Significance
This study provides in vivo evidence that Lrp4 loss in osteoblast (OB)-lineage cells results in high–bone-mass deficit. Lrp4 in OB-lineage cells is required to reduce serum levels of sclerostin and to facilitate sclerostin inhibition of OB differentiation and function. These results demonstrate that Lrp4 is a critical player in bone-mass homeostasis and support a model that Lrp4 acts as a sclerostin receptor, inhibiting Wnt/β-catenin signaling and promoting receptor activator of nuclear kappa B ligand (RANKL)-induced osteoclastogenesis. As mutations in the Lrp4 gene have been identified in high–bone-mass patients, this study provides pathophysiological insight into relevant high–bone-mass disorders.
Keywords: LRP4, sclerostin, osteoblasts, osteoclasts, β-catenin
Abstract
Bone mass is maintained by balanced activity of osteoblasts and osteoclasts. Lrp4 (low-density lipoprotein receptor related protein 4) is a member of the LDL receptor family, whose mutations have been identified in patients with high–bone-mass disorders, such as sclerosteosis and van Buchem diseases. However, it remains unknown whether and how Lrp4 regulates bone-mass homeostasis in vivo. Here we provide evidence that Lrp4-null mutation or specific mutation in osteoblast-lineage cells increased cortical and trabecular bone mass, which was associated with elevated bone formation and impaired bone resorption. This phenotype was not observed in osteoclast-selective Lrp4 knockout mice. Mechanistic studies indicate that loss of Lrp4 function in osteoblast-lineage cells increased serum levels of sclerostin, a key factor for bone-mass homeostasis that interacts with Lrp4, but abolished the inhibition of Wnt/β-catenin signaling and osteoblastic differentiation by sclerostin. Concomitantly, sclerostin induction of RANKL (receptor activator of nuclear kappa B ligand) was impaired, leading to a lower ratio of RANKL over OPG (osteoprotegerin) (a key factor for osteoclastogenesis). Taken together, these results support the view for Lrp4 as a receptor of sclerostin to inhibit Wnt/β-catenin signaling and bone formation and identify Lrp4 as a critical player in bone-mass homeostasis.
Bone remodeling is a dynamic process essential for maintenance of skeletal integrity and bone homeostasis (1). Bone mass is tightly regulated by bone-forming osteoblasts (OBs) and bone-resorbing osteoclasts (OCs). OBs are differentiated from bone marrow stromal cells (BMSCs) or mesenchymal progenitor cells, whereas OCs are derived from hematopoietic bone marrow macrophages or myeloid monocytes (BMMs). The balance of bone formation and resorption is critical for maintenance of healthy bone mass. The imbalance of bone formation and resorption could result in high–bone-mass disorders such as sclerosteosis and van Buchem disease or bone loss such as osteoporosis.
The canonical Wnt/β-catenin signaling is critical to regulate bone-mass homeostasis (1, 2). Binding of Wnt ligands to a dual-receptor complex of frizzled and Lrp5/6 leads to accumulation of cytoplasmic β-catenin and translocation of β-catenin into the nucleus to regulate gene expression. This pathway is required for commitment of mesenchymal stem cells to the OB lineage, OB precursor cell proliferation and differentiation, and OC genesis and activation (1–3). Clinically, Lrp5 mutations are associated with the osteoporosis–pseudoglioma syndrome, a low–bone-mass disorder (4), as well as with high–bone-mass disorders (5, 6).
Lrp4 is a member of LDL family protein, containing a large extracellular region with multiple LDLa, EGF-like, and β-propeller repeats, a transmembrane domain, and a short C-terminal region. Lrp4 is a receptor of agrin (7, 8), critical for neuromuscular junction formation. Mice lacking Lrp4 (null allele) die at birth because of inability to breathe (9). Lrp4 is also highly related to Lrp5/6 and interacts with sclerostin, a key extracellular factor for bone remodeling (10–13). Mutations have been identified in Lrp4 and in sclerostin in patients with high–bone-mass disorders, such as sclerosteosis and van Buchem disease (10–16). However, how Lrp4 regulates bone-mass homeostasis remains unclear. Mice harboring a stop codon upstream of the transmembrane domain of Lrp4 exhibit increased bone formation and elevated bone resorption, but decreased bone mineral density (11). Because the extracellular domain of Lrp4 remains intact, it is unclear whether this mutant mouse model represents a gain or loss of Lrp4 function.
Here we provide evidence that Lrp4 in OB-lineage cells suppresses bone overgrowth. Both muscle-rescued Lrp4-null (mr-Lrp4mitt) and OB-selective Lrp4 knockout mice (Lrp4Ocn-cko) showed increased cortical and trabecular bone mass, elevated bone formation, and impaired bone resorption. In contrast, these phenotypes were not observed in OC-selective Lrp4 knockout (Lrp4LysM-cko) mice. Loss of Lrp4 function in OB-lineage cells abolished sclerostin inhibition of Wnt/β-catenin signaling and OB differentiation, despite the fact that serum sclerostin was increased. In addition, Lrp4 deficiency in OB-lineage cells impaired sclerostin induction of receptor activator of nuclear kappa B ligand (RANKL), thus reducing the ratio of RANKL over osteoprotegerin (OPG). These results suggest that Lrp4 in OB-lineage cells is necessary to prevent bone formation and to promote bone resorption, therefore maintaining adequate bone homeostasis. Loss of Lrp4 function in OB-lineage cells results in high bone mass, providing a pathophysiological mechanism of relevant high–bone-mass disorders.
Results
High–Bone-Mass Deficit in Muscle-Rescued Lrp4-Null Mice.
