Significance
IgG fragment crystallizable domain (Fc) sialylation has emerged as an important but controversial concept for regulating anti-inflammatory activity of antibodies. Moreover, translating this concept to potent anti-inflammatory therapeutics has been hampered by the difficulty of generating suitable sialylated products for human use. We describe for the first time, to our knowledge, the development of a robust, scalable process to generate a sialylated intravenous immunoglobulin (IVIg) drug candidate with maximum Fc sialylation devoid of unwanted modifications. By using a wide panel of physicochemical analytics and in vivo models, we have validated the quality and potent anti-inflammatory activity of this clinical candidate. This report not only confirms the controversial anti-inflammatory activity of IgG-Fc sialylation, it also represents the first sialylated IVIg preparation, to our knowledge, with consistent anti-inflammatory potency suitable for clinical development.
Keywords: IVIg, sialylation, antibody, inflammation, autoimmune disease
Abstract
Despite the beneficial therapeutic effects of intravenous immunoglobulin (IVIg) in inflammatory diseases, consistent therapeutic efficacy and potency remain major limitations for patients and physicians using IVIg. These limitations have stimulated a desire to generate therapeutic alternatives that could leverage the broad mechanisms of action of IVIg while improving therapeutic consistency and potency. The identification of the important anti-inflammatory role of fragment crystallizable domain (Fc) sialylation has presented an opportunity to develop more potent Ig therapies. However, translating this concept to potent anti-inflammatory therapeutics has been hampered by the difficulty of generating suitable sialylated products for clinical use. Therefore, we set out to develop the first, to our knowledge, robust and scalable process for generating a well-qualified sialylated IVIg drug candidate with maximum Fc sialylation devoid of unwanted alterations to the IVIg mixture. Here, we describe a controlled enzymatic, scalable process to produce a tetra-Fc–sialylated (s4-IVIg) IVIg drug candidate and its qualification across a wide panel of analytic assays, including physicochemical, pharmacokinetic, biodistribution, and in vivo animal models of inflammation. Our in vivo characterization of this drug candidate revealed consistent, enhanced anti-inflammatory activity up to 10-fold higher than IVIg across different animal models. To our knowledge, this candidate represents the first s4-IVIg suitable for clinical use; it is also a valuable therapeutic alternative with more consistent and potent anti-inflammatory activity.
Intravenous immunoglobulin (IVIg) is a therapeutic blood product prepared from the pooled plasma of 3,000–60,000 healthy donors per batch (1–3). It is a complex heterogeneous mixture of IgG subclasses and low amounts of IgA, IgM, and other plasma proteins (4). IVIg contains a wide array of antibodies expected to be present in human serum, and the large number of donors ensures diversity in the Ig repertoire that far exceeds that of an individual donor (5).
IVIg has been used for more than 30 years for the treatment of a variety of acute and chronic autoimmune and systemic inflammatory diseases (2–5). Although efficacious in these diseases, the precise mechanism of action of IVIg is not well-understood. Various studies have documented a series of nonmutually exclusive mechanisms modulating components of the innate and adaptive immune system (4, 6). For example, IVIg has been shown to mediate anti-inflammatory responses through its action on dendritic cells, natural killer cells, regulatory T cells, B cells, and the monocyte/macrophage system, and through its suppression or neutralization of soluble factors, such as inflammatory cytokines, chemokines, and pathogenic autoantibodies (5–7).
Although beneficial in numerous indications, IVIg preparations have distinct limitations, such as variable efficacy, clinical risks, high costs, and finite supply (2, 3, 8). Different IVIg preparations are frequently treated as interchangeable products clinically, but it is well-known that significant differences in product preparations exist that may impact tolerability and activity in selected clinical applications (9). At the current maximal dosing regimens, only partial and unsustained responses are obtained in many instances (2, 4). In addition, the long infusion times (4–6 h) associated with the high volume of IVIg treatment consume significant resources at infusion centers (8) and negatively affect patient-reported outcomes, such as convenience and quality of life (10). Developing therapeutic alternatives that could leverage the broad biological activities of IVIg and simultaneously, provide more consistent and potent anti-inflammatory activity with minimal inconvenience would be highly valuable to clinicians and patients.
The IgGs in IVIg are composed of a fragment antigen-binding domain (Fab fragment) that facilitates the selective interaction with specific antigens and a fragment crystallizable domain (Fc fragment) that interacts with cellular receptors (Fc receptors) known to play critical functions in modulating the activation state of immune cells (11). The IgG Fc fragment contains a conserved N-linked glycan at position N297. The core of the N297 N-linked glycan is composed of two GlcNAc and three mannose residues. Typically, this core can be further extended with fucose, galactose, sialic acid, and bisecting GlcNAc monosaccharides through selective enzymatic glycosylation reactions (12, 13). Alterations to the N297 glycan composition have been shown to have a significant impact in modulating the interaction between the IgG Fc fragment and the Fc receptors. For example, removal of fucose from the IgG N-glycan core has been shown to increase its affinity for Fc gamma receptor IIIa (FcγRIIIa), leading to enhanced antibody-dependent cellular cytotoxicity (14). Sialylation, the addition of terminal sialic acid to N297 glycan, has also been shown to decrease the affinity for type I Fc receptors and increase the affinity for type II Fc receptors (11).
In the past three decades, a wealth of reports has documented alterations in antibody glycosylation associated with different diseases in humans. Among these reports, changes in antibody sialylation have been associated with the evolution of autoimmune and inflammatory diseases. For example, rheumatoid arthritis and juvenile idiopathic arthritis are associated with decreased levels of IgG sialylation (15, 16). Additional studies have shown that this translates particularly to the pathogenic antibodies in inflamed joints of arthritis patients (17). It has also been shown that IgG sialylation increases during pregnancy and that this increase may be associated with the remission of rheumatoid arthritis during pregnancy (18, 19). In addition, pathogenic proteinase 3 autoantibodies are less sialylated in patients with active Wegener’s vasculitis (granulomatosis with polyangiitis) (20).
Alterations in endogenous IgG sialylation have been associated with treatment response in inflammatory/autoimmune diseases. For example, in patients with Kawasaki disease treated with IVIg, increased levels of endogenous human IgG sialylation decreased the likelihood of IVIg treatment resistance (defined as persistent or recrudescent fever at least 36 h after the completion of IVIg infusion) (21). Similarly, in patients with Guillain–Barré syndrome, those with more severe forms of the disorder showed a lower level of IgG sialylation, despite IVIg treatment (22).
Translation of these natural observations in humans to therapeutic options was first realized in 2006, when it was proposed that high doses (>1 g/kg) of unfractionated IVIg were required to elicit sufficient anti-inflammatory activity because of a limited concentration of sialylated IgG in the total IVIg preparation. This theory was substantiated in a mouse model of arthritis that showed similar levels of anti-inflammatory activity when using 1 g/kg IVIg and 0.1 g/kg sialic acid-enriched IVIg, therefore indicating a 10-fold enhancement with the addition of terminal sialic acids (23, 24). Additional results supporting this theory were subsequently reported in other independent studies and other animal models (25, 26). It was further shown that the anti-inflammatory activity of sialylation can be recapitulated using a sialylated Fc fragment derived from IVIg or an IgG1 recombinant antibody (after in vitro sialylation of the Fc) at a 30-fold lower dose than IVIg (24, 27). These results opened the possibility of developing sialylated IVIg and other sialylated antibodies with enhanced anti-inflammatory properties.
