Significance
Mind wandering is a spontaneous and self-generated behavior believed to be important for many mental functions, including creativity and future planning. Can the propensity to mind-wander be modulated externally? If so, this observation would mean that directly modifying spontaneous neural activity can change internally directed thought. To answer this question, we used noninvasive transcranial direct current stimulation (tDCS) to stimulate the prefrontal cortex. Our results showed, for the first time to our knowledge, that mind wandering, probably the most omnipresent internal cognitive function, can be enhanced by external stimulation. In addition, we showed that the frontal lobes play a causal role in mind wandering. We furthermore suggest that the executive control system, and specifically the dorsolateral prefrontal cortex, might play an important role in mind-wandering behavior.
Keywords: mind wandering, spontaneous activity, brain stimulation, tDCS, frontal lobes
Abstract
Humans mind-wander quite intensely. Mind wandering is markedly different from other cognitive behaviors because it is spontaneous, self-generated, and inwardly directed (inner thoughts). However, can such an internal and intimate mental function also be modulated externally by means of brain stimulation? Addressing this question could also help identify the neural correlates of mind wandering in a causal manner, in contrast to the correlational methods used previously (primarily functional MRI). In our study, participants performed a monotonous task while we periodically sampled their thoughts to assess mind wandering. Concurrently, we applied transcranial direct current stimulation (tDCS). We found that stimulation of the frontal lobes [anode electrode at the left dorsolateral prefrontal cortex (DLPFC), cathode electrode at the right supraorbital area], but not of the occipital cortex or sham stimulation, increased the propensity to mind-wander. These results demonstrate for the first time, to our knowledge, that mind wandering can be enhanced externally using brain stimulation, and that the frontal lobes play a causal role in mind-wandering behavior. These results also suggest that the executive control network associated with the DLPFC might be an integral part of mind-wandering neural machinery.
Humans spend a lot of their daytime engaged in mind wandering (or daydreaming). According to some reports, mind wandering can occupy up to half of our waking hours (1). Mind wandering is believed to be important for future planning and simulations (2–4), personal problem solving and decision making (5, 6), creative thinking (7), and learning (8). A notable property of mind wandering is that it is spontaneous and self-generated (5), but could mind wandering be modulated externally by means of brain stimulation? If so, this observation would mean that directly modifying spontaneous neural activity can change internally directed thought. Using brain stimulation, it is also possible to establish the causal role of particular brain regions in mind wandering. Previous functional MRI (fMRI) studies found that mind wandering is associated with activations in the default mode network (9), including the medial frontal cortex (10–15). In addition, fMRI studies found that the executive control network (16) and the dorsolateral prefrontal cortex (DLPFC) in particular have also been activated during mind-wandering tasking (10, 12, 17). These latter results have been taken as evidence to support the role of the executive control network in mind wandering (18, 19). The extent to which executive function is involved in mind wandering has been debated over recent years (5, 20), because executive function is associated with an external task focus (antithetical to mind wandering). Critically, all of these previous studies used fMRI, which does not allow for a causal link to be established between mind wandering and any of these specific brain regions and cognitive processes.