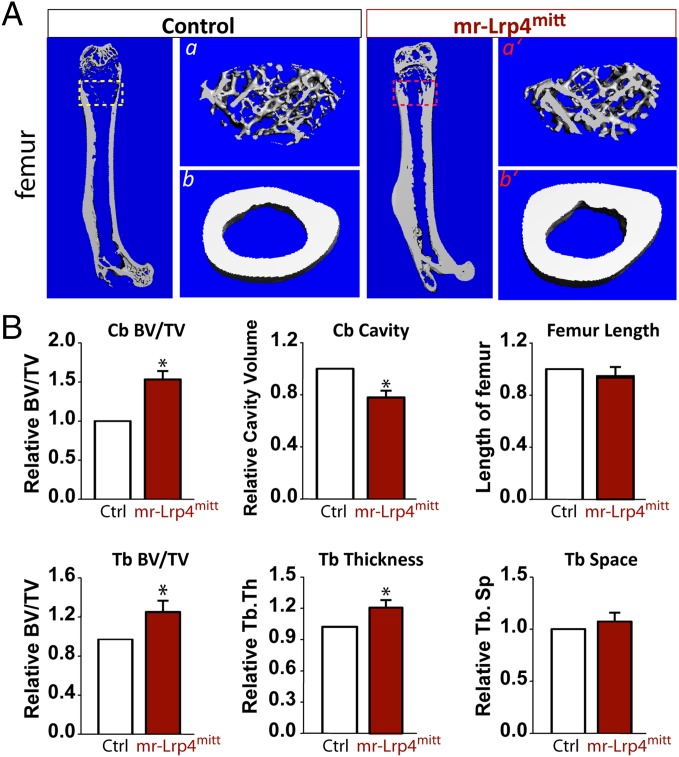
Lrp4mitt is considered as a null allele because the mutation results in a stop codon at the amino acid 377 (Fig. S1A) (9). Lrp4mitt mice die immediately after birth, due to the absence of neuromuscular junction and hence inability to breathe (9, 17, 18). To investigate functions of Lrp4 in bone remodeling, we crossed Lrp4mitt mice with HSA (human skeletal α-actin) promoter-driven Lrp4 transgenic mice (HSA–Lrp4), where Lrp4 was expressed specifically in skeletal muscles (Fig. S1 A and B). Western blot analysis indicated that in muscle-rescued Lrp4mitt (thus referred to as mr-Lrp4mitt), Lrp4 was detected only in skeletal muscle, but not in other tissues (Fig. S1 C and D). In agreement, muscle expression of Lrp4 restores neuromuscular junction formation (19), but not digit development (Fig. S1 E and F). The syndactyly-like digit deficit was detected in mr-Lrp4mitt mice (Fig. S1 E and F), as it was in Lrp4mitt mice (9, 17, 18). However, the mr-Lrp4mitt mice were viable and had relatively normal body size with a slightly reduced body weight (Fig. S1E). Intriguingly, the mutant long bones (e.g., tibia and femurs) appeared to be white/pale in color (Fig. S1F); that may be due to reduced bone marrow cells. Microcomputer tomographic (μCT) analysis of femurs from 3-mo-old mr-Lrp4mitt mice showed decreased bone marrow cavity and increased cortical bone volumes/total volumes (Cb, BV/TV), compared with that of transgenic control littermates (HSA–Lrp4) (Fig. 1 A and B). In addition, the trabecular bone volume (Tb, BV/TV) and trabecular bone thickness (Tb.Th) were increased, but trabecular numbers (Th.N) were decreased (Fig. 1 A and B). These results indicate that both cortical and trabecular bone volumes were increased in Lrp4 mutant mice, suggesting a negative role of Lrp4 in regulating bone mass.
Fig. 1.
High–bone-mass deficit in young adult mr-Lrp4mitt mice. The μCT analysis of femurs from 3-mo-old control (HSA–Lrp4) and mr-Lrp4mitt littermates. Five males per genotype were examined blindly. Representative images are shown in A. The 3D images shown at Right (a, a’, b, and b’) were derived from the marked corresponding regions of the femurs shown at Left. Quantification analyses (mean ± SD, n = 5) are presented in B. *P < 0.05, significant difference.
High–Bone-Mass Deficit in OB-, but Not OC-, Selective Lrp4 Knockout Mice.
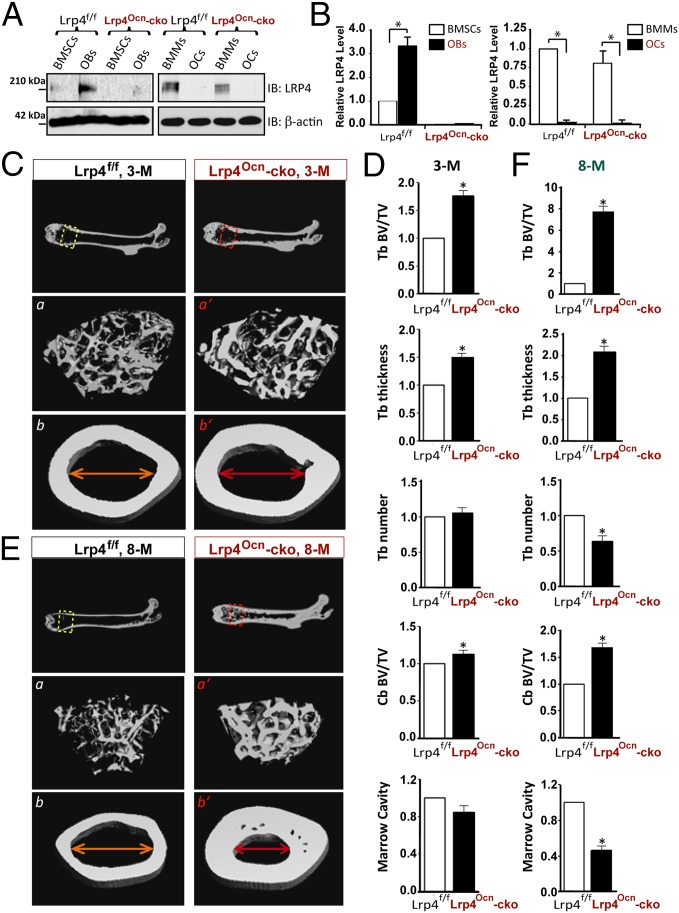
To understand how Lrp4 regulates bone mass, we examined its expression in OB, OC, and precursor cells (BMSC and BMM, respectively). Lrp4 level in BMSC was moderate, but increased in OBs; on the other hand, Lrp4 in BMMs was relatively high, but diminished in OCs (Fig. 2 A and B). To determine whether Lrp4 in OBs is crucial, we crossed floxed Lrp4 (Lrp4f/f) mice with osteocalcin (Ocn)–Cre to generate Lrp4Ocn-cko mice (Fig. S2). In Ocn–Cre mice, Cre expression, under the control of Ocn promoter, is active at mature OB-lineage cells including OBs, osteocytes, and BMSCs (20). In agreement, Lrp4 expression was diminished specifically in BMSCs and OBs, but not BMMs, in young adult Lrp4Ocn-cko mice (Fig. 2 A and B). Note that adult Lrp4Ocn-cko mice were slightly larger in body size and heavier in body weight and had normal digit development (Fig. S2B). As shown in Fig. 2, μCT analysis of 3-mo-old Lrp4Ocn-cko mice showed increased trabecular and cortical bone volumes, compared with littermate controls (Lrp4f/f). The trabecular thickness was also increased in Lrp4Ocn-cko femurs (Fig. 2 C and D). These phenotypes appeared to be more severe in Lrp4Ocn-cko mice at the age of 8 mo old (Fig. 2 E and F). Together, these results indicate that Lrp4 in OB-lineage cells is critical for decrease of bone mass. This notion was further supported by studies of Lrp4LysM-cko mice, which were generated by crossing Lrp4f/f with lysozyme M (LysM)–Cre that expresses Cre selectively in mature macrophages (precursors of OCs) (Fig. S2 C and D) (21). As expected, Lrp4 level was diminished in BMMs in Lrp4LysM-cko mice (Fig. S3 A and B). Compared with the controls, no significant change was observed in cortical bone volume and bone marrow cavity in Lrp4LysM-cko femurs (Fig. S3 C and D). However, there appeared to be decreases in trabecular bone volume and thickness in Lrp4LysM-cko femurs, instead of increases observed in mr-Lrp4mitt or Lrp4Ocn-cko mice (Fig. S3 C and D).