In recent years, different reports have debated the anti-inflammatory benefits of sialylation. For example, studies using desialylated IVIg in immune thrombocytopenic purpura (ITP) models (28) and Sambucus nigra agglutinin (SNA) -enriched IVIg in arthritis models (29) have shown that sialylation does not enhance IVIg activity or that it may even be dispensable for its therapeutic effects. On the contrary, more comprehensive studies performed by several independent laboratories testing desialylated or hypersialylated IVIg across different animal models under preventive and therapeutic treatment modalities have shown that sialylation is critical for the anti-inflammatory activity of IVIg (24–27, 30). Furthermore, T cell-independent vaccinations resulted in the generation of hypersialylated immunomodulatory antibodies that were able to modulate other immune responses, establishing a broad relevance of these sialylated IgG glycoforms as modulators of immune responses (31). Notably, none of the previous studies used precisely the same protocols for enriching or depleting sialic acid-containing IgG glycoforms, which may partially explain the discrepancies of these studies. Thus, the major aim of this study was to use rigorous, controlled processes and quality controls to investigate the potential of hypersialylated IVIg as a drug candidate with enhanced therapeutic activity.
We describe a robust, controlled sialylation process to generate tetra-Fc–sialylated IVIg and show that this process yields a product with consistent enhanced anti-inflammatory activity. Specifically, we first observed that the sialylated IVIg was at least 10 times more potent than the parent IVIg product in a model of collagen antibody-induced arthritis (CAIA) using a prophylactic dose. We further confirmed this enhanced anti-inflammatory activity with therapeutic dosing in models of K/BxN serum-induced arthritis and ITP, and a prophylactic model of skin-blistering disease. Importantly, we noted that, without a tightly controlled process of sialylation, unwanted side products can accumulate that may be responsible for the inconsistent activity observed in previous studies. Therefore, the process that we describe combined with sensitive controls to generate a tetra-Fc–sialylated IVIg (s4-IVIg) devoid of undesired modifications has been critical to obtain the consistent enhanced anti-inflammatory activity and serves as the first example, to our knowledge, of a therapeutic candidate for product development.
Results
Optimization of Sialylation Process: Maximizing Fc Sialylation.
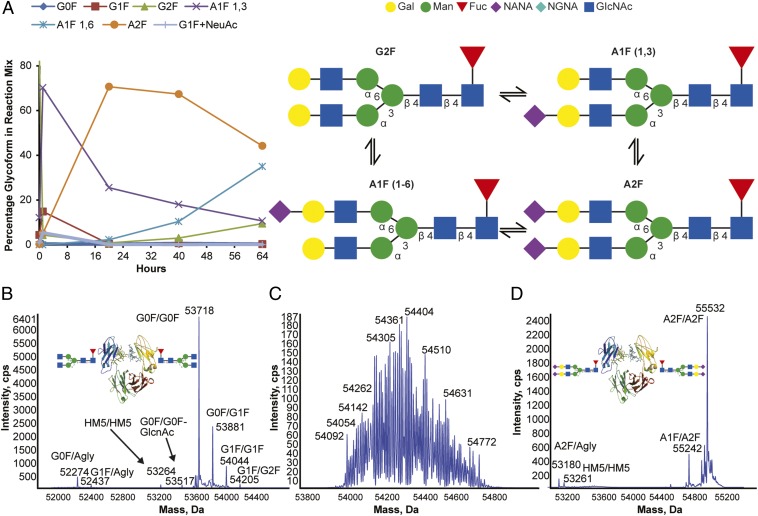
In our goal to develop a sialylated IVIg product with drug properties suitable for clinical development, we focused on establishing a robust sialylation process with one primary goal: to maximize the enzymatic incorporation of sialic acids while limiting the introduction of undesired alterations to the product. To achieve this goal, we focused on optimizing the enzymatic reactions to maximize the disialylated [i.e., the disialylated and fucosylated glycan (A2F)] content in the product, leading to the introduction of four sialic acids per Fc fragment (two sialic acids per A2F glycan in each chain). For this optimization, we used a recombinant human IgG1 Fc as a surrogate substrate for initial development. As described previously (24), α2–6 Fc sialylation can be achieved by reacting an Fc-containing substrate molecule with β1,4 galactosyl transferase-1 (B4GalT) and α2,6-sialyltransferase (ST6Gal1) enzymes in the presence of the corresponding sugar nucleotide substrates [uridine diphosphate galactose (UDP-Gal) and cytidine 5′ monophospho-N-acetyl neuraminic acid (CMP-NANA)]. Interestingly, we rapidly realized that, under our initial reaction conditions, ST6Gal1 can not only catalyze the transfer of the sialic acid from CMP-NANA sugar nucleotide to the Fc glycan but also, facilitate the removal of the sialic acid from the sialylated product. As shown in Fig. 1A, the sialylation of the α1,3 branch of the biantennary glycan (to form A1F-1,3) was rapid and essentially complete by 30 min, whereas the doubly sialylated species (A2F) formed at an ∼10× slower rate and stopped accumulating by 24 h. After 24 h, a monosialylated species with the sialic acid on the α1,6 branch (A1F-1,6) started to form and continued accumulating steadily, reaching ∼35% of glycosylated species by 64 h. Concomitantly, A2F exhibited a steady decline from 71% at 20 h to 44% at 64 h, suggesting that the A1F-1,6 glycoform was generated from the removal of sialic acid residues on the more exposed α1,3 branch of disialylated A2F. Additional incubation led to the cleavage of the 1,6 sialic acid in the A1F-1,6 glycoform, generating the asialylated but fully galactosylated and fucosylated species G2F. G2F, which was present in trace amounts at the beginning of the reaction, appeared at measurable amounts at 40 h and continued increasing, reaching 15% by 64 h as the levels of A2F and A1F-1,6 declined. This result further showed that extended incubation results in cleavage of the α1,6 sialic acid (Fig. 1A).
Fig. 1.
Optimization of sialylation process leads to a sialylated Fc with symmetrical A2F/A2F Fc glycoforms (four sialic acids per Fc) devoid of undesired modifications. (A) Time course of recombinant human IgG1 Fc-sialylated N-glycoform formation in the presence of CMP-NANA and ST6GalT1. Galactosylated recombinant human Fc was incubated with 20 mM CMP-NANA and 0.3 U/mg ST6GalT1 at 37 °C. Aliquots were removed at different time points, and the relative proportions of Fc glycoforms were determined by liquid chromatography–MS/MS. (B) Intact size exclusion chromatography (SEC) -MS of recombinant Fc before sialylation. (C) Intact SEC-MS of sialylated recombinant Fc after suboptimal sialylation reaction. Product is sialylated, but significant modifications lead to undesirable heterogeneity. (D) Intact SEC-MS of sialylated recombinant Fc after optimal sialylation reaction. cps, Cycles per second; NGNA, N-glycolylneuraminic acid.
