Abstract
BACKGROUND
Stillbirths (≥ 20 weeks’ gestation), which account for about 1 in 200 U.S. pregnancies, may grieve parents deeply. Unresolved grief may lead to persistent depression.
METHODS
We compared depressive symptoms in 2009 (6–36 months after index delivery) among consenting women in the Stillbirth Collaborative Research Network’s population-based case-control study conducted 2006–2008 (N=275 who delivered a stillbirth and N=522 who delivered a healthy live birth (excluding live births < 37 weeks, infants who had been admitted to a neonatal intensive care unit or who died). Women scoring > 12 on the Edinburgh Depression Scale were classified as currently depressed. Crude (cOR) and adjusted (aOR) odds ratios and 95% confidence intervals [CI] were computed from univariate and multivariable logistic models, with weighting for study design and differential consent. Marginal structural models examined potential selection bias due to low follow-up.
RESULTS
Current depression was more likely in women with stillbirth (14.8%) vs. healthy live birth (8.3%, cOR 1.90 [95% CI 1.20, 3.02]). However, after control for history of depression and factors associated with both depression and stillbirth, the stillbirth association was no longer significant (aOR 1.35 [95% CI 0.79, 2.30]). Conversely, for the 76% of women with no history of depression a significant association remained after adjustment for confounders (aOR 1.98 [95% CI 1.02, 3.82]).
CONCLUSIONS
Improved screening for depression and referral may be needed for women’s health care. Research should focus on defining optimal methods for support of women suffering stillbirth so as to lower the risk of subsequent depression.
Few events are as emotionally challenging for families as stillbirth (fetal death at ≥ 20 weeks’ gestation), affecting one in 200 pregnancies in the U.S.1 In 2012, U.S. vital statistics recorded 24,073 stillbirths -- representing 6.1 per 1,000 deliveries2 -- more than the U.S. infant mortality rate.
Stillbirth imposes a substantial, immediate burden of grief.3–4 While symptoms of depression may be “normal” expressions of this grief, depressive symptoms that do not resolve into mourning – “the process of recovery, with gradual lessening of distress and return to normal patterns of living”5 – within 6 months of the loss can become persistent and debilitating.5–7 Women with a history of depression are especially vulnerable to experiencing persistent depression after perinatal loss and stillbirth,3,8–10 even after the subsequent birth of a healthy child.8 Less is known about persistent depression in women with stillbirth who have no prior history of depression or about the prevalence and predictors of persistent depression. Further, many of the studies of post-stillbirth depression have lacked comparable groups of women post-live birth, to determine whether depression differs by pregnancy outcome. Our objective was to determine if depression, as defined by a score >12 on the Edinburgh Depression Scale (EDS) at one time point 6–36 months after the index event, is greater among women whose index delivery was a stillbirth compared to women with a healthy live birth.
METHODS
Study Design and Sample
The Stillbirth Collaborative Research Network (SCRN)1, a population-based case-control study that enrolled women in-hospital immediately after delivery, included populations of Rhode Island and selected counties in Massachusetts, Georgia, Texas, and Utah. Investigators selected 59 hospitals to ensure access to at least 90% of all pregnancies to residents ending in live birth or stillbirth. Recruitment and enrollment of 663 cases and 1932 controls occurred between March 2006 and September 2008. Study personnel followed a standardized protocol including maternal interview, medical record abstraction, placental pathology, biospecimen testing, and, for stillbirths, postmortem examination.12 Also, for each participant, staff requested contact information and requested written consent for further contact.
The SCRN-Outcomes after Study Index Stillbirth (OASIS) study was approved by Institutional Review Boards at all participating sites. Women were contacted in 2009 (between 6 months and 3 years after their index delivery) if they had provided written informed consent in SCRN. A letter marked confidential and addressed specifically to the participant was sent to her last known address. If the letter requesting permission for a telephone interview was returned or there was no response, site staff tried calling the participant and utilized other contact information to locate the woman.
Located women were given an explanation of the follow-up study procedures, after which they provided verbal consent for the telephone interview, conducted in either English or Spanish at their choice. Because women with losses were asked about their grief experience, interviewers were not blind to the index pregnancy outcome. Interviewers were trained to recognize symptoms of immediate distress or indications that the respondent might be contemplating harm to herself or others. In this event, interviewers followed a detailed referral protocol.
All women were queried about subsequent pregnancies and complications, and about life-course stresses hypothesized to be associated with an increased stillbirth risk. Psychosocial instruments were selected based on four criteria: 1) validity for telephone interviewing; 2) a validated Spanish translation for the population under study; 3) use for multiple race/ethnicities; and 4) where relevant, use in other perinatal loss studies.
History of depression
A woman was classified with history of depression if in her initial interview she reported that she had ever been depressed and had sought help, or reported having used an antidepressant during this pregnancy, or if a prescription for antidepressant was noted in her prenatal or hospital record. While 48% of women reported having ever been “down in the dumps,” 22% met the depression history definition. For 2430 women with initial interview and chart abstraction, the crude odds ratio [cOR] for risk of stillbirth associated with depression history was 0.85, 95% CI [0.67, 1.08].
Spielberger Trait Anxiety Scale of the State-Trait Anxiety Inventory (STAI)
The original interview included the trait anxiety scale of STAI.13 We conducted psychometric analyses of the STAI to test for differences by race/ethnicity, language of the interview, and outcome of the index pregnancy (see supplemental data). A single trait anxiety score was computed as an average score using 18 items and allowing no more than one to be missing. The score was then categorized using quartiles established from SCRN women with live birth deliveries.
Spielberger Trait Anger Scale of the State-Trait Anger Expression Inventory (STAXI-2)
The initial interview also included the trait anger scale of STAXI-214, assessing the frequency of anger experience, expression and control. In psychometric analyses, we focused only on the frequency of anger experience, with temperament and reaction subscales (see supplemental data). The scale performed well, and a single trait anger score was computed according to the specific instructions from the developer and then categorized using quartiles established from SCRN women with live birth deliveries.
Stressful Life Events (SLE)
The original interview included the 13-item SLE scale from PRAMS.15 Psychometric properties of the SLE for SCRN participants have been previously reported.16 Based on our previous findings, we grouped the scale into 4 factors: financial, emotional, traumatic, and partner-related events. A factor was categorized as “yes” if any of the items in the factor was present and “no” otherwise, with possible scores on the number of SLE factors ranging from 0 to 4.