Fig. 2.
High–bone-mass deficit in Lrp4Ocn-cko mice. (A and B) Western blot analysis of Lrp4 expression in BMSCs, OBs, BMMs, and OCs derived from 2-mo-old Lrp4f/f (control) and Lrp4Ocn-cko mice. Quantification analysis (mean ± SD, n = 3) is presented in B. *P < 0.05, significant difference. (C–F) The μCT analysis of femurs of control (Lrp4f/f) and Lrp4Ocn-cko littermates at ages of 3 mo (C and D) and 8 mo old (E and F). Five males per genotype per age group were examined blindly. Representative images are shown in C and E. The 3D images shown at Bottom (a, a’, b, and b’) were derived from the marked corresponding regions shown at Top. Quantification analyses (mean ± SD, n = 5) are presented in D and F. *P < 0.05, significant difference.
Increased Bone Formation in mr-Lrp4mitt and Lrp4Ocn-cko Mice.
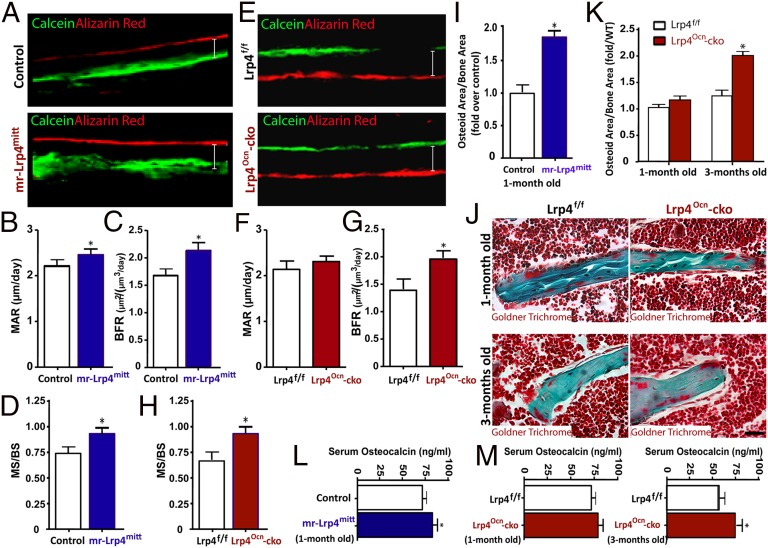
The bone-mass increase in both mr-Lrp4mitt and Lrp4Ocn-cko mice may result from enhanced bone formation and/or decreased bone resorption. To address this question, we asked whether bone formation is altered by loss of Lrp4 in OB-lineage cells. Neonatal mice were injected with two doses (separated by a 12-d interval) of calcein and alizarin red, both fluorescent markers of bone formation. Mineral apposition rate (MAR), bone formation rate (BFR), and mineral surface (MS) / bone surface (BS) were assessed in nondecalcified histological sections of femurs and tibia. As shown in Fig. 3 A–D, the MAR, BFR, and MS/BS were all increased in 1-mo-old mr-Lrp4mitt mice, compared with littermate controls, suggesting that Lrp4 deficiency promotes bone formation. Similar increases in BFR and MS/BS were observed in 1-mo-old Lrp4Ocn-cko mice; MAR in the mutant mice showed trend of increase, which, however, did not reach statistical significance (Fig. 3 E–H). To verify these results, we examined bone formation in mice at different ages by Goldner’s trichrome staining, which labels newly formed osteoids (the unmineralized bone matrix). Again, a robust increase in osteoid numbers per unit of bone surface was observed in 1-mo-old mr-Lrp4mitt mice (Fig. 3I). For Lrp4Ocn-cko mice, the increase was not significant until the age of 3 mo old (Fig. 3 J and K). This age-dependent progression was also observed with serum levels of osteocalcin, a marker of bone formation. As shown in Fig. 3 L and M, serum osteocalcin level was increased in 1-mo-old mr-Lrp4mitt and 3-mo-old Lrp4Ocn-cko mice. Taken together, these results demonstrate increased bone formation in mice lacking Lrp4 in OB-lineage cells and suggest that Lrp4 may serve as a negative regulator of bone formation, identifying a possible cellular mechanism.
Fig. 3.
Increased bone formation in both mr-Lrp4mitt and Lrp4Ocn-cko mice. (A–H) Increased bone formation in both mr-Lrp4mitt (A–D) and Lrp4Ocn-cko (E–H) mice detected by dynamic histomorphometric measurements of calcein and alizarin red double-labeled femurs (SI Materials and Methods). (A and E) Representative images of histologic sections showing calcein and alizarin red double-labeling of endocortical bone in femur mid diaphysis of Lrp4 mutant and their control mice. Ec. MAR (endocortical mineral apposition rate) (B and F), Ec. BFR (endocortical bone formation rate) (C and G), and MS (mineral surface) / BS (bone surface) (D and H) are presented. The values of mean ± SD from five males per genotype are shown. *P < 0.05, significant difference. (I–K) Increased bone formation in both mr-Lrp4mitt (I) and Lrp4Ocn-cko (J and K) femurs detected by Goldner trichrome-staining analyses (SI Materials and Methods). Representative images are shown in J. Quantification analyses are presented in I and K. The values of mean ± SD from three males per genotype are shown. *P < 0.05, significant difference. (L and M) Increased serum osteocalcin levels in 1-mo-old mr-Lrp4mitt and 3-mo-old Lrp4Ocn-cko mice. The serum osteocalcin levels were measured by ELISAs (SI Materials and Methods). The values of mean ± SD from five males per genotype are shown. *P < 0.05, significant difference. Note that HSA–Lrp4 and Lrp4f/f serve as controls for mr-Lrp4mitt and Lrp4Ocn-cko, respectively.
Decreased Bone Resorption in Both mr-Lrp4mitt and Lrp4Ocn-cko Mice.