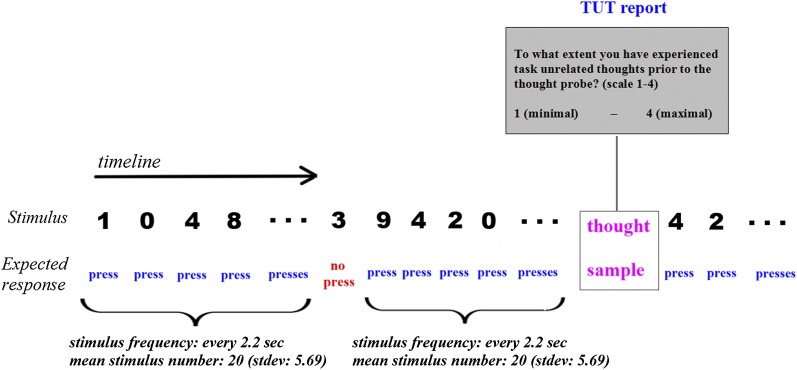
Here, we therefore used transcranial direct current stimulation (tDCS) to explore the neural mechanisms of mind wandering. The tDCS method is a noninvasive stimulation of the brain with a low electrical current using anode (current entry point) and cathode (current exit point) electrodes (reviewed in refs. 21 and 22). During stimulation, both anodal stimulation and cathodal stimulation are thought to change primarily the resting membrane potential, without introducing synaptic changes. The aftereffect of anodal stimulation is thought to include modulation of GABA and glutamate synapses, whereas the aftereffect of cathodal stimulation is thought to include modulation of glutamatergic synapses (reviewed in ref. 23). The three goals of the study were as follows: (i) to examine whether the propensity to mind-wander can be modulated externally using stimulation, (ii) to establish whether frontal lobes play a causal role in mind wandering, and (iii) to explore the role of executive function in mind wandering using a causal method. Participants performed the Sustained Attention to Response Task (SART) (24), which required them to press a keyboard spacebar every time a digit other than “3” appeared on a screen (Fig. 1). This extremely monotonous task has been widely used in mind-wandering research, because it effectively promotes task-unrelated thoughts (TUTs) (e.g., refs. 10 and 25). To estimate the level of TUTs, the paradigm included periodic thought sampling questions (Materials and Methods). During the first half of the experimental task (20 min), participants underwent tDCS stimulation at the prefrontal cortex [Fig. 2; anode electrode at the left DLPFC, cathode electrode at the right supraorbital area (bilateral anodal/cathodal tDCS)]. As the control conditions, we used sham stimulation (the same montage as for prefrontal cortex stimulation) and stimulation of the occipital cortex (anode electrode at the occipital cortex, cathode electrode at the right supraorbital area). We focused on the frontal lobes because this brain region has been previously implicated in mind wandering (reviewed in refs. 18 and 19). In addition, the DLPFC is a central locus of the executive control network (16), permitting us to test potential involvement of executive function in mind wandering.
Fig. 1.
Experimental paradigm. The SART paradigm that was used in the experiment is shown. Participants were asked to press a keyboard spacebar when a stimulus (digit) other than 3 appeared on a screen. For the digit 3, they had to restrain themselves from pressing the spacebar. The periodic thought probe consisted of a question regarding propensity to mind-wander (i.e., TUTs).
Fig. 2.
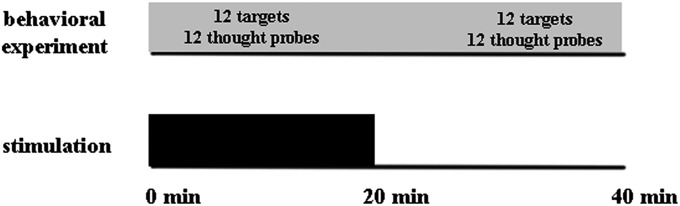
tDCS design. The stimulation started with the beginning of the experimental task. The first half of the experimental task was executed during the stimulation (20 min), and the second half of the experimental task was executed after the stimulation (20 min).
Results
Experiment 1.
The experiment used a within-subjects design. Participants performed the exact same experiment twice (two sessions on different days, counterbalanced order of sessions across participants). The session included either prefrontal cortex stimulation (anode electrode at the left DLPFC, cathode electrode at the right supraorbital area) or sham stimulation (the same positions of the electrodes). Total duration of the experimental task was 40 min, whereas the stimulation of the prefrontal cortex was applied during the first 20 min (Fig. 2 and Materials and Methods).
