Abstract
Using MDCK cells as a model system, evidence is presented demonstrating that the signaling pathways mammalian target of rapamycin (mTOR) and phosphoinositide 3-kinase (PI 3-kinase) play important roles in the regulation of epithelial tubule formation. Incubation of cells with collagen gel overlays induced early (4–8 h) reorganization of cells (epithelial remodeling) into three-dimensional multicellular tubular structures over 24 h. An MDCK cell line stably expressing the PH domain of Akt, a PI 3-kinase downstream effector, coupled to green fluorescent protein (GFP-Akt-PH) was used to determine the distribution of phosphatidyl inositol-3,4,5-P3 (PIP3), a product of PI 3-kinase. GFP-Akt-PH was associated with lateral membranes in control cells. After incubation with collagen gel overlays, GFP-Akt-PH redistributed into the lamellipodia of migrating cells suggesting that PIP3 plays a role in epithelial remodeling. Using the small molecule inhibitor LY-294002 that inhibits both mTOR and PI 3-kinase, we demonstrated that kinase activity was required for epithelial remodeling, disruption of cell junctions and subsequent modulation of tubule formation. Since the mTOR signaling pathway is downstream of PI 3-kinase, the effects of rapamycin, a specific mTOR inhibitor, on tubule formation were assessed. Rapamycin did not affect epithelial remodeling or GFP-Akt-PH redistribution but inhibited elongated tubule formation that occurred later (24 h) in morphogenesis. These results were further supported by using RNA interference to down-regulate mTOR and inhibit tubule formation. Our studies demonstrate that PI 3-kinase regulates early epithelial remodeling stages while mTOR modulates latter stages of tubule development.
The organization of epithelial cells into three-dimensional tubular structures is an important process that occurs during development of many organs (Bissell et al., 2003; Lubarsky and Kransnow, 2003). Epithelial tubules are polarized structures composed of the apical membrane that lines the tubule lumen and the basolateral membrane found between adjacent cells and contacting the extracellular matrix (ECM) substratum (Rodriguez-Boulan et al., 2005; Halbleibe and Nelson, 2006). Association of epithelial cells with the ECM is mediated through basal membrane receptors termed integrins, a family of transmembrane proteins that bind to specific ECM components (Damsky and Ilic, 2002; Miranti and Brugge, 2002). Integrin binding to ECM induces phosphorylation of integrin associated protein kinases which regulate cell signaling through well established downstream signal transduction pathways (Damsky and Ilic, 2002; Luo et al., 2003). The majority of these studies were done on non-polarized cells such as fibroblasts and leukocytes. However, there is some evidence for integrin regulation of signal transduction events specific for epithelia (Damsky and Ilic, 2002; Miranti and Brugge, 2002; O'Brien et al., 2002). This signaling has not been fully elucidated during epithelial tubule morphogenesis. Polarized epithelial cells provide a unique model for studying such regulation since intracellular signaling initiated at one membrane domain could have profound effects on regulatory events in the opposite membrane domain allowing molecular cross-talk between apical and basolateral membranes.
Madin-Darby canine kidney (MDCK) and mammary epithelial cells have been extensively utilized as model systems for studying epithelial polarity development and tubule formation (Hall et al., 1982; Wang et al., 1990a,b; Montesano et al., 1991; O'Brien et al., 2002). Incubation of MDCK and mammary epithelial cell monolayers with collagen gel overlays induced the formation of tubular structures within 24 h (Hall et al., 1982; Ojakian et al., 2001). This process can be divided into an early phase termed epithelial remodeling in which cell rearrangements occur over 4–8 h followed by a late phase which is characterized by the reorganization of migrating cells into tubular structures with distinct apical lumens (12–24 h; see Ojakian and Schwimmer, 1994; Schwimmer and Ojakian, 1995; Zuk and Matlin, 1996; Ojakian et al., 2001). These three-dimensional structures are composed of polarized epithelial cells that have established adherens and tight junctions (Hinck et al., 1994; Jou et al., 1998; Wheelock and Johnson, 2003; Matter et al., 2005). In contrast, MDCK cells grown in suspension within collagen gels form polarized epithelial cysts (Wang et al., 1990a,b; O'Brien et al., 2002; Yu et al., 2003). Treatment of these cysts with hepatocyte growth factor (HGF) induces formation of polarized, tubular membrane extensions making this an attractive model for the study of epithelial tubule formation (Montesano et al., 1991; O'Brien et al., 2002; Yu et al., 2003).
The PI 3-kinase signaling pathway is an excellent candidate for regulation of epithelial tubule formation since integrin-ECM interactions have been shown to modulate PI 3-kinase activity in cell binding studies (Potempa and Ridley, 1998; Watton and Downward, 1999; Kovacs et al., 2002). Indeed, using the small molecule inhibitor LY-294002, it was demonstrated that PI 3-kinase plays an important role in the regulation of kidney tubule branching morphogenesis during development (Tang et al., 2002). In support of these observations, Mostov's laboratory reported that LY-294002 prevented HGF-induced tubular extension formation from MDCK cysts (Yu et al., 2003, 2005). More recent studies by these investigators have provided good evidence that phosphatidyl inositol-3,4-P2 (PIP2) and PIP3 are involved in biogenesis of the apical and basolateral membranes, respectively (Gassama-Diagne et al., 2006; Martin-Belmonte et al., 2007). Surprisingly, stable expression of the PI 3-kinase p110 subunit also induced formation of cell extensions from MDCK cysts in collagen (Khwaja et al., 1998). However, evidence was not presented demonstrating that cell extensions were polarized tubular structures with junctional complexes (Khwaja et al., 1998). Furthermore, expression of activated Akt an important downstream effector of PI 3-kinase (Pece et al., 1999; Cantley, 2002; Miranti and Brugge, 2002; Luo et al., 2003), did not induce formation of cell extensions (Khwaja et al., 1998) suggesting that other signaling pathways are involved.
Another downstream signaling target that is regulated by changes in PI 3-kinase activity levels is the mammalian target for rapamycin (mTOR). The mTOR protein complex is involved in regulation of cell growth and proliferation (Fingar et al., 2002; Sawyers, 2003; Inoki et al., 2005; Martin and Hall, 2005; Sarbassov et al., 2005b) as well as in cytoskeletal function (Jacinto et al., 2004; Sarbassov et al., 2004). Furthermore, recent studies have suggested that excessive mTOR activity plays an important regulatory role in epithelial cyst formation during polycystic kidney disease (Shillingford et al., 2006). These observations led us to determine the role of mTOR in modulation of epithelial tubule formation. In our study, we provide evidence that the PI 3-kinase and mTOR signaling pathways have important functions in regulating epithelial tubule formation.
Materials and Methods
Cell culture
MDCK II cells were cultured in Delbecco's minimal essential medium (DMEM) containing 10% fetal bovine serum (FBS) in an atmosphere of 5% CO2–95% air at 37°C. For tubule formation experiments, cells were plated at 2.5 × 105 cells/ml on type I collagen-coated Falcon 35 mm culture wells (for SDS–PAGE and immunoblotting), cover glasses (for confocal microscopy) or Millipore filters (0.45 μm pores; for transepithelial electrical resistance measurements) and cultured in DMEM-FBS for 2 days. Epithelial tubule formation was initiated by incubation with type I collagen gel overlays as described previously (Ojakian et al., 1997, 2001). Kinase inhibitors were added to the collagen solution at their final concentrations prior to gel formation. After incubation for the appropriate times, collagen gels were removed by aspiration and the cell monolayers prepared for immunoblotting, tight junction permeability measurements or confocal microscopy. MDCK cells stably expressing green fluorescent protein (GFP) coupled to the pleckstrin homology (PH) domain of Akt (GFP-Akt-PH) were generously provided by Drs. Wei Yu and Keith Mostov (University of California, San Francisco) and grown under the same conditions as wild type cells (Yu et al., 2003).
