Abstract
Background
Anopheles sinensis has become an important malaria vector in China. The long-term extensive utilization of pyrethroids for ITNs and IRS for mosquito control in the last three decades has resulted in the occurrence of resistant An. sinensis populations in many regions. Knockdown resistance (kdr), caused by point mutations in the VGSC gene, is one of the mechanisms that confer resistance to DDT and pyrethroids. Recently, several investigations revealed the kdr occurrence in some An. sinensis populations, however, no kdr data were available earlier than 2009. A survey tracking the dynamics of the kdr mutations in past decades would provide invaluable information to understand how the kdr alleles spread in mosquito populations temporally and spatially.
Methods
A survey was conducted on the kdr alleles at condon 1014 of the VGSC gene and their distributions in 733 specimens of An. sinensis and 232 specimens of the other eight member species of the Anopheles hyrcanus group that were collected from 17 provinces in China in 1996–2014.
Results
A total of three kdr alleles, TTT (F), TTG (F) and TGT (C) were detected, and TGT (C) and TTT (F) were already present in the specimens from Jiangsu and Shandong as early as 1997. The TTT (F) was the most frequent mutant allele, and largely distributed in central China, namely Shandong, Jiangsu, Anhui, Henan, Shanghai, Jiangxi and Hubei. When data were analysed in three time intervals, 1996–2001, 2005–2009, 2010–2014, the prevalence of kdr alleles increased progressively over time in the populations in central China. In contrast, the kdr alleles were less frequent in the samples from other regions, especially in Yunnan and Hainan, despite the documented presence of pyrethroid resistant populations in those regions. Interestingly, no mutant alleles were detected in all 232 specimens of eight other species in the An. hyrcanus group.
Conclusion
The survey revealed that the kdr occurrence and accumulation in the An. sinensis populations were more frequent in central China than in the other regions, suggesting that the kdr mutations may contribute significantly to the pyrethroid resistance in the mosquitoes in central China.
Electronic supplementary material
The online version of this article (doi:10.1186/s12936-015-0644-0) contains supplementary material, which is available to authorized users.
Keywords: Anopheles sinensis, Anopheles lesteri, Pyrethroids, Knockdown resistance, Kdr allele
Background
Mosquito control is one of the integrated programmes to prevent transmission of mosquito-borne diseases such as malaria, filariasis, and dengue fever. Chemical insecticides have been extensively used for vector management since the 1940s. Four major categories of insecticides have been utilized: organochlorines, organophosphates, carbamates and pyrethroids [1]. DDT and pyrethroids function as neurotoxins that target voltage-gated sodium channels (VGSC) and interfere electronic signaling in the nervous system, which results in paralysis and death, an effect known as knockdown [2]. One of the mechanisms that mosquitoes have developed for the resistance to DDT and pyrethroids is the target insensitivity, which is caused by mutations in the VGSC gene. A prominent mutation is the substitution of leucine at residue position 1014 in mosquitoes with the knockdown resistance (kdr) [1]. A positive correlation between the kdr mutation and the resistant phenotype to pyrethroids and DDT was well documented in various Anopheles populations [3-10].
Anopheles sinensis is an Oriental species with wide distributions in China. Anopheles sinensis is one of the principal malaria vectors in many malaria-endemic regions, especially in the central China, due to its abundant population size [11]. In China, DDT has been widely used for conventional indoor residue sprays (IRS) since 1950s, and pyrethroids have been applied for IRS and insecticide-treated nets (ITNs) since 1980s [12]. These measures have been effective in reducing malaria transmission [13]. However, the long-term applications of insecticides have resulted in the development of resistance in mosquito populations. For instance, the DDT resistance in An. sinensis was documented in Yunnan as early as 1981 [14]; the permethrin resistance was reported in Sichuan in 1989 [15]; and in Fujian in 1989–1993, resistant populations occurred two years after IRS and ITNs applications, and the resistance spread in more populations three years after applications [13].
Recent years, the kdr genotyping has been included in monitoring pyrethroid resistance in An. sinensis in China. Several investigations have been made on the distributions of kdr alleles in various An. sinensis populations, such as provinces Jiangsu, Henan, Hunan, Anhui, Jiangxi, Yunnan, and Hainan [7,16-21]. However, these studies were conducted in the last five years, the kdr data were obtained from the mosquito specimens that were sampled in 2009 and after. No earlier kdr data were available. A historical survey tracking the dynamics of the kdr mutations in the past decades would provide invaluable information to understand how the kdr mutations occurred and spread in mosquito populations temporally and spatially. Therefore, a study was conducted to investigate the genotypes of the codon 1014 of the VGSC gene in the specimens of An. sinensis and the other eight member species of the An. hyrcanus group that were collected from 17 provinces in 1996–2014. The data revealed that the kdr alleles were already present in the specimens sampled in 1997, and kdr alleles progressively increased over decades in the An. sinensis populations in central China. The kdr were much less prevalent in the other populations, particularly in Yunnan and Hainan.
Methods
Mosquito collections and species identification
Wild mosquito adults were collected in 1996–2014 from 31 sampling sites in 17 provinces in China. Mosquitoes were caught by using light traps at livestock corrals or human landing catches, with consent of the owners and persons involved in the study. The collection information was summarized in Tables 1, 2 and Figure 1. Mosquitoes of the Anopheles hyrcanus group were sorted out in the field by morphology using the identification keys [11], and brought back to the lab. The species identity was molecularly determined by a diagnostic PCR assay based on the ribosomal DNA (rDNA) second internal transcribed spacer (ITS2) markers [22] or by the ITS2 sequencing [23].
Table 1.
