In PNAS, Daloso et al. (1) show that, in plant cells, the activities of two enzymes, succinate dehydrogenase and fumarase, in the tricarboxylic acid (TCA) cycle in the mitochondria, as well as one cytosolic enzyme associated with the cycle (ATP-citrate lyase), are regulated by reversible reduction-oxidation mediated by interaction with thioredoxin.
Plant metabolism is very complex. Plant cells synthesize all of the metabolic building blocks known from the well-studied animal cells, sugars, lipids, amino acids, and their polymers. In addition, they synthesize all of the vitamins (which are actually coenzymes that we humans cannot synthesize and therefore need to acquire from the food we eat, i.e., mainly from plants), as well as a huge variety of so-called secondary metabolites such as alkaloids and terpenoids. Intermediary metabolism in plant cells therefore needs to have a tight, but flexible, regulation to deliver the correct building blocks in the right amounts at the right place and the right time.
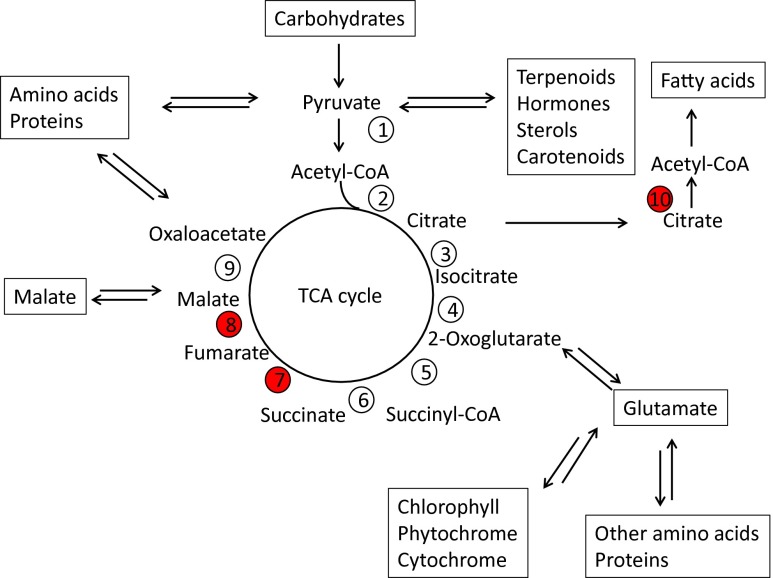
The TCA cycle (also called the citric acid cycle or the Krebs cycle to honor its discoverer, Sir Hans Krebs), which is located in the mitochondria of all eukaryotic organisms, animal, plants, and fungi, is usually depicted as being in the center of cellular metabolism on metabolic maps (Fig. 1). It has a dual purpose: (i) to harness the energy released during the degradation of carbohydrates, fatty acids, and proteins, in the cellularly useful forms, reducing equivalents (mainly NADH but also NADPH) and ATP and (ii) to produce a number of central metabolites used in biosynthesis (Fig. 1). Because the degradation of carbohydrates via glycolysis, proteins via amino acids, and fatty acids (via acetyl-CoA) feeds into the TCA cycle at several different points, the cycle must be able to cope with delivery and removal of intermediates simultaneously at multiple points. The input and output of the cycle changes dynamically with time (developmental processes) and environmental conditions (Fig. 1), and this would be expected to require multiple layers of regulation; some are long term like protein biosynthesis/degradation, whereas others are much more rapid to be able to adjust to sudden metabolic changes, such as the onset/disappearance of light or attack by pathogens (2).
Fig. 1.
The TCA cycle and its integration into plant cellular metabolism. (1) Pyruvate dehydrogenase; (2) citrate synthase; (3) aconitase; (4) isocitrate dehydrogenase; (5) 2-oxoglutarate dehydrogenase; (6) succinyl-CoA synthase; (7) succinate dehydrogenase; (8) fumarase; (9) malate dehydrogenase; (10) ATP-citrate lyase. The three enzymes that Daloso et al. showed to be regulated by thioredoxin are highlighted in red.
