West Chester, PA) was used for monocyte depletion of hPBMC and preparation of accessory cells for the studies described in Subheading 3.4.
Fig. 2.
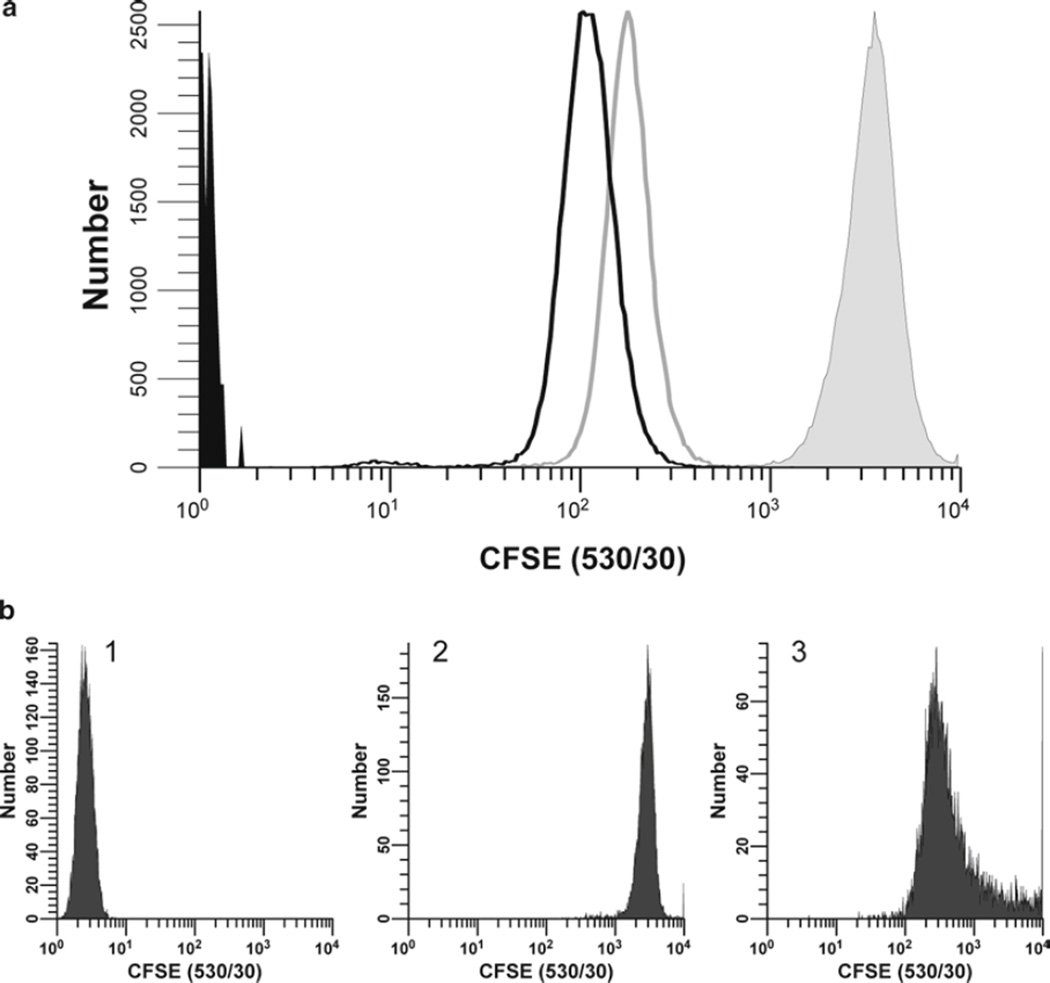
Additional factors affecting intensity and heterogeneity of CFSE labeling. (a) Proliferation-independent sources of intensity loss. hPBMC isolated from peripheral blood were stained with CFSE as described in Subheading 3.1 (final concentrations: 1 × 107 cells/mL, 5 µM CFSE). Unfixed samples were analyzed immediately post-staining (filled gray histogram; gMFI = 3,350, gCV = 35.2%) and after 24 h in culture at 37°C without stimulus; either unfixed (unfilled histogram, gray line; gMFI = 182, gCV = 33.0%) or fixed in 2% methanol-free formaldehyde (unfilled histogram, black line; gMFI = 109, gCV = 45.0%). Data were collected on an LSR II flow cytometer using a lymphocyte scatter gate and with CFSE detector voltage set so that the unfixed T = 0 day (T0) sample remained fully on scale in the last decade, a voltage at which the unstained T0 sample (filled black histogram) was not fully on scale in the first decade. Due to the substantial (>tenfold) proliferation-independent intensity loss characteristically seen during the first 24 h, T0 samples should not be used as compensation controls, and a stabilization period in culture is required before labeled cells are used for in vitro cytotoxicity or proliferation assays (see Notes 20–22). Further intensity losses due to fixation are less pronounced but must be taken into account when selecting optimal staining conditions if fixed samples are to be analyzed in batch mode (see Note 23). (b) Effect of staining conditions on CFSE fluorescence distributions. Replicate samples of logarithmically growing cultured U937 cells were stained at 1 × 107 cells/mL with 0.5 µM CFSE for 5 min at either 37°C with occasional mixing after dye addition or at ambient temperature without further mixing (see Subheading 3.1 and Note 16). Stained cells were washed and then analyzed on a CyAn flow cytometer (Beckman Coulter, Miami, FL) using constant instrument settings (HV = 351). Histogram 1: unstained control with CFSE detector voltage adjusted to place all cells on scale in the first decade, with few/no cells accumulating in the first channel. Histogram 2: cells labeled at 37°C with immediate mixing gave an ideal staining profile, with a bright, homogenously stained, symmetrical population falling in the fourth decade and very few cells accumulating in the last channel (gMFI = 2,817, gCV = 23.4%). Histogram 3: Cells labeled at ambient temperature without further mixing were suboptimally labeled at this relatively low concentration as evidenced by their dim, asymmetric, right skewed, staining pattern (gMFI = 468, gCV = 274%).
Fig. 4.
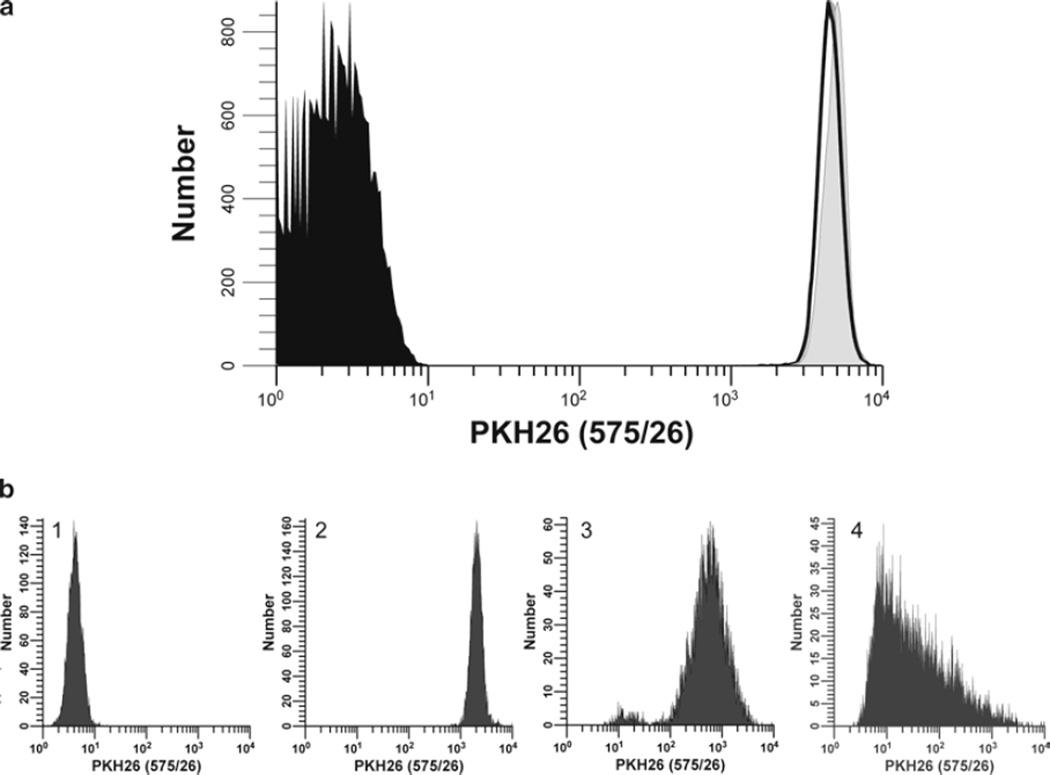
Additional factors affecting intensity and heterogeneity of PKH26 staining. (a) Stability of PKH26 intensity during the first 24 h of culture and after fixation. hPBMC isolated from peripheral blood were prepared and labeled with PKH26 in Diluent C as described in Subheading 3.2 (final concentrations: 5 × 107 cells/mL, 10 µM PKH26). Unfixed samples were analyzed immediately post-staining (T0) (filled gray histogram; gMFI = 4,874, gCV = 16.0%) and after 24 h in culture (T24) at 37°C without stimulus; either unfixed (unfilled histogram, gray line; gMFI = 4,557, gCV = 17.9%) or fixed in 2% methanol-free formaldehyde (unfilled histogram, black line; gMFI = 4,536, gCV = 17.6%). Data were collected on an LSR II flow cytometer using a lymphocyte scatter gate, and with PKH26 detector voltage set so that the unfixed T0 sample remained fully on scale in the last decade and the unstained control mostly on scale in the first decade. In contrast to CFSE (Fig. 2a), intensity and distribution differences between T0 and T24 fixed and unfixed samples were minimal. (b) Effect of staining conditions on PKH26 fluorescence distributions. Replicate samples of logarithmically growing, cultured U937 cells were stained with PKH26 (final concentrations: 1 × 107 cells/mL, 12.5–15 µM PKH26) for 3 min at ambient temperature, with or without trituration after addition of 2× cells to 2× dye (see Subheading 3.2 and Notes 29–31). Stained cells were washed and then analyzed on a CyAn flow cytometer using constant instrument settings (HV = 547). Histogram 1: unstained control with PKH26 detector voltage adjusted to place all cells on scale in the first decade, with few/no cells accumulating in the first channel. Histogram 2: staining with 15 µM dye by addition of 2× cells to 2× dye with immediate trituration resulted in a bright, homogenously stained symmetrical population of cells placed in the fourth decade, with no cells accumulating in the last channel (gMFI = 2,548, gCV = 26.2%). Histogram 3: staining with 15 µM dye by addition of 2× cells to 2× dye without immediate trituration resulted in a reduced intensity and a broader CV (gMFI = 505, gCV = 116%) as well as a dimly stained subpopulation, possibly due to a drop of cells dispensed down the wall of the tube and not well-mixed with the final staining solution. Histogram 4: a staining error led to 3 µL of concentrated ethanolic dye stock being added directly to 2× cells in Diluent C without further trituration rather than being used to prepare a 2× dye solution in Diluent C. This resulted in a final dye concentration of 12 µM but yielded extremely dim and heterogenous staining (gMFI = 32.9, gCV = 1,020%). The observed right skewing most likely reflects poor mixing due to the combined effects of widely disparate cell and dye volumes, lack of trituration, and the fact that cells closest to the dye-dispensing point would be exposed to a higher concentration of dye than those farther away.
Fig. 7.
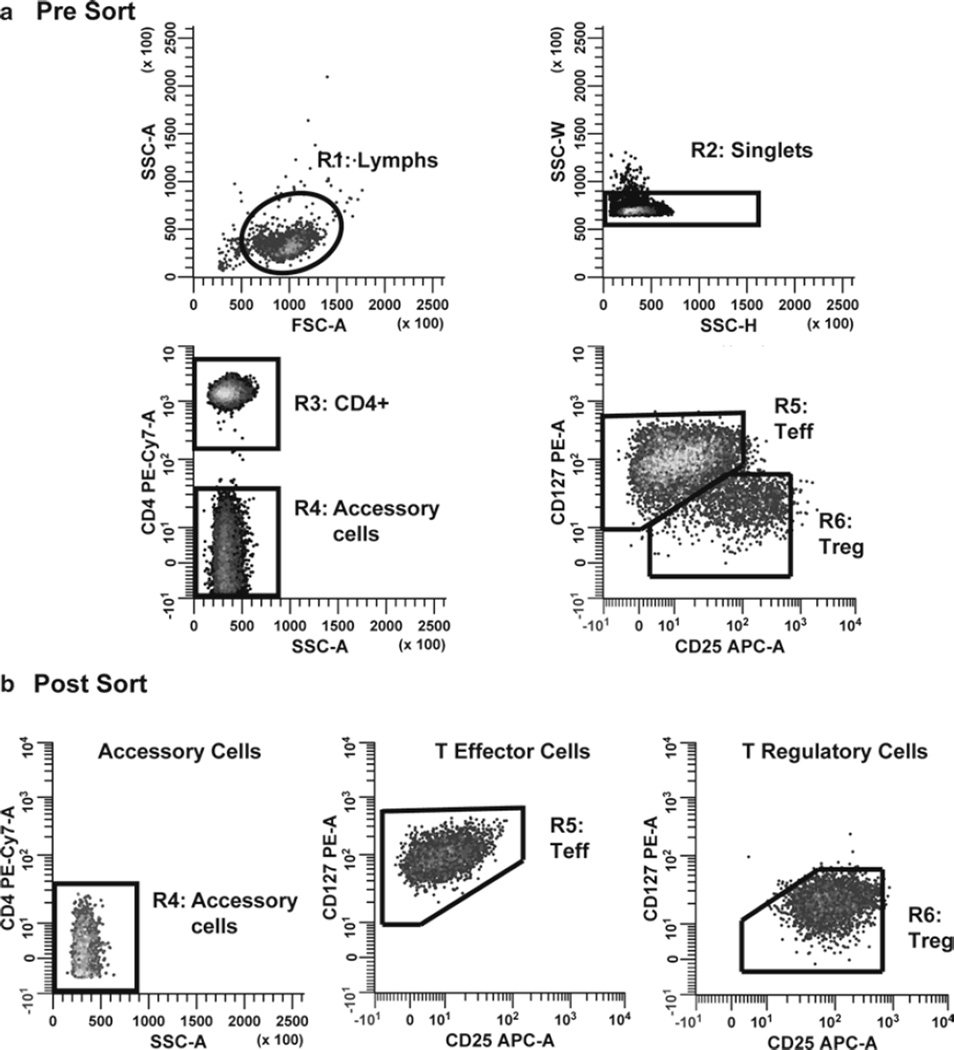
Sorting logic for Teff, Treg, and accessory cells. Monocyte-depleted lymphocytes prepared from TRIMA filters were stained with CD127 PE, CD4 PE-Cy7, and CD25 APC and then sorted into populations of Teff, Treg, and accessory cells to study the effects of Teff and Treg on each other’s proliferative response. (a) Histograms were sequentially gated by applying the lymphocyte scatter region (R1 on FSC vs. SSC plot) and side scatter-based multiplet exclusion criteria (R2 on SSC-H vs. SSC-W plot); R1 and R2 to the SSC-A versus CD4 PECy7-A plot; and R1, R2, and R3 to the CD25 APC-A versus CD127 PE-A plot. Teff cells were defined as CD4+ CD127bright CD25dim singlet lymphocytes. Treg cells were defined as CD4+ CD127dim CD25+ singlet lymphocytes. Accessory cells were defined as CD4− singlet lymphocytes. (b) After sorting, purity was assessed for each population and found to be greater than 97% in all cases.