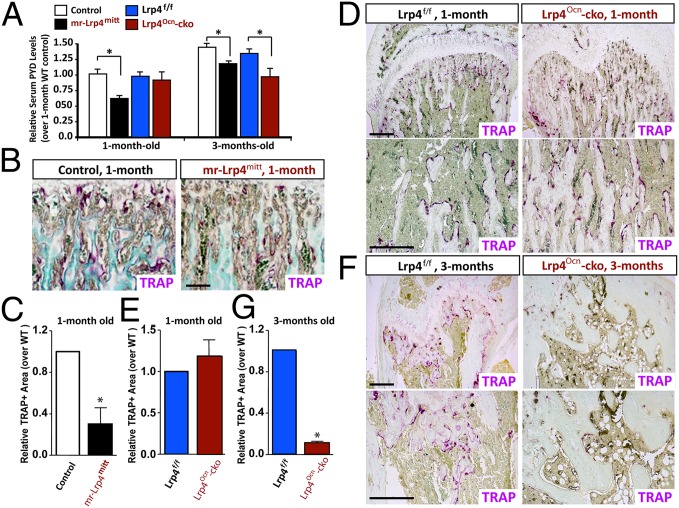
We next determined whether Lrp4 deficiency alters bone resorption by measuring serum levels of deoxy-pyridinoline (PYD), a collagen cross-link molecule that provides valuable information on bone resorption. At the age of 3 mo old, serum PYD was significantly decreased in both mr-Lrp4mitt and Lrp4Ocn-cko mice, compared with littermate controls (Fig. 4A). As with bone formation deficit, PYD reduction was detected in 1-mo-old mr-Lrp4mitt, but not Lrp4Ocn-cko mice (Fig. 4A). The reduction appears to be specific because it was not observed in OC-selective Lrp4 knockout (Lrp4LysM-cko) mice (Fig. S4). In fact, serum PYD was increased in Lrp4LysM-cko mice (Fig. S4A). These results suggest an age-dependent inhibition of bone resorption by loss of Lrp4 in OB-lineage cells. Altered bone resorption could be due to a change in OC number and/or function. We thus asked if OC genesis is impaired in Lrp4 mutant mice. As shown in Fig. 4 B and C, the number of tartrate-resistant acid phosphatase positive (TRAP+) OCs in femurs was lower in mr-Lrp4mitt mice than in littermate controls at the age of 1 mo old, suggesting that Lrp4 is necessary for proper control of OC genesis. Intriguingly, TRAP+ OCs were also lower in 3-mo-old, but not 1-mo-old, Lrp4Ocn-cko mice than in controls (Fig. 4 D and E). In contrast, the number of TRAP+ OCs in Lrp4lysM-cko mice was slightly greater than that of littermate controls (Fig. S4 B–F). These observations suggest that OC genesis appears to be positively regulated by Lrp4 in OB-lineage cells, but negatively regulated by Lrp4 in OC-lineage cells.
Fig. 4.
Decreased serum levels of PYD and reduced TRAP+ OCs in both mr-Lrp4mitt and Lrp4Ocn-cko mice. (A) Measurements of serum levels of PYD in 1-mo-old and 3-mo-old controls and Lrp4 mutant mice by ELISA analyses (SI Materials and Methods). The values of mean ± SD from five males per genotype are shown. *P < 0.05, significant difference. (B–G) TRAP-staining analysis of femur sections from different aged control and Lrp4 mutant littermates (five mice per age group per genotype, both males and females). Representative images of TRAP staining are shown in B, D, and F. (Scale bars, 100 μm.) The quantitative analysis of TRAP+ cells per unit bone surface (BS) was carried out in trabecular bones of femurs from different aged control and indicated Lrp4 mutant mice, and the values of mean ± SD from five mice are shown in C, E, and G. *P < 0.05, significant difference. Note that HSA–Lrp4 and Lrp4f/f serve as controls for mr-Lrp4mitt and Lrp4Ocn-cko, respectively.
Requirement of Lrp4 in OB-Lineage Cells for Sclerostin to Inhibit OB Differentiation.
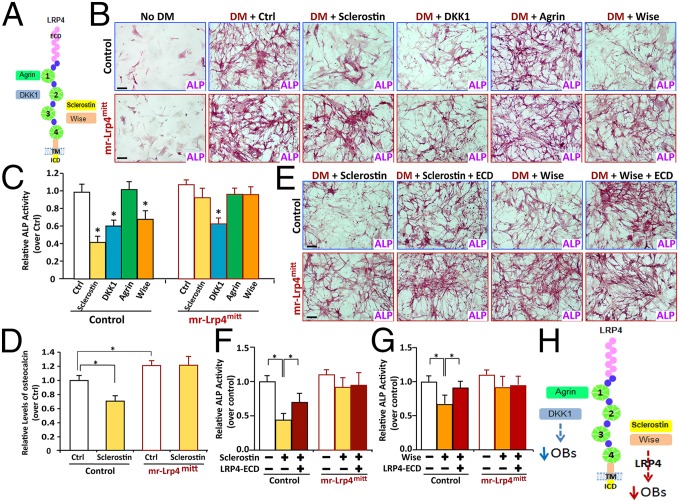
Lrp4 interacts with multiple extracellular proteins, including sclerostin (SOST), Wise (a paralog of sclerostin that is also called Sostdc1), dickkopf-1 (DKK1), and agrin (Fig. 5A) (7, 8, 11, 22). Sclerostin, Wise, and DKK1 are negative regulators of Wnt/β-catenin signaling and/or bone formation (1, 13, 23, 24). To determine whether their effect requires Lrp4, we cultured BMSCs from mr-Lrp4mitt and control littermates and examined in vitro OB differentiation by ALP (alkaline phosphatase) staining and osteocalcin expression. No difference of ALP activity was detected between control and Lrp4-deficient BMSCs under basal OB culture condition (Fig. S5 A–D). The mRNA of collagen X, an OB marker, was at a similar level between Lrp4 mutant and control BMSCs (Fig. S5E). However, the expression of osteocalcin, a marker of bone formation, was increased in Lrp4 mutant BMSCs (Fig. S5F). These results suggest that Lrp4 may have little effect on OB differentiation, but reduces OB function/bone formation. We then determined whether sclerostin, DKK1, and agrin affect OB differentiation and, if yes, whether the effect requires Lrp4. Condition media (CMs) containing sclerostin, DKK1, or agrin were collected from respectively transfected HEK293 cells (Fig. S6 A–C). Control and Lrp4 mutant BMSCs were induced for OB differentiation in the presence of the CM. As shown in Fig. 5 B and C, both sclerostin and DKK1, but not agrin, reduced ALP activity (an indicator for OB differentiation), in agreement with previous reports (1, 13, 23, 24). Interestingly, the inhibitory effect of sclerostin was diminished in mr-Lrp4mitt BMSCs (Fig. 5 B and C), suggesting that Lrp4 is necessary for sclerostin inhibition of OB differentiation. To further test this view, we examined osteocalcin expression in BMSCs treated with or without sclerostin. Indeed, sclerostin reduced osteocalcin expression in control, but not Lrp4 mutant, BMSCs (Fig. 5D). Together, these results indicate the requirement of Lrp4 for sclerostin inhibition of OB differentiation/function. Notice that Lrp4 is also necessary for Wise inhibition of OB differentiation, but not for the regulation by DKK1 (Fig. 5 B and C), indicating the specificity of the effect. Moreover, the inhibitory effect of sclerostin and Wise was attenuated by Lrp4–extracellular domain (Lrp4–ECD), the polypeptide containing the entire Lrp4 extracellular domain that is able to interact with sclerostin, Wise, and agrin (7, 8, 11, 18, 22). This suggested that sclerostin/Wise inhibition is mediated by direct binding to the extracellular region of Lrp4, supporting Lrp4 as a receptor.
