Significance
Climate change impacts on wildlife populations are likely to be accentuated by pollution. Small (inbred) populations may be more vulnerable to these effects, but empirical data supporting these hypotheses are lacking. We present the first substantial empirical evidence, to our knowledge, for interactive effects on population viability of elevated temperature (climate); an endocrine disrupting chemical, clotrimazole (pollution); and inbreeding. Using the zebrafish (Danio rerio) as a model, we show these three factors interact to skew population sex ratios toward males and that this interaction can lead to increased risk of extinction. Our results suggest that climate change and pollution impacts are likely to pose significant extinction risks for small, endangered populations exhibiting environmental sex determination and/or differentiation.
Keywords: stressors, additive, interaction, population, viability
Abstract
Endocrine disrupting chemicals (EDCs) are potent environmental contaminants, and their effects on wildlife populations could be exacerbated by climate change, especially in species with environmental sex determination. Endangered species may be particularly at risk because inbreeding depression and stochastic fluctuations in male and female numbers are often observed in the small populations that typify these taxa. Here, we assessed the interactive effects of water temperature and EDC exposure on sexual development and population viability of inbred and outbred zebrafish (Danio rerio). Water temperatures adopted were 28 °C (current ambient mean spawning temperature) and 33 °C (projected for the year 2100). The EDC selected was clotrimazole (at 2 μg/L and 10 μg/L), a widely used antifungal chemical that inhibits a key steroidogenic enzyme [cytochrome P450(CYP19) aromatase] required for estrogen synthesis in vertebrates. Elevated water temperature and clotrimazole exposure independently induced male-skewed sex ratios, and the effects of clotrimazole were greater at the higher temperature. Male sex ratio skews also occurred for the lower clotrimazole exposure concentration at the higher water temperature in inbred fish but not in outbred fish. Population viability analysis showed that population growth rates declined sharply in response to male skews and declines for inbred populations occurred at lower male skews than for outbred populations. These results indicate that elevated temperature associated with climate change can amplify the effects of EDCs and these effects are likely to be most acute in small, inbred populations exhibiting environmental sex determination and/or differentiation.
Climate change is seen globally as a major environmental risk, and in combination with environmental degradation via chemical pollution and habitat loss, it has the potential to have a severe impact on wildlife populations (1), particularly in freshwater ecosystems (2, 3). Combinations of environmental stressors have been shown to have interactive effects on the health of individuals in many taxa (4), including fish (5, 6), amphibians (7, 8), and reptiles (9, 10), with indications of interactive effects at the population level for some invertebrates (11, 12). It is also recognized that small, isolated, and inbred wildlife populations are potentially more vulnerable to environmental change because of their lower adaptive capabilities (13, 14). However, our understanding of the interactive effects of chemical pollutants and projected increases in global temperature is limited, and nothing is known concerning the interactive effects of these stressors on small, inbred populations that typify endangered species.
Species with temperature-dependent sex determination are likely to be particularly susceptible to climate change. In some reptiles, including tortoises and turtles, warmer temperatures during incubation generally induce female development (15, 16). Feminization also occurs in crocodilians at temperatures both above and below temperatures that are optimal for male development (17, 18). Female-biased populations are not necessarily at greater risk of extinction, provided some males are present (19), since numbers of females, rather than males, largely determine the reproductive output of a population (19, 20). However, in lizards (17, 18), fish (21), and some amphibians (22), temperature elevation tends to induce male development and male-biased sex ratios can dramatically reduce population fitness (23, 24). Sexual differentiation in these animals is also strongly influenced by chemicals that disrupt hormone systems (25–28). Some endocrine disrupting chemicals (EDCs) are highly potent pollutants, and exposure concentrations occurring in natural surface waters have been shown to affect reproductive development negatively in reptiles (29), amphibians (28), and fish (30, 31).
The negative effects of environmental stressors, including elevated temperature and EDC pollutants, are likely to be greatest in endangered species because endangered species are often typified by small, isolated populations, which not only increases the negative impacts of stochastic fluctuations in both numbers of individuals and sex ratios, but also increases the risk of inbreeding (32, 33). Inbreeding has two related effects. First, it generates inbreeding depression, a reduction in fitness in inbred individuals, with effects felt most acutely in key life history traits, such as fecundity and fertility (34, 35). Second, inbreeding also leads to reductions in genetic variation and the loss of heterozygosity, both of which are crucial in buffering genetic systems against environmental perturbations (13, 14). Associated reductions in buffering capacity could mean that endangered taxa have increasing susceptibility to EDC exposure and global increases in temperature.
We investigated the combined effects of elevated temperature and clotrimazole, which is representative of a broad class of EDCs (aromatase inhibitors, which affect sex hormone biosynthesis), on sexual differentiation and development in both inbred and outbred zebrafish (Danio rerio). The zebrafish is a freshwater species native to the Indian subcontinent and has been studied widely as a model animal in biomedical and environmental science (36–38). Importantly, the sex-determining mechanism in zebrafish is known to be modified by environmental factors, and independent exposures to elevated water temperatures (21, 39), and to chemicals that inhibit aromatase, have been shown to induce male development in this species (39, 40). The enzyme aromatase [cytochrome P450(CYP)19] mediates the conversion of male hormones (androgens) to female hormones (estrogens), and thus plays a pivotal role in sex assignment in zebrafish and other vertebrates (41). Studies in the European sea bass (Dicentrarchus labrax) have also shown silencing of the aromatase gene (cyp19a1) via methylation of its promoter region in response to elevated water temperatures, resulting in male sexual development (42). However, combined impacts of temperature and aromatase-inhibiting EDCs on fish sex and on the viability of inbred vs. outbred populations have not been studied. Here, we found elevated temperature and exposure to the aromatase inhibitor clotrimazole combined additively to affect sexual differentiation and development in zebrafish. Furthermore, male skews occurred in inbred fish at lower clotrimazole concentrations than for outbred fish. According to population viability analysis (PVA), the observed male skews are likely to lead to dramatic reductions in population growth and viability, particularly in inbred populations.
Results
Characterization of Inbred and Outbred Families.
Fertilization, hatching success, and juvenile survivorship differed between inbred and outbred fish (n = 20 families) before the start of the laboratory exposure study [multivariate ANOVA (MANOVA): F(3,15) = 11.70, P < 0.001]. Two of the 20 inbred lines failed to recruit beyond swim-up and exogenous feeding [5–10 d postfertilization (dpf)], and post hoc comparison of mean survivorship per family from 0 to 30 dpf for inbreds vs. outbreds revealed significant differences, with both the inclusion (F(1,19) = 34.45, P < 0.001) and exclusion (F(1,19) = 65.95, P < 0.001) of inbred families, which failed to recruit (SI Appendix, Fig. S1). Considering all families, mean inbreeding depression on survivorship was δ = 2.7 (Eq. 1) (43), with five lethal equivalent (LE) recessive alleles per diploid genome (Eq. 2) (44):
| [1] |
| [2] |
where XI is mean inbred survivorship, XO is mean outbred survivorship (to 30 dpf), and F is the inbreeding coefficient [here, it was assumed to match the theoretical inbreeding coefficient (FIT) = 0.25] (Methods and SI Appendix, Fig. S1).
Developmental Effects in Exposure Treatments.