Definition of Current Depression
Current depression was a score of > 12 on the EDS administered at the time of follow-up interview. The EDS, a 10-item self-report questionnaire with possible scores ranging from 0 – 30,17 has high sensitivity and specificity for the detection of major depression in pregnancy.18–19 It has been widely used with pregnant, postpartum, and non-pregnant women,19–20 has been validated for telephone use,21–22 and has a validated Spanish version.23
Like several other survey measures of depressive symptoms, the EDS may be less sensitive to depression among black than white women (measured for Black Caribbean and White English women).24 In psychometric analyses (see supplemental data), the scale demonstrated good internal consistency with Cronbach’s alpha of 0.80 for the overall sample.
Intervening Pregnancy
To explore whether an intervening pregnancy might affect the EDS score at the follow-up interview, we classified women into four categories: no intervening pregnancy, intervening pregnancy occurred just prior to interview (pregnancy outcome not yet known), outcome(s) of intervening pregnancy(ies) were only live birth(s), and outcome(s) of intervening pregnancy(ies) included at least one loss.
Analysis
All results were weighted for oversampling and other aspects of the study design as well as for differential consent based on characteristics recorded on all eligible deliveries that were screened for the SCRN study.11 Analyses were performed using SUDAAN version 11.0.25 We calculated the relative odds of depression at the time of the follow-up interview given the outcome of the index pregnancy (stillbirth or healthy live birth) with cOR and adjusted (aOR) odds ratios and 95% confidence intervals [CI] from univariate and multivariable logistic models. Women were excluded from the “healthy” live-born comparison group if their infant was preterm (gestation < 37 weeks), had been admitted to a neonatal intensive care unit, or died.
Since depression is known to be recurrent26 we included history of prior depression in the analysis, hypothesizing that stillbirth might increase recurrence risk. We also conducted a separate analysis excluding women with history of depression to test whether stillbirth experience increases the risk of depression among women without a prior history.
We tested history of depression, STAI, STAXI, and all variables known early in pregnancy (such as maternal and paternal socio-demographic factors, and maternal behavioral and medical risks) previously reported to be associated with stillbirth risk27 for both potential confounding and interaction with index pregnancy outcome. Finally, we considered time from index pregnancy to interview.
We selected variables for the multivariable analysis if they were potential confounders (i.e., modified the cOR by >10%), using a forward selection procedure with stillbirth / healthy live birth included at the initial step, followed by the potential confounding factors with p≤0.15 for inclusion. We tested for effect modification with time from the index pregnancy by assessing for interaction at p<0.05 in the final model. Also, as anxiety trait is known to co-vary with depression, we decided a priori to test for anxiety trait as a mediator of effect in the final model.
Sensitivity Analysis
Loss to follow up was substantial and any resulting bias due to this loss cannot be measured. However, the potential impact was explored using marginal structural models28–30 to construct two additional weighting components with variables known for the entire sample of interviewed women with prenatal records.
The first component made use of maternal variables theoretically associated with exposure (in this instance, case/control status) and outcome (depression at follow-up): age, race, ethnicity, education, marital status, method of insurance payment, moved during index pregnancy, pre-pregnancy BMI, pregnancy history prior to index, whether the index pregnancy was wanted, and whether it was a multifetal pregnancy. The numerator of the component was taken as the estimated probability of stillbirth from a logistic regression using these variables on the original weighted data for the 597 women with stillbirth and the 1,287 women with healthy term live births targeted for the Edinburgh analysis (see Figure 1). The denominator was the estimated probability of stillbirth using the same variables plus time from delivery of the index pregnancy to attempted follow-up.
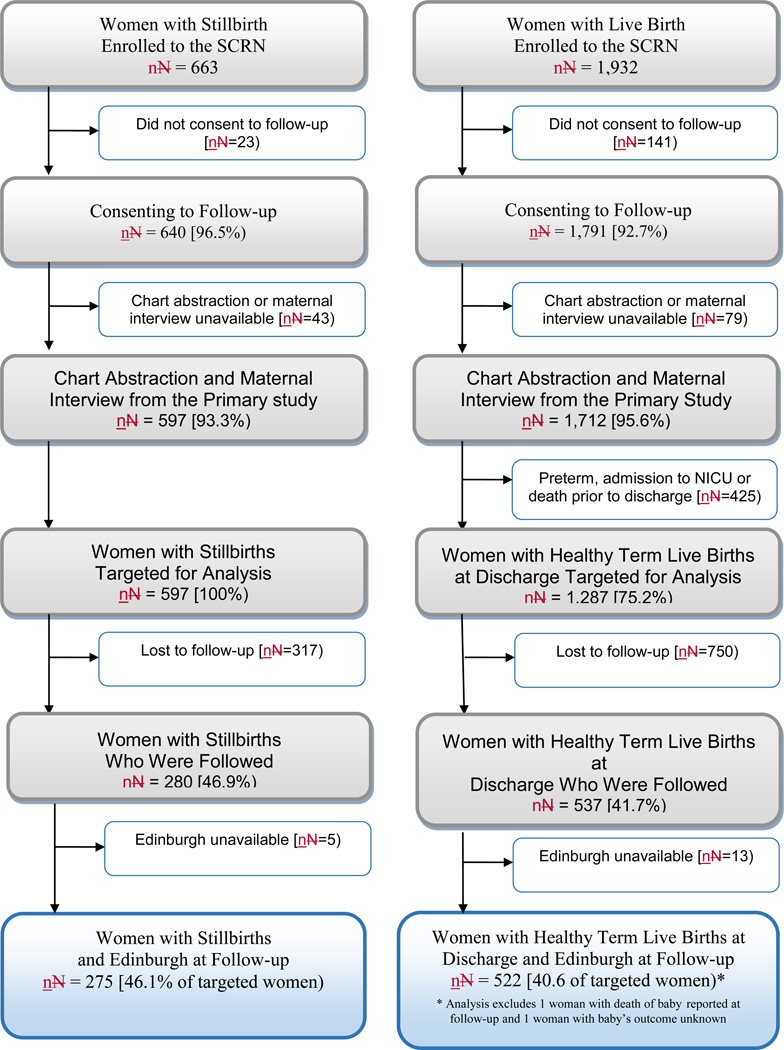
Figure 1. Study Enrollment and Inclusion in Edinburgh Analysis.
