Significance
Because India—home to one fifth of all births—has no monitoring system for maternal health, basic facts about maternal nutrition are unknown. Using statistically adjusted nationally representative survey data, this paper presents, to my knowledge, the first estimates of prepregnancy body mass and weight gain during pregnancy in India and compares them with sub-Saharan Africa: 42.2% of Indian women are underweight when they begin pregnancy compared with 16.5% of African women. In both regions, women gain little weight during pregnancy, but because of prepregnancy deficits, Indian women end pregnancy weighing less than African women do at the beginning. Deficits in maternal nutrition could help explain the Asian enigma, the puzzle of why Indian children are much smaller than their relative wealth predicts.
Keywords: maternal health, nutrition, India, sub-Saharan Africa
Abstract
Despite being wealthier, Indian children are significantly shorter and smaller than African children. These differences begin very early in life, suggesting that they may in part reflect differences in maternal health. By applying reweighting estimation strategies to the Demographic and Health Surveys, this paper reports, to my knowledge, the first representative estimates of prepregnancy body mass index and weight gain during pregnancy for India and sub-Saharan Africa. I find that 42.2% of prepregnant women in India are underweight compared with 16.5% of prepregnant women in sub-Saharan Africa. Levels of prepregnancy underweight for India are almost seven percentage points higher than the average fraction underweight among women 15–49 y old. This difference in part reflects a previously unquantified relationship among age, fertility, and underweight; childbearing is concentrated in the narrow age range in which Indian women are most likely to be underweight. Further, because weight gain during pregnancy is low, averaging about 7 kg for a full-term pregnancy in both regions, the average woman in India ends pregnancy weighing less than the average woman in sub-Saharan Africa begins pregnancy. Poor maternal health among Indian women is of global significance because India is home to one fifth of the world’s births.
Children in India are significantly shorter and smaller than children in sub-Saharan Africa. Because Indian children are much richer, on average, than African children, scholars have described anthropometric differences between Indians and Africans as an “Asian enigma” (1–4). Although there are likely many reasons why Indian children are shorter than African children (5, 6) and why Indian children are shorter than economic indicators predict, demographic and health surveys show that physical differences between Indian and African children begin very early in life, suggesting that the Asian enigma may in part reflect differences in maternal health. That Indian women have worse health during pregnancy than African women is also consistent with an anomalously high rate of neonatal mortality in India, as well as high rates of low birth weight, even among relatively privileged groups (7). Poor maternal health and nutrition among Indian women is of global significance because India is home to one sixth of the world’s population and one fifth of the world’s births.
In recent decades, India has experienced rapid economic growth and significant reductions in poverty. Despite this economic success, however, measures of women’s nutrition remain exceptionally poor. The latest Demographic and Health Survey (DHS), in 2005, showed that 35.5% of women aged 15–49 y are underweight, suggesting that maternal health and nutrition are extremely also poor. India's high rate of underweight among women is worrisome in light of mounting evidence that nutrition during pregnancy is important not only for neonatal survival but also for birth weight (8, 9), which is associated with height and health in childhood and adulthood (10–13), as well cognition and productivity (14–17).
Prepregnancy body mass and weight gain during pregnancy are useful measures of maternal nutrition. These factors interact to determine birth weight: on average, women with lower prepregnancy body mass need to gain more weight during pregnancy to deliver infants of the same birth weight as women who start pregnancy with higher body mass (18, 19). The Institute of Medicine guidelines for women in the United States recommend higher weight gain during pregnancy for women with a prepregnancy body mass index (BMI) of less than 18.5 kg/m2 (19).
What are average prepregnancy BMI and weight gain during pregnancy in India, and how do these figures compare with those in sub-Saharan Africa? These basic nutrition facts are unknown because no representative longitudinal monitoring systems exist for these regions. This paper’s primary contribution is to produce, to my knowledge, the first representative estimates of prepregnancy BMI and weight gain during pregnancy for India and sub-Saharan Africa.
To estimate prepregnancy BMI, I adjust the BMIs of nonpregnant women for selection into pregnancy based on observable characteristics of pregnant women. I find that in India, 42.2% of prepregnant women are underweight; this figure is about seven percentage points higher than the average fraction underweight among women 15–49 y old. These estimates constitute a significant contribution to the literature because they show that commonly cited figures for average women’s nutrition in India (1, 20, 21) significantly underestimate the fraction of prepregnant women who are underweight and overestimate average prepregnancy BMI. About half of the difference between the prevalence of women’s underweight and the prevalence of prepregnancy underweight can be attributed to previously unquantified age patterns of fertility and undernutrition that are likely due to pronounced sex and age hierarchies in Indian households. Such hierarchies have been documented and studied by demographers, sociologists, and anthropologists (22–24). In contrast, in the much poorer African sample, only 16.5% of prepregnant women are underweight.
Empirical distributions of the weights of pregnant women show that Indian women do not compensate for low prepregnancy body mass by gaining adequate weight during pregnancy. Indeed, on average, women in India end pregnancy weighing less than women in sub-Saharan Africa begin pregnancy.
Women in both India and sub-Saharan Africa gain only about 7 kg, on average, for a full-term pregnancy. Two different methods confirm these results. Such a small weight gain is only about half of the minimum recommended gain for underweight women in the United States, for whom national guidelines recommend gaining between 12.5 and 18 kg during pregnancy. It is only about 60% of the minimum recommended gain for normal weight women, for whom the guidelines recommend gaining between 11.5 and 16 kg (19).