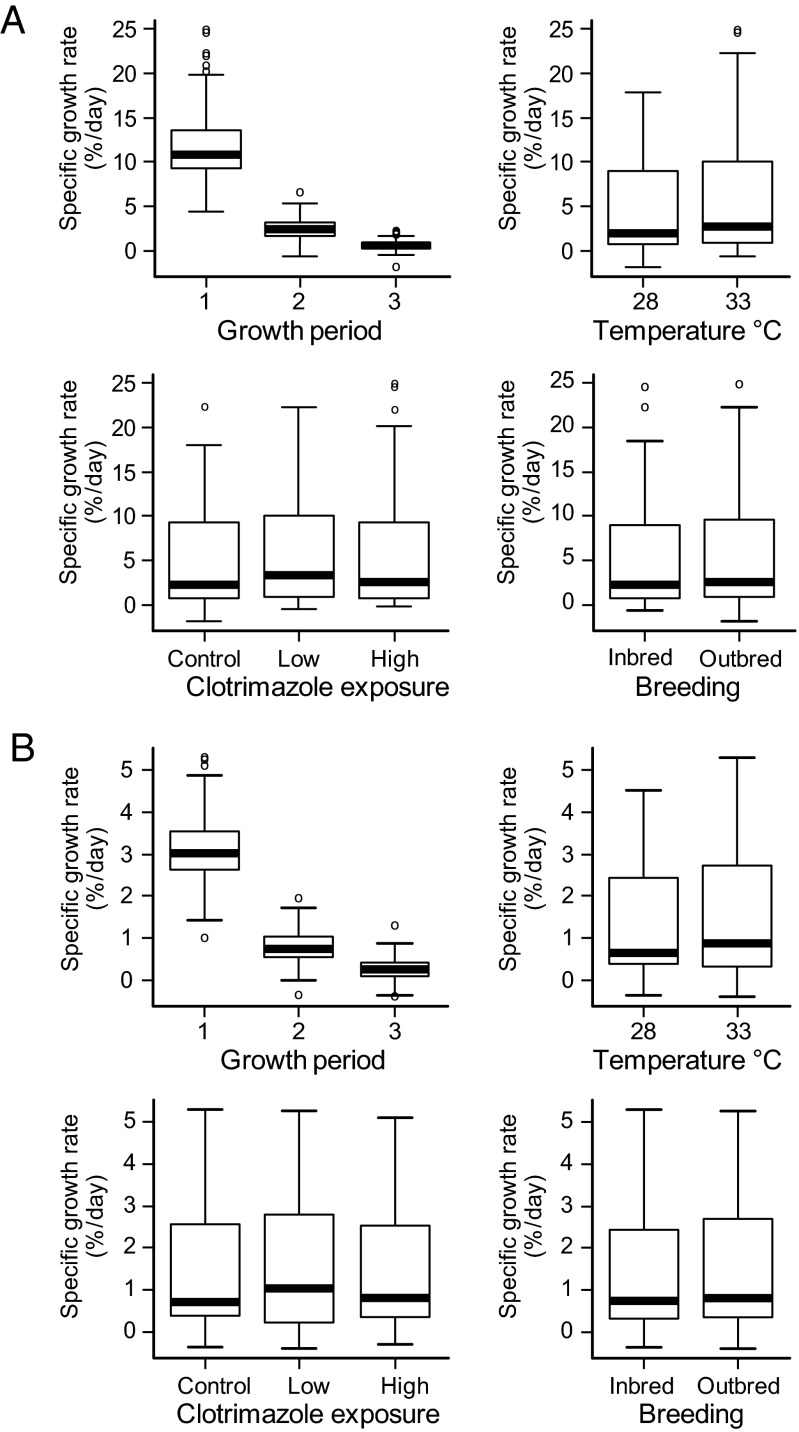
During the exposure study (treatments are summarized in Table 1), mean growth rates in both inbred and outbred families were greater at projected (year 2100) mean temperatures for indigenous spawning zebrafish (33 °C) than at current ambient mean temperatures (28 °C). Growth rate declined with maturation (growth period) and increased with temperature (Fig. 1): both P < 0.015 according to the best-fit linear mixed effects (lme) models (SI Appendix, Table S1.1 A and B). A higher growth rate at 33 °C would normally be expected to induce female-biased sex ratios, because female zebrafish are generally larger than male zebrafish (45, 46). However, despite an increased growth rate, inbred fish in the higher temperature treatments exhibited male skews of ∼72%, compared with ∼48% in equivalent outbred treatments. High-level (8 μg/L) clotrimazole exposure also induced male-skewed sex ratios of ∼80% and ∼60% in inbred and outbred fish, respectively. In combined high temperature and high clotrimazole treatments, skews of ∼97% males were recorded in inbred fish, compared with ∼82% males in outbred fish. Male skews of ∼82% also occurred in inbred fish at lower, more environmentally realistic, clotrimazole concentrations in combination with elevated temperature, but not in the equivalent outbred treatments (∼49% males). The fixed effects of breeding (inbred versus outbred families), clotrimazole exposure, and temperature all contributed significantly (additively) to reductions in the proportion of females per family per treatment (Fig. 2 and Table 2): all P ≤ 0.0003 according to the best-fit lme model (SI Appendix, Table S1.2).
Table 1.
Experimental treatments
| Clotrimazole nominal (and measured) exposure concentrations, μg/L | Inbred zebrafish families (FIT = n + 0.25) | Outbred zebrafish families (FIT = n) | ||
| 28 °C | 33 °C | 28 °C | 33 °C | |
| 0 (0) | ✔ | ✔ | ✔ | ✔ |
| 2 (1.7) | ✗ | ✔ | ✗ | ✔ |
| 10 (8.0) | ✔ | ✔ | ✔ | ✔ |
FIT = n (unknown quantity) for outbreds and FIT = n + 0.25 for inbreds following one generation of full-sibling mating.
✔Replication for each treatment was n = 18 inbred families and n = 20 outbred families. Each family replicate per treatment was represented by n = 4 fish.
✗This “low dose × low temperature” treatment was not included in our analyses because we knew, from our previous work (40, 83), that the low-dose clotrimazole (2 μg/L) alone would not affect population-relevant end points (survival, growth, gonadal development or fecundity), and we sought to maximize replication for the other treatment regimes.
Fig. 1.
Specific growth rates vs. growth (exposure) period, temperature, breeding, and clotrimazole treatment. Specific growth rate (% per day) for n = 18 inbred and n = 20 outbred zebrafish families (each stocked with n = 4 individuals per treatment) was based on the following: body weight (A; wet mass in grams) and body length (B; standard length in centimeters). Growth periods are as follows: (i) prepubescence = 40–54/55 dpf; (ii) sexual differentiation = 54/55–74/80 dpf; and (iii) completion of sexual development = 74/80–91/100 dpf for the 33 °C and 28 °C exposures, respectively. Paired time points (x/y dpf) delineate equivalent degree-day exposure periods, thus accounting for increased growth and development rates at elevated temperature. Boxes represent the interquartile range and median (line), and whiskers show the full range of data, excluding outliers (o). Growth rate declined with maturation (growth period) and increased with temperature (both P < 0.015) according to the best-fit lme model (SI Appendix, Table S1.1 A and B).
Fig. 2.
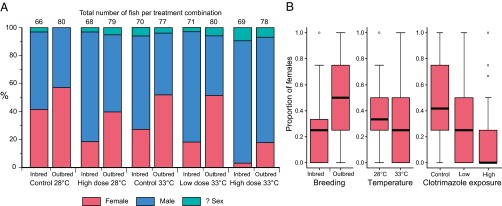
Gonadal sex in the different treatments. Overall sex ratios for each treatment combination (A) and proportion of females by individual treatment (B) for n = 18 inbred and n = 20 outbred zebrafish families (each stocked with n = 4 individuals per treatment). Boxes represent the interquartile range and median (line), and whiskers show the full range, excluding outliers. The fixed effects of breeding, clotrimazole exposure, and temperature all contributed significantly to reductions in the proportion of females (arcsine square root-transformed) per family per treatment (all P ≤ 0.0003) according to the best-fit lme model (SI Appendix, Table S1.2).
Table 2.
Model scenarios based on sex ratio skews in combined clotrimazole exposure, temperature, and breeding treatments
| No. | Emergent inbreeding depression | Exposure scenario | Age 0+ survivorship | Overall proportion of females per treatment, % | Mean proportion of females per family per tank compartment per treatment, % (SD) |
| 1a | ✔ | Inbred, zero clotrimazole, 28 °C | Low | 42 | 41 (28) |
| 1b | ✗ | (Inbred control) | |||
| 2a | ✔ | Outbred, zero clotrimazole, 28 °C | High | 57 | 58 (30) |
| 2b | ✗ | (Outbred control) | |||
| 3a | ✔ | Inbred, zero clotrimazole, 33 °C | Low | 27 | 28 (33) |
| 3b | ✗ | ||||
| 4a | ✔ | Outbred, zero clotrimazole, 33 °C | High | 52 | 52 (34) |
| 4b | ✗ | ||||
| 5a | ✔ | Inbred, high clotrimazole, 28 °C | Low | 19 | 18 (18) |
| 5b | ✗ | ||||
| 6a | ✔ | Outbred, high clotrimazole, 28 °C | High | 40 | 40 (30) |
| 6b | ✗ | ||||
| 7a | ✔ | Inbred, low clotrimazole, 33 °C | Low | 18 | 18 (22) |
| 7b | ✗ | ||||
| 8a | ✔ | Outbred, low clotrimazole, 33 °C | High | 51 | 51 (26) |
| 8b | ✗ | ||||
| 9a | ✔ | Inbred, high clotrimazole, 33 °C | Low | 3 | 3 (8) |
| 9b | ✗ | ||||
| 10a | ✔ | Outbred, high clotrimazole, 33 °C | High | 18 | 17 (26) |
| 10b | ✗ |
Exposure scenarios represent treatment combinations in the exposure study. Clotrimazole geometric mean measured concentrations: control (0 μg/L), low (1.7 μg/L), high (8 μg/L). First-year (age 0+) survivorship: low = 4 ± 3% for inbreds, high = 9 ± 3% for outbred zebrafish (SI Appendix, Table S7). Proportions of females per family (tank compartment) per treatment were used for statistical analysis (arcsine-square root transformed data) and PVA.