RNA interference (RNAi)
We initiated RNAi studies by developing small, inhibitory RNA (siRNA) against dog mTOR since MDCK cells are canine in origin. mRNA sequences for mTOR (XM 860431) were downloaded from the Dog Genome site (www.ensembl.org). These sequences were used to design and commercially synthesize mTOR siRNA (Ambion, Austin, TX). The sequences used in this study were as follows; siRNA-1: CUUUCAUUGGCAUCUGAGCTG; siRNA-2 AAUGUGAUGGUUCAGCUGGTC. For RNAi experiments, MDCK cells were plated at 4 × 104 cells/ml in six well culture plates and grown for 24 h prior to the addition of 10 nM mTOR siRNA-1 or siRNA-2 mixed with the cell permeabilization reagent Lipofectamine 2000 as recommended by the manufacturer (Invitrogen, Carlsbad, CA). To determine if mTOR protein down-regulation had occurred, after an additional 2 days growth, the cells were extracted with TX-100 and levels of mTOR analyzed by SDS–PAGE and Western blotting using a commercial rabbit antibody against total mTOR (Cell Signaling Technology, Danvers, MA). As a protein loading control, we determined that β-catenin levels were unaffected by these treatments.
Antibodies and inhibitors
The antibodies used in this study were: mouse monoclonal antibody (mAb) 3F2 developed by our laboratory against the MDCK apical membrane glycoprotein gp135 (Ojakian and Schwimmer, 1988), mAb against β-catenin (BD Transduction Laboratories, San Jose, CA), mAb 3G8 against E-cadherin (from Dr. W. James Nelson, Stanford University); rabbit antibodies were used against the following proteins: ZO-1 (Zymed Laboratories, South San Francisco, CA), mTOR and phosphorylatedserine2448-mTOR (P-Ser2448-mTOR; Cell Signaling Technologies). Fluorescent-labeled secondary antibodies goat anti-rabbit IgG (GAR) Alexa 488 and goat anti-mouse IgG (GAM) Alexa 594 were purchased from Molecular Probes (Carlsbad, CA). Protein kinase inhibitors used were: LY-294002 and rapamycin (Calbiochem, La Jolla, CA).
Confocal microscopy
After termination of experiments, MDCK cells were fixed in methanol at −20°C for 5 min. GFP-Akt-PH cells were fixed in 3.7% formaldehyde in phosphate buffered saline (PBS) for 30 min at 4°C, then permeabilized with 0.1% saponin for 10 min at 4°C. Fixed cells were washed with PBS then incubated in PBS containing 3% BSA and 1% goat serum (BSA-GS) to block non-specific binding. Antibodies were made up in BSA-GS at the following dilutions: anti-gp135 (1:10), β-catenin (1:50), ZO-1 (1:20), GAM-IgG Alexa 488 (1:100), and GAR-Alexa 594 (1:100). Primary and secondary antibodies were incubated for 1 h each. The stained cells were mounted in 10% glycol-PBS containing 12% diethyldiamine (Sigma Chemical Co., St. Louis, MO) to prevent bleaching. Confocal microscopy was done with Biorad 1024 and Radiance 2000 laser scanning confocal microscopes. Beginning at the apical surface, Z-series images were captured at 1 μm intervals through the cell monolayers (10 μm) then recombined and presented as three-dimensional projections.
Immunoblotting
To quantify the levels of β-catenin association with the cytoskeleton a differential detergent extraction procedure was used (Hinck et al., 1994; Ojakian et al., 2001). We use a detergent solubility assay in which the adherens junction protein β-catenin is extracted from epithelial cells in sequential steps. The first extraction uses a Triton-X-100 (TX-100; 0.5%) containing buffer. This extract has β-catenin that is not tightly bound to the cytoskeleton. The β-catenin associated with the remaining cytoskeleton fraction is then extracted with a radioimmunoassay buffer (RIPA buffer) that contains TX-100 (1%), sodium deoxycholate (1%) and SDS (0.1%). Both samples are separated by SDS-PAGE and the β-catenin levels measured by immunoblotting. The β-catenin solubility is determined by dividing the TX-100 fraction by the RIPA fraction. To determine the levels of mTOR activity, samples were detergent-extracted with mTOR extraction buffer (1% TX-100, 120 mM NaCl, 1 mM EDTA, 40 mM HEPES, pH 7.5) according to published procedures (Sarbassov et al., 2004, 2005a). Detergent extracted samples were separated by SDS–PAGE on 10% gels then transferred to nitrocellulose and analyzed by immunoblotting using a mAb against β-catenin (1:250) or rabbit antibodies against mTOR (1:500) and P-Ser2448-mTOR (1:500). Proteins transferred to nitrocellulose were detected by using GAM-IgG or GAR-IgG coupled to alkaline phosphatase (1:1,000) and enhanced chemiluminance (Amersham, Piscataway, NJ). Quantitation of β-catenin and mTOR was done using NIH Image Scion Software.
Transepithelial electrical resistance (TER) measurements
Tight junction permeability was determined as described previously (Ojakian et al., 2001; Eisen et al., 2004). MDCK cells grown on micropore filters were incubated for 6 h with, or without, collagen gel overlays lacking, or containing, protein kinase inhibitors. Prior to the start of each experiment, initial TER (ωcm2) measurements were taken using Hg-HgCl electrodes to pass 10 μA of current across the monolayers. Initial TER readings (~150 ωcm2) were compared to those taken at the end of the experiment and presented as % change in TER.
Statistical evaluation
Due to the very small sample sizes, non-parametric tests of differences among conditions were applied. First, the omnibus Kruskal-Wallis test was conducted of the null hypothesis that all four conditions have the same distribution; if this test proved statistically significant, post-hoc Mann-Whitney tests were conducted for pair-wise comparisons. Exact two-tailed P values coming from StatXact software (Cytel, Inc., Cambridge, MA) are reported.
Results
The role of PI 3-kinase in epithelial tubule formation
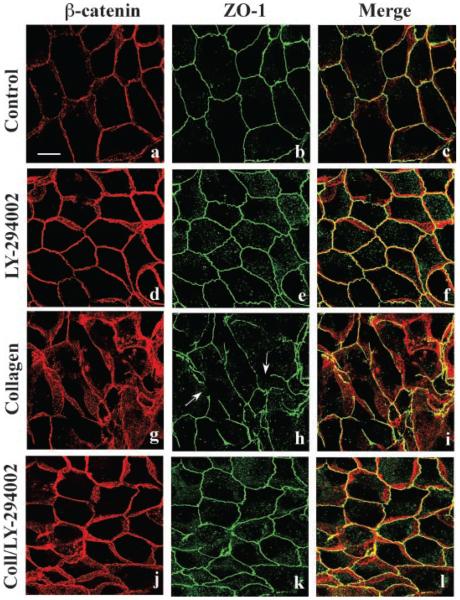
There is evidence that expression of activated PI 3-kinase in MDCK cells stimulated tubule formation in collagen gel (Khwaja et al., 1998). More recently, others (Yu et al., 2003) provided further data that PI 3-kinase plays a role in biogenesis of epithelial tubules. To obtain new insight into the cellular mechanisms regulating epithelial tubule formation, we initiated experiments with the PI 3-kinase inhibitor LY-294002 (Brunn et al., 1996; Pece et al., 1999; Cantley, 2002; Luo et al., 2003). Changes in cell morphology and the distribution of cell junctions during the early stages of epithelial remodeling and tubule formation were studied. MDCK cells were incubated for 6 h in the absence, or presence, of collagen gel overlays containing, or lacking, 20 μM LY-294002. Fixed cells were doubled-labeled with mouse mAb against β-catenin (red staining) and rabbit antibody against ZO-1 (green staining) to localize adherens and tight junctions (Eisen et al., 2004, 2006), respectively, and monitor junctional complex organization during epithelial remodeling. Confocal microscopy demonstrated that β-catenin and ZO-1 were localized to the lateral membranes of both control cells (Fig. 1a–c) and those incubated for 6 h with LY-294002 (Fig. 1d–f). After incubation with collagen gel for 6 h, extensive epithelial remodeling had occurred and lateral membrane β-catenin and ZO-1 staining patterns were dispersed (Fig. 1g–i) indicating that tight and adherens junctions were disrupted. Inclusion of LY-294002 in collagen gel overlays inhibited cell rearrangements and normal β-catenin and ZO-1 distributions were observed indicating that cell junctions appeared to be unaltered (Fig. 1j–l). These data suggest that PI 3-kinase plays an important role in collagen-mediated tight and adherens junction disassembly and epithelial remodeling.
Fig. 1.