This analysis compares Edinburgh results at follow up interview for women with stillbirth and women with healthy term live birth pregnancies. A pregnancy was categorized as a stillbirth pregnancy if there were any stillbirths delivered and a live birth pregnancy if all live births were delivered. Women could have been eligible for enrollment to the Stillbirth Collaborative Research Network (SCRN) for one or more deliveries occurring during the enrollment period for the study. However, the flow chart accounts for each individual woman once, as a woman enrolled to the study was eligible for only one follow-up interview based on her last enrollment to the SCRN. A fetal death was defined by Apgar scores of 0 at 1 and 5 minutes and no signs of life by direct observation. Fetal deaths were classified as stillbirths if the best clinical estimate of gestational age at death was 20 or more weeks. Fetal deaths at 18 and 19 weeks without good dating were also included as stillbirths.
Similarly, the second component used variables theoretically associated with likelihood of follow-up and outcome (depression at follow-up): study site, age, race, ethnicity, education, marital status, method of insurance payment, moved during index pregnancy, pregnancy history prior to index, whether the index pregnancy was wanted, whether the index pregnancy was multifetal, history of depression prior to index, and number of SLE factors prior to index. The numerator of the component was taken as the estimated probability of follow-up from a logistic regression using these variables. The denominator was the estimated probability using the same variables plus time from delivery of the index pregnancy to attempted follow-up.
Finally, on the subset of women included in the Edinburgh analysis, the weight components were combined (original analysis weight × component 1 × component 2) and multiplied by constants for cases and for controls such that the sum of the weights is the same as found using the original analysis weights. This final adjustment results in overall weighted counts that reflect the size of the study after accounting for the oversampling.
RESULTS
Comparing interviewed with non-interviewed women
Figure 1 presents SCRN study enrollment and SCRN-OASIS study follow-up status. We included information for 797 women, of whom 51 were interviewed in Spanish. Seventeen women were referred by interviewers because of signs of immediate distress (12 whose index pregnancy outcome was stillbirth and 5 controls).
Differences were observed between women who were located and interviewed and those who were lost to follow-up (Table 1). There was no difference in follow up status by gestational length of the index pregnancy or by time from the index pregnancy to follow-up attempt.
Table 1.
Characteristics of Women Consenting to the SCRN Study and Targeted for Edinburgh Analysis by Availability of Edinburgh at Follow-up
| Characteristic - Weighted %a | Women Followed and Administered Edinburgh | |||||
|---|---|---|---|---|---|---|
| Stillbirths | Healthy Live Births | |||||
| No | Yes | P-value | No | Yes | P-value | |
| Unweighted sample size, N | 322 | 275 | 763 | 524 | ||
| Weighted sample size, Nw | 326 | 270 | 678 | 463 | ||
| Sociodemographic | ||||||
| Age at delivery, years | ||||||
| <20 | 16.5 | 10.1 | 0.146 | 12.6 | 7.0 | <.001 |
| 20–34 | 67.4 | 71.1 | 77.3 | 74.2 | ||
| 35–39 | 11.5 | 13.9 | 8.1 | 16.2 | ||
| 40+ | 4.5 | 4.9 | 1.9 | 2.6 | ||
| Race/ethnicity | ||||||
| Non-Hispanic white | 25.0 | 45.1 | <.001 | 36.4 | 61.5 | <.001 |
| Non-Hispanic black | 26.4 | 18.8 | 12.1 | 10.1 | ||
| Hispanic | 40.4 | 29.9 | 42.8 | 21.3 | ||
| Other | 8.3 | 6.1 | 8.7 | 7.2 | ||
| Education, grade | ||||||
| 0–11 [none/primary/some secondary] | 30.3 | 13.4 | <.001 | 24.8 | 8.0 | <.001 |
| 12 [completed secondary] | 30.4 | 29.8 | 29.4 | 20.3 | ||
| 13+ [college] | 39.3 | 56.8 | 45.8 | 71.7 | ||
| Marital status/cohabitating | ||||||
| Not married or cohabitating | 30.5 | 18.2 | <.001 | 18.3 | 10.3 | <.001 |
| Cohabitating | 28.6 | 23.2 | 30.6 | 15.4 | ||
| Married | 40.9 | 58.5 | 51.2 | 74.3 | ||
| Insurance/method of payment | ||||||
| No insurance | 7.3 | 3.0 | <.001 | 4.5 | 1.1 | <.001 |
| Any public/private assistance | 63.0 | 43.3 | 58.8 | 32.3 | ||
| VA/commercial health ins/ HMO | 29.7 | 53.7 | 36.7 | 66.5 | ||
| Pregnancy-Associated | ||||||
| Moved during pregnancy | 34.3 | 24.4 | 0.009 | 33.1 | 26.2 | 0.015 |
| Pregnancy wanted ever, before pregnancy | 93.4 | 94.5 | 0.586 | 93.8 | 96.5 | 0.027 |
| Gestational age at delivery, weeks | ||||||
| 18–19 | 3.1 | 1.6 | 0.080 | 0.0 | 0.0 | 0.133 |
| 20–23 | 30.1 | 37.4 | 0.0 | 0.0 | ||
| 24–27 | 14.4 | 17.7 | 0.0 | 0.0 | ||
| 28–31 | 15.1 | 9.1 | 0.0 | 0.0 | ||
| 32–36 | 20.2 | 18.9 | 6.2 | 4.3 | ||
| 37+ | 17.2 | 15.3 | 93.8 | 95.7 | ||
| Psychosocial | ||||||
| History of depression | 22.1 | 20.9 | 0.721 | 22.6 | 27.6 | 0.085 |
| STAI trait-anxiety scale scoreb | ||||||
| ≤ 25.8 | 9.5 | 20.2 | <.001 | 20.9 | 28.7 | 0.005 |
| 25.8–32.1 | 11.7 | 20.7 | 22.1 | 25.5 | ||
| 32.1–38.9 | 25.7 | 23.2 | 28.2 | 27.6 | ||
| >38.9 | 53.0 | 36.0 | 28.8 | 18.2 | ||
| STAXI-2 trait-anger scale scoreb | ||||||
| ≤ 12.3 | 17.0 | 20.3 | 0.101 | 21.4 | 19.6 | 0.145 |
| 12.3–14.9 | 16.6 | 17.6 | 19.9 | 22.4 | ||
| 14.9–18.9 | 25.3 | 31.0 | 25.6 | 30.5 | ||
| >18.9 | 41.1 | 31.1 | 33.1 | 27.5 | ||
| Follow-up | ||||||
| Months to follow-up attempt | ||||||
| < 12 | 14.7 | 11.3 | 0.650 | 4.2 | 5.0 | 0.149 |
| 12–23 | 45.6 | 47.6 | 46.0 | 51.3 | ||
| 24–35 | 39.4 | 40.5 | 48.2 | 41.3 | ||
| 36+ | 0.3 | 0.6 | 1.6 | 2.4 | ||
Weighted percentages and p-values are shown by analysis inclusion status, i.e., whether the Edinburgh was available at follow-up among women consenting to the Stillbirth Collaborative Research Network [SCRN] study and targeted for Edinburgh analysis. The weights take into account the study design and differential consent based on characteristics recorded on all eligible deliveries that were screened for the study. Unweighted and weighted samples sizes are also provided. Sample sizes vary slightly by characteristic included in the table.