These observations were critical in optimizing the parameters to maximize the yield of the A2F species while minimizing the A1F-1,3, A1F-1,6, and G2F glycoforms. By evaluating a matrix of parameters affecting the transient state of the G2FA1F-1,3A2FA1F-1,6G2F glycoform distribution, we found that the desialylation component of the reaction (A2FA1F-1,6G2F) was facilitated by spontaneous decomposition of CMP-NANA in the reaction. Therefore, we envisioned that replenishing the CMP-NANA in the reaction could help maximize the A2F yield and found that periodic dosing of fresh CMP-NANA maximized the A2F glycan levels after 24 h without the formation of A1F-1,6 or G2F species. These findings were subsequently confirmed in reactions with IVIg (Fig. S1).
Optimization of Sialylation Process: Minimizing Undesired Modifications.
Another main goal of the process optimization was to minimize the introduction of undesired modifications to the product during the enzymatic sialylation. Not only is this critical for the development of a product destined for clinical use, but it is important when aiming to develop a complex mixture product with consistent anti-inflammatory activity. For example, one of the key outstanding questions raised in previous publications is whether the process used to enrich for sialylated Igs could affect other important attributes of product quality.
During our optimization of the enzymatic sialylation process, we observed significant heterogeneity in the sialylated recombinant Fc product, suggesting that additional modifications beyond sialylation were imprinted onto the molecule during the process (Fig. 1 B and C). More detailed peptide liquid chromatography–tandem MS analyses identified several modifications, including different advanced glycation end products (AGEs) that have modified arginine and lysine residues (Fig. S2). The most abundant of these modifications is 5-methylimidazol-4–one, although other modifications, such as carboxymethyl arginine and imidazolidine, were also observed. In addition, aggregates were observed in the protein along with increased internal fluorescence. This internal fluorescence is consistent with the presence of cross-linking AGEs, such as pentosidine, which cross-link lysine and arginine. Given the well-documented negative effects of AGEs on human health (32, 33), we set out to investigate the source of these modifications.
The sialylation process requires two sugar nucleotides (UDP-Gal and CMP-NANA), two enzymes (B4-GalT and ST6-Gal1), and buffer systems containing the cofactor manganese chloride; therefore, we evaluated the influence of these reagents and reaction conditions for the imprinting of undesired modifications onto the product backbone. Although we found that different reagents used in the sialylation reaction (such as UDP-Gal and manganese chloride) could promote backbone modifications, enzyme quality was the predominant factor responsible for these modifications. Based on the nature of the modifications imprinted onto the product, we sought to investigate the host cell proteome of the recombinant-derived enzymes used in our original nonoptimized sialylation reaction. Proteomic analysis of the sialyltransferase enzyme preparations identified different glycolytic enzymes as background proteins in addition to the sialyltransferase that, according to previous reports (34), could contribute to the formation of AGEs found on the sialylated recombinant human IgG1 Fc product (Table S1). Based on these observations, we optimized the preparation of B4GalT and ST6Gal1 enzymes with high purity and activity. As described in Materials and Methods, the enzymes were effectively produced in a transient HEK expression system and purified through cation exchange and affinity chromatography. The optimized enzyme production process resulted in highly active enzymes devoid of host cell proteins that could facilitate the introduction of undesired backbone modifications to the product. After optimization of these parameters, the achieved product had a predominantly tetrasialylated Fc glycoform (disialylated glycans on both heavy chains) and was devoid of undesired modifications (Fig. 1D).
Large-Scale Preparation and Physicochemical Characterization of s4-IVIg.
The optimized sialylation process was subsequently applied to IVIg for the production of an s4-IVIg. Given that previous approaches to generate sialic acid-enriched IVIg by enrichment of the sialylated IgG fraction have been shown to induce specific enrichment/depletion of selected antibodies within IVIg (35), we used a flow-through purification workflow to eliminate non-IVIg components introduced during the sialylation reaction. As described in Materials and Methods, the purification workflow was composed of a cation exchange column and a Blue Trisacryl (PALL Life Sciences) column connected in series. The Blue Trisacryl column is composed of a Cibacron Blue affinity chromatography reagent that enables the retention of enzymes and sugar nucleotides. The optimized process was applied to IVIg and scaled up from low microgram to multigram, with yields of up to 94%.
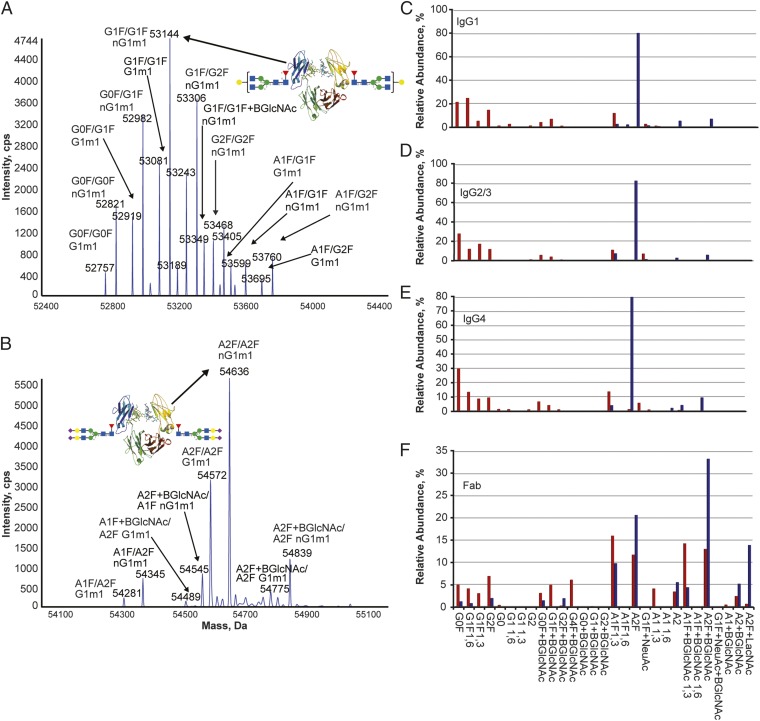
Given the broad heterogeneity of IVIg and the limited physicochemical description typically used in the industry to depict these products, we developed a comprehensive characterization panel to clearly qualify the sialylated IVIg product. IVIg was extensively analyzed before and after sialylation. We first confirmed that application of the optimized sialylation process resulted in a sialylated IVIg with symmetrical tetrasialylated Fc similar to results obtained with the recombinant Fc prototype (Fig. 2 A and B). The intact mass spectrometric data of the sialylated Fc also reflected the distribution of sialylated Fc on the IgG allotypes found naturally in IVIg. More detailed analyses of glycosylation were performed in an isotype- and site-specific manner to discriminate the different Fc isotypes and Fab glycosylation. As shown in Fig. 2 C–E, Fc glycosylation was changed from predominantly asialylated species (e.g., G0F and G1F) in the starting IVIg material to more than 90% disialylated species (e.g., A2F, A2, and A2F+BGlcNAc) in each IgG isotype. Analysis of the Fab glycans further revealed that, although the IVIg starting material contained a significant distribution of monosialylated (∼35%) and disialylated (∼35%) glycans in the Fab, the distribution shifted to a higher level of disialylated glycans upon sialylation (∼75%) (Fig. 2F).
Fig. 2.