The degradation of carbohydrates in glycolysis, a cytosolic pathway, gives rise to pyruvate, which is transported into the mitochondria. Here pyruvate is converted into acetyl-CoA by the pyruvate dehydrogenase complex that is, strictly speaking, not part of the TCA cycle. Energy released by this reaction is conserved in the form of one molecule of NADH. The first reaction of the actual TCA cycle is the condensation of acetyl-CoA with oxaloacetate (OAA) catalyzed by the enzyme citrate synthase to give the tricarboxylic acid citrate. In a series of seven consecutive reactions catalyzed by the enzymes aconitase, isocitrate dehydrogenase, 2-oxoglutarate dehydrogenase, succinyl-CoA synthase, succinate dehydrogenase, fumarase, and malate dehydrogenase, OAA is regenerated from citrate. In these reactions two molecules of CO2 are released and three molecules of NADH, one molecule of FADH2, and one molecule of ATP (or GTP depending on the organism) are synthesized, thus conserving about 50% the chemical energy released when citrate is oxidized. The net result of the oxidation of one molecule of pyruvate to three molecules of CO2 is four molecules of NADH and one molecule of ATP. The NADH can either be used directly or it can be converted into ATP by oxidative phosphorylation catalyzed by the electron transport chain and ATP synthase located in the inner mitochondrial membrane. The result of this conversion of one form of chemical energy to another is 2.5 ATP per NADH, whereas FADH2 yields 1.5 ATP. Thus, the total yield of ATP can be as high as 12.5 ATP per pyruvate molecule oxidized (including one ATP formed by so-called substrate level phosphorylation) (3). Most of the intermediates of the TCA cycle are important starting compounds for major biosynthetic pathways as illustrated in Fig. 1, whereas the NADH and the ATP are used for biosynthesis or maintenance in the mitochondria or elsewhere in the cell.
The plant TCA cycle is known to be regulated at the entry point—the activity of pyruvate dehydrogenase is regulated by protein phosphorylation (4)—as well as at several points in the cycle by allosteric effects, modification of activity by molecules such as NADH, NAD+, and ADP. In general, the cycle enzymes are activated when both the reduction level ([NAD(P)H]/[NAD(P)+] ratio) and the phosphorylation level ([ATP]/[ADP]) fall below threshold levels. This makes sense because under these conditions the mitochondria and the cell need to step up the rate of NADH and ATP synthesis to maintain homeostasis (2, 5).
One very important biochemical regulatory mechanism of enzyme activity is cysteine-cystine redox interconversions (-SH + HS- ↔ -S-S-). In photosynthesis in the chloroplasts, CO2 is fixed in the light to form sugars and eventually starch. It has long been known that several key enzymes in the CO2 fixation pathway called the Calvin cycle are activated by thioredoxin (6). The oxidized form can be converted back into the reduced form by interaction with thioredoxin, which in turn becomes oxidized. The reduced form of thioredoxin is regenerated by an enzyme called thioredoxin reductase using NADPH. In sunlight, NADPH and therefore thioredoxin are both kept very reduced, which means that the enzymes regulated by thioredoxin are also kept in the reduced form. It therefore makes good sense that the reduced forms of the Calvin cycle enzymes regulated by thioredoxin are much more active than the oxidized forms because the rate of CO2 fixation should be high in the light, which creates reducing conditions. In contrast, in darkness, NADPH and thioredoxin, as well as the enzymes regulated by thioredoxin, are all relatively oxidized, and the Calvin cycle comes to a halt.