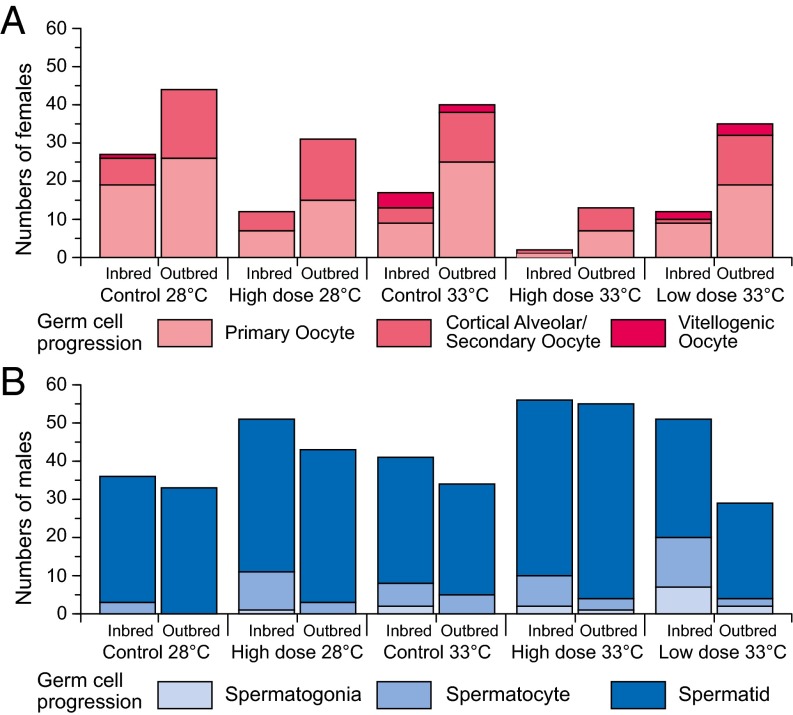
There were no significant treatment effects on gonad weight (SI Appendix, Fig. S2 and Table S1.3). However, the fixed effects of breeding and gonadal sex both influenced germ cell development (additively). According to the best-fit lme model (SI Appendix, Table S1.4), there were significant differences (P ≤ 0.0017) for breeding and gonadal sex in germ cell development in inbred males (79 ± 0.8% spermatids) compared with outbred males (91 ± 0.4% spermatids), but not in inbred females (42 ± 3.4% ≥ cortical alveolar stage) compared with outbred females (44 ± 2.8% ≥ cortical alveolar stage) (Fig. 3). No treatment-related effects were detectable for the expression of the gene cyp19a1a, encoding aromatase in testes, because expression levels were at or below detection limits across all treatments. In contrast, there were significant effects of inbreeding and clotrimazole exposure on cyp19a1a expression in ovaries. High-level clotrimazole exposure (8 μg/L) was shown to be a reliable predictor (P = 0.0003) of elevated ovarian cyp19a1a expression (Fig. 4), and there was a significant (more than additive) interaction between high clotrimazole exposure and inbreeding (P = 0.0147) according to the best-fit lme model (SI Appendix, Table S1.5). Thus, the few remaining females in the high-level clotrimazole, inbred treatments appeared to compensate for P450(CYP)19 aromatase inhibition by up-regulating cyp19a1a expression (Fig. 4).
Fig. 3.
Germ cell progression in individuals: females (A) and males (B) for n = 18 inbred and n = 20 outbred zebrafish families (each stocked with n = 4 individuals per treatment). Individuals were classified by their most advanced germ cell developmental stage (advancing stages 1–3 are indicated by increasingly darker shading). According to the best-fit lme model (SI Appendix, Table S1.4), there were significant differences, due to breeding and gonadal sex (both P ≤ 0.0017), in germ cell development in inbred males (79 ± 0.8% spermatids) vs. outbred males (91 ± 0.4% spermatids), but not in inbred females (42 ± 3.4% ≥cortical alveolar stage) compared with outbred females (44 ± 2.8% ≥cortical alveolar stage).
Fig. 4.
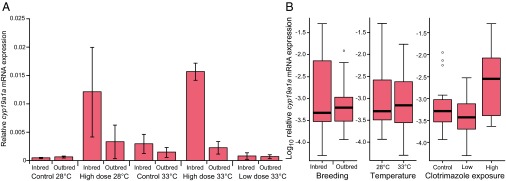
Relative aromatase (cyp19a1a) gene expression in ovaries (primary oocyte stage). Relative gene expression for each treatment combination (A) and log10 relative gene expression by individual treatment (B) for n = 6 females per treatment. Relative gene expression = (E ref)Ct ref/(E target)Ct target, where ref is the housekeeping gene (rpl8), target is the gene of interest (cyp19a1a), E is PCR amplification efficiency, and Ct is cycle threshold (number of temperature cycles yielding above-background expression) for that particular gene. High-level clotrimazole exposure (8 μg/L) was shown to be a reliable predictor (P = 0.0003) of elevated ovarian cyp19a1a expression, and there was a significant interaction with inbreeding (P = 0.0147) according to the best-fit lme model (SI Appendix, Table S1.5).
PVA.
PVA indicated that inbred zebrafish populations composed of different age classes had a greater probability of extinction (PE2ac = 0.91–1.0, PE3ac = 0.56–1.0) compared with outbred populations (PE2ac = 0.05–0.19, PE3ac = 0) (Fig. 5). Both inbred and outbred populations, when composed of two age classes (2ac: age 0+ juveniles and age 1+ adults), were more vulnerable to stochastic effects on recruitment and generally had higher PEs, which tended to obscure the effect of sex ratio skew on PE2ac. Nevertheless, extreme male skews in outbreds increased PE2ac from 0.08 to 0.19, for 80 and 90% male skews, respectively. Equivalent skews had no effect on outbred populations composed of three age classes (including older, age 2+ adults), but in inbred populations, PE3ac increased from 0.56 to >0.9 and the mean time to extinction (MTE) decreased by at least a decade (Fig. 5). Per capita population growth rate (r) was responsive to sex ratio skews in both inbred and outbred populations, regardless of age class composition. A male skew of >82% in inbred zebrafish resulted in a threefold reduction in r, whereas in outbred fish, a larger >90% male skew led to a smaller, twofold reduction in r (Fig. 5). The additional simulation of inbreeding depression on age 0+ survivorship, based on five LEs, further reduced mean per capita population growth rate from a baseline of r = 2.4 to 0.2 in both inbreds and outbreds, irrespective of sex ratio (Fig. 5). To evaluate the likely consequences of these reductions in r on population viability, we extrapolated our results using an established regression model (47) of minimum viable adult population size (ln MVPA) vs. r (r = lnR0). These extrapolations predicted MVPA to be 500 vs. 10,000 adults for r = 2.4 and r = 0.2, respectively.
Fig. 5.
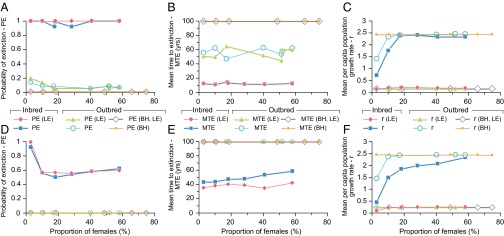
PE, MTE, and r vs. proportion of females in inbred and outbred zebrafish populations. Results from n = 100 repeat simulations for populations with two age classes (0+, 1+): PE (A), MTE (B), and mean r (C). Results for populations with three age classes (0+, 1+, 2+): PE (D), MTE (E), and mean r (F). Emergent inbreeding depression (on age 0+ survivorship) was simulated based on five LE alleles per diploid genome. Compensatory density dependence (of age 0+ survivorship) was simulated using the BH model. All scenarios were based on a maximum carrying capacity of 5,000 ± 1,000 individuals, including circa 100–200 ≥age 1+ adults capable of spawning on alternate days during the 120-d monsoon spawning season. Fecundity was 50 ± 20 eggs per female per spawn (these and other input parameter reference values and ranges are provided in SI Appendix, Table S7).
After assessing the importance of sex ratio and age class composition for population viability, sensitivity analysis was performed on other PVA model input parameters to assess their effects on PE, MTE, and r (SI Appendix, Fig. S3). Crucially, this analysis showed that a greater than fivefold reduction in female fecundity would be required to reduce r by threefold and twofold, as observed for maximum sex ratio skews, in inbreds and outbreds, respectively. Furthermore, a fivefold increase in the variation in individual fecundity had no effect on any of the PVA outputs, including r, indicating a considerable buffering potential of the highly fecund females. First-year survivorship and inbreeding depression on this vital rate were more influential on population viability compared to the influence of sex ratio and female fecundity. Sensitivity analysis showed that the 2.25-fold lower survivorship in first-year (age 0+) inbred fish (4%), compared with outbreds (9%), corresponded with a five- to 10-fold higher PE and a sixfold lower MTE. Furthermore, r was reduced by 1.25-fold, and this reduction was more than 12-fold greater following the simulation of emergent inbreeding depression on first-year survivorship. Using the Beverton–Holt (BH) model (48) parameterized for zebrafish (49), a simulated increase in first-year survivorship at low population densities highlighted the importance of density-dependent survival in the first year, which may offer some additional compensatory reserve for buffering population growth (Fig. 5).
Discussion
Wildlife populations are often exposed to a wide range of physical, chemical, and biological stressors, some of which can act together to increase the prevalence or severity of an effect. Examples include interactions between temperature and metals impairing development of individual zebrafish (5); temperature and pesticides affecting physiological and sexual development in fish (6) and in reptiles (10); and UV-B radiation in combination with various other physical, chemical, or biological stressors having an impact on individual amphibian survival (8). However, there have been very few studies of population-level impacts due to interactions between chemicals and other environmental stressors (4, 11, 12). Environmental pressures on wildlife will increase with predicted scenarios for climate change, human population growth, and economic development (1). Many wildlife populations are likely to be exposed simultaneously to rising temperatures (50), increasing isolation due to habitat loss, diminishing freshwater reserves, and increasing pollution due to concentrating chemical residues (2, 3).