3. Methods
Virtually any eukaryotic cell can be stained with either class of tracking dye after a single cell suspension has been obtained (see Notes 1 and 2). The labeling conditions described below have been successfully used to stain hPBMC and cultured cell targets used for the immune function assays discussed here, but are likely to require modification for other cell types, assay systems, or dye combinations (see Notes 3–5). Although CFSE is used herein to represent a typical protein-labeling dye, and PKH26, PKH67, and CellVue® Claret to represent typical membrane-labeling dyes, many other tracking dyes are available (see Note 6) and the principles described in Subheadings 3.1 and 3.2 also apply to optimization of conditions for use of those dyes.
3.1. hPBMC Staining with CFSE
Prepare a 5-mM stock solution of CFSE (MW 557.47 g/mol) in anhydrous DMSO (see Notes 7–9).
Wash cells to be labeled twice in serum-free PBS (or HBSS) and resuspend in serum-free buffer at a final concentration of 5 × 107 cells per mL (range 0.5–50 × 106 cells/mL; see Notes 5, 10, and 11), using a tube that will hold at least six times the volume of the cell suspension.
Immediately prior to cell labeling, prepare a 50-µM working CFSE solution by diluting the 5-mM stock solution of CFSE in DMSO from step 1 into PBS (see Note 12).
For a final staining concentration of 5 µM CFSE, add 100 µL of working CFSE solution per mL of cell suspension (e.g., for 2 mL of cells at 5 × 107 cells/mL, add 200 µL of 50 µM CFSE; see Notes 13–15).
Immediately vortex the tube briefly to disperse CFSE throughout the cell suspension. Incubate at ambient temperature (~21°C) for 5 min, with occasional mixing either manually or on a rotator (see Notes 16 and 17).
Stop the reaction by adding a 5× volume of CM (containing 10% FBS) or a 1× volume of FBS, and mixing well (see Note 18).
Wash the cells twice with 5–10 volumes of CM, centrifuge at 400 × g for 5 min at ~21°C, and discard the supernatant. After resuspension of the cell pellet for the second wash, remove an aliquot for cell counting. After the final wash, adjust the cell concentration to 5 × 105 cells/mL during the final resuspension in CM.
Assess recovery, viability, and fluorescence intensity profile of labeled cells immediately post-staining to determine whether to proceed with the assay setup (see Note 19 and Figs. 1 and 2).
At 24-h post-labeling, verify that labeled but non-proliferating cells (e.g., unstimulated control) are resolved well enough from unstained cells for purposes of the assay to be performed (Figs. 1 and 2) and that CFSE fluorescence can be adequately compensated in adjacent spectral windows used for measurement of other probes such as PE and RFP (see Notes 6 and 20–22). If samples are to be fixed and analyzed in batch mode, verify that loss of intensity due to fixation does not compromise the ability to distinguish desired number of daughter generations (see Note 23 and Fig. 2).
Verify that labeled cells are functionally equivalent to unlabeled cells (see Note 24).
Fig. 1.
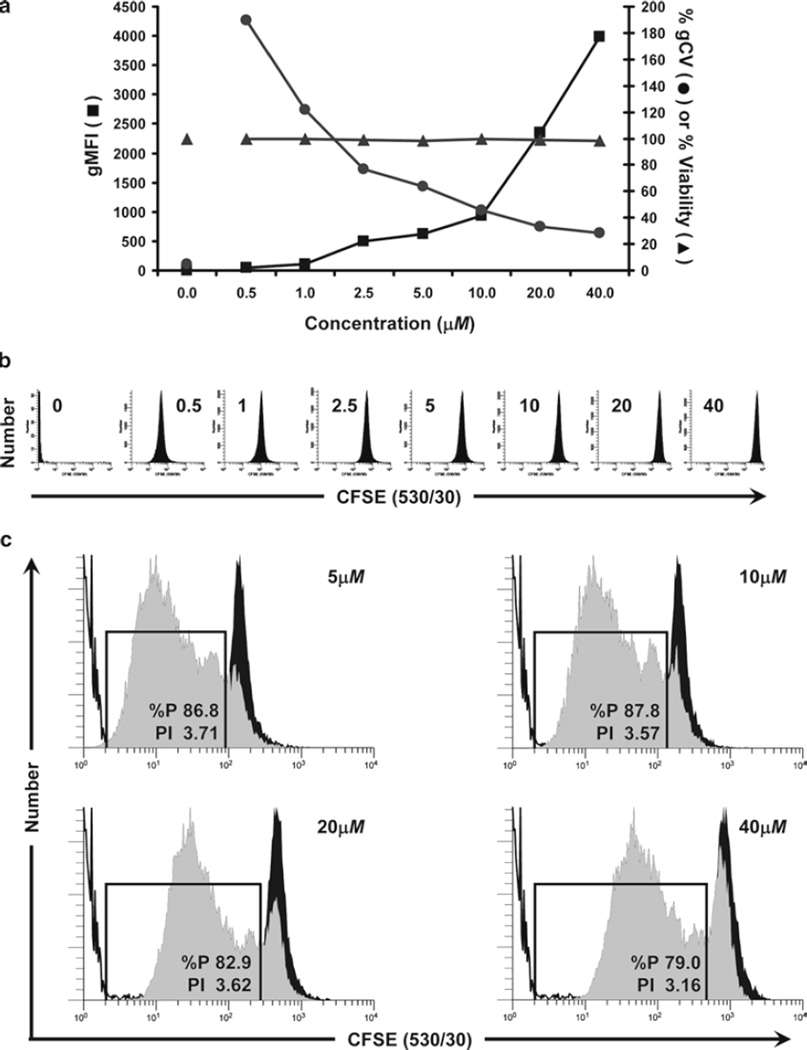
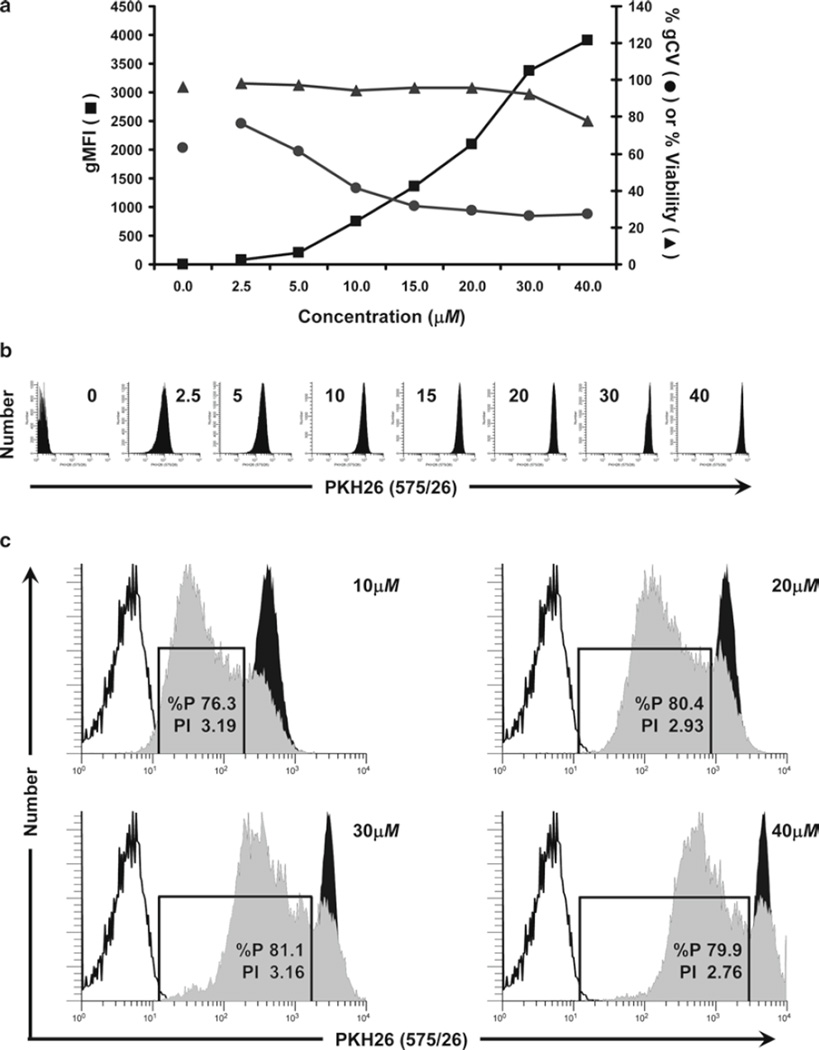
Considerations for optimization of hPBMC staining with CFSE. The optimal concentration for any tracking dye is that which yields cells that are as brightly and homogeneously stained as possible, while also exhibiting good viability, unaltered cell function, and the ability to compensate adequately for color overlap with other probes to be used. In this study, the maximum tolerated concentration of CFSE was determined for hPBMC isolated from peripheral blood, labeled as described in Subheading 3.1 (final concentrations: 5 × 107 cells/mL and 0.5–40 µM CFSE) and analyzed by flow cytometry immediately upon completion of staining (T0). (a) The relationships between dye concentration, initial staining intensity (geometric mean fluorescence intensity; gMFI), peak width (calculated as % gCV = geometric SD/gMFI × 100), and viability (% of cells able to exclude trypan blue) are shown. Viability was minimally affected at all concentrations of CFSE tested. Increasing CFSE concentrations led to increasing intensities (gMFI) and decreasing peak widths (gCV). (b) Individual histograms for each test sample shown in (a) were collected at a constant CFSE detector voltage, which was set so that the 40-µM sample remained fully on scale in the last decade. Note that at this voltage, unstained cells were not fully on scale in the first decade. (c) Samples stained with the indicated concentrations of CFSE were cultured in the presence of anti-CD3 and anti-CD28 for 4 days (gray histograms) and compared to controls that were CFSE stained but unstimulated (black histograms) or unstained (unfilled histograms) to determine the effect of CFSE concentration on proliferative potential (see Subheading 3.4.5 for description of methods for quantifying the extent of proliferation). Although post-staining viabilities were similar at all concentrations (Fig. 1a), both the proliferative fraction (% proliferating cells; %P) and the proliferative index (fold increase in cell number; PI) decreased at the highest concentration (40 µM) and %P also decreased at 20 µM, indicating that some inhibition of proliferation was occurring at higher concentrations of CFSE.
3.2. hPBMC Staining with PKH26, PKH67, or CellVue Claret® Membrane Dyes
Wash cells to be labeled twice in serum-free PBS or HBSS (see Note 5), using a conical polypropylene tube (see Note 25) sufficient to hold at least six times the final staining volume in step 5. After resuspension of the cell pellet for the second wash, remove an aliquot for cell counting (see Note 15) and determine the volume needed to prepare a 2× working cell solution at a concentration of 2 × 106 cells per mL in step 3 (range 2–100 × 106 cells/mL; see Table 1 and Note 26).
Following the second wash in step 1, aspirate the supernatant, taking care to minimize the amount of remaining buffer (no more than 15–25 µL) while avoiding aspiration of cells from the pellet (see Notes 27 and 28). Flick the tip of the conical tube once or twice with a finger to loosen/resuspend the cell pellet in the small amount of fluid remaining, but avoid significant aeration since this reduces cell viability.
To a second conical polyproplene tube (see Note 25), add a volume of Diluent C staining vehicle (provided with each membrane dye kit) equal to that calculated in step 1 for the preparation of the 2× cell solution. Prepare a 2× PKH26 or CellVue Claret working dye solution by adding the appropriate amount of 1 mM ethanolic dye stock to the Diluent C (e.g., add 2 µL of dye to 1.0 mL of Diluent C for a 2× working dye solution for a 2-µM working stock and a final dye concentration of 1 µM after admixture with 2× cells in step 5). Immediately triturate several times, then flick or gently vortex the tube to ensure complete dispersion of dye in the diluent, avoiding deposition of fluid in cap or as droplets on walls. Proceed with steps 4 and 5 as rapidly as possible (see Notes 29 and 30).
Prepare a 2× cell suspension by adding the volume of Diluent C calculated in step 1 to the partially resuspended cell pellet from step 2. Triturate three to four times to obtain a single-cell suspension and proceed immediately to step 5. Excessive mixing should be avoided since this reduces cell viability.
Rapidly admix the 2× cell suspension prepared in step 4 into the 2× working dye solution prepared in step 3, triturating three to four times immediately upon completion of addition in order to achieve as nearly instantaneous exposure of all cells to the same amount of dye as is possible (see Note 31).
After 3 min, stop the labeling by adding a 5× volume of CM (containing 10% FBS) or a 1× volume of FBS or other cell-compatible protein, and mixing well (i.e., if 1 mL of cells was combined with 1 mL of dye, then add 10 mL of CM or 2 mL of FBS) (see Note 32).
Centrifuge the stained cells (400 × g for 5 min at ~21°C) and then wash twice in CM. After the first wash, resuspend the cells and transfer them to a clean conical polypropylene tube (see Note 33). After the final wash, count and resuspend the cells to 1.5 × 106 cells/mL in CM.
Assess recovery, viability, and fluorescence intensity profile of labeled cells immediately post-staining to determine whether to proceed with assay setup (see Note 19 and Figs. 3 and 4).
Verify that labeled but non-proliferating cells (e.g., unstimulated control) are resolved well enough from unstained cells for purposes of the assay to be performed (see Figs. 3 and 4) and that membrane dye fluorescence can be adequately compensated in adjacent spectral windows used for measurement of other probes (see Notes 6, 34, and 35).
Verify that labeled cells are functionally equivalent to unlabeled cells (see Note 24).
Table 1.