Plant mitochondria are known to contain the thioredoxin system, and one of the enzymes in the mitochondrial respiratory chain, the alternative oxidase, has been shown to be regulated by thioredoxin-dependent reversible oxidation reduction of cysteins (7–10). In several studies, a number of potential thioredoxin-regulated enzymes have been identified (10, 11), including several TCA cycle enzymes, but there has been no previous demonstration that the activity of any TCA cycle enzymes was regulated in vivo. That is the reason the results published by Daloso et al. (1) are so interesting. They compared extractable enzyme activities in WT Arabidopsis with those of mutant plants where either the mitochondrial thioredoxin (trxo1) or both the mitochondrial and the cytosolic forms of thioredoxin reductase (ntra ntrb) had been knocked out. They also measured metabolite pool sizes and followed the fate of 13C in experiments where leaves were fed different labeled intermediates. Finally, they conducted in vitro complementation experiments where they measured enzyme activities in mitochondrial extracts or whole leaf extracts and the effect of adding thioredoxin and thioredoxin reductase. The conclusion from all these experiments is that two TCA cycle enzymes, succinate dehydrogenase and fumarase, are deactivated by thioredoxin, whereas the cytosolic enzyme ATP-citrate lyase is activated.
Both of the TCA cycle enzymes have been reported to be potential thioredoxin target proteins (10, 11), and the results by Daloso et al. (1) establish that their activities are indeed regulated by thioredoxin in vivo. This regulation might assist in adjusting the cycle to serve in one of its noncyclic modes where only parts of the cycle are used (2, 12).
ATP-citrate lyase is involved in delivering acetyl-CoA to fatty acid biosynthesis using citrate deriving from the TCA cycle and exported from the mitochondria. Because the thioredoxin control is exerted under reducing conditions (high [NAD(P)H]/[NAD(P)+] ratio), a deceleration of the TCA cycle and a draining off of intermediates (and reducing equivalents) for fatty acid biosynthesis—a noncyclic mode of TCA function—makes excellent sense.
An open question is the origin of the NADPH required specifically by thioredoxin reductase to reduce thioredoxin. The TCA cycle enzymes make NADH, but unlike mammalian mitochondria, plant mitochondria do not contain an energy-dependent transhydrogenase able to keep the NADP pool reduced by transfer of reducing equivalents from NADH to NADP+ (13). NADPH is most likely synthesized either from NADH by an NADH kinase (14) or by a matrix NADP-specific isocitrate dehydrogenase, which is separate from the NAD-specific isocitrate dehydrogenase that is part of the TCA cycle (15, 16). The regulation of these two complementary enzymes is still not understood.
As a result of the study of Daloso et al. (1), we now know that the regulation of the TCA cycle activity has several layers involving both allosteric regulation and posttranslational modifications such as protein phosphorylation and thioredoxin reduction/oxidation. However, it is likely that we still have much to learn about TCA cycle regulation by posttranslational modifications. A number of TCA cycle enzymes are candidates for additional regulation as they are either phosphorylated (17) or acetylated (18) or both. This includes both of the abovementioned NAD- and NADP-isocitrate dehydrogenases, which means that the thioredoxin regulation could be overlayed by still other regulatory mechanisms. Detailed in vivo studies, similar to the present one, using KO mutants and metabolic profiling are required to establish which of these posttranslational modifications has physiological importance.
Footnotes
The author declares no conflict of interest.
See companion article on page E1392.