More than 800 chemicals discharged into aquatic ecosystems are reported to have endocrine activity, affecting hormone synthesis and metabolism and/or acting as agonists or antagonists of sex hormone receptors (51). Some of these chemicals have been shown to alter sexual programming and reduce reproductive fitness in wild fish at environmentally relevant exposure concentrations (31, 52, 53). Additive effects have been shown for EDCs acting as estrogen receptor agonists (54), but less consideration has been given to the combined effects of exposure to EDCs with other modes of action, including modulators of other steroid hormone receptors (e.g., androgen receptor agonists or antagonists) and disruptors of hormone synthesis and metabolism. Here, we focus on the priority hazardous substance clotrimazole (55), an aromatase inhibitor that blocks estrogen synthesis in vertebrates, including fish (56). Limited data are available quantifying environmental concentrations of clotrimazole, but in Europe, they have been measured in wastewater treatment plant effluents up to 0.17 μg/L (57, 58) and in receiving river and estuarine waters up to 0.047 μg/L (58, 59). The lower clotrimazole concentration (1.7 μg/L) adopted in our study is therefore higher than those clotrimazole concentrations reported for effluent discharges. However, clotrimazole is only one of a wide range of aromatase-inhibiting compounds used in crop protection and veterinary and human medicine, some of which have been detected at concentrations ranging between 0.05 and 9.0 μg/L in agricultural (60) and urban (58) catchments, and some of these compounds have been shown to act additively to inhibit aromatase (61).
We investigated the effects of temperature, chemical (EDC) pollution, and inbreeding in fish using the zebrafish as a model and found that elevated temperature, the EDC clotrimazole, and inbreeding all interacted and reduced population viability via their effects on population sex ratios. Sex ratios were skewed significantly toward males, with maximum skews of over 97% in inbred fish at elevated temperature and clotrimazole exposure. There was also a significant male skew in inbred populations following low-level clotrimazole exposure at elevated temperature, but not in the equivalent outbred treatment. Thus, inbreeding significantly increased the impact of clotrimazole on zebrafish sex ratios, particularly at the higher temperature, which is predicted to occur with climate change.
PVA showed that population growth rates declined sharply in response to male skews, and declines for inbred populations occurred at lower male skews than for outbred populations. Simulated year-on-year inbreeding further exacerbated population growth rate declines by depressing first-year survival, and, consequently, the minimum viable population size was predicted to increase by 20-fold, from 500 to 10,000 breeding adults. In the wild, zebrafish are commonly found in pond ecosystems containing only circa 100 adults (62), highlighting the importance of seasonal connections between metapopulations in their native flood plains during the monsoon rains. Climatic instability, including the periodic onset of El Niño events and drier monsoon seasons (63) accompanying increasing temperatures in South East Asia, may therefore augment population impacts on zebrafish. Other environmental stressors likely to accompany projected climate change, including reduced nutrition, overcrowding, and, in aquatic environments, increasing acidification and reduced dissolved oxygen, may also generate male-skewed sex ratios in fish (25, 26) and other taxa, including amphibians (22) and reptiles (51).
More generally, induction of male-biased sex ratios in wildlife exhibiting sexual size dimorphism has been linked to metabolic effects, which culminate in reduced growth. This mechanism has been observed in birds (e.g., the Arctic glaucous gull, Larus hyperboreus) (64), but it appears to be more prevalent in fish (65). In our study, we quantified somatic growth as an integrative measure of physiological/metabolic status, because larger size favors female development in zebrafish (45, 46). We found that elevating the temperature from 28 °C to 33 °C increased the somatic growth rate, but this growth increase was not the key determinant of sexual differentiation in our study (i.e., it did not favor the development of females). Instead, male-skewed sex ratios in the elevated temperature and/or clotrimazole exposure treatments appeared to be mediated by the inhibition of P450(CYP)19 aromatase, as has been found in other studies (39, 40, 42). Gene expression of cyp19a1a encoding aromatase was up-regulated in the few females remaining in the inbred, elevated temperature, and/or high-level clotrimazole exposure treatments, and we hypothesize that this up-regulation was the result of a compensatory response to P450(CYP)19 aromatase inhibition in these individuals. Although many other genes have been implicated in sex determination and differentiation in zebrafish (66, 67), these genes appear to vary in importance between different strains (68) and under different environmental conditions (69). In contrast, cyp19a1a (and cyp19a1b) plays the pivotal role in the production of estrogen, ultimately controlling sexual phenotype in zebrafish (41) and in all animals exhibiting environmental/temperature-dependent sex determination/differentiation (18).
The long-term impacts of global environmental change on wildlife species will depend upon niche specificity, plasticity, and dispersal (70); dependence on environmental cues, ecological interactions (including sex-determining mechanisms), physiological tolerance, and adaptive capacity (71), each underpinned by genetic diversity (13, 14). Sexual reproduction has the potential to promote genetic diversity in populations by accelerating the production of novel genotypes and limiting the accumulation of deleterious mutations (72–75). However, these benefits will be limited in small populations, particularly those populations with imbalanced sex ratios. Furthermore, the scope for directional selection to maintain sex ratios in species exhibiting environmental sex determination during climate change (76, 77) is likely to diminish, because temperature fluctuations are predicted to increase along with mean global temperatures (50). Therefore, more immediate phenotypic responses are likely to be of greater importance, including population dynamic responses buffering recruitment (as illustrated in our sensitivity analysis) and phenotypic plasticity in sexual development (78). Due to its rapid life cycle, sexual plasticity as a juvenile, and potential to undergo sex change during adulthood (78), although not proven (79), the zebrafish would appear to be a sensitive model for illustrating the potential impacts of climate change. However, future impacts may be greater in less fecund species, whose sex is determined entirely by environmental cues during early life and subsequently becomes fixed.
Anthropogenic pressures from accelerated climate change, pollution, and habitat destruction have increased significantly in recent decades and are projected to continue to do so throughout this century (1). Considerable scientific effort has been devoted to gathering evidence of globally coherent trends in ecological responses to climate change, and research has generally sought to distinguish these climate change impacts from those impacts of nonclimatic influences (80). Here, we show that the interactions of different environmental factors associated with climate change, physical, chemical, and biological, are fundamental to future impact predictions. Our study indicates that interactions between elevated temperature and a chemical that modifies sex can have profound impacts, and that these impacts are likely to be greater on inbred populations. Thus, endangered species, particularly those endangered species exhibiting irreversible environmental sex determination and low fecundity, are likely to suffer the interactive effects of multiple environmental insults most acutely.
Methods
Generation of Inbred and Outbred Zebrafish.
Inbred and outbred families of zebrafish (n = 20) were derived from reciprocal pairings of individual males and females taken randomly from wild populations collected from two geographically distinct areas (Mozahadi and Kechuri) in the Brahmaputra river basin in Bangladesh (SI Appendix, Fig. S4 and Table S2.1). Brood stocks were maintained in separate full-sibling families under standard laboratory conditions (28 °C, 12-h/12-h light/dark photoperiod with a 20-min dawn/dusk transition and food provided ad libitum twice daily). For each subsequent generation (F1–F3), outcrosses were performed, generating 20 outbred (F3) families with theoretical inbreeding coefficient FIT = n, where n is the background inbreeding coefficient in the wild source populations (SI Appendix, Table S2.2 and S2.3). Full-sibling mating was also performed using randomly selected brothers and sisters from each of the F2 brood stock family lines, resulting in 20 inbred (F3) families with theoretical inbreeding coefficient FIT = n + 0.25 (SI Appendix, Table S2.4).
Fertilization success (at 4–6 h postfertilization), hatching success (at 5 dpf), and survivorship (to 30 dpf) were recorded for each family before the exposure study to enable the assessment of inbreeding depression on these traits (Eq. 1).
EDC Exposure Simulation.
Clotrimazole (Chemical Abstracts Service CAS no. 23593-75-1), our model EDC pollutant, was obtained from Sigma–Aldrich (98% pure). Clotrimazole is a widely used imidazole fungicide, which inhibits the enzyme P450(CYP)51 lanosterol 14α-demethylase and the conversion of lanosterol to ergosterol in fungal cell wall synthesis (81). The compound is also a potent ligand and inhibitor of P450(CYP)19 aromatase, which catalyses the conversion of androgens to estrogens in vertebrates, including fish (56). Our nominal aqueous exposure concentrations of 2 and 10 μg of clotrimazole per liter exceeded measured (57–59) and predicted (55) environmental concentrations for this priority hazardous chemical (55); however, clotrimazole is only one of a wide range of similarly acting chemicals (aromatase inhibitors) present in the aquatic environment, as previously discussed.
Experimental Design and Exposure Treatments.
