Abstract
Macrophages (Mø) ingest apoptotic cells with unique effects on their cytokine production, but the signaling pathways involved are virtually unknown. Signal transduction in response to recognition of apoptotic thymocytes by resident murine alveolar (AMø) or peritoneal (PMø) Mø was studied by in vitro phagocytosis assay. Phagocytosis was decreased in a dose-dependent and non-toxic fashion by inhibiting phosphatidylinositol 3 kinase (wortmannin and LY294002), protein tyrosine phosphorylation (herbimycin A, genistein, piceatannol and, for AMø only, PP2), and protein kinase C (staurosporine, Gö 6976 and calphostin C). Exposure of Mø to apoptotic or heat-killed thymocytes, but not to viable thymocytes, rapidly activated ERK1/2, as detected by specific phosphorylation, but did not activate NF-κB or MAP kinases p38 or JNK. Mø phagocytosis of apoptotic T cells requires tyrosine, serine/threonine, and lipid phosphorylation. Mø recognition of apoptotic T cells triggers rapid but limited MAP kinase activation.
Keywords: Apoptosis, Phagocytosis, Lung, Signal transduction, Protein Kinases/Phosphatases
Introduction
Apoptotic cells must be cleared by phagocytosis during ontogeny and in the resolution of inflammation [1, 2]. Almost any cell can eliminate the shrunken remnants of adjacent apoptotic cells, but only macrophages (Mø) can expediently clear large numbers of apoptotic leukocytes dying during waning immune responses [3-6]. In most organs, this clearance process is believed to function with great efficiency, so that even in the thymus where millions of thymocytes are eliminated daily, it has been difficult to demonstrate uningested apoptotic cells in vivo [7]. The case appears to be different, however, in the lungs of mice, where apoptotic lymphocytes are easily found, both in normal mice and during a secondary pulmonary immune response [8]. This defect in clearance appears to result because the principal resident lung phagocytes, alveolar macrophages (AMø), exhibit markedly reduced phagocytosis of apoptotic leukocytes, either compared to inflammatory lung Mø (in rabbits) [9], or to resident peritoneal Mø (PMø) (in mice) [10]. In the latter system, the disparity between the two Mø types was not due to kinetic differences, was seen with seven inbred mouse strains, and was not detected using two other particles (carboxylate-modified polystyrene microbeads and opsonized zymosan), excluding a global defect in phagocytosis by AMø [10]. Notably, the AMø defect was also observed in vivo [10]. Defining the basis and significance of this altered phagocytosis could provide fundamental insights into the regulation of regional immunity in the lungs, a site of frequent exposure to pathogens and of many immunologic diseases.
A variety of surface receptors have been implicated in recognition and phagocytosis of apoptotic cells {reviewed in [11]}. Altered expression of one or more of these receptors is a potential explanation for the observed deficit in phagocytosis of apoptotic thymocytes by murine AMø. However, although we [10] and others [12] have identified a number of disparities between AMø and PMø by analysis of surface receptors implicated in this process, our previous blocking experiments did not show any of these differences to be responsible for the phagocytic defect [10]. Decreased ingestion could also result from differences in post-receptor signal transduction in AMø. Relatively little is known about signal transduction following recognition of apoptotic cells by mammalian phagocytes [13-15].
The goal of this study was to identify potential signal transduction pathways necessary for phagocytosis of apoptotic cells by resident murine tissue Mø. To this purpose, we took two complementary approaches. First, we used pharmacological inhibitors of enzymes in three pathways previously identified to be involved in Mø phagocytosis mediated by the better studied FcγR system [16, 17]. The inhibitors used were wortmannin and LY294002 for phosphatidylinositol 3 kinase (PI-3K); herbimycin A, genistein, PP2 and piceatannol for protein tyrosine kinases (PTK); and staurosporine, Gö 6976, and calphostin C for protein kinase C (PKC). Although none of these inhibitors is absolutely specific for a single enzyme family, they are nevertheless useful screening reagents that have been used as an initial step in the definition of many signaling systems. Second, we examined possible consequences of apoptotic cell recognition on the downstream signaling components NF-κB and the three families of MAP kinases (SAPK/JNKs, p38 kinase and ERK 1/2), all of which are activated by FcγR-mediated phagocytosis [18-21]. We found marked decrease in phagocytosis using enzyme inhibitors of the three relatively upstream signaling components (PI-3K, PTK, and PKC), providing evidence for multiple signal transduction events during Mø phagocytosis of apoptotic cells. Additionally, we found that exposure to apoptotic thymocytes (or to a small percentage of necrotic thymocytes), but not to viable thymocytes, rapidly induced activation of ERKs 1/2, but not NF-κB, JNKs or p38 kinase.
MATERIALS AND METHODS
Reagents
Herbimycin A, genistein, and staurosporine were purchased from Sigma (St. Louis, MO). The protein tyrosine kinase inhibitor PP2 (4-Amino-5-(4-chlorophenyl)-7-( t-butyl)pyrazolo {3,4-d}pyrimidine), and its inactive control PP3 (4-Amino-7-phenylpyrazolo {3,4-d}pyrimidine), piceatannol, calphostin C, Gö 6976, PD98059 and SB203580 were purchased from Calbiochem Novabiochem Corp. (San Diego, CA). LY294002 was purchased from Biomol (Plymouth Meeting, PA). Calphostin was light-activated before use, as recommended by the manufacturer.
Mice
Pathogen-free C57BL/6 female mice were used in all experiments. Mice were purchased from Charles River Laboratory Inc. (Wilmington, MA) at 7-8 weeks of age and used at 8-14 weeks of age. Mice were housed in the Animal Care Facility at the Ann Arbor VA Medical Center, which is fully accredited by the American Association for Accreditation of Laboratory Animal Care, where they were fed standard animal chow (Rodent Lab chow 5001, Purina; St. Louis, MO) and chlorinated tap water ad libitum. This study complied with the NIH “Guide for the Care and Use of Laboratory Animals” {Department of Health, Education, & Welfare Publication No. (NIH) 80-23} and followed a protocol approved by the Animal Care Committee of the local Institutional Review Board.
Isolation and culture of Mø
Mice were euthanized by asphyxia in a high CO2 environment, which we have previously shown does not impair the capacity of AMø to ingest apoptotic thymocytes compared to mice euthanized by exsanguination while anesthetized using pentobarbital [10]. Resident AMø and PMø were harvested and cultured as previously described in detail [10]. PMø among the peritoneal lavage cells were first enriched by negative selection using CD19- and CD90-conjugated paramagnetic beads (Miltenyi Biotec; Auburn, CA) according to the manufacturer's instructions. Mø were plated at 2 ×105 cells/well in sterile 8-well Lab-Tek slides (Nalge Nunc International; Naperville, IL) and, after 1 hour incubation at 37° C, nonadherent cells were removed by gentle washing. Mø monolayers were cultured overnight in complete medium {RPMI 1640 containing 10% heat-inactivated FBS, 25 mM HEPES, 2 mM L-glutamine, 1 mM pyruvate, 100 units/ml penicillin/streptomycin (all obtained from GIBCO-BRL) and 55 μM 2-mercaptoethanol (Sigma; St. Louis, MO)} in a 5% CO2 environment at 37° C before use in the phagocytosis assay.
Isolation and apoptosis induction in thymocytes and cloned T cells
Thymuses were harvested from normal mice and minced to yield a single cell suspension. To induce apoptosis, thymocytes were resuspended with RPMI 1640 containing 10% heat-inactivated FBS at the concentration of 1 × 106/ml and incubated for 6 h with a final concentration of 10−6 M dexamethasone (Sigma). This treatment yields a population with a low percentage of late apoptotic (11.4 +/− 1.6%, mean ± SEM, n=7 experiments) as judged by positivity for both annexin V and PI staining.