Application of sialylation process to IVIg alters Fc glycosylation of all IgG isotypes and Fab glycans to generate IgG Fc bearing primarily four sialic acid residues. IVIg is shown in red, and s4-IVIg is shown in blue. (A) Deconvoluted mass spectrum for papain-digested IVIg. (B) Deconvoluted mass spectrum for papain-digested s4-IVIg. (C) Glycosylation profile of IgG1 isotypes in IVIg and s4-IVIg. (D) Glycosylation profile of IgG2/3 isotypes in IVIg and s4-IVIg. (E) Glycosylation profile of IgG4 isotypes in IVIg and s4-IVIg. (F) Fab glycosylation profile for IVIg and s4-IVIg. cps, Cycles per second.
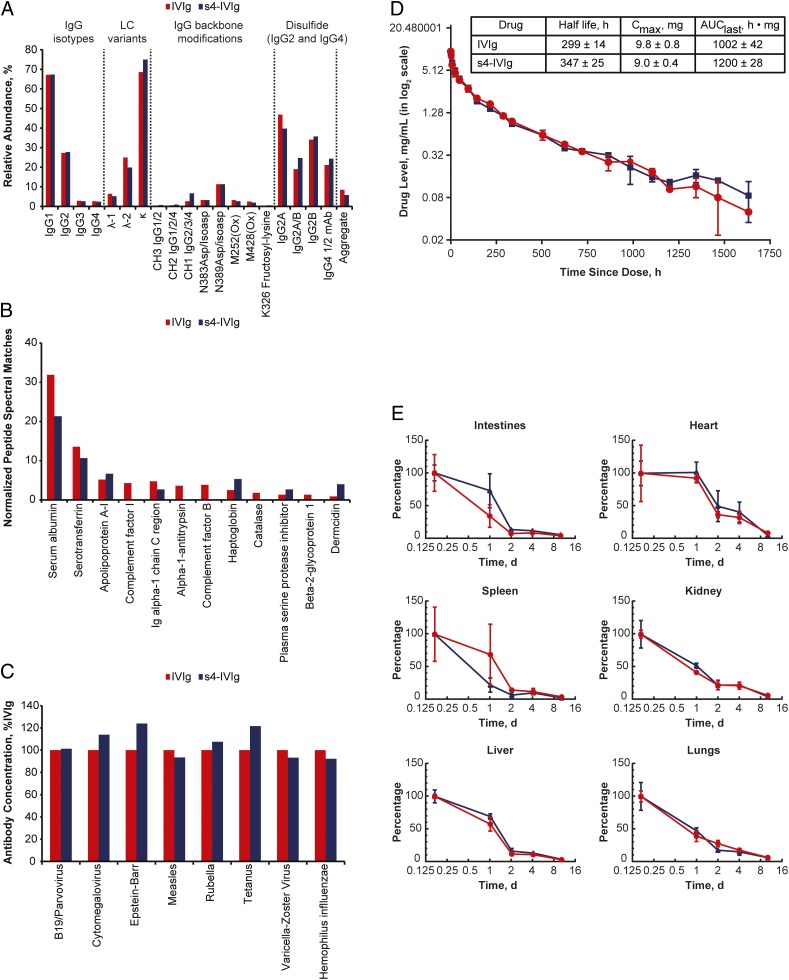
As discussed previously, we also wanted to ensure that other properties of the IVIg mixture were not negatively impacted during the sialylation process. To this end, we performed a detailed analysis of a wide panel of quality attributes in the starting material and the product, including distribution of IgG isotypes, distribution of κ- and λ-chain IgGs, IgG4 half-antibodies, IgG2 disulfide isoforms, and site-specific backbone modifications, such as oxidation and deamidation, aggregation state, and proteolytic cleavage. As shown in Fig. 3A, optimization of the process performed with the recombinant Fc material translated effectively to generation of the s4-IVIg, because all of the proteinaceous components of the IVIg mixture remained relatively unchanged during the process. Given that IVIg is known to contain other non-IgG protein impurities, we also monitored for the impact of the sialylation process on the non-IgG proteome. This analysis primarily showed a small decrease in most of the non-IgG proteins in the s4-IVIg product that reflected the effects of additional purification steps in the process (Fig. 3B). Because large changes in IVIg antigen-binding specificity have been observed for SNA-enriched IVIg preparations (28), we evaluated the impact of our sialylation process on antigen-binding specificity. As shown in Fig. 3C, no major differences were observed between the parent and sialylated IVIg in terms of antigen-binding specificity. Furthermore, because the process is always performed under aseptic conditions, low endotoxin levels are consistently achieved in the final product (<7 EU/g), s4-IVIg.
Fig. 3.
Sialylation reaction does not substantially alter the composition or the pharmacokinetics and biodistribution parameters of IVIg. IVIg is shown in red, and s4-IVIg is shown in blue. (A) Comparison of IgG protein backbone variants and posttranslational modifications for IVIg and s4-IVIg. (B) Distribution of non-IgG proteins in IVIg after depletion of IgG by protein G. Proteins other than monomeric IgG represent less than 2% of the total IVIg mixture. (C) Comparison of antigen-binding specificity for IVIg and s4-IVIg. (D) Pharmacokinetics profiles of IVIg and s4-IVIg dosed at 0.6 g/kg i.v. (E) Organ biodistribution analysis of IVIg and s4-IVIg in WT mice. AUC, area under the curve; LC, light chain.
Pharmacokinetics and Biodistribution of s4-IVIg.
As part of our interest in minimizing negative alterations to the IVIg drug, we first evaluated the pharmacokinetics and biodistribution properties of s4-IVIg. IVIg and s4-IVIg were administered as a single dose at 0.6 g/kg, and blood samples were collected for 68 d. Human Ig levels were determined by ELISA. Results showed no significant differences in pharmacokinetics parameters between IVIg and s4-IVIg (Fig. 3D). Additional analyses comparing the organ-specific biodistribution of fluorescence-labeled IVIg and s4-IVIg in mice also confirmed similar tissue accumulation between IVIg and s4-IVIg at the organ-specific level (Fig. 3E). These results are consistent with the fact that s4-IVIg is not significantly modified from the parent IVIg but that the overall sialylation content has been increased in the IgG mixture.
Biological Efficacy of s4-IVIg Candidate in Different Arthritis Models.