Non-Perturbing Membrane-Dye Staining Conditions for Selected Cell Types
| Cell type | Final cell concentration | Final dye concentration | Reference(s) |
|---|---|---|---|
| hPBMCa(high cell #) | 1 × 107cells/mL | 2 µM PKH67 | (49) |
| 3 × 107cells/mL | 4 µM CellVue Claret | (45) | |
| 5 × 107cells/mL | 5 µM CellVue Claret | Fig. 6 | |
| hPBMCa(low cell #) | 5 × 106cells/mL | 2 µM PKH26 | (50) |
| 1 × 106cells/mL | 1 µM CellVue Claret | Figs. 8 and 10 | |
| Cultured cell lines | 1 × 107/mL | 15 µM PKH26 (U937) | Fig. 4b |
| 1 × 107/mL | 12.5–15 µM PKH26 (U937) | (41) | |
| 1 × 107/mL | 1 µM PKH67 (K562) | Fig. 5 | |
| 1 × 107/mL | 1 µM PKH67 (polyclonal T cell lines) | (15) | |
| 1 × 107/mL | 10 µM CellVue Claret (YAC-1) | E. Breslin, personal communication | |
A low-speed wash (300 × g) post-Ficoll–Hypaque was used to minimize platelet contamination (see Note 11)
Fig. 3.
Considerations for optimization of hPBMC staining with PKH26. In this study, monocyte-depleted lymphocytes isolated from TRIMA filters (Subheading 3.4.1) were labeled with the indicated concentrations of PKH26 in Diluent C as described in Subheading 3.2 (final cell concentration: 5 × 107/mL) to determine the maximum tolerated concentration. Cells were analyzed by flow cytometry immediately upon completion of staining (T0). (a) The effect of PKH26 concentration on staining intensity, CV, and viability was determined in a titration study similar to that described in Fig. 1a. Viability was minimally affected at concentrations up to 30 µM but decreased at 40 µM, most likely due to the ethanol vehicle present in the 1-mM PKH26 dye stock (final concentration in staining step: 4% at 40 µM). As with CFSE, increasing PKH26 concentrations yielded increasing fluorescence intensities and decreasing peak widths. (b) Individual histograms for each test sample shown in (a) were collected at a constant PKH26 detector voltage, which was set so that the 40-µM sample remained fully on scale in the last decade. Note that at this voltage, unstained cells were mostly on scale in the first decade. (c) Samples stained with the indicated concentrations of PKH26 were cultured in the presence of anti-CD3 and anti-CD28 for 4 days (gray histograms) and compared to unstimulated stained (black histograms) and unstained controls (unfilled histograms). The proliferative fraction (%P) was essentially identical at the three highest concentrations but slightly lower at the lowest concentration (10 µM) due to the overlap of highly proliferated cells with the unstained cell region. Proliferative index (PI) was somewhat reduced at the highest PKH26 concentration (40 µM), suggesting that the rate of expansion had decreased and that the proliferative potential as well as viability had been compromised.
3.3. Total Cytotoxicity: Quantitation of Cell-Mediated Killing Using Multiple Tracking Dyes
The radioactive chromium (51Cr)-release assay has traditionally been considered the gold standard for determining the cytolytic potential of effector cells (19–21). Although the assay is reliable, it has a number of disadvantages and functional limitations. The major disadvantage is the use of radioactivity, which is potentially hazardous and impractical for some laboratories. Other limitations include difficulty in labeling targets with 51Cr and the spontaneous release of 51Cr from targets, causing extremely high background levels, which makes resolution of effector-mediated lysis difficult. High backgrounds can be particularly problematic for longer term assays (18 h–10 days), which are sometimes required to detect low-frequency effectors (22) or to measure antibody-dependent cytotoxicity (23). The use of flow cytometry and cell tracking dyes to measure cytotoxicity does not require radioactivity and has the distinct advantage of being able to measure killing at the single cell level even when targets and effectors cannot be distinguished on the basis of light scatter. In the simplest format, target cells are labeled with a tracking dye and incubated for a period of time, after which viability is assessed by flow cytometry. However, a wide variety of in vitro and in vivo cytotoxicity assays have been described, in which different combinations of tracking dyes, viability probes, and antibody reagents are used to further characterize effectors, targets, and mechanisms of killing (6, 21–29). The protocol described here uses killing of a cultured cell line (K562) by lymphokine (IL-2) activated killer (LAK) cells as a model system, but the principles and general procedures are applicable to virtually any effector–target combination. In addition to illustrating that a new far-red cell tracking dye (CellVue Claret) does not alter LAK functionality, we discuss two different methods for measuring target cell death: (a) on a relative basis by determining percentage of targets deemed dead based on their inability to exclude 7-AAD, and (b) on an absolute basis by using counting beads to enumerate the number of viable target cells that remain when effectors are present versus when they are absent. The latter method is unaffected by cells lost due to complete lysis and, therefore, is particularly useful for longer term cytotoxicity assays.
3.3.1. Generation of Stained LAK Effector Cells
Prepare hPBMC from heparinized peripheral blood using the laboratory’s standard density gradient fractionation protocol, with the addition of a final low-speed wash step (300 × g) to minimize platelet contamination (see Note 11). Count and adjust to 1 × 108 hPBMC/mL.
Stain hPBMC with CellVue Claret at a final dye concentration of 5 µM and a final cell concentration of 5 × 107 cells/mL, according to the procedures described in Subheading 3.2 (see Note 19).
Assess recovery, viability, and fluorescence intensity profile of labeled cells immediately post-staining to determine whether to proceed with assay setup (see Note 19).
Resuspend labeled hPBMC in CM at 3 × 106 cells/mL (typically 5–10 mL total volume) and incubate upright in a T25 flask with 1,000 IU/mL of IL-2 at 37°C for 4 days to generate LAK effector cells. Set up a parallel flask of unstained hPBMC for use as assay and instrument setup controls (see Subheadings 3.3.3 and 3.3.4).
On day 4, harvest LAK effector cells, triturating to disperse any cell clusters into a single cell suspension. Wash once with 50 mL of CM, count, and resuspend at 1 × 107 cells/mL in CM.
3.3.2. Labeling K562 Target Cells
On day 4 of the LAK induction period, harvest logarithmically growing K562 targets (see Note 37). Wash twice with 50 mL of HBSS, count, and adjust to 2 × 107 cells/mL in Diluent C for staining.
Stain K562 cells with PKH67 at a final dye concentration of 1 µM and a final cell concentration of 1 × 107 cells/mL, according to the procedures described in Subheading 3.2 (see Note 38).
Assess recovery, viability, and fluorescence intensity profile of labeled cells immediately post-staining to determine whether to proceed with assay setup (see Note 19).
Wash PKH67-labeled K562 targets twice in 15 mL of CM. Count and adjust to 1 × 105 cells/mL in CM.
3.3.3. Cytotoxicity Assay
In a 96-well round-bottom plate, make triplicate serial 1:2 dilutions of the LAK effectors as follows: Pipet 200 µL of the stained LAK cell suspension into the first well, and 100 µL of CM into each of seven adjacent wells. Serially transfer 100 µL of LAK cells from the first well to the second, then from the second to the third, etc., ending with a transfer of 100 µL from the seventh well to the eighth well and removal of 100 µL of cell suspension from the eighth well.
Add 100 µL of stained K562 targets to each well, creating effector-to-target ratios of 100:1, 50:1, 25:1, 12.5:1, 6.2:1, 3.1:1, 1.6:1, and 0.8:1 (total volume per well: 200 µL).
Add 100 µL of targets and 100 µL of effectors to the target-only and effector-only wells, respectively, followed by 100 µL of CM (see Note 39). Incubate the plate at 37°C for 4 h (see Note 40).
After the incubation period has elapsed, label test wells directly in the 96-well plate with a saturating amount of anti-CD45 PacBlue on ice for 30 min (see Note 41).
Transfer the contents of each well into individually labeled 12 × 75 mm round-bottom tubes compatible with the laboratory’s flow cytometer. Wash each well with 200 µL of cold FCM buffer and transfer the wash fluid to the appropriate tube.
Wash each sample once with 3 mL of cold FCM buffer and resuspend in 150 µL of FCM buffer.
Add 8 µL of 7-AAD (100 µg/mL stock) and 50 µL of Spherotech enumeration beads (stock concentration ~1 × 106 beads/mL; final concentration in tube ~2.4 × 105 beads/mL) using reverse pipetting technique. Let the setup stand for 30 min on ice so 7-AAD can equilibrate before initiating acquisition of flow cytometric data.
3.3.4. Flow Cytometric Acquisition and Analysis
Establish appropriate voltage settings using autofluorescence and single color controls from Table 2 (see Notes 39, 41, and 42).
Using the single color controls from Table 2, adjust compensation settings according to your laboratory and/or instrument manufacturer’s standard procedures (see Note 39).
Acquire data on the flow cytometer using the gating strategy summarized in Fig. 5.
Calculate cytotoxicity using the method described in step 5 or 6 (see Fig. 6 and Notes 43 and 44).
-
Method 2: This alternative method uses a calculation comparable to the approach used in a standard 51Cr release assay, using regions R7 (singlet beads) and R8 (live K562 targets) defined on plots 4 and 8 of Fig. 5.For example, using the numbers of events obtained from the data shown in Fig. 5 gives the following result:
Table 2.
Recommended Assay and Instrument Controls for Measuring Cytotoxicity
| Cells | Label | Treatment | Comments | |
|---|---|---|---|---|
| Assay controlsa | LAK effectors only | Claret | Incubate with assay samples | Negative control: used to calculate spontaneous LAK cell death |
| K562 targets only | PKH67 | Incubate with assay samples | Negative control: used to calculate spontaneous K562 cell death | |
| K562 (heat killed) + LAK effectors | PKH67 Claret | Incubate with assay samples | Positive control: only appropriate for assessment by 7-AAD exclusion, not by bead enumeration | |
| LAK cells | None | Same E:T ratios as test samples | Staining control: used to verify that tracking dye-labeled cells kill equivalently to unlabeled cellse | |
| Instrument controlsb | K562 cells | None | None | Select voltage for LAK/Claret channelf |
| K562 cells | PKH67 | None | Select voltage for K562/PKH67 channel; set color compensation for all other channels; set negative region in LAK/Claret channel (Fig. 5, plot 3) | |
| K562 cells | CD45 PacBlue | None | Set CD45 threshold or gate to include both targets and effectors (K562 MFI < LAK MFI) | |
| LAK cells | Nonec | None | Select voltage for K562/PKH67 channelg | |
| LAK cells | Claret | None | Select voltage for LAK/Claret channel; set color compensation in all other channels; set negative region in K562/PKH67 channel (Fig. 5, plot 5) | |
| LAK cells | CD45 PacBlue | None | Color compensation | |
| LAK cells | 7-AAD | Heat killedd | Color compensation | |
Negative and positive assay controls are included in the experimental plate with test samples, or set up in parallel with the experimental plate, to verify that the expected biological outcomes can be recognized using the chosen instrument conditions
Instrument controls are used to establish instrument voltages and compensation settings
For unstained LAK cell controls, it will be necessary to set up a separate culture of unstained PBMC with IL-2 at the same time as the CellVue Claret-stained PBMC
To heat kill, incubate at 56°C for 30 min. K562 cells could also be used but light scatter properties after heat killing differ substantially from those seen after LAK killing
Needed only to establish optimized staining conditions for tracking dye when assay is first being implemented in the laboratory; not required on a routine basis
Use of unstained LAK to select high voltage for CellVue Claret detector would place unstained K562 cells midscale due to their much greater autofluorescence. Therefore, unstained K562 cells were used instead to maximize dynamic range
Use of unstained K562 to select high voltage for the PKH67 detector would place unstained LAK cells offscale low due to their much lower autofluorescence. Therefore, unstained LAK cells were used instead to maximize dynamic range
Fig. 5.
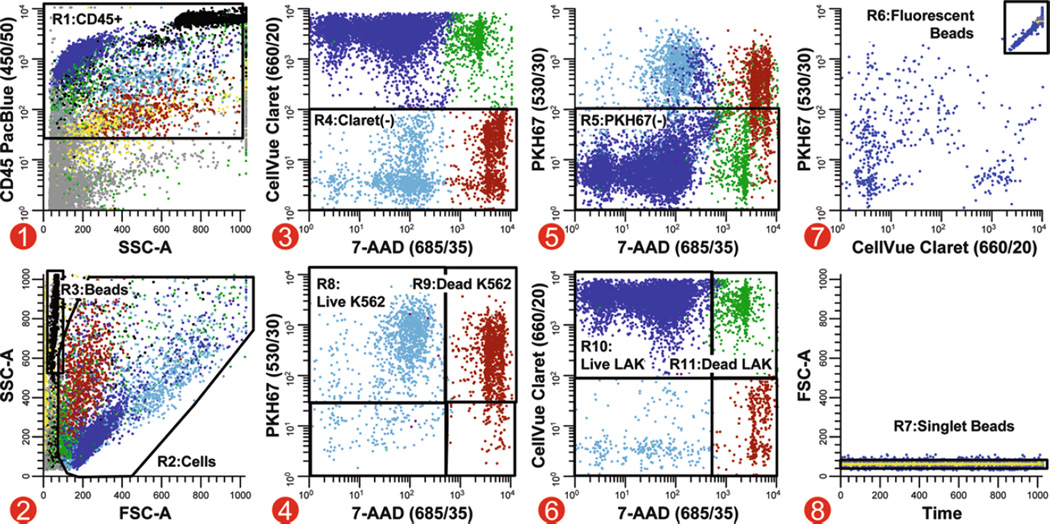
Analysis strategy for simultaneous quantitation of target and effector viability in a direct cytotoxicity assay. In this multicolor cytotoxicity assay, LAK effector cells stained with CellVue Claret (final concentrations: 5 × 107 cells/mL, 5 µM dye) and K562 targets stained with PKH67 (final concentrations: 1 × 107 cells/mL, 1 µM dye) were co-cultured at 37°C to assess LAK cell-mediated killing. Killing was assessed using two different metrics: (1) as % of targets able to exclude a viability probe (7-AAD) and (2) using counting beads to enumerate the number of viable target cells that remained when effectors were present versus when they were absent. Because these beads have very low forward scatter (R3, plot 2), it was not possible to set an acquisition threshold on this parameter and reliably ensure that all bead events were collected. Side scatter was, therefore, used as the thresholding parameter, with anti-CD45 PacBlue being used to include all leukocytes and beads (plot 1; see Note 41). A reciprocal gating strategy was applied to assess target and effector cell numbers and viability. A two-parameter plot of CellVue Claret versus 7-AAD fluorescence, gated on CD45+ events (R1, plot 1) with cell-like light scatter (R2, plot 2) that were not beads (not R6, plot 7), was used to identify target cells as events that were not CellVue Claret positive (R4, plot 3). Live versus dead target cells were then enumerated on a two-parameter plot of PKH67 versus 7-AAD fluorescence (plot 4, gated on R1&R2&R4 and not R6). Similarly, CellVue Claret-stained LAK cells were identified as PKH67 negative (R5) on a plot of CellVue Claret versus 7-AAD fluorescence (plot 5, gated on R1&R2 and not R6. This strategy was used because substantial differences in autofluorescence between targets and effectors (see Table 2) made it difficult to establish instrument settings that gave complete resolution between PKH67+ K562 targets and PKH67 negative (CellVue Claret+) LAK cells (plot 5), whereas much better resolution was possible between CellVue Claret + LAK cells and CellVue Claret negative (PKH67+) K562 cells (plot 3). Counting beads (R6), which exhibit broad-spectrum fluorescence (plot 7, gated on R3), were enumerated in plot 8 (gated on R3&R6) after gating on R7 to exclude doublets and larger aggregates. Extent of cytotoxic killing was assessed as described in Subheading 3.3.4, steps 5 and 6. Color codes for plots 1–6: blue = viable LAK effectors; green = nonviable LAK effectors; light blue = viable K562 targets; red-brown = dead K562 targets; black = enumeration beads; gray = noise/debris; yellow = very low forward scatter events.