11 women with stillbirth (4%) and 18 women with healthy live birth (4%) were missing STAI and STAXI-2 scales.
The STAI scale is scored if there were responses for at least 17 of 18 items used in the summary score. An average of the non-missing items is computed [using the appropriate numeric coding for each item] and multiplied by 20 [the number of items that constitutes the scale as originally developed]. STAXI-2 is scored according to the specific instructions from the developer. Cutpoints given in the table are based on quartiles from the weighted distribution, using live birth controls.
Association of stillbirth with depression at follow-up interview
Among women whose index pregnancy was a stillbirth, 14.8% had EDS > 12 at follow up compared to 8.3% of women who had a healthy live birth (8.3%) (Table 2). Depression history increased the strength of the association between having a stillborn and an EDS>12 6–36 months later by at least 10%, while adjustment with other characteristics either reduced the strength of the association by at least 10% or did not alter the association by at least 10% (Table 2). The STAXI-2 trait anger score confounded the stillbirth / current depression association by more than 10% (Table 2).
Table 2.
Edinburgh Scorea > 12 at Follow-up by Characteristics for Stillbirths and Healthy Live Births
| Characteristic Used for Adjustment to Odds Ratio |
Edinburgh Score > 12 − % [Nw]b | Adjusted Odds Ratio [95% CI]c Stillbirth vs Healthy Live Birth cOR [95% CI]: 1.90 [1.20, 3.02] |
|
|---|---|---|---|
| Stillbirth 14.8 [270] |
Healthy Live Birth 8.3 [461] |
||
| Sociodemographic | |||
| Maternal age at delivery, years | 1.9 [1.2, 3.0] | ||
| < 20 | 18.7 [27] | 17.8 [32] | |
| 20–34 | 15.7 [192] | 7.0 [342] | |
| 35–39 | 9.9 [38] | 10.0 [75] | |
| 40+ | 7.1 [13] | 10.5 [12] | |
| Maternal race/ethnicity | 1.8 [1.1, 2.8] | ||
| Non-Hispanic white | 13.8 [122] | 6.1 [283] | |
| Non-Hispanic black | 15.4 [51] | 13.4 [47] | |
| Hispanic | 16.1 [81] | 11.6 [98] | |
| Other | 13.5 [17] | 10.9 [33] | |
| Maternal education, grade | 1.7 [1.0, 2.7] | ||
| 0–11 [none/primary/some secondary] | 28.4 [36] | 26.0 [37] | |
| 12 [completed secondary] | 17.7 [80] | 9.4 [92] | |
| 13+ [college] | 10.3 [152] | 6.1 [331] | |
| Paternal education, grade | 1.6 [1.0, 2.5] | ||
| 0–11 [none/primary/some secondary] | 25.3 [48] | 14.6 [51] | |
| 12 [completed secondary] | 18.8 [90] | 12.5 [90] | |
| 13+ [college] | 8.1 [123] | 6.2 [305] | |
| Marital status/cohabitating | 1.7 [1.0, 2.7] | ||
| Not married or cohabitating | 17.8 [49] | 25.4 [48] | |
| Cohabitating | 18.1 [62] | 9.5 [69] | |
| Married | 12.6 [157] | 5.8 [343] | |
| Insurance/method of payment | 1.7 [1.0, 2.7] | ||
| No insurance | 27.2 [8] | 30.8 [5] | |
| Any public/private assistance | 19.3 [116] | 14.4 [148] | |
| VA/commercial health ins/HMO | 10.5 [144] | 5.0 [306] | |
| Maternal Self-Reported Substance Abuse | |||
| Average no. of cigarettes during 3 months prior to pregnancy | 1.8 [1.1, 2.9] | ||
| Did not smoke | 12.4 [235] | 7.3 [419] | |
| < 10 | 35.5 [21] | 27.2 [22] | |
| ≥ 10 | 25.9 [13] | 10.7 [18] | |
| Alcohol consumption during 3 months prior to pregnancy | 2.0 [1.2, 3.1] | ||
| Did not drink | 12.3 [166] | 8.4 [257] | |
| Drank, no binging | 21.6 [59] | 7.6 [124] | |
| Binged | 15.8 [44] | 9.6 [79] | |
| Lifetime drug use | 1.9 [1.2, 3.1] | ||
| Never used drugs | 13.6 [189] | 7.3 [320] | |
| Ever used drugs w/o addiction | 19.9 [71] | 10.8 [131] | |
| Ever used drugs w/ addiction | 0.0 [7] | 26.6 [4] | |
| Maternal Medical / Physiologic | |||
| Body mass index, kg/m2 | 1.7 [1.0, 2.7] | ||
| < 18.5 [underweight] | 15.0 [9] | 14.4 [14] | |
| 18.5–24.9 [normal weight] | 10.5 [98] | 5.7 [252] | |
| 25–29.9 [overweight] | 14.7 [73] | 10.1 [96] | |
| 30–34 [obese] | 17.0 [45] | 12.0 [53] | |
| ≥ 35 [morbidly obese] | 22.4 [44] | 13.8 [44] | |
| Blood type | 1.7 [1.0, 2.7] | ||
| A | 15.7 [79] | 8.2 [167] | |
| B | 10.9 [32] | 10.3 [51] | |
| O | 9.8 [124] | 8.3 [221] | |
| AB | 38.8 [17] | 7.0 [17] | |
| Pregnancy-Associated | |||
| Pregnancy history | 1.8 [1.2, 2.9] | ||
| • Nulliparous; never pregnant or only elective terminations | 15.7 [95] | 7.4 [139] | |
| • Nulliparous with previous losses | 13.4 [35] | 6.4 [24] | |
| • Multiparous with no previous losses at < 20 wk or stillbirths | 10.8 [89] | 7.6 [202] | |
| • Multiparous with no stillbirth but previous losses at < 20 wk | 13.6 [36] | 11.5 [88] | |
| • Multiparous with stillbirth | 39.6 [14] | 12.3 [9] | |
| Pregnancy wanted ever, before pregnancy | 1.9 [1.2, 3.0] | ||
| Yes | 13.9 [252] | 7.7 [441] | |
| No | 27.3 [15] | 22.7 [16] | |
| Multi-fetal pregnancy | 2.0 [1.3, 3.2] | ||
| Yes | 6.6 [21] | 0.0 [11] | |
| No | 15.5 [249] | 8.5 [450] | |
| Gestational age at delivery, weeks | 1.9 [1.0, 3.7] | ||
| 18–24 | 16.5 [105] | -- [0] | |
| 24–31 | 12.2 [72] | -- [0] | |
| 32+ | 14.9 [92] | 8.3 [461] | |
| Psychosocial | |||
| History of depressiond | 2.1 [1.3, 3.3] | ||
| Yes | 21.2 [57] | 17.1 [127] | |
| No | 13.1 [214] | 5.0 [334] | |