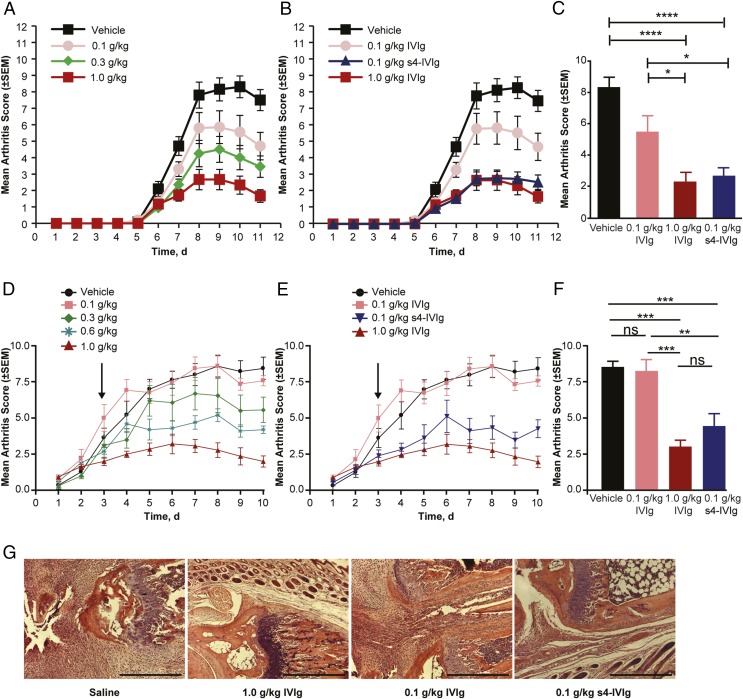
To evaluate the anti-inflammatory activity of the s4-IVIg drug candidate, we first tested this product in two different models of arthritis. In the first model (K/BxN), IVIg activity was reported to be sialylation-dependent (23, 24). The second model (CAIA) was recently reported to be sialylation-independent (29). For these and all in vivo studies, the s4-IVIg product was compared with the same lot of IVIg used as starting material across different models (Figs. 2 and 3 show physicochemical characterization of both materials). To compare these materials, we first performed a detailed dose–response study in each model to identify the linear range of the dose–response. Optimal doses within the linear range of the response were used to compare IVIg and s4-IVIg. This dose optimization is an important element of these studies, because in some previous reports, IVIg has been compared with SNA-enriched or desialylated IVIg outside the linear range of the response; therefore, any effect of IVIg sialylation would be difficult to conclude (29). As shown in Fig. 4 A and D, when a prophylactic or therapeutic dose of IVIg was administered in the CAIA or K/BxN model, IVIg showed a good linear dose–response between 0.1 g/kg and 1 g/kg. As shown in Fig. 4 B and C, s4-IVIg at 0.1 g/kg showed protection similar to that of IVIg at 1 g/kg when dosed prophylactically in the CAIA model. Similar results were observed in the K/BxN arthritis model when s4-IVIg and IVIg were dosed therapeutically (Fig. 4 E and F). H&E staining of ankle joints of K/BxN-induced arthritis mice treated with IVIg and s4-IVIg also revealed extensive neutrophil infiltration in animals treated with saline and IVIg at 0.1 g/kg but limited infiltration in groups treated with IVIg at 1.0 g/kg and s4-IVIg at 0.1 g/kg (Fig. 4G).
Fig. 4.
s4-IVIg drug candidate shows enhanced anti-inflammatory potency in two different models of arthritis. (A–C) Results from the CAIA arthritis model. (D–G) Results from the K/BxN arthritis model. (A) IVIg dose–response in CAIA. (B and C) Comparison of 0.1 g/kg s4-IVIg with IVIg at 0.1 and 1 g/kg in CAIA dosed prophylactically (on day 1). (C) Mean arthritis score measured at day 10. (D) IVIg dose–response in the K/BxN model. (E and F) Comparison of 0.1 g/kg s4-IVIg with IVIg at 0.1 and 1 g/kg in the K/BxN model dosed in therapeutic mode. Arrows indicate time of dosing for IVIg/s4-IVIg. (F) Mean arthritis score measured at day 10. (G) Representative H&E staining of ankle joints of K/BxN-induced arthritis mice treated with saline, IVIg (1.0 and 0.1 g/kg), and s4-IVIg (0.1 g/kg). Extensive neutrophil infiltration is observed in the saline-treated and 0.1 g/kg IVIg-treated mice, but limited infiltration is observed in the groups treated with IVIg at 1.0 g/kg and s4-IVIg at 0.1 g/kg. ns, Not significant. *P < 0.05; **P < 0.01; ***P < 0.001; ****P < 0.0001.
Biological Efficacy of s4-IVIg Candidate in the ITP Model.
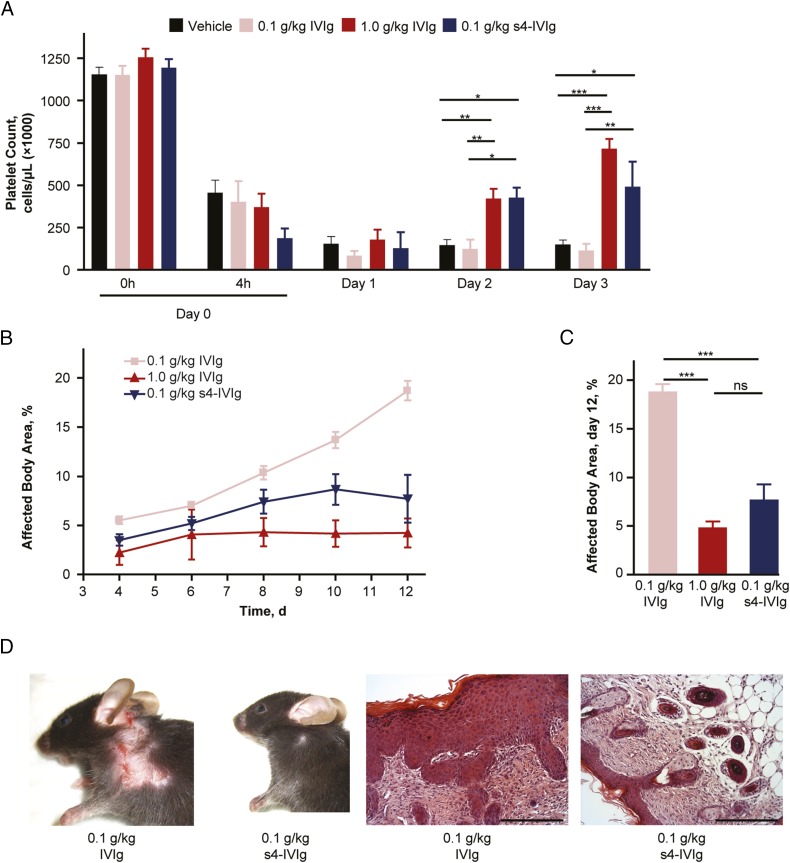
As performed in the arthritis models, we first established the dose–response profile for IVIg in the ITP model (Fig. S3). After maximum platelet depletion with the antiplatelet antibody, mice were treated with IVIg from 0.1 to 1 g/kg, and platelet levels were measured on the day of the IVIg treatment (day 1) and the two subsequent days after treatment (days 2 and 3 of the experiment). A clear dose–response was observed in this model within this range (Fig. S3). Complete activity of IVIg was lost at 0.1 g/kg. Similar to the effects observed in the arthritis models, comparing s4-IVIg and IVIg in the ITP model, a clear enhanced efficacy was observed. As shown in Fig. 5, therapeutic treatment with s4-IVIg at 0.1 g/kg returned platelet levels at days 2 and 3 to levels similar to those obtained with IVIg at 1 g/kg.
Fig. 5.
s4-IVIg shows enhanced potency in ITP when treated therapeutically and epidermolysis bullosa acquisita (EBA) pemphigoid mouse models when treated prophylactically. (A) Comparison of therapeutic dosing of 0.1 g/kg s4-IVIg with IVIg at 0.1 and 1 g/kg. (B and C) Comparison of 0.1 g/kg s4-IVIg with IVIg at 0.1 and 1 g/kg in the EBA pemphigoid mouse model. (D, Left) Representative day 12 pictures and (D, Right) H&E-stained skin sections are shown for IVIg- and s4-IVIg–treated groups at 0.1 g/kg. ns, Not significant. (Scale bar: 100 μm.) *P < 0.05; **P < 0.01; ***P < 0.001.