Summary Statistics
Table 1 shows that the 2005 Indian gross domestic product (GDP) per capita was twice the GDP per capita of sub-Saharan Africa, and the Indian population was 1.45 times as large (25). The 2005 poverty headcount ratio, using the World Bank’s $1.25 per day poverty line, was lower in India than sub-Saharan Africa (26). Further, the 2005 total fertility rate (TFR) in India was considerably lower than in sub-Saharan Africa; the Population Reference Bureau estimates an India TFR of 3.0 compared with 5.6 for sub-Saharan Africa (27).
Table 1.
Summary statistics
| 2005 population (in millions)* | 2005 GDP per capita (in international dollars)* | Poverty headcount ratio at $1.25/d PPP† | 2005 total fertility rate (region)‡ | Number of countries | Sample size women 15–49 y§ | ||||||
| Region | Sample | Region | Sample | Region | Sample | Region | Sample | Nonpregnant, not using contraception | 3+ mo pregnant | ||
| India | 1,091 | 1,091 | 2,492 | 2,492 | 41.6% | 41.6% | 3.0 | 1 | 1 | 69,543 | 5,055 |
| Sub-Saharan Africa | 752 | 597 | 1,480 | 990 | 52.8% | 56.8% | 5.6 | 47 | 29 | 148,964 | 17,601 |
Computations made using data from the Penn World Tables (25), using real GDP per capita.
Year 2005 World Development Indicators (26) data are used. These estimates use purchasing power parity (PPP) figures from the 2005 International Comparison Program. The estimate for sub-Saharan Africa sample countries is computed using country-level poverty headcount ratio data from the years, between 2001 and 2007, that is closest to 2005. Data for Zimbabwe are missing.
Estimates are taken from the Population Reference Bureau (27).
Sample sizes of women whose anthropometry was measured are shown.
Table 1 also provides information on the samples used for the analysis. The sample of African countries is restricted to those 29 countries with a DHS that measured women’s weights between 2000 and 2010; these are listed in Table S1. Sample countries from sub-Saharan Africa represent about 80% of the population of the region and are poorer than the region as a whole.
What Fraction of Prepregnant Women Are Underweight?
It is common to cite the fraction of women who are underweight (BMI < 18.5 kg/m2) as a proxy for prepregnancy undernutrition. However, if women who get pregnant are younger, less educated, or otherwise different from those who do not, and if these differences are correlated with body mass, then average BMI and fraction underweight among nonpregnant women of childbearing age in a representative cross section will give biased estimates of prepregnancy nutrition measures.
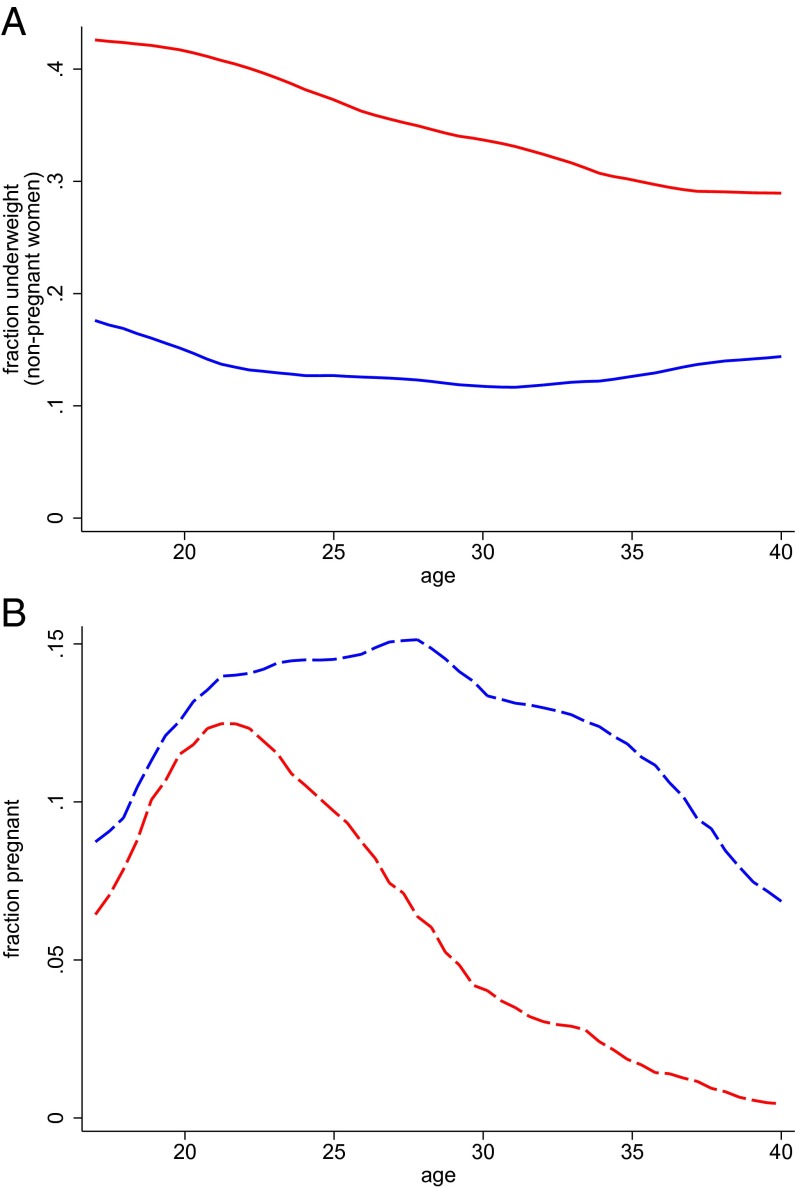
Are Indian women who become pregnant different from those who do not, in ways that correlate with nutrition? Fig. 1A plots the fraction of nonpregnant women who are underweight at each age. Fig. 1B plots the fraction of women who are pregnant at each age. The Indian data, in red, show a statistically significant negative relationship between age and the probability of being underweight: women in their early 20s are almost 15 percentage points more likely to be underweight than 40-y-old women. The early 20s are also the time when Indian women are most likely to be pregnant, leading to a concentration of childbearing during a nutritionally vulnerable period. This finding coincides with a prior literature that suggests that young Indian women have particularly low social status early in their marriages (23). This has negative consequences for their own health and the health of their children (22).