PI3-kinase regulation of adherens and tight junction structure during epithelial remodeling. MDCK cells were incubated for 6 h with DMEM (a–c; Control), DMEM containing 20 μM LY-294002 (d–f), collagen gel overlays (g–i), or collagen gel overlays containing LY-294002 (j–l). Methanolfixed cells were double-labeled for β-catenin (red staining) and ZO-1 (green staining) to visualize adherens and tight junctions, respectively, and examined by confocal microscopy. Merged 3-D projection images through the cell monolayer demonstrate that adherens and tight junctions reside adjacent to each other at the interface of the apical and basolateral membrane (Merge). Incubation with collagen gel overlays induced cell rearrangement and tight junction fragmentation (g–i; arrows) disrupting the normal staining pattern. Inclusion of LY-294002 in the collagen gels inhibited cell rearrangements and control (a–c) cell junction staining patterns were observed (j–l). Scale bar, 10 μm.
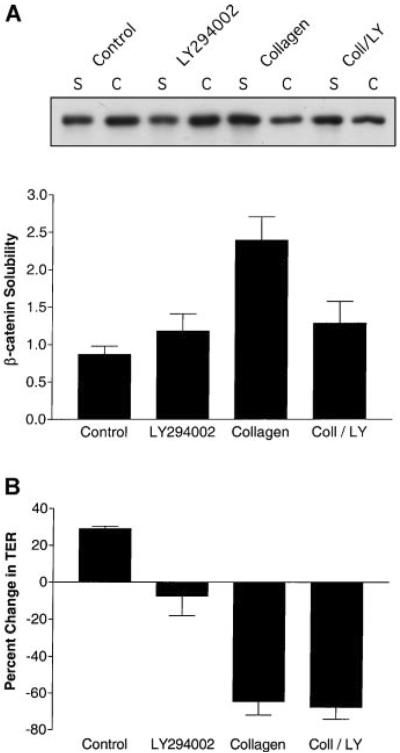
To study the biochemical and physiological effects of PI 3-kinase inhibition during epithelial remodeling, the association of β-catenin with the actin cytoskeleton was determined using the differential detergent solubility method (Hinck et al., 1994). This quantative procedure has been used as an indicator of adherens junction assembly and stability (Hinck et al., 1994; Ojakian et al., 2001; Ehrlich et al., 2002). After 6 h incubation with collagen gel overlays, SDS-PAGE and immunoblotting analysis demonstrated that extensive dissociation of β-catenin from the cytoskeleton had occurred (Fig. 2A; also see Ojakian et al., 2001; Eisen et al., 2004, 2006). However, the presence of LY-294002 in collagen gel overlays inhibited β-catenin dissociation from the cytoskeleton (Fig. 2A) providing biochemical evidence that PI 3-kinase may be playing an active role in adherens junction disassembly during epithelial tubule formation.
Fig. 2.
Inhibition of PI 3-kinase affected β-catenin association with the cytoskeleton but not tight junction permeability during epithelial remodeling. MDCK cells were incubated for 6 h with DMEM (Control), DMEM containing 20 μM LY-294002, collagen gel overlays (Collagen) or collagen gel overlays containing LY-294002 (Coll/LY; n = 4). A: β-catenin association with the cytoskeleton was determined by differentially detergent solubility, SDS-PAGE and immunoblotting. A representative β-catenin immunoblot is presented that includes the TX-100 soluble (s) fractions and the RIPA cytoskeletal (c) extracts. Quantitative data are presented in graph form as the levels of TX-100/RIPA soluble extracts of β-catenin (β-catenin solubility; mean ± SE). Statistical analysis demonstrated that β-catenin ratios for collagen-treated cells (Coll) were significantly different (P < 0.04) from cells incubated in medium alone. B: Tight junction permeability was determined by TER measurements (n = 3). TER (Ω cm2) is presented as % change from initial readings (mean ± SE). The mean starting TER for these experiments was ~150 Ω cm2. When Collagen is compared to the Control there are significant differences (P < 0.01). However, when Collagen/LY-294002 (Coll/LY) is compared to the Control there is no significant difference. This indicates that LY-294002 did not affect collagen-mediated increases in tight junction permeability.
Changes in tight junction permeability were monitored by TER measurements after collagen-mediated epithelial remodeling. Collagen gel overlays increased the permeability (Fig. 2B) indicating that alterations in tight junctions had occurred. LY-294002 in collagen gel overlays did not inhibit the increase in tight junction permeability normally observed in collagen (Fig. 2B). The normal staining pattern of the tight junction protein ZO-1 remained intact during incubation with collagen gel overlays containing LY-294002 (Fig. 1j–l). Therefore, it is possible that signaling regulating epithelial permeability was independent of changes in PI 3-kinase activity.
The PI 3-kinase signaling pathway appears to be an important regulator of epithelial cyst biogenesis in collagen gel (Yu et al., 2003; Gassama-Diagne et al., 2006; Martin-Belmonte et al., 2007). Previous work has demonstrated that GFP-Akt-PH is translocated to plasma membrane regions enriched in PIP3 (Yu et al., 2003; Gassama-Diagne et al., 2006; Martin-Belmonte et al., 2007) making it an excellent probe for temporal and spatial studies of tubule formation. In control monolayers, β-catenin and GFP-Akt-PH were co-localized to the basolateral membrane (Fig. 3a–c). However, after 6 h incubation with collagen gel overlays, GFP-Akt-PH is found predominantly at the leading edge of migrating lamellipodia indicating that extensive rearrangements had occurred (Fig. 3d–f; arrows). LY-294002 prevented formation of lamellipodia with GFP-Akt-PH at the leading edge (data not shown; Eisen and Ojakian) suggesting that PI 3-kinase is involved in spatial signaling events that regulate cell migration during epithelial tubule formation.
Fig. 3.
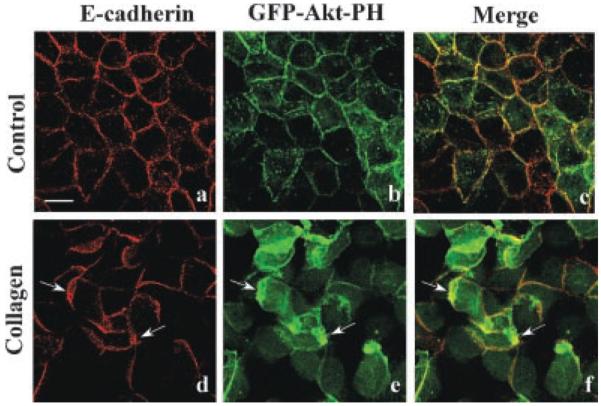
Spatial regulation of PI3-kinase. MDCK cells stably expressing GFP-Akt-PH were incubated for 6 h with DMEM (Control) or collagen gel overlays. Confocal microscopy demonstrated that GFP-Akt-PH (green staining) and E-cadherin (red staining) are localized to the basolateral membrane (a–c). After incubation with collagen gel overlays, epithelial remodeling had occurred as demonstrated by the formation of numerous lamellipodia (d–f). Under these conditions GFP-Akt-PH and E-cadherin are localized to the lamellipodial leading edge (d–f, arrows). Scale bar, 10 μm.
Long term regulation of tubule formation by mTOR
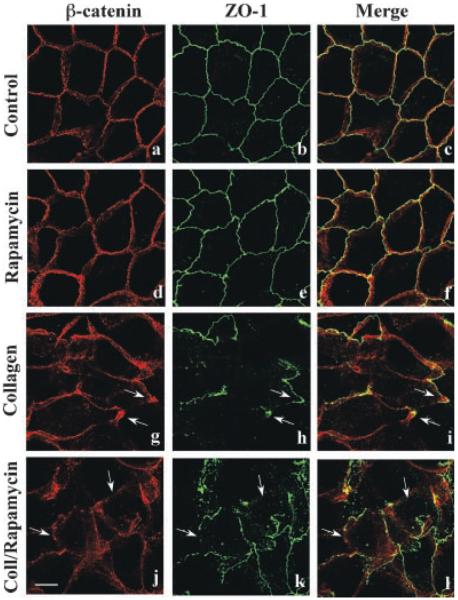
A prominent downstream effector regulated by changes in PI 3-kinase activity levels is the mTOR signaling pathway. The mTOR protein complexes are involved in regulation of cell growth and proliferation (Fingar et al., 2002; Edinger et al., 2003; Jacinto et al., 2004; Sarbassov et al., 2004, 2005a,b; Inoki et al., 2005; Martin and Hall, 2005). There is evidence that mTOR regulates PI 3-kinase pathway function during formation of polarized mammary epithelial cysts (Debnath et al., 2003). This important observation led us to investigate if mTOR signaling was involved in regulation of tubule formation. MDCK cells were incubated with collagen gel overlays for 6 h to determine the effects of the mTOR specific inhibitor rapamycin (Edinger et al., 2003; Jacinto et al., 2004; Sarbassov et al., 2004) on cellular dynamics. Confocal analysis determined that 20 nM rapamycin did not inhibit collagen-mediated epithelial remodeling, formation of lamellipodia or disruption of the β-catenin and ZO-1 staining patterns (Fig. 4).