Biological Efficacy of s4-IVIg Candidate in Autoimmune Skin-Blistering Epidermolysis Bullosa Acquisita Pemphigus.
We also used an Fcγ receptor-dependent skin-blistering disease caused by collagen type VII-specific autoantibodies (36) to evaluate the efficacy of s4-IVIg. Prophylactic dosing with IVIg between 0.1 and 1 g/kg at the onset of the disease (day 4) resulted in a clear dose–response within this dose range, which was measured by the percentage of affected body area (Fig. S3). In a separate experiment, s4-IVIg was compared with IVIg across the doses in the linear range of the response. As shown in Fig. 5 B and C, although IVIg lost its activity between 0.3 and 0.1 g/kg, s4-IVIg displayed good efficacy at these doses, thus showing a stronger effect than that of IVIg in this model. Furthermore, marked reduction of skin inflammation and recruitment of inflammatory effector cells were evident in animals treated with the s4-IVIg preparation (Fig. 5D).
Discussion
As the interplay between specific carbohydrate structures and protein function continues to be unraveled, promising examples of the translation of this evolving field into actual therapeutics have begun to emerge. Two prime examples of successful therapeutic development leveraging the effects of protein glycosylation are the development of a long-acting erythropoietin (darbepoetin alfa; Aranesp; Amgen) with increased sialylation content, which reduces the clearance facilitated by the asialoglycoprotein receptor (37), and the development of an anti-CD20 antibody (obinutuzumab; GAZYVA; Genentech) that is devoid of Fc fucosylation, which has enhanced effector function and antitumor properties (38). Identification of the important role of terminal sialic acid-containing IgG glycoforms for IVIg activity in 2006 (23) and its confirmation by several independent laboratories in the past few years in a variety of independent in vivo models (23–27) have opened the door to the development of new anti-inflammatory therapeutics.
Despite this convincing evidence, some studies using different or identical in vivo model systems showed sialic acid-independent pathways of IVIg activity (28, 29). However, none of these studies can be compared directly because of the use of different protocols and experimental conditions. For example, the use of desialylated IVIg generated with unqualified desialylation processes (potentially leading to IVIg preparations with residual sialylation content) or alteration to the composition of the IVIg mixture during desialylation or purification could potentially explain the lack of requirement of sialylation in the model of inflammatory arthritis (28, 29). Additionally, some studies comparing the biological activity of IVIg and desialylated IVIg used fairly high doses outside the linear range of dose–response, which complicates interpretation of the results because of the complex and multiple mechanisms of IVIg (29). Furthermore, some of these studies used lectin columns to enrich intact IVIg preparations for sialylation, resulting in a predominant enrichment of specific antibodies and IgG glycovariants with enhanced Fab2 and not Fc sialylation, providing yet another potential explanation for a lack of activity (28). Some of these issues, such as ensuring that the properties of the IVIg mixture were not negatively affected during the sialylation and purification processes, were addressed in our study. Furthermore, optimal doses within the linear range of the response were used to compare IVIg and s4-IVIg, thus enabling the effect of IVIg sialylation to be accurately assessed.
Despite these discrepancies, translating the beneficial effects of Fc sialylation to a real therapeutic alternative has been limited by the lack of a robust industrial process enabling large-scale production of sialylated Igs with desired features essential for additional clinical development. In fact, none of the previous studies used industry-level quality control standards to ensure that the resultant hypersialylated product did not contain undesired alterations that could interfere with its activity. As has been shown here, detailed analysis of IgG glycoforms arising through a standard assay using a 2,6 sialic acid transferase established that a variety of AGEs, including 5-methylimidazol-4-one and aggregates, can be easily introduced if using unqualified processes. All of these side products would impede the use of this preparation in the clinic, because AGEs have the potential to be immunogenic (32, 33, 39–41), and the formation of aggregates may result in the development of immune-related safety concerns, such as cytokine-release syndrome (7, 42). In addition, these side products could diminish the sialic acid-dependent immunomodulatory activity.
By establishing industrial-scale protocols and quality control steps, we generated for the first time, to our knowledge, a tetrasialylated IVIg preparation with an unprecedented level of purity. This s4-IVIg showed ∼10-fold enhancement of activity in four independent in vivo model systems, thus confirming the initial results published in 2006 (23). In contrast to other studies that failed to show enhanced activity of a sialic acid-enriched IVIg in the ITP model and that used IVIg enriched by SNA affinity chromatography (28), we were able to show 10-fold improved activity with our s4-IVIg in this model. This finding is of special interest, because there is evidence that enriching IgG with the use of SNA does not increase amounts of the rare sialylated IgG glycoforms containing two sialic acid residues per sugar moiety (43). In contrast, our protocol results in more than 90% glycovariants with two sialic acid residues per sugar moiety, which may suggest that this tetrasialylated IgG glycovariant has the highest anti-inflammatory activity.
In summary, our results show that highly pure and homogeneous s4-IVIg preparations are critical to obtain a consistently higher level of activity across a variety of animal models under preventive and therapeutic treatment conditions. Nonetheless, we agree with the conclusions of other studies suggesting that different pathways of IVIg activity may be operative, depending on the type of autoimmune disease (5, 7, 44). To maintain all possible Fab- and Fc-dependent functions, we chose to hypersialylate the intact IVIg molecule and ensured that the breadth of antibody specificities and isotypes were maintained in this preparation. Taken together, our results provide the basis for large-scale production of an IVIg product that has enhanced anti-inflammatory activity, whereas it maintains other possible functions. Based on our in vivo studies, patients with skin-blistering diseases or ITP may be attractive candidates for the first in-human trial. Patients with chronic inflammatory demyelinating polyneuropathy may also be an interesting target population, because they respond to IVIg therapy with an up-regulation of the inhibitory FcγR (45), which was shown to be a critical IVIg-triggered effector pathway to dampen autoantibody activity in murine models (46, 47). Furthermore, given the documented effects of Fc sialylation in reducing IgG antibody-dependent cellular cytotoxicity activity, it may also be possible to use this enzymatic sialylation strategy to develop therapeutic antibodies with reduced effector functions.
Materials and Methods
Full details are in SI Experimental Procedures.
B4GalT and ST6Gal1.
Human B4GalT1 and ST6Gal1 were produced by transient expression methods in HEK293 cells. More details are in SI Experimental Procedures.
Preparation of s4-IVIg.
Commercial-grade IVIg (GAMMAGARD; Baxter) was buffer-exchanged into 50 mM 3-(N-morpholino)propanesulfonic acid (Mops) from its formulation buffer. Buffer exchange was performed on a SciLog tangential flow filtration setup using a 30-kDa membrane, and the product was concentrated to ∼125 mg/mL for the sialylation reaction. Buffer-exchanged IVIg was incubated from 40 to 48 h at 37 °C with 50 mM Mops (pH 7.4), 8 mM MnCl2, 5 mM UDP-Gal, and B4GalT (15 mU/mg protein) at an IVIg concentration of ∼110 mg/mL. Galactosylation was confirmed by peptide liquid chromatography/MS. Galactosylated IVIg was further incubated for 72 h at 37 °C with 50 mM Mops (pH 7.4), 48 mM CMP-sialic acid, and ST6Gal1 (17 mU/mg protein). s4-IVIg was buffer-exchanged and concentrated for downstream purification by a multicolumn setup to remove enzymes and sugar nucleotides used in the process. This purification was accomplished by passing the sialylated IVIg mixture in a flow-through mode over a Poros XS Cation Exchange Column (Applied Biosystems), a Blue Trisacryl Column (PALL Life Sciences), and a diatomaceous earth filter, all of which were connected in series on an AKTA Purifier. Purified s4-IVIg was buffer-exchanged into the formulation buffer (250 mM glycine, pH 4.5) and concentrated to >80 mg/mL on a 30-kDa polyethersulfone membrane.