Fig. 6.
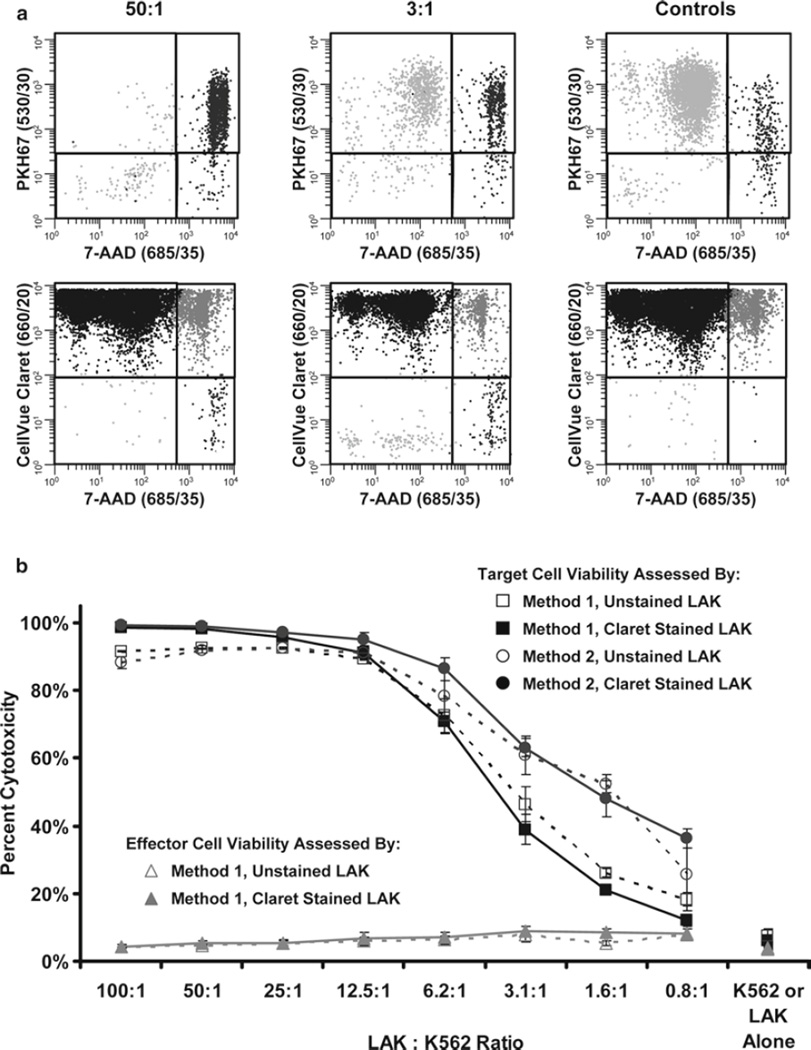
LAK cell-mediated killing of K562 targets is unaffected by staining with CellVue Claret. LAK cells were labeled with CellVue Claret and incubated with PKH67-labeled K562 cells at effector to target (E:T) ratios ranging from 100:1 to 0.8:1 for 4 h at 37°C. Test samples and controls (Table 2) were analyzed using the gating strategies described in Fig. 5. (a) Representative plots from test samples with 50:1 and 3:1 E:T ratios and from the K562 target only and LAK cell only controls. (b) LAK-induced cytotoxicity of K562 cells was assessed for each condition as described in Fig. 5 using either Method 1 (based on percent of target cells that took up 7-AAD, Subheading 3.3.4, step 5; squares) or Method 2 (based on number of viable target cells remaining in the presence versus absence of effectors, Subheading 3.3.4, step 6; circles). As an internal control, percentage of dead LAK effectors (triangles) was assessed by Method 1 at each E:T ratio and verified to be acceptably low and relatively constant. To determine whether CellVue Claret staining affected their cytolytic potential, parallel studies were performed using CellVue Claret-stained (solid lines) and unstained (dashes) LAK effectors. The data indicate that LAK cells kill K562 cells in a concentration-dependent manner and that the tracking dye did not affect function. Interestingly, Method 2 was slightly more sensitive at detecting target cell loss (Note 43). Representative data from one of two replicate experiments are shown; data points signify the mean ± 1 standard deviation of triplicate samples.
3.4. Tracking Proliferation: Inhibitory and Enhancing Effects of Treg and Teff Cell Interactions
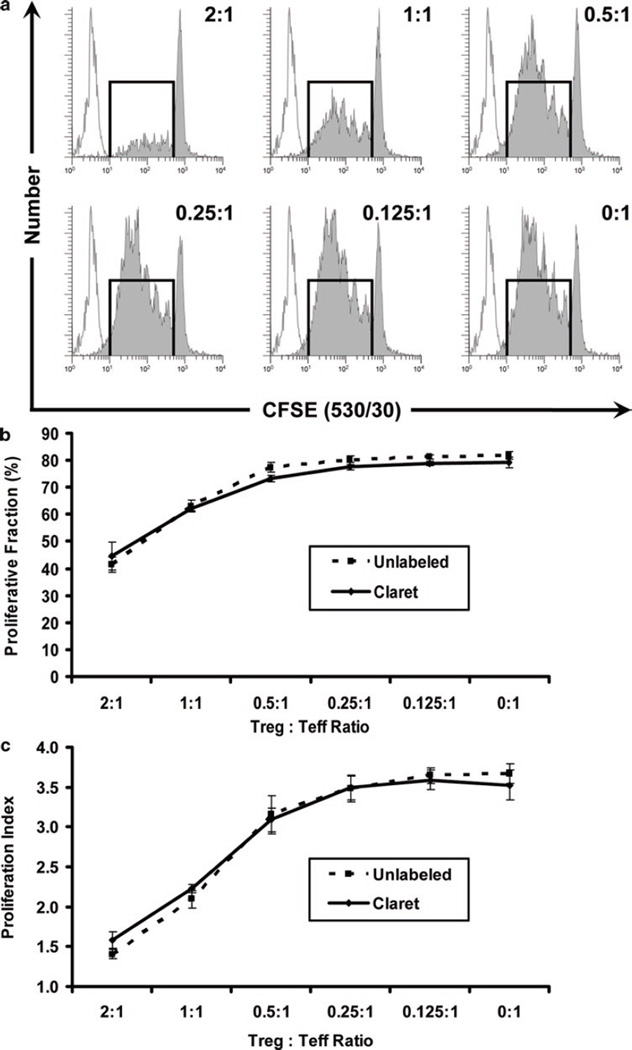
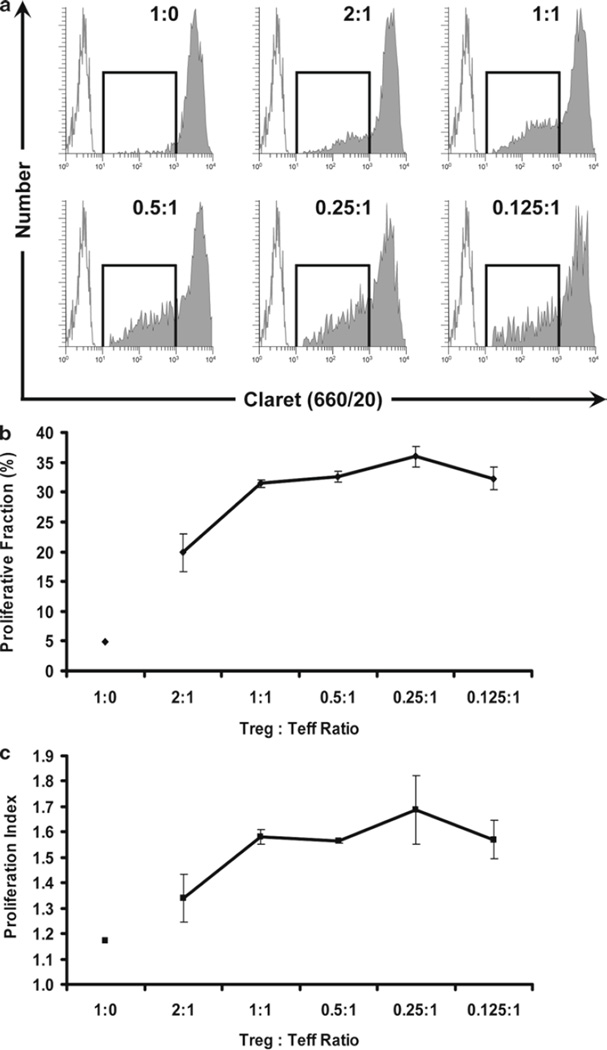
Regulatory T cells (Treg) exert potent immunosuppressive effects in autoimmune diseases, transplantation, and graft-versus-host disease (30), inhibiting proliferation of effector T cells (Teff) primarily by downregulating induction of their IL-2 mRNA (31). Phenotypically, Treg are defined by their co-expression of CD3, CD4, CD25, the transcription factor FOXP3, and dim expression of CD127, along with several other surface markers shared with activated T cells such as GITR and CTLA-4 (30, 32, 33). As reviewed by Brusko et al. (34), assays that use tracking dyes to monitor Treg suppression of anti-CD3 plus IL-2-induced effector T-cell (Teff) proliferation have significant advantages over in vitro suppression assays using 3H-thymidine, a standard measure of Treg activity. In particular, although they require approximately tenfold more cells, tracking dye-based assays reflect total Teff proliferation throughout the 4-day culture period rather than simply measuring DNA synthesis during the final hours of the response and can be extended to enable simultaneous monitoring of low level Treg proliferation as well (34). Our experience with a single-color in vitro suppression assay has been that it can be difficult to reliably distinguish highly proliferated CFSEdim Teff from unlabeled Treg, since both populations express similar levels of CD4. Use of a second tracking dye has the advantage of not only simplifying discrimination between Treg and CFSEdim Teff, but also allowing assessment of whether increasing numbers of Teff in the assay have any effect on Treg proliferation. In the variation described here, isolation of CD4+ Treg and Teff by sorting was combined with CFSE labeling of Teff and CellVue Claret labeling of Treg to ascertain the effect of Treg:Teff ratio on the proliferative response of each cell type (see Note 45). Parallel studies using unstained Treg confirmed that their ability to suppress Teff proliferation was unaltered by labeling with CellVue Claret.
3.4.1. Preparation of Monocyte-Depleted Lymphocytes (see Note 46)
Prepare TRIMA filtrate by draining TRIMA filter into a 50-mL conical tube, followed immediately by rinsing the filter with 40 mL of 10% ACD in PBS to dislodge trapped cells (35) (see Note 2).
Isolate hPBMC from the TRIMA filtrate using the laboratory’s standard density gradient fractionation protocol, with the addition of a final low-speed wash (300 × g) to minimize platelet contamination (see Note 11).
To separate lymphocytes from monocytes via cold aggregation (36, 37), resuspend hPBMC in 50 mL of cold CM and dispense 12.5 mL each into four 15-mL conical polypropylene tubes. Affix the tubes onto the fins of tube rotator and rotate along their horizontal axis, parallel to the benchtop, at 18 rpm at 4°C to induce monocyte aggregation (see Note 47). After 30–45 min, visible 1–3 mm aggregates will form that contain primarily monocytes.
Remove the tubes from the rotator and place vertically on ice for 15 min, permitting aggregated cells to precipitate at 1 × g to the bottom of each tube.
Harvest supernatant containing the monocyte-depleted lymphocytes, wash twice with cold HBSS, and use for isolation of Treg, Teff, and accessory cells (see Subheading 3.4.2 and Notes 48 and 49).
3.4.2. Isolation of Treg, Teff, and Accessory Cells by Flow Cytometry and Sorting (see Note 50)
Adjust monocyte-depleted lymphocytes from Subheading 3.4.1, step 5, to 5 × 107 cells/mL in HBSS and incubate for 10 min with 600 µg/mL of human IgG to block Fc receptor binding.
Add a mAb cocktail containing anti-CD127 PE, anti-CD4 PECy7, and anti-CD25 APC to the IgG-blocked lymphocytes and incubate on ice for 30 min (see Note 51).
Wash the cells twice with HBSS and resuspend at 1.5 × 107 cells/mL in HBSS.
Sort antibody-labeled cells on a fluorescence-activated cell sorter (e.g., FACSAria II or equivalent) into glass tubes containing CM at a rate that provides for purities of 95% or greater (see Note 52). The gating logic used to sort Treg, Teff, and accessory cells is illustrated in Fig. 7.
3.4.3. Proliferation Protocol
Stain sorted Treg with CellVue Claret (final cell concentration of 1 × 106/mL; final dye concentration, 1 µM) according to the procedures described in Subheading 3.2. Wash in CM, count, and adjust to 1 × 106 cells/mL.
Stain sorted Teff cells with CFSE (final cell concentration of 5 × 107/mL; final dye concentration, 5 µM) according to the procedures described in Subheading 3.1. Wash in CM, count, and adjust to 5 × 105 cells/mL.
In a 96-well round-bottom plate, make triplicate serial 1:2 dilutions of the Treg as follows: Pipet 200 µL of the stained Treg suspension into the first well and 100 µL of CM into an adjacent set of four wells. Serially transfer 100 µL of Treg from the first well to the second, then from the second to the third, etc., ending with the transfer of 100 µL from the fourth well to the fifth well and removal of 100 µL of cell suspension from the fifth well (see Note 40).
Add 100 µL of stained Teff to each well, creating Treg-to-Teff ratios of 2:1, 1:1, 0.5:1, 0.25:1, and 0.125:1 (see Note 53).
Add 50 µL of Treg and 100 µL of Teff cell to the Treg-only and Teff-only wells, respectively (see Notes 54 and 55).