Sampling information of Anopheles sinensis in China
| Collection site | Latitude/longitude coordinates | Code | Sample size | Date | |
|---|---|---|---|---|---|
| Anhui | Hefei | N:31°52′, E:117°16′ | HF06 | 19 | 07/2006 |
| Huangshan | N:30°08′, E:118°10′ | HS13 | 60 | 07/2013 | |
| Xuancheng | N:30°57′, E:118°44′ | XC13 | 30 | 07/2013 | |
| Chongqing | Kaixian | N:31°10′, E:108°23′ | CQ08 | 16 | 07/2008 |
| Fujian | Jianyang | N:27°24′, E:118°03′ | FJ97 | 20 | 09/1997 |
| Guangdong | Zhuhai | N:22°15′, E:113°33′ | GD07 | 26 | 10/2007 |
| Guangxi | Tiane | N:25°01′, E:106°59′ | GX05 | 22 | 07/2005 |
| Guizhou | Sinan | N:27°51′, E:108°08′ | SN97 | 16 | 05/1997 |
| Kaili | N:26°37′, E:107°56′ | KL07 | 20 | 08/2007 | |
| Hainan | Chengmai | N:19°45′, E:110°00′ | CM97 | 7 | 05/1997 |
| Qiongzhong | N:19°03′, E:109°50′ | QZ10 | 12 | 08/2010 | |
| Wenchang | N:19°36′, E:110°43′ | WC11 | 14 | 08/2011 | |
| Changjiang | N:19°15′, E:109°02′ | CJ13 | 7 | 06/2013 | |
| Haikou | N:20°01′, E:110°20′ | HK13 | 5 | 08/2013 | |
| Henan | Zhengzhou | N:34°45′, E:113°38′ | ZZ97 | 9 | 07/1997 |
| ZZ07 | 10 | 08/2007 | |||
| Nanyang | N:33°00′, E:112°31′ | NY01 | 23 | 06/2001 | |
| Hubei | Wuhan | N:30°34′, E:114°18′ | WH06 | 24 | 08/2006 |
| Suizhou | N:31°43′, E:113°22′ | SZ07 | 13 | 07/2007 | |
| Jiangsu | Xuyi | N:32°58′, E:118°31′ | XY97 | 20 | 07/1997 |
| Wujing | N:31°40, E:119°56′ | WJ97 | 24 | 07/1997 | |
| Jiangxi | Yongxiu | N:29°08′, E:115°44′ | JX09 | 16 | 09/2009 |
| Liaoning | Huludao | N:40°44′, E:120°51′ | LN08 | 10 | 08/2008 |
| Shandong | Jining | N:35°24′, E:116°35 | JN97 | 3 | 07/1997 |
| JN00 | 31 | 07/2000 | |||
| JN07 | 22 | 08/2007 | |||
| JN12 | 12 | 07/2012 | |||
| Caoxian | N:34°49′, E:115°32′ | CX12 | 30 | 07/2012 | |
| Shanghai | Jiading | N:31°22′, E:121°14′ | JD12 | 24 | 08/2012 |
| Shaanxi | Ningshan | N:33°32′, E:108°26′ | NS96 | 2 | 08/1996 |
| NS13 | 36 | 07/2013 | |||
| Sichuan | Pujiang | N:30°14′, E:102°29′ | PJ96 | 20 | 07/1997 |
| PJ97 | 18 | 07/1997 | |||
| Jiuzhaigou | N:33°16′, E:104°14′ | JZ14 | 46 | 07/2014 | |
| Yunnan | Puer | N:22°47′, E:100°58′ | PR97 | 10 | 05/1997 |
| PR05 | 10 | 08/2005 | |||
| PR10 | 17 | 07/2010 | |||
| Zhaotong | N:27°20′, E:103°43′ | ZT06 | 14 | 07/2006 | |
| Yingjiang | N:24°51′, E:97°55′ | YJ13 | 15 | 08/2013 | |
Table 2.
Sampling information of member species in the Anopheles hyrcanus group in China
| Collection site | Latitude/longitude coordinates | Species | Sample size | Date | |
|---|---|---|---|---|---|
| Guangdong | Zhuhai | N:22°15′, E:113°33′ | An. lesteri | 2 | 07/2001 |
| 9 | 10/2007 | ||||
| Guangxi | Lab colony | -- | An. lesteri | 1 | - |
| Hainan | Wenchang | N:19°36′, E:110°43′ | An. lesteri | 11 | 12/2001 |
| 6 | 08/2010 | ||||
| Henan | Nanyang | N:33°00′, E:112°31′ | An. lesteri | 5 | 06/2001 |
| An. yatsushiroensis | 3 | ||||
| Hubei | Suizhou | N:31°43′, E:113°22′ | An. lesteri | 4 | 07/2007 |
| Jiangsu | Lab colony | -- | An. lesteri | 17 | - |
| Liaoning | Zhangwu | N:42°31′, E:122°28′ | An. lesteri | 1 | 08/2007 |
| Zhuanghe | N:39°51′, E:122°55′ | An. lesteri | 2 | 08/1999 | |
| Faku | N:42°24′, E:123°14′ | An. lesteri | 1 | 08/1996 | |
| 12 | 08/1998 | ||||
| An. yatsushiroensis | 5 | ||||
| Sujiatun | N:41°34′, E:123°25′ | An. lesteri | 1 | 08/1997 | |
| 8 | 08/1998 | ||||
| 2 | 08/2002 | ||||
| An. yatsushiroensis | 1 | ||||
| Donggang | N:39°58′, E:123°52′ | An. lesteri | 18 | 07/1997 | |
| An. yatsushiroensis | 6 | ||||
| An. lesteri | 11 | 07/1999 | |||
| An. yatsushiroensis | 1 | ||||
| An. lesteri | 44 | 08/2004 | |||
| An. yatsushiroensis | 3 | ||||
| Shandong | Rongchen | N:37°10′, E:122°29′ | An. yatsushiroensis | 3 | 08/2004 |
| Jining | N:35°24′, E:116°35 | An. belenrae | 3 | 08/1997 | |
| 7 | 08/2012 | ||||
| Caoxian | N:34°49′, E:115°32′ | An. belenrae | 3 | 08/2012 | |
| Shaanxi | Yulin | N:38°16′, E:109°44′ | An. keleini | 16 | 08/2009 |
| 3 | 08/2013 | ||||
| Sichuan | Junlian | N:28°02′, E:104°35′ | An. lesteri | 6 | 07/1996 |
| Pujiang | N:30°14′, E:102°29′ | An. lesteri | 2 | 07/1998 | |
| An. yatsushiroensis | 2 | ||||
| Liangshan | N:27°53′, E:102°35′ | An. liangshanensis | 3 | 08/1997 | |
| Yunnan | Zhaotong | N:27°20′, E:103°43′ | An. lesteri | 2 | 08/1999 |
| 1 | 08/2006 | ||||
| Kunming | N:25°03′, E:102°42′ | An. kunmingensis | 2 | 08/1997 | |
| Puer | N:22°47′, E:100°58′ | An. lesteri | 1 | 08/1999 | |
| An. peditaeniatus | 2 | 08/2007 | |||
| An. crawfordi | 2 | ||||
Figure 1.