Characterization of IVIg and s4-IVIg.
More details on the physicochemical and biological characterizations of s4-IVIg are in SI Experimental Procedures.
Supplementary Material
Acknowledgments
We thank Maurice Hains, Radouane Zouaoui, Jason Wang, and Sal Marchese for their contributions to the preparation of different batches of s4-IVIg and Humberto Tavares and Isaac Aldrich for assistance with the collagen antibody-induced arthritis studies. We thank Sandra Sipsey for assistance with enzyme preparations and Tanmoy Ganguly and Lakshmanan Thiruneelakantapillai for insightful discussions. Writing/editorial support was provided by Paul Miller (Momenta) and ApotheCom.
Footnotes
Conflict of interest statement: N.W., D.O., J.C.L., D.M., H.S., R.M., J.W.M., L.R., B.C.S., L.L., G.V.K., and C.J.B. are employees of Momenta Pharmaceuticals with stock compensation and have a patent pending. N.B., A.M., S.T., E.C., K.G., W.A., and A.M.M. are employees of Momenta Pharmaceuticals with stock compensation. F.N. has a patent issued. I.S. has nothing to disclose.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1422481112/-/DCSupplemental.
References
- 1.Imbach P. Treatment of immune thrombocytopenia with intravenous immunoglobulin and insights for other diseases. A historical review. Swiss Med Wkly. 2012;142:w13593. doi: 10.4414/smw.2012.13593. [DOI] [PubMed] [Google Scholar]
- 2.Feasby T, et al. Guidelines on the use of intravenous immune globulin for neurologic conditions. Transfus Med Rev. 2007;21(2 Suppl 1):S57–S107. doi: 10.1016/j.tmrv.2007.01.002. [DOI] [PubMed] [Google Scholar]
- 3.Anderson D, et al. Guidelines on the use of intravenous immune globulin for hematologic conditions. Transfus Med Rev. 2007;21(2 Suppl 1):S9–S56. doi: 10.1016/j.tmrv.2007.01.001. [DOI] [PubMed] [Google Scholar]
- 4.Prins C, Gelfand EW, French LE. Intravenous immunoglobulin: Properties, mode of action and practical use in dermatology. Acta Derm Venereol. 2007;87(3):206–218. doi: 10.2340/00015555-0249. [DOI] [PubMed] [Google Scholar]
- 5.Negi VS, et al. Intravenous immunoglobulin: An update on the clinical use and mechanisms of action. J Clin Immunol. 2007;27(3):233–245. doi: 10.1007/s10875-007-9088-9. [DOI] [PubMed] [Google Scholar]
- 6.Bayry J, Negi VS, Kaveri SV. Intravenous immunoglobulin therapy in rheumatic diseases. Nat Rev Rheumatol. 2011;7(6):349–359. doi: 10.1038/nrrheum.2011.61. [DOI] [PubMed] [Google Scholar]
- 7.Schwab I, Nimmerjahn F. Intravenous immunoglobulin therapy: How does IgG modulate the immune system? Nat Rev Immunol. 2013;13(3):176–189. doi: 10.1038/nri3401. [DOI] [PubMed] [Google Scholar]
- 8.Hartung HP, et al. Clinical applications of intravenous immunoglobulins (IVIg)—beyond immunodeficiencies and neurology. Clin Exp Immunol. 2009;158(Suppl 1):23–33. doi: 10.1111/j.1365-2249.2009.04024.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gelfand EW. Differences between IGIV products: Impact on clinical outcome. Int Immunopharmacol. 2006;6(4):592–599. doi: 10.1016/j.intimp.2005.11.003. [DOI] [PubMed] [Google Scholar]
- 10.Kallenberg CG. A 10% ready-to-use intravenous human immunoglobulin offers potential economic advantages over a lyophilized product in the treatment of primary immunodeficiency. Clin Exp Immunol. 2007;150(3):437–441. doi: 10.1111/j.1365-2249.2007.03520.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pincetic A, et al. Type I and type II Fc receptors regulate innate and adaptive immunity. Nat Immunol. 2014;15(8):707–716. doi: 10.1038/ni.2939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jefferis R. Glycosylation as a strategy to improve antibody-based therapeutics. Nat Rev Drug Discov. 2009;8(3):226–234. doi: 10.1038/nrd2804. [DOI] [PubMed] [Google Scholar]
- 13.Bieberich E. Synthesis, processing, and function of N-glycans in N-glycoproteins. Adv Neurobiol. 2014;9:47–70. doi: 10.1007/978-1-4939-1154-7_3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Umaña P, Jean-Mairet J, Moudry R, Amstutz H, Bailey JE. Engineered glycoforms of an antineuroblastoma IgG1 with optimized antibody-dependent cellular cytotoxic activity. Nat Biotechnol. 1999;17(2):176–180. doi: 10.1038/6179. [DOI] [PubMed] [Google Scholar]
- 15.Parekh RB, et al. Association of rheumatoid arthritis and primary osteoarthritis with changes in the glycosylation pattern of total serum IgG. Nature. 1985;316(6027):452–457. doi: 10.1038/316452a0. [DOI] [PubMed] [Google Scholar]
- 16.Parekh RB, et al. Galactosylation of IgG associated oligosaccharides: Reduction in patients with adult and juvenile onset rheumatoid arthritis and relation to disease activity. Lancet. 1988;1(8592):966–969. doi: 10.1016/s0140-6736(88)91781-3. [DOI] [PubMed] [Google Scholar]
- 17.Scherer HU, et al. Glycan profiling of anti-citrullinated protein antibodies isolated from human serum and synovial fluid. Arthritis Rheum. 2010;62(6):1620–1629. doi: 10.1002/art.27414. [DOI] [PubMed] [Google Scholar]
- 18.van de Geijn FE, et al. Immunoglobulin G galactosylation and sialylation are associated with pregnancy-induced improvement of rheumatoid arthritis and the postpartum flare: Results from a large prospective cohort study. Arthritis Res Ther. 2009;11(6):R193. doi: 10.1186/ar2892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Anthony RM, Kobayashi T, Wermeling F, Ravetch JV. Intravenous gammaglobulin suppresses inflammation through a novel T(H)2 pathway. Nature. 2011;475(7354):110–113. doi: 10.1038/nature10134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Espy C, et al. Sialylation levels of anti-proteinase 3 antibodies are associated with the activity of granulomatosis with polyangiitis (Wegener’s) Arthritis Rheum. 2011;63(7):2105–2115. doi: 10.1002/art.30362. [DOI] [PubMed] [Google Scholar]
- 21.Ogata S, et al. Treatment response in Kawasaki disease is associated with sialylation levels of endogenous but not therapeutic intravenous immunoglobulin G. PLoS ONE. 2013;8(12):e81448. doi: 10.1371/journal.pone.0081448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fokkink WJ, et al. IgG Fc N-glycosylation in Guillain-Barré syndrome treated with immunoglobulins. J Proteome Res. 2014;13(3):1722–1730. doi: 10.1021/pr401213z. [DOI] [PubMed] [Google Scholar]