Centrifuge sorted accessory cells (400 × g for 5 min at ~21°C), pool into a 50-mL conical tube, adjust to 1 × 106 cells/mL with CM, and irradiate with 3,000 rad of gamma irradiation to inhibit proliferation. After irradiation, adjust the concentration to 5 × 105 cells/mL in CM.
To an aliquot of accessory cells commensurate with the size of the experiment, add azide-free anti-CD3 (clone OKT3) to a final concentration of 3 µg/mL and anti-CD28 (clone 28.2) to a final concentration of 1.5 µg/mL. Add 0.1 mL of this preparation to each test well from step 4, yielding a final concentration of 1 µg/mL of anti-CD3 and 0.5 µg/mL of anti-CD28 in a final volume of 0.3 mL/well.
Add CM to bring each well to a final volume of 0.3 mL and incubate in a humidified 37°C incubator with 5% CO2 for 96 h (see Note 56).
After the 96-h incubation, remove the plate from the incubator and harvest cells from each well into individually labeled 12 × 75 mm round-bottom tubes compatible with the laboratory’s flow cytometer and place on ice. Rinse each well with 200 µL of cold HBSS, adding with the appropriate tube. QS each tube to 3 mL with HBSS.
Centrifuge at 400 × g for 5 min at ~21°C and resuspend each pellet in 100 µL of cold HBSS buffer, adding 10 µL of human IgG to block Fc receptor binding.
Incubate for 10 min on ice and then label with anti-CD4 PECy7 (clone SK3) and 5 µL of LIVE/DEAD® Fixable Violet reagent, diluted 1:50 from frozen DMSO stock (see Note 57).
Incubate for 30 min on ice and then wash two times with FCM buffer. Resuspend the cells in 300 µL of FCM buffer for flow cytometric analysis.
3.4.4. Flow Cytometric Acquisition and Analysis
Establish appropriate voltage settings using autofluorescence and single-color controls from Table 3 (see Notes 54 and 58).
Using the single-color controls from Table 3, adjust compensation settings according to your laboratory and/or instrument manufacturer’s standard procedures (see Notes 55 and 58).
Acquire data on the flow cytometer using the gating strategy shown in Fig. 8 (see Note 59).
Table 3.
Recommended Assay and Instrument Controls for Measuring Immune Cell Proliferation
| Cells | Label | Treatment | Notes | |
|---|---|---|---|---|
| Assay controlsa | Teff | CFSE | +acc, no stimulusd | Negative control: spontaneous Teff proliferation |
| Teff | CFSE | +acc + stimulus | Positive control: maximum Teff proliferation | |
| Treg | Claret | +acc, no stimulus | Negative control: spontaneous T regulatory proliferation | |
| Treg | Claret | +acc + stimulus | Define Treg proliferation | |
| Teff | CFSE | PHA | Positive control: verify that Teff are able to proliferate when cell surface receptors are bypassed | |
| acc | None | None | Optional: confirm that they remain CD4− throughout assay and will not be confused with highly divided Teff | |
| Teff | Varying CFSE | +acc + stimulus | Staining control: used during assay setup to verify that concentration of tracking dye chosen does not affect Teff proliferation (Fig. 1c)e | |
| Treg | None | +acc + stimulus | Staining control: used during assay setup to verify that tracking dye labeled cells proliferate equivalently to unlabeled cells (Fig. 9)e | |
| Instrument controlsb | Teff | None | Unstimulated, accessory only | Set voltage and negative region in CFSE channel |
| Teff | CFSE | Unstimulated | Set voltage in CFSE channel; set color compensation in other channels; estimate location of undivided Teff | |
| Treg | None | Unstimulated | Set voltage and negative region in Claret channel | |
| Treg | Claret | Unstimulated | Set voltage in Claret channel; set color compensation in other channels; estimate location of undivided Treg | |
| Teff (no Claret) | CD4 PE-Cy7 | Unstimulated | Set compensation | |
| acc + Teff (no CFSE)c | LIVE/DEAD Fixable Violet | Unstimulated | Set compensation | |
Assay controls are included in the experimental plate with test samples to verify that the expected biological outcomes can be recognized using the chosen instrument conditions
Instrument controls are used to establish instrument voltages and settings
Irradiated accessory cells will be nonviable and should be 100% positive for this viability probe
Acc = accessory cells (CD4 negative lymphocytes); stimulus = anti-CD3 plus anti-CD28
Needed only to establish optimized staining conditions for tracking dye when assay is first being implemented in the laboratory; not required on a routine basis
Fig. 8.
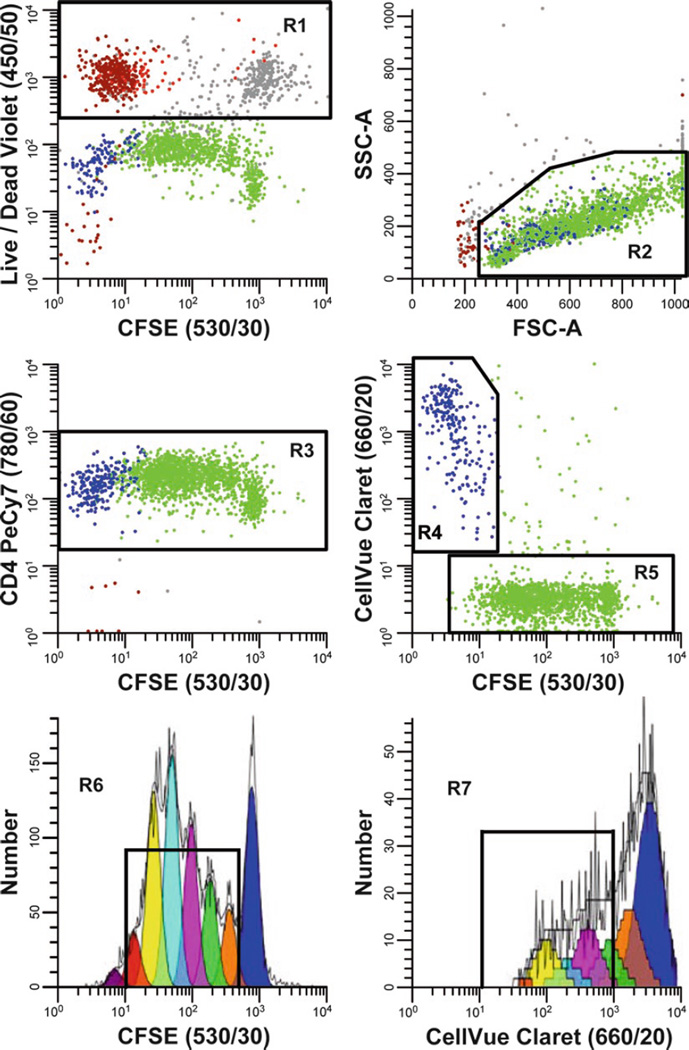
Simultaneous analysis of Teff and Treg proliferation during an in vitro suppression assay. After isolation by sorting as described in Fig. 7, Teff cells were stained with CFSE (final concentrations: 5 × 107 cells/mL, 5 µM dye) and co-incubated for 4 days with sorted Treg stained with CellVue Claret (final concentrations: 1 × 106 cells/mL, 1 µM dye) in the presence of anti-CD3, anti-CD28, and sorted, irradiated accessory cells (see Subheading 3.4.3 for details). Representative data are shown for one of three triplicate samples at a Treg:Teff ratio of 0.25:1. LIVE/DEAD Fixable Violet reagent was used to exclude dead cells (R1, upper left plot; in the electronic version: accessory cells = red-brown, nonviable Teff = gray and nonviable Treg = red) from all other data plots. CellVue Claret staining was used to distinguish viable Treg (R4, center right plot) from viable but highly proliferated Teff (R5, center right plot). A single parameter CFSE (530/30) proliferation profile for Teff (lower left plot) was generated by gating on cells that were CFSE + (R5), CD4+ (R3), viable (not R1), and had lymphocyte scatter properties (R2). A single parameter CellVue Claret (660/20) proliferation profile for Treg was generated by gating on cells that were CellVue Claret + (R4), CD4+ (R3), viable (not R1), and had lymphocyte scatter properties (R2). Note the generous lymphocyte region (R2) defined to include lymphocyte blasts. Proliferative fractions, representing the percent of cells that have undergone one or more divisions, were calculated as described in Subheading 3.4.5 (R6 = 78.6% and R7 = 37.6% for Teff and Treg, respectively). Proliferative indices (PI), representing fold expansion of Teff and Treg populations during the culture period, were calculated as described in Subheading 3.4.5 using Gaussians to model each of the generational peaks (e.g., in the electronic version: blue = parental generation, orange = first daughter generation, etc.).
3.4.5. Calculation of Proliferative Fraction and Proliferative Index
Either Proliferative Fraction (%P), a semi-quantitative estimate of percent proliferating cells, or Proliferative Index (PI), a more quantitative estimate of fold population expansion, may be used to analyze the extent of proliferation. In either approach, the starting point is a single parameter tracking dye dilution profile for the appropriate subpopulation of viable lymphocytes (here CFSE for Teff and CellVue Claret for Treg), created using the gating strategy described in Fig. 8.
Calculation of %P. To calculate %P, a stained, unstimulated control is used to set the upper boundary for enumeration of daughter cells, selecting an intensity that gives an acceptably low value for dividing cells in the absence of stimulus (e.g., 1–5%; see Figs. 9 and 10). An unstained control is used to define the lower boundary for the enumeration of proliferating cells, selecting an intensity that gives an acceptably low value for dividing cells in the absence of proliferation dye. %P is then defined as the percentage of proliferating cells with fluorescence intensity less than that of the stained but unstimulated control and more than that of the unstained control.
Calculation of PI. To calculate PI, a specifically designed peak-modeling software such as ModFit LT (Verity Software House, Topsham, ME), FCS Express (De Novo Software, Los Angeles, CA), or FlowJo (TreeStar, Ashland, OR) is used to fit the viable, lymphocyte-gated, single-parameter CFSE and CellVue Claret data. These programs use a nonlinear least squares analysis to find iteratively the best fit to the raw data by changing the position, height, and CV of each generational Gaussian. After loading and gating the histogram, users define the location of the parental generation, its spacing, and if necessary its SD. When modeling lipophilic dyes, an equal spacing between generations is assumed, whereas when modeling CFSE, an unequal spacing must be assumed to adjust for observed nonlinearities in peak spacing (possibly due to continued slow dye loss even after 24 h). The area under each Gaussian is taken as a measure of the relative number of cells in that generation and the sum of all Gaussians corresponds to the relative number of cells in the total population. These values are then used internally by the software to calculate the PI.
- Calculation of percent suppression. The degree of suppression observed when Treg cells are co-cultured with Teff cells is calculated using one of the two following methods:
-
Method 1This method is appropriate when the proliferation metric (Px) is %P or any other measure for which the value goes to 0 when the proliferative response is fully suppressed.
-
Method 2This method is appropriate when the Px is PI or any other measure for which the value goes to 1 when the proliferative response is fully suppressed (51).
-
Method 1
Fig. 9.
Inhibition of Teff proliferation by Treg cells. Teff cells were stained with CFSE and incubated with graded ratios of Treg cells in the presence of anti-CD3, anti-CD28, and accessory cells, as described in Fig. 8. The maximum proliferative potential of Teff was assessed in the absence of Treg cells (Treg:Teff ratio of 0:1). As increasing numbers of Tregs were added to the culture system, increasing inhibition of Teff cell proliferation was observed, as expected. Similar results were obtained with both CellVue Claret-stained (solid line) or unstained (dashed line) Treg, indicating that staining with the CellVue Claret tracking dye did not affect the potency of inhibition by Treg cells. Proliferative fraction (%P) and Proliferative Index (PI) were determined as described in Fig. 8 and Subheading 3.4.5. Data points in (b) and (c) represent the mean ± 1 standard deviation of triplicate samples. (a) Representative proliferation profiles for Teff at varying Treg:Teff ratios (filled histograms), showing increasing inhibition of proliferation at higher ratios. Unstained, unstimulated cells (unfilled histograms) are overlaid for reference. Stained, unstimulated cells largely overlapped with the stimulated parental population (not shown in this figure; see Fig. 1c). (b) Effect of Treg:Teff ratio on Proliferative Fraction of Teff. (c) Effect of Treg:Teff ratio on Proliferative Index of Teff.
Fig. 10.
Enhanced Proliferation of Treg cells at low Treg:Teff ratios. The use of a two-dye system allows for the discrimination of Treg and Teff from accessory cells and from each other, and the simultaneous measurement of proliferation in both subsets. In the same samples as shown in Fig. 9, the proliferation of CellVue Claret-stained Treg cells was monitored at different Treg:Teff ratios. Treg are generally anergic and, as expected, did not proliferate when incubated with anti-CD3, anti-CD28, and accessory cells in the absence of Teff cells (Treg:Teff ratio of 1:0). However, as the proportion of Teff in the cultures was increased (i.e., as the Treg:Teff ratio decreased), the extent of Treg proliferation also increased. Proliferative fraction (%P) and Proliferative Index (PI) were determined as described in Fig. 8 and Subheading 3.4.5. Data points in (b) and (c) represent the mean ± 1 standard deviation of triplicate samples. (a) Representative proliferation profiles for Treg at varying Treg:Teff ratios showing increasing proliferation at lower ratios (increased proportion of Teff in cultures) (filled histograms). Unstained, unstimulated cells (unfilled histograms) are overlaid for reference. (b) Effect of Treg:Teff ratio on Proliferative Fraction of Treg. (c) Effect of Treg:Teff ratio on Proliferative Index of Treg.
Acknowledgments
The authors have had the opportunity to work with many wonderful people on the development of these techniques over the years. In particular, they would like to acknowledge the technical and intellectual contributions of Bruce Bagwell (Verity Software House), Drew Bantly (University of Pennsylvania), Nadège Bercovici (IDM), Lizanne Breslin (PTI Research and SciGro), Jan Fisher (Dartmouth Medical School), Alice Givan (Dartmouth Medical School), Brian Gray (Molecular Targeting Technologies), Jonni Moore (University of Pennsylvania), Betsy Ohlsson-Wilhelm (SciGro), Feng Qian (Roswell Park), Earl Timm, Jr. (Roswell Park), and Mary Waugh (Dartmouth Medical School). They would also like to thank the Bowdoin class of 2006 from the Annual Courses in Flow Cytometry (Research Methods and Applications) who generated the data for Figs. 2b and 4b.
Flow cytometry was performed at Roswell Park Cancer Institute’s Flow Cytometry Laboratory, which was established in part by equipment grants from the NIH Shared Instrument Program, and receives support from the Core Grant (5 P30 CA016056-29) from the National Cancer Institute to the Roswell Park Cancer Institute.