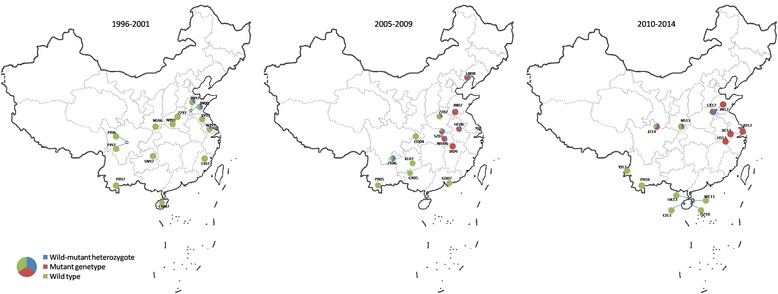
A schematic map of sampling sites for Anopheles sinensis in China and the composition of the kdr genotypes in the samples in the three time intervals, 1996–2001, 2005–2009 and 2010–2014. The genotype composition in each location was displayed by a pie chart with blue as wild/mutant heterozygote, red as mutant genotype and green as wildtype genotype (see Additional file 2: Table S2 for details).
kdr gene amplification and sequencing
To identify kdr alleles, a partial sequence of S6 segment of domain II of the VGSC gene was amplified from 20-50 ng genomic DNA from single specimens using CD1 (5′-TGA TCG TGT TTC GCG TGC TG-3′) and CD2 (5′-GTC TCG TTA TCC GCC GTT GG-3′) primers [3]. The PCR kit was from Aidlab, China. The PCR reaction was carried out in Verity 96 well Thermal Cycler (Applied Biosystems, USA) included an initial step of denaturation at 94°C for 1 min, followed by 35 cycles of amplification at 94°C for 30 s, 55°C for 30 s, and 68°C for 30 s, with a final extension step at 68°C for 7 min. After electrophoresis, PCR products were purified and used for sequencing in both directions with the CD1 and CD2 primers, respectively. There were 37 specimens, of which the PCR products were cloned into plasmids (pGEMX-T Easy Vector, Aidlab, China), and then sequenced, due to the double peaks at two positions of the codon 1014.
Statistical analyses
The codon 1014 was examined by sequence analysis, and genotypes were determined. In each sample, for a particular allele, the allele frequency was calculated as: number of alleles/(sample size × 2). The mutation frequency was defined as frequency of sum of wildtype/mutant heterozygotes and mutant/mutant homozygotes, which was calculated as: (sum of wildtype/mutant and mutant/mutant individuals)/sample size. The maximum likely frequency (y) of an allele present or absent in a sample of a given size (x) was obtained from the upper of 95% confidence limit of binomial distribution, given by y = 1–0.051/x, following the example of Post and Millest [24].
Results
Taxonomic composition of the mosquito collections
In the current study, 733 specimens of An. sinensis were used in the kdr allele survey. Some of these samples were from mosquito collections of our previous work on the molecular identification and phylogeny of the An. hyrcanus group [22,23] and the population genetics of An. sinensis [25] and Anopheles lesteri [26] since 1996. The samples included specimens from 31 sampling sites in 17 provinces in China from 1996 to 2014 (Figure 1 and Table 1). In addition, 232 mosquitoes of eight other species in the An. hyrcanus group were identified in the samples collected in 19 locations from 11 provinces in China in 1996–2013, including 167 of Anopheles lesteri, 24 of Anopheles yatsushiroensis, 13 of Anopheles belenrae, 19 of Anopheles kleini, three of Anopheles liangshanensis, two of Anopheles peditaeniatus, two of Anopheles kunmingensis and two of Anopheles crawfordi.
Frequency and distribution of kdr mutations in the An. sinensis populations
In order to detect kdr alleles, a 343 bp fragment of the IIS6 domain of the VGSC gene was PCR amplified and sequenced directly. At codon 1014, four alleles were identified in the An. sinensis samples. In addition to the wildtype codon TTG encoding leucine (L), three mutant alleles were detected. Codons TTT and TTC both code for phenylalanine (F), and codon TGT codes for cysteine (C). A total of 10 genotypes were detected, including wildtype homozygote TTG/TTG (55.25%), mutant homozygotes TTT/TTT (17.60%), TTC/TTC (0.27%), TGT/TGT (1.91%), wildtype/mutant heterozygotes TTG/TTT (8.19%), TTG/TTC (0.82%), TTG/TGT (4.50%), TTT/TTC (0.68%), TTT/TGT (10.23%), and TTC/TGT (0.55%). Overall, the frequency of mutant genotypes (F/F, C/C and F/C) accounted for 31.24%, and the frequency of heterozygote genotypes L/F and L/C was 13.51%. The kdr allele frequency was presented in Additional file 1: Table S1.The geographic distribution of genotypes, i.e. wildtype homozygotes, wildtype/mutant heterozygotes, and mutant homozygotes, were depicted in Figure 1. The mutant alleles (1014F and 1014C) were highly prevalent in seven regions in central China, namely Shandong, Henan, Jiangsu, Shanghai, Anhui, Jiangxi and Hubei. The mutant alleles were less prevalent in the samples from other regions, including Sichuan, Chongqing, Shaanxi, Yunnan, Guizhou, and Hainan Island (Additional file 1: Table S1).
