Significance
Status epilepticus (SE) is defined as a state of continuous unremitting seizures that often exhibits underlying deficits in neuronal inhibition mediated by GABAA receptors. The efficacy of neuronal inhibition is critically dependent on the activity of the K+/Cl– cotransporter KCC2, which allows neurons to maintain low intracellular Cl– levels. KCC2 activity is enhanced by phosphorylation of residue serine 940, and here we show that SE leads to rapid dephosphorylation of this key regulatory residue. Moreover, we demonstrate that deficits in S940 phosphorylation directly contribute to the onset and severity of SE. Collectively, our results suggest that deficits in KCC2 activity directly contribute to the pathophysiology of SE.
Keywords: KCC2, epilepsy, kainate, phosphorylation, chloride
Abstract
The K+/Cl– cotransporter (KCC2) allows adult neurons to maintain low intracellular Cl– levels, which are a prerequisite for efficient synaptic inhibition upon activation of γ-aminobutyric acid receptors. Deficits in KCC2 activity are implicated in epileptogenesis, but how increased neuronal activity leads to transporter inactivation is ill defined. In vitro, the activity of KCC2 is potentiated via phosphorylation of serine 940 (S940). Here we have examined the role this putative regulatory process plays in determining KCC2 activity during status epilepticus (SE) using knockin mice in which S940 is mutated to an alanine (S940A). In wild-type mice, SE induced by kainate resulted in dephosphorylation of S940 and KCC2 internalization. S940A homozygotes were viable and exhibited comparable basal levels of KCC2 expression and activity relative to WT mice. However, exposure of S940A mice to kainate induced lethality within 30 min of kainate injection and subsequent entrance into SE. We assessed the effect of the S940A mutation in cultured hippocampal neurons to explore the mechanisms underlying this phenotype. Under basal conditions, the mutation had no effect on neuronal Cl– extrusion. However, a selective deficit in KCC2 activity was seen in S940A neurons upon transient exposure to glutamate. Significantly, whereas the effects of glutamate on KCC2 function could be ameliorated in WT neurons with agents that enhance S940 phosphorylation, this positive modulation was lost in S940A neurons. Collectively our results suggest that phosphorylation of S940 plays a critical role in potentiating KCC2 activity to limit the development of SE.
Fast synaptic inhibition in the adult brain is largely mediated via the activation of γ-aminobutyric acid receptors (GABAARs). The ability of neurons to maintain low intracellular Cl– is dependent upon the activity of the K+/Cl– cotransporter KCC2. KCC2 expression in the rodent brain is developmentally regulated with low levels evident before birth that then dramatically increase from postnatal day 7 (P7) onwards and correlate with the appearance of hyperpolarizing GABAAR currents (1). KCC2 null mice die shortly after birth, exhibit high levels of intracellular Cl– and anomalous excitatory actions of GABA and glycine, highlighting the vital role of KCC2 (2). In addition to regulating Cl– transport, KCC2 exhibits transporter-independent properties that affect glutamate receptors and dendritic spines (3–6).
Status epilepticus (SE) is a state of continuous seizures that is highly lethal and leads to long-term neurological deficits in survivors. A key feature of SE is impaired GABAergic inhibition (7) that manifests clinically as resistance to the GABAA modulator diazepam (8). The prevailing hypothesis of impaired GABAergic signaling during SE is displacement of GABAA receptors from the synapse and a reduction in the number of receptors on the cell surface (9). However, an additional component that could contribute to the compromised inhibition during SE is a buildup of intracellular Cl– due to ionic plasticity and deficits in KCC2 function. KCC2 dysfunction is thought to contribute to traumatic brain injury, neuropathic pain, and acute stress (10). Consistent with this, deficits in the activity and expression levels of KCC2 develop rapidly in animal models of SE and persist after its termination (11–13).
Recent evidence indicates that the transport activity and membrane trafficking of KCC2 are modulated by protein kinase C (PKC)-dependent phosphorylation of residue S940 within its cytoplasmic domain. S940 phosphorylation leads to increased KCC2 transport activity as well as increased accumulation on the plasma membrane due to a reduction in endocytosis (14, 15). Using phospho-specific antibodies we demonstrate that SE induced by the chemoconvulsant kainate results in rapid S940 dephosphorylation. Furthermore, genetically ablating S940 phosphorylation accelerates the development and lethality of SE. Thus, our results provide to our knowledge the first direct evidence that deficits in KCC2 activity directly contribute to the pathophysiology of SE.
Results
Status Epilepticus Induced Dephosphorylation of S940 and KCC2 Internalization.
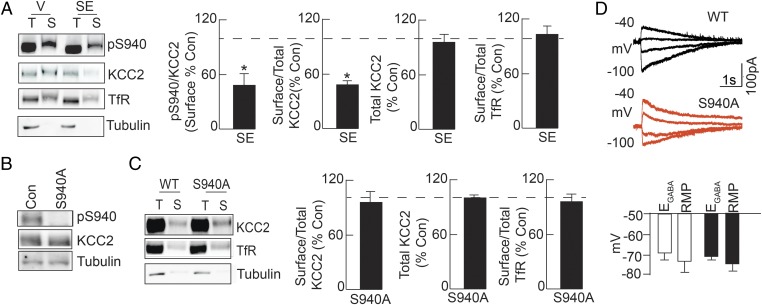
Consistent with its essential role in regulating neuronal Cl– homeostasis, deficits in KCC2 activity are seen in patients with intractable epilepsy and in animal models of SE (9). However, the mechanisms by which persistent elevations in neuronal activity lead to KCC2 inactivation remain unknown. We used the chemoconvulsant kainate to induce SE in C57/BL6 mice due to the strain’s consistently robust behavioral and electrographic seizure profiles. Mice were injected with kainate and hippocampal slices were prepared after 30 min, which was sufficient to induce SE in wild-type (WT) mice as defined by the development of continuous seizures with stage 5 convulsions (16). Hippocampal slices were incubated for 60 min at 32 °C and subject to biotinylation followed by immunoblotting. Comparing the ratio of surface pS940:KCC2 immunoreactivity revealed that 30 min of SE induced significant dephosphorylation of S940 on the plasma membrane relative to vehicle-treated mice (52 ± 15% of control, n = 4, P = 0.032) (Fig. 1A). Additionally, SE reduced the ratio of surface to total KCC2 expression levels (51 ± 0.2% of control, n = 4, P = 0.004) (Fig. 1A). In contrast to the deficits in KCC2 surface levels, no deficits in the levels of the transferrin receptor (TfR) were observed after kainate exposure (saline: 129 ± 54; kainate: 84 ± 38, n = 4, P = 0.800) (Fig. 1A). These results suggested that deficits in KCC2 surface activity could contribute to elevated levels of intracellular Cl– that develop rapidly in animal models of SE and persist after its termination (11–13).