Fig. 5.
Impairment of sclerostin and Wise suppression of OB differentiation in Lrp4-deficient BMSCs. (A) Schematic of Lrp4 structure and its potential ligands. (B and C) Sclerostin and Wise, but not DKK1, reduced ALP activity in an Lrp4-dependent manner. The control, sclerostin, DKK1, and agrin condition medium (CM) were mixed with the OB differentiation medium (DM) at a ratio of 1:1 and incubated with control and Lrp4 mutant BMSCs to induce OB differentiation for 1 wk. The Wise recombinant protein (100 ng/mL) was tested. OB-like cells were subjected to ALP-staining analysis. Representative images are shown in B. (Scale bars, 80 μm.) Quantitative analyses (mean ± SD from three different cultures) are presented in C. Data were normalized by WT controls. *P < 0.05, significant difference. (D) Sclerostin reduced osteocalcin expression in an Lrp4-dependent manner. BMSCs were treated with control CM or sclerostin CM for 24 h. mRNAs were subjected to real-time PCR analyses. The values were normalized to β-actin and WT controls. Means ± SD values from three different experiments are presented. *P < 0.05, significant difference. (E–G) Reduction of the sclerostin/Wise inhibitory effects by Lrp4–ECD. As in B, the indicated CM were mixed with the DM (1:1), and then incubated with Lrp4 mutant and control BMSCs to induce OB differentiation for 1 wk. Representative images (E) and quantitative analyses (mean ± SD from three different cultures) (F and G) are shown. Data were normalized by WT controls. *P < 0.05, significant difference. (Scale bars, 80 μm.) (H) Illustration of a working model for Lrp4-binding protein’s effect on OB differentiation. Note that sclerostin and Wise inhibit OB in an Lrp4-dependent manner; DKK1 inhibition of OB is Lrp4-independent; and agrin has little to no effect on this event.
Necessity of Lrp4 in OB-Lineage Cells for Sclerostin to Suppress Wnt/β-Catenin Signaling.
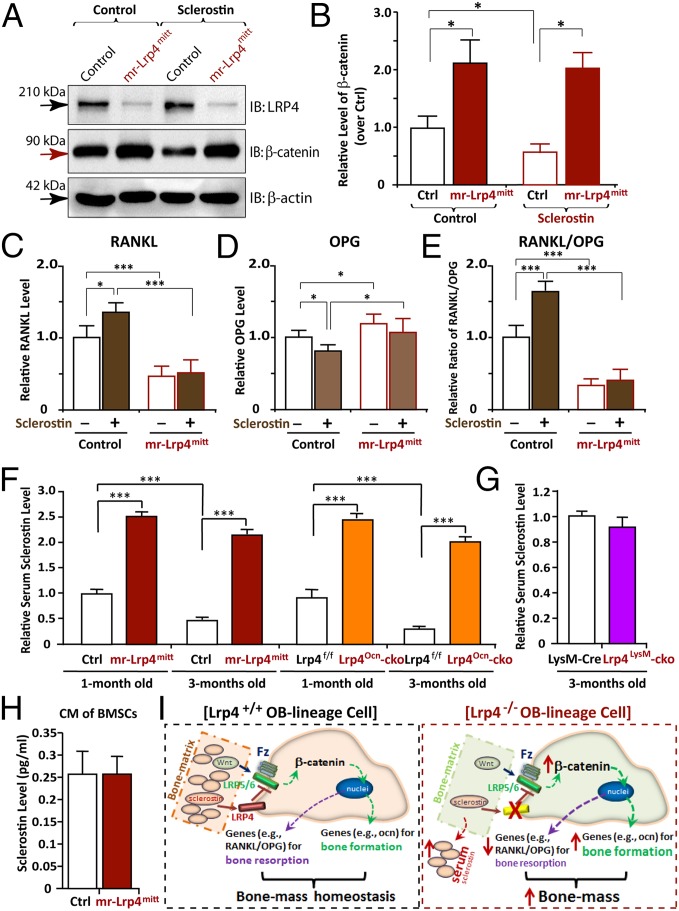
Sclersotin is believed to be an antagonist of Wnt/β-catenin signaling to inhibit OB differentiation/function (1). We asked whether Lrp4 in BMSCs is required for sclerostin to inhibit Wnt/β-catenin signaling. The level of β-catenin, a key indicator of Wnt canonical signaling, was examined in control and Lrp4-deficient BMSCs treated with or without sclerostin. A marked increase in β-catenin was detected in Lrp4-deficient BMSCs, compared with the WT controls (Fig. 6 A and B). In response to sclerostin, β-catenin was decreased in control, but not Lrp4 mutant, BMSCs (Fig. 6 A and B). Notice that transcripts of SOST (sclerostin) and Wnt5a were unchanged, and those of DKK1 (an inhibitor of Wnt signaling) were increased in Lrp4 mutant BMSCs (Fig. S7 A and B), suggesting that the β-catenin alteration is not due to the change in Wnt5a activation or DKK1 inactivation. These results thus support the view for Lrp4 as a receptor of sclerostin to negatively regulate Wnt/β-catenin signaling.
Fig. 6.