The specimens were collected in a time span of 1996–2014. To trace back to the temporal occurrence of kdr genotypes, the data were analysed in three time intervals, 1996–2001, 2005–2009 and 2010–2014 (Additional file 2: Table S2). In1996-2001, 203 specimens in 13 samples from 10 provinces were examined, the mutant alleles TGT (C) and TTT (F) were detected in 21 specimens, and seven of these specimens were found in Shandong and Jiangsu as early as 1997. These specimens were all wildtype/mutant heterozygotes, L/F and L/C. The mutation frequency was 5.0-45.16% in the populations of 1996–2001 (Additional file 2: Table S2). In 2005–2009, 222 specimens in 13 samples from 11 provinces were screened, three mutant genotypes, TTT/TTT, TGT/TGT, and TTC/TGT, occurred with frequency of 0.45-21.62% in the seven samples from six provinces, Shandong, Henan, Anhui, Hubei, Jiangxi, and Liaoning. The mutation frequency rose to 30-100%. No mutant alleles were detected in the samples from the other five regions (Additional file 1: Table S1 and Additional file 2: Table S2). In 2010–2014, 308 specimens in 13 samples from seven regions were investigated. The kdr alleles were found in Shandong, Shanghai, Anhui, Shaanxi, and Sichuan (Additional file 1: Table S1 and Additional file: 2: Table S2). In addition to the mutant genotypes mentioned above, two more mutant genotypes, TTC/TTC and TTT/TTC, were detected in Anhui and Sichuan. The mutation frequency was 41.67-100%. No mutant alleles were detected in the samples from Yunnan and Hainan (Additional file 2: Table S2).
Geographically, the kdr alleles were distributed largely in the populations from central China. Therefore, the samples from central China were further analysed. As shown in Table 3, the frequency of wildtype genotype dropped over time, from 80.91% in 1996–2001 to 0.64% in 2010–2014, whilst the frequency of mutant genotypes rose from 0 to 87.18% (Table 3, Figure 2A). Furthermore, the mutant genotypes F/F and F/C were enriched in Shandong, Shanghai and Anhui in 2012–2014 (Table 3). In the regions other than central China, no mutant alleles were detected in the three of four samples from Yunnan (1996–2013) and five samples from Hainan (1997, 2010–2013), and two samples from Guizhou (1997, 2007), one sample from Guangdong (2007), one sample from Fujian (1997) and one sample from Guangxi (2005) (Table 4). Overall, the frequency of wildtype homozygotes was 100% in 1997–2001, and still relatively high as 71.71% in 2010–2014 in these regions (Figure 1, Figure 2B). The analysis revealed that the kdr alleles were enriched in the An. sinensis populations in central China with a trend of progressive increase over time. The kdr alleles were less prevalent in the other regions of China.
Table 3.
The frequency of kdr mutations in the Anopheles sinensis samples in central China
| Sample code | Sample location | Sample size | Wildtype | Wildtype/mutant heterozygote | Mutant genotype | Mutation frequency (%) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mutant homozygote | Mutant heterozygote | ||||||||||||
| TTG(L)/ TTG(L) | TTG(L)/ TTT(F) | TTG(L)/ TTC(F) | TTG(L)/ TGT(C) | TTT(F)/ TTT(F) | TTC(F)/ TTC(F) | TGT(C)/ TGT(C) | TTT(F)/ TTC(F) | TTT(F)/ TGT(C) | TTC(F)/ TGT(C) | ||||
| 1997-2001 | |||||||||||||
| ZZ97 | Henan | 9 | 9 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0.00 |
| XY97 | Jiangsu | 20 | 19 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 5.00 |
| WJ97 | Jiangsu | 24 | 19 | 3 | 0 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 20.83 |
| JN97 | Shandong | 3 | 2 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 33.33 |
| JN00 | Shandong | 31 | 17 | 4 | 0 | 10 | 0 | 0 | 0 | 0 | 0 | 0 | 45.16 |
| NY01 | Henan | 23 | 23 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0.00 |
| Total | 110 | 89 | 7 | 0 | 14 | 0 | 0 | 0 | 0 | 0 | 0 | 19.09 | |
| Genotype frequency(%) | 80.91 | 6.36 | 0.00 | 12.73 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | |||
| 2005-2009 | |||||||||||||
| HF06 | Anhui | 19 | 1 | 4 | 0 | 3 | 8 | 0 | 0 | 0 | 3 | 0 | 94.74 |
| WH06 | Hubei | 24 | 0 | 3 | 0 | 4 | 5 | 0 | 5 | 0 | 7 | 0 | 100.00 |
| SZ07 | Hubei | 13 | 3 | 4 | 0 | 0 | 2 | 0 | 0 | 0 | 4 | 0 | 76.92 |
| ZZ07 | Henan | 10 | 7 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 2 | 0 | 30.00 |
| JN07 | Shandong | 22 | 1 | 0 | 0 | 0 | 16 | 0 | 0 | 0 | 5 | 0 | 95.45 |
| JX09 | Jiangxi | 16 | 0 | 0 | 0 | 0 | 12 | 0 | 0 | 0 | 3 | 1 | 100.00 |
| Total | 104 | 12 | 11 | 0 | 8 | 43 | 0 | 5 | 0 | 24 | 1 | 88.46 | |
| Genotype frequency(%) | 11.54 | 10.58 | 0.00 | 7.69 | 41.35 | 0.00 | 4.81 | 0.00 | 23.08 | 0.96 | |||
| 2010-2014 | |||||||||||||
| JN12 | Shandong | 12 | 0 | 0 | 0 | 0 | 7 | 0 | 1 | 0 | 4 | 0 | 100.00 |
| CX12 | Shandong | 30 | 1 | 11 | 0 | 6 | 10 | 0 | 0 | 0 | 2 | 0 | 96.67 |
| JD12 | Shanghai | 24 | 0 | 0 | 0 | 0 | 12 | 0 | 1 | 0 | 10 | 1 | 100.00 |
| HS13 | Anhui | 60 | 0 | 2 | 0 | 0 | 28 | 1 | 4 | 3 | 20 | 2 | 100.00 |
| XC13 | Anhui | 30 | 0 | 0 | 0 | 0 | 15 | 0 | 2 | 0 | 13 | 0 | 100.00 |
| Total | 156 | 1 | 13 | 0 | 6 | 72 | 1 | 8 | 3 | 49 | 3 | 99.36 | |
| Genotype frequency(%) | 0.64 | 8.33 | 0.00 | 3.85 | 46.15 | 0.64 | 5.13 | 1.92 | 31.41 | 1.92 | |||
Figure 2.