Fig. 1.
Underweight by age (among nonpregnant women) and pregnancy by age in India and sub-Saharan Africa. Epanechnikov kernel-weighted local polynomial regressions. Data from India are shown in red; data from sub-Saharan Africa are shown in blue. Underweight women have a BMI < 18.5 kg/m2.
The Indian data contrast sharply with those for sub-Saharan Africa. The solid blue curve in Fig. 1 shows a quadratic relationship between age and underweight among African women. African women in their early 20s are about 25 percentage points less likely to be underweight than Indian women in the same age range. In sub-Saharan Africa, fertility levels, shown with dashed curves, are much higher, and childbearing is spread out between the ages of about 17 and 35, rather than being concentrated in the early 20s.
To estimate prepregnancy BMI and fraction underweight, I construct a nonparametric reweighting function to compute what the BMIs of nonpregnant women would be if they had the same distribution of observable characteristics as pregnant women. Table 2 presents means and cluster bootstrapped confidence intervals resulting from statistical reweighting. For comparison, the first rows of the “body mass index” and “fraction underweight” results show averages for nonpregnant women aged 15–49 y. The second rows show the results of reweighting by only the age structure of pregnant women, in years. Age reweighting makes a difference for India: BMI adjusted for the age profile of pregnancy is 0.7 points lower than that of the average woman, and the adjusted fraction underweight is 3.5 percentage points higher than the unadjusted fraction. Adjustment for the age pattern of pregnancy in sub-Saharan Africa shows that nonpregnant women with the same age distribution as pregnant women have similar average anthropometric outcomes as nonpregnant women.
Table 2.
Estimates of prepregnancy BMI, fraction underweight and weight, and weight gain during pregnancy for India and sub-Saharan Africa
| Nutrition indicator | India | Sub-Saharan Africa | ||
| Mean | 95% CI | Mean | 95% CI | |
| Body mass index | ||||
| Nonpregnant women, 15–49 y | 20.47 | [20.41, 20.53] | 21.90 | [21.82, 21.98] |
| Prepregnant women, reweighted by age (CU dropped) | 19.81 | [19.73, 19.90] | 21.75 | [21.66, 21.84] |
| Prepregnant women, extended reweighting (CU dropped) | 19.54 | [19.46, 19.62] | 21.49 | [21.39, 21.59] |
| Prepregnant women, extended reweighting (CU included) | 19.57 | [19.50, 19.63] | . | . |
| Fraction underweight (BMI < 18:5 kg/m2) | ||||
| Nonpregnant women, 15–49 y | 0.355 | [0.349, 0.360] | 0.152 | [0.147, 0.158] |
| Prepregnant women, reweighted by age (CU dropped) | 0.390 | [0.382, 0.399] | 0.146 | [0.140, 0.153] |
| Prepregnant women, extended reweighting (CU dropped) | 0.422 | [0.410, 0.432] | 0.165 | [0.157, 0.174] |
| Prepregnant women, extended reweighting (CU included) | 0.418 | [0.410, 0.432] | . | . |
| Weight (kg) | ||||
| Nonpregnant women, 15–49 y | 47.31 | [47.17, 47.46] | 54.58 | [54.38, 54.81] |
| Prepregnant women, reweighted by age (CU dropped) | 45.81 | [45.62, 46.02] | 54.29 | [54.05, 54.52] |
| Prepregnant women, extended reweighting (CU dropped) | 44.92 | [44.71, 45.19] | 53.45 | [53.22, 53.74] |
| Prepregnant women, extended reweighting (CU included) | 45.04 | [44.87, 45.21] | . | . |
| Prepregnant women, extended reweighting, using women who delivered last year (CU dropped) | 44.85 | [44.69, 45.02] | 53.08 | [52.84, 53.33] |
| Weight gain for a full-term pregnancy (kg) | ||||
| Method 1, no controls | 7.13 | [6.48, 7.77] | 6.47 | [5.95, 6.99] |
| Method 1, extended controls | 7.00 | [6.35, 7.64] | 6.27 | [5.88, 6.66] |
| Method 2, reweight with pregnant women | 6.88 | [6.18, 7.69] | 7.44 | [6.32, 8.44] |
| Method 2, reweight with women who delivered last year | 6.95 | [6.23, 7.76] | 7.84 | [6.73, 8.93] |
CU, contraceptive users.
In Table 2, the third rows of results for “body mass index” and “fraction underweight” show the results of an extended statistical reweighting, which splits the sample of nonpregnant women in each region into mutually exclusive bins to account for differences between pregnant and nonpregnant women in age, education, urban/rural residence, the number of living children, the age of the youngest living child, whether she has at least one living son, and whether one or more of her children has died in the last 5 y. These factors were chosen for inclusion in the reweighting function because demographers have linked them to fertility (28–34), and they are also plausibly correlated with body mass. Summary statistics for these covariates, as well as linear probability regressions showing that they predict pregnancy are shown in Tables S2 and S3.