Footnotes
Lymphocytes and monocytes are typically isolated from anticoagulated blood using standard Ficoll-Hypaque density centrifugation techniques prior to labeling, but cryopreserved PBMCs, adherent cell lines (harvested using trypsinization), and non-adherent lines are also suitable for staining. Cells may be labeled while adherent by flooding the culture dish or flask with dye solution. However, this typically gives considerably more heterogeneous intensity distributions, especially for membrane dyes (38), and makes their interpretation in dye dilution proliferation assays more complex. Labeling of single cell suspensions is, therefore, generally preferred.
Labeled cells are typically placed back into culture for in vitro assays or injected into animal models for in vivo functional studies. Standard sterile technique should, therefore, be followed throughout the labeling protocols described in Subheadings 3.1–3.4.
Amount of dye required for bright but nontoxic staining will in general increase as total number and/or size of cells to be stained increases. However, exact concentrations resulting in over-labeling and loss of function will vary depending on cell type and class of tracking dye used (e.g., Table S1 in (1)). Therefore, appropriateness of final cell concentration and final tracking dye concentration used for labeling should always be verified by comparing viability and functionality of labeled versus unlabeled cells. Similarly, both cell and dye concentrations used for labeling should be reported in any publication.
Total number of cells to be stained will depend on the number of replicates and controls required by the experimental protocol. Staining intensities are most easily reproduced when staining is done in volumes ranging from 0.5 to several milliliters. Once an approximate cell concentration has been established based on these factors, a preliminary dye titration experiment is recommended to determine (or verify) the optimal concentration of the tracking dye (39, 40).
Exogenous protein reduces labeling efficiency for both protein and membrane dyes and is, therefore, normally removed by washing the cells with a protein-free buffer such as PBS or HBSS prior to staining. However, when labeling must be done at relatively low cell concentrations due to limited number of cells or other experimental concerns, addition of exogenous protein may aid in avoiding over-labeling and resultant loss of cell viability or functionality (see Note 10 and (4)).
Selection of proliferation tracking dye(s) for a given study is typically based on spectral compatibility with other fluorochromes to be used in the study (1, 41), and the ability to achieve acceptable starting intensity without adverse effect on function (1, 4, 42–44). CFSE, PKH26, and PKH67 can be excited using a 488-nm laser line (absorption maxima: 492, 551, and 490 nm, respectively) and have emission maxima of 516, 565, and 504 nm, respectively. CellVue Claret (absorption maximum: 654 nm) can be excited using red diode or HeNe laser lines at 633–635 nm or a 647-nm Kr laser line and has an emission maximum of 677 nm.
CFSE is highly lipophilic but poorly soluble in ethanol or other polar organic solvents. If CFSE is purchased as a bulk powder, it should be accurately weighed out and made up as a 5-mM stock solution (MW 557.47 g/mol) in freshly opened anhydrous DMSO. Although the entire contents of dye vial can be dissolved in a calculated volume of DMSO (4), the final dye concentration should be confirmed spectrophotometrically (e.g., by absorption at 490 nm) and adjusted as needed for consistency, since exact weights contained may otherwise vary sufficiently from vial to vial to require re-titration of new versus old dye stocks in order to avoid toxicity (E. Tenorio, personal communication).
Aliquots of 5 mM CFSE dye stock in DMSO can be stored in a dessicator at −20°C for several months but repeated freezing and thawing of a given aliquot should be avoided since DMSO is hygroscopic and takes up moisture from the air. The presence of water leads to reduced labeling efficiency due to hydrolysis of both the diacetate ester moieties required for entry into cells and the succinimidyl ester moieties required for covalent reaction with amino groups under physiologic conditions.
If the necessary weighing or spectrophotometric instrumentation is not available in the laboratory, fresh 5 mM stock may be prepared for use in step 1 of Subheading 3.1, by adding anhydrous DMSO to commercially available single-use vials containing pre-weighed amounts of CFSE. However, it should be noted that cost per milligram of dye is greater for such vials than for dye purchased in bulk powder form.
When staining cells at concentrations <1 × 107/mL, inclusion of exogenous protein (e.g., 5% v/v FBS or 1% v/v serum albumin) in the resuspension buffer is suggested to avoid over-labeling and loss of cell function (see Notes 5 and 14). Resuspension in a serum-free culture medium will also reduce labeling efficiency and potential for over-labeling, due to the presence of free amino acids that compete for reaction with CFSE. Alternatively, if addition of exogenous protein must be avoided due to other experimental considerations, the working stock of CFSE prepared in Subheading 3.1, step 3 may be further diluted in buffer prior to initiation of cell labeling in Subheading 3.1, step 4. The time between initial dilution and initiation of cell labeling should be minimized since hydrolysis begins immediately upon dilution of the DMSO stock into aqueous solution and proceeds very rapidly.
Platelets present in variable amounts act as “hidden” sources of added protein or membrane that can affect labeling efficiency even when hPBMC and dye concentrations are carefully reproduced. Addition of a final low-speed wash step (5 min at 300 × g) minimizes platelet contamination of hPBMC and improves consistency of staining with both protein- and membrane-labeling dyes.
Ensure that the 5 mM CFSE stock in DMSO is completely thawed prior to preparation of the working stock, but minimize the length of time that the DMSO stock is exposed to ambient conditions to limit the uptake of moisture. The CFSE working stock solution should be clear and colorless. If there is any sign of yellowing, it should not be used, since this indicates conversion to carboxyfluorescein, the charged fluorescent hydrolysis product which will not enter cells.
This concentration was chosen such that following a 24-h stabilization period, the fluorescence intensity of non-dividing lymphocytes should fall within the third and fourth decade of a four-decade log amplifier when unstained cells are placed in the first decade (see also Notes 20–22).
For an hPBMC concentration of 1 × 107/mL, staining at a final concentration of 0.5–1.0 µM CFSE is recommended to avoid over-labeling. CFSE labels proteins indiscriminately and if the function of critical residues is modified by labeling, it can interfere with signal transduction pathways, proliferation, and other cell functions even when cell viability remains acceptable (see Table S1 in (1)). More extensive labeling increases the likelihood of altered cell function(s) and the extent of labeling is a function of dye concentration, cell concentration, labeling time, and labeling conditions (temperature, mixing, etc.). Concentrations given here for both cells and dye should, therefore, be taken only as a starting point and verified in each user’s experimental system.
Obtaining reproducible starting intensities from study to study requires accurately reproducing both dye and cell concentrations. Therefore, cell counting using a Coulter Counter or other automated cell counter rather than manual counting using a hemocytometer is recommended, since results of replicate hemocytometer counts often vary by as much as 15–20%.
Since uptake of CFSE into cells and reaction with free amino groups occur very rapidly, it is critical to disperse the dye solution quickly and evenly throughout the cell suspension immediately after addition.
Once formed by hydrolysis, carboxyfluorescein is sensitive to photobleaching. Therefore, covering with aluminum foil or placing in a dark location is recommended to protect tubes or wells containing CFSE-labeled cells from exposure to high intensity light or prolonged exposure to room light.
Inclusion of protein in the stop solution is essential, since it reacts with and inactivates free CFSE. Free amino acids in culture medium further aid in the inactivation. Alternatively, PBS or HBSS containing 1–2% serum albumin may be used as a stop solution.
For starting cell numbers of 107 or more, recoveries of at least 85% and viabilities of at least 90% should be obtained for freshly drawn hPBMC (e.g., Fig. 1a and Table 3 in (45)). However, recoveries typically decrease at lower cell numbers and may also be lower for preparations in which the cells are older or have been subjected to other stresses (e.g., pheresis, elutriation, or cryopreservation and thawing). Staining intensity and CV will vary for different cell types, but a bright symmetrical fluorescence intensity profile coupled with poor recovery and/or viability usually indicates substantial overlabeling and the need to increase cell concentration, decrease dye concentration, or both. Conversely, heterogeneous and/or dim staining (<2 log separation from unstained control) coupled with good recovery and viability suggests under-labeling and the need to decrease cell concentration, increase dye concentration, or both.
The great majority of cell-associated CFSE is lost within the first 24–48 h as cells export and/or degrade unreacted dye and labeled proteins/peptides that are short-lived, damaged by the covalent labeling process, destined for secretion, etc. This typically results in (a) up to a 1-log decrease in mean fluorescence intensity and (b) an approximately 5–10% decrease in CV, since T0 intensities reflect primarily variations in cell size, whereas T24–48 intensities reflect primarily variations in cellular content of stable long-lived proteins (see Fig. 2a).
Some labeling protocols recommend a washout period in which freshly stained cells are incubated in CM at 37°C for 30–60 min to hasten fluorescence stabilization. Our experience has been that a relatively short washout period of this type is not sufficient to prevent transfer of exported dye to unlabeled cells present in a co-culture, suggesting that labeled cells continue to export labeled proteins beyond the washout period (41). Therefore, at least a 24-h stabilization period in culture is recommended before labeled cells are used for in vitro cytotoxicity or proliferation assays. For in vivo cytotoxicity or proliferation assays, if labeled cells are to be reinfused within less than 24 h post-staining, it is critical to include control animals in which minimal killing or proliferation is expected in order to verify that ex vivo labeling conditions resulted in adequate resolution between stably labeled cells and unlabeled cells.
T0 CFSE-labeled samples are NOT appropriate for use as compensation controls because they are so much brighter than T24 samples that they typically cannot be run on the same intensity scale when unstained cells are placed within the first decade (see Note 20 and Fig. 2a). For proliferation assays based on CFSE dye dilution, it is, therefore, critical to select a staining concentration that gives adequate separation from unstained cells at 24 h without unacceptable fluorescence overlap into spectral channels used to detect other reagents. It is also essential to ensure that sufficient cells are prepared to provide unstimulated T24 samples for use as compensation controls. For an example of typical controls to set up for a proliferation assay, see Table 2 in ref. (45). Inability to achieve adequate color compensation for a CFSE-labeled but unstimulated (i.e., non-proliferating) T24 sample indicates the need to reduce CFSE concentration, increase cell concentration, or both during the staining step, while recognizing that this may reduce the number of daughter cell generations that can be resolved from unstained cells.
Fixation of CFSE-labeled cells in EtOH (46) or methanol-free formaldehyde (Fig. 2a) leads to further loss of cell-associated CFSE (30–50% decrease in fluorescence intensity), most likely due to leakage of small but stably labeled peptides or proteins out of the cells as membrane permeabilization occurs. Fortunately, the decrease from fresh to fixed cells does not appear to affect the shape of dye dilution profiles, which can therefore still be used to deduce cell proliferation history so long as the weaker fluorescence of fixed cells does not compromise the ability to resolve the desired number of generations from unstained cells.
For dye dilution proliferation assays using hPBMC (or PBMC from other species), it may be necessary to use an independent method such as 3H-thymidine incorporation (see Table S1 in (1)) to verify that cell function is unaltered by labeling with tracking dye at the chosen concentration.
Like most membrane-intercalating dyes, PKH26, PKH67, and CellVue Claret contain not only aromatic chromophores but also lipophilic alkyl tails, all of which readily adsorb to the walls of polystyrene tubes or plates. This can substantially reduce labeling efficiency, particularly when working at relatively low dye concentrations (2 µM or less). Use of polypropylene tubes is strongly recommended to minimize adsorptive dye loss and maximize reproducibility of labeling.
The PKH and CellVue dyes are incorporated into membranes based on strong hydrophobic forces that drive partitioning from the aqueous phase, in which the dyes are highly insoluble, into cell membranes, where they remain stably intercalated due to noncovalent interactions with the lipid bilayer. This means the final staining intensity is strongly affected by both the amount of dye and the amount of membrane present in the staining step. As shown in Table 1, dye concentrations required for bright but nonperturbing staining, therefore, vary with cell type/size as well as cell concentration, making it important to publish and accurately reproduce both dye and cell concentrations in order to obtain reproducible results.
Membrane-intercalating dyes are even more lipophilic than CFSE due to long chain alkyl moieties that mediate stable insertion into the membrane bilayer. Although the dyes are modestly soluble in polar organic solvents such as ethanol, the alkyl chains tend to self-associate in aqueous solutions, reducing membrane-labeling efficiency. The presence of salts substantially increases the extent of micelle and aggregate formation and decreases general membrane-labeling efficiency, although phagocytic cells can become differentially labeled by taking up dye aggregates. Use of conical rather than round-bottom tubes is highly recommended, since this facilitates more complete removal of salt-containing media or buffers prior to cell labeling.
The technique used to remove supernatant prior to resuspension in Diluent C or during wash steps can, in our experience, substantially impact both quality of staining and cell recovery when labeling with membrane dyes. Tube inversion and blotting typically leaves ~100 µL of supernatant, leading to significant salt remaining in Subheading 3.2, step 2 and reduced labeling efficiency in Subheading 3.2, step 5. Subsequent aspiration to reduce the amount of fluid risks loss of cells at the top of the pellet that have been loosened as the fluid drains back down the side of the tube. The best method that we have observed for maximizing fluid removal while minimizing cell loss is to fit a sterile disposable 200-µL pipette tip at the end of a vacuum aspirator, which reduces the aperture size and makes it easier to position the point of suction accurately relative to the cell pellet.
The fact that the PKH and CellVue dyes label cells by partitioning into membranes has two other significant consequences beyond the negative effect of salts on staining efficiency. First, staining occurs even more rapidly than for CFSE, with the partitioning of dye into membranes being essentially complete within 15–30 s after admixing 2× cells and 2× dye solutions. Second, to obtain bright, homogeneous labeling, it is critically important that admixing of cells and dye be done so that all cells are exposed to the same concentration of dye at the same time. This is much more easily achieved by admixing similar volumes of dye and cells than by trying to disperse a small volume of ethanolic dye into a much larger volume of cell suspension (see Fig. 4b).
Diluent C is an aqueous isotonic, iso-osmotic, salt-free staining vehicle that contains neither organic solvents nor physiologic salts. Although Diluent C is designed to maximize dye solubility and minimize cell toxicity for short periods (5–15 min), the longer cells and dye stand in Diluent C, the more likely that (a) staining efficiency will decrease due to dye aggregation and (b) decreased cell viability or function may result from lack of physiologic salts. Subheading 3.2, steps 2–5 should, therefore, be completed in as short a period as possible, preferably 1–2 min. When multiple samples are to be labeled, it is highly recommended that processing through the first wash of Subheading 3.2, step 7 be completed for each sample before the next sample is stained. However, remaining steps may then be carried out in parallel for all samples.
In theory, results should be the same whether 2× cells are admixed with 2× dye or vice versa. However, our experience has been that adding cells to dye makes it easier to reliably obtain homogeneous staining. Adding dye to cells runs the risk that any cells on the wall of the tube may remain unstained if they are above the level of admixed solution or stain at lower intensity if they do not mix with bulk staining solution until after trituration is completed (see Fig. 4b).