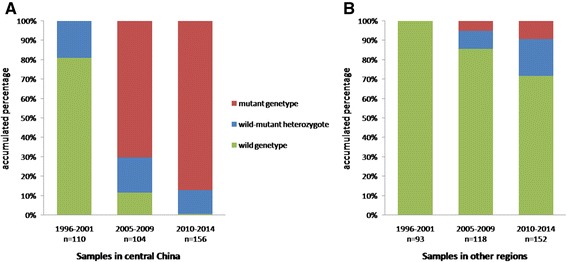
Temporal and spatial trend of kdr genotypes in Anopheles sinensis in China in 1997–2014. (A) Mosquitoes were sampled in Shandong, Jiangsu, Anhui, Shanghai, Hubei, Henan and Jiangxi (data in Table 3). (B) Mosquitoes were sampled in Liaoning, Shaanxi, Guizhou, Sichuan, Chongqing, Yunnan, Hainan, Fujian, Guangdong, and Guangxi (data in Table 4). See Figure 1 for sampling sites on the map.
Table 4.
The frequency of kdr mutations in the Anopheles sinensis samples in other regions
| Sample code | Sample location | Sample size | Wildtype | Wildtype/mutant heterozygote | Mutant genotype | Mutation frequency (%) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mutant homozygote | Mutant heterozygote | ||||||||||||
| TTG(L)/ TTG(L) | TTG(L)/ TTT(F) | TTG(L)/ TTC(F) | TTG(L)/ TGT(C) | TTT(F)/ TTT(F) | TTC(F)/ TTC(F) | TGT(C)/ TGT(C) | TTT(F)/ TTC(F) | TTT(F)/ TGT(C) | TTC(F)/ TGT(C) | ||||
| 1997-2001 | |||||||||||||
| NS96 | Shaanxi | 2 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0.00 |
| PJ96 | Sichuan | 20 | 20 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0.00 |
| SN97 | Guizhou | 16 | 16 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0.00 |
| PR97 | Yunnan | 10 | 10 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0.00 |
| CM97 | Hainan | 7 | 7 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0.00 |
| PJ97 | Sichuan | 18 | 18 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0.00 |
| FJ97 | Fujian | 20 | 20 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0.00 |
| Total | 93 | 93 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0.00 | |
| Genotype frequency(%) | 100.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | |||
| 2005-2009 | |||||||||||||
| GX05 | Guangxi | 22 | 22 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0.00 |
| PR05 | Yunnan | 10 | 10 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0.00 |
| ZT06 | Yunnan | 14 | 6 | 4 | 2 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 57.14 |
| KL07 | Guizhou | 20 | 20 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0.00 |
| GD07 | Guangdong | 26 | 26 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0.00 |
| CQ08 | Chongqing | 16 | 16 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0.00 |
| LN08 | Liaoning | 10 | 1 | 2 | 0 | 1 | 5 | 0 | 0 | 0 | 1 | 0 | 90.00 |
| Total | 118 | 101 | 6 | 2 | 3 | 5 | 0 | 0 | 0 | 1 | 0 | 14.41 | |
| Genotype frequency(%) | 85.59 | 5.08 | 1.69 | 2.54 | 4.24 | 0.00 | 0.00 | 0.00 | 0.85 | 0.00 | |||
| 2010-2014 | |||||||||||||
| PR10 | Yunnan | 17 | 17 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0.00 |
| QZ10 | Hainan | 12 | 12 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0.00 |
| WC11 | Hainan | 14 | 14 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0.00 |
| CJ13 | Hainan | 7 | 7 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0.00 |
| NS13 | Shaanxi | 36 | 21 | 7 | 1 | 1 | 5 | 0 | 1 | 0 | 0 | 0 | 41.67 |
| YJ13 | Yunnan | 15 | 15 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0.00 |
| HK13 | Hainan | 5 | 5 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0.00 |
| JZ14 | Sichuan | 46 | 18 | 16 | 3 | 1 | 4 | 1 | 0 | 2 | 1 | 0 | 60.87 |
| Total | 152 | 109 | 23 | 4 | 2 | 9 | 1 | 1 | 2 | 1 | 0 | 28.29 | |
| Genotype frequency(%) | 71.71 | 15.13 | 2.63 | 1.32 | 5.92 | 0.66 | 0.66 | 1.32 | 0.66 | 0.00 | |||
The allele of codon 1014 in other species of the An. hyrcanus group
A total of 232 specimens from eight other species in the An. hyrcanus group were screened. No kdr alleles were detected. All of these specimens were the homozygotes of the wildtype allele TTG.
Discussion
In China, DDT was largely used in 1950s-1970s, and pyrethroids have been utilized since 1980s when DDT was banned [27]. The applications of pyrethroids for IRS and ITNs have greatly contributed to the success of reducing malarial transmission. The occurrence of pyrethroid resistance in An. sinensis has been documented in many regions since 1980s [28,29]. For example, in Zhejiang [30], Hubei [31], Jiangsu [32], Shandong [33], Yunnan [34], Henan [34], Fujian [35], and Hainan [36]. Recently, Wang et al. conducted a survey of pyrethroid susceptibility in the An. sinensis populations from eight malaria endemic regions including Hubei, Henan, Hunan, Jiangsu, Jianxi, Sichuan, Shanghai and Yunnan [37]. They found that the An. sinensis populations in all examined regions were resistant to deltamethrin. The mortalities were in a range of 5.96-64.54% upon exposure to the diagnostic concentration of 0.25% deltamethrin [37]. The relationship of kdr genotypes and pyrethroid resistance has been investigated in recent years. At codon 1014 of the VGSC gene, five mutant alleles, TTT(F), TTG(F), TGT(C), TCG(S) and TGG(W), have been detected. The TTT(F) allele was the most prevalent mutant, followed by the alleles TGT(C) and TCG(S); the allele TGG(W) occurred rarely [7,16-19,21,34,38,39]. The kdr alleles were present with high frequency largely in the populations in central China, such as Jiangsu, Anhui, Shandong, Hubei, and Henan. For example, Qi and Cui detected the kdr alleles 1014F and 1014C in three populations from Henan, and the kdr allele frequency was associated with the resistant phenotype. However, no kdr alleles were detected in the two populations from Yunnan [34]. In the five populations collected from Jiangsu in 2009–2010, the kdr alleles 1014F and 1014C were detected and the frequency of allele 1014F was correlated with the resistance to beta-cypermethrin in these populations [16]. In the Guangxi collections, the kdr alleles 1014S, 1014F and 1014W were found [20]. In an investigation reported by Zhong et al. the kdr alleles 1014F and 1014C were present with high frequency (88.5-94.8%) in the resistant populations from Hunan, Hubei and Jiangsu in central China [17]. Both kdr alleles and the monooxygenase activity were significantly associated with the deltamethrin resistance, but the monooxygenase activity played a stronger role. On the other hand, no kdr alleles were detected in the two resistant populations in Yunnan, where the resistance was correlated with the monooxygenase activity [17]. A similar pattern was found in another study, in which the kdr mutation L1014F (70.0-88.9%) and L1014C (11.1-26.7%) were detected in the Anhui populations with higher frequency in the resistant mosquitoes [19]. Again, no kdr alleles were found in the mosquitoes from Yunnan. Based on a CART statistical analysis, metabolic detoxification enzymes (monooxygenases, glutathion S-transferase and carboxylesterases) played major roles in resistance to pyrethroids and DDT while kdr alleles weighed less in the context [19]. In Hainan Island, the L/F heterozygotes were present with low frequency (6.7-9.5%) in the DDT and pyrethroid-resistant individuals of the two An. sinensis populations, but no kdr alleles were detected in the sympatric An. vagus [18]. Overall, aforementioned studies demonstrated that the kdr mutations occurred largely in the An. sinensis populations in central China, where the resistance to DDT and pyrethroids was conferred primarily by the metabolic detoxification mechanisms as well as the kdr mutation. In the populations in other regions, the kdr alleles were less prevalent, and the pyrethroid resistance was conferred by the metabolic mechanisms.