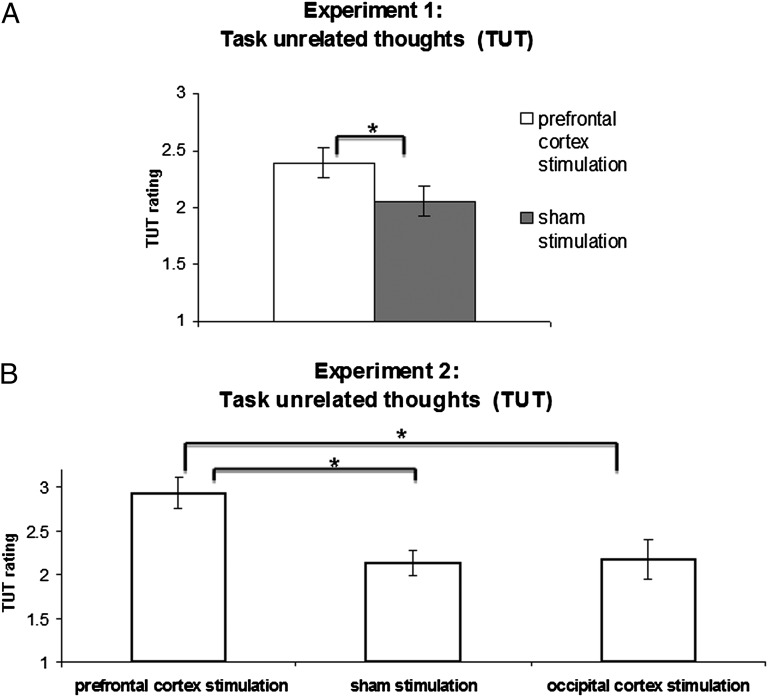
We start by reporting the results of the full experimental length (40 min; 24 targets and 24 thought probes). Stimulation of the prefrontal cortex significantly increased the self-reported rating of TUTs compared with the sham stimulation (Fig. 3A) {prefrontal cortex stimulation = 2.39 [mean square error (MSE) = 0.19], sham stimulation = 2.05 (MSE = 0.17), t(13) = 2.63, P = 0.021(paired two-tailed t test), Cohen’s d = 0.7}. In addition to TUT ratings, we tested two measures related to execution of the external task. Correct detection rates were slightly, but not significantly, higher in the prefrontal cortex compared with sham stimulation [prefrontal cortex stimulation = 64.7% (MSE = 4.7%), sham stimulation = 61% (MSE = 4.7%), t(13) = 1.06, P = 0.31, Cohen’s d = 0.28]. No difference between the two conditions for average response time for nontarget stimuli was found [prefrontal cortex stimulation = 0.41 s (MSE = 0.03), sham stimulation = 0.4 s (MSE = 0.02), t(13) < 1, Cohen’s d = 0.2].
Fig. 3.
TUT results of experiments 1 and 2. (A) Results of experiment 1. The experiment using a within-subjects design included two conditions: prefrontal cortex stimulation and sham stimulation. (B) Results of experiment 2, The experiment using a between-subjects design included three conditions: prefrontal cortex stimulation, sham stimulation, and occipital cortex stimulation. A TUT rating scale ranging from 1 (minimal TUT) to 4 (maximal TUT) was used for both experiments. Analysis of the full experimental length (40 min) is shown. The asterisk denotes a significant difference (P = 0.021 in experiment 1 and P < 0.016 in experiment 2). The error bars denote the SEM.
The analysis so far was based on the full experimental length, which combined effects of the online (first 20 min) and offline (last 20 min) stimulation. However, because the behavioral effects of online and offline stimulation may differ (e.g., ref. 26), we were interested in examining the effects of tDCS stimulation on mind wandering before the task and during the task. To this end, we analyzed separately the first half of the experiment (“online stimulation”: first 20 minutes, 12 targets and 12 thought probes) and the second half of the experiment (“offline stimulation”: last 20 minutes, 12 targets and 12 thought probes). The pattern of results for the two parts analyzed separately was similar to the pattern of results of the full experiment. For the first half of the experiment, stimulation of the prefrontal cortex increased the TUT rating compared with the sham stimulation [prefrontal cortex stimulation = 2.23 (MSE = 0.18), sham stimulation = 1.99 (MSE = 0.16)], and the effect was marginally significant [t(13) = 1.82, P = 0.09, Cohen’s d = 0.49]. For the second half of the experiment, stimulation of the prefrontal cortex significantly increased the TUT rating compared with the sham stimulation [prefrontal cortex stimulation = 2.50 (MSE = 0.2), sham stimulation = 2.15 (MSE = 0.19), t(13) = 2.59, P = 0.022, Cohen’s d = 0.60]. To compare the results of the two halves, we conducted two-way repeated measures ANOVA with type of stimulation (stimulation, sham) and part of the experiment (first half, second half) as factors. We found a significant main effect of type of stimulation [F(1,13) = 6.86, P = 0.021] and a significant main effect of time of stimulation [F(1,13) = 5.920, P = 0.03], but no significant interaction between the two [F(1,13) < 1]. Thus, in the second part of the experiment, there was an increase in the level of mind wandering, but this effect was unrelated to the brain stimulation. For the correct detection rate of the target stimuli and response time for nontarget stimuli measures, no significant effects were found (results are reported in SI Text). Thus, we conclude that the effects of online and offline stimulation did not differ.