| Significant Life Events: # of factorse | 1.7 [1.0, 2.7] | ||
| 0 | 13.4 [62] | 6.0 [129] | |
| 1 | 7.3 [83] | 5.2 [146] | |
| 2 | 10.4 [62] | 4.6 [115] | |
| 3 | 30.5 [43] | 21.7 [48] | |
| 4 | 31.1 [17] | 39.1 [15] | |
| STAI trait-anxiety scale scoref | 1.6 [0.9, 2.6] | ||
| ≤ 25.8 | 11.2 [53] | 3.0 [129] | |
| 25.8–32.1 | 7.8 [53] | 3.3 [114] | |
| 32.1–38.9 | 7.8 [60] | 6.8 [121] | |
| > 38.9 | 26.2 [94] | 23.0 [80] | |
| STAXI-2 trait-anger scale scoref | 2.1 [1.3, 3.4] | ||
| ≤ 12.3 | 11.9 [53] | 5.8 [88] | |
| 12.3–14.9 | 12.9 [46] | 5.8 [99] | |
| 14.9–18.9 | 15.8 [80] | 7.2 [135] | |
| > 18.9 | 17.9 [81] | 11.2 [122] | |
| Follow-up | |||
| Months to follow-up attempt | 1.8 [1.2, 2.9] | ||
| < 6 | -- [0] | -- [0] | |
| 6–12 | 25.1 [31] | 12.0 [23] | |
| 12–18 | 9.7 [66] | 8.6 [109] | |
| 18–24 | 8.7 [63] | 9.5 [127] | |
| 24+ | 18.4 [111] | 7.0 [202] | |
Nw=weighted sample size, 95% CI = 95% confidence interval, cOR=crude odds ratio
The Edinburgh is scored if there were responses for all 10 items.
Weighted percentages and sample sizes are given. The weights take into account the study design and differential consent based on characteristics recorded on all eligible deliveries that were screened for the study.
Odds ratios are taken from weighted logistic regression models with Edinburgh score > 12 (yes versus no) as the dependent variable. A separate model is fit for each row characteristic shown in the table as a covariate along with SCRN case status. The odds ratios reported are for stillbirth versus healthy live birth, adjusted for the covariate.
Women are classified as having a history of depression if they had taken antidepressant medications or if they indicated they had sought help for depression.
Significant life events are grouped as four factors: financial, emotional, traumatic, and partner. Factors that were noted are summed.
11 women with stillbirth (4%) and 18 women with healthy live birth (4%) were missing STAI and STAX-2 scales. The STAI scale is scored if there were responses for at least 17 of 18 items used in the summary score. An average of the non-missing items is computed [using the appropriate numeric coding for each item] and multiplied by 20 [the number of items that constitutes the scale as originally developed]. STAXI-2 is scored according to the specific instructions from the developer. Cutpoints given in the table are based on quartiles from the weighted distribution, using live birth controls.
The time between delivery and the first follow-up interview was not a confounder of the association between EDS > 12 and delivery of a stillbirth. When time to interview was treated as an ordinal variable, there appeared to be a U-shaped trend for EDS > 12 among stillbirths by time since index, but not among live births (Table 2). However, this trend did not represent a significant interaction between the index delivery outcome and the time of the follow-up interview (data not shown).
Women with stillbirth were much more likely to have had an intervening pregnancy: 172 (62.5%) for index stillbirth versus 143 (27.4%) for index live birth. However, there was no statistically significant difference among the categories for women with index live birth or stillbirth (Table 3).
Table 3.
Edinburgh Scorea > 12 at Follow-up by Classification of Intervening Pregnanciesb For Stillbirths and Healthy Live Births
| Intervening Pregnancy | Weightedc | Unweighted | ||
|---|---|---|---|---|
| Edinburgh Score > 12 − % [Nw] | Edinburgh Score > 12 − % [N] | |||
| Stillbirths | Healthy Live Births | Stillbirths | Healthy Live Births | |
| No | 17.8 [106] | 8.5 [335] | 18.4 [103] | 9.8 [378] |
| Yes - Only incompleted | 5.2 [29] | 8.1 [38] | 6.9 [29] | 7.1 [42] |
| Yes - At least one live birth | 12.6 [119] | 7.4 [77] | 12.0 [125] | 8.0 [87] |
| Yes - No live births | 28.0 [16] | 13.0 [9] | 27.8 [18] | 14.3 [14] |
| P-value | 0.072 | 0.940 | 0.144 | 0.836 |
Nw=weighted sample size.
The Edinburgh is scored if there were responses for all 10 items.
Excludes one healthy live birth with no information provided on intervening pregnancies.
The weights take into account the study design and differential consent based on characteristics recorded on all eligible deliveries that were screened for the study.
The woman was pregnant at the time of the follow-up interview.
Among the subset of women without a history of depression (N = 609, 76.4% of the sample), 13.1% of those with stillbirth scored > 12 compared to 5.0% of these women who had a live birth. Maternal race/ethnicity, maternal and paternal education, marital status, availability and type of health insurance, BMI, blood type, the number of SLE factors, and the STAI anxiety score reduced the strength of this association by at least 10%. In this subgroup, a significant interaction (P = 0.014) was observed between delivery outcome and time to follow-up. At 24–36 months post-delivery 17.6% of women who delivered a stillbirth and 1.9% of women who delivered a live birth had an EDS > 12.