Fig. 4.
Rapamycin does not inhibit epithelial remodeling. MDCK cells were incubated for 6 h with DMEM (a–c), DMEM containing 20 nM rapamycin (d–f), collagen gel overlays (g–i), or collagen gel overlays containing rapamycin (j–l). Cells were double-labeled for β-catenin (red staining) and ZO-1 (green staining) to visualize adherens and tight junctions, respectively, and examined by confocal microscopy. The presence of rapamycin did not prevent collagen-mediated epithelial remodeling as demonstrated by disrupted β-catenin and ZO-1 staining (j–l; arrows). Scale bar, 10 μm.
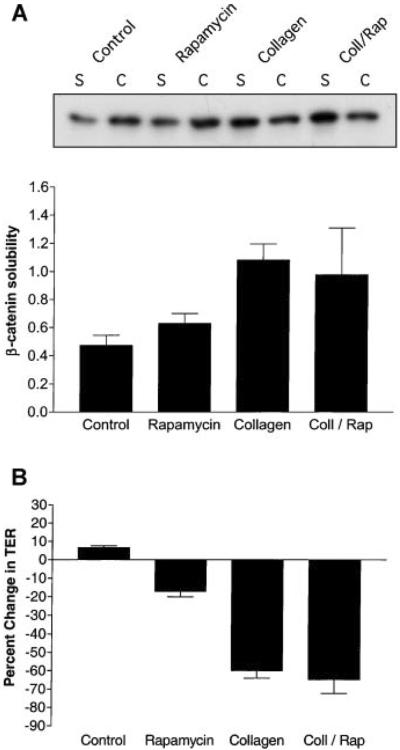
Biochemical analysis using the differential detergent solubility assay to assess adherens junction function determined that rapamycin had no effect on β-catenin association with the cytoskeleton in the presence of collagen gel (Fig. 5A). Furthermore, TER measurements demonstrated that rapamycin did not prevent increases in tight junction permeability induced by collagen (Fig. 5B). These results provide evidence that mTOR does not regulate cell junctions during the early stages of epithelial tubule formation.
Fig. 5.
Rapamycin does not affect adherens and tight junction function during epithelial remodeling. MDCK cells were incubated for 6 h with DMEM (Control), DMEM containing 20 nM rapamycin (Rapamycin), collagen gel overlays (Collagen), or collagen gel overlays containing rapamycin (Coll/Rap). A: β-catenin association with the actin cytoskeleton was determined by differentially detergent solubility, SDS-PAGE and immunoblotting. Quantitative data (n = 3) are presented as levels of soluble-to-insoluble β-catenin ratios (mean ± SE). Statistical analysis demonstrated that β-catenin ratios for control cells were significantly different from cells incubated in collagen (P < 0.05) but not from cells incubated with collagen containing rapamycin. B: Tight junction permeability was determined by TER measurements (n = 3). TER (Ω cm2) is presented as % change from initial readings (mean ± SE). These data demonstrated that rapamycin did not have a significant effect on collagen-mediated increases in tight junction permeability (Coll/Rap).
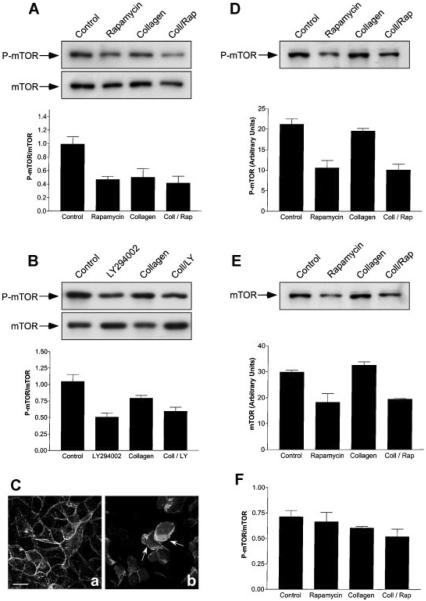
To further study the role of mTOR in epithelial tubule morphogenesis, experiments were carried out to determine if incubation of MDCK cells with collagen gel overlays has any effect on mTOR activity. Cells were incubated for 6 h with, or without, collagen gel overlays containing or lacking rapamycin. The cells were detergent extracted according to published procedures (Sarbassov et al., 2004, 2005a) and mTOR activity determined by immunoblotting using an antibody against mTOR P-serine2448 (P-Ser2448-mTOR). Our studies demonstrated that incubation with rapamycin, collagen gel, and collagen gel containing rapamycin all inhibited (~40%) mTOR activity after 6 h (Fig. 6A). No changes in the levels of total mTOR protein were observed over this time period. An identical analysis after 1 h incubation with collagen gel overlays demonstrated that mTOR phosphorylation was not affected (Eisen and Ojakian, unpublished work). Since rapamycin does not appear to inhibit collagen-mediated cell rearrangements or adherens and tight junction function (Figs. 4 and 5), these results provided evidence that mTOR activity was not essential for regulation of cell motility and epithelial remodeling during the early phases of epithelial tubule formation. The observation that epithelial remodeling is not inhibited by rapamycin suggests that the cells are not inhibited by an ~40% reduction in P-mTOR (Fig. 6A). Another possibility is that mTOR requires spatial redistribution into signaling structures such as lamellipodia in order for it to be effective in regulation of epithelial remodeling. However, attempts to localize P-mTOR and mTOR by confocal microscopy with available antibodies were not successful.
Fig. 6.
Inhibition of mTOR activity during epithelial remodeling. A: MDCK cells were incubated for 6 h with DMEM (Control), DMEM containing 20 nM rapamycin, collagen gel overlays (Collagen), or collagen gel overlays containing rapamycin (Coll/Rap). The cells were detergent-extracted and the levels of total mTOR and activated mTOR (P-mTOR) determined by SDS-PAGE and immunoblotting (n = 5). Representative immunoblots are presented. Graphic representation of P-mTOR/mTOR (arbitrary units ± SE) to determine mTOR activation levels demonstrated that Collagen (P < 0.02) and Coll/Rap (P < 0.004) were significantly different from control cells. B: Cells were treated in a manner identical to that described above except that they were incubated for 6 h with, or without, collagen gel overlays containing, or lacking, 20 μM LY-294002. SDS-PAGE and immunoblotting (see representative immunoblots) demonstrated a ~50% inhibition of P-mTOR activity (n = 4; P < 0.002) with collagen gel overlays containing, or lacking, LY-294002. C: Spatial regulation of activated PI3-kinase was not inhibited by rapamycin. MDCK cells stably expressing GFP-Akt-PH were incubated with 20 nM rapamycin or collagen gel overlays containing rapamycin for 6 h. Confocal microscopy demonstrated that GFP-Akt-PH was localized to the basolateral membrane in the presence of rapamycin alone (a). After incubation with collagen geloverlays containing rapamycin, epithelial remodeling occurred as demonstrated by the formation of lamellipodia (b). Under these conditions GFP-Akt-PH was localized to the lamellipodial leading edge (b, arrows). Scale bar, 10 μm. D–F: Long-term incubation with rapamycin induced down-regulation of mTOR. Cells were incubated with DMEM (Control), DMEM containing 20 nM rapamycin, collagen gel overlays (Collagen), or collagen gel overlays containing rapamycin (Coll/Rap) for 24 h (n = 3) and prepared for immunoblotting. D: P-Ser2448-mTOR (P-mTOR) levels (arbitrary units ± SEM). A representative immunoblot demonstrates that treatment with rapamycin or collagen/rapamycin decreased P-mTOR protein levels when compared to the control. Quantitative measurements demonstrated that the level of P-mTOR in Collagen/Rap-treated cells was significantly lower that those in collagen gel alone (P = 0.003). E: Total mTOR levels (arbitrary units ± SE). Treatment with collagen gel did not produce significant decreases in mTOR protein when compared to the control. However, mTOR levels in Collagen/Rap-treated cells were significantly lower that those in control cells (P < 0.005). F: Graphic representation of P-mTOR/mTOR to calculate mTOR activation levels demonstrated that all treatments were not significantly different from control cells.