The main result of experiment 1 was that stimulation of the prefrontal cortex compared with sham stimulation increased the TUTs (i.e., propensity for mind wandering); however, the experiment was unable to consider whether this effect was region-specific or, instead, a nonspecific effect of tDCS, because the stimulation site was not varied. To address the issue of stimulation specificity, we conducted a second experiment in which, in addition to prefrontal cortex and sham stimulation, we stimulated a control site in the occipital cortex.
Experiment 2.
This experiment used a between-subjects design (a single session per participant). Participants comprising a new group were randomly allocated to one of three groups: prefrontal cortex stimulation (anode at the left DLPFC, cathode at the right supraorbital area), sham stimulation (the same position of the electrodes), and stimulation of the occipital cortex (anode at the occipital cortex, cathode at the right supraorbital area; Materials and Methods). The experimental paradigm and other stimulation parameters were as in experiment 1.
We start with the analysis of the full experimental length. Results of the TUT rating are shown in Fig. 3B, where we can see that the TUT rating for the prefrontal stimulation condition was substantially higher than in the sham stimulation and in occipital cortex stimulation conditions [prefrontal cortex stimulation = 2.93 (MSE = 0.18), sham stimulation = 2.12 (MSE = 0.14), occipital cortex stimulation = 2.17 (MSE = 0.22)]. Statistically, one-way ANOVA with three levels (prefrontal cortex stimulation, sham stimulation, and occipital cortex stimulation) revealed a significant main effect [F(2,28) = 5.85, P = 0.007, η2 = 0.29]. A post hoc two-sample t test indicated that stimulation of the prefrontal cortex increased the TUTs compared with sham stimulation [t(18) = 3.51, P = 0.002, Cohen’s d = 1.24] and compared with the occipital cortex [t(19) = 2.62, P = 0.016, Cohen’s d = 1]. Thus, using a between-subjects design, we reproduced the effect of experiment 1 by showing that stimulation of the prefrontal cortex, but not sham stimulation, increased the TUTs. Critically, we also showed that this effect was not a result of the global effect of tDCS, because no effect was found when the occipital control site was stimulated. For the external task measures, similar to experiment 1, the correct detection rate of the target stimuli in the prefrontal cortex stimulation condition was higher than in other conditions, but the effect did not reach significance [prefrontal cortex stimulation = 69.9% (MSE = 4.6%), sham stimulation = 55.9% (MSE = 6.6%), occipital cortex stimulation = 64.6% (MSE = 4.6%), F(2,28) = 1.63, P = 0.21, η2 = 0.1]. There was no significant difference between the three conditions for the average response time for nontarget stimuli [prefrontal cortex stimulation = 0.42 (MSE = 0.24), sham stimulation = 0.41 (MSE = 0.14), occipital cortex stimulation = 0.41 (MSE = 0.14), F(2,28) < 1, η2 = 0].