Inbred and outbred zebrafish were subject to aqueous clotrimazole exposures (nominally 2 or 10 μg/L) at two water temperatures (28 °C or 33 °C) in a multifactorial design (Table 1). The temperatures of 28 °C and 33 °C were selected to represent current ambient and future mean temperatures across India and Bangladesh during the monsoon season, which coincides with the main spawning season for zebrafish (38). Temperature elevation to 33 °C was based on worst-case predictions (82) according to Intergovernmental Panel on Climate Change scenario A2 for the Indian subcontinent in the year 2100. Our primary aim here was to assess how the elevated temperatures predicted under future climate change trajectories could augment the impact of clotrimazole on inbred and outbred populations. It was for this reason that we focused on temperature increases and did not include a low-dose 2 μg of clotrimazole per liter × low-temperature (28 °C) exposure treatment. Furthermore, in our previous work, exposure of inbred and outbred zebrafish to a moderately higher dose of clotrimazole (2.9 μg/L) at 28 °C reduced the expression of several steroidogenic enzymes and perturbed plasma concentrations of the male hormone 11-ketotestosterone, but there were no effects on population-relevant end points: survival, growth, gonadal development (40), or fecundity (83).
Chemical exposures of the zebrafish were conducted in a continual flow-through test system from 40 to 100 dpf (12–130 mean wet weight in milligrams), which includes the period of gonadal sex differentiation. Subdivided exposure tanks supplied with flow-through water provided a total of 200 compartments (measuring 7.5 L each) accommodating replicate zebrafish families (n = 18 for inbreds and n = 20 for outbreds) for each of five clotrimazole × temperature treatment combinations (Table 1). Each exposure tank was separated by a sealed glass partition into two lanes, each with four screened flow-through compartments containing either inbred or outbred fish families. This arrangement avoided any possible exchange of pheromones secreted into the water between inbred and outbred fish (40). Each compartment was stocked with four fish taken at random from any given inbred or outbred family, and assignment of tanks to each treatment was also randomized. Compartments were stocked in three consecutive blocks determined by the availability of fish generated by the pair breeding program (SI Appendix, Table S2).
Water quality parameters were monitored and maintained within guideline limits for fish sexual development tests (84) (SI Appendix, Table S3). Water temperature was measured hourly by an automated monitoring system (Facility Monitoring Systems) that was calibrated weekly with thermometer readings in each exposure tank. Temperature was maintained at 28 ± 1.5 °C or 33 ± 1.5 °C throughout the exposure study. In the latter case, temperature was increased gradually from 28 °C to 33 °C during the first 48 h to facilitate acclimation.
Clotrimazole concentrations were quantified throughout the exposure study using tandem liquid chromatography and mass spectrometry. The geometric mean-measured concentrations of clotrimazole were 0 μg/L for the control, 1.7 ± 0.2 μg/L (85%) for the nominal of 2 μg/L, and 8.0 ± 0.8 μg/L (80%) for the nominal of 10 μg/L (SI Appendix, Table S4). The limit of analytical detection for clotrimazole was 0.2 μg/L. Analytical standards and spiked chemical recoveries were consistently greater than 95% of nominals.
Sampling and Analysis of Developmental Effects End Points.
Fish growth rate (85) was assessed throughout the exposure study, because larger size tends to favor female development in zebrafish (45, 46). Individual body lengths were measured in vivo via scaled photographs. All fish (n = 4) were taken from each tank compartment and placed one at a time in decanted tank water in partitioned Petri dishes, and overhead photographs were taken with a Nikon D90 camera (Nikon Imaging) followed by image analysis [ImageJ; NIH (86)] (±1 mm). Body weights were measured in the Petri dishes using a chemical balance (±10 mg). Measurements were performed on exposure days 0/0 (40 dpf), 14/15 (54/55 dpf), 34/40 (74/80 dpf), and 51/60 (91/100 dpf) for the 33 °C and 28 °C exposures, respectively. Paired time points (x/y dpf) delineated equivalent degree-day (87) exposure periods, thus accounting for increased growth rate at elevated temperature. The first intervening period covered prepubescence, the second spanned the period of sexual differentiation of the gonad, and the final period represented completion of sexual development (88). Growth rate measurements were assumed to be affected insignificantly by the low incidence of mortality [13 inbred fish and three outbred fish (16 from a total of 760 fish)] or physical deformity (three inbred fish and one outbred fish with dorsoventral curvature) occurring during the exposure study.
All fish were sampled terminally on exposure day 60, and individual standard length (±0.1 mm) and wet weight (±1 mg) were measured. Fish then underwent necropsy, and the right gonad was excised, weighed (±0.05 mg), snap-frozen in liquid nitrogen, and stored at −80 °C for subsequent analysis of the expression of the aromatase gene (cyp19a1a). The left gonad was left in situ, and all fish remaining at the end of the exposure were fixed in Bouin’s solution (Sigma–Aldrich) and embedded in paraffin wax for histological processing (SI Appendix, Table S5). For each fish, four or five 5-μm transverse serial sections were obtained (taken 500 μm apart along the gonad), stained on separate glass slides using haematoxylin and eosin (H&E), and examined using a Leitz Diaplan light microscope (magnification of 10–100×). At the end of the exposure study, the gonads of all fish were analyzed for histopathology (nominally n = 20 outbred families × n = 4 fish for each of five treatment combinations = 400 fish and n = 18 inbred families × n = 4 fish for each of five treatment combinations = 360 fish). Gonadal sex was recorded, and the most advanced sex cell development stage was used as a measure of the progression of gonadal development [male stages: (i) spermatogonia, (ii) spermatocytes, (iii) spermatids/spermatozoa; female stages: (i) primary oocytes, (ii) cortical alveolar/secondary oocytes, (iii) vitellogenic oocytes]. Expression of the aromatase gene [cyp19a1a; National Center for Biotechnology Information (NCBI) RefSeq database, www.ncbi.nlm.nih.gov/refseq, accession no. NM_131154.2] was quantified in mRNA extracted from the preserved right gonads of a subset of n = 6 males and n = 6 females from each treatment combination (Methods, Statistical Analysis). Quantification of cyp19a1a mRNA was performed by real-time quantitative PCR relative to the housekeeping gene, ribosomal protein l8 (rpl8; NCBI RefSeq database, www.ncbi.nlm.nih.gov/refseq, accession no. NM_200713.1) (SI Appendix, Table S6).
Statistical Analysis.
Gonad weight and relative gene expression were log10-transformed, and all proportional data, including the proportion of eggs fertilized at 0 dpf, proportion hatching at 5 dpf, proportion of larvae surviving to 30 dpf (survivorship), and proportion of females per tank compartment (family) per treatment, were arcsine square root-transformed to improve normality (Anderson–Darling tests). Data were then assessed for equality of variances between treatment groups using Bartlett’s test (for normal data), or Levene’s test, before statistical analysis. MANOVA was used to compare mean fertilization, hatching, and survivorship in inbred vs. outbred families before the start of the exposure study (0–30 dpf). The size spectra (standard length and wet weight) of inbred and outbred families at the start of the exposure study (40 dpf) were also compared in each treatment using MANOVA. All these tests were run in Minitab 16 (Minitab).
Individual families were treated as statistical replicates for the various exposure study end points. For the developmental end points (except for relative cyp19a1a gene expression), there were n = 20 families for outbreds and n = 18 families for inbreds (two inbred families failed to recruit), and families were stocked with n = 4 fish (per tank compartment) in each exposure treatment. For cyp19a1a gene expression, measurements were made on a subset of n = 6 males and n = 6 females from each treatment combination. The individuals selected for the analysis of cyp19a1a gene expression were those individuals with the most frequently occurring gonadal sex cell development stage (i.e., primary oocyte stage in females, spermatid stage in males), and they were taken from different families at random. Log10 relative ovarian cyp19a1a expression was analyzed statistically, but this statistical analysis was not possible for testes because cyp19a1a expression in testes was below or at detection limits. Statistical analysis of developmental end points was performed using lme models (R-statistics, version 2.15.2; R Foundation for Statistical Computing), and all models included family as a random effect. For log10 gonad weight and germ cell progression, individual was also included as a random effect, to account for sex. Specific lme models are defined in SI Appendix, Table S1. The statistical significance of fixed effects (temperature, clotrimazole exposure, and breeding) was determined by ANOVA comparisons of successively simpler models, with improved Akaike information criterion (AIC) scores.
Modeling Population-Level Impacts Using PVA.
PVA models provide insights into how intrinsic and extrinsic factors interact to determine the fate of populations and enable assessments of extinction risk (89). PVA also allows for examining interactions between “deterministic” processes or trends (e.g., anthropogenic climate change, pollution, habitat loss) and “stochastic” processes (i.e., random environmental and demographic fluctuations, random genetic drift, natural catastrophes) (90). We used the individual-based PVA modeling software Vortex, version 9.99c (89, 91) to model the population-level impact of sex ratio skews determined by temperature, clotrimazole exposure, and breeding in our experimental exposures and emergent inbreeding depression on first-year (juvenile) survivorship. Mean adult survivorship and fecundity did not differ between inbreds and outbreds (83), but these and all other vital rates were allowed to vary within typical ranges (SDs) accounting for natural fluctuations (due to demographic and environmental stochasticity) measured, or estimated, for wild zebrafish (38, 46, 49, 92, 93). The parameterization of Vortex is summarized in Fig. 6 and described in more detail in SI Appendix (Table S7). Two approaches were used to model asymptotic, density-dependent population growth: (i) a combined ceiling carrying capacity/breeding probability model (89) and (ii) the BH recruitment model (48) parameterized for zebrafish (49). Population viability was measured in terms of PE and MTE, as well as r and MVPA (47). The use of these alternative approaches and also two population age structures, comprising two and three age classes, respectively, provided increased confidence in PVA predictions. This PVA follows the general assumption that zebrafish live in the wild for up to 2 or 3 y (38, 49).