Although LY-294002 is considered a specific PI 3-kinase inhibitor, there is evidence that it also affects mTOR signaling. Previous work has demonstrated that LY-294002 inhibits mTOR activity in the same concentration range that it inhibits PI 3-kinase (Brunn et al., 1996; Sarbassov et al., 2005a). These observations bring into question whether LY-294002 inhibition of epithelial tubule formation is due to regulation of either the PI 3-kinase or mTOR pathways, or, a combination of both. To resolve this issue, MDCK cells were incubated for 6 h with, or without, collagen gel overlays either lacking, or containing 20 μM LY-294002. This concentration is frequently used to inhibit PI 3-kinase activation (Ehrlich et al., 2002; Yu et al., 2003). Immunoblotting demonstrated that LY-294002 alone, or in collagen gel overlays, inhibited mTOR activity (Fig. 6B).
To study the role of mTOR in greater detail, the effect of rapamycin on PI 3-kinase activation during epithelial remodeling was determined. MDCK cells expressing GFP-Akt-PH were incubated with collagen gel overlays for 6 h in the presence, or absence, of rapamycin. Incubation with collagen gel overlays containing rapamycin did not inhibit formation of lamellipodia containing GFP-Akt-PH (Fig. 6C). Conversely, inclusion of LY-294002 in collagen gel overlays inhibited formation of lamellipodia containing GFP-Akt-PH (Eisen and Ojakian, unpublished observations) providing evidence that PI 3-kinase, and probably not mTOR, signaling has distinct regulatory effects on the early phase of epithelial tubule formation. Rapamycin has been shown to have different short (2 h) and long-term (24 h) effects in a variety of cells (Sarbassov et al., 2006). Therefore, further experiments were done with MDCK cells to determine the activation state of mTOR after prolonged incubation (24 h) with collagen gel overlays containing, or lacking, rapamycin. These data demonstrated that there were μ40% statistically significant decreases in P-Ser2448-mTOR (Fig. 6D) and total mTOR protein (Fig. 6E) levels over 24 h. It was determined that P-mTOR/mTOR ratios did not change (Fig. 6F) suggesting that mTOR activity was regulated by the levels of total mTOR protein.
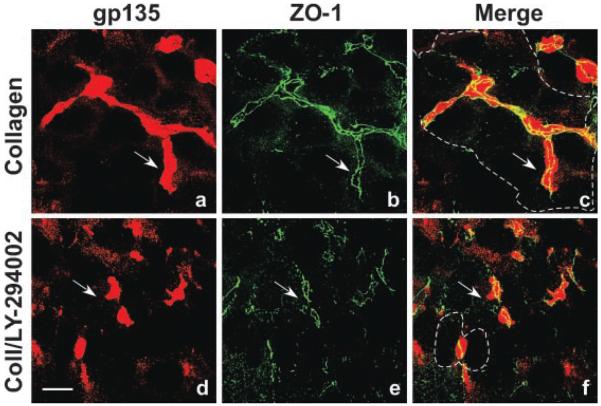
To determine if the PI 3-kinase signaling pathway was involved in regulation of tubule formation, MDCK cells were incubated for 24 h with collagen gel overlays lacking, or containing, LY-294002. This long-term incubation with collagen gel overlays induced formation of elongated multicellular tubules with gp135-positive apical lumens (Fig. 7a–c). These large tubules were frequently composed of 10–15 cells and had an elaborate network of associated tight junctions as demonstrated by ZO-1 staining (Fig. 7a–c). However, after 24 h incubation with collagen gel overlays containing LY-294002, only small gp135-positive apical lumens (between adjacent cell pairs) with associated ring-like tight junctions were observed (Fig. 7d–f). These data suggest that PI 3-kinase and probably mTOR regulate common downstream effectors involved in later stages of epithelial tubule morphogenesis.
Fig. 7.
PI3-kinase regulates formation of elongated, multicellular epithelial tubules. MDCK cells were incubated for 24h with collagen gel overlays lacking (a–c; Control) or containing 20 μM LY-294002 (d–f; Coll/LY-294002). Methanol-fixed cells were double-labeled for gp135 (red staining) and ZO-1 (green staining) to visualize apical membranes and tight junctions, respectively, and examined by confocal microscopy. Incubation with collagen gel overlays induced the formation of elongated, multicellular epithelial tubules with gp135-positive apical lumens and associated ZO-1 positive tight junctions (a–c;arrows). The basal membranes of cells comprising this tubule are outlined (c;dashed line). Incubation with collagen gel overlays containing LY-294002 only allowed the formation of small gp135-positive apical lumens with associated ring-like tight junctions (d–f; arrows). The membranes of the cell pair contributing to this tubule are outlined (f; dashed line). Scale bar, 10 μm.
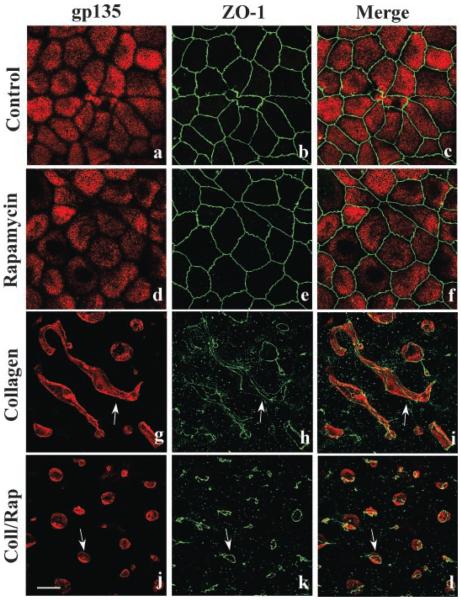
Previous studies have demonstrated that long-term incubation (24 h) with rapamycin down-regulated components of the mTOR signaling pathway (Edinger et al., 2003; Sarbassov et al., 2006). Therefore, we initiated collagen gel overlay experiments to study the effects of long-term rapamycin exposure on tubule formation. Cells were incubated for 24 h with, or without, collagen gel overlays then analyzed by confocal microscopy. Cells incubated with either DMEM alone (Fig. 8a–c) or DMEM containing rapamycin (Aig. 8d–f) were unaffected having apical membrane gp135 and ZO-1 positive tight junctions. Cells incubated with collagen alone formed elongated, multicellular tubules (Fig. 8g–i) with gp135-positive apical lumens and numerous ZO-1 positive tight junctions. However, cells incubated with collagen gel containing rapamycin had small apical lumens with associated simple ring-like tight junctions (Fig. 8j–l). These data, and those presented above (Fig. 6), suggest that that rapamycin inhibits the late stage of epithelial tubule formation.
Fig. 8.
mTOR regulates the formation of elongated, multicellular epithelial tubules. MDCK cells were incubated with DMEM (a–c; Control), DMEM containing 20 nM rapamycin (d–f), collagen gel overlays (g–i), or collagen gel overlays containing rapamycin (j–l) for 24 h. Cells were double-labeled for gp135 (red staining) and ZO-1 (green staining) to visualize apical membranes and tight junctions, respectively, and examined by confocal microscopy. These experiments demonstrated that 24 h incubation without (a–c) or with rapamycin (d–f) had no effect on gp135 apical staining or tight junction integrity. Incubation with collagen gel overlays induced the formation of large, elongated epithelial tubules with gp135-positive apical lumens (g–i; arrows) and associated ZO-1 positive tight junctions. Incubation with collagen gel overlays containing rapamycin only allowed the formation of small gp135-positive apical lumens with associated ring-like tight junctions (j–l; arrows). Scale bar, 10 μm.