The results of the extended reweighting of fraction underweight in Table 2 show that 42.2% of prepregnant women in India are underweight, cluster bootstrapped 95% CI = [0.410, 0.432], compared with 16.5% in sub-Saharan Africa, cluster bootstrapped 95% CI = [0.157, 0.174]. The average prepregnant woman in India is about seven percentage points more likely to be underweight than the average woman between the ages of 15 and 49 y. In sub-Saharan Africa, in contrast, the average fraction underweight and the fraction of prepregnant women who are underweight are very similar.
Only those nonpregnant women who are not using modern contraception at the time of the interview are used to compute the results of the extended reweighting. Because the Indian DHS collected data on contraceptive use histories, it is possible to check the assumption of a zero failure rate of contraception by statistically reweighting over the characteristics described above, as well as an indicator for whether nonpregnant women are using modern contraception. The results, shown in Table 2, change very little, which is expected considering that only 4% of pregnant women in the Indian sample were using modern contraception in the month of conception. This specification is not shown for the African sample, because only 9 of the 29 surveys collected contraceptive use histories. In those countries where contraceptive use histories were collected, only 5% of pregnant women were using modern contraception in the month of conception.
Materials and Methods provides further details about the reweighting functions used to generate the results in Table 2.
Do Indian Women Compensate for Low Prepregnancy Body Mass with Weight Gain During Pregnancy?
Prior research on weight gain during pregnancy in India studies small samples of women, which are not intended to be representative of the population (35–37). To estimate average weight gain for full-term pregnancies in the population, this paper uses two strategies: the first compares the weights of women in early pregnancy with those of women in late pregnancy, and the second compares the weights of nonpregnant women, adjusted for selection, with weights of women in late pregnancy.
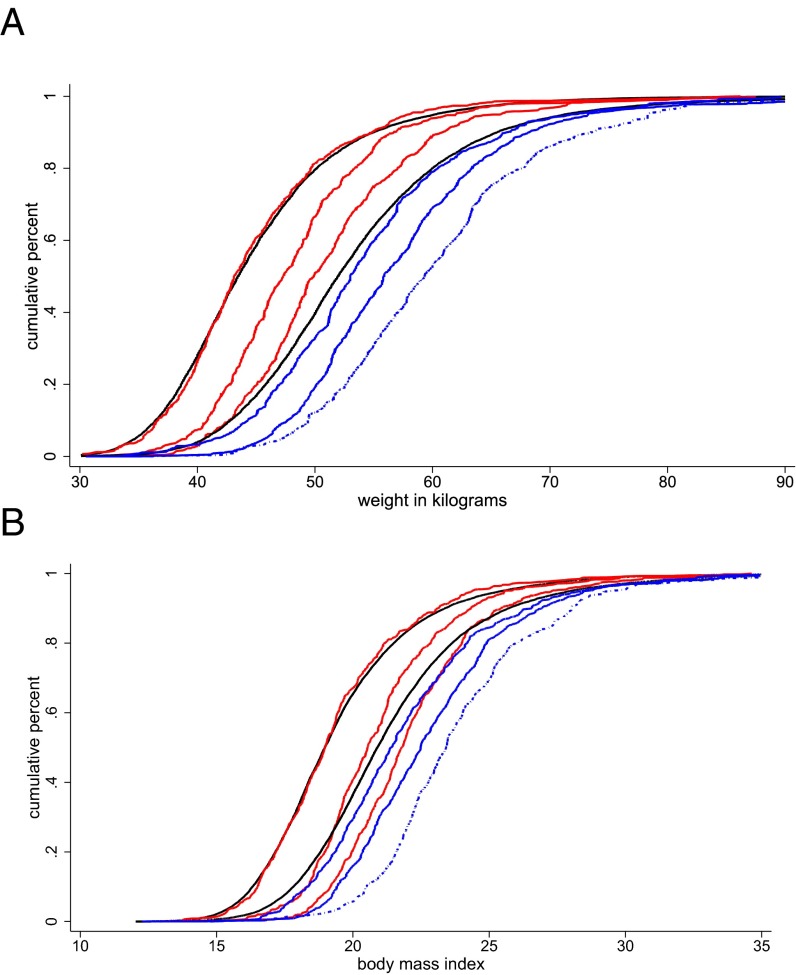
Before turning to model-based estimates of weight gain during pregnancy, Fig. 2 plots distributions of weights of pregnant and prepregnant women in India and sub-Saharan Africa. Everywhere in the weight distribution, Indian women end pregnancy weighing less than African women do when they begin pregnancy. Each red or blue curve in Fig. 2 shows an empirical cumulative distribution (CDF) of weight in kilograms (Fig. 2A) or BMI (Fig. 2B) for women at 3, 6, or 9+ mo of gestation. If, except for differences in gestational age, women who report early pregnancy are similar to those who report late pregnancy, then the horizontal distance between CDFs of the same color provides a visual description of weight gain during the second and third trimesters.
Fig. 2.
Estimated CDFs for prepregnant women and empirical CDFs for 3, 6, and 9+ mo of gestation. Estimated CDFs for prepregnant women are shown in black for in India (Left) and sub-Saharan Africa (Right). These are estimated using the extended reweighting function described in Materials and Methods. Red curves represent empirical CDFs for Indian women at 3, 6, and 9+ mo of gestation (left to right). Blue curves present empirical CDFs for African women at 3, 6, and 9+ mo of gestation.