Some literature protocols suggest the use of CM, which contains 10% FBS, as the stop reagent. However, our experience has been that use of neat FBS results in more efficient removal of unbound dye (due to its higher protein concentration) and also reduced likelihood of forming dye aggregates large enough to sediment with cells during subsequent wash steps (due to its lower ionic strength). If CM is used, a larger volume (5× rather than 1×) is recommended to ensure sufficient protein is present to adsorb all unbound dye.
Even when polypropylene tubes are used (see Note 25), some dye adsorption to tube walls may occur. Therefore, washing efficiency is improved if cells are transferred to a fresh polypropylene tube after aspiration of the stop solution and resuspension of the cell pellet for the first wash in Subheading 3.2, step 6. This is particularly important if CM is used as the stop reagent (see Note 32), since carryover of dye particles may result in inadvertent labeling of other cell types present in the culture.
In contrast to CFSE, the fluorescence intensity of PKH26, PKH67, or CellVue Claret labeled cells does not decrease significantly in the absence of cell proliferation, and the intensity is also stable to fixation with neutral methanol-free formaldehyde (e.g., 1–2% paraformaldehyde dissolved in PBS). A small aliquot of cells fixed at T0 can, therefore, be used as a compensation control to evaluate the overlap of membrane dye signal into spectral regions used to detect other reagents. For an example of typical controls to set up for a lymphocyte proliferation assay, see Table 2 in ref. (45). As with CFSE, the inability to achieve adequate color compensation indicates the need to reduce dye concentration, increase cell concentration, or both, during the staining step (see Note 22).
Paradoxically, our experience and that of others suggest that while over-staining with membrane dyes may not reduce viability or functionality, it can sometimes result in increased T24 fluorescence intensity compared with T0 intensity, either in the absence (see Fig. 1 in (1) and Fig. 1 in (45)) or presence (B. Lahti, personal communication) of cell proliferation. The most likely explanation is stacking and self-quenching of dye in the plasma membrane at T0 that is relieved as the dye redistributes into intracellular membranes via normal membrane trafficking mechanisms (38). Dye dilution appears to proceed linearly with cell division once stacking/quenching is relieved, presumably because total number of molecules per cell does not change, and membrane dyes are typically pH insensitive in physiologic ranges. However, for assay systems where proliferation monitoring is to be initiated immediately (e.g., continuously growing cell lines or transfectants), cell and/or dye concentration(s) in the staining step should be chosen to ensure that dye dilution proceeds linearly from T0. Finally, it should be noted that although CFSE over-labeling may also result in stacking/quenching, any modest increase from T0 to T24 would be completely undetectable in the face of the substantial division-independent dye loss seen during the same period.
Regardless of which class of tracking dyes is used, labeling prior to LAK induction with IL-2 is preferable to post-induction labeling. This is particularly true if CFSE or other protein dyes are used, since staining immediately before assay initiation runs the risk that early dye efflux could result in unintended transfer of label to target cells (41). It is also recommended when labeling with membrane dyes, since it gives the cells time to recover from thermal or other stresses incurred during the staining procedure.
Use of target cells obtained from high-density cultures should be avoided, due to the presence of significant numbers of apoptotic and/or dead cells. Such cells will readily stain with tracking dyes but will give highly variable staining with 7-AAD or other dyes used to determine viability by dye exclusion, making it much more difficult to establish the intensity limit above which a target cell is to be considered nonviable.
Cell and dye concentrations given in Subheading 3.3.1, step 2 and Subheading 3.3.2, step 2 were selected to give staining intensities that (a) were on scale in the last decade of a 4-log intensity scale when unstained cells were placed in the first decade, and (b) could readily be compensated in adjacent spectral channels. It should be noted that a slight peak asymmetry is of somewhat less concern when labeling targets or effectors for a cytotoxicity assay than when labeling hPBMC for a proliferation assay. It is however, important to ensure that 100% of cells are labeled with the chosen tracking dye so that a clear distinction can be made between cells that are labeled, and cells that are unlabeled or those that are labeled with a different dye.
Table 2 summarizes recommended assay and staining/instrument setup controls, as well as additional controls required at the time of initial assay setup or useful for troubleshooting (if sufficient cells are available to accommodate their inclusion).
For longer term assays, it may be desirable to set up the plate with a border of CM-filled wells around the periphery in order to minimize evaporation-associated variability in cell and cytokine concentrations in the assay wells.
CD45 was used here as a gating parameter because both LAK and K562 are CD45+ (although K562 are dimmer than LAK). The PacBlue conjugate was selected here for spectral compatibility with the other probes used in the assay. Because the enumeration beads also fluoresce in the PacBlue channel (450/50), they also will be included in the “CD45” gate (Fig. 5, plot 1). A low SSC threshold was used to reduce debris but required substantial care to ensure that all events corresponding to both dead and live targets and effectors were included above the threshold. An alternative strategy would be to set a threshold on CD45 PacBlue that included all bead and cellular events (live and dead), avoiding the necessity for a side scatter threshold.
Choice of optimized labeling conditions for tracking dye(s) should have already established that stained cells will be on scale, without events accumulating in the highest channel. This condition should be established when compensation is set to 0% and voltages are adjusted to place unstained control cells in the first decade, but sufficiently above the left axis such that events do not accumulate in the lowest channel. In addition, it is critical to recognize that cells labeled with tracking dyes can be extremely bright, requiring substantially decreased detector voltage settings compared with those used for the detection of immunofluorescence. If the tracking dye signal in a secondary channel (set to a higher detector voltage) is greater than its intensity in the primary channel, it will be impossible to compensate properly for tracking dye overlap in the secondary channel (41). In this case, staining with concentrations of tracking dye(s) may be required.
As shown in Fig. 6, CellVue Claret staining does not affect the cytolytic potential of LAK cells. Somewhat surprisingly for a 4-h assay, the use of 7-AAD to quantify dead target cells (Method 1) appears to be less sensitive than the use of enumeration beads to count remaining viable target cells (Method 2). To test the hypothesis that a too-stringent CD45 region or scatter region (R1 or R2 of Fig. 5, respectively) might have excluded some dead K562 targets from the PKH67 + 7-AAD + gate in Method 1, we evaluated the effect of extending region R1 to include events with lower CD45 intensity, or expanding region R2 to incorporate events with lower forward scatter. These manipulations (especially in combination) gave decreased rather than increased values for percent cytotoxicity. This was true using either Method 1 or 2 and did not reduce the differential between the two. In order to enumerate dead cells with a viability dye, Method 1 requires that cells remain intact enough to contain a nucleus. It has been demonstrated in longer term assays that killed cells may fragment, enucleate, and thus become uncountable by this method (23). The data of Fig. 6 suggest that even in a relatively short-term assay, Method 2 – which relies on enumeration of live cells remaining in the sample – is inherently a more accurate way to quantify cytotoxicity. An alternate hypothesis considered was that Method 1 might be more sensitive to the quadstat region used to determine % dead targets than Method 2, due to a consistent decrease in PKH67 intensity observed for dead versus live K562 targets, which was most pronounced at low E:T ratios. For example, in the center panel of Fig. 6a (3:1 E:T ratio), gMFI = 299 for PKH67 + 7-AAD+ events, gMFI = 498 for PKH67 + 7-AAD− events, and approximately 8% of PKH67 + 7-AAD+ events fall in the lower right quadrant (i.e., are PKH67dim). Lowering the quadstat boundary to include ≥98% of PKH67dim 7-AAD+, however, actually resulted in decreased values for percent cytotoxicity, not increased values, due to increased levels of noise in the live K562 region. Further analysis of the reduced PKH67 intensity observed for 7-AAD+ targets suggested that it was at least partially due to membrane transfer from K562 targets to LAK effectors, as evidenced by increases in both PKH67 and CD45 intensity observed for live CellVue Claret-positive LAK cells at low E:T ratios (data not shown).
Number of non-viable effectors present in the assay can be calculated similarly, using a CellVue Claret versus 7-AAD histogram as gated in Fig. 5, plot 6 (gated on R1&R2&R5 and not R6) and then dividing the number of dead effectors (CellVue Claret and 7-AAD dual positive; R11) by the total number of effectors (CellVue Claret positive; R10 + R11). This may be helpful in troubleshooting if target cell killing is lower than expected and/or in longer term assays in which some effector cell death is expected.
During the analysis of suppression assays, fluorescent monoclonal antibodies (mAb) such as anti-CD4 can be used to discriminate Teff and Treg from accessory cells, which are required to maximize Teff proliferation if soluble anti-CD3 is used for stimulation rather than plate-bound anti-CD3 (data not shown).
Anti-coagulated peripheral blood or apheresis packs used for stem or mononuclear cell collections (47) are suitable sources of both lymphocytes and accessory cells. WBC-retaining TRIMA filters were used as a starting point for the studies described here because they represent a ready source of large numbers of hPBMC (average recovery: 1.2 ± 0.4 × 109 cells; n = 39), an important consideration when using multiparameter flow cytometry and sorting to isolate low-frequency subpopulations such as Treg cells.
Be sure to rotate tubes on their horizontal axis to avoid foaming, sloshing from side to side, or end-over-end mixing, which will inhibit monocyte aggregation.
Typical lymphocyte recoveries from a TRIMA filter are 7.1 ± 3.1 × 108 cells. Typical composition is 71.8 ± 7.1% T cells, 3.3 ± 3.6% monocytes, and 24.7 ± 6.4% B/NK cells as determined by CD45 FITC/CD14 PE/CD3 PerCP staining and flow cytometric analysis (n = 39).
Though not further used in the studies described here, the cell pellet obtained after cold aggregation and 1 × g fractionation is an excellent source of relatively pure monocytes. These may be harvested with a 10-mL pipette, being careful not to disrupt the aggregates. To remove platelets, overlay the aggregates onto 5 mL of FBS and let them to sediment at 1 × g on ice for 15 min. Recover pellet, and wash twice with 15 mL of Versene to remove platelets and once with 15 mL of CM. Typical recoveries are 2.1 ± 1.2 × 108 monocytes with 74 ± 10% purity (n = 39).
All procedures should be carried out on ice with cold reagents.
All mAbs should be used at concentrations previously determined to be saturating. In general, we have found that the concentration of mAb determined to be saturating for 100 µL of whole blood will also be saturating when staining up to 3–5 × 106 lymphocytes in a volume of 100 µL.
After sorting, we routinely obtain 1–4 × 106 Treg and 1–2 × 107 Teff cells from a starting preparation of 1.5 × 108 monocyte-depleted lymphocytes. Accessory cells were simultaneously sorted with the Treg and Teff cells until 1.5 × 107 cells had been recovered.
Ideally, given sufficient cell numbers, samples should be prepared in triplicate.
Table 3 summarizes recommended assay and staining/instrument setup controls, as well as additional controls required at the time of initial assay setup or useful for troubleshooting (if sufficient cells are available to accommodate their inclusion).
Single-color compensation controls are best prepared from cells treated in the same fashion as the test samples. This is particularly important for CFSE, which exhibits rapid non-proliferation-related intensity losses during the first 24 h and more slowly thereafter (see Fig. 2 and (1, 48)). If insufficient numbers of CellVue Claret-stained Treg cells are available for both test and control samples, it is possible, although not ideal, to stain other lymphocytes (e.g., excess Teff) under identical cell concentration and dye conditions for use as Treg surrogates in establishing appropriate detector voltages and compensation settings.
Since many wells in the 96-well plate will remain empty, it is recommended that wells surrounding test and control cells for the assay be filled with CM to minimize evaporation in the assay wells.
Although 7-AAD can be used as an alternative to LIVE/DEAD Fixable Violet reagent (45), color compensation for CellVue Claret is simpler with the latter since there is greater spectral separation than with 7-AAD. In our experience, labeling cells with LIVE/DEAD Fixable Violet reagent can successfully be performed in the presence of low levels of exogenous protein, although this will reduce the separation between viable and nonviable populations. Here we chose to incubate simultaneously with human IgG, anti-CD4 PECy7, and LIVE/DEAD reagent in order to minimize cell losses associated with sequential staining and additional wash steps.
Choice of optimized labeling conditions for both Treg (here CellVue Claret stained) and Teff (here CFSE stained) should have already established that stained cells will be on scale, without events accumulating in the highest channel. This condition should be established when compensation is set to 0% and voltages are adjusted to place unstained control cells in the first decade, but sufficiently above the left axis so that events do not accumulate in the lowest channel.
Total number of cells to be collected depends upon the actual population of interest. In a cell proliferation experiment, the population of interest may be distributed over a broad range of intensities representing up to seven or eight generations. Therefore, to model and calculate the number of cells in each generation accurately, a large number of cells may need to be collected. It is most appropriate to set a stop region on the specific population of interest and collect sufficient cells for analysis within this region. In this example, if the population of interest is Treg cells, it is recommended that a stop region be set on viable Treg cells and a minimum of 5,000 events be collected within this region. When studying rare cells, it may be necessary to simply run the sample tube nearly dry in order to collect the maximum possible number of events.