Even though rapamycin is considered a specific inhibitor, there is the possibility that it could be inhibiting other signaling pathways. Therefore, RNAi was used as an alternative procedure to morphogenesis. Cells incubated with mTOR-siRNA had significantly lower levels of mTOR protein compared to cells incubated with scrambled siRNA (Fig. 9A). In contrast, β-tubulin levels were similar in both conditions indicating a specific reduction in mTOR protein. We demonstrated that two different mTOR-siRNAs inhibited elongated tubule formation while scrambled siRNA did not when cells were overlaid with collagen (Fig. 9B). Importantly, the small apical lumens produced by siRNA treatment appeared similar in size and morphology to those produced when MDCK cells were incubated for 24 h with collagen gel overlays containing rapamycin (Fig. 8). These results suggested that rapamycin and mTOR-siRNA are inhibiting the same components in the mTOR signal transduction pathway.
Fig. 9.
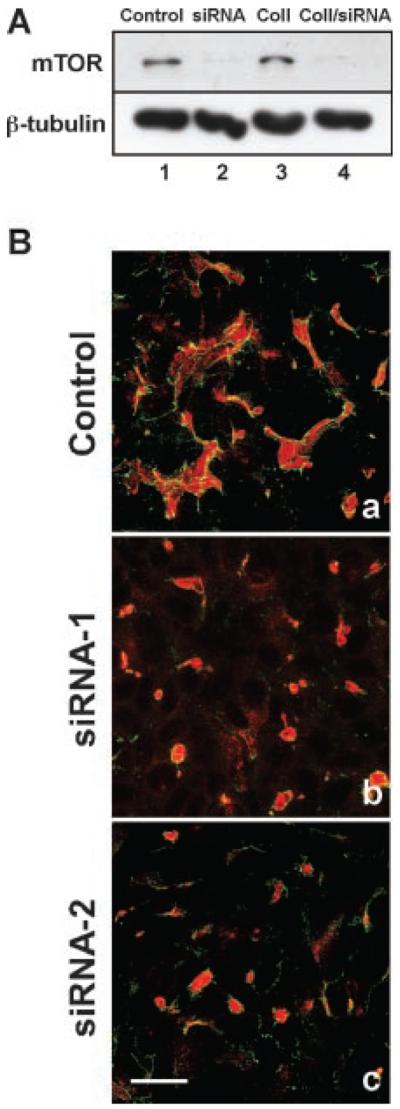
RNAi down-regulation of mTOR inhibits epithelial tubule formation. A: Western blot analysis of mTOR down-regulation. MDCK cells were incubated for 48 h with mTOR siRNA followed by 24 h incubation with, or without, collagen gel overlays. Control (lane 1) and collagen gel (Coll) treated cells (lane 3) cells were incubated with medium containing mTOR siRNA with a scrambled sequence. For mTOR down-regulation, cells were incubated in either medium (lane 2) or with collagen gel containing mTOR siRNA (lane 4). Our data demonstrate that mTOR-siRNA induced a significant down-regulation of mTOR in the presence, or absence, of collagen gel overlays (lanes 2, 4). β-tubulin was used as a protein loading control. B: Morphological analysis of epithelial tubule formation. Control cells incubated with scrambled siRNA formed elongated, multicellular tubules in collagen gel (a) identical to those lacking the scrambled RNA. However, inclusion of mTOR siRNA-1 (b) or mTOR siRNA-2 (c) in the collagen gel overlays inhibited elongated tubule formation, only allowing the biogenesis of small lumens that stained positive for gp135 (b, c; red staining) with associated ring-like tight junctions containing ZO-1 (green staining). Scale Bar, 8 μm.
Discussion
Using MDCK cells as a model system, our laboratory previously demonstrated that the Rho family GTPase signal transduction pathway plays an important role in regulation of epithelial tubule formation (Eisen et al., 2004, 2006). In this report we present evidence that the PI 3-kinase and mTOR signaling pathways, upstream regulators of Rho family GTPase signaling, are important modulators of MDCK cell epithelial tubule formation. The major observations made in this study are that the PI 3-kinase inhibitor LY-294002 inhibited both epithelial remodeling and tubule formation, while rapamycin and mTOR-siRNA inhibited only epithelial tubule formation. Furthermore, collagen-mediated disruption of adherens and tight junctions accompanied by increased cell motility appeared to play an important regulatory role in modulation of epithelial tubule assembly (Ojakian et al., 2001; Eisen et al., 2004, 2006). In studies presented here, we demonstrated that inhibition of PI 3-kinase prevented collagen-mediated cellular rearrangements and dissociation of adherens junction β-catenin from the cytoskeleton. In addition, changes in TER were not observed suggesting that PI 3-kinase regulates adherens junction structure and function but not tight junction permeability during epithelial remodeling.
We have obtained evidence that incubation of MDCK cells with collagen gel overlays induced redistribution of myosin II, Rho-kinase and Rac1 to lamellipodia of migrating cells (Eisen et al., 2004, 2006), producing a spatially distinct membrane domain that could serve as a site for the regulation of cellular events. This hypothesis is supported by observations on MDCK cysts demonstrating that PIP2 and PIP3 have polarized plasma membrane distributions and functions. Recent observations provide evidence that PIP2 and PIP3 are involved in the regulation of apical and basolateral membrane biogenesis, respectively (Gassama-Diagne et al., 2006; Martin-Belmonte et al., 2007). We demonstrated that after incubation with collagen gel overlays, GFP-Akt-PH was primarily localized to the leading edge of lamellipodia suggesting that temporal and spatial rearrangement of PI 3-kinase and PIP3 had occurred. These observations support a proposal that lamellipodia can serve as signaling platforms during epithelial remodeling. Our hypothesis is further supported by live cell imaging studies demonstrating that signaling components are involved in temporal and spatial regulation of cell motility (Kraynov et al., 2001; Fukada et al., 2003; Ridley et al., 2004) and formation of adherens and tight junctions in epithelial cells (Potempa and Ridley, 1998; Ehrlich et al., 2002; Wheelock and Johnson, 2003; Matter et al., 2005).
These studies provided evidence that PI 3-kinase regulation of epithelial tubule morphogenesis utilizes other signal transduction pathways to modulate these developmental processes. This hypothesis is supported by observations that LY-294002 at concentrations used for PI 3-kinase inhibition also decreased mTOR activity bringing into question this inhibitor's specificity (Brunn et al., 1996; Sarbassov et al., 2005a). We also observed that LY-294002 inhibits mTOR phosphorylation in MDCK cells (see Fig. 6B). In the present study we demonstrate that rapamycin inhibited mTOR activity over 6 h but did not inhibit epithelial remodeling suggesting that mTOR is involved in regulation of late steps of epithelial tubule formation. Furthermore, there are studies demonstrating that MDCK cells stably expressing constitutively activated Akt, a major downstream effector of PI 3-kinase, did not form tubular extensions in collagen gel (Khwaja et al., 1998). In the same study, MDCK cysts that stably expressed constitutively activated PI 3-kinase spontaneously formed tubular extensions. Together, these results suggest that other downstream effectors of PI 3-kinase are also involved in regulation of tubule formation.
The mTOR-signaling pathway is downstream of PI 3-kinase and plays an important role in regulation of cell growth and proliferation (Inoki et al., 2005; Martin and Hall, 2005; Sarbassov et al., 2005b). Elevated mTOR activity has been demonstrated in human PKD epithelial cysts and rapamycin inhibited kidney cyst formation in a mouse PKD model (Shillingford et al., 2006). However, to our knowledge, the studies presented in the current report are the first to describe rapamycin and siRNA down-regulation of mTOR-mediated inhibition of epithelial tubule formation. Although rapamycin and mTOR-siRNA did not have an effect on epithelial remodeling during the early developmental phase (6 h) of tubule formation, prolonged (24 h) mTOR inhibition prevented formation of elongated, multicellular tubules suggesting that mTOR signaling modulates late regulatory pathways during epithelial tubule morphogenesis. This suggested the possibility that there are two separate mechanisms regulating mTOR activity during epithelial remodeling and tubule formation.