Multivariate analysis
In the overall cohort, the experience of stillbirth was no longer statistically associated with depression at follow-up n a multivariable model that included number of SLE factors, history of depression, maternal education, health insurance, BMI, and blood type (Table 4).
Table 4.
Edinburgh Scorea > 12 Crude and Adjusted Odds Ratios for Stillbirth - All Women and Women with No History of Depression –
| OR Typeb | All Women | Women with No History of Depressionc |
|---|---|---|
| OR [95% CI] | OR [95% CI] | |
| Crude | 1.9 [1.2, 3.0] | 2.8 [1.6, 5.2] |
| Adjusted | 1.4 [0.8, 2.3] | 2.0 [1.0, 3.8] |
95% CI = 95% confidence interval, OR=odds ratio
The Edinburgh is scored if there were responses for all 10 items.
Odds ratios are from weighted logistic regression models with Edinburgh score > 12 (yes versus no) as the dependent variable. Sample sizes for the crude odds ratios were N=797 (overall) and N=609 (among women with no history of depression). For the adjusted odds ratios, the covariates included in each model were taken from a forward selection procedure with stillbirth / healthy live birth included at the initial step. A P-value ≤0.15 was required for a covariate to be retained. Sample sizes for the final models were N=757 (overall) and N=597 (among women with no history of depression). The potential covariates for the overall model were, in order of selection: number of significant life event factors, history of depression, education, method of payment for health insurance maternal, body mass index, blood type, and marital status (not selected). The potential covariates for the subset without a history of depression were, in order of selection: education, number of significant life event factors, body mass index, method of payment for health insurance, marital status (not selected), blood type (not selected), and race/ethnicity (not selected). The weights take into account the study design and differential consent based on characteristics recorded on all eligible deliveries that were screened for the study.
Women are classified as having a history of depression if they had taken antidepressant medications or if they indicated they had sought help for depression.
Among the subset of women with no history of depression, experience of stillbirth in the index pregnancy was associated with a nearly 3-fold elevated risk of depressive symptoms measured at follow-up interview (Table 4). This association was attenuated in the multivariable model. Time from the index pregnancy to follow-up interacted with the outcome of the index pregnancy in predicting depression at follow-up for women with no prior history of depression in a pattern similar to the total group (P-value for interaction term = 0.029). There was a significant high risk for depression at 24+ months after delivery of a stillbirth (aOR 6–12 months post-index = 2.21 95% CI [0.45, 10.77]; 12–18 months = 0.93 95% CI [0.23, 3.74]; 18–24 months = 0.31 95% CI [0.03, 3.55]; 24+ months = 11.93 95% CI [3.06, 46.53]).
Sensitivity Analysis
Re-weighted results adjusting for loss to follow-up through marginal structural models were very similar to our previous results, with the exception that the confidence limits were slightly wider (see supplemental data). For the multivariable analysis, the variables that entered the models were identical, although the order of entry was slightly different.
COMMENT
Stillbirth represents a major loss often engendering traumatic grief; further, stillbirth can trigger persistent depression. While the stillbirth association in our study was not statistically significant overall, it remained statistically significant after adjustment for confounders among the majority of women with no prior history of depression.
In a recent study of postpartum depression measured at first postpartum visit 2–6 weeks after the delivery, stillbirth was associated with a 9-fold odds ratio for EDS >12 (adjusted OR 9.4; 95% CI 6.0, 14.8).31 Some questions in the EDS mirror grief processes, and thus use of this screening measure to distinguish between normal grief and clinical depression requiring treatment is difficult within the first 6 months of the event. In another study at 6 months post-delivery, the relative risk of depressive symptoms was less than 3 (23% of 378 women with stillbirth and 8% of a matched control group of 232 women with live birth).4 In our study of women 6–36 months after the index event, the relative risk was less than 2 (14.8% vs. 8.3%). In another study of a national sample of women interviewed 9 months after a live birth, history of multiple loss prior to the index pregnancy was associated with only a slight elevation in depressive symptoms.32 Taken together with previous research,5our observations support the concept of a grieving period in the 6 months following delivery of a stillbirth followed by a small, but significant increased risk of incident depression among women with no previous history of depression.
All told, half of reproductive-age women with major depressive episodes go untreated for failure to diagnose, lack of resources or stigma associated with mental health conditions.4,33 African American women represent a higher-risk demographic group for depression during their reproductive career because of their greater risk of experiencing stillbirth4,30 and their greater exposure to significant life events.16 Notably, however, depressed African American women are also less likely to receive adequate treatment.4 Our data indicate that women with stillbirth may be screened for subsequent depression for at least 3 years postpartum. The subsequent pregnancy may be a particularly vulnerable period for women with previous pregnancy loss.9,34 Assessment of lack of support during the grieving process, particularly from the father35 and family, may help identify those at particular risk of an extended grieving period.
Our study had several weaknesses
Among women targeted for this analysis, 57% could not be contacted for follow-up interview. While the percentage followed is similar to other studies of postpartum women,4 the combined losses beginning with attempt to enroll in the original study to successful follow up interview (Figure 1) may affect the generalizability of our findings.
Characteristics of women lost to follow-up suggest that they might be at higher risk of depression 6–36 months’ post-index delivery than the sample that was followed. They were more likely to be mobile during pregnancy, less-educated, with no health insurance, and of minority status. Thus, this analysis may underestimate depression for the total SCRN sample. However, since the sensitivity analysis with marginal structural models was essentially the same as the analysis controlling for variables in the original study known to affect loss to follow-up, the substantial loss between index event and interview may not have affected the odds ratio estimates that contrast women with stillbirth versus women with healthy live births.
Health conditions during pregnancy were included as potential confounders. However, postpartum health conditions that might affect later depression, such as chronic hypertension or diabetes diagnosed after delivery, were not assessed.
While a score of >12 on the EDS has been found in numerous studies to have high predictive positive values when compared with clinical depression,18–20 we did not conduct clinical assessments in this study. We also did not ask women whether they had received treatment for depression at any time after the initial interview. In addition to resolving their grief over time, if a substantial number of women experiencing postpartum depression had also resolved their depression through treatment, our estimates of current depression may be lower than estimates of having ever-experienced postpartum depression would have been. However, the association between experience of postpartum depression relative to experience of stillbirth would likely not have been affected unless there was a different likelihood of treatment for women experiencing both stillbirth and postpartum depression. Other limitations include the evaluation of women at only one point in time and a lack of professional psychiatric evaluation to assess depression. It is possible that some of the women who reported having felt depressed in the past but who had not sought professional help had been clinically depressed prior to the index pregnancy event. However, since our definition of prior history of depression yielded a prevalence estimate of lifetime depression that is similar to other cross-sectional studies, we decided that inclusion of the larger percentage of women who had not sought professional help would introduce severe misclassification bias. Nevertheless, that decision undoubtedly excluded some women who were clinically depressed but for reasons of lack of resources or aversion to care had not been seen by a professional.