References
- 1.Wallace PK, Tario JD, Jr, Fisher JL, Wallace SS, Ernstoff MS, Muirhead KA. Tracking antigen-driven responses by flow cytometry: monitoring proliferation by dye dilution. Cytometry A. 2008;73:1019–1034. doi: 10.1002/cyto.a.20619. [DOI] [PubMed] [Google Scholar]
- 2.Parish CR. Fluorescent dyes for lymphocyte migration and proliferation studies. Immunol Cell Biol. 1999;77:499–508. doi: 10.1046/j.1440-1711.1999.00877.x. [DOI] [PubMed] [Google Scholar]
- 3.Poon RY, Ohlsson-Wilhelm BM, Bagwell CB, Muirhead KA. Use of PKH membrane intercalating dyes to monitor cell trafficking and function. In: Diamond RA, DeMagio S, editors. In Living Color: Flow Cytometry and Cell Sorting Protocols. New York: Springer; 2000. pp. 302–352. [Google Scholar]
- 4.Quah BJ, Warren HS, Parish CR. Monitoring lymphocyte proliferation in vitro and in vivo with the intracellular fluorescent dye carboxyfluorescein diacetate succinimidyl ester. Nat Protoc. 2007;2:2049–2056. doi: 10.1038/nprot.2007.296. [DOI] [PubMed] [Google Scholar]
- 5.Wallace PK, Muirhead KA. Cell tracking 2007: a proliferation of probes and applications. Immunol Invest. 2007;36:527–561. doi: 10.1080/08820130701812584. [DOI] [PubMed] [Google Scholar]
- 6.Fuse S, Underwood E. Simultaneous analysis of in vivo CD8+ T cell cytotoxicity against multiple epitopes using multicolor flow cytometry. Immunol Invest. 2007;36:829–845. doi: 10.1080/08820130701683753. [DOI] [PubMed] [Google Scholar]
- 7.Schafer R, Wiskirchen J, Guo K, Neumann B, Kehlbach R, Pintaske J, Voth V, Walker T, Scheule AM, Greiner TO, Hermanutz-Klein U, Claussen CD, Northoff H, Ziemer G, Wendel HP. Aptamer-based isolation and subsequent imaging of mesenchymal stem cells in ischemic myocard by magnetic resonance imaging. Rofo. 2007;179:1009–1015. doi: 10.1055/s-2007-963409. [DOI] [PubMed] [Google Scholar]
- 8.Flexman JA, Minoshima S, Kim Y, Cross DJ. Magneto-optical labeling of fetal neural stem cells for in vivo MRI tracking. Conf Proc IEEE Eng Med Biol Soc. 2006;1:5631–5634. doi: 10.1109/IEMBS.2006.259982. [DOI] [PubMed] [Google Scholar]
- 9.Stroh A, Boltze J, Sieland K, Hild K, Gutzeit C, Jung T, Kressel J, Hau S, Reich D, Grune T, Zimmer C. Impact of magnetic labeling on human and mouse stem cells and their long-term magnetic resonance tracking in a rat model of Parkinson disease. Mol Imaging. 2009;8:166–178. [PubMed] [Google Scholar]
- 10.Modo M, Beech JS, Meade TJ, Williams SC, Price J. A chronic 1 year assessment of MRI contrast agent-labelled neural stem cell transplants in stroke. Neuroimage. 2009;47(Suppl 2):T133–T142. doi: 10.1016/j.neuroimage.2008.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sun N, Lee A, Wu JC. Long term non-invasive imaging of embryonic stem cells using reporter genes. Nat Protoc. 2009;4:1192–1201. doi: 10.1038/nprot.2009.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schierling W, Kunz-Schughart LA, Muders F, Riegger GA, Griese DP. Fates of genetically engineered haematopoietic and mesenchymal stem cell grafts in normal and injured rat hearts. J Tissue Eng Regen Med. 2008;2:354–364. doi: 10.1002/term.104. [DOI] [PubMed] [Google Scholar]
- 13.Cicalese A, Bonizzi G, Pasi CE, Faretta M, Ronzoni S, Giulini B, Brisken C, Minucci S, Di Fiore PP, Pelicci PG. The tumor suppressor p53 regulates polarity of self-renewing divisions in mammary stem cells. Cell. 2009;138:1083–1095. doi: 10.1016/j.cell.2009.06.048. [DOI] [PubMed] [Google Scholar]
- 14.Daubeuf S, Aucher A, Sampathkumar S-G, Preville X, Yarema KJ, Hudrisier D. Chemical labels metabolically installed into the glycoconjugates of the target cell surface can be used to track lymphocyte/target cell interplay via trogocytosis: comparisons with lipophilic dyes and biotin. Immunol Invest. 2007;36:687–712. doi: 10.1080/08820130701674596. [DOI] [PubMed] [Google Scholar]
- 15.Gertner-Dardenne J, Poupot M, Gray BD, Fournié J-J. Lipophilic fluorochrome trackers of membrane transfers between immune cells. Immunol Invest. 2007;36:665–685. doi: 10.1080/08820130701674646. [DOI] [PubMed] [Google Scholar]
- 16.Hawkins ED, Hommel M, Turner ML, Battye FL, Markham JF, Hodgkin PD. Measuring lymphocyte proliferation, survival and differentiation using CFSE time-series data. Nat Protoc. 2007;2:2057–2067. doi: 10.1038/nprot.2007.297. [DOI] [PubMed] [Google Scholar]
- 17.Kusumbe AP, Bapat SA. Cancer stem cells and aneuploid populations within developing tumors are the major determinants of tumor dormancy. Cancer Res. 2009;69:9245–9253. doi: 10.1158/0008-5472.CAN-09-2802. [DOI] [PubMed] [Google Scholar]
- 18.Hamelik RM, Krishan A. Click-iT assay with improved DNA distribution histograms. Cytometry A. 2009;75:862–865. doi: 10.1002/cyto.a.20780. [DOI] [PubMed] [Google Scholar]
- 19.Roder JC, Haliotis T, Klein M, Korec S, Jett JR, Ortaldo J, Heberman RB, Katz P, Fauci AS. A new immunodeficiency disorder in humans involving NK cells. Nature. 1980;284:553–555. doi: 10.1038/284553a0. [DOI] [PubMed] [Google Scholar]
- 20.Morales A, Ottenhof PC. Clinical application of a whole blood assay for human natural killer (NK) cell activity. Cancer. 1983;52:667–670. doi: 10.1002/1097-0142(19830815)52:4<667::aid-cncr2820520417>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- 21.Zaritskaya L, Shurin MR, Sayers TJ, Malyguine AL. New flow cytometric assays for monitoring cell-mediated cytotoxicity. Expert Rev. Vaccines. 2010;9:601–616. doi: 10.1586/erv.10.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sheehy ME, McDermott AB, Furlan SN, Klenerman P, Nixon DF. A novel technique for the fluorometric assessment of T lymphocyte antigen specific lysis. J Immunol Methods. 2001;249:99–110. doi: 10.1016/s0022-1759(00)00329-x. [DOI] [PubMed] [Google Scholar]
- 23.Flieger D, Spengler U, Beier I, Sauerbruch T, Schmidt-Wolf IG. Prestimulation of monocytes by the cytokines GM-CSF or IL-2 enhances the antibody dependent cellular cytotoxicity of monoclonal antibody 17-1A. Z Gastroenterol. 2000;38:615–622. doi: 10.1055/s-2000-7511. [DOI] [PubMed] [Google Scholar]
- 24.Aubry JP, Blaecke A, Lecoanet-Henchoz S, Jeannin P, Herbault N, Caron G, Moine V, Bonnefoy JY. Annexin V used for measuring apoptosis in the early events of cellular cytotoxicity. Cytometry. 1999;37:197–204. doi: 10.1002/(sici)1097-0320(19991101)37:3<197::aid-cyto6>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 25.Ely P, Wallace PK, Givan AL, Graziano RF, Guyre PM, Fanger MW. Bispecific-armed, interferon gamma-primed macrophage-mediated phagocytosis of malignant non-Hodgkin’s lymphoma. Blood. 1996;87:3813–3821. [PubMed] [Google Scholar]
- 26.Lee-MacAry AE, Ross EL, Davies D, Laylor R, Honeychurch J, Glennie MJ, Snary D, Wilkinson RW. Development of a novel flow cytometric cell-mediated cytotoxicity assay using the fluorophores PKH-26 and TO-PRO-3 iodide. J Immunol Methods. 2001;252:83–92. doi: 10.1016/s0022-1759(01)00336-2. [DOI] [PubMed] [Google Scholar]
- 27.Schutz C, Fischer K, Volkl S, Hoves S, Halbritter D, Mackensen A, Fleck M. A new flow cytometric assay for the simultaneous analysis of antigen-specific elimination of T cells in heterogeneous T cell populations. J Immunol Methods. 2009;344:98–108. doi: 10.1016/j.jim.2009.03.008. [DOI] [PubMed] [Google Scholar]
- 28.Wilkinson RW, Lee-MacAry AE, Davies D, Snary D, Ross EL. Antibody-dependent cell-mediated cytotoxicity: a flow cytometry-based assay using fluorophores. J Immunol Methods. 2001;258:183–191. doi: 10.1016/s0022-1759(01)00474-4. [DOI] [PubMed] [Google Scholar]
- 29.Chen X, Wang B, Chang LJ. Induction of primary anti-HIV CD4 and CD8 T cell responses by dendritic cells transduced with self-inactivating lentiviral vectors. Cell Immunol. 2006;243:10–18. doi: 10.1016/j.cellimm.2006.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Liu W, Putnam AL, Xu-Yu Z, Szot GL, Lee MR, Zhu S, Gottlieb PA, Kapranov P, Gingeras TR, Fazekas de St Groth B, Clayberger C, Soper DM, Ziegler SF, Bluestone JA. CD127 expression inversely correlates with FoxP3 and suppressive function of human CD4+ T reg cells. J Exp Med. 2006;203:1701–1711. doi: 10.1084/jem.20060772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shevach EM. Mechanisms of FOXP3+ T regulatory cell-mediated suppression. Immunity. 2009;30:636–645. doi: 10.1016/j.immuni.2009.04.010. [DOI] [PubMed] [Google Scholar]
- 32.Boros P, Bromberg JS. Human FOXP3+ regulatory T cells in transplantation. Am J Transplant. 2009;9:1719–1724. doi: 10.1111/j.1600-6143.2009.02704.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Corthay A. How do regulatory T cells work? Scand J Immunol. 2009;70:326–336. doi: 10.1111/j.1365-3083.2009.02308.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Brusko TM, Hulme MA, Myhr CB, Haller MJ, Atkinson MA. Assessing the in vitro suppressive capacity of regulatory T cells. Immunol Invest. 2007;36:607–628. doi: 10.1080/08820130701790368. [DOI] [PubMed] [Google Scholar]
- 35.Neron S, Thibault L, Dussault N, Cote G, Ducas E, Pineault N, Roy A. Characterization of mononuclear cells remaining in the leukoreduction system chambers of apheresis instruments after routine platelet collection: a new source of viable human blood cells. Transfusion. 2007;47:1042–1049. doi: 10.1111/j.1537-2995.2007.01233.x. [DOI] [PubMed] [Google Scholar]
- 36.Wallace PK, Keler T, Coleman K, Fisher J, Vitale L, Graziano RF, Guyre PM, Fanger MW. Humanized mAb H22 binds the human high affinity Fc receptor for IgG (FcgammaRI), blocks phagocytosis, and modulates receptor expression. J Leukoc Biol. 1997;62:469–479. doi: 10.1002/jlb.62.4.469. [DOI] [PubMed] [Google Scholar]
- 37.Mentzer SJ, Guyre PM, Burakoff SJ, Faller DV. Spontaneous aggregation as a mechanism for human monocyte purification. Cell Immunol. 1986;101:312–319. doi: 10.1016/0008-8749(86)90144-9. [DOI] [PubMed] [Google Scholar]
- 38.Rousselle C, Barbier M, Comte VV, Alcouffe C, Clement-Lacroix J, Chancel G, Ronot X. Innocuousness and intracellular distribution of PKH67: a fluorescent probe for cell proliferation assessment. In Vitro Cell Dev Biol Anim. 2001;37:646–655. doi: 10.1290/1071-2690(2001)037<0646:iaidop>2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 39.Horan PK, Melnicoff MJ, Jensen BD, Slezak SE. Fluorescent cell labeling for in vivo and in vitro cell tracking. Methods Cell Biol. 1990;33:469–490. doi: 10.1016/s0091-679x(08)60547-6. [DOI] [PubMed] [Google Scholar]
- 40.Wallace PK, Palmer LD, Perry-Lalley D, Bolton ES, Alexander RB, Horan PK, Yang JC, Muirhead KA. Mechanisms of adoptive immunotherapy: improved methods for in vivo tracking of tumor-infiltrating lymphocytes and lymphokine-activated killer cells. Cancer Res. 1993;53:2358–2367. [PubMed] [Google Scholar]
- 41.Tario JD, Jr, Gray BD, Wallace SS, Muirhead KA, Ohlsson-Wilhelm BM, Wallace PK. Novel lipophilic tracking dyes for monitoring cell proliferation. Immunol Invest. 2007;36:861–885. doi: 10.1080/08820130701712933. [DOI] [PubMed] [Google Scholar]
- 42.Givan AL. A flow cytometric assay for quantitation of rare antigen-specific T-cells: using cell-tracking dyes to calculate precursor frequencies for proliferation. Immunol Invest. 2007;36:563–580. doi: 10.1080/08820130701683803. [DOI] [PubMed] [Google Scholar]
- 43.Givan AL, Fisher JL, Waugh MG, Bercovici N, Wallace PK. Use of cell-tracking dyes to determine proliferation precursor frequencies of antigen-specific T cells. Methods Mol Biol. 2004;263:109–124. doi: 10.1385/1-59259-773-4:109. [DOI] [PubMed] [Google Scholar]
- 44.Lyons AB, Doherty KV. Flow cytometric analysis of cell division by dye dilution. In: Robinson JP, Darzynkiewicz Z, Hoffman R, Nolan JP, Orfao A, Rabinovitch PS, Watkins S, editors. Current Protocols in Cytometry. Vol. 9.11. New York: John Wiley & Sons, Inc.; 2004. [DOI] [PubMed] [Google Scholar]
- 45.Bantly AD, Gray BD, Breslin E, Weinstein EG, Muirhead KA, Ohlsson-Wilhelm BM, Moore JS. Cellvue Claret, a new far-red dye, facilitates polychromatic assessment of immune cell proliferation. Immunol Invest. 2007;36:861–885. doi: 10.1080/08820130701712461. [DOI] [PubMed] [Google Scholar]
- 46.Matera G, Lupi M, Ubezio P. Heterogeneous cell response to topotecan in a CFSE-based proliferation test. Cytometry A. 2004;62:118–128. doi: 10.1002/cyto.a.20097. [DOI] [PubMed] [Google Scholar]
- 47.Wallace PK, Romet-Lemonne JL, Chokri M, Kasper LH, Fanger MW, Fadul CE. Production of macrophage-activated killer cells for targeting of glioblastoma cells with bispecific antibody to FcgammaRI and the epidermal growth factor receptor. Cancer Immunol Immunother. 2000;49:493–503. doi: 10.1007/s002620000142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lyons AB, Parish CR. Determination of lymphocyte division by flow cytometry. J Immunol Methods. 1994;171:131–137. doi: 10.1016/0022-1759(94)90236-4. [DOI] [PubMed] [Google Scholar]
- 49.Bercovici N, Givan AL, Waugh MG, Fisher JL, Vernel-Pauillac F, Ernstoff MS, Abastado JP, Wallace PK. Multiparameter precursor analysis of T-cell responses to antigen. J Immunol Methods. 2003;276:5–17. doi: 10.1016/s0022-1759(03)00059-0. [DOI] [PubMed] [Google Scholar]
- 50.Givan AL, Fisher JL, Waugh M, Ernstoff MS, Wallace PK. A flow cytometric method to estimate the precursor frequencies of cells proliferating in response to specific antigens. J Immunol Methods. 1999;230:99–112. doi: 10.1016/s0022-1759(99)00136-2. [DOI] [PubMed] [Google Scholar]
- 51.Munson ME. An improved technique for calculating relative response in cellular proliferation experiments. Cytometry. 2010;77A:909–910. doi: 10.1002/cyto.a.20935. [DOI] [PubMed] [Google Scholar]