Analysis of online and offline stimulation as two parts revealed similar results to the full experiment pattern of the results. For the first part of the experiment, the TUT rating for the prefrontal stimulation condition was higher than in the other two conditions: prefrontal cortex stimulation = 2.74 (MSE = 0.26), sham stimulation = 1.94 (MSE = 0.11), and occipital cortex stimulation = 2.02 (MSE = 0.21). Statistically, one-way ANOVA with three levels (prefrontal cortex stimulation, sham stimulation, and occipital cortex stimulation) revealed a significant main effect [F(2,28) = 4.35, P = 0.02, η2 = 0.23]. A post hoc two-sample t test indicated that stimulation of the prefrontal cortex increased the TUTs compared with sham stimulation [t(18) = 2.8, P = 0.011, Cohen’s d = 1.07] and compared with the occipital cortex [t(19) = 2.12, P = 0.047, Cohen’s d = 0.85]. For the second part of the experiment, the TUT rating for the prefrontal stimulation condition was higher than in the other two conditions [prefrontal cortex stimulation = 3.25 (MSE = 0.19), sham stimulation = 2.37 (MSE = 0.22), occipital cortex stimulation = 2.42 (MSE = 0.24)]. Statistically, one-way ANOVA with three levels (prefrontal cortex stimulation, sham stimulation, and occipital cortex stimulation) revealed a significant main effect [F(2,28) = 4.94, P = 0.014, η2 = 0.26]. A post hoc two-sample t test indicated that stimulation of the prefrontal cortex increased the TUTs compared with sham stimulation [t(18) = 3, P = 0.007, Cohen’s d = 1.12] and compared with the occipital cortex [t(19) = 2.68, P = 0.014, Cohen’s d = 1.02]. To compare the results of the two halves, we conducted mixed repeated measures ANOVA with a within-subject factor “part of the experiment” (first half, second half) and a between-subject factor “type of stimulation” (prefrontal cortex, sham, occipital cortex). We found a significant main effect of part of the experiment [F(1,28) = 16.355, P < 0.001], but no significant interaction between part of the experiment and type of stimulation [F(2,28) < 1]. For the correct detection rate of the target stimuli and response time for nontarget stimuli measures, no significant effects were found (results are reported in SI Text). Thus, similar to experiment 1, the effects of online and offline stimulation did not differ significantly.
Discussion
In the current study, we showed that tDCS stimulation of the prefrontal cortex, but not of the occipital cortex or sham stimulation, increased the propensity of participants to mind-wander (i.e., TUTs). We conclude that (i) mind wandering, probably the most omnipresent internal cognitive function, can be modulated by means of external stimulation and (ii) the frontal lobes play a causal role in mind-wandering behavior. In addition, the observation that stimulation of the frontal lobes increased mind wandering without impairing external task performance (but slightly improving it) supports the notion that the executive control network, and the DLPFC in particular, might be involved in mind wandering. The implications of these findings are discussed below.
Mind wandering is a ubiquitous phenomenon. A notable property of mind wandering is that it is self-generated and internally triggered; that is, at some point in time, the brain (mind) becomes more preoccupied with internal thoughts and starts to pay less attention to the external environment. Mind wandering is a spontaneous process, but it can nonetheless be modulated by the external environment. For example, in an experimental situation, mind wandering is usually less frequent when the external task is more difficult (reviewed in refs. 5, 20). What has not been known previously is whether it is possible to modulate the propensity to mind-wander using external influence on the brain. In the present study, we were able to augment the self-reported level of mind wandering through the use of tDCS. Remarkably, the effect was replicated in two independent groups of participants using within- and between-subjects designs. Thus, we conclude that spontaneous brain neural activity can be modulated externally, and this effect is reflected in a change of internally directed thought.
What are the neural correlates of mind wandering? This question has been previously explored mainly through the use of fMRI, and the prominent loci of activations have been found in the frontal lobes (reviewed in refs. 18, 19). However, fMRI studies are necessarily correlational and cannot establish a causal link between behavior and the neural substrate. Here, in two experiments, we showed that stimulation of the lateral prefrontal cortex, but not sham stimulation, increased the propensity to mind-wander. In addition, the fact that stimulation of the control site (occipital cortex) did not increase the mind wandering rules out the possibility of unspecific (global) tDCS influence. Thus, we provide the first causal evidence, to our knowledge, that the frontal lobes are involved in mind wandering. It is worth noting that our findings do not imply that the frontal lobes are the only region of the brain causally involved in mind wandering, because we did not examine any regions other than the occipital lobe control site.