The study also had numerous strengths
We assessed a large cohort of women with well-characterized stillbirth and appropriate controls. The study was population-based and had considerable racial, ethnic, and geographic diversity. We utilized generally accepted and validated instruments to assess depression, anxiety and stressful life events. Finally, we utilized a wide range of characteristics from the index pregnancies in a multivariable model to identify the independent contributions of stillbirth.
In summary, depression at 6–36 months’ postpartum, as defined by an EDS score >12, was not significantly associated with stillbirth. However, women with no prior depression and stillbirth had about a two-fold increased odds of having depressive symptoms 6 to 36 months later compared to women with healthy live births. Further studies should focus on defining optimal methods for the emotional management of women suffering from stillbirth so as to lower the risk of subsequent depression.
Supplementary Material
Acknowledgments
We acknowledge the contribution of the Stillbirth Collaborative Research Network: University of Texas Health Science Center at San Antonio: Donald J. Dudley, Deborah Conway, Josefine Heim-Hall, Karen Aufdemorte, Angela Rodriguez; University of Utah School of Medicine: Robert M. Silver, Michael W. Varner, Kristi Nelson; Emory University School of Medicine and the Rollins School of Public Health: Carol J. Rowland Hogue, Barbara J. Stoll, Janice Daniels Tinsley, Bahig Shehata, Carlos Abromowsky; Brown University: Donald Coustan, Halit Pinar, Marshall Carpenter, Susan Kubaska; University of Texas Medical Branch at Galveston: George R. Saade, Radek Bukowski, Jennifer Lee Rollins, Hal Hawkins, Elena Sbrana; RTI International: Corette B. Parker, Matthew A. Koch, Vanessa R. Thorsten, Holly Franklin, Pinliang Chen; Pregnancy and Perinatology Branch, Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Institutes of Health: Marian Willinger, Uma M. Reddy; Columbia University Medical Center: Robert L. Goldenberg.
We also acknowledge the following members of the National Institute of Child Health and Human Development Scientific Advisory and Safety Monitoring Board for their review of the study protocol, materials, and progress: Reverend Phillip Cato; James W. Collins Jr.; Terry Dwyer; William P. Fifer; John Ilkekis; Marc Incerpi; George Macones; Richard M. Pauli; Raymond W. Redline; Elizabeth Thorn(chair), as well as all of the other physicians, study coordinators, research nurses, and patients who participated in the Stillbirth Collaborative Research Network. None of the individuals named received any compensation for their contributions.
Funding: This study was supported by grant funding from the Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Institutes of Health and with supplemental funding from the Office of Research in Women’s Health, National Institutes of Health: U10-HD045953 (Brown University, Rhode Island); U10-HD045925 (Emory University, Georgia); U10-HD045952 (University of Texas Medical Branch at Galveston); U10-HD045955 (University of Texas Health Sciences Center at San Antonio); UK10-HD045944 (University of Utah Health Sciences Center); U10-HD045954 (RTI International, North Carolina).
References
- 1.MacDorman MF, Kirmeyer SE, Wilson EC. Fetal and perinatal mortality, United States, 2006. National Vital Statistics Reports. 2012;60(8) [PubMed] [Google Scholar]
- 2.Centers for Disease Control, Reproductive Statistics Branch, Division of Vital Statistics, NCHS. [Accessed June 30, 2014];User guide to the 2012 fetal death public use file. http://www.cdc.gov/nchs/data_access/Vitalstatsonline.htm.
- 3.Neugebauer R, Kline J, Shrout P, Skodol A, O’Connor P, Geller PA, et al. Major depressive disorder in the 6 months after miscarriage. Journal of the American Medical Association. 1997;277:383–388. [PubMed] [Google Scholar]
- 4.Gold KJ, Johnson TR. Mothers at risk: maternal mental health outcomes after perinatal death. Obstetrics and Gynecology. 2014;123(Suppl 1):6S. [Google Scholar]
- 5.Badenhorst W, Hughes P. Psychological aspects of perinatal loss. Best Practice and Research in Clinical Obstetrics and Gynaecology. 2007;21:249–259. doi: 10.1016/j.bpobgyn.2006.11.004. http://www.sciencedirect.com/science/article/pii/S1521693406001416. [DOI] [PubMed] [Google Scholar]
- 6.Cacciatore J, Bushfield S. Stillbirth: the mother’s experience and implications for improving care. Journal of Social Work in End-of-Life and Palliative Care. 2007;3:59–79. doi: 10.1300/J457v03n03_06. [DOI] [PubMed] [Google Scholar]
- 7.Saflund K, Wredling R. Differences within couples’ experience of their hospital care and well-being three months after experiencing a stillbirth. Acta Obstetrica et Gynecologica Scandinavica. 2006;85:1193–1199. doi: 10.1080/00016340600804605. [DOI] [PubMed] [Google Scholar]
- 8.Blackmore EM, Cȏté-Arsenault D, Tang W, Glover V, Evans J, Golding J, et al. Previous prenatal loss as a predictor of perinatal depression and anxiety. The British Journal of Psychiatry. 2011;198:373–378. doi: 10.1192/bjp.bp.110.083105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hughes PM, Turton P, Evans CD. Stillbirth as a risk factor for depression and anxiety in the subsequent pregnancy: cohort study. BMJ. 1999;318:1721–1724. doi: 10.1136/bmj.318.7200.1721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vance JC, Boyle FM, Najman JM, Thearle MJ. Couple distress after sudden infant or perinatal death: a 30 month followup. Journal of Paediatric and Child Health. 2002;38:368–372. doi: 10.1046/j.1440-1754.2002.00008.x. [DOI] [PubMed] [Google Scholar]