The black curves in Fig. 2 show estimated CDFs of prepregnancy weight and BMI, computed using the extended reweighting function on the sample of nonpregnant women not using contraception discussed in the previous section. For Indian women, the estimated prepregnancy CDFs are very close to the empirical distributions for women at 3 mo of gestation. For African women, the CDFs of estimated prepregnancy weight and BMI are slightly to the left of the 3-mo distributions. The larger gap between the prepregnancy distribution and the 3-mo distribution for sub-Saharan Africa suggests that African women may gain more weight in the first trimester than Indian women. Fig. S1 presents additional estimates of prepregnancy weight distributions that were calculated by modeling the association between body weight and gestational age, individual characteristics, and their interactions in cross sections of pregnant women.
Table 2 presents several estimates for mean prepregnancy weight, computed using the same methods described above for prepregnancy BMI and underweight, as well as estimated means and confidence intervals for weight gain during pregnancy for India and sub-Saharan Africa. The confidence intervals on weight gain for all four estimation strategies overlap within and across regions, demonstrating that, despite very different prepregnancy BMIs, women in India and sub-Saharan Africa gain similar amounts of weight during pregnancy.
Estimates labeled “method 1” are computed using ordinary least squares (OLS) regressions of weight in kilograms on month of gestation for women three or more months pregnant. These regressions estimate average monthly weight gain. Linear modeling of these data is appropriate because weight gain during pregnancy is known to follow a linear pattern at these gestational ages (38–40). Assuming that first trimester gain is 10% of second and third trimester gain (19), average weight gain for a full-term pregnancy is estimated as , where is the coefficient on month of gestation from the regression.
Table 2 presents two ways of estimating weight gain during pregnancy using OLS. The first estimates weight gain using uncontrolled regressions of weight on month of gestation. The second estimates weight gain using regressions that control for factors related to selection into reported month of gestation, including age, years of schooling, number of living children, urban residence, and wealth quintile interacted with country. Dummy variables for the interaction of DHS sample region with month of interview are also included in the controlled regressions. Table S5 shows the regression table associated with the method 1 estimates from Table 2, as well as weight gain estimates from additional regression specifications.
Estimates labeled “method 2” in Table 2 compute weight gain by comparing the weights of pregnant women who report 9+ mo of gestation with the weights of nonpregnant women, adjusted for selection into pregnancy using the extended reweighting function used to compute prepregnancy BMI and fraction underweight. To account for the fact that women who report 9+ mo of pregnancy will continue to gain weight until they deliver, these differences are adjusted upward by , where is average monthly gain estimated from the controlled regression described above. In Table 2, the results labeled “method 2, reweight with pregnant women” use the characteristics of pregnant women in the reweighting function; the results labeled “method 2, reweight with women who delivered last year” use characteristics, from the time of conception, of women who delivered a live infant in the year before the survey. The results of these two strategies would differ if rates of terminated pregnancies (due either to miscarriage or abortion) or preterm births are high and if characteristics that correlate with terminated pregnancies and preterm births also correlate with weight. However, Table 2 shows that the results are similar.
The results in Table 2 suggest that Indian and African women gain similar amounts of weight during pregnancy; the confidence intervals for all eight weight gain estimates overlap. Women in both regions gain only about 7 kg during pregnancy, which is far less than what is recommended for women in the United States with similar prepregnancy body mass. The estimation of these means and confidence intervals, as well as the strengths and weaknesses of each approach, are discussed further in Materials and Methods.
Discussion
This paper provides, to my knowledge, the first estimates of prepregnancy BMI, fraction underweight, and weight gain during pregnancy for India, the country with the highest number of births in the world and serious challenges for population health, and for sub-Saharan Africa, a region that is significantly poorer.
By a novel application of reweighting estimation strategies, I find that 42.2% of prepregnant women in India are underweight, which is more than 25 percentage points higher than the comparable figure for sub-Saharan Africa. Women in both regions gain very little weight during pregnancy, but because of prepregnancy deficits, women in India end pregnancy weighing even less than women in sub-Saharan Africa begin it. Because prepregnancy BMI interacts with weight gain to produce birth weight, one would expect Indian infants to be born at significantly lower birth weights than African infants, a prediction that is supported by differences in anthropometric outcomes very early in life measured in the DHS. Because birth weight is a determinant of height, these differences in in utero nutrition may help explain the Asian enigma that Indians are shorter than Africans, despite their relatively better economic circumstances.
This study also found that in India, the prevalence of underweight among prepregnant women is higher than estimates for the average woman. About half of the gap between average underweight and prepregnancy underweight is explained by a previously unquantified relationship among age, the prevalence of underweight, and pregnancy, shown in Fig. 1.
These results suggest that further research is needed to understand why health during pregnancy in India is so poor and how it might be improved. Strong economic arguments exist as to why India should invest in pregnancy to improve infant health (14, 41). However, to the extent that poor health during pregnancy is caused by low intrahousehold status among young women, which imposes a heavy burden of manual labor and restricts food intake (23), government intervention may prove extremely difficult. Indeed, prior literature on maternal health care has observed that the coincidence of childbearing with the restricted mobility and low intrahousehold status of young women limits their use of health services (23, 42).
Although certainly important, discrimination against young women is not the only reason why maternal health is so poor. Indeed, India’s most recent DHS shows that the prevalence of underweight is 25% among men aged 40–50 y; these are the household members with the highest intrahousehold status. Exposure to infectious disease, poor sanitation, and poor diets all contribute to low body mass among both men and women. In the context of strong intrahousehold inequalities, investments in public goods that improve the disease environment similarly for everyone may be particularly useful.