- 23.Kaneko Y, Nimmerjahn F, Ravetch JV. Anti-inflammatory activity of immunoglobulin G resulting from Fc sialylation. Science. 2006;313(5787):670–673. doi: 10.1126/science.1129594. [DOI] [PubMed] [Google Scholar]
- 24.Anthony RM, et al. Recapitulation of IVIG anti-inflammatory activity with a recombinant IgG Fc. Science. 2008;320(5874):373–376. doi: 10.1126/science.1154315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schwab I, Biburger M, Krönke G, Schett G, Nimmerjahn F. IVIg-mediated amelioration of ITP in mice is dependent on sialic acid and SIGNR1. Eur J Immunol. 2012;42(4):826–830. doi: 10.1002/eji.201142260. [DOI] [PubMed] [Google Scholar]
- 26.Schwab I, et al. Broad requirement for terminal sialic acid residues and FcγRIIB for the preventive and therapeutic activity of intravenous immunoglobulins in vivo. Eur J Immunol. 2014;44(5):1444–1453. doi: 10.1002/eji.201344230. [DOI] [PubMed] [Google Scholar]
- 27.Anthony RM, Wermeling F, Karlsson MC, Ravetch JV. Identification of a receptor required for the anti-inflammatory activity of IVIG. Proc Natl Acad Sci USA. 2008;105(50):19571–19578. doi: 10.1073/pnas.0810163105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Leontyev D, et al. Sialylation-independent mechanism involved in the amelioration of murine immune thrombocytopenia using intravenous gammaglobulin. Transfusion. 2012;52(8):1799–1805. doi: 10.1111/j.1537-2995.2011.03517.x. [DOI] [PubMed] [Google Scholar]
- 29.Campbell IK, et al. Therapeutic effect of IVIG on inflammatory arthritis in mice is dependent on the Fc portion and independent of sialylation or basophils. J Immunol. 2014;192(11):5031–5038. doi: 10.4049/jimmunol.1301611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Massoud AH, et al. Dendritic cell immunoreceptor: A novel receptor for intravenous immunoglobulin mediates induction of regulatory T cells. J Allergy Clin Immunol. 2014;133(3):853–863.e5. doi: 10.1016/j.jaci.2013.09.029. [DOI] [PubMed] [Google Scholar]
- 31.Hess C, et al. T cell-independent B cell activation induces immunosuppressive sialylated IgG antibodies. J Clin Invest. 2013;123(9):3788–3796. doi: 10.1172/JCI65938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Basta G, Schmidt AM, De Caterina R. Advanced glycation end products and vascular inflammation: Implications for accelerated atherosclerosis in diabetes. Cardiovasc Res. 2004;63(4):582–592. doi: 10.1016/j.cardiores.2004.05.001. [DOI] [PubMed] [Google Scholar]
- 33.Tóbon-Velasco JC, Cuevas E, Torres-Ramos MA. Receptor for AGEs (RAGE) as mediator of NF-kB pathway activation in neuroinflammation and oxidative stress. CNS Neurol Disord Drug Targets. 2014;13(9):1615–1626. doi: 10.2174/1871527313666140806144831. [DOI] [PubMed] [Google Scholar]
- 34.Hamada Y, et al. Rapid formation of advanced glycation end products by intermediate metabolites of glycolytic pathway and polyol pathway. Biochem Biophys Res Commun. 1996;228(2):539–543. doi: 10.1006/bbrc.1996.1695. [DOI] [PubMed] [Google Scholar]
- 35.Käsermann F, et al. Analysis and functional consequences of increased Fab-sialylation of intravenous immunoglobulin (IVIG) after lectin fractionation. PLoS ONE. 2012;7(6):e37243. doi: 10.1371/journal.pone.0037243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sitaru C, et al. Induction of complement-fixing autoantibodies against type VII collagen results in subepidermal blistering in mice. J Immunol. 2006;177(5):3461–3468. doi: 10.4049/jimmunol.177.5.3461. [DOI] [PubMed] [Google Scholar]
- 37.Egrie JC, Browne JK. Development and characterization of novel erythropoiesis stimulating protein (NESP) Br J Cancer. 2001;84(Suppl 1):3–10. doi: 10.1054/bjoc.2001.1746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Salles G, et al. Phase 1 study results of the type II glycoengineered humanized anti-CD20 monoclonal antibody obinutuzumab (GA101) in B-cell lymphoma patients. Blood. 2012;119(22):5126–5132. doi: 10.1182/blood-2012-01-404368. [DOI] [PubMed] [Google Scholar]
- 39.Ahmad S, Moinuddin, Ali A. Immunological studies on glycated human IgG. Life Sci. 2012;90(25-26):980–987. doi: 10.1016/j.lfs.2012.05.002. [DOI] [PubMed] [Google Scholar]
- 40.Schmidt AM, Yan SD, Yan SF, Stern DM. The multiligand receptor RAGE as a progression factor amplifying immune and inflammatory responses. J Clin Invest. 2001;108(7):949–955. doi: 10.1172/JCI14002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bozhinov A, et al. Advanced glycation end products contribute to the immunogenicity of IFN-β pharmaceuticals. J Allergy Clin Immunol. 2012;129(3):855–858, e6. doi: 10.1016/j.jaci.2011.10.035. [DOI] [PubMed] [Google Scholar]
- 42.Sathish JG, et al. Challenges and approaches for the development of safer immunomodulatory biologics. Nat Rev Drug Discov. 2013;12(4):306–324. doi: 10.1038/nrd3974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Stadlmann J, et al. A close look at human IgG sialylation and subclass distribution after lectin fractionation. Proteomics. 2009;9(17):4143–4153. doi: 10.1002/pmic.200800931. [DOI] [PubMed] [Google Scholar]
- 44.Svetlicky N, et al. The advantage of specific intravenous immunoglobulin (sIVIG) on regular IVIG: Experience of the last decade. J Clin Immunol. 2013;33(Suppl 1):S27–S32. doi: 10.1007/s10875-012-9842-5. [DOI] [PubMed] [Google Scholar]
- 45.Tackenberg B, et al. Impaired inhibitory Fcgamma receptor IIB expression on B cells in chronic inflammatory demyelinating polyneuropathy. Proc Natl Acad Sci USA. 2009;106(12):4788–4792. doi: 10.1073/pnas.0807319106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Samuelsson A, Towers TL, Ravetch JV. Anti-inflammatory activity of IVIG mediated through the inhibitory Fc receptor. Science. 2001;291(5503):484–486. doi: 10.1126/science.291.5503.484. [DOI] [PubMed] [Google Scholar]
- 47.Crow AR, et al. IVIg-mediated amelioration of murine ITP via FcgammaRIIB is independent of SHIP1, SHP-1, and Btk activity. Blood. 2003;102(2):558–560. doi: 10.1182/blood-2003-01-0023. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.