Fig. 6.
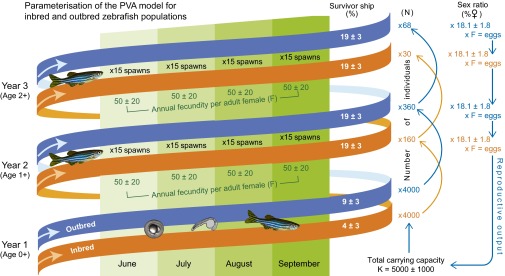
Parameterization of the PVA model for inbred and outbred zebrafish populations. Simulations were run for two age classes (age 0+ juveniles and age 1+ adults) and, subsequently, for three age classes (0+, 1+, and 2+). There was assumed to be no difference in male and female survivorship: age 0+ = 4 ± 3% for inbreds and 9 ± 3% for outbreds; ≥age 1+ = 19 ± 3%. Sex ratio (e.g., 18% females) was varied according to experimental exposure results (Table 2). Annual fecundity (F) per adult female (≥age 1+) = 50 ± 20 eggs × 60 spawning events during the 120-d monsoon season. Ceiling carrying capacity (K) was set at 5,000 ± 1,000 individuals. Other parameters, including simulation of emergent inbreeding depression, are provided in SI Appendix, Table S7.
“Control” population models were set up representing inbred and outbred populations under no chemical exposure and normal ambient temperature (0 μg of clotrimazole per liter, 28 °C) to account for breeding-related differences in first-year survivorship and surviving adult sex ratios in inbred vs. outbred fish (Table 2 and SI Appendix, Table S7). Full-sibling mating in inbred fish simulated the occurrence of an acute genetic bottleneck [e.g., a dry “El Niño” monsoon (63)]. Control models were also run with and without simulating emergent inbreeding depression [compare genetic stochasticity (89)] on age 0+ survivorship over successive generations of inbreeding (including between more distant relatives). Emergent inbreeding depression was parameterized according to an estimated mean of five LE recessive alleles per diploid genome in our zebrafish (Eq. 2). Similar LE estimates have been made for other wild-caught zebrafish from the Ganges River basin (94, 95). All stochastic simulations were run for 100 generations, with input parameter values selected at random from a standard distribution of values (SI Appendix, Table S7). Simulations were repeated 100 times for each scenario, from which the PE, MTE, and mean r were generated. Control models were verified by comparing generated age distributions with observed counts in natural populations (49). Sensitivity analysis was also performed on inbred and outbred control models to confirm that the input parameter values (SI Appendix, Table S8) represented reasonable worst-case values, thus providing a relevant basis for a conservative risk assessment of the population effects of our experimental exposure treatments.
Based on the findings of our experimental exposure study, we then explored the population impacts of a range of sex ratio skews induced by elevated temperature and/or clotrimazole exposure (Table 2). Inbred treatment effects on sex ratio were input to the inbred, “low” juvenile survivorship control model, whereas outbred treatment effects were input to the outbred, “high” juvenile survivorship control model (Fig. 6). All other model input parameters were the same as the inbred and outbred control models, including female fecundity, which is unaffected in zebrafish at the adopted clotrimazole exposure concentrations (83). The 10 exposure scenarios (Table 2) were then rerun, this time also simulating emergent inbreeding depression over consecutive generations. All simulations were run for populations consisting of two and three age classes (Figs. 5 and 6).
Supplementary Material
Acknowledgments
We thank the collective staff at the Brixham Laboratory (AstraZeneca) for their support during the exposure study and Sue Rouillard at the drawing office (University of Exeter) for the production of our figures. We also thank the anonymous referees, whose searching comments helped to improve our manuscript. This research was supported financially by the UK Natural Environment Research Council (Grant NE/F007787/1), the UK Biotechnology and Biological Sciences Research Council (Grant BB/L01548X/1), the University of Exeter, and AstraZeneca’s Global Safety Health and Environment (SHE) Research Programme.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1416269112/-/DCSupplemental.
References
- 1.United Nations Environment Programme 2012 Biodiversity. Global Environment Outlook Report GEO-5. Available at www.unep.org/geo/geo5.asp. Accessed November 16, 2014.
- 2.Bates BC, Kundzewicz ZW, Wu S, Palutikof JP. Climate Change and Water. Technical Paper of the Intergovernmental Panel on Climate Change. IPCC Secretariat; Geneva: 2008. [Google Scholar]
- 3.Vörösmarty CJ, et al. Global threats to human water security and river biodiversity. Nature. 2010;467(7315):555–561. doi: 10.1038/nature09440. [DOI] [PubMed] [Google Scholar]
- 4.Holmstrup M, et al. Interactions between effects of environmental chemicals and natural stressors: A review. Sci Total Environ. 2010;408(18):3746–3762. doi: 10.1016/j.scitotenv.2009.10.067. [DOI] [PubMed] [Google Scholar]
- 5.Hallare AV, Schirling M, Luckenbach T, Köhler H-R, Triebskorn R. Combined effects of temperature and cadmium on developmental parameters and biomarker responses in zebrafish (Danio rerio) embryos. J Therm Biol. 2005;30(1):7–17. [Google Scholar]
- 6.Quinn AL. The Impacts of Agricultural Chemicals and Temperature on the Physiological Stress Response in Fish. University of Lethbridge; Leithbridge, AB, Canada: 2007. [Google Scholar]
- 7.Sih A, Bell AM, Kerby JL. Two stressors are far deadlier than one. Trends Ecol Evol. 2004;19(6):274–276. doi: 10.1016/j.tree.2004.02.010. [DOI] [PubMed] [Google Scholar]
- 8.Bancroft BA, Baker NJ, Blaustein AR. A meta-analysis of the effects of ultraviolet B radiation and its synergistic interactions with pH, contaminants, and disease on amphibian survival. Conserv Biol. 2008;22(4):987–996. doi: 10.1111/j.1523-1739.2008.00966.x. [DOI] [PubMed] [Google Scholar]
- 9.Gibbons JW, et al. The global decline of reptiles, déjà vu amphibians. Bioscience. 2000;50(8):653–666. [Google Scholar]
- 10.Willingham EJ. The effects of atrazine and temperature on turtle hatchling size and sex ratios. Front Ecol Environ. 2005;3(6):309–313. [Google Scholar]
- 11.Moe SJ, et al. Combined and interactive effects of global climate change and toxicants on populations and communities. Environ Toxicol Chem. 2013;32(1):49–61. doi: 10.1002/etc.2045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gergs A, Zenker A, Grimm V, Preuss TG. Chemical and natural stressors combined: From cryptic effects to population extinction. Sci Rep. 2013;3:2036. doi: 10.1038/srep02036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lande R, Shannon S. The role of genetic variation in adaptation and population persistence in a changing environment. Evolution. 1996;50(1):434–437. doi: 10.1111/j.1558-5646.1996.tb04504.x. [DOI] [PubMed] [Google Scholar]
- 14.Reed DH, Lowe EH, Briscoe DA, Frankham R. Fitness and adaptation in a novel environment: Effect of inbreeding, prior environment, and lineage. Evolution. 2003;57(8):1822–1828. doi: 10.1111/j.0014-3820.2003.tb00589.x. [DOI] [PubMed] [Google Scholar]
- 15.Kamel SJ, Mrosovsky N. Deforestation: Risk of sex ratio distortion in hawksbill sea turtles. Ecol Appl. 2006;16(3):923–931. doi: 10.1890/1051-0761(2006)016[0923:drosrd]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 16.Hawkes LA, Broderick AC, Godfrey MH, Godley BJ. Investigating the potential impacts of climate change on a marine turtle population. Glob Change Biol. 2007;13(5):923–932. [Google Scholar]
- 17.Head G, May RM, Pendleton L. Environmental determination of sex in the reptiles. Nature. 1987;329(6136):198–199. [Google Scholar]
- 18.Pieau C, Dorizzi M, Richard-Mercier N. Temperature-dependent sex determination and gonadal differentiation in reptiles. Cell Mol Life Sci. 1999;55(6-7):887–900. doi: 10.1007/s000180050342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wapstra E, et al. Climate effects on offspring sex ratio in a viviparous lizard. J Anim Ecol. 2009;78(1):84–90. doi: 10.1111/j.1365-2656.2008.01470.x. [DOI] [PubMed] [Google Scholar]
- 20.Warner RR. Sperm allocation in coral reef fish, strategies for coping with demands on sperm production. Bioscience. 1997;47(9):561–564. [Google Scholar]
- 21.Ospina-Alvarez N, Piferrer F. Temperature-dependent sex determination in fish revisited: Prevalence, a single sex ratio response pattern, and possible effects of climate change. PLoS ONE. 2008;3(7):e2837. doi: 10.1371/journal.pone.0002837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Eggert C. Sex determination: The amphibian models. Reprod Nutr Dev. 2004;44(6):539–549. doi: 10.1051/rnd:2004062. [DOI] [PubMed] [Google Scholar]
- 23.Andersson M, Simmons LW. Sexual selection and mate choice. Trends Ecol Evol. 2006;21(6):296–302. doi: 10.1016/j.tree.2006.03.015. [DOI] [PubMed] [Google Scholar]
- 24.Rankin DJ, Kokko H. Do males matter? The role of males in population dynamics. Oikos. 2007;116(2):335–348. [Google Scholar]
- 25.Baroiller JF, D’Cotta H, Saillant E. Environmental effects on fish sex determination and differentiation. Sex Dev. 2009;3(2-3):118–135. doi: 10.1159/000223077. [DOI] [PubMed] [Google Scholar]
- 26.Delvin RH, Nagahama Y. Sex determination and sex differentiation in fish: An overview of genetic, physiological and environmental influences. Aquaculture. 2002;208(3-4):191–364. [Google Scholar]
- 27.Kohno S, Guillette LJ. Endocrine disruption and reptiles: Using the unique attributes of temperature-dependent sex determination to assess impacts. In: Matthiessen P, editor. Endocrine Disrupters: Hazard Testing and Assessment Methods. Wiley; Hoboken, NJ: 2013. pp. 245–271. [Google Scholar]
- 28.Orton F, Tyler CR. Do hormone-modulating chemicals impact on reproduction and development of wild amphibians? Biol Rev Camb Philos Soc. 2014 doi: 10.1111/brv.12147. [DOI] [PubMed] [Google Scholar]
- 29.Hamlin HJ, Guillette LJ., Jr Birth defects in wildlife: The role of environmental contaminants as inducers of reproductive and developmental dysfunction. Syst Biol Reprod Med. 2010;56(2):113–121. doi: 10.3109/19396360903244598. [DOI] [PubMed] [Google Scholar]
- 30.Harris CA, et al. The consequences of feminization in breeding groups of wild fish. Environ Health Perspect. 2011;119(3):306–311. doi: 10.1289/ehp.1002555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kidd KA, et al. Collapse of a fish population after exposure to a synthetic estrogen. Proc Natl Acad Sci USA. 2007;104(21):8897–8901. doi: 10.1073/pnas.0609568104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Brook BW, Tonkyn DW, O’Grady JJ, Frankham R. Contribution of inbreeding to extinction risk in threatened species. Conserv Ecol. 2002;6(1):16. [Google Scholar]
- 33.Charlesworth D, Willis JH. The genetics of inbreeding depression. Nat Rev Genet. 2009;10(11):783–796. doi: 10.1038/nrg2664. [DOI] [PubMed] [Google Scholar]
- 34.Roff DA. Inbreeding depression: Tests of the overdominance and partial dominance hypotheses. Evolution. 2002;56(4):768–775. doi: 10.1111/j.0014-3820.2002.tb01387.x. [DOI] [PubMed] [Google Scholar]
- 35.Wright LI, Tregenza T, Hosken DJ. Inbreeding, inbreeding depression and extinction. Conserv Genet. 2008;9(4):833–843. [Google Scholar]
- 36.Spitsbergen JM, Kent ML. The state of the art of the zebrafish model for toxicology and toxicologic pathology research—Advantages and current limitations. Toxicol Pathol. 2003;31(Suppl):62–87. doi: 10.1080/01926230390174959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rubinstein AL. Zebrafish assays for drug toxicity screening. Expert Opin Drug Metab Toxicol. 2006;2(2):231–240. doi: 10.1517/17425255.2.2.231. [DOI] [PubMed] [Google Scholar]
- 38.Spence R, Gerlach G, Lawrence C, Smith C. The behaviour and ecology of the zebrafish, Danio rerio. Biol Rev Camb Philos Soc. 2008;83(1):13–34. doi: 10.1111/j.1469-185X.2007.00030.x. [DOI] [PubMed] [Google Scholar]
- 39.Uchida D, Yamashita M, Kitano T, Iguchi T. An aromatase inhibitor or high water temperature induce oocyte apoptosis and depletion of P450 aromatase activity in the gonads of genetic female zebrafish during sex-reversal. Comp Biochem Physiol A Mol Integr Physiol. 2004;137(1):11–20. doi: 10.1016/s1095-6433(03)00178-8. [DOI] [PubMed] [Google Scholar]
- 40.Brown AR, et al. Are toxicological responses in laboratory (inbred) zebrafish representative of those in outbred (wild) populations? A case study with an endocrine disrupting chemical. Environ Sci Technol. 2011;45(9):4166–4172. doi: 10.1021/es200122r. [DOI] [PubMed] [Google Scholar]
- 41.Guiguen Y, Fostier A, Piferrer F, Chang C-F. Ovarian aromatase and estrogens: A pivotal role for gonadal sex differentiation and sex change in fish. Gen Comp Endocrinol. 2010;165(3):352–366. doi: 10.1016/j.ygcen.2009.03.002. [DOI] [PubMed] [Google Scholar]
- 42.Navarro-Martín L, et al. DNA methylation of the gonadal aromatase (cyp19a) promoter is involved in temperature-dependent sex ratio shifts in the European sea bass. PLoS Genet. 2011;7(12):e1002447. doi: 10.1371/journal.pgen.1002447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Falconer DS, Mackay TFC. Introduction to Quantitative Genetics. 4th Ed Longman; New York: 1996. [Google Scholar]
- 44.Morton NE, Crow JF, Muller HJ. An estimate of the mutational damage in man from data on consanguineous marriages. Proc Natl Acad Sci USA. 1956;42(11):855–863. doi: 10.1073/pnas.42.11.855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lawrence C, Ebersole JP, Kesseli RV. Rapid growth and out-crossing promote female development in zebrafish (Danio rerio) Environ Biol Fishes. 2008;81(2):239–246. [Google Scholar]
- 46.Uusi-Heikkilä S, Wolter C, Meinelt T, Arlinghaus R. Size-dependent reproductive success of wild zebrafish Danio rerio in the laboratory. J Fish Biol. 2010;77(3):552–569. doi: 10.1111/j.1095-8649.2010.02698.x. [DOI] [PubMed] [Google Scholar]
- 47.Reed DH, O’Grady JJ, Brook BW, Ballou JD, Frankham R. Estimates of minimum viable population sizes for vertebrates and factors influencing those estimates. Biol Conserv. 2003;113(1):23–34. [Google Scholar]
- 48.Beverton RJH, Holt SJ. 1957. On the Dynamics of Exploited Fish Populations, Fishery Investigations Series II (Ministry of Agriculture, Fisheries, and Food, HMSO, London), Vol XIX.
- 49.Hazlerigg CRE, Tyler CR, Lorenzen K, Wheeler JR, Thorbek P. Population relevance of toxicant mediated changes in sex ratio in fish: An assessment using an individual-based zebrafish (Danio rerio) model. Ecol Modell. 2014;280(2014):76–88. [Google Scholar]
- 50.Intergovernmental Panel on Climate Change . Summary for policymakers. In: Stocker TF, et al., editors. Climate Change 2013: The Physical Science Basis. Contribution of Working Group I to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change. Cambridge Univ Press; Cambridge, UK: 2013. [Google Scholar]
- 51.United Nations Environment Programme, World Health Organization 2013. State of the Science of Endocrine Disrupting Chemicals—2012: An Assessment of the State of the Science of Endocrine Disruptors Prepared by a Group of Experts for the United Nations Environment Programme and World Health Organization, eds Bergman Å, Heindel JJ, Jobling S, Kidd KA, Zoeller RT (UNEP/WHO, Geneva)
- 52.Jobling S, et al. Wild intersex roach (Rutilus rutilus) have reduced fertility. Biol Reprod. 2002;67(2):515–524. doi: 10.1095/biolreprod67.2.515. [DOI] [PubMed] [Google Scholar]
- 53.Orlando EF, et al. Endocrine-disrupting effects of cattle feedlot effluent on an aquatic sentinel species, the fathead minnow. Environ Health Perspect. 2004;112(3):353–358. doi: 10.1289/ehp.6591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kortenkamp A. Ten years of mixing cocktails: A review of combination effects of endocrine-disrupting chemicals. Environ Health Perspect. 2007;115(1) Suppl 1:98–105. doi: 10.1289/ehp.9357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Oslo Paris Commission . OSPAR Background Document on Clotrimazole 2013 update. London: 2013. [Google Scholar]
- 56.Hinfray N, Porcher J-M, Brion F. Inhibition of rainbow trout (Oncorhynchus mykiss) P450 aromatase activities in brain and ovarian microsomes by various environmental substances. Comp Biochem Physiol C Toxicol Pharmacol. 2006;144(3):252–262. doi: 10.1016/j.cbpc.2006.09.002. [DOI] [PubMed] [Google Scholar]
- 57.Kahle M, Buerge IJ, Hauser A, Müller MD, Poiger T. Azole fungicides: Occurrence and fate in wastewater and surface waters. Environ Sci Technol. 2008;42(19):7193–7200. doi: 10.1021/es8009309. [DOI] [PubMed] [Google Scholar]
- 58.Macikova P, Groh KJ, Ammann AA, Schirmer K, Suter MJ-F. Endocrine disrupting compounds affecting corticosteroid signaling pathways in czech and swiss waters: Potential impact on fish. Environ Sci Technol. 2014;48(21):12902–12911. doi: 10.1021/es502711c. [DOI] [PubMed] [Google Scholar]
- 59.Roberts PH, Thomas KV. The occurrence of selected pharmaceuticals in wastewater effluent and surface waters of the lower Tyne catchment. Sci Total Environ. 2006;356(1-3):143–153. doi: 10.1016/j.scitotenv.2005.04.031. [DOI] [PubMed] [Google Scholar]
- 60.Berenzen N, et al. A comparison of predicted and measured levels of runoff-related pesticide concentrations in small lowland streams on a landscape level. Chemosphere. 2005;58(5):683–691. doi: 10.1016/j.chemosphere.2004.05.009. [DOI] [PubMed] [Google Scholar]
- 61.Jacobsen NW, Halling-Sørensen B, Birkved FK. Inhibition of human aromatase complex (CYP19) by antiepileptic drugs. Toxicol In Vitro. 2008;22(1):146–153. doi: 10.1016/j.tiv.2007.09.004. [DOI] [PubMed] [Google Scholar]
- 62.Hazlerigg CRE. 2012 Fish population ecology and ecological risk assessment. PhD thesis (University of Exeter, Exeter, UK). Available at ethos.bl.uk/OrderDetails.do?uin=uk.bl.ethos.555911. Accessed November 16, 2014.