Materials and Methods
Data.
This paper uses data from the DHS, which are publicly available from www.dhsprogram.com. For sub-Saharan Africa, I construct a dataset that includes all countries in which a survey collected data on women’s weights and pregnancy duration between 2000 and 2010. If a country had more than one such DHS in this time window, the survey that took place closest to 2005 is chosen. A list of 29 countries and survey years included in the sub-Saharan Africa sample is presented in Table S1. Anthropometry data are missing for 4.3% of pregnant women and 4.6% of nonpregnant women in the India sample and for 2.4% of pregnant women and 2.4% of nonpregnant women in the sub-Saharan Africa sample; these observations are dropped from the analysis.
Design Weights.
Design weights are used to compute all results. For India, I use the women’s sampling weights provided by the DHS. For sub-Saharan Africa, I construct a weight, , for each woman i in country c. , where is the population of country c in 2005 from the Penn World Tables (25). is the sampling weight for woman i within a given survey.
Estimation of Prepregnancy Indicators.
This paper applies a nonparametric reweighting function similar to that described by DiNardo et al. (43) and Geruso (44). The reweighting function used to generate many of the results reported in Table 2, , is defined as , where is a single set of indicators for the intersections of categorical indicators assigned from observable characteristics that are correlated with pregnancy and body size. With the exception of education and urban residence, which are assigned for all women based on what is true at the time of survey, pregnant women are assigned characteristics based on what was true in the month of conception, and nonpregnant women are assigned characteristics based on what is true when they are surveyed. In Table 2, results labeled “reweighted by age” reweight only by age in years, and nonpregnant women who are using modern contraception are dropped from the reweighting procedure. The results of the extended reweighting likewise omit women using contraception and include a larger set of characteristics in the reweighting function: dummy variables for age groups (15–19, 20–24, 25–30, 30–40, and 40–50 y); education level (primary or less, some secondary or more); residence (urban, rural); age of the youngest child (no living children, youngest child is <1 y old, youngest child is between 1 and 2 y old, youngest child is >2 y old); presence of a living son; number of living children (one, two, three, and four or more); and whether any of the woman’s children died in the 5 y before the interview. Table S2 presents summary statistics for these covariates for pregnant and nonpregnant women. Table S3 shows linear probability regressions of an indicator for being 3+ mo pregnant on these covariates. The results in Table 2 include nonpregnant women who are using modern contraception and add to the extended reweighting function an indicator for the use of modern contraception at the time of conception.
The probability of reporting pregnancies of 1 or 2 mo of gestation is low relative to the probability of reporting pregnancies of other months of gestation. Table S4 shows the fraction of women reporting pregnancies of each gestational age. To avoid biasing the results due to selection into reporting of early pregnancies, only women reporting 3 mo or more since their last menstrual period are included in the sample of pregnant women used to compute the reweighting function. Throughout the paper, month of gestation is calculated based on the respondent’s reported time since her last menstrual period, which is asked of all respondents, and is rounded up or down to the nearest month if reported in weeks. Where data on time since last menstrual period is missing, the response to a question asked only of pregnant women (“How many months pregnant are you?”) is used.
Mean prepregnancy BMI, , is , where i indexes nonpregnant women and is the number of nonpregnant women in the sample. For India, the results in Table 2 for the age-only reweighting and the extended reweighting without contraceptive users drop less than 1% of the sample of pregnant women due to lack of support in the distribution of nonpregnant women. For the extended reweighting that includes contraceptive users, 2% of the sample of pregnant women are dropped before calculating the reweighting function. For sub-Saharan Africa, no pregnant women are dropped to compute the results of the age-only reweighting; for the extended reweighting, about a tenth of a percent of the sample of pregnant women is dropped.
To compute the bootstrapped confidence intervals for results about body mass, fraction underweight, and weight Table 2, I resample clusters from the original data sources (DHS primary sampling units), stratifying within urban residence and state for the India sample and urban residence and country for the African sample.
Estimation of Weight Gain During Pregnancy.
Method 1.
Estimates labeled method 1 in Table 2 present average weight gain for a full-term pregnancy estimated as , where is the coefficient on month of gestation from an OLS regression of weight on month of gestation for women 3+ mo pregnant. This method for computing weight gain assumes that women in both regions gain an additional 10% of their second and third trimester weight gain in the first trimester. Table S5 presents estimates of weight gain in pregnancy computed using several different estimates of from OLS regressions of the form
| [1] |
where is the weight in kilograms of pregnant woman i. is month of gestation, calculated as described above. Controls, , are added to the regression in stages to correct for possible selection into gestational age reporting, which could bias negatively if disadvantaged women fail to report early pregnancies or positively if disadvantaged women are more likely to miscarry or terminate the pregnancy. Column 3 in Table S5 controls for age fixed effects, years of schooling fixed effects, number of living children fixed effects, an urban fixed effect, and dummy variables for the interaction of wealth quintile with country. Column 3 also adds controls for place interacted with month of interview. Places are countries in the Africa sample and states in the India sample. Columns 4 and 5 restrict the regression to women reporting 4–9 and 3–8 mo of pregnancy, respectively, to test whether the results are sensitive to omitting the months that are most likely affected by pregnancy underreporting and prematurity, respectively. The results are not sensitive to these respecifications; 95% confidence intervals of weight gain for method 1 results in Table 2 and Table S5 are calculated as .
Method 2.