Apoptosis assay
Leukocyte apoptosis was measured by flow cytometric analysis of surface expression of phosphatidylserine (PS) and exclusion of propidium iodide (PI), a sensitive and specific measure of early apoptosis [22, 23]. For this purpose, 100 μl aliquots were stained with annexin V-FITC (Apoptosis Detection Kit; R & D Systems; Minneapolis, MN) according to the manufacturer’s protocol. Cells were analyzed without fixation by flow cytometry within 1 hour of staining.
Opsonization of Ig-SRBC
SRBC (Colorado Serum; Boulder, CO) (1 ml in Alsever’s solution) were washed twice in 15 ml PBS without cations. SRBC were resuspended (1.6 × 107 cells in 1.6 ml final volume) in PBS containing rabbit anti-SRBC antisera (Cedarlane Laboratories Ltd.; Hornby, ON, Canada) (1 μg/2 × 106 SRBC), and were incubated for 20 min at 37°C. These conditions were determined to be optimal by agglutination. SRBC were washed twice in 15 ml PBS without cations, resuspended at 1 × 107/ml in complete medium, and then 200 μl/well was added to the Mø monolayers.
Phagocytosis assays
Phagocytosis of apoptotic thymocytes in vitro was assayed by co-incubation with adherent Mø monolayers in complete medium as previously described [10]. Results were expressed as percentage of Mø containing at least one ingested thymocyte (percent phagocytic Mø), and as phagocytic index, which was generated by multiplying the percent phagocytic Mø by the mean number of phagocytosed cells per Mø. Phagocytosis of Ig-SRBC was performed in exactly the same manner, except that Ig-SRBC were substituted for apoptotic thymocytes.
Western analysis of signaling intermediaries
Mø isolated as above were seeded at a density of 4 × 105 cells/well in complete medium in 24-well tissue culture plate (Becton-Dickinson) and purified by overnight adherence. This method results in >95% pure Mø populations as determined by morphological and surface marker expression analysis. Apoptotic thymocyte (4 × 106/well) were added and cultures were incubated in 5% CO2 at 37°C for various times. Next, Mø were washed twice in D-PBS containing 100 mM sodium orthovanadate, and then lysed in 50 μl of ice-cold lysis buffer (50 mM HEPES pH 7.5, 150 mM NaCl, 1 mM EDTA, 2 mM EGTA, 1% Triton X-100, 10 mM sodium fluoride, 1 mM sodium orthovanadate and 1x protease inhibitor cocktail (Set III, Calbiochem-Novabiochem). Cytoplasmic lysates were electrophoresed in a 12.5% SDS-PAGE under reducing condition, and proteins were transferred to a solid support membrane (PVDF, polyvinylidene difluoride, Millipore) using 10 mM CAPS {3-(Cyclohexylamino)-1-propanesulfonic acid} (Calbiochem-Novabiochem) pH 10.0, 5% methanol as transfer buffer, as previously described [24].
After incubating membranes in blocking buffer (5% protease-free, immunoglobulin-free BSA (Sigma) in TBST (100 mM Tris-HCl pH 7.5, NaCl 145 mM, 0.05% Tween 20), primary antibodies were added and membranes were incubated overnight at 4°C. The antibodies used were anti-pan ERK, anti-pan JNKs and anti-pan p-38 (Santa Cruz Biotech., Santa Cruz, CA), anti-IkB-α, anti-phospho-IkB-α, anti-phospho-ERK1/2 and anti-phospho-p38 (Cell Signaling, Beverly, MA) and anti-phospho-JNKs (Promega, Madison, WI). Membranes, washed twice in TBST, were incubated with the appropriate horseradish peroxidase-conjugated secondary antibody (Pierce, Rockford, IL). Chemiluminescence was developed by adding a peroxidase/luminol-based substrate (SuperSignal West Femto Maximum sensitivity substrate, Pierce). Signals were detected using radiographic film (X-Omat, Kodak, NJ). For reprobing, blots were washed twice in TBST and incubated for 30 min at 55°C in a buffer containing 10 mM Tris-HCl, pH 6.8, 2% SDS and 100 mM 2-mercaptoethanol.
Statistical analysis
Data were expressed as mean ± SEM. Statistical calculations were performed using Statview and SuperANOVA programs (Abacus Concepts, Inc.; Berkeley, CA) on a Macintosh PowerPC G3 computer. Continuous ratio scale data were evaluated by unpaired Student t test (for two samples); use of this parametric statistic was deemed appropriate, as phagocytosis of apoptotic thymocytes by PMø has been shown to follow a Gaussian distribution [25]. Significant differences were defined as p<0.05. The IC50 of pharmacological inhibitors was calculated from dose-response curves using the phagocytic index as the reference variable.
RESULTS
FcR-mediated phagocytosis by murine AMø and by PMø is equivalent
Because the best studied mechanism of Mø phagocytosis utilizes FcγR, we first compared resident murine AMø and PMø for ingestion of opsonized SRBC to assure that these two cell types did not differ in this receptor system. We have previously shown equivalent phagocytosis by AMø and PMø using opsonized zymosan [10], but that result is a less rigorous test of FcR-mediated ingestion, as zymosan clearance also involves receptors for β-glucan and mannose. AMø showed the same ability as PMø to ingest Ig-SRBC (21.7 ± 3.8% phagocytic for AMø versus 17.0 ± 2.1% for PMø, p = 0.29, unpaired t test; phagocytic index 0.3 ± 0.1 for AMø versus 0.2 ± 0.0 for PMø, p = 0.13, unpaired t test; mean ± SEM of 8 mice per group assayed individually). Together with our previous finding that these two Mø types have equivalent capacity to ingest carboxylate-modified polystyrene microbeads [10], these results indicate that the defect in ingestion of apoptotic thymocytes by murine AMø is highly specific.
Inhibitors of PI-3K and of PKC activity block phagocytosis of apoptotic thymocytes by resident tissue Mø
Profound and uniform inhibition of ingestion was seen using the chemically unrelated PI-3K inhibitors wortmannin (IC50 5 nM for PMø, 8 nM for AMø) and LY294002 (IC50 ≤30 μM for PMø, ≤ 23 μM for AMø) (Fig. 1). Careful microscopic examination of these slides disclosed that with both agents Mø bound but did not fully engulf the apoptotic thymocytes, extending only short pseudopodia as has previously been described with PI-3K inhibition in FcγR-mediated phagocytosis [17, 26]. Measurement of Mø viability by annexin V-FITC and PI staining confirmed that neither of these inhibitors, nor any of the others used in this study, induced significant Mø toxicity at the concentrations used (data not shown).
Figure 1.
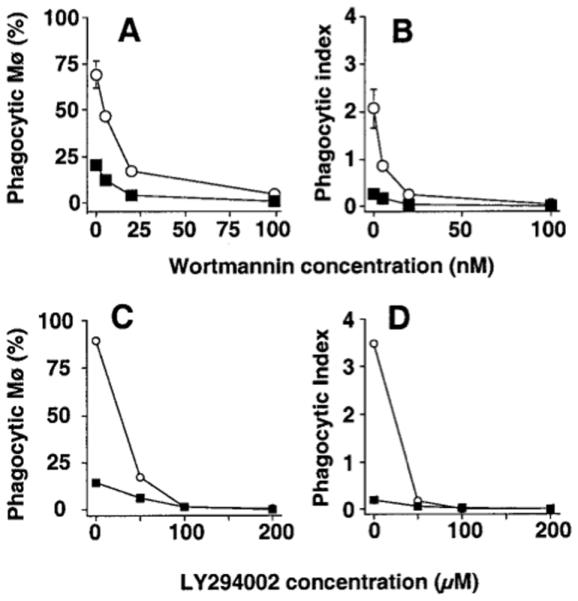
Inhibition of PI-3K blocks Mø ingestion of apoptotic thymocytes. A, B. Wortmannin dose-response. Resident PMø (open circles) and AMø (closed squares) from normal C57BL/6 mice (2 × 105 cell in 400 μl) were pre-incubated with various concentration of wortmannin for 60 min at 37°C in chamber slides and then co-incubated with 2 × 106 apoptotic thymocytes in the same concentration of the inhibitor for 90 minutes. Slides then were washed, fixed, and stained with H & E before phagocytosis was determined. Data are percentage phagocytosis positive Mø (A) and phagocytic index (B) as mean ± SEM of at least three replicates in a single experiment for each inhibitor. C, D. LY294002 dose-response. AMø or PMø from normal mice were pre-incubated with various concentrations of LY294002 for 30 min and then assayed as described for wortmannin-treated cells. Data are mean ± SEM of 4-8 replicates.
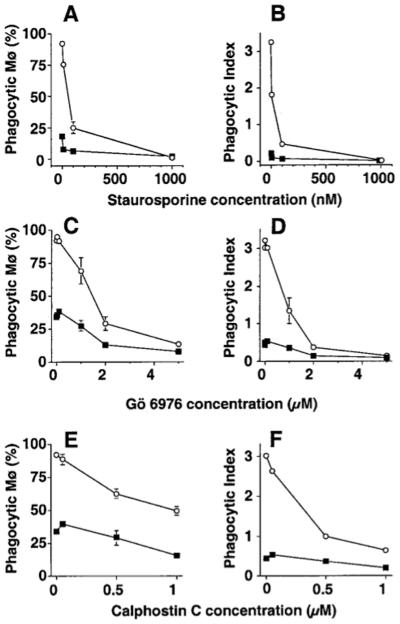
Suppression of phagocytosis was also seen using PKC inhibitors, although the degree of inhibition varied with the individual inhibitor (Fig. 2). Profound suppression was seen using the non-specific inhibitor staurosporine (IC50 16 nM for PMø, 10 nM for AMø) and with the nonglycosidic indolocarbazole Gö 6976 (IC50 1 μM for PMø, 1.7 μM for AMø), whereas less marked inhibition was seen in both Mø types using calphostin C (IC50 0.33 μM for PMø, 0.92 μM for AMø).
Figure 2.
Inhibition of PKC blocks Mø ingestion of apoptotic thymocytes. Resident PMø (open circles) and AMø (closed squares) from normal C57BL/6 mice were pre-incubated with various concentration of staurosporine (A, B), Gö 6976 (C, D), or Calphostin C (E, F) for 30 min at 37°C, were co-incubated with apoptotic thymocytes in the same concentration of the inhibitor for 90 minutes, and then were assayed for phagocytosis. (A, C, E) Percentage of phagocytic Mø; (B, D, F) phagocytic index. Data are mean ± SEM of at least three wells per point in a single experiment.
Inhibitors of protein tyrosine phosphorylation block phagocytosis of apoptotic thymocytes by resident tissue Mø
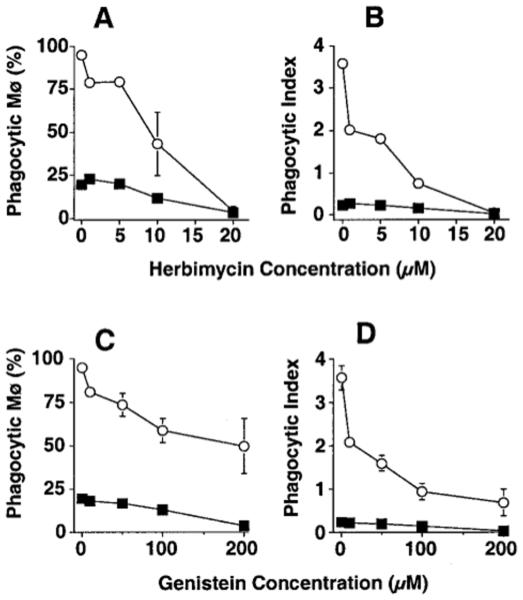
We also observed a dose-dependent decrease in phagocytosis of apoptotic cells by both types of tissue Mø using the broad spectrum PTK inhibitors herbimycin A (IC50 3.6 μM for PMø, 12.2 for AMø) and genistein (IC50 33 μM for PMø, 124 μM for AMø) (Fig. 3). Maximal inhibition by herbimycin A was somewhat greater than by genistein (e.g., for percent phagocytic PMø, 68.1 ± 11.3% inhibition using herbimycin 15 μM versus 20.0 ± 2.7% using genistein 100 μM, p=0.015, unpaired t test; for phagocytic index 83.0± 6.3% inhibition using herbimycin 15 μM versus 49.0 ± 3.3% using genistein 100 μM; p<0.0001, unpaired t test; mean ± SEM, n = 8).
Figure 3.
Inhibition of protein tyrosine phosphorylation blocks Mø ingestion of apoptotic thymocytes. Resident PMø (open circles) and AMø (closed squares) from normal C57BL/6 mice (2 × 105 cell in 400 μl) were pre-incubated with various concentration of herbimycin A (A, B) or genistein (C, D) for 30 min at 37°C, were co-incubated with apoptotic thymocytes in the same concentration of the inhibitor for 90 minutes, and then were assayed for phagocytosis. Data are percentage phagocytosis positive Mø (A, C) and phagocytic index (B, D) as mean ± SEM of at least three replicates in a single experiment.
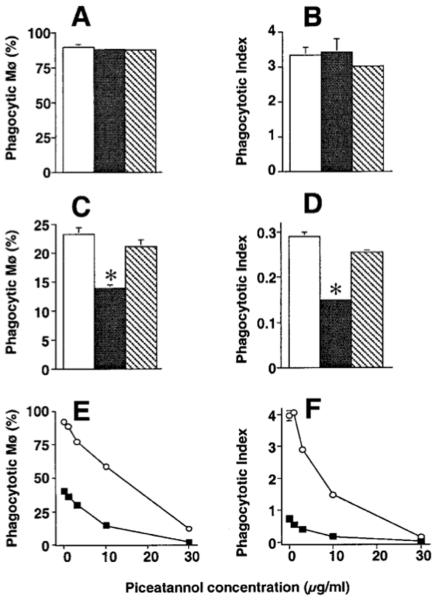
To further investigate the role of PTKs, we used more selective PTK inhibitors, basing our choices on the results in T cells, where TCR ligation leads first to activation of the Src family members Lck and Fyn, followed by activation of the Syk family member ZAP-70. Surprisingly, PP2, a specific inhibitor of Src family PTKs, revealed a difference between the two Mø types. No inhibition of ingestion by PMø was seen at 30 μM (Fig 4A & 4B) or in a separate experiment at 50 μM (not shown) when compared to the inactive control substance PP3, whereas in both experiments ingestion by AMø was significantly inhibited by roughly half by PP2 (Fig. 4C, D). In control experiments, these doses of PP2 inhibited uptake of Ig-SRBC by both AMø and PMø (data not shown), confirming the potency of the inhibitor preparation. By contrast, marked and dose-dependent inhibition was seen in both types of Mø using the Syk-specific inhibitor piceatannol (IC50 32 nM for PMø, corresponding to a dose of 8 μg/ml, 48 nM for AMø, corresponding to a dose of 12 μg/ml) (Fig 4E & 4F).
Figure 4.
Effect of specific PTK family inhibitors on Mø ingestion of apoptotic thymocytes. A-D: Src family inhibitors. Resident PMø (A, B) and AMø (C, D) from normal C57BL/6 mice were pre-incubated with medium (open squares) or with medium containing 30 μM PP2 (dark stippled) or the inactive control PP3 (light cross-hatched) for 10 min at 37°C and then co-incubated with apoptotic thymocytes as previously described in the same concentration of the inhibitor for 90 minutes. Note differences in scales between PMø and AMø. Similar results were obtained in a separate experiment using PP2 and PP3 at 50 μM. E, F: Syk family inhibitor. Resident PMø (open circles) and AMø (closed squares) from normal C57BL/6 mice were pre-incubated with various concentration of piceatannol for 10 min at 37°C and then co-incubated with apoptotic thymocytes in the same concentration of the inhibitor for 90 minutes. Data are percentage phagocytosis positive Mø (A, C, E) and phagocytic index (B, D, F) as mean ± SEM of at least three replicates in a single experiment. Similar results were found in a separate experiment. *, p<0.05, unpaired t test.
Preliminary experiments in which we analyzed adhesion of apoptotic thymocytes by the two types of Mø (B. Hu and J.L. Curtis, manuscript in preparation) rather than phagocytosis indicated that the current results were not due to an effect on binding to the thymocytes by any of the inhibitors used here (data not shown).
Phagocytosis of apoptotic thymocytes by resident tissue Møs specifically activates ERK MAP kinases
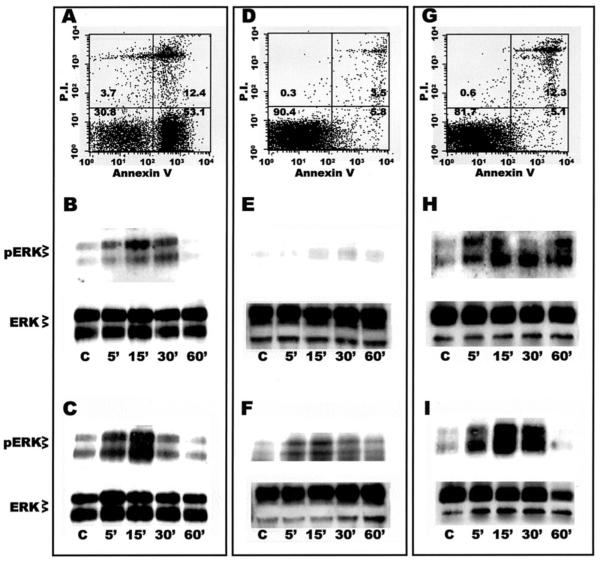
To examine more downstream signaling events proceeding from phagocytosis of apoptotic thymocytes, we next assayed the phosphorylation state of MAP kinases by Western analysis. Flow cytometric analysis confirmed that the majority of the thymocytes in the mixture were in early apoptosis. In the example shown (Fig. 5A), 53.1% were positive for annexin-FITC but negative for PI, whereas double-positivity, indicating cells in late apoptosis, was seen in only 12.4%. Exposure of both types of Mø to these apoptotic cells induced specific phosphorylation of ERK1/2, which is necessary for its activation and for more distal signal transduction. Phosphorylation of both the p42 and p44 forms of ERK was seen in both AMø (Fig. 5B) and in PMø (Fig. 5C), and, interestingly, was observed at 5 min and maximal at 15 min, before appreciable phagocytosis was detected {[10] and unpublished observation}. In both Mø types, ERK phosphorylation then decreased to basal levels by 60 min. ERK phosphorylation was more rapid and somewhat greater in PMø, probably reflecting differences in the kinetics of interaction of the two types of Mø with apoptotic cells (B. Hu and J.L. Curtis, manuscript in preparation). No ERK phosphorylation was detected in an equivalent number of the apoptotic thymocytes themselves (data not show).
Figure 5.
Mø exposure to apoptotic or necrotic, but not viable, thymocytes rapidly activates ERK 1/2. A, D, G. Representative flow cytometric analysis of thymocyte viability. Thymocyte preparations were stained with annexin V-FITC and PI; numbers indicate the percentage of cells in each quadrant. A, thymocytes made apoptotic by 6 hour treatment with dexamethasone; D, freshly-isolated thymocytes (note virtual absence of cells showing annexin V-FITC or PI staining); G, mixture of 12% necrotic thymocytes (30 minute incubation at 56°C) and freshly-isolated thymocytes (note double staining with annexin V-FITC and PI, and virtual absence of cells staining for PI alone). B, C, E, F, H, I. Western analysis of Mø phospho-ERK (pERK) and total ERK (ERK) expression during exposure to various types of thymocytes. Resident AMø (B, E, H) and PMø (C, F, I) from normal C57BL/6 mice (4 × 105 cells per well in 24 well plates) were incubated for the indicated times with 4 × 106 thymocytes which were either apoptotic (B, C), freshly-isolated (E, F), or a mixture of necrotic and freshly isolated (H, I). Cytoplasmic lysates, corresponding to 400,000 Mø/lane, were electrophoresed using 12.5% SDS-PAGE run under reducing conditions, transferred by electrophoration to PVDF membranes, and immunoblotted using phospho-specific anti-ERK 1/2 Ab (top row in each panel), and then stripped and reprobed with an anti-pan ERK 1/2 Ab as a loading control (bottom row in each panel). These data are representative of two separate experiments with similar results.
By contrast, exposure to apoptotic cells did not induce specific phosphorylation of either of the other MAP kinase species, p38 kinase, or JNKs (not shown). We next assessed activation of NF-κB, measuring phosphorylation and degradation of IkB, which is necessary and sufficient to release NF-κB from the cytoplasm and permit its nuclear translocation [27]. Ingestion of apoptotic thymocytes did not induce activation of NF-κB in either type of resident murine Mø. Control experiments using LPS stimulation confirmed the ability of these assay systems to detect phosphorylation of IκB and of all three MAP kinases in both Mø types (data not shown). Thus, the activation of downstream serine-threonine kinases on recognition of apoptotic cells appears limited to ERK.
The prompt phosphorylation of ERK1/2 on contact with apoptotic thymocytes raised the question if viable thymocytes in the mixture {e.g., 30.8% in the experiments shown (Fig 5A)} contributed to ERK activation. To test this possibility, Mo monolayers were incubated for various times with freshly-isolated thymocytes (Fig. 5D), and then Mø lysates were tested for time-dependent phosphorylation of MAP kinases or of IκB. Minimal ERK phosphorylation (Figs. 5E & 5F) and no evidence of activation of the other signaling intermediaries (data not shown) was seen. It should be noted that even these freshly-isolated thymocytes contained 5.8% annexin V-positive, PI-negative cells and 3.5% double-positive cells (presumably as the result of cell death during isolation). These results implied that recognition of an apoptotic cell, rather than the simple process of cell contact, induced the transient ERK phosphorylation seen in the earlier experiments.
However, the preparations of apoptotic thymocytes used in those experiment inevitably contained some late apoptotic cells {e.g., 12.4% in Fig 5.A}. To address the possibility that ERK activation might result from this small fraction, rather than from the much larger fraction of early apoptotic cells, we performed a variety of experiments. We attempted to induce pure necrosis in thymocytes by previously described methods, including freeze-thaw cycles and heating to 56°C [28, 29]. In our hands, the former process never yielded intact cells, but instead resulted almost entirely in cell fragments, the vast majority of which were annexin V-positive. Heating the cells for a variety of times from 15-60 minutes did produce a uniform preparation of intact cells which were PI-positive. However, all thymocytes which were PI-positive were also annexin V-positive, indicating that they had externalized phosphatidylserine (Fig. 5G). Given the importance of the PS receptor for ingestion of apoptotic cells [30] (and unpublished data), it was thus not surprising that in control experiments these double-positive thymocytes were readily ingested by PMø (data not shown).
Nevertheless, we attempted to determine whether such “necrotic” cells could account for the ERK activation seen in our earlier experiments. To this purpose, we mixed viable thymocytes with a final concentration of 12% thymocytes rendered necrotic by heating to 56°C for 30 minutes. This fraction of necrotic cells was chosen to mimic the percentage of late apoptotic cells seen in our earlier experiments. Western analysis showed induction of ERK1/2 activation that was identical in both magnitude and kinetics in both types of tissue Mø (Figs. 5H & 5I) to that seen using dexamethasone-treated thymocytes (Fig. 5B & 5C). Hence, we cannot formally exclude the possibility that ERK activation results from contact with the late apoptotic cells alone. As in the experiments involving thymocytes assayed 6 hours after dexamethasone treatment, no phosphorylation of p38, JNK, or IκB was seen on exposure to the mixture of viable and necrotic thymocytes (not shown).
The very early timing of ERK phosphorylation led us to question whether ERK activation might be required for the phagocytic process itself. To test this possibility, we pre-incubated Mø with PD98059 (5-50 μM), which specifically blocks ERK phosphorylation, or as a control with SB203580 (1-10 μM), a specific inhibitor of p38 kinase activation. Neither treatment decreased subsequent phagocytosis of apoptotic thymocytes by either type of Mø (not shown), indicating that ERK activation is a consequence rather than a participant in the phagocytic process, in agreement with previous findings for FcγR-mediated phagocytosis [31].
DISCUSSION
The findings of this study define in broad outlines the signal transduction events involved in phagocytosis of apoptotic cells. Phagocytosis of apoptotic thymocytes by resident murine tissue Mø was severely decreased by pharmacological inhibitors of PI-3K activity, of PKC activity, or of protein tyrosine phosphorylation. These effects were seen both with PMø, which avidly ingest apoptotic thymocytes, and with AMø, which do not. Inhibitory effects were not due to Mø toxicity, and with the single exception of the Src inhibitor PP2, both Mø cell types showed similar dose-responses. Incubation with apoptotic thymocytes, but not with viable thymocytes, induced rapid yet transient activation of ERK1/2, but not of p38 kinase, JNKs, or NF-κB. These results provide a springboard for deciphering the complex signal transduction network controlling Mø clearance of apoptotic cells.
Our inhibitor results are significant because they demonstrate a requirement for multiple protein and lipid phosphorylation reactions during Mø phagocytosis of apoptotic cells. The observed IC50s are generally comparable to or less than those previously seen in studies of phagocytosis by Mø and Mø cell lines [14-17, 32], supporting the view that our results derive from pharmacological effects on specific enzymes, rather than generalized toxicity. While it may appear counter-intuitive that the non-specific PKC inhibitor staurosporine is often used to induce apoptosis, yet it did not lead to increased Mø apoptosis here, we believe that this finding results from the brief duration of our experiments. Results similar to ours with regard to PI-3K inhibitors during phagocytosis of apoptotic leukocytes by bone marrow-derived murine Mø have recently been reported by Leverrier and Ridley, who also co-localized tyrosine phosphorylation to the phagocytic cup [15]. Our results complement their morphologic findings by demonstrating the functional importance of protein tyrosine phosphorylation for phagocytosis, and by examining potentially involved PTK families.
Blocking each of these three phosphorylation pathways (PI-3K, PKC, and PTK) has also been found in some studies to inhibit FcR-mediated phagocytosis [16, 17, 26, 32-34], although two groups found that PKC inhibition affected FcR-mediated phagocytosis by murine PMø only minimally [16, 35]. Our results are compatible with the possibility that some or all of these signaling pathways are shared during phagocytosis of these two particle types because they are necessary for the mechanical process of particle engulfment itself. This is particularly likely for PI-3K inhibition, which has previously been shown to block phagosome closure during FcR-mediated phagocytosis [17, 26]. However, it is also possible that the requirement for PI-3K action in the current study additionally reflects the need for its product, PIP3, to recruit to the membrane and thus activate more downstream signaling components (e.g., PKC, or a Tec family PTK, as is seen in signaling through TCR and BCR [36]).
Precise definition of the specific enzymes, adapters and linkers involved in signal transduction during phagocytosis of apoptotic cells will clearly require considerable additional study. Based on the analogies with FcγR-mediated phagocytosis and T cell activation, the conventional interpretation of our PTK inhibition data would be sequential activation first of a Src-like PTK which then activates a piceatannol-inhibitable PTK, likely Syk itself [37, 38]. This hypothesis is supported by the recent observation that phagocytosis of apoptotic cells by immature human dendritic cells was inhibited by herbimycin A, in agreement with our findings, and by another cell-permeable PTK inhibitor, Lavendustin A [14]. Moreover, in human monocytes, the Src family member Lyn associates with CD14 [39], a receptor that contributes to clearance of apoptotic cells [40]. However, the findings that PMø were not inhibited by PP2 and AMø were inhibited only at doses of 30-50 μM suggests that Src family members may not be involved in this process, since their IC50 for PP2 is typically in the 5 μM range. Alternatively, our data are compatible with involvement in apoptotic cell recognition of a non-Src PTK that is also inhibited by herbimycin A and genistein. The Axl/Sky family of receptor tyrosine kinases, especially Mer, has been implicated in clearance of apoptotic cells via their ligand, Gas6 [41-43], but the sensitivity of this PTK family to inhibitors has not yet been assessed. The disparity we observed in the effect of PP2 on the two Mø types is compatible with the possibility that PMø have alternative means of activating Syk that are lacking in AMø. Supporting this possibility, in mice genetically deficient in the three members of the Src family present in Mø (Hck, Lyn, Fgr), both FcγR-mediated Syk activation and phagocytosis of Ig-opsonized particles is decreased but not abolished [44]. Pursuing this lead will be important to defining the basis of the specific phagocytic defect in murine AMø and in understanding the overall process of apoptotic cell clearance.
The role of PKC in this phagocytic process is also likely to be complex and potentially revealing, although not specific for this particle type. Involvement of PKC has been demonstrated in FcγR-mediated phagocytosis [32, 45, 46], but has not previously been studied during ingestion of apoptotic cells. PKC is a family of serine/threonine kinases comprising at least twelve isoforms that differ in substrate utilization and mechanisms of activation {reviewed in [47]}. Gö 6976 has been reported to act as a partially selective inhibitor of the classical PKC α and βI isoforms at nM concentrations that did not affect kinase activity of the novel or atypical PKC δ-, ε-, and ζ-isoforms even at micromolar concentrations [48]. Our results using this inhibitor argue for involvement of classical PKC isoforms in phagocytosis of apoptotic cells. This interpretation would also be compatible with the incomplete inhibition of phagocytosis seen using calphostin C, which has greater activity against novel rather than classical PKCs [49]. However, current data on the specificity of the inhibitors we used for various isoforms are too inconclusive [47] to allow us to predict with certainty which isoforms are involved from the current data alone. Human AMø have been shown to differ from monocytes in expression of both classical and atypical isoforms [50], suggesting that Mø in different tissues may use different PKC isoforms for the same purpose. Which PKC isoforms are expressed by primary murine Mø is undefined. We believe it likely that more than one PKC isoform will be involved in Mø phagocytosis of apoptotic cells, and we are actively investigating that possibility.
Our finding that Mø phagocytosis of apoptotic thymocytes did not induce activation of NF-κB agrees with previous findings in a transformed murine Mø line, J774 [51], which we extend by showing that neither p38 kinase nor JNKs are activated by this stimulus. NF-κB, when released from the cytoplasm where it is bound by unphosphorylated IκB, binds to and activates the promoters of many proinflammatory genes. MAP kinases phosphorylate and thereby activate a variety of transcription factors including ELK1, ATF-2 and c-Jun, and also stabilize mRNAs of inflammatory genes [52]. Collectively, the absence of activation of these signaling intermediaries supports previous observations that Mø ingestion of apoptotic cells does not lead to pro-inflammatory cytokine production [51, 53, 54]. However, based on those previous studies, the observation that both apoptotic cells and heat-killed cells activated ERK1/2 was unanticipated.
Several points about the observed ERK activation merit discussion. First, the rapidity of its initiation (i.e., by 5 minutes, well before any ingestion occurs) indicates that the process is triggered when the Mø recognizes alterations of the target cell surface. One possible candidate for such an alteration is surface PS expression, which our annexin V-staining data suggests is shared both by heat-killed cells as well as by early and late apoptotic cells. Although it remains formally possible that annexin-V simply gained access to PS within the inner membrane leaflet in the heat-killed cells in our experiments, this possibility appears unlikely based on the molecular size of annexin V. It is also possible that other surface changes contribute to recognition of apoptosis in a manner that triggers ERK activation [56]. Second, as shown by the negative results using PD98059, ERK activation is unnecessary for phagocytosis itself, raising the question of the point at which it diverges from cytoskeletal rearrangements needed for ingestion. Third, based on the results of the mixing experiment (Fig. 5G-I), we cannot exclude the possibility that the ERK activation we observed using thymocytes treated for 6 hours with dexamethasone was attributable solely to the small fraction of late apoptotic cells it contained. However, that interpretation would require that Mø ERK activation depends on rapid and specific detection of additional signals of cell death common both to late apoptotic cells and to cells killed rapidly by heating, but absent from early apoptotic cells. A molecular basis by which that could occur is currently unknown. A more likely alternative is that Mø detection of surface PS on the apoptotic cells, suggested by several groups [30, 57] to be central to recognition of cell death, also induces ERK activation. Thus, all PS-positive cells, whether in early or late apoptosis, or even in secondary necrosis, would trigger ERK activation. This model plus our data suggests that ERK activation in response to apoptotic cells is non-linear, increasing from minimal at a total of 9.4% annexin-V positive thymocytes (Fig 5D, right lower panel + right upper panel), to readily detectable at a total of 17.4% annexin-V positive thymocytes (Fig 5G, right lower panel + right upper panel), without further increase at a total of 65.5% annexin-V positive thymocytes (Fig 5A, right lower panel + right upper panel). Although the current data indicate that ERK activation in the absence of p38 cannot be the sole explanation for the anti-phlogistic state induced by ingestion of apoptotic cells, an intriguing possibility remains that it contributes to the process. Interestingly, activation of ERK in the absence of p38 activation has been shown to suppress IL-12 production, an effect that is apparently exploited by Leishmania species to thwart development of protective immunity [55].
Our data support the viewpoint that techniques purported to induce pure necrosis (as opposed to apoptosis) should be interpreted with caution unless the resulting cells are characterized thoroughly [58]. Originally considered antithetic forms of cell death, necrosis and apoptosis are now considered by many to be closely related processes that differ in the completeness with which the internal death program is executed [59, 60]. We found that whereas freeze-thaw cycling fragmented the majority of cells, heating resulted almost entirely in intact thymocytes that stained with both annexin V and PI. Thus, these cells were indistinguishable by these criteria from late apoptotic cells, although they were produced by a method felt classically to induce necrosis.
In summary, we have shown that recognition and ingestion of apoptotic T cells by resident Mø at two distinct epithelial surfaces activates multiple signal transduction events that have prominent similarities but also subtle differences both with FcγR-mediated Mø phagocytosis and with aspects of T cell activation. It may be possible to exploit these differences to devise localized therapeutic means to combat immunosuppressive effects of apoptotic cell clearance that are counter-productive to host defense [29, 51, 54, 61].
ACKNOWLEDGMENTS
Supported by RO1 HL56309 and RO-1 HL6157 from the USPHS; and by Merit Review funding and a Research Enhancement Award Program (REAP) grant from the Department of Veterans Affairs.
We thank Drs. Bethany Moore, Joel A. Swanson and all the members of the Ann Arbor VAMC REAP for helpful suggestions, and Joyce O’Brien for secretarial support.
Portions of this work have been presented previously at the Autumn Immunology Conference, Chicago, IL, November 20, 2000.
References
- 1.Cohen JJ, Duke RD, Fadok VA, Sellins KS. Apoptosis and programmed cell death in immunity. Annu Rev Immunol. 1992;10:267–293. doi: 10.1146/annurev.iy.10.040192.001411. [DOI] [PubMed] [Google Scholar]
- 2.Savill J, Fadok V, Henson P, Haslett C. Phagocyte recognition of cells undergoing apoptosis. Immunol Today. 1993;14:131–136. doi: 10.1016/0167-5699(93)90215-7. [DOI] [PubMed] [Google Scholar]
- 3.Savill JS, Wyllie AH, Henson JE, Walport MJ, Henson PM, Haslett C. Macrophage phagocytosis of aging neutrophils in inflammation: programmed cell death in the neutrophil leads to its recognition by macrophages. J Clin Invest. 1989;83:865–875. doi: 10.1172/JCI113970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Akbar AN, Savill J, Gombert W, Bofill M, Borthwick NJ, Whitelaw F, Grundy J, Janossy G, Salmon M. The specific recognition by macrophages of CD8+,CD45RO+ T cells undergoing apoptosis: A mechanism for T cell clearance during resolution of viral infections. J Exp Med. 1994;180:1943–1947. doi: 10.1084/jem.180.5.1943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Stern M, Savill J, Haslett C. Human monocyte-derived macrophage phagocytosis of senescent eosinophils undergoing apoptosis. Mediation by alpha v beta 3/CD36/thrombospondin recognition mechanism and lack of phlogistic response. Am J Pathol. 1996;149:911–921. [PMC free article] [PubMed] [Google Scholar]
- 6.Haslett C. Granulocyte apoptosis and its role in the resolution and control of lung inflammation. Am J Respir Crit Care Med. 1999;160:S5–11. doi: 10.1164/ajrccm.160.supplement_1.4. [DOI] [PubMed] [Google Scholar]
- 7.Surh CD, Sprent J. T-cell apoptosis detected in situ during positive and negative selection in the thymus. Nature. 1994;372:100–103. doi: 10.1038/372100a0. [DOI] [PubMed] [Google Scholar]
- 8.Milik AM, Beuchner-Maxwell VA, Kim S, Sonstein J, Seitzman GD, Beals TF, Curtis JL. Lung lymphocyte elimination by apoptosis in the murine response to intratracheal particulate antigen. J Clin Invest. 1997;99:1082–1091. doi: 10.1172/JCI119236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Newman SL, Henson JE, Henson PM. Phagocytosis of senescent neutrophils by human monocyte-derived macrophages and rabbit inflammatory macrophages. J Exp Med. 1982;156:430–442. doi: 10.1084/jem.156.2.430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hu B, Sonstein J, Christensen PJ, Punturieri A, Curtis JL. Deficient in vitro and in vivo phagocytosis of apoptotic T cells by resident murine alveolar macrophages. J Immunol. 2000;165:2124–2133. doi: 10.4049/jimmunol.165.4.2124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Aderem A, Underhill DM. Mechanisms of phagocytosis in macrophages. Annu Rev Immunol. 1999;17:593–623. doi: 10.1146/annurev.immunol.17.1.593. [DOI] [PubMed] [Google Scholar]
- 12.Platt N, da Silva RP, Gordon S. Class A scavenger receptors and the phagocytosis of apoptotic cells. Immunol Lett. 1999;65:15–19. doi: 10.1016/s0165-2478(98)00118-7. [DOI] [PubMed] [Google Scholar]
- 13.Zocchi MR, Poggi A, Rubartelli A. The RGD-containing domain of exogenous HIV-1 Tat inhibits the engulfment of apoptotic bodies by dendritic cells. AIDS. 1997;11:1227–1235. doi: 10.1097/00002030-199710000-00005. [DOI] [PubMed] [Google Scholar]
- 14.Albert ML, Kim JI, Birge RB. αvβ5 integrin recruits the CrkII-Dock180-rac1 complex for phagocytosis of apoptotic cells. Nat Cell Biol. 2000;2:899–905. doi: 10.1038/35046549. [DOI] [PubMed] [Google Scholar]
- 15.Leverrier Y, Ridley AJ. Requirement for Rho GTPases and PI 3-kinases during apoptotic cell phagocytosis by macrophages. Curr Biol. 2001;11:195–199. doi: 10.1016/s0960-9822(01)00047-1. [DOI] [PubMed] [Google Scholar]
- 16.Greenberg S, Chang P, Silverstein SC. Tyrosine phosphorylation is required for Fc receptor-mediated phagocytosis in mouse macrophages. J Exp Med. 1993;177:529–534. doi: 10.1084/jem.177.2.529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cox D, Tseng CC, Bjekic G, Greenberg S. A requirement for phosphatidylinositol 3-kinase in pseudopod extension. J Biol Chem. 1999;274:1240–1247. doi: 10.1074/jbc.274.3.1240. [DOI] [PubMed] [Google Scholar]
- 18.Muroi M, Muroi Y, Suzuki T. The binding of immobilized IgG2a to Fc gamma 2a receptor activates NF-kappa B via reactive oxygen intermediates and tumor necrosis factor-alpha 1. J Biol Chem. 1994;269:30561–30568. [PubMed] [Google Scholar]
- 19.Durden DL, Kim HM, Calore B, Liu Y. The Fc gamma RI receptor signals through the activation of hck and MAP kinase. J Immunol. 1995;154:4039–4047. [PubMed] [Google Scholar]
- 20.Trotta R, Kanakaraj P, Perussia B. Fc gamma R-dependent mitogen-activated protein kinase activation in leukocytes: a common signal transduction event necessary for expression of TNF-alpha and early activation genes. J Exp Med. 1996;184:1027–1035. doi: 10.1084/jem.184.3.1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rose DM, Winston BW, Chan ED, Riches DW, Gerwins P, Johnson GL, Henson PM. Fc gamma receptor cross-linking activates p42, p38, and JNK/SAPK mitogen-activated protein kinases in murine macrophages: role for p42MAPK in Fc gamma receptor-stimulated TNF-alpha synthesis. J Immunol. 1997;158:3433–3438. [PubMed] [Google Scholar]
- 22.Fadok VA, Voelker DR, Campbell PA, Cohen JJ, Braton DL, Henson PM. Exposure of phophatidylserine on the surface of apoptotic lymphocytes triggers specific recognition and removal by macrophages. J Immunol. 1992;148:2207–2216. [PubMed] [Google Scholar]
- 23.Vermes I, Haanen C, Steffens-Nakken H, Reutelingsperger C. A novel assay for apoptosis. Flow cytometric detection of phosphatidylserine expression on early apoptotic cells using fluorescein labelled Annexin V. J Immunol Methods. 1995;184:39–51. doi: 10.1016/0022-1759(95)00072-i. [DOI] [PubMed] [Google Scholar]
- 24.Punturieri A, Filippov S, Allen E, Caras I, Murray R, Reddy V, Weiss SJ. Regulation of elastinolytic cysteine proteinase activity in normal and cathepsin K-deficient human macrophages. J Exp Med. 2000;192:789–800. doi: 10.1084/jem.192.6.789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Licht R, Jacobs CW, Tax WJ, Berden JH. An assay for the quantitative measurement of in vitro phagocytosis of early apoptotic thymocytes by murine resident peritoneal macrophages. J Immunol Methods. 1999;223:237–248. doi: 10.1016/s0022-1759(98)00212-9. [DOI] [PubMed] [Google Scholar]
- 26.Araki N, Johnson MT, Swanson JA. A role for phosphoinositide 3-kinase in the completion of macropinocytosis and phagocytosis by macrophages. J Cell Biol. 1996;135:1249–1260. doi: 10.1083/jcb.135.5.1249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Brown K, Gerstberger S, Carlson L, Franzoso G, Siebenlist U. Control of I kappa B-alpha proteolysis by site-specific, signal-induced phosphorylation. Science. 1995;267:1485–1488. doi: 10.1126/science.7878466. [DOI] [PubMed] [Google Scholar]
- 28.Griffith TS, Yu XH, Herndon JM, Green DR, Ferguson TA. CD95-induced apoptosis of lymphocytes in an immune privileged site induces immunological tolerance. Immunity. 1996;5:7–16. doi: 10.1016/s1074-7613(00)80305-2. [DOI] [PubMed] [Google Scholar]
- 29.Freire-de-Lima CG, Nacimento DO, Soares MBP, Bozza PT, Castro-Faria-Neto HC, de Mello FG, DosReis GA, Lopes MF. Uptake of apoptotic cells drives the growth of a pathogenic trypanosome in macrophages. Nature. 2000;403:199–203. doi: 10.1038/35003208. [DOI] [PubMed] [Google Scholar]
- 30.Fadok VA, Bratton DL, Rose DM, Pearson A, Ezekewitz RA, Henson PM. A receptor for phosphatidylserine-specific clearance of apoptotic cells. Nature. 2000;405:85–90. doi: 10.1038/35011084. [DOI] [PubMed] [Google Scholar]
- 31.Karimi K, Lennartz MR. Mitogen-activated protein kinase is activated during IgG-mediated phagocytosis, but is not required for target ingestion. Inflammation. 1998;22:67–82. doi: 10.1023/a:1022347808042. [DOI] [PubMed] [Google Scholar]
- 32.Karimi K, Lennartz MR. Protein kinase C activation precedes arachidonic acid release during IgG-mediated phagocytosis. J Immunol. 1995;155:5786–5794. [PubMed] [Google Scholar]
- 33.Zheleznyak A, Brown EJ. Immunoglobulin-mediated phagocytosis by human monocytes requires protein kinase C activation. Evidence for protein kinase C translocation to phagosomes. J Biol Chem. 1992;267:12042–12048. [PubMed] [Google Scholar]
- 34.Swanson JA, Johnson MT, Beningo K, Post P, Mooseker M, Araki N. A contractile activity that closes phagosomes in macrophages. J Cell Sci. 1999;112:307–316. doi: 10.1242/jcs.112.3.307. [DOI] [PubMed] [Google Scholar]
- 35.Roubey RA, Ross GD, Merrill JT, Walton F, Reed W, Winchester RJ, Buyon JP. Staurosporine inhibits neutrophil phagocytosis but not iC3b binding mediated by CR3 (CD11b/CD18) J Immunol. 1991;146:3557–3562. [PubMed] [Google Scholar]
- 36.Schaeffer EM, Schwartzberg PL. Tec family kinases in lymphocyte signaling and function. Curr Opin Immunol. 2000;12:282–288. doi: 10.1016/s0952-7915(00)00088-1. [DOI] [PubMed] [Google Scholar]
- 37.Greenberg S, Chang P, Silverstein SC. Tyrosine phosphorylation of the gamma subunit of Fc gamma receptors, p72syk, and paxillin during Fc receptor-mediated phagocytosis in macrophages. J Biol Chem. 1994;269:3897–3902. [PubMed] [Google Scholar]
- 38.Crowley MT, Costello PS, Fitzer-Attas CJ, Turner M, Meng F, Lowell C, Tybulewicz VL, DeFranco AL. A critical role for Syk in signal transduction and phagocytosis mediated by Fcgamma receptors on macrophages. J Exp Med. 1997;186:1027–1039. doi: 10.1084/jem.186.7.1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Stefanova I, Corcoran ML, Horak EM, Wahl LM, Bolen JB, Horak ID. Lipopolysaccharide induces activation of CD14-associated protein tyrosine kinase p53/56lyn. J Biol Chem. 1993;268:20725–20728. [PubMed] [Google Scholar]
- 40.Devitt A, Moffatt OD, Raykundalia C, Capra JD, Simmons DL, Gregory CD. Human CD14 mediates recognition and phagocytosis of apoptotic cells. Nature. 1998;392:505–509. doi: 10.1038/33169. [DOI] [PubMed] [Google Scholar]
- 41.Ishimoto Y, Ohashi K, Mizuno K, Nakano T. Promotion of the uptake of PS liposomes and apoptotic cells by a product of growth arrest-specific gene, gas6. J Biochem (Tokyo) 2000;127:411–417. doi: 10.1093/oxfordjournals.jbchem.a022622. [DOI] [PubMed] [Google Scholar]
- 42.Gal A, Li Y, Thompson DA, Weir J, Orth U, Jacobson SG, Apfelstedt-Sylla E, Vollrath D. Mutations in MERTK, the human orthologue of the RCS rat retinal dystrophy gene, cause retinitis pigmentosa. Nat Genet. 2000;26:270–271. doi: 10.1038/81555. [DOI] [PubMed] [Google Scholar]
- 43.Scott RS, McMahon EJ, Pop SM, Reap EA, Caricchio R, Cohen PL, Earp HS, Matsushima GK. Phagocytosis and clearance of apoptotic cells is mediated by MER. Nature. 2001;411:207–211. doi: 10.1038/35075603. [DOI] [PubMed] [Google Scholar]
- 44.Fitzer-Attas CJ, Lowry M, Crowley MT, Finn AJ, Meng F, DeFranco AL, Lowell CA. Fcgamma receptor-mediated phagocytosis in macrophages lacking the Src family tyrosine kinases Hck, Fgr, and Lyn. J Exp Med. 2000;191:669–682. doi: 10.1084/jem.191.4.669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zheng L, Zomerdijk TP, Aarnoudse C, van Furth R, Nibbering PH. Role of protein kinase C isozymes in Fc gamma receptor-mediated intracellular killing of Staphylococcus aureus by human monocytes. J Immunol. 1995;155:776–784. [PubMed] [Google Scholar]
- 46.Larsen EC, DiGennaro JA, Saito N, Mehta S, Loegering DJ, Mazurkiewicz JE, Lennartz MR. Differential requirement for classic and novel PKC isoforms in respiratory burst and phagocytosis in RAW 264. cells. J Immunol. 2000;165:2809–2817. doi: 10.4049/jimmunol.165.5.2809. [DOI] [PubMed] [Google Scholar]
- 47.Way KJ, Chou E, King GL. Identification of PKC-isoform-specific biological actions using pharmacological approaches. Trends Pharmacol Sci. 2000;21:181–187. doi: 10.1016/s0165-6147(00)01468-1. [DOI] [PubMed] [Google Scholar]
- 48.Martiny-Baron G, Kazanietz MG, Mischak H, Blumberg PM, Kochs G, Hug H, Marme D, Schachtele C. Selective inhibition of protein kinase C isozymes by the indolocarbazole Go 6976. J Biol Chem. 1993;268:9194–9197. [PubMed] [Google Scholar]
- 49.Keenan C, Goode N, Pears C. Isoform specificity of activators and inhibitors of protein kinase C gamma and delta. FEBS Lett. 1997;415:101–108. doi: 10.1016/s0014-5793(97)01104-6. [DOI] [PubMed] [Google Scholar]
- 50.Monick MM, Carter AB, Gudmundsson G, Geist LJ, Hunninghake GW. Changes in PKC isoforms in human alveolar macrophages compared with blood monocytes. Am J Physiol. 1998;275:L389–3971. doi: 10.1152/ajplung.1998.275.2.L389. [DOI] [PubMed] [Google Scholar]
- 51.McDonald PP, Fadok VA, Bratton D, Henson PM. Transcriptional and translational regulation of inflammatory mediator production by endogenous TGF-β in macrophages that have ingested apoptotic cells. J Immunol. 1999;163:6164–6172. [PubMed] [Google Scholar]
- 52.Rao A, Luo C, Hogan PG. Transcription factors of the NFAT family: regulation and function. Annu Rev Immunol. 1997;15:707–747. doi: 10.1146/annurev.immunol.15.1.707. [DOI] [PubMed] [Google Scholar]
- 53.Voll RE, Herrmann M, Roth EA, Stach C, Kalden JR, Girkonstaite I. Immunosuppressive effects of apoptotic cells. Nature. 1997;390:350–351. doi: 10.1038/37022. [DOI] [PubMed] [Google Scholar]
- 54.Fadok VA, McDonald PP, Bratton DL, Henson PM. Regulation of macrophage cytokine production by phagocytosis of apoptotic and post-apoptotic cells. Biochem Soc Trans. 1998;26:653–656. doi: 10.1042/bst0260653. [DOI] [PubMed] [Google Scholar]
- 55.Feng GJ, Goodridge HS, Harnett MM, Wei XQ, Nikolaev AV, Higson AP, Liew FY. Extracellular signal-related kinase (ERK) and p38 mitogen-activated protein (MAP) kinases differentially regulate the lipopolysaccharide-mediated induction of inducible nitric oxide synthase and IL-12 in macrophages: Leishmania phosphoglycans subvert macrophage IL-12 production by targeting ERK MAP kinase. J Immunol. 1999;163:6403–6412. [PubMed] [Google Scholar]
- 56.Zhuang J, Ren Y, Snowden RT, Zhu H, Gogvadze V, Savill JS, Cohen GM. Dissociation of phagocyte recognition of cells undergoing apoptosis from other features of the apoptotic program. J Biol Chem. 1998;273:15628–15632. doi: 10.1074/jbc.273.25.15628. [DOI] [PubMed] [Google Scholar]
- 57.Pradhan D, Krahling S, Williamson P, Schlegel RA. Multiple systems for recognition of apoptotic lymphocytes by macrophages. Mol Biol Cell. 1997;8:767–778. doi: 10.1091/mbc.8.5.767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Renvoize C, Biola A, Pallardy M, Breard J. Apoptosis: identification of dying cells. Cell Biol Toxicol. 1998;14:111–120. doi: 10.1023/a:1007429904664. [DOI] [PubMed] [Google Scholar]
- 59.Leist M, Single B, Castoldi AF, Kuhnle S, Nicotera P. Intracellular adenosine triphosphate (ATP) concentration: a switch in the decision between apoptosis and necrosis. J Exp Med. 1997;185:1481–1486. doi: 10.1084/jem.185.8.1481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kroemer G, Dallaporta B, Resche-Rigon M. The mitochondrial death/life regulator in apoptosis and necrosis. Annu Rev Physiol. 1998;60:619–642. doi: 10.1146/annurev.physiol.60.1.619. [DOI] [PubMed] [Google Scholar]
- 61.Erwig LP, Gordon S, Walsh GM, Rees AJ. Previous uptake of apoptotic neutrophils or ligation of integrin receptors downmodulates the ability of macrophages to ingest apoptotic neutrophils. Blood. 1999;93:1406–1412. [PubMed] [Google Scholar]