We propose that early (6 h) regulation is due to disruption of cell junctions accompanied by increased actinomyosin-regulated cell motility, epithelial remodeling and a corresponding decrease in mTOR signaling. However, at later stages of development, long-term incubation with rapamycin (24 h) induced a down-regulation of mTOR producing an inhibition of both mTOR activity and formation of elongated, multicellular tubules. Rapamycin-induced mTOR protein down-regulation has also been observed in PC3 cells using long-term incubation (24–72 h) with rapamycin (Sarbassov et al., 2006). Furthermore, these investigators demonstrated that a 24 h rapamycin incubation induced dissociation of mTOR complexes in a variety of cell types. It is possible that a similar rapamycin-induced disruption of mTOR protein complexes occurs in MDCK cells during 24 h incubation with collagen gel overlays. If so, this could disrupt actin function of mTOR complex 2 (Jacinto et al., 2004; Sarbassov et al., 2004) producing an inhibition of epithelial tubule formation. Such a determination will require targeted down-regulation of mTOR complex components using RNA interference and related procedures.
Acknowledgments
We thank Dr. Jim Marrs for critically reading the manuscript, Dr. Bill Chirico for helpful discussion, Dr. W. James Nelson for providing antibodies to E-cadherin, Drs. Wei Yu and Keith Mostov for the GFP-Akt-PH MDCK cells, Una Yearwood for typing the manuscript, Wei Min and Bill Oxberry for assistance with confocal microscopy and Vincent Garofalo for graphic arts coordination. We would also like to thank Dr. Jeremy Weedon, Senior Statistician (SUNY Downstate Medical Center), for his expert help in doing the statistical analysis of our data. This project was supported by a grant from the National Institute of Diabetes and Digestive and Kidney Diseases DK-60570.
Contract grant sponsor: National Institute of Diabetes and Digestive and Kidney Diseases;
Contract grant number: DK-60570.
Literature Cited
- Bissell MJ, Rizki A, Mian IS. Tissue architecture: The ultimate regulator of breast epithelial function. Curr Opin Cell Biol. 2003;15:753–762. doi: 10.1016/j.ceb.2003.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunn GJ, Williams J, Sabers C, Wiederrcht G, Lawrence JC, Jr., Abraham RT. Direct inhibition of the signaling functions of the mammalian target of rapamycin by the phosphoinositide 3-kinase inhibitors wortmannin and LY294002. EMBO J. 1996;15:5256–5267. [PMC free article] [PubMed] [Google Scholar]
- Cantley LC. The phosphoinositide 3-kinase pathway. Science. 2002;296:1655–1657. doi: 10.1126/science.296.5573.1655. [DOI] [PubMed] [Google Scholar]
- Damsky CH, Ilic D. Integrin signaling: It's where the action is. Curr Opin Cell Biol. 2002;14:594–602. doi: 10.1016/s0955-0674(02)00368-x. [DOI] [PubMed] [Google Scholar]
- Debnath J, Walker SJ, Brugge JS. Akt activation disrupts mammary acinar architecture and enhances proliferation in an mTOR-dependent manner. J Cell Biol. 2003;163:315–326. doi: 10.1083/jcb.200304159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edinger AL, Linardic C, Chang GG, Thompson CB, Abraham RT. Differential effects of rapamycin on mammalian target of rapamycin signaling functions in mammalian cells. Cancer Res. 2003;64:8451–8460. [PubMed] [Google Scholar]
- Ehrlich JS, Hansen MDH, Nelson WJ. Spatio-temporal regulation of Rac localization and lamellipodia dynamics during epithelial cell-cell adhesion. Dev Cell. 2002;3:259–270. doi: 10.1016/s1534-5807(02)00216-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisen R, Ratcliffe DR, Ojakian GK. Modulation of epithelial tubule formation by Rho kinase. Am J Physiol. 2004;286:C857–C866. doi: 10.1152/ajpcell.00246.2003. [DOI] [PubMed] [Google Scholar]
- Eisen R, Walid S, Ratcliffe DR, Ojakian GK. Modulation of epithelial tubule formation by Rho family GTPases. Am J Physiol. 2006;286:C857–C866. doi: 10.1152/ajpcell.00246.2003. [DOI] [PubMed] [Google Scholar]
- Fingar DC, Salama S, Tsou C, Harlow E, Blenis J. Mammalian cell size is controlled by mTOR and its downstream targets S6K1 and 4EBP1/eIF4E. Genes Dev. 2002;16:1472–1487. doi: 10.1101/gad.995802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukada M, Nakagawa M, Kaibuchi K. Roles of Rho-family GTPases in cell polarization and directional migration. Curr Opin Cell Biol. 2003;15:590–597. doi: 10.1016/s0955-0674(03)00097-8. [DOI] [PubMed] [Google Scholar]
- Gassama-Diagne A, Yu W, ter Beest M, Martin-Belmonte F, Kierbel A, Engel J, Mostov K. Phosphatidylinositol-3,4,5-trisphosphate regulates the formation of the basolateral plasma membrane in epithelial cells. Nat Cell Biol. 2006;8:963–970. doi: 10.1038/ncb1461. [DOI] [PubMed] [Google Scholar]
- Halbleibe J, Nelson WJ. Cadherins in development: Cell adhesion, sorting, and tissue morphogenesis. Genes Dev. 2006;20:3199–3214. doi: 10.1101/gad.1486806. [DOI] [PubMed] [Google Scholar]
- Hall A. Rho GTPases and the actin cytoskeleton. Science. 1998;279:509–514. doi: 10.1126/science.279.5350.509. [DOI] [PubMed] [Google Scholar]
- Hall HG, Farson DA, Bissell MJ. Lumen formation by epithelial cell lines in response to collagen gel overlay: A morphogenetic model in culture. Proc Nat Acad Sci USA. 1982;79:4672–4676. doi: 10.1073/pnas.79.15.4672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinck L, Näthke IS, Papkoff J, Nelson WJ. Dynamics of cadherin/catenin complex formation: Novel protein interactions and pathways of complex formation. J Cell Biol. 1994;125:1327–1340. doi: 10.1083/jcb.125.6.1327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoki K, Ouyang H, Li Y, Guan K-L. Signaling by target of rapamycin proteins in cell growth control. Micro Mol Biol Rev. 2005;69:79–100. doi: 10.1128/MMBR.69.1.79-100.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacinto E, Loewith R, Schmidt A, Lin S, Ruegg MA, Hall A, Hall MN. Mammalian TOR complex 2 controls the actin cytoskeleton and is rapamycin insensitive. Nat Cell Biol. 2004;6:1122–1128. doi: 10.1038/ncb1183. [DOI] [PubMed] [Google Scholar]
- Jou T-S, Schneeberger EE, Nelson WJ. Structural and functional regulation of tight junctions by RhoA and Rac1 small GTPases. J Cell Biol. 1998;142:101–115. doi: 10.1083/jcb.142.1.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khwaja A, Lehmann K, Marte BM, Downward J. Phosphoinositide 3-kinase induces scattering and tubulogenesis in epithelial cells through a novel pathway. J Biol Chem. 1998;273:18793–18801. doi: 10.1074/jbc.273.30.18793. [DOI] [PubMed] [Google Scholar]
- Kovacs E, Ali RG, McCormack AJ, Yap AS. E-cadherin homophilic ligation directly signals through Rac and PI3-K to regulate adhesive contacts. J Biol Chem. 2002;277:6708–6718. doi: 10.1074/jbc.M109640200. [DOI] [PubMed] [Google Scholar]
- Kraynov VS, Chamberlain C, Bokoch GM, Schwartz MA, Slabaugh S, Hahn KM. Localized Rac activation dynamics visualized in lining cells. Science. 2001;290:333–337. doi: 10.1126/science.290.5490.333. [DOI] [PubMed] [Google Scholar]
- Lubarsky B, Kransnow MA. Tube morphogenesis: Making and shaping biological tubes. Cell. 2003;112:19–28. doi: 10.1016/s0092-8674(02)01283-7. [DOI] [PubMed] [Google Scholar]