A known property of the tDCS is its relatively limited spatial resolution (23, 27). Therefore, our study was not designed to establish unequivocally what regions within the frontal lobes are involved in mind wandering. Having stated that fact, the montage we used (anode at F3 EEG 10–20 system location and cathode at contralateral supraorbital area) has been widely used to stimulate the DLPFC cortical region (e.g., refs. 28–32). In addition, using a volume conductance method, it was recently shown that the brain current density is higher in cortical regions that are closer to the stimulation electrode (33). Therefore, it is plausible that the left DLPFC, which has been previously implicated in mind wandering (e.g., refs. 10, 18), was stimulated in our study. In addition to the DLPFC, neural activity in the medial frontal lobes, the region implicated in different types of internal processing (9), could potentially be modulated as a result of frontal lobe stimulation. This thesis could be supported by two combined tDCS-fMRI studies that stimulated the DLPFC (the same montage as ours) and reported changes after stimulation in functional connectivity of the default mode network, including the medial frontal cortex (34, 35). Finally, it cannot be ruled out that the right frontopolar cortex (36), under the cathode electrode, was also stimulated (21, 27, 37), contributing to the increased level of mind wandering. Taken together, the present results suggest that several regions within the prefrontal cortex could be potentially stimulated. Future studies, using our paradigm and setup of simultaneous fMRI and tDCS (38), might be able to provide a more direct answer to the question of stimulation spatial specificity.
An additional interesting observation was that the increase in mind wandering as a result of stimulation of the frontal lobes was not accompanied by a decrease in detection of targets; that is, without stimulation, mind wandering and external task performance are usually anticorrelated (reviewed in ref. 19). Our result is unlikely to reflect an insufficient statistical power, because there was a consistent trend toward increase in detection of targets as a result of stimulation in both experiments. Thus, to some extent, the brain stimulation caused an enhancement (extension) of cognitive capacity, so that the increase in mind wandering did not compromise performance of the external task. This effect could possibly be mediated by the DLPFC, a region that plays a central role in executive processing, cognitive control, and working memory (16). Previous tDCS studies have shown that anodal stimulation of the DLPFC (a montage identical to ours) enhances cognitive control (39) and working memory (29, 32). In the context of our experiment, cognitive control is needed for detection of targets, yet there is accumulated evidence that cognitive control is also important for mind wandering (5, 10, 18; cf. ref. 20). Thus, it is possible that tDCS stimulation of the DLPFC increased the total capacity of the executive control system. Taken together, our results support the notion that executive control is an integral part of the mind-wandering cognitive mechanism.
In the present study, we used tDCS stimulation, which was applied during the first 20 min of the experimental task, and we used no stimulation during the remaining 20 min of the experimental task. Our main analysis was conducted for the full experimental length (40 min: 24 targets and 24 thought probes). In addition, we analyzed separately the periods of the online stimulation (first 20 min of the experiment) and the offline stimulation (last 20 min of the experiment). The general pattern of results for both online and offline stimulation in both experiments 1 and 2 was similar to the patterns revealed in the full experiment. In particular, only prefrontal cortex stimulation increased the propensity of participants to mind-wander. Direct comparison between the two parts of the experiment for all conditions revealed a higher level of mind wandering in the second part (i.e., offline stimulation) compared with the first part (i.e., online stimulation) of the experiment. This result is in line with previous reports of an increased level of mind wandering over time (e.g., refs. 40, 41); a possible explanation of this observation is an increased level of fatigue during the experiment (20). Critically, this increased level of mind wandering in the second part of our experiment was unrelated to prefrontal cortex stimulation, because the increase was found for all stimulation conditions, including a sham condition. Thus, the behavioral effects of online and offline stimulation did not differ. This result is apparently at odds with the notion that behavioral effects (26, 42, 43) and physiological mechanisms (23) of online and offline stimulation may differ. However, it should be noted that the number of studies that directly (within the same study) compared online and offline stimulation is limited, and their results do not provide a clear picture. In particular, two studies found enhanced effects for online compared with offline stimulation in a motor learning task (montage: anode electrode at motor cortex, cathode electrode over the contralateral supraorbital ridge) (26) and in an n-back working memory task (montage: anode electrode at left F3 electrode site, cathode electrode positioned extracephalically on the right upper arm) (42). Another study found enhanced effects for online compared with offline stimulation in a picture-naming task for older adults, but not for young adults [montage: anode electrode at left DLPFC, cathode electrode at central zero (Cz) EEG site] (43). Finally, in a visual perceptual learning task, the opposite pattern was found: The effects of offline stimulation were stronger compared with online stimulation (montage: anode electrode at the occipital cortex, cathode electrode positioned extracephalically on the right arm) (44). Therefore, it is possible that the differences between online and offline effects are task- and montage-dependent. More studies are needed to understand the differences between online and offline stimulation effects.