Fig. 1.
SE induces dephosphorylation of S940 and KCC2 internalization. (A) Hippocampal slices were prepared from WT mice 30 min after 20 mg/kg kainate injection, and subsequent entrance into SE, and subjected to biotinylation. Surface (S) and total (T) fractions were immunoblotted with pS940, KCC2, transferrin receptor (TfR), and tubulin antibodies and normalized to values in vehicle-treated mice. (B) Immunoblots of hippocampal lysates show that reactivity of the phospho-specific antibody to S940 was abolished by the S940A mutation. (C) Hippocampal slices prepared from WT and S940A mice were subject to biotinylation to determine basal surface (S) and total (T) KCC2 expression normalized to TfR. (D) EGABA and RMP values were compared in DGGCs from both genotypes. *P < 0.05, significantly different than the control. Values are mean ± SEM.
Generation and Characterization of S940A Knockin Mice.
To determine whether deficits in S940 phosphorylation contribute to the pathophysiology of SE, we generated mice in which S940 was mutated to an alanine residue (S940A) via homlogous recombination (Fig. S1A). The presence of the mutation within exon 22 of the KCC2 gene was confirmed by DNA sequencing (Fig. S1B). S940A homozygous mice were viable, survived through adulthood, and did not exhibit any overt phenotypes. However, consistent with its mutation, the pS940 antibody did not exhibit immunoreactivity in S940A mice (n = 5, P < 0.001) (Fig. 1B). Immunoblotting coupled with biotinylation revealed that the cell surface expression levels of KCC2 were similar between WT and S940A mice (n = 4, P > 0.05) (Fig. 1C). Moreover, expression of the Na+/K+/Cl– cotransporter NKCC1, which mediates Cl– uptake, was unchanged in S940A mice [WT: 118.6 ± 7.6 arbitrary units (AU), S940A: 99.9 ± 11.9 AU, n = 4 per genotype, P > 0.05] (Fig. S1C). Similar expression levels of NKCC1 and KCC2 are also reflected by unchanged GABAA reversal potential (EGABA) values measured in dentate gyrus granule cells in hippocampal slices (WT: −70 ± 5 mV, n = 7; S940A: −68 ± 4 mV, n = 7, P = 0.770), suggesting that the S940A mutation did not alter basal KCC2 activity (Fig. 1D).
Importantly, the S940A mutation did not alter the levels of the GABAA receptor β3 subunit (WT: 114 ± 14 AU, S940A: 108 ± 21 AU, n = 4, P = 0.408) (Fig. S1C), the GABAA receptor γ2 subunit (WT: 102 ± 2 AU, S940A: 111 ± 11 AU, n = 4, P = 0.571), or that of the GABAergic/glycinergic synapse scaffolding protein gephyrin (WT: 92 ± 11, S940A: 112 ± 18, n = 4, P = 0.186) (Fig. S1C). Moreover, no changes were observed in the expression of the postsynaptic density protein 95 (PSD-95) (WT: 0.42 ± 0.11 AU, n = 3; S940A: 0.59 ± 0.14 AU, n = 4, P = 0.213) or the vesicular GABA transporter (VGAT) (WT: 8.18 ± 0.53 AU, n = 3; S940A: 6.7 ± 0.45 AU, n = 4, P = 0.258) (Fig. S1C). These data indicated that essential markers of excitatory and inhibitory synapses in the hippocampus were not affected by the S940A mutation.
The cellular distribution of KCC2 in the brains of S940A and WT mice was compared using immunohistochemistry (Fig. S1D). DAB (3, 3′-diaminobenzidine) staining did not reveal any deficits in KCC2 expression between genotypes. Higher magnification analysis was performed in the hippocampus and dentate gyrus (Fig. S1D). In agreement with previous observations (17), KCC2 was heavily expressed in the stratum moleculare with lower expression in the cell body layer of the dentate gyrus and in the pyramidal layer of the hippocampus (Fig. S1D). Consistent with our cell surface studies, KCC2 expression in S940A mice was indistinguishable in these regions from the hippocampus from WT littermates.
Finally, we compared the behavior of S940A and WT mice. Littermates were observed in the open field arena for 30 min and showed no significant stereotyped behavior or motor deficits (n = 13 WT; n = 9 S940A; P > 0.05) (Fig. S2A). S940A mice also performed normally in the rotarod test: No differences were observed in latency to fall between genotypes (P > 0.05) (Fig. S2B). Collectively these results suggested that mutation of S940 does not impact the basal levels of KCC2 expression and activity or induce any overt behavioral phenotypes in vivo.
Mutation of S940 Increases the Onset and Lethality of SE.
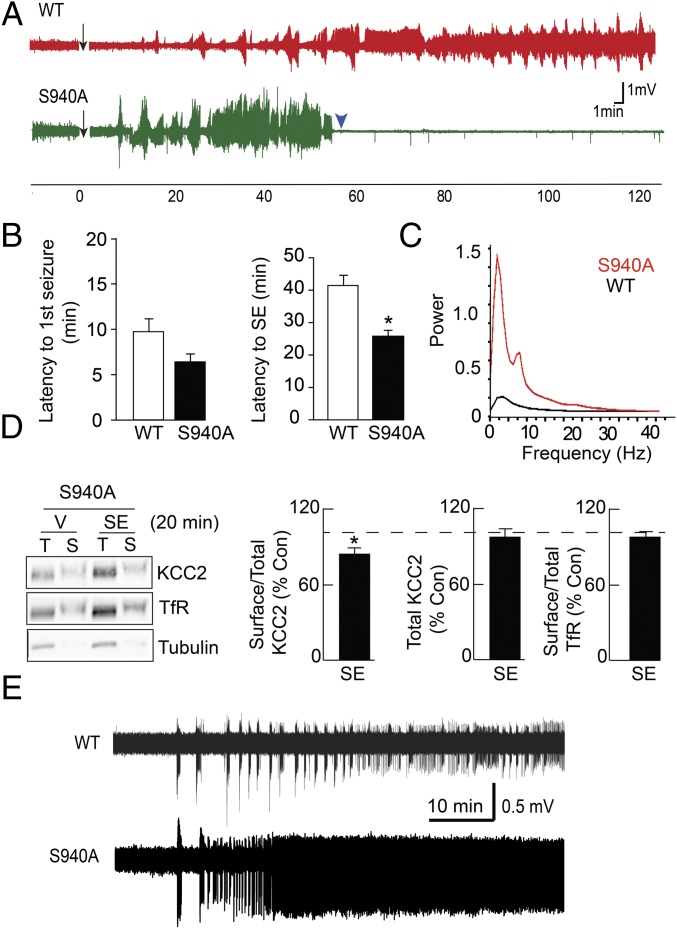
We examined the effects of mutating S940 on the ability of 20 mg/kg kainate to induce SE. Strikingly, whereas both genotypes experienced stage 1 seizures and eventually progressed to stage 5 convulsions, all S940A mice died within 26 ± 2 min after the first stage 5 seizure. In contrast, no lethality was seen in WT littermates up to 120 min after the first stage 5 seizure (n = 6 per genotype). We also performed EEG recordings to further analyze the effects of kainate (Fig. 2A). Interestingly, the S940A mutation did not cause a significant difference in the latency to onset of the first seizure (WT: 9.7 ± 1 min; S940A: 6.3 ± 1 min; n = 6, P = 0.208). To gauge the development of SE, we defined SE onset as the point when all subsequent epileptiform discharges occurred less than 2 min apart. As such, the time period between the first seizure and SE onset was significantly reduced in S940A mice (WT: 41.3 ± 3.0 min; S940A: 25.8 ± 1.5 min; P = 0.005) (Fig. 2B), indicating that the progression from discontinuous seizure events to sustained seizure activity was accelerated by the S940A mutation. Moreover, analysis of the fast Fourier transform indicated that the seizure power was increased at all frequencies between 1 and 30 Hz in the S940A mice (Fig. 2C), suggesting an increase of seizure severity (Fig. S3).
Fig. 2.
The KCC2-S940A mutation increases the onset and lethality of SE. (A) Representative EEG recordings for WT and S940 mice before and after injection with 20 mg/kg kainate. The arrow represents kainate injection, whereas the arrowhead indicates the death of the mouse. (B) Latency of seizure onset and SE onset following kainate injection was compared for WT and S940A mice. (C) EEG power spectra are shown for WT and S940A mice during SE. (D) Hippocampal slices prepared from S940A mice after kainate injection and subsequent entrance into SE were subject to biotinylation to determine surface (S) and total (T) KCC2 expression normalized to TfR. (E) Field potential recordings in the entorhinal cortex of slices obtained from WT and S940A mice. The traces begin at the onset of 0-Mg2+ perfusion. *P < 0.05, significantly different than the control. Values are mean ± SEM.
We then examined the effects of kainate-induced SE on KCC2 surface expression in S940A mice. To account for the increased lethality of kainate in S940A mice, we performed biochemical assays at early time points. In contrast to an ∼50% loss in KCC2 surface levels in WT mice (51 ± 0.2% of control; n = 4 per treatment, P = 0.004), hippocampal slices prepared from S940A littermates undergoing SE only showed an ∼20% loss in KCC2 surface levels (79 ± 11% of control, n = 4, P = 0.042) (Fig. 2D), indicating that the S940A mutation partially blocked the SE-induced internalization of KCC2 protein. Importantly, this effect on internalization was consistent with our previous studies of the S940A mutant protein expressed in nonneuronal cell lines (14). These results suggested that the key deficit in the mutant was the loss of S940 phosphorylation, and not the internalization of the protein. Together, our results indicated that loss of S940 phosphorylation results in a dramatic increase in both the onset and lethality of kainate-induced SE.
To support our EEG findings, we analyzed the effects of the S940A mutation in the low Mg2+ model of in vitro epileptiform activity in acute entorhinal–hippocampal slices. This model results in repetitive ictal-like activity in layers III/IV of the entorhinal cortex (EC) that in some slices degenerates into a late recurrent discharge pattern that corresponds to SE (18). Field recordings in the EC of WT slices demonstrated that perfusion of 0-Mg2+ solution induced seizure-like events (SLEs) in 23.6 ± 9.7 min (mean ± SD, n = 14) (Fig. 2E). However, recordings in S940A slices led to 0-Mg2+ induced SLEs within 8.9 ± 2.7 min (mean ± SD, n = 6, P = 0.0841), indicating that the S940A mutation did not accelerate the development of the first SLE. In 8 of 14 WT slices, the SLE pattern of activity degenerated into late recurrent discharges within 61.0 ± 32.0 min (SD, n = 8) of 0-Mg2+ onset (Fig. 2E). In sharp contrast, six of six S940A mutant slices developed a recurrent discharge pattern at earlier times (27.9 ± 14.4 min, SD, n = 6, P = 0.0371) (Fig. S4). Together with our in vivo measurements, our data clearly indicated that the S940A mutant mice exhibited faster progression into SE.
Mutation of S940 Does Not Modify Cl– Extrusion Under Basal Conditions.
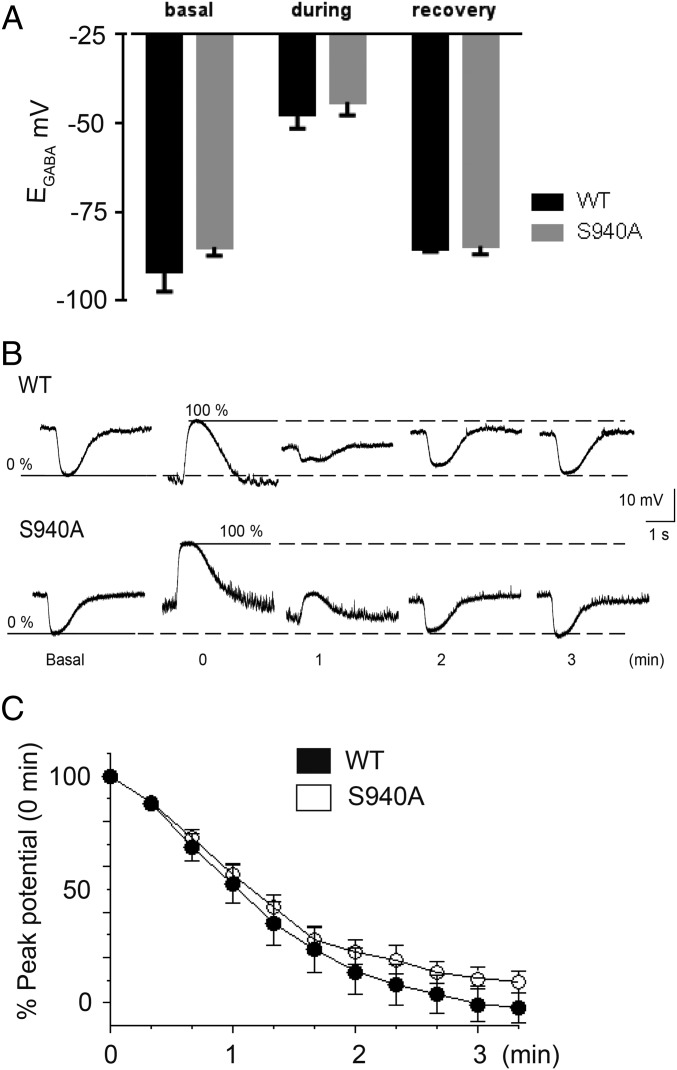
To investigate the effects of the S940A mutation on Cl– homeostasis, we used the gramicidin perforated patch clamp technique to measure KCC2 activity in 18–25 days in vitro (DIV) hippocampal neurons from S940A mice. The baseline EGABA values measured in the presence of tetrodotoxin (TTX, 500 nM) were similar in both WT and S940A hippocampal neurons: −92 ± 6 mV for WT (n = 11) and −85 ± 2 mV (n = 12, P = 0.480), respectively (Fig. 3A). These values were more negative than those obtained in dentate gyrus granule cells (DGGCs) because we used a Hepes-based buffer, which removes the contribution of depolarizing HCO3– currents to EGABA (19). These data indicated that in the absence of network activity, and particularly GABAergic interneuron activity, the S940A mutation had no effect on resting Cl– levels.
Fig. 3.
Mutation of S940 does not affect resting EGABA values or Cl– extrusion rates. (A) EGABA values obtained from WT and S940A neurons under basal conditions, during exposure to furosemide, and after the recovery from furosemide washout. (B) Current clamp recordings of muscimol responses by WT (Upper) and S940A (Lower) cultured neurons were hyperpolarizing under basal conditions (0% value). After exposure to furosemide, the muscimol responses switched to depolarizing (100% value) and then recovered back toward the basal values upon removal of furosemide over the time course indicated. (C) Summary graph of the replicate data obtained from experiments depicted in B. For each cell tested, the data points during the recovery phase were normalized to the difference between the 0% and 100% values (SI Materials and Methods). All experiments were performed using the perforated patch technique. Values are mean ± SEM.
To confirm our basal measurements, we exposed neurons to the nonselective KCC2 inhibitor furosemide (500 μM) in the absence of TTX, which results in activity-dependent Cl– loading that is independent of NKCC1 activity (20). A 3-min exposure to furosemide resulted in a similar positive shift in EGABA values for both WT (−48 ± 4 mV, n = 11) and S940A (−44 ± 4 mV, n = 12, P = 0.2334) neurons (Fig. 3A). To measure the rate of KCC2 dependent Cl– extrusion, furosemide was rapidly removed and the amplitude and polarity of muscimol responses were measured every 20 s in the presence of TTX over a time course of 4 min. The rate of recovery of stable hyperpolarizing responses to muscimol was not affected by the S940A mutation (WT: −37 ± 4% recovery per minute, n = 11; S940A: −34 ± 2% recovery per minute, n = 12, P = 0.5517) (Fig. 3 B and C). Both genotypes reached equilibrium within 4 min, and reestablished similar EGABA values (WT: −85 ± 1 mV, n = 11; S940A: −85 ± 2 mV, n = 12, P = 0.3759) that were comparable to the initial baseline values (paired t tests: WT: P = 0.4151; S940A: P = 0.4743) (Fig. 3A). Consistent with measurements in DGGCs and our previous results using Rb+ flux assays (14), these experiments indicated that mutation of S940 does not impair the basal activity of KCC2 in neurons.
S940A Mice Exhibit a Selective Deficit in KCC2 Activity After Glutamate Exposure.
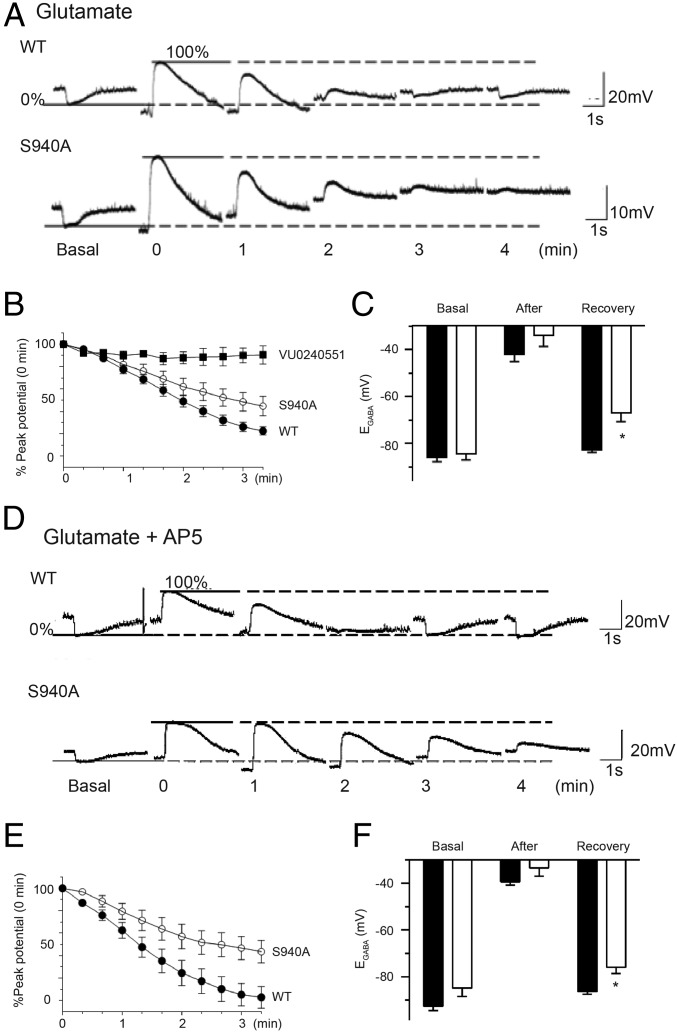
To simulate the effects of neuronal hyperexcitability that are evident during SE, cultured neurons were transiently exposed to glutamate, a treatment that induces NMDAR-mediated Ca2+-dependent dephosphorylation of S940, leading to KCC2 inactivation (15, 20). After a 30-s exposure to glutamate, S940A neurons had slower rates of recovery (WT: −22 ± 1% recovery per minute, n = 15; S940A: −15 ± 2% recovery per minute, n = 12, P = 0.0102) (Fig. 4 A and B). Furthermore, EGABA values obtained 6 min after glutamate exposure were more positive in S940A neurons (WT: −82 ± 1 mV, n = 15; S940A: −70 ± 4 mV, n = 12, P = 0.0037) (Fig. 4C). Importantly, the recovery of hyperpolarizing responses after glutamate exposure was completely blocked by the selective KCC2 inhibitor VU0240551 (21), indicating that our assays measure the KCC2-dependent Cl– extrusion capacity of neurons (Fig. 4B). Further analysis revealed that the glutamate-induced deficits in Cl– extrusion were blocked by the NMDA receptor antagonist AP5 in WT neurons but not in S940A neurons (WT: −26 ± 3% recovery per minute, n = 11; S940A: −16 ± 3% recovery per minute, n = 11, P = 0.0141) (Fig. 4 D and E). Unlike WT neurons, which exhibited full recovery of EGABA within 6 min of the glutamate/AP5 challenge (basal: −93 ± 2 mV, recovery: −86 ± 1 mV, n = 11, P = 0.0525), S940A neurons did not completely recover (basal: −85 ± 4 mV, recovery: −76 ± 3 mV, n = 11, P = 0.0281), indicating that S940 phosphorylation is a limiting factor for NMDA receptor-dependent inactivation of KCC2 (Fig. 4F). These results demonstrated that S940A mice have a selective deficit in KCC2 activity during neuronal hyperexcitability, an observation that is likely to contribute to the lethal seizure phenotype of S940A mice.
Fig. 4.
Mutation of S940 causes a Cl– extrusion deficit after glutamate exposure. (A) Current clamp recordings of muscimol responses by WT (Upper) and S940A (Lower) cultured neurons were hyperpolarizing under basal conditions (0% value). After exposing the neurons to three consecutive 10-s pulses of glutamate, the muscimol responses switched to depolarizing (100% value) and then recovered back toward the basal values over the time course indicated. (B) Summary graph of the replicate data obtained from experiments depicted in A. For each cell tested, the data points during the recovery phase were normalized to the difference between the 0% and 100% values (SI Materials and Methods). A set of experiments were performed in the presence of the KCC2 inhibitor VU0240551 to confirm that the observed recovery from the glutamate challenge was KCC2 dependent. (C) EGABA values obtained from WT and S940A neurons under basal conditions, immediately after exposure to glutamate, and upon recovery from the glutamate challenge. (D) Similar to the experiments depicted in A, these exemplar traces were obtained from WT and S940A neurons exposed to glutamate and AP5. (E) Similar to B, the graph summarizes the normalized data of the recovery of muscimol responses of WT and S940A neurons after exposure to glutamate/AP5. (F) EGABA values obtained from WT and S940A neurons under basal conditions, immediately after exposure to glutamate/AP5, and upon recovery from the glutamate challenge. *P < 0.05, significantly different than the control. Values are mean ± SEM.
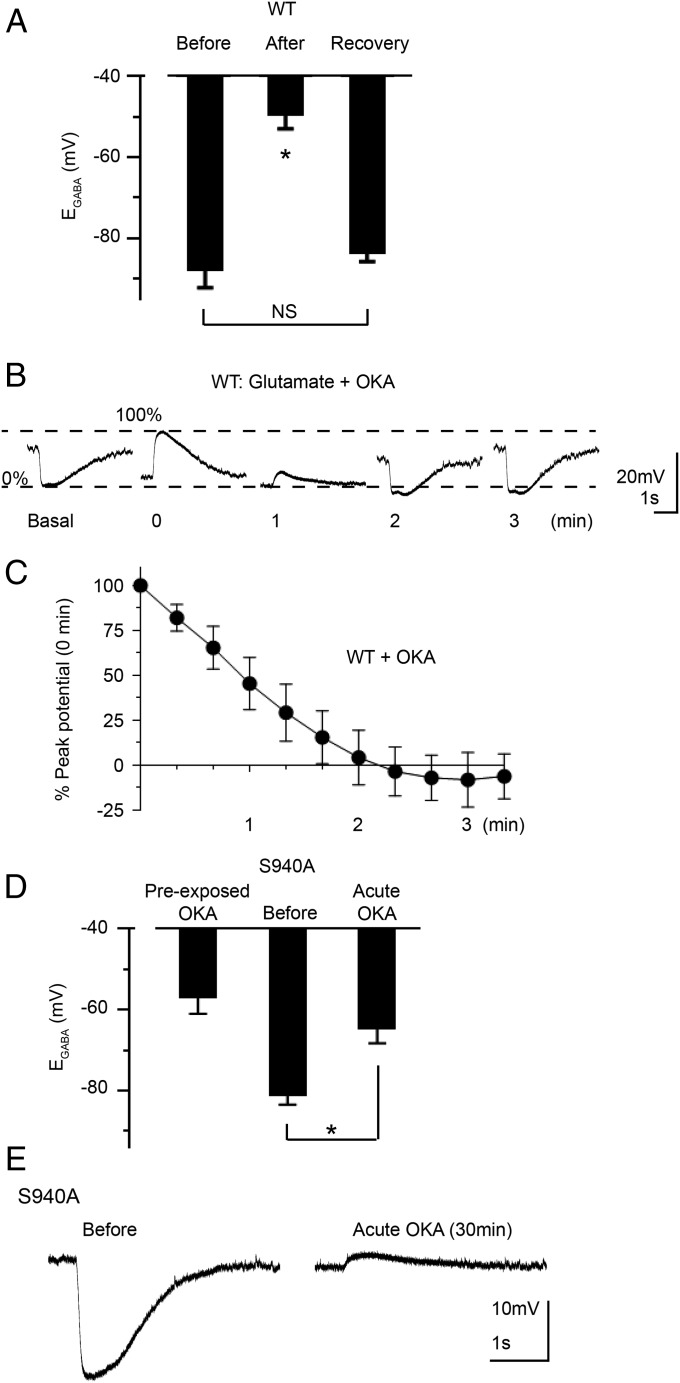
Positive Modulation of KCC2 Function upon Inhibition of Protein Phosphatase 1 Was Lost in S940A Mice.
Glutamate/NMDAR-dependent inactivation of KCC2 can be ameliorated by application of okadaic acid (OKA) which, in parallel, blocks protein phosphatase 1 (PP1)-dependent dephosphorylation of S940 (15). We examined the effects of pretreating neurons with OKA (1 μM; 30 min) on KCC2 recovery after exposure to glutamate. In WT neurons, baseline EGABA after preexposure to OKA was −88 ± 5 mV (n = 9), which was not statistically different from untreated controls (P = 0.2536, unpaired t test), indicating that OKA exposure did not alter the resting Cl– extrusion capacity of neurons (Fig. 5A). However, OKA significantly accelerated the rate of recovery after exposure to glutamate (−40 ± 4% recovery per minute, n = 9, P = 0.0011 compared with glutamate alone) (Fig. 5 A–C). These neurons also exhibited EGABA values that were similar to basal levels (−83 ± 2 mV, n = 9, P = 0.1654). Together these findings indicated that whereas OKA pretreatment did not alter the basal equilibrium EGABA, it significantly accelerated the rate of recovery from glutamate-induced EGABA shifts.
Fig. 5.
Mutation of S940 abolishes positive modulation of KCC2 activity by PP1 inhibition. (A) EGABA values obtained from WT neurons that were preexposed to OKA (30 min) under basal conditions, immediately after exposure to glutamate, and upon recovery from the glutamate challenge. (B) Current clamp recordings of muscimol responses by WT cultured neurons that were preexposed to OKA (30 min) were hyperpolarizing under basal conditions (0% value). After exposing the neurons to three consecutive 10-s pulses of glutamate, the muscimol responses switched to depolarizing (100% value) and then recovered back toward the basal values over the time course indicated. (C) Summary graph of the replicate data obtained from experiments depicted in B. For each cell tested, the data points during the recovery phase were normalized to the difference between the 0% and 100% values. (D) EGABA values obtained from S940A neurons under the indicated conditions. Data depicted in the Left column were obtained from a different set of neurons than the data depicted in the Center and Right columns. (E) Exemplar current clamp traces of a hyperpolarizing muscimol response by an S940A neuron under basal conditions (Left), which switched to a depolarizing response after a 30-min OKA exposure (Right). *P < 0.05, significantly different than the control. Values are mean ± SEM.
In contrast, KCC2-S940A neurons preexposed to OKA exhibited strongly depolarized EGABA values compared with untreated controls −57 ± 4 mV (n = 15, P < 0.0001, unpaired t test) (Fig. 5D), and their membrane potential responses to muscimol application were either depolarizing or purely shunting (silent responses). This effect precluded our analysis of postglutamate responses of S940A neurons pretreated with OKA. We therefore examined an acute exposure to OKA (1 μM) alone for 30 min, which induced a depolarizing shift of EGABA in S940A neurons (before: −81 ± 2 mV; after −65 ± 4 mV; n = 6, P = 0.0009, paired t test) (Fig. 5D). These positive shifts in EGABA values converted the baseline hyperpolarizing muscimol responses to depolarizing or silent responses (Fig. 5E). These OKA experiments demonstrated that the S940A mutation converted OKA from an activator to an inhibitor of KCC2 function. These studies revealed that phosphorylation of S940 preserved the Cl– extrusion capacity of neurons and selectively potentiated KCC2 activity during neuronal hyperexcitability.
Discussion
SE is a medical emergency that leads to significant mortality and morbidity, usually arising from gross decreases in the efficacy of GABAergic inhibition. Consistent with their role in mediating GABAergic inhibition, deficits in the cell surface levels of GABAARs and the amplitudes of miniature inhibitory postsynaptic currents are evident in animal models of SE (22–24). KCC2 deficits are also clear in patients with intractable epilepsy and animal models of seizures (11, 13, 25). Here we have examined the mechanisms that underlie KCC2 inactivation during neuronal hyperexcitability and whether they contribute to the pathophysiology of SE.
The activity of KCC2 and its membrane trafficking are subject to positive modulation via phosphorylation of S940 within its C-terminal domain (14, 15). To determine the significance of S940 in vivo, we created a S940A knockin mouse using homologous recombination. Significantly, S940A homozygotes were viable, bred normally, and did not exhibit any behavioral deficits in the rotarod or open field tests. S940A mice exhibited comparable KCC2 distribution and levels of Cl– extrusion to their WT littermates. This finding is in agreement with measurements of transporter activity in HEK-293 cells where the S940A mutation had minimal effects on KCC2-mediated Rb+ flux (14). In contrast, kainate caused rapid death in S940A mice due to an accelerated development of SE and more severe electrographic activity. To confirm the increased excitability seen in our EEG measurements, we exposed hippocampal slices to Mg2+-free artificial cerebrospinal fluid (ACSF). In this accepted in vitro model of seizures, the conversion from SLEs into SE-like recurrent discharges was accelerated in slices derived from S940A mice (26). Therefore, whereas S940 does not appear to be a prerequisite for basal KCC2 expression levels, it plays a critical role in limiting the onset and severity of SE.
To further examine the role that S940 plays in limiting the development of SE, we compared the effects of KCC2 cell surface expression between genotypes. SE-induced internalization of KCC2 was reduced in S940A mice compared with WT, which suggested that deficits in S940 phosphorylation and not the number of transporters on the cell surface underlie the seizure phenotype of mutant mice. To precisely measure KCC2 function in a neuronal system, we exposed cultured neurons to excess glutamate, a principle mediator of neuronal hyperexcitability during seizures (27). In agreement with our previous HEK cell studies, basal levels of Cl– extrusion were equivalent in WT and S940A neurons. However, transient exposure of neurons to glutamate revealed a selective deficit in KCC2 activity in S940A cultures. Moreover, whereas glutamate-induced impairment of KCC2 in WT neurons could be ameliorated by OKA, exposure of S940A neurons to OKA resulted in KCC2 inactivation even without prior glutamate exposure. OKA limits NMDAR-dependent PP1-mediated dephosphorylation of S940, and our results demonstrated that the ability of OKA to potentiate KCC2 activity was directly mediated by this residue. Presumably, the inhibitory effects of OKA in the absence of S940 may reflect enhanced phosphorylation of other residues in KCC2 such as T906 and T1007, which have been postulated to cause transporter inactivation (28, 29). Of note, S940 is not conserved in the other KCCs that are inhibited by OKA under basal conditions (30), suggesting a unique role for this residue in regulating neuronal Cl– homeostasis.
Importantly, recent genetic studies of patients suffering from idiopathic epilepsy identified mutations within KCC2 (31, 32) that decrease S940 phosphorylation and compromise transporter activity. Thus, changes in S940 phosphorylation may contribute to epileptogenesis in humans. Our results suggested that phosphorylation of KCC2-S940 was a critical mechanism that limited the progression of seizures into SE. As such, agents that potentiate KCC2 activity or prevent S940 dephosphorylation may be novel therapeutic strategies for seizure-related disorders. Finally, the role KCC2 plays in regulating neuronal Cl– homeostasis is contentious, as a recent study suggests that this parameter in adult neurons is set by impermeant anions, rather than KCC2 activity (33). However, our results suggest that under pathological conditions, KCC2 activity plays a critical role in limiting the onset and severity of SE.
Materials and Methods
More detailed information on materials and methods is provided in SI Materials and Methods.
Creation of S940A Mouse.
The S940A mice were created by genOway using homologous recombination as detailed previously (34).
Biochemical Measurements.
Antibodies used in this study have been described previously as have the methods for immunoblotting, and immunoprecipitation (15).
Electrophysiology and EEG.
KCC2 function was assessed using the perforated patch clamp technique (15). The pinnacle system and Labchart 7 were used for EEG recording and analysis.
Behavior.
Measurements using the rotarod and activity in the open field were assessed as described previously (34).
Supplementary Material
Acknowledgments
This work was supported by Grant 206026 from the Simons Foundation (to S.J.M.); National Institutes of Health (NIH)–National Institute of Neurological Disorders and Stroke Grants NS051195, NS056359, NS081735, and NS087662 (to S.J.M.); and NIH–National Institute of Mental Health Grant MH097446 (to P.A.D. and S.J.M.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1415126112/-/DCSupplemental.
References
- 1.Blaesse P, Airaksinen MS, Rivera C, Kaila K. Cation-chloride cotransporters and neuronal function. Neuron. 2009;61(6):820–838. doi: 10.1016/j.neuron.2009.03.003. [DOI] [PubMed] [Google Scholar]
- 2.Hübner CA, et al. Disruption of KCC2 reveals an essential role of K-Cl cotransport already in early synaptic inhibition. Neuron. 2001;30(2):515–524. doi: 10.1016/s0896-6273(01)00297-5. [DOI] [PubMed] [Google Scholar]
- 3.Li H, et al. KCC2 interacts with the dendritic cytoskeleton to promote spine development. Neuron. 2007;56(6):1019–1033. doi: 10.1016/j.neuron.2007.10.039. [DOI] [PubMed] [Google Scholar]
- 4.Gauvain G, et al. The neuronal K-Cl cotransporter KCC2 influences postsynaptic AMPA receptor content and lateral diffusion in dendritic spines. Proc Natl Acad Sci USA. 2011;108(37):15474–15479. doi: 10.1073/pnas.1107893108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mahadevan V, et al. Kainate receptors coexist in a functional complex with KCC2 and regulate chloride homeostasis in hippocampal neurons. Cell Reports. 2014;7(6):1762–1770. doi: 10.1016/j.celrep.2014.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fiumelli H, et al. An ion transport-independent role for the cation-chloride cotransporter KCC2 in dendritic spinogenesis in vivo. Cereb Cortex. 2013;23(2):378–388. doi: 10.1093/cercor/bhs027. [DOI] [PubMed] [Google Scholar]
- 7.Milgram NW, Yearwood T, Khurgel M, Ivy GO, Racine R. Changes in inhibitory processes in the hippocampus following recurrent seizures induced by systemic administration of kainic acid. Brain Res. 1991;551(1-2):236–246. doi: 10.1016/0006-8993(91)90938-r. [DOI] [PubMed] [Google Scholar]
- 8.Goodkin HP, Kapur J. The impact of diazepam’s discovery on the treatment and understanding of status epilepticus. Epilepsia. 2009;50(9):2011–2018. doi: 10.1111/j.1528-1167.2009.02257.x. [DOI] [PubMed] [Google Scholar]
- 9.Deeb TZ, Maguire J, Moss SJ. Possible alterations in GABAA receptor signaling that underlie benzodiazepine-resistant seizures. Epilepsia. 2012;53(Suppl 9):79–88. doi: 10.1111/epi.12037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Medina I, et al. Current view on the functional regulation of the neuronal K(+)-Cl(-) cotransporter KCC2. Front Cell Neurosci. 2014;8:27. doi: 10.3389/fncel.2014.00027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pathak HR, et al. Disrupted dentate granule cell chloride regulation enhances synaptic excitability during development of temporal lobe epilepsy. J Neurosci. 2007;27(51):14012–14022. doi: 10.1523/JNEUROSCI.4390-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lee HH, Jurd R, Moss SJ. Tyrosine phosphorylation regulates the membrane trafficking of the potassium chloride co-transporter KCC2. Mol Cell Neurosci. 2010;45(2):173–179. doi: 10.1016/j.mcn.2010.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Barmashenko G, Hefft S, Aertsen A, Kirschstein T, Köhling R. Positive shifts of the GABAA receptor reversal potential due to altered chloride homeostasis is widespread after status epilepticus. Epilepsia. 2011;52(9):1570–1578. doi: 10.1111/j.1528-1167.2011.03247.x. [DOI] [PubMed] [Google Scholar]
- 14.Lee HH, et al. Direct protein kinase C-dependent phosphorylation regulates the cell surface stability and activity of the potassium chloride cotransporter KCC2. J Biol Chem. 2007;282(41):29777–29784. doi: 10.1074/jbc.M705053200. [DOI] [PubMed] [Google Scholar]
- 15.Lee HH, Deeb TZ, Walker JA, Davies PA, Moss SJ. NMDA receptor activity downregulates KCC2 resulting in depolarizing GABAA receptor-mediated currents. Nat Neurosci. 2011;14(6):736–743. doi: 10.1038/nn.2806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Racine RJ. Modification of seizure activity by electrical stimulation. II. Motor seizure. Electroencephalogr Clin Neurophysiol. 1972;32(3):281–294. doi: 10.1016/0013-4694(72)90177-0. [DOI] [PubMed] [Google Scholar]
- 17.Gulyás AI, Sík A, Payne JA, Kaila K, Freund TF. The KCl cotransporter, KCC2, is highly expressed in the vicinity of excitatory synapses in the rat hippocampus. Eur J Neurosci. 2001;13(12):2205–2217. doi: 10.1046/j.0953-816x.2001.01600.x. [DOI] [PubMed] [Google Scholar]
- 18.Dreier JP, Heinemann U. Late low magnesium-induced epileptiform activity in rat entorhinal cortex slices becomes insensitive to the anticonvulsant valproic acid. Neurosci Lett. 1990;119(1):68–70. doi: 10.1016/0304-3940(90)90757-z. [DOI] [PubMed] [Google Scholar]
- 19.Farrant M, Kaila K. The cellular, molecular and ionic basis of GABA(A) receptor signalling. Prog Brain Res. 2007;160:59–87. doi: 10.1016/S0079-6123(06)60005-8. [DOI] [PubMed] [Google Scholar]
- 20.Deeb TZ, Nakamura Y, Frost GD, Davies PA, Moss SJ. Disrupted Cl(-) homeostasis contributes to reductions in the inhibitory efficacy of diazepam during hyperexcited states. Eur J Neurosci. 2013;38(3):2453–2467. doi: 10.1111/ejn.12241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Delpire E, et al. Small-molecule screen identifies inhibitors of the neuronal K-Cl cotransporter KCC2. Proc Natl Acad Sci USA. 2009;106(13):5383–5388. doi: 10.1073/pnas.0812756106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Naylor DE, Liu H, Wasterlain CG. Trafficking of GABA(A) receptors, loss of inhibition, and a mechanism for pharmacoresistance in status epilepticus. J Neurosci. 2005;25(34):7724–7733. doi: 10.1523/JNEUROSCI.4944-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Goodkin HP, Joshi S, Mtchedlishvili Z, Brar J, Kapur J. Subunit-specific trafficking of GABA(A) receptors during status epilepticus. J Neurosci. 2008;28(10):2527–2538. doi: 10.1523/JNEUROSCI.3426-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Terunuma M, et al. Deficits in phosphorylation of GABA(A) receptors by intimately associated protein kinase C activity underlie compromised synaptic inhibition during status epilepticus. J Neurosci. 2008;28(2):376–384. doi: 10.1523/JNEUROSCI.4346-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Huberfeld G, et al. Perturbed chloride homeostasis and GABAergic signaling in human temporal lobe epilepsy. J Neurosci. 2007;27(37):9866–9873. doi: 10.1523/JNEUROSCI.2761-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dreier JP, Heinemann U. Regional and time dependent variations of low Mg2+ induced epileptiform activity in rat temporal cortex slices. Exp Brain Res. 1991;87(3):581–596. doi: 10.1007/BF00227083. [DOI] [PubMed] [Google Scholar]
- 27.During MJ, Spencer DD. Extracellular hippocampal glutamate and spontaneous seizure in the conscious human brain. Lancet. 1993;341(8861):1607–1610. doi: 10.1016/0140-6736(93)90754-5. [DOI] [PubMed] [Google Scholar]
- 28.Rinehart J, et al. Sites of regulated phosphorylation that control K-Cl cotransporter activity. Cell. 2009;138(3):525–536. doi: 10.1016/j.cell.2009.05.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.de Los Heros P, et al. The WNK-regulated SPAK/OSR1 kinases directly phosphorylate and inhibit the K+-Cl- co-transporters. Biochem J. 2014;458(3):559–573. doi: 10.1042/BJ20131478. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 30.Jennings ML, Schulz RK. Okadaic acid inhibition of KCl cotransport. Evidence that protein dephosphorylation is necessary for activation of transport by either cell swelling or N-ethylmaleimide. J Gen Physiol. 1991;97(4):799–817. doi: 10.1085/jgp.97.4.799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Puskarjov M, et al. A variant of KCC2 from patients with febrile seizures impairs neuronal Cl- extrusion and dendritic spine formation. EMBO Rep. 2014;15(6):723–729. doi: 10.1002/embr.201438749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kahle KT, et al. Genetically encoded impairment of neuronal KCC2 cotransporter function in human idiopathic generalized epilepsy. EMBO Rep. 2014;15(7):766–774. doi: 10.15252/embr.201438840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Glykys J, et al. Local impermeant anions establish the neuronal chloride concentration. Science. 2014;343(6171):670–675. doi: 10.1126/science.1245423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tretter V, et al. Deficits in spatial memory correlate with modified gamma-aminobutyric acid type A receptor tyrosine phosphorylation in the hippocampus. Proc Natl Acad Sci USA. 2009;106(47):20039–20044. doi: 10.1073/pnas.0908840106. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.