- 11.Parker CB, Hogue CJ, Koch MA, Willinger M, Reddy U, Thorsten VR, et al. for the Stillbirth Collaborative Research Network. Stillbirth Collaborative Research Network: Design, methods and recruitment experience. Paediatric and Perinatal Epidemiology. 2011;25:425–435. doi: 10.1111/j.1365-3016.2011.01218.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pinar H, Koch MA, Hawkins H, Heim-Hall J, Abramowsky CR, Thorsten VR, et al. for the Stillbirth Collaborative Research Network. The stillbirth collaborative research network postmortem examination protocol. American Journal of Perinatology. 2012;29:187–202. doi: 10.1055/s-0031-1284228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Spielberger CD, Gorsuch RL, Lushene R, Vagg PR, Jacobs GA. Manual for the State-Trait Anxiety Inventory. Palo Alto, CA: Consulting Psychologists Press; 1983. [Google Scholar]
- 14.Spielberger CD. The State-Trait Anger Expression Inventory-2 (STAXI-2): Professional manual. Odessa, FL: Psychological Assessment Resources (PAR); 1999. [Google Scholar]
- 15.U.S. Centers for Disease Control and Prevention, Division of Reproductive Health. Pregnancy Risk Assessment Monitoring System (PRAMS). Phase 5 Core Questionnaire. Topic Reference. 2004 [Google Scholar]
- 16.Hogue CJR, Parker CB, Willinger M, Temple JR, Bann CM, Silver RM, et al. for the Eunice Kennedy Shriver National Institute of Child Health and Human Development Stillbirth Collaborative Research Network Writing Group. A population-based case-control study of stillbirth: The relationship of significant life events to the racial disparity for African Americans. American Journal of Epidemiology. 2013;177:755–767. doi: 10.1093/aje/kws381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cox J, Holden J, Sagovsky R. Detection of postnatal depression: Development of the 10-item Edinburgh Postnatal Depression Scale. British Journal of Psychiatry. 1987;150:782–786. doi: 10.1192/bjp.150.6.782. [DOI] [PubMed] [Google Scholar]
- 18.Murray D, Cox J. Screening for depression during pregnancy with the Edinburgh Depression Scale (EPDS) Journal of Reproductive and Infant Psychology. 1990;8:99–107. [Google Scholar]
- 19.Ji S, Long Q, Newport DJ, Na H, Knight B, Zach EB, et al. Validity of depression rating scales during pregnancy and the postpartum period: impact of trimester and parity. Journal of Psychiatric Research. 2011;45:213–219. doi: 10.1016/j.jpsychires.2010.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pitanupong J, Liabsuetrakul T, Vittayanont A. Validation of the Thai Edinburgh postnatal depression scale for screening postpartum depression. Psychiatry Research. 2007;149:253–259. doi: 10.1016/j.psychres.2005.12.011. [DOI] [PubMed] [Google Scholar]
- 21.Cox JL, Chapman G, Murray D, Jones P. Validations of the Edinburgh postnatal depression scale (EPDS) in non-postnatal women. Journal of Affective Disorders. 1996;39:185–189. doi: 10.1016/0165-0327(96)00008-0. [DOI] [PubMed] [Google Scholar]
- 22.Zelkowitz P, Milet TH. Screening for post-partum depression in a community sample. Canadian Journal of Psychiatry. 1995;40:80–86. doi: 10.1177/070674379504000205. [DOI] [PubMed] [Google Scholar]
- 23.Chaudron LH, Kitzman HJ, Peifer KL, Morrow S, Perez LM, Newman MC. Prevalence of maternal depressive symptoms in low-income Hispanic women. Journal of Clinical Psychiatry. 2005;66:418–423. doi: 10.4088/jcp.v66n0402. [DOI] [PubMed] [Google Scholar]
- 24.Edge D, Baker D, Rogers A. Perinatal depression among black Caribbean women. Health and Social Care in the Community. 2004;12:430–438. doi: 10.1111/j.1365-2524.2004.00513.x. [DOI] [PubMed] [Google Scholar]
- 25.Research Triangle Institute. SUDAAN Language Manual, Release 11.0. Research Triangle Park, NC: Research Triangle Institute; 2012. [Google Scholar]
- 26.Burcusa SL, Lacono WG. Risk for recurrence in depression. Clinical Psychology Review. 2007;27:959–985. doi: 10.1016/j.cpr.2007.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.The Stillbirth Collaborative Research Network Writing Group. Association between stillbirth and risk factors known at the time of pregnancy confirmation. Journal of the American Medical Association. 2011;306:2469–2479. doi: 10.1001/jama.2011.1798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Robins JM, Hernán MA, Brumback B. Marginal structural models and causal inference in epidemiology. Epidemiology. 2000 Sep;11(5):550–560. doi: 10.1097/00001648-200009000-00011. [DOI] [PubMed] [Google Scholar]
- 29.Hernán MA, Brumback B, Robins JM. Marginal structural models to estimate the causal effect of zidovudine on the survival of HIV-positive men. Epidemiology. 2000 Sep;11(5):561–570. doi: 10.1097/00001648-200009000-00012. [DOI] [PubMed] [Google Scholar]
- 30.Cole SR, Hernán MA. Constructing inverse probability weights for marginal structural models. Am J Epidemiol. 2008 Sep 15;168(6):656–664. doi: 10.1093/aje/kwn164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nelson DB, Freeman MP, Johnson NL, McIntire DD, Leveno KJ. A prospective study of postpartum depression in 17,648 parturients. Journal of Maternal-Fetal and Neonatal Medicine. 2013 Mar 21;:1–7. doi: 10.3109/14767058.2013.777698. Early Online: [DOI] [PubMed] [Google Scholar]
- 32.Price SK. Stepping back to gain perspective: pregnancy loss history, depression, and parenting capacity in the Early Childhood Longitudinal Study, Birth Cohort (ECLS-B) Death Studies. 2008;32:97–122. doi: 10.1080/07481180701801170. [DOI] [PubMed] [Google Scholar]
- 33.Ko JY, Farr SL, Dietz PM, Robbins CL. Depression and treatment among U.S. pregnant and nonpregnant women of reproductive age, 2005–2009. Journal of Women’s Health. 2012;21:830–836. doi: 10.1089/jwh.2011.3466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chojenta C, Harris S, Reilly N, Forder P, Austin MP, Loxton D. History of pregnancy loss increases the risk of mental health problems in subsequent pregnancies but not in the postpartum. PLoS One. 2014;9:e95038. doi: 10.1371/journal.pone.0095038. cCollection 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Surkan PJ, Radestad I, Cnattingius S, Steineck G, Dickman PW. Social support after stillbirth for prevention of maternal depression. Acta Obstetrica Gynecologica Scandinavica. 2009;88:1358–1364. doi: 10.3109/00016340903317974. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.