It is useful to validate weight gain estimates from method 1 using a second method. Estimates produced using method 1 may overestimate weight gain during pregnancy if women gain less than 10% of second and third trimester gain in the first trimester. The black curve representing the distribution of weights among prepregnant women in India in Fig. 2 suggests that, for India, this may be true. However, if gestational ages are misreported, then the coefficients on gestational age would be attenuated, and method 1 would underestimate weight gain in pregnancy. Method 2 estimates average gain as the difference between average prepregnant weight computed using the nonparametric reweighting method described above for BMI and the average weight of women in late pregnancy. This method does not make assumptions about first trimester gain, nor does it potentially suffer from attenuation bias. However, method 2 will only produce unbiased estimates of weight gain in pregnancy insofar as the nonparametric reweighting accounts for all important endogeneity in selection into pregnancy. Fig. S1, which plots the estimated prepregnancy weight distribution from the nonparametric reweighting of nonpregnant women against estimates of the distribution of prepregnant weight based on correcting the weights of individual pregnant women for gestational age, observable characteristics, and their interactions, provides evidence that the distributions resulting from the nonparametric reweighting are good estimates of the prepregnancy distributions.
Method 2 estimates average gain as where is the average weight of women reporting 9+ mo of gestation; is an estimate of prepregnancy weight from the nonparametric reweighting; and is the average estimated monthly gain from the controlled regression described for method 1. The inclusion of a half a month of linear weight gain assumes that pregnant women who report 9+ mo since their last menstrual period are, on average, at the midpoint of the final month of pregnancy. Average prepregnancy weight is computed using the extended reweighting function described above, dropping contraceptive users. The first row of method 2 results in Table 2 reweights over characteristics of pregnant women; the second row of method 2 results reweights over characteristics, from the time of conception, of women who delivered a live birth in the year before the survey.
Sampling error contributes to the variance of both and and to the estimation of . Therefore, to calculate 95% CIs for this estimate of weight gain, I bootstrap the entire calculation, stratifying within urban residence and state for the India sample and urban residence and country for the African sample. I cluster at the primary sampling unit level.
Supplementary Material
Acknowledgments
I thank Hoyt Bleakley, Anne Case, Robert Chase, Janet Currie, Angus Deaton, Thomas Espenshade, Dennis Feehan, Aashish Gupta, Jeffrey Hammer, Payal Hathi, Sara McLanahan, Franco Peracchi, Sangita Vyas, Charles Westoff, data specialists at ICF International, and especially Dean Spears for helpful comments and discussions. Partial support for this research was provided by Eunice Kennedy Shriver National Institute of Child Health and Human Development Grant 5R24HD047879.
Footnotes
The author declares no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1416964112/-/DCSupplemental.
References
- 1.Bhutta ZA. Why has so little changed in maternal and child health in south Asia? BMJ. 2000;321(7264):809–812. doi: 10.1136/bmj.321.7264.809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nubé M. The Asian enigma: Predisposition for low adult BMI among people of South Asian descent. Public Health Nutr. 2009;12(4):507–516. doi: 10.1017/S1368980008002826. [DOI] [PubMed] [Google Scholar]
- 3.Deaton A. Height, health, and development. Proc Natl Acad Sci USA. 2007;104(33):13232–13237. doi: 10.1073/pnas.0611500104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Underwood BA. Health and nutrition in women, infants, and children: Overview of the global situation and the Asian enigma. Nutr Rev. 2002;60(5 Pt 2):S7–S13. doi: 10.1301/00296640260130425. [DOI] [PubMed] [Google Scholar]
- 5.Coffey D, Deaton A, Drèze J, Spears D, Tarozzi A. Stunting among children: Facts and implications. Econ Polit Wkly. 2013;XLVIII(34):68–70. [Google Scholar]
- 6.Spears D. 2013. How much international variation in child height can sanitation explain? World Bank Policy Research working paper 6351.
- 7.WHO, UNICEF . Low Birth Weight: Country, Regional and Global Estimates. UNICEF; New York: 2004. [Google Scholar]
- 8.Ludwig DS, Currie J. The association between pregnancy weight gain and birthweight: A within-family comparison. Lancet. 2010;376(9745):984–990. doi: 10.1016/S0140-6736(10)60751-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hytten F, Leitch I. The Physiology of Human Pregnancy. Blackwell Scientific Publications; Oxford: 1971. [Google Scholar]
- 10.Almond D, Currie J. Killing me softly: The fetal origins hypothesis. J Econ Perspect. 2011;25(3):153–172. doi: 10.1257/jep.25.3.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Barker DJ. The fetal and infant origins of disease. Eur J Clin Invest. 1995;25(7):457–463. doi: 10.1111/j.1365-2362.1995.tb01730.x. [DOI] [PubMed] [Google Scholar]
- 12.Currie J, Vogl T. Early-life health and adult circumstance in developing countries. Annu Rev Econ. 2013;5(1):1–36. [Google Scholar]