References
- 1.Daloso DM, et al. Thioredoxin, a master regulator of the tricarboxylic acid cycle in plant mitochondria. Proc Natl Acad Sci USA. 2015;112:E1392–E1400. doi: 10.1073/pnas.1424840112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Millar AH, Whelan J, Soole KL, Day DA. Organization and regulation of mitochondrial respiration in plants. Annu Rev Plant Biol. 2011;62:79–104. doi: 10.1146/annurev-arplant-042110-103857. [DOI] [PubMed] [Google Scholar]
- 3.Møller IM, Rasmusson AG, Browse JJ. Plant respiration and lipid metabolism. In: Taiz L, Zeiger E, Murphy A, Møller IM, editors. Plant Physiology. 6th Ed. Sinauer Associates; Sunderland, MA: 2014. pp. 317–352. [Google Scholar]
- 4.Tovar-Méndez A, Miernyk JA, Randall DD. Regulation of pyruvate dehydrogenase complex activity in plant cells. Eur J Biochem. 2003;270(6):1043–1049. doi: 10.1046/j.1432-1033.2003.03469.x. [DOI] [PubMed] [Google Scholar]
- 5.Nunes-Nesi A, Araújo WL, Obata T, Fernie AR. Regulation of the mitochondrial tricarboxylic acid cycle. Curr Opin Plant Biol. 2013;16(3):335–343. doi: 10.1016/j.pbi.2013.01.004. [DOI] [PubMed] [Google Scholar]
- 6.Buchanan BB, Balmer Y. Redox regulation: A broadening horizon. Annu Rev Plant Biol. 2005;56:187–220. doi: 10.1146/annurev.arplant.56.032604.144246. [DOI] [PubMed] [Google Scholar]
- 7.Banze M, Follmann H. Organelle specific NADPH thioredoxin reductase in plant mitochondria. J Plant Physiol. 2000;156(1):126–129. [Google Scholar]
- 8.Laloi C, et al. Identification and characterization of a mitochondrial thioredoxin system in plants. Proc Natl Acad Sci USA. 2001;98(24):14144–14149. doi: 10.1073/pnas.241340898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gelhaye E, et al. A specific form of thioredoxin h occurs in plant mitochondria and regulates the alternative oxidase. Proc Natl Acad Sci USA. 2004;101(40):14545–14550. doi: 10.1073/pnas.0405282101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yoshida K, Noguchi K, Motohashi K, Hisabori T. Systematic exploration of thioredoxin target proteins in plant mitochondria. Plant Cell Physiol. 2013;54(6):875–892. doi: 10.1093/pcp/pct037. [DOI] [PubMed] [Google Scholar]
- 11.Balmer Y, et al. Thioredoxin links redox to the regulation of fundamental processes of plant mitochondria. Proc Natl Acad Sci USA. 2004;101(8):2642–2647. doi: 10.1073/pnas.0308583101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sweetlove LJ, Beard KFM, Nunes-Nesi A, Fernie AR, Ratcliffe RG. Not just a circle: Flux modes in the plant TCA cycle. Trends Plant Sci. 2010;15(8):462–470. doi: 10.1016/j.tplants.2010.05.006. [DOI] [PubMed] [Google Scholar]
- 13.Bykova NV, Rasmusson AG, Igamberdiev AU, Gardeström P, Møller IM. Two separate transhydrogenase activities are present in plant mitochondria. Biochem Biophys Res Commun. 1999;265(1):106–111. doi: 10.1006/bbrc.1999.1627. [DOI] [PubMed] [Google Scholar]
- 14.Outten CE, Culotta VC. A novel NADH kinase is the mitochondrial source of NADPH in Saccharomyces cerevisiae. EMBO J. 2003;22(9):2015–2024. doi: 10.1093/emboj/cdg211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Møller IM, Rasmusson AG. The role of NADP in the mitochondrial matrix. Trends Plant Sci. 1998;3(1):21–27. [Google Scholar]
- 16.Møller IM. Plant mitochondria and oxidative stress. Electron transport, NADPH turnover and metabolism of reactive oxygen species. Annu Rev Plant Physiol Plant Mol Biol. 2001;52:561–591. doi: 10.1146/annurev.arplant.52.1.561. [DOI] [PubMed] [Google Scholar]
- 17.Havelund JF, Thelen JJ, Møller IM. Biochemistry and proteomics of mitochondria from non-photosynthetic tissues. Front Plant Sci. 2013;4:51. doi: 10.3389/fpls.2013.00051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.König AC, Hartl M, Boersema PJ, Mann M, Finkemeier I. The mitochondrial lysine acetylome of Arabidopsis. Mitochondrion. 2014;19(Pt B):252–260. doi: 10.1016/j.mito.2014.03.004. [DOI] [PubMed] [Google Scholar]