Impairment of sclerostin reduction of β-catenin and induction of RANKL/OPG in Lrp4-deficient BMSCs and increase of serum sclerostin in both mr-Lrp4mitt and Lrp4Ocn-cko, but not Lrp4LysM-cko, mice. (A and B) Increase of β-catenin level and impairment of sclerostin reduction of β-catenin in Lrp4-deficient BMSCs. BMSCs were treated with control CM or sclerostin CM for 12 h. Cell lysates were subjected to Western blot analyses using indicated antibodies. Quantification analysis by ImageJ software is shown in B. The values of mean ± SD from three different experiments were presented. Data were normalized by β-actin loading and WT controls. *P < 0.05, significant difference. (C–E) Failure of sclerostin induction of RANKL/OPG in Lrp4-deficient BMSCs. BMSCs were treated with control CM or sclerostin CM for 24 h. The mRNAs were subjected to real-time PCR analyses. The values are normalized to β-actin and WT controls. Means ± SD values from three different experiments are presented. *P < 0.05; ***P < 0.01, significant difference. (F and G) Increased serum levels of sclerostin in both mr-Lrp4mitt and Lrp4Ocn-cko, but not Lrp4LysM-cko, mice. (H) A comparable level of CM–sclerostin between WT control and Lrp4 mutant BMSCs. In F–H, the serum and CM–sclerostin were measured by ELISA analysis (SI Materials and Methods). Means ± SD values from three different animals per genotype or three different cultures are presented. Data were normalized to their controls. Note that the control values of serum sclerostin in HSA–Lrp4 mice (1 mo old), Lrp4f/f (1 mo old), and LysM–Cre (3 mo old) were 750, 700, and 350 pg/mL, respectively. ***P < 0.01, significant difference. (I) Illustration of a working model for Lrp4 in OB-lineage cells to serve as a receptor of sclerostin, sequestering sclerostin into bone matrix, suppressing Wnt/β-catenin signaling and bone formation, and promoting RANKL-induced OC genesis and bone resorption, thus maintaining bone-mass homeostasis.
Requirement of Lrp4 in OB-Lineage Cells for Sclerostin to Induce RANKL Expression.
To understand how Lrp4 in OB-lineage cells regulates OC genesis and activation, we determined whether Lrp4 is necessary for expressing proteins crucial for OC genesis. They included macrophage colony-stimulating factor (M-CSF), which is critical for OC precursor cell proliferation and survival; RANKL, which is essential for OC genesis and activation; and OPG, an antagonist of RANKL (25, 26). M-CSF mRNA in Lrp4-deficient BMSCs was comparable to that of control cells (Fig. S7C). In contrast, RANKL was markedly reduced, but OPG was slightly increased, in mutant cells (Fig. S7C). This resulted in a significant decrease in the ratio of RANKL over OPG (Fig. S7D). Intriguingly, sclerostin increased RANKL, but reduced OPG, in control BMSCs, resulting in an elevation of the ratio of RANKL over OPG (Fig. 6 C–E). This sclerostin’s effect was also abolished in Lrp4-deficient BMSCs (Fig. 6 C–E), demonstrating the necessity of Lrp4 for sclerostin to induce RANKL/OPG expression.
Elevated Serum Sclerostin in Both mr-Lrp4mitt and Lrp4Ocn-cko, but Not Lrp4LysM-cko, Mice.
The requirement of Lrp4 for sclerostin to suppress β-catenin signaling and OB differentiation and to induce RANKL/OPG expression supports the view for Lrp4 in OB-lineage cells as a receptor of sclerostin. Sclerostin is a soluble protein in the blood/serum as well as in bone matrix (1). To determine whether Lrp4 regulates its distribution, we measured serum sclerostin by ELISA analysis. Serum sclerostin levels were markedly elevated in both mr-Lrp4mitt and Lrp4Ocn-cko mice, compared with control littermates (Fig. 6 F and G). The increase was detected at neonatal age of Lrp4 mutant mice (Fig. 6F), and not due to the increased expression, because SOST transcripts in Lrp4 mutant BMSCs were comparable to those of controls (Fig. S7A). Sclerostin levels in CMs of Lrp4 mutant BMSCs were similar to those of controls (Fig. 6H), excluding altered secretion or uptake of sclerostin in Lrp4-deficient BMSCs. Finally, serum sclerostin levels in Lrp4LysM-cko mice were similar to those of controls, indicating the specific function of Lrp4 in OB-lineage cells. Together, these results suggest that Lrp4 in OB-lineage cells is critical to control serum sclerostin levels, probably by interacting and sequestering sclerostin into the bone matrix.
Discussion
We showed that Lrp4 loss in OB-lineage cells increased cortical and trabecular bone mass. The deficits were observed in Lrp4-null and OB-selective Lrp4 knockout mice, but not in OC-specific mutant mice. The increased bone mass in Lrp4 mutant mice was associated with elevated bone formation and impaired OC genesis and bone resorption. Loss of Lrp4 in OB-lineage cells abolished sclerostin inhibition of Wnt/β-catenin signaling and OB differentiation and sclerostin induction of the ratio of RANKL over OPG (a key factor for osteoclastogenesis). These results thus identify Lrp4 as a key player in bone-mass homeostasis and support a working model depicted in Fig. 6I. In this model, Lrp4 in OB-lineage cells suppresses bone formation and promotes bone resorption probably by sequestering sclerostin into the bone matrix and serving as a sclerostin receptor to negatively regulate Wnt/β-catenin signaling and promote RANKL-induced osteoclastogenesis.
The high–bone-mass phenotype in mr-Lrp4mitt and Lrp4Ocn-cko mice was remarkably similar to that of SOST mutant mice and/or animals treated with anti-sclerostin antibodies, supporting the view for Lrp4 as a receptor of sclerostin in vivo. The high–bone-mass phenotypes in Lrp4 mutant mice also resemble clinical features of sclerosteosis and van Buchem disease (10, 14–16). Interestingly, mutations in both Lrp4 and SOST genes have been identified in patients with sclerosteosis and van Buchem diseases (10, 14–16). SOST’s gene product, sclerostin, binds to Lrp4 (10, 11). Lrp4 loss in OB-lineage cells impaired sclerostin inhibition of Wnt/β-catenin signaling and OB differentiation (Figs. 5 and 6) and increased serum levels of sclerostin (Fig. 6). These observations suggest that osteoblastic Lrp4 serves as a receptor for sclerostin, and impaired sclerostin–Lrp4 pathway in OB-lineage cells might be a pathophysiological mechanism for sclerosteosis and van Buchem diseases.
Note that in addition to high–bone-mass deficit, young adult mr-Lrp4mitt mice display additional phenotypes that were not observed in Lrp4Ocn-cko or LrpLysM-cko mice. For example, the digit development was impaired in mr-Lrp4mitt, but not Lrp4Ocn-cko or LrpLysM-cko, mice (Figs. S1 and S2), suggesting that Lrp4 loss in other cell types (e.g., chondrocytes) may contribute to this deficit. Interestingly, the digit deficit resembles the hand defect for sclerosteosis patients as well as for patients with Cenani–Lenz syndrome (27–29). Lrp4 mutations are also identified in patients with Cenani–Lenz syndrome, in addition to bone-related diseases (27–29). A similar digit defect is also reported in SOST−/−, but not in Wise−/− mice (30). These observations thus suggest that the sclerostin–Lrp4 pathway plays an important role not only in adult bone-mass homeostasis, but also in limb development.