In conclusion, we showed that mind wandering can be modulated externally by means of stimulation, and that the frontal lobes play a causal role in mind wandering. Our results shed new light on the neural mechanisms of mind wandering and spontaneous activity in general; furthermore, they suggest that the executive control system might play an important role in mind wandering.
Materials and Methods
Participants.
Forty-seven healthy volunteers participated in the two experiments, which were approved by the Department of Psychology, Bar Ilan University review board committee; written informed consent was provided by all participants. Data of two participants were excluded due to the participants’ inability to follow the instructions (one participant in experiment 1 and another in experiment 2). After exclusion, in experiment 1, 14 participants (average age = 24.4 y, eight male) took part in two experimental sessions (two laboratory visits) separated by at least 1 wk (mean time between sessions = 8.3 d). Participants were randomly allocated to receive either prefrontal cortex or sham stimulation during the first session (seven participants per group, four male) and subsequently received the other type of stimulation in the second session. In experiment 2, new participants took part in the experiment, which required only a single session. Participants were randomly allocated to one of three groups: prefrontal cortex stimulation (10 participants; average age = 25.3, seven male), sham stimulation (10 participants; average age = 26.1 y, six male), or occipital cortex stimulation (11 participants; average age = 24.5 y, six male). The sample size was determined a priori based on previous tDCS studies that stimulated DLPFC (a meta-analysis is provided in ref. 29).
Apparatus.
Participants sat in a comfortable chair at a distance of 60 cm from the monitor. Stimuli were presented using MATLAB 7.6 (MathWorks) with Psychtoolbox (45). Participants wore earplugs to minimize the influence of external noise.
Experimental Design and Stimuli.
The SART paradigm was used (10, 24) (Fig. 1). Digits from “0” to “9” were shown on the screen in a random order. Stimuli were black [Red–Green–Blue (RGB): 0, 0, 0], and the background was gray (RGB: 104, 104, 104). The stimuli were presented at the center of the screen (3° of visual angle). Stimulus duration and interstimulus interval were 1 s and 1.2 s, respectively. Duration parameters were chosen in accordance with previous studies (10, 25). For all digits except 3 (i.e., nontargets), participants had to press a keyboard spacebar as soon as they detected the stimulus. For the digit 3 (i.e., the target), they had to refrain from responding. Participants were instructed to balance speed (response for nontargets) and accuracy (no response for targets). From time to time, participants were presented with thought probes (Fig. 1). The thought probe question asked “To what extent have you experienced task-unrelated thoughts prior to the thought probe?” The answer scale ranged from “1” (minimal TUTs) to “4” (maximal TUTs). All of the text in the experiment was presented in Hebrew. Both the target and thought probes appeared after a long sequence of nontargets (average number of nontargets = 20; SD = 5.69, minimum = 12, maximum = 29). The experiment included 24 targets and 24 thought probes. The order of targets and thought probe blocks was random (the same for all participants). The duration of the experiment was 40 min.
The definition of TUTs varies from study to study (e.g., refs. 10, 12, 13). In our study, TUTs were defined for the participants as thoughts that were irrelevant to the experiment and did not help with task execution (e.g., thoughts that were related to personal memories or future plans). Participants were asked to base their TUT ratings on the last several seconds before the probe. All participants confirmed that they understood the instructions. In addition, before the experiment, participants underwent a short training session that included two targets and two thought probes. At the end of the experiment, during the informal debriefing, participants indicated that their TUTs were related to their personal lives.
Brain Stimulation.