- Luo J, Manning BD, Cantley LC. Targeting the PI3K-Akt pathway in human cancer: Rationale and promise. Cancer Cell. 2003;4:257–262. doi: 10.1016/s1535-6108(03)00248-4. [DOI] [PubMed] [Google Scholar]
- Martin-Belmonte F, Gassama A, Datta A, Yu W, Rescher U, Gerke V, Mostov K. PTEN-mediated apical segregation of phosphoinositides controls epithelial morphogenesis through Cdc42. Cell. 2007;128:383–397. doi: 10.1016/j.cell.2006.11.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin DE, Hall MN. The expanding TOR signaling network. Curr Opin Cell Biol. 2005;17:158–166. doi: 10.1016/j.ceb.2005.02.008. [DOI] [PubMed] [Google Scholar]
- Matter K, Aijaz S, Traparam A, Balda MS. Mammalian tight junctions in the regulation of epithelial differentiation and proliferation. Curr Opin Cell Biol. 2005;17:453–458. doi: 10.1016/j.ceb.2005.08.003. [DOI] [PubMed] [Google Scholar]
- Miranti CK, Brugge JS. Sensing the environment: A historical perspective on integrin signal transduction. Nat Cell Biol. 2002;4:83–90. doi: 10.1038/ncb0402-e83. [DOI] [PubMed] [Google Scholar]
- Montesano R, Schaller G, Orci L. Identification of a fibroblast-derived epithelial morphogen as hepatocyte growth factor. Cell. 1991;67:901–908. doi: 10.1016/0092-8674(91)90363-4. [DOI] [PubMed] [Google Scholar]
- O'Brien LE, Zegers MMP, Mostov KE. Building epithelial architecture: Insights from three-dimensional culture models. Nat Rev Mol Cell Biol. 2002;3:531–537. doi: 10.1038/nrm859. [DOI] [PubMed] [Google Scholar]
- Ojakian GK, Schwimmer R. The polarized distribution of an apical cell surface glycoprotein is maintained by interactions with the cytoskeleton of Madin-Darby canine kidney cells. J Cell Biol. 1988;107:2377–2388. doi: 10.1083/jcb.107.6.2377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ojakian GK, Schwimmer R. Regulation of epithelial cell surface polarity reversal by β1 integrins. J Cell Sci. 1994;107:561–576. [PubMed] [Google Scholar]
- Ojakian GK, Nelson WJ, Beck KA. Mechanisms for de novo biogenesis of an apical membrane compartment in groups of simple epithelial cells surrounded by extracellular matrix. J Cell Sci. 1997;110:2781–2794. doi: 10.1242/jcs.110.22.2781. [DOI] [PubMed] [Google Scholar]
- Ojakian GK, Ratcliffe D, Schwimmer R. Integrin regulation of cell-cell adhesion during epithelial tubule formation. J Cell Sci. 2001;114:941–952. doi: 10.1242/jcs.114.5.941. [DOI] [PubMed] [Google Scholar]
- Pece S, Chiariello M, Murga C, Gutkind JS. Activation of the protein kinase Akt/PKB by the formation of E-cadherin-mediated cell-cell junctions. J Biol Chem. 1999;274:19347–19351. doi: 10.1074/jbc.274.27.19347. [DOI] [PubMed] [Google Scholar]
- Potempa S, Ridley AJ. Activation of both MAP kinase and phosphatidylinositide 3-kinase by Ras is required for hepatocyte growth factor/scatter factor-induced adherens junction disassembly. Mol Biol Cell. 1998;9:2185–2200. doi: 10.1091/mbc.9.8.2185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ridley AJ, Schwartz MA, Burridge K, Firtel RA, Ginsburg MH, Borisy G, Parsons JT, Horwitz AR. Cell migration: Integrating signals from front to back. Science. 2004;302:1704–1709. doi: 10.1126/science.1092053. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Boulan E, Kreitzer G, Müsch A. Organization of vesicular trafficking in epithelia. Nat Rev Mol Cell Biol. 2005;6:233–247. doi: 10.1038/nrm1593. [DOI] [PubMed] [Google Scholar]
- Sarbassov DD, Ali SM, Kim DH, Guertin DA, Latek RR, Erdjument-Bromage H, Tempst P, Sabatini DM. Rictor, a novel binding partner of mTOR, defines a rapamycin-insensitive and raptor-independent pathway that regulates the cytoskeleton. Curr Biol. 2004;14:1296–1302. doi: 10.1016/j.cub.2004.06.054. [DOI] [PubMed] [Google Scholar]
- Sarbassov DD, Guertin DA, Ali SM, Sabatini DM. Phosphorylation and regulation of Akt/PKB by the rictor-mTOR complex. Science. 2005a;307:1098–1101. doi: 10.1126/science.1106148. [DOI] [PubMed] [Google Scholar]
- Sarbassov DD, Ali SM, Sabatini DM. Growing roles for the mTOR pathway. Curr Opin Cell Biol. 2005b;17:596–603. doi: 10.1016/j.ceb.2005.09.009. [DOI] [PubMed] [Google Scholar]
- Sarbassov DD, Ali SM, Sengupta S, Sheen J-H, Hsu PP, Bagley AF, Markhard AL, Sabatini DM. Prolonged rapamycin treatment inhibits mTORC2 assembly and Akt/PKB. Mol Cell. 2006;22:159–168. doi: 10.1016/j.molcel.2006.03.029. [DOI] [PubMed] [Google Scholar]
- Sawyers CL. Will mTOR inhibitors make it as cancer drugs? Cancer Cell. 2003;4:343–348. doi: 10.1016/s1535-6108(03)00275-7. [DOI] [PubMed] [Google Scholar]
- Schwimmer R, Ojakian GK. The α2β1 integrin regulates collagen-mediated MDCK epithelial membrane remodeling and tubule formation. J Cell Sci. 1995;108:2487–2498. doi: 10.1242/jcs.108.6.2487. [DOI] [PubMed] [Google Scholar]
- Shillingford JM, Murcia NS, Larson CH, Low SH, Hedgepeth R, Brown N, Flask CA, Novick AC, Goldfarb DA, Kramer-Zucker A, Walz G, Piontek KB, Geromp GG, Weimbs T. The mTOR pathway is regulated by polycystin-1, and its inhibition reverses renal cystogenesis in polycystic kidney disease. Proc Nat Acad Sci. 2006;103:5466–5471. doi: 10.1073/pnas.0509694103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang M-J, Cai Y, Tsai S-J, Wang Y-K, Dressler GR. Ureteric bud outgrowth in response to RET activation is mediated by phosphatidylinositol 3-kinase. Dev Biol. 2002;243:128–136. doi: 10.1006/dbio.2001.0557. [DOI] [PubMed] [Google Scholar]
- Wang AZ, Ojakian GK, Nelson WJ. Steps in the morphogenesis of a polarized epithelium. I. Uncoupling the roles of cell-cell and cell-substratum contact in establishing plasma membrane polarity in multicellular epithelial (MDCK) cysts. J Cell Sci. 1990a;95:137–151. doi: 10.1242/jcs.95.1.137. [DOI] [PubMed] [Google Scholar]
- Wang AZ, Ojakian GK, Nelson WJ. Steps in the morphogenesis of a polarized epithelium. II. Disassembly and assembly of plasma membrane domains during reversal of epithelial cell polarity in multicellular epithelial (MDCK) cysts. J Cell Sci. 1990b;95:153–165. doi: 10.1242/jcs.95.1.153. [DOI] [PubMed] [Google Scholar]
- Watton SJ, Downward J. Akt/PKB localisation and 3′phosphoinositide generation at sites of epithelial cell-matrix and cell-cell interaction. Curr Biol. 1999;9:433–436. doi: 10.1016/s0960-9822(99)80192-4. [DOI] [PubMed] [Google Scholar]
- Wheelock MJ, Johnson KR. Cadherin-mediated cellular signaling. Curr Opin Cell Biol. 2003;15:509–514. doi: 10.1016/s0955-0674(03)00101-7. [DOI] [PubMed] [Google Scholar]
- Yu W, O'Brien LE, Wang F, Bourne H, Mostov KE, Zegers MP. Hepatocyte growth factor switches orientation of polarity and mode of movement during morphogenesis of multicellular epithelial structures. Mol Biol Cell. 2003;14:748–763. doi: 10.1091/mbc.E02-06-0350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu W, Datta A, Leroy P, O'Brien LE, Mak G, Jou T-S, Matlin KS, Mostov KE, Zegers MMP. β1 integrin orients epithelial polarity via Rac1 and laminin. Mol Biol Cell. 2005;16:433–445. doi: 10.1091/mbc.E04-05-0435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuk A, Matlin K. β1 integrin in polarized MDCK cells mediates tubulocyst formation in response to type I collagen overlay. J Cell Sci. 1996;109:1875–1889. doi: 10.1242/jcs.109.7.1875. [DOI] [PubMed] [Google Scholar]