In the current study, codon 1014 was examined in a large collection of the An. sinensis samples covering 17 provinces in a time span of 1996 to 2014. The kdr alleles 1014C and 1014F were found in the specimens sampled from Jiangsu and Shandong as early as 1997 (Additional file 1: Table S1). This clearly indicated that the kdr alleles already existed in the An. sinensis populations in 1990s. In line with the findings in the other reports mentioned above, the kdr alleles were more prevalent in central China. The occurrence of kdr alleles has been progressively increasing over time (Figures 1 and 2). For example, in the samples from Shandong that were collected in 1997, 2007, and 2012, the mutation frequency increased from 33.3% in 1997, 95.45% in 2007 to 100% in 2012 (Table 3). In the other regions, the kdr genotypes frequency was lower in Sichuan (0 in 1997 and 60.78% in 2014). Moreover, no kdr alleles were detected in the samples from Yunnan (2005–2013, except ZT06), Guangxi (2005), Fujian (1997), and Hainan (1997–2013) (Table 4). Apparently the pyrethroid resistance in these regions was conferred majorly by the mechanisms other than kdr mutations. Similar situations have been reported in An. gambiae in Africa. In south-western Nigeria, the kdr alleles were not found in the pyrethroid-resistant individuals of the molecular M form of An. gambiae [40]. In a survey conducted in south-western Chad, central Africa, the allele 1014F was frequently present in the resistant S form of An. gambiae, but was not found in the M form or An. arabiensis [41]. The frequent occurrence and accumulation of the kdr alleles in An. sinensis in central China may be explained by their large population size and wide distribution range. The mutant alleles may have had better chances to be selected and maintained in the An. sinensis populations in central China.
In this study, no kdr alleles were detected in all 232 specimens of the other eight member species of the An. hyrcanus group, including 167 specimens of An. lesteri. This resembles a finding reported by Kang et al. [42], in which the kdr alleles were only found in An. sinensis, not in the other five species (Anopheles pullus, Anopheles kleini, An. lesteri and An. belenrae) in the An. hyrcanus group from the Republic of Korea [42]. As a primary malaria vector in China, An. lesteri (previously known as An. anthropaphagus, which was rectified as An. lesteri based on the rDNA ITS2 sequences [23]), has been a major target of the vector control programmes. The extensive implementation of IRS and ITNs in 1990s-2010s has led to a remarkable reduction in An. lesteri abundance in China, which has resulted in a significant drop in malaria morbidity [43-45]. It is intriguing that the low kdr occurrence in some An. sinensis populations and absence in An. lesteri (in this study) given that both mosquitoes had been exposed to strong pyrethroid pressure. Further study is needed to investigate the resistant status and underlying mechanisms in these populations.
Conclusions
The longitudinal survey of the historical samples revealed that the kdr mutations in An. sinensis largely occurred and accumulated progressively over time in central China. The differential kdr mutation distribution patterns suggest that diverse resistance mechanisms occur in different populations. Further studies are required to understand how the kdr alleles disperse among populations.
Acknowledgements
A number of people provided field assistance, mosquito specimens and valuable information used in the study. We are especially grateful to Hongning Zhou and Jinyong Jiang (Yunnan), Manni Yang (Chongqing, Guangdong and Shandong), Guangquan Huang and Guoying Chen (Hubei), Jin Wu (Guangdong), Yaming Huang (Guangxi), YaohuaShi (Guizhou), Peng Li (Henan), Jibo Zhang and Zhe Chen (Liangning), Xixin Chen and Maoqing Gong (Shandong), Xintian Lei and Yu Ha (Sichuan), WeiZhao, Linhai Zeng, Zhonglin Lin and Xiaohua Wang (Hainan), Jiafang Gao (Jiangsu), Lin Lin and Ying Ma (Jiangxi), Youxiang Xu, XiangyuLi, Heng Peng and TongWu (Shanghai) and Baohai Xu (Fujian). This work was funded byYM’s grants from National Natural Science Foundation of China (81371848) and National Natural Science Foundation of China-Yunnan Joint Fund (U0932604). JX was supported by the grants SC2GM092789 and SC1AI112786 from the National Institutes of Health, and the grant DMS-1222592 from National Science Foundation, USA.
Additional files
The frequency of kdr alleles in the Anopheles sinensis samples in China.
The distribution of kdr alleles in the Anopheles sinensis samples in China.
Footnotes
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
All authors made contribution to the collection of mosquitoes. YM and JX designed the study. YM identified specimens by morphological characters. YW and WY did PCR and sequence comparison. YM, JX, YW and ZY did data analysis, YM, JX and YW wrote the manuscript. All authors read and approved the final version of the manuscript.
Contributor Information
Yan Wang, Email: wang1yu2yan@126.com.
Wanqin Yu, Email: ivyyu@nmsu.edu.
Hua Shi, Email: placdc@139.com.
Zhenzhou Yang, Email: pcochina@hotmail.com.
Jiannong Xu, Email: jxu@nmsu.edu.
Yajun Ma, Email: yajun_ma@163.com.
References
- 1.Hemingway J, Ranson H. Insecticide resistance in insect vectors of human disease. Annu Rev Entomol. 2000;45:371–91. doi: 10.1146/annurev.ento.45.1.371. [DOI] [PubMed] [Google Scholar]
- 2.Narahashi T. Neuronalion channels as the target sites of insecticides. Pharmacol Toxicol. 1996;79:1–14. doi: 10.1111/j.1600-0773.1996.tb00234.x. [DOI] [PubMed] [Google Scholar]
- 3.Martinez-Torres D, Chandre F, Williamson MS, Darriet F, Berge JB, Devonshire AL, et al. Molecular characterization of pyrethroid knockdown resistance (kdr) in the major malaria vector Anopheles gambiae s.s. Insect Mol Biol. 1998;7:179–84. doi: 10.1046/j.1365-2583.1998.72062.x. [DOI] [PubMed] [Google Scholar]
- 4.Ndiath MO, Cailleau A, Diedhiou SM, Gaye A, Boudin C, Richard V, et al. Effects of the kdr resistance mutation on the susceptibility of wild Anopheles gambiae populations to Plasmodium falciparum: a hindrance for vector control. Malar J. 2014;13:340. doi: 10.1186/1475-2875-13-340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ibrahim SS, Manu YA, Tukur Z, Irving H, Wondji CS. High frequency of kdr L1014F is associated with pyrethroid resistance in Anopheles coluzzii in Sudan savannah of northern Nigeria. BMC Infect Dis. 2014;14:441. doi: 10.1186/1471-2334-14-441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dabire RK, Namountougou M, Diabate A, Soma DD, Bado J, Toe HK, et al. Distribution and frequency of kdr mutations within Anopheles gambiae s.l. populations and first report of the ace 1 G119S mutation in Anopheles arabiensis from Burkina Faso (West Africa) PLoS One. 2014;9:e101484. doi: 10.1371/journal.pone.0101484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bai L, Zhu GD, Zhou HY, Tang JX, Li JL, Xu S, et al. Development and application of an AllGlo probe-based qPCR assay for detecting knockdown resistance (kdr) mutations in Anopheles sinensis. Malar J. 2014;13:379. doi: 10.1186/1475-2875-13-379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Aizoun N, Aikpon R, Akogbeto M. Evidence of increasing L1014F kdr mutation frequency in Anopheles gambiae s.l. pyrethroid resistant following a nationwide distribution of LLINs by the Beninese National Malaria Control Programme. Asian Pac J Trop Biomed. 2014;4:239–43. doi: 10.1016/S2221-1691(14)60238-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Witzig C, Parry M, Morgan JC, Irving H, Steven A, Cuamba N, et al. Genetic mapping identifies a major locus spanning P450 clusters associated with pyrethroid resistance in kdr-free Anopheles arabiensis from Chad. Heredity (Edinb) 2013;110:389–97. doi: 10.1038/hdy.2012.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Namountougou M, Diabate A, Etang J, Bass C, Sawadogo SP, Gnankinie O, et al. First report of the L1014S kdr mutation in wild populations of Anopheles gambiae M and S molecular forms in Burkina Faso (West Africa) Acta Trop. 2013;125:123–7. doi: 10.1016/j.actatropica.2012.10.012. [DOI] [PubMed] [Google Scholar]
- 11.Lu B, editor. Fauna Sinaca, Insecta, Vol.9, Diptera: Culicidae II. 1. Beijing, China: Science Press; 1997. [Google Scholar]
- 12.Lu B, editor. Integrated mosquito management Second edition. Beijing, China: Science Press; 1999. [Google Scholar]
- 13.Xu LS, Wu QJ, Huang BF, Zhang SY, He QH, Li LS, et al. Observation on the efficacy of deltamethrin against mosquitos in a large scale for malaria control. Strait J Preventive Med. 1995;1:9–11. [Google Scholar]
- 14.Xu JW, Liu H. The development of resistance to the DDT of Anopheles sinensis and Anopheles minimus in Yunnan. Chin J Parasitic Dis Control. 1998;11:358. [Google Scholar]
- 15.Lei XT, Chen JX, Wu YF, Wei HY, Lan YH, Wang Y, et al. Investigation report of Anopheles sinensis Kdr-likes factors detection on high concentrations of pyrethroids. Sichuan Parasitic Dis Control. 1992;20:7–10. [Google Scholar]
- 16.Tan WL, Wang ZM, Li CX, Chu HL, Xu Y, Dong YD, et al. First report on co-occurrence knockdown resistance mutations and susceptibility to beta-cypermethrin in Anopheles sinensis from Jiangsu Province China. PLoS One. 2012;7:e29242. doi: 10.1371/journal.pone.0029242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhong D, Chang X, Zhou G, He Z, Fu F, Yan Z, et al. Relationship between knockdown resistance, metabolic detoxification and organismal resistance to pyrethroids in Anopheles sinensis. PLoS One. 2013;8:e55475. doi: 10.1371/journal.pone.0055475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Qin Q, Li Y, Zhong D, Zhou N, Chang X, Li C, et al. Insecticide resistance of Anopheles sinensis and An. vagus in Hainan Island, a malaria-endemic area of China. Parasit Vectors. 2014;7:92. doi: 10.1186/1756-3305-7-92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chang X, Zhong D, Fang Q, Hartsel J, Zhou G, Shi L, et al. Multiple resistances and complex mechanisms of Anopheles sinensis mosquito: a major obstacle to mosquito-borne diseases control and elimination in China. PLoS Negl Trop Dis. 2014;8:e2889. doi: 10.1371/journal.pntd.0002889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tan WL, Li CX, Wang ZM, Liu MD, Dong YD, Feng XY, et al. First detection of multiple knockdown resistance (kdr)-like mutations in voltage-gated sodium channel using three new genotyping methods in Anopheles sinensis from Guangxi Province, China. J Med Entomol. 2012;49:1012–20. doi: 10.1603/ME11266. [DOI] [PubMed] [Google Scholar]
- 21.Wu S, Zhang G, Xu X, Wang D, Yang F, Zhang S, et al. Knockdown resistance mutations found in voltage-gated sodium channel genes of Anopheles sinensis in Anhui Province. J Pathol Biol. 2010;5:922–4. [Google Scholar]
- 22.Ma YJ, Qu FY, Cao YC, Yang BJ. On molecular identification and taxonomic status of Anopheles lesteri and Anopheles anthropophagus in China (Diptera: Culicidae) Chin J Parasitol Parasit Dis. 2000;18:325–8. [PubMed] [Google Scholar]
- 23.Ma Y, Xu J. The Hyrcanus group of Anopheles (Anopheles) in China (Diptera: Culicidae): species discrimination and phylogenetic relationships inferred by ribosomal DNA internal transcribed spacer 2 sequences. J Med Entomol. 2005;42:610–9. doi: 10.1093/jmedent/42.4.610. [DOI] [PubMed] [Google Scholar]
- 24.Post RJ, Millest AL. Sample size in parasitological and vector surveys. Parasitol Today. 1991;7:141. doi: 10.1016/0169-4758(91)90279-W. [DOI] [PubMed] [Google Scholar]
- 25.Ma Y, Yang M, Fan Y, Wu J, Xu J. Population structure of the malaria vector Anopheles sinensis (Diptera: Culicidae) in China: two gene pools inferred by microsatellites. PLoS One. 2011;6:e22219. doi: 10.1371/journal.pone.0022219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yang M, Ma Y, Wu J. Mitochondrial genetic differentiation across populations of the malaria vector Anopheles lesteri from China (Diptera: Culicidae) Malar J. 2011;10:216. doi: 10.1186/1475-2875-10-216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hua X, Shan Z. Production, usage and environment pollution factor of agrochemical in China. Adv Environm Sci. 1996;4:33–45. [Google Scholar]
- 28.Cui F, Raymond M, Qiao CL. Insecticide resistance in vector mosquitoes in China. Pest Manag Sci. 2006;62:1013–22. doi: 10.1002/ps.1288. [DOI] [PubMed] [Google Scholar]
- 29.Liu S, Cui F, Yan S, Qiao C. Investigation of organophosphate and pyrethroid resistance in vector mosquitoes in China. Chin J Vector Biol Control. 2011;22:184–9. [Google Scholar]
- 30.Wang J. Resistance to two pyrethroids in Anopheles sinensis from Zhejiang, China. J Am Mosq Control Assoc. 1999;15:308–11. [PubMed] [Google Scholar]
- 31.Yu P, Xu B, Wu K, Xia S. Resistance monitoring of three dominant vector mosquito species in Shashi, Hubei Province. Hubei J Prevent Med. 1993;4:13–5. [Google Scholar]
- 32.Li J, Zhou H, Cao G, Wang W, Gu Y, Liu Y, et al. Sensitivity of Anopheles sinensis to insecticides in Jiangsu Province. Chin J Schisto Control. 2011;23:296–300. [PubMed] [Google Scholar]
- 33.Liu H, Cheng P, Wang H, Liu L, Huang X, Dai Y, et al. Habits and insecticide resistance of Anopheles sinensis in Shandong province, China, 2008–201 1. Chin J Vector Biol Contr. 2013;24:17–8. [Google Scholar]
- 34.Qi X, Cui J. Investigation on the resistance of Anopheles sinensis to deltamethrin and its association with the kdr genotypes in Yunnan province and parts of Henan province. Chin J Vector Biol Contr. 2012;23:98–104. [Google Scholar]
- 35.Wu J, He X, Liu Q. Study of the insecticide sensitivity of malaria vectors in northwest area of Fujian Province. Chin J Vector Biol Control. 1994;5:71–3. [Google Scholar]
- 36.Zeng L, Wang S, Sun D, Zhao W, Li S, Yang X. Resistance assay of malaria vectors to four kinds of common insecticides in some endemic areas of Hainan Province. Chin J Parasitol Parasit Dis. 2011;29:200–3. [PubMed] [Google Scholar]
- 37.Wang DQ, Xia ZG, Zhou SS, Zhou XN, Wang RB, Zhang QF. A potential threat to malaria elimination: extensive deltamethrin and DDT resistance to Anopheles sinensis from the malaria-endemic areas in China. Malar J. 2013;12:164. doi: 10.1186/1475-2875-12-164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fu FY, Chen B, Zhong D. The association between deltamethrin resistance and kdr mutation in Anopheles sinensis in Chongqing, China. Acta Parasitol Med Entomol Sinica. 2013;20:25–30. [Google Scholar]
- 39.Chang XL, Xue YQ, Zhang AD, Zhu GD, Fang Q. Deltamethrin resistance, metabolic detoxification enzyme and kdr mutation in Anopheles sinensis in region along Huaihe River in Anhui Province. Chinese J Schisto Control. 2013;25:263–7. [PubMed] [Google Scholar]
- 40.Awolola TS, Brooke BD, Koekemoer LL, Coetzee M. Absence of the kdr mutation in the molecular ‘M’ form suggests different pyrethroid resistance mechanisms in the malaria vector mosquito Anopheles gambiae s.s. Trop Med Int Health. 2003;8:420–2. doi: 10.1046/j.1365-3156.2003.01034.x. [DOI] [PubMed] [Google Scholar]
- 41.Kerah-Hinzoumbe C, Peka M, Nwane P, Donan-Gouni I, Etang J, Same-Ekobo A, et al. Insecticide resistance in Anopheles gambiae from south-western Chad. Central Africa Malar J. 2008;7:192. doi: 10.1186/1475-2875-7-192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kang S, Jung J, Lee S, Hwang H, Kim W. The polymorphism and the geographical distribution of the knockdown resistance (kdr) of Anopheles sinensis in the Republic of Korea. Malar J. 2012;11:151. doi: 10.1186/1475-2875-11-151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Xu BL, Su YP, Shang LY, Zhang HW. Malaria control in Henan Province, People’s Republic of China. Am J Trop Med Hyg. 2006;74:564–7. [PubMed] [Google Scholar]
- 44.Tang LH. Anopheles anthropophagus in China: Biology and control. 1. Shanghai, China: Shanghai Science and Technical Publishers; 2008. [Google Scholar]
- 45.Liu XZ, Xu BL. Malaria situation and evaluation on the control effect in Henan Province during 1990–2005. Chin J Parasitol Parasit Dis. 2006;24:226–9. [PubMed] [Google Scholar]