For tDCS, a battery-driven, constant-current stimulator was used (Magstim). A saline-soaked pair of surface sponge electrodes (7 × 5-cm cathode, 4 × 4-cm anode) were used to transfer the current. To ensure a more focused stimulation effect, the stimulating electrode (anode) was smaller than the reference electrode (cathode) (22). For the real stimulation, the current was ramped up to 1 mA over 30 s, remained at 1 mA for 20 min, and was ramped down to 0 mA over 30 s. For the sham stimulation, the procedure was the same but the stimulation continued for only 2 min (22). Most participants did not feel the electrical current in the case of either real or sham stimulation. Those who felt it reported an itching sensation during ramping up, but not during the experiment. Thus, this procedure allowed for participants to remain blinded as to their stimulation condition. To stimulate the prefrontal cortex, the anode was positioned at F3 (EEG 10–20 system) and the cathode at the right supraorbital area. This montage is widely used to stimulate the DLPFC cortical location (e.g., refs. 28–32). The same montage was used for the sham stimulation. To stimulate the occipital cortex (control montage), the anode electrode was at the occipital zero (Oz) position and the cathode was at the Cz position. This control montage was chosen because it does not stimulate the frontal lobes and the electrodes are far enough from default mode network regions (e.g., temporoparietal junction). The stimulation and the experimental task started at the same time (Fig. 2). For stimulation conditions, we used a combination of online and offline stimulations (e.g., refs. 46, 47), where the first half of the experimental task was executed during the stimulation (20 min) and the second half of the experimental task was executed after the stimulation (20 min). None of the participants noticed or felt a change in stimulation over the course of the experiment.
Data Analysis.
Custom MATLAB code and SPSS 17 software (IBM Corporation) were used in data analysis. The main analysis was based on the full experimental length (40 min, 24 targets and 24 thought probes). Given that the timing of the tDCS stimulation relative to the experimental paradigm might influence the behavioral effects (26, 42–44), we conducted an additional analysis for the first (online stimulation) and second (offline stimulation) parts of the experiment separately (20 min, 12 targets and 12 thought probes each).
Data were analyzed within subjects (experiment 1; comparison between two stimulation conditions of the same participant) and between subjects (experiment 2; comparison between stimulation conditions of different participants). The significance of any differences between conditions was estimated using a two-tailed, paired t test (experiment 1); one-way ANOVA (experiment 2); and a post hoc two-tailed, two-sample t test (experiment 2). The effects of online stimulation (first half of the experiment) and offline stimulation (second half of the experiment) were compared using repeated measures two-way ANOVA (experiment 1) and mixed repeated measures ANOVA (experiment 2). For all of the tests, assumption of normality of the data was first validated using the Lilliefors test (lillietest MATLAB function). In addition, we repeated the analyses using nonparametric tests, and the significant effects were qualitatively the same.
Supplementary Material
Acknowledgments
We thank Micah Allen for helpful advice, and Karine Jospe and Hadas Pick for technical assistance. This work was supported by the Israeli Center of Research Excellence in Cognitive Sciences (M.B., Program 51/11 for M.L.) and the Wellcome Trust (G.R.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission. M.C.C. is a guest editor invited by the Editorial Board.
See Commentary on page 3182.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1421435112/-/DCSupplemental.
References
- 1.Killingsworth MA, Gilbert DT. A wandering mind is an unhappy mind. Science. 2010;330(6006):932. doi: 10.1126/science.1192439. [DOI] [PubMed] [Google Scholar]
- 2.Stawarczyk D, Majerus S, Maj M, Van der Linden M, D’Argembeau A. Mind-wandering: Phenomenology and function as assessed with a novel experience sampling method. Acta Psychol (Amst) 2011;136(3):370–381. doi: 10.1016/j.actpsy.2011.01.002. [DOI] [PubMed] [Google Scholar]
- 3.Bar M, Aminoff E, Mason M, Fenske M. The units of thought. Hippocampus. 2007;17(6):420–428. doi: 10.1002/hipo.20287. [DOI] [PubMed] [Google Scholar]
- 4.Bar M. The proactive brain: Memory for predictions. Philos Trans R Soc Lond B Biol Sci. 2009;364(1521):1235–1243. doi: 10.1098/rstb.2008.0310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Smallwood J, Schooler JW. The restless mind. Psychol Bull. 2006;132(6):946–958. doi: 10.1037/0033-2909.132.6.946. [DOI] [PubMed] [Google Scholar]
- 6.Smallwood J, Ruby FJ, Singer T. Letting go of the present: Mind-wandering is associated with reduced delay discounting. Conscious Cogn. 2013;22(1):1–7. doi: 10.1016/j.concog.2012.10.007. [DOI] [PubMed] [Google Scholar]
- 7.Baird B, et al. