Granzier et al. (1) recently made a mouse model with modified titin to test the proposal that “titin is a protein ruler that determines thick filament length.” Fourteen domains spanning 56 nm were deleted from titin at the end of the thick filament (IA junction) between the thick filament bound (A-band) and elastic (I-band) parts of the molecule. No change in thick filament length was observed, from which it was concluded that “Findings disproved that titin at the IA junction is crucial for thick filament length control, settling a long-standing hypothesis.”
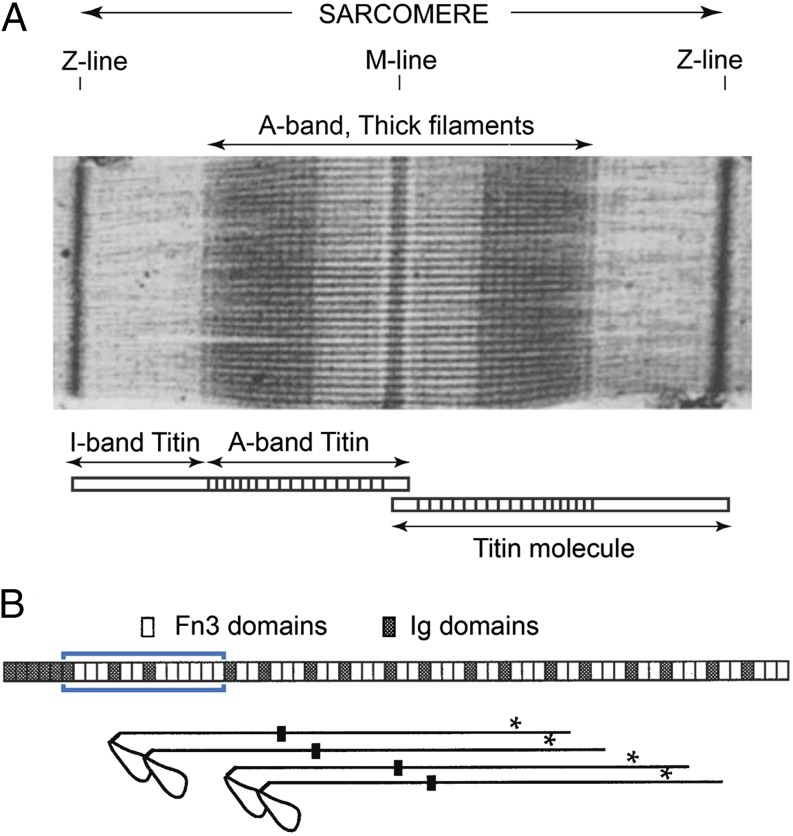
Vertebrate striated muscle thick filaments are remarkably uniform in length, 1.6 μm, and probably contain precisely 294 myosin molecules (Fig. 1A). There have been proposals for how the filament length is exactly controlled, but none has been proven. Individual titin molecules span between the Z- and M-lines and in the A-band are thought to lie on the surface of the filament shaft. This suggested an obvious mechanism for exact length control, with titin acting as a molecular ruler (2), which is a widely accepted idea. Interactions between myosin and titin are indicated by close correspondence between the helical structure of the filament and patterning of the A-band titin Ig (Ig) and fibronectin (Fn3) domains (Fig. 1B) (3), although these interactions are not fully understood. Furthermore, titin A-band epitope distances to the M-line do not vary with sarcomere length, indicating that titin is firmly associated with the thick filament. This contrasts with I-band titin, which contains only Ig domains and unique sequences and behaves elastically.
Fig. 1.
(A) Sharp sarcomere A-band edges indicate thick filaments are all exactly the same length (micrograph reprinted from ref. 5). The extent of the titin molecule is shown below. (B) Titin and myosin arrangement near the A-band end. (Upper) Titin domain arrangement around I-band/A-band transition region [blue box indicates deletion made by Granzier et al. (1)]. (Lower) Stagger of the last four myosin registers at the thick filament tip, spaced 14.3 nm, to same length scale as the titin domains. Black boxes indicate the N-terminal ends of LMM; asterisks indicate a major titin binding site. Note the missing register of myosin in the middle of the four shown, which results in a pale line near the A-band edge in A. Diagram adapted from ref. 3.
We do not contest the conclusion that the IA junction part of titin is not critical for thick filament length control. However, the claim that this disproves the ruler hypothesis is unjustified. Myosin is a long molecule, ∼180 nm, and evidence indicates that its main interaction sites for assembly into filaments and titin attachment are in the C-terminal tail, light meromyosin (LMM), more than 100 nm away from the IA junction (Fig. 1B). Polymer length is therefore unlikely to be affected by deletion of titin domains from the filament tip region. Furthermore, the suggestion in ref. 3 that Ig-Fn3-Fn3 domain motifs form a “myosin head motif” that regulates the packing and order of myosin heads remains untested by these experiments, as the impact of the IA junction deletion on myosin head order and the “cross-bridge gap” at the thick filament end (3) has not been investigated and would require much better resolved EM images than those presented by Granzier et al. (1). Whether the ruler hypothesis is correct can be questioned (4), but these recent data do not provide a critical test.
Footnotes
The authors declare no conflict of interest.
References
- 1.Granzier HL, et al. Deleting titin’s I-band/A-band junction reveals critical roles for titin in biomechanical sensing and cardiac function. Proc Natl Acad Sci USA. 2014;111(40):14589–14594. doi: 10.1073/pnas.1411493111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Whiting A, Wardale J, Trinick J. Does titin regulate the length of muscle thick filaments? J Mol Biol. 1989;205(1):263–268. doi: 10.1016/0022-2836(89)90381-1. [DOI] [PubMed] [Google Scholar]
- 3.Bennett PM, Gautel M. Titin domain patterns correlate with the axial disposition of myosin at the end of the thick filament. J Mol Biol. 1996;259(5):896–903. doi: 10.1006/jmbi.1996.0367. [DOI] [PubMed] [Google Scholar]
- 4.Tskhovrebova L, Trinick J. Molecular rulers? Curr Biol. 2012;22(9):R317–R318. doi: 10.1016/j.cub.2012.02.049. [DOI] [PubMed] [Google Scholar]
- 5.Huxley HE. Recent x-ray diffraction and electron microscope studies of striated muscle. J Gen Physiol. 1967;50(6, Suppl):71–83. doi: 10.1085/jgp.50.6.71. [DOI] [PMC free article] [PubMed] [Google Scholar]