How does Lrp4 in OB-lineage cells regulate bone-mass homeostasis? High bone mass is temporally associated with increased bone formation and decreased bone resorption in both mr-Lrp4mitt and Lrp4Ocn-cko mutant mice, suggesting that Lrp4 in OB-lineage cells acts as an inhibitor of osteoblastic function, but a stimulator of osteoclastic activation, thus promoting homeostasis of bone mass. This view is supported by in vitro BMSC-OB culture studies. Lrp4 in OB-lineage cells is required for sclerostin inhibition of OB differentiation and function (Fig. 5), as well as sclerostin induction of RANKL/OPG (Fig. 6). Thus, the increased bone formation in Lrp4 mutant mice may result from loss of sclerostin’s inhibitory effect.
How does Lrp4 in OB-lineage cells promote osteoclastic function in a cell nonautonomous manner? Several mechanisms may underlie this event. First, the reduced RANKL/OPG (Fig. 6E and Fig. S7D) may impair OC genesis and bone resorption in mr-Lrp4mitt and Lrp4Ocn-cko mutant mice because the ratio of RANKL over OPG is essential for OC genesis and activation (25, 26). Second, the increased DKK1 (Fig. S7A) may inhibit Wnt5a-induced OC genesis (1). Third, the increased osteoid in the mutant mice (Fig. 3 I–K) may also contribute to the reduction of bone resorption. Whereas these are possible underlying mechanisms, it remains unclear exactly how Lrp4 in OB-lineage cells regulates RANKL/OPG expression and osteoid formation. Note that Wnt/β-catenin signaling in OB-lineage cells is critical for OPG expression and osteoid formation (1). We thus propose a working model such that Lrp4 in OB-lineage cells suppresses bone formation and promotes OC genesis by inhibiting Wnt/β-catenin signaling (Fig. 6I).
It remains unclear exactly how Lrp4 mediates sclerostin inhibition of Wnt/β-catenin signaling and bone formation. Sclerostin, Wise, and DKK1 are soluble factors that inhibit Wnt signaling presumably by binding LRP5/6 (12, 13, 31). Why do sclerostin/Wise, but not DKK1, inhibit OB in an Lrp4-dependent manner? It is noteworthy that the central core region of sclerostin is critical for binding to the first β-propeller domain of LRP5/6, not for binding to Lrp4 (32). The interaction of sclerostin with Lrp4 is impaired by two sclerosteosis-associated mutations, R1170W and W1186S (10), suggesting that the 3rd β-propeller domain of Lrp4 may interact with sclerostin. Moreover, serum levels of sclerostin are markedly increased in both mr-Lrp4mitt and Lrp4Ocn-cko mice (Fig. 6 E and F). In light of these observations, we speculate that Lrp4 interaction with sclerostin may result in local enrichment of sclerostin in bone matrix and change sclerostin conformation to expose its central core binding site for LRP5/6 (Fig. 6I). However, future work will be needed to investigate which domain or motif in Lrp4 interacts with sclerostin/Wise and how the interaction is regulated. Such knowledge may help to explain the complexity of Lrp4 behavior in maintaining bone-mass homeostasis.
Materials and Methods
Animals.
All experimental procedures were approved by the Animal Subjects Committee at the Georgia Health Sciences University, according to US National Institutes of Health guidelines. Generation of Lrp4mitt and Lrp4-floxed mice were described previously (9, 18). Additional information is provided in SI Materials and Methods.
Measurements of Serum Levels of Osteocalcin, PYD, and Sclerostin.
Mouse serum samples were collected and subjected to ELISA analysis. Additional information is provided in SI Materials and Methods.
In Vitro OB Culture and Treatments with Lrp4 Ligands: Sclerostin, DKK1, Agrin, Wise, and Lrp4–ECD.
Whole bone marrow cells were isolated from lone bones of 8-wk-old WT and Lrp4 mutant mice. Additional information is provided in SI Materials and Methods.
RNA Isolation and Real-Time PCR.
Total RNA was isolated from BMSCs by TRIzol extraction (Invitrogen). Additional information is provided in SI Materials and Methods.
In addition, microcomputed tomography (μCT), bone histomorphometric analysis, dynamic bone histomorphometry to measure the bone formation rate in vivo, RNA isolation and real-time PCR; plasmids and transient transfection, and statistical analysis are described in the SI Materials and Methods.
Note.
Since the submission of our paper, Chang et al. report that ablation of Lrp4 in OB increases bone formation and bone mass and elevates serum sclerostin levels (33).
Supplementary Material
Acknowledgments
We are grateful to Dr. Tom Clemens (Johns Hopkins Medical School) and Dr. L. A. Niswander (University of Colorado) for valuable mouse lines. We thank Ms. Xue-Mei Cao (University of Alabama at Birmingham) for μCT analysis, Drs. Xu Feng (University of Alabama at Birmingham), Xingming Shi (Georgia Regents University), and Mei Wan (Johns Hopkins Medical School) for reagents, and members of the W.-C.X. and L.M. laboratories for helpful discussions. This study was supported in part by grants from the National Institutes of Health (to W.-C.X. and L.M.), Department of Veterans Affairs (to W.-C.X. and L.M.), and Beijing Nova Program (Z131102000413033 to H.W.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1419714112/-/DCSupplemental.
References
- 1.Baron R, Kneissel M. WNT signaling in bone homeostasis and disease: From human mutations to treatments. Nat Med. 2013;19(2):179–192. doi: 10.1038/nm.3074. [DOI] [PubMed] [Google Scholar]
- 2.Day TF, Guo X, Garrett-Beal L, Yang Y. Wnt/beta-catenin signaling in mesenchymal progenitors controls osteoblast and chondrocyte differentiation during vertebrate skeletogenesis. Dev Cell. 2005;8(5):739–750. doi: 10.1016/j.devcel.2005.03.016. [DOI] [PubMed] [Google Scholar]
- 3.Glass DA, 2nd, et al. Canonical Wnt signaling in differentiated osteoblasts controls osteoclast differentiation. Dev Cell. 2005;8(5):751–764. doi: 10.1016/j.devcel.2005.02.017. [DOI] [PubMed] [Google Scholar]
- 4.Gong Y, et al. LDL receptor-related protein 5 (LRP5) affects bone accrual and eye development. Cell. 2001;107(4):513–523. doi: 10.1016/s0092-8674(01)00571-2. [DOI] [PubMed] [Google Scholar]
- 5.Little RD, et al. A mutation in the LDL receptor-related protein 5 gene results in the autosomal dominant high-bone-mass trait. Am J Hum Genet. 2002;70(1):11–19. doi: 10.1086/338450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Boyden LM, et al. High bone density due to a mutation in LDL-receptor-related protein 5. N Engl J Med. 2002;346(20):1513–1521. doi: 10.1056/NEJMoa013444. [DOI] [PubMed] [Google Scholar]
- 7.Zhang B, et al. LRP4 serves as a coreceptor of agrin. Neuron. 2008;60(2):285–297. doi: 10.1016/j.neuron.2008.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kim N, et al. Lrp4 is a receptor for Agrin and forms a complex with MuSK. Cell. 2008;135(2):334–342. doi: 10.1016/j.cell.2008.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Weatherbee SD, Anderson KV, Niswander LA. LDL-receptor-related protein 4 is crucial for formation of the neuromuscular junction. Development. 2006;133(24):4993–5000. doi: 10.1242/dev.02696. [DOI] [PubMed] [Google Scholar]
- 10.Leupin O, et al. Bone overgrowth-associated mutations in the LRP4 gene impair sclerostin facilitator function. J Biol Chem. 2011;286(22):19489–19500. doi: 10.1074/jbc.M110.190330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Choi HY, Dieckmann M, Herz J, Niemeier A. Lrp4, a novel receptor for Dickkopf 1 and sclerostin, is expressed by osteoblasts and regulates bone growth and turnover in vivo. PLoS ONE. 2009;4(11):e7930. doi: 10.1371/journal.pone.0007930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Semënov M, Tamai K, He X. SOST is a ligand for LRP5/LRP6 and a Wnt signaling inhibitor. J Biol Chem. 2005;280(29):26770–26775. doi: 10.1074/jbc.M504308200. [DOI] [PubMed] [Google Scholar]
- 13.Li X, et al. Sclerostin binds to LRP5/6 and antagonizes canonical Wnt signaling. J Biol Chem. 2005;280(20):19883–19887. doi: 10.1074/jbc.M413274200. [DOI] [PubMed] [Google Scholar]
- 14.Brunkow ME, et al. Bone dysplasia sclerosteosis results from loss of the SOST gene product, a novel cystine knot-containing protein. Am J Hum Genet. 2001;68(3):577–589. doi: 10.1086/318811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Balemans W, et al. Identification of a 52 kb deletion downstream of the SOST gene in patients with van Buchem disease. J Med Genet. 2002;39(2):91–97. doi: 10.1136/jmg.39.2.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Loots GG, et al. Genomic deletion of a long-range bone enhancer misregulates sclerostin in van Buchem disease. Genome Res. 2005;15(7):928–935. doi: 10.1101/gr.3437105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ahn Y, Sims C, Logue JM, Weatherbee SD, Krumlauf R. Lrp4 and Wise interplay controls the formation and patterning of mammary and other skin appendage placodes by modulating Wnt signaling. Development. 2013;140(3):583–593. doi: 10.1242/dev.085118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wu H, et al. Distinct roles of muscle and motoneuron LRP4 in neuromuscular junction formation. Neuron. 2012;75(1):94–107. doi: 10.1016/j.neuron.2012.04.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yumoto N, Kim N, Burden SJ. Lrp4 is a retrograde signal for presynaptic differentiation at neuromuscular synapses. Nature. 2012;489(7416):438–442. doi: 10.1038/nature11348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhao G, et al. Targeted overexpression of insulin-like growth factor I to osteoblasts of transgenic mice: Increased trabecular bone volume without increased osteoblast proliferation. Endocrinology. 2000;141(7):2674–2682. doi: 10.1210/endo.141.7.7585. [DOI] [PubMed] [Google Scholar]
- 21.Cross M, Mangelsdorf I, Wedel A, Renkawitz R. Mouse lysozyme M gene: isolation, characterization, and expression studies. Proc Natl Acad Sci USA. 1988;85(17):6232–6236. doi: 10.1073/pnas.85.17.6232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kumar J, et al. LRP4 association to bone properties and fracture and interaction with genes in the Wnt- and BMP signaling pathways. Bone. 2011;49(3):343–348. doi: 10.1016/j.bone.2011.05.018. [DOI] [PubMed] [Google Scholar]
- 23.van Bezooijen RL, et al. Sclerostin is an osteocyte-expressed negative regulator of bone formation, but not a classical BMP antagonist. J Exp Med. 2004;199(6):805–814. doi: 10.1084/jem.20031454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ott SM. Sclerostin and Wnt signaling—the pathway to bone strength. J Clin Endocrinol Metab. 2005;90(12):6741–6743. doi: 10.1210/jc.2005-2370. [DOI] [PubMed] [Google Scholar]
- 25.Yasuda H, et al. Osteoclast differentiation factor is a ligand for osteoprotegerin/osteoclastogenesis-inhibitory factor and is identical to TRANCE/RANKL. Proc Natl Acad Sci USA. 1998;95(7):3597–3602. doi: 10.1073/pnas.95.7.3597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Teitelbaum SL. Bone resorption by osteoclasts. Science. 2000;289(5484):1504–1508. doi: 10.1126/science.289.5484.1504. [DOI] [PubMed] [Google Scholar]
- 27.Khan TN, et al. Cenani-Lenz syndrome restricted to limb and kidney anomalies associated with a novel LRP4 missense mutation. Eur J Med Genet. 2013;56(7):371–374. doi: 10.1016/j.ejmg.2013.04.007. [DOI] [PubMed] [Google Scholar]
- 28.Kariminejad A, et al. Severe Cenani-Lenz syndrome caused by loss of LRP4 function. Am J Med Genet A. 2013;161A(6):1475–1479. doi: 10.1002/ajmg.a.35920. [DOI] [PubMed] [Google Scholar]
- 29.Lindy AS, et al. Truncating mutations in LRP4 lead to a prenatal lethal form of Cenani-Lenz syndrome. Am J Med Genet A. 2014;164A(9):2391–2397. doi: 10.1002/ajmg.a.36647. [DOI] [PubMed] [Google Scholar]
- 30.Collette NM, et al. Sost and its paralog Sostdc1 coordinate digit number in a Gli3-dependent manner. Dev Biol. 2013;383(1):90–105. doi: 10.1016/j.ydbio.2013.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Balemans W, et al. Novel LRP5 missense mutation in a patient with a high bone mass phenotype results in decreased DKK1-mediated inhibition of Wnt signaling. J Bone Miner Res. 2007;22(5):708–716. doi: 10.1359/jbmr.070211. [DOI] [PubMed] [Google Scholar]
- 32.Holdsworth G, et al. Characterization of the interaction of sclerostin with the low density lipoprotein receptor-related protein (LRP) family of Wnt co-receptors. J Biol Chem. 2012;287(32):26464–26477. doi: 10.1074/jbc.M112.350108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chang MK, et al. Disruption of Lrp4 function by genetic deletion or pharmacological blockade increases bone mass and serum sclerostin levels. Proc Natl Acad Sci USA. 2014;111(48):E5187–5195. doi: 10.1073/pnas.1413828111. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.