- 63.Ropelewski CF, Halpert MS. Global and regional scale precipitation patterns associated with the El Niño/Southern Oscillation. Monthly Weather Review. 1987;115(8):1606–1626. [Google Scholar]
- 64.Erikstad KE, Moum T, Bustnes JO, Reiertsen TK. High levels of organochlorines may affect hatching sex ratio and hatchling body mass in arctic glaucous gulls. Funct Ecol. 2011;25(1):289–296. [Google Scholar]
- 65.Parker GA. The evolution of sexual size dimorphism in fish. J Fish Biol. 1992;41(sB):1–20. [Google Scholar]
- 66.von Hofsten J, Olsson P-E. Zebrafish sex determination and differentiation: Involvement of FTZ-F1 genes. Reprod Biol Endocrinol. 2005;3:63. doi: 10.1186/1477-7827-3-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Bradley KM, et al. An SNP-based linkage map for zebrafish reveals sex determination. G3 (Bethesda) 2011;1(1):3–9. doi: 10.1534/g3.111.000190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wilson CA, et al. Wild sex in zebrafish: Loss of the natural sex determinant in domesticated strains. Genetics. 2014;198(3):1291–1308. doi: 10.1534/genetics.114.169284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Anderson JL, et al. Multiple sex-associated regions and a putative sex chromosome in zebrafish revealed by RAD mapping and population genomics. PLoS ONE. 2012;7(7):e40701. doi: 10.1371/journal.pone.0040701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Perry AL, Low PJ, Ellis JR, Reynolds JD. Climate change and distribution shifts in marine fishes. Science. 2005;308(5730):1912–1915. doi: 10.1126/science.1111322. [DOI] [PubMed] [Google Scholar]
- 71.Foden WB, et al. Identifying the world’s most climate change vulnerable species: A systematic trait-based assessment of all birds, amphibians and corals. PLoS ONE. 2013;8(6):e65427. doi: 10.1371/journal.pone.0065427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Rice WR, Chippindale AK. Sexual recombination and the power of natural selection. Science. 2001;294(5542):555–559. doi: 10.1126/science.1061380. [DOI] [PubMed] [Google Scholar]
- 73.Colegrave N. Sex releases the speed limit on evolution. Nature. 2002;420(6916):664–666. doi: 10.1038/nature01191. [DOI] [PubMed] [Google Scholar]
- 74.Becks L, Agrawal AF. The evolution of sex is favoured during adaptation to new environments. PLoS Biol. 2012;10(5):e1001317. doi: 10.1371/journal.pbio.1001317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Roze D. Disentangling the benefits of sex. PLoS Biol. 2012;10(5):e1001321. doi: 10.1371/journal.pbio.1001321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Tucker JK, Dolan CR, Lamer JT, Dustman EA. Climatic warming, sex ratios and red-eared sliders (Trachemys scripta elegans) in Illinois. Chelonian Conserv Biol. 2008;7(1):60–69. [Google Scholar]
- 77.Conover DO, Voorhees DAV, Ehtisham A. Sex ratio selection and changes in environmental sex determination in laboratory populations of Menidia menidia. Evolution. 1992;46(6):1722–1730. doi: 10.1111/j.1558-5646.1992.tb01164.x. [DOI] [PubMed] [Google Scholar]
- 78.Le Page Y, et al. Aromatase, brain sexualization and plasticity: The fish paradigm. Eur J Neurosci. 2010;32(12):2105–2115. doi: 10.1111/j.1460-9568.2010.07519.x. [DOI] [PubMed] [Google Scholar]
- 79.Fenske M, Segner H. Aromatase modulation alters gonadal differentiation in developing zebrafish (Danio rerio) Aquat Toxicol. 2004;67(2):105–126. doi: 10.1016/j.aquatox.2003.10.008. [DOI] [PubMed] [Google Scholar]
- 80.Parmesan C, Yohe G. A globally coherent fingerprint of climate change impacts across natural systems. Nature. 2003;421(6918):37–42. doi: 10.1038/nature01286. [DOI] [PubMed] [Google Scholar]
- 81.Rodrigues AD, Gibson GG, Ioannides C, Parke DV. Interactions of imidazole antifungal agents with purified cytochrome P-450 proteins. Biochem Pharmacol. 1987;36(24):4277–4281. doi: 10.1016/0006-2952(87)90670-8. [DOI] [PubMed] [Google Scholar]
- 82.Rupa Kumar KR, et al. High-resolution climate change scenarios for India for the 21st century. Curr Sci. 2006;90(3):334–345. [Google Scholar]
- 83.Bickley LK, et al. Interactive effects of inbreeding and endocrine disruption on reproduction in a model laboratory fish. Evol Appl. 2013;6(2):279–289. doi: 10.1111/j.1752-4571.2012.00288.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Organisation for Economic Cooperation and Development . OECD Guideline for Testing of Chemicals, Guideline 234: Fish, Sexual Development Test. OECD; Paris: 2011. [Google Scholar]
- 85.Bolger T, Connolly P. The selection of suitable indices for the measurement and analysis of fish condition. J Fish Biol. 1989;34(2):171–182. [Google Scholar]
- 86.Schneider CA, Rasband WS, Eliceiri KW. NIH Image to ImageJ: 25 years of image analysis. Nat Methods. 2012;9(7):671–675. doi: 10.1038/nmeth.2089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Neuheimer AB, Taggart CT. The growing degree day and fish size at age: The overlooked metric. Can J Fish Aquat Sci. 2007;64(2):375–385. [Google Scholar]
- 88.Maack G, Segner H. Morphological development of the gonads in zebrafish. J Fish Biol. 2003;62(4):895–906. [Google Scholar]
- 89.Miller PS, Lacy RC. 2005. VORTEX: A Stochastic Simulation of the Extinction Process. Version 9.50 User’s Manual [Conservation Breeding Specialist Group (SSC/IUCN), Apple Valley, MN]
- 90.Shaffer ML. Minimum population sizes for species conservation. Bioscience. 1981;31(2):131–134. [Google Scholar]
- 91.Lacy RC, Borbat M, Pollak JP. 2005. VORTEX: A Stochastic Simulation of the Extinction Process. Version 9.50 (Chicago Zoological Society, Brookfield, IL)
- 92.Hazlerigg CRE, Lorenzen K, Thorbek P, Wheeler JR, Tyler CR. Density-dependent processes in the life history of fishes: Evidence from laboratory populations of zebrafish Danio rerio. PLoS ONE. 2012;7(5):e37550. doi: 10.1371/journal.pone.0037550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Uusi-Heikkilä S, Kuparinen A, Wolter C, Meinelt T, O’Toole AC, Arlinghaus R. Experimental assessment of the probabilistic maturation reaction norm: Condition matters. Proc Royal Soc Lond B Biol Sci. 2011;278(1706):709–717. doi: 10.1098/rspb.2010.1507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.McCune AR, et al. A low genomic number of recessive lethals in natural populations of bluefin killifish and zebrafish. Science. 2002;296(5577):2398–2401. doi: 10.1126/science.1071757. [DOI] [PubMed] [Google Scholar]
- 95.McCune AR, Houle D, McMillan K, Annable R, Kondrashov AS. Two classes of deleterious recessive alleles in a natural population of zebrafish, Danio rerio. Proc Biol Sci. 2004;271(1552):2025–2033. doi: 10.1098/rspb.2004.2787. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.