- 13.Adair LS. Size at birth and growth trajectories to young adulthood. Am J Hum Biol. 2007;19(3):327–337. doi: 10.1002/ajhb.20587. [DOI] [PubMed] [Google Scholar]
- 14.Behrman J, Rosenzweig M. Returns to birthweight. Rev Econ Stat. 2004;86(2):586–601. [Google Scholar]
- 15.Grantham-McGregor S, et al. International Child Development Steering Group Developmental potential in the first 5 years for children in developing countries. Lancet. 2007;369(9555):60–70. doi: 10.1016/S0140-6736(07)60032-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Susser M, Stein Z. Timing in prenatal nutrition: A reprise of the Dutch Famine Study. Nutr Rev. 1994;52(3):84–94. doi: 10.1111/j.1753-4887.1994.tb01395.x. [DOI] [PubMed] [Google Scholar]
- 17.Black S, Devereux P, Salvanes K. From the cradle to the labor market? The effect of birth weight on adult outcomes. Q J Econ. 2007;122(1):409–439. [Google Scholar]
- 18.Institute of Medicine . Nutrition During Pregnancy: Part I, Weight Gain. National Academies Press; Washington, DC: 1990. [Google Scholar]
- 19.Yaktine A, Rasmussen K, editors. Weight Gain During Pregnancy: Reexamining the Guidelines. National Academies Press; Washington, DC: 2009. [PubMed] [Google Scholar]
- 20.Bhutta ZA, et al. Maternal and Child Undernutrition Study Group What works? Interventions for maternal and child undernutrition and survival. Lancet. 2008;371(9610):417–440. doi: 10.1016/S0140-6736(07)61693-6. [DOI] [PubMed] [Google Scholar]
- 21.Black RE, et al. Maternal and Child Undernutrition Study Group Maternal and child undernutrition: Global and regional exposures and health consequences. Lancet. 2008;371(9608):243–260. doi: 10.1016/S0140-6736(07)61690-0. [DOI] [PubMed] [Google Scholar]
- 22.Das Gupta M. Life course perspectives on women’s autonomy and health outcomes. Am Anthropol. 1995;97(3):481–491. [Google Scholar]
- 23.Jeffery P, Jeffery R, Lyon A. Labour Pains and Labour Power: Women and Childbearing in India. Zed Books; London; 1989. [Google Scholar]
- 24.Dyson T, Moore M. On kinship structure, female autonomy, and demographic behavior in India. Popul Dev Rev. 1983;9(1):35–60. [Google Scholar]
- 25.Heston A, Summers R, Aten B. Penn World Table. Center for International Comparisons at the Univ of Pennsylvania; Philadelphia: 2002. [Google Scholar]
- 26.World Bank . World Development Indicators 1960-2013. World Bank; Washington, DC: 2014. [Google Scholar]
- 27.Population Reference Bureau . 2005 World Population Datasheet. Population Reference Bureau; Washington, DC: 2005. [Google Scholar]
- 28.Castro Martín T. Women’s education and fertility: Results from 26 Demographic and Health Surveys. Stud Fam Plann. 1995;26(4):187–202. [PubMed] [Google Scholar]
- 29.Bongaarts J. The proximate determinants of fertility. Technol Soc. 1987;9:243–260. [Google Scholar]
- 30.Easterlin R. Does human fertility adjust to the environment? Am Econ Rev. 1971;61(3):399–407. [Google Scholar]
- 31.Bongaarts J, Frank O, Lesthaeghe R. The proximate determinants of fertility in sub-Saharan Africa. Popul Dev Rev. 1984;10:511–537. [Google Scholar]
- 32.Das N. Sex preference and fertility behavior: A study of recent Indian data. Demography. 1987;24(4):517–530. [PubMed] [Google Scholar]
- 33.Montgomery M, et al. From Death to Birth: Mortality Decline and Reproductive Change. National Academies Press; Washington, DC: 1997. [PubMed] [Google Scholar]
- 34.Preston S, et al. The Effects of Infant and Child Mortality on Fertility. Academic Press, New York; 1978. [Google Scholar]
- 35.Yajnik CS, et al. Neonatal anthropometry: The thin-fat Indian baby. The Pune Maternal Nutrition Study. Int J Obes Relat Metab Disord. 2003;27(2):173–180. doi: 10.1038/sj.ijo.802219. [DOI] [PubMed] [Google Scholar]
- 36.Agarwal DK, Agarwal KN, Satya K, Agarwal S. Weight gain during pregnancy—a key factor in perinatal and infant mortality. Indian Pediatr. 1998;35(8):733–743. [PubMed] [Google Scholar]
- 37.Hutter I. Being Pregnant in Rural South India: Nutrition of Women and Well-Being of Children. Purdue Univ Press; West Lafayette, IN: 1994. [Google Scholar]
- 38.Billewicz WC, Thomson AM. Clinical significance of weight trends during pregnancy. BMJ. 1957;1(5013):243–247. doi: 10.1136/bmj.1.5013.243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Siega-Riz AM, Adair LS, Hobel CJ. Institute of Medicine maternal weight gain recommendations and pregnancy outcome in a predominantly Hispanic population. Obstet Gynecol. 1994;84(4):565–573. [PubMed] [Google Scholar]
- 40.Abrams B, Selvin S. Maternal weight gain pattern and birth weight. Obstet Gynecol. 1995;86(2):163–169. doi: 10.1016/0029-7844(95)00118-b. [DOI] [PubMed] [Google Scholar]
- 41.Alderman H, Behrman J. Reducing the incidence of low birth weight in low-income countries has substantial economic benefits. World Bank Res Obs. 2006;21(1):25–48. [Google Scholar]
- 42.Bloom SS, Wypij D, Das Gupta M. Dimensions of women’s autonomy and the influence on maternal health care utilization in a north Indian city. Demography. 2001;38(1):67–78. doi: 10.1353/dem.2001.0001. [DOI] [PubMed] [Google Scholar]
- 43.DiNardo J, Fortin M, Lemieux T. Labor market institutions and the distribution of wages, 1973-1992: A semiparametric approach. Econometrica. 1996;64(5):1001–1044. [Google Scholar]
- 44.Geruso M. Black-white disparities in life expectancy: How much can the standard SES variables explain? Demography. 2012;49(2):553–574. doi: 10.1007/s13524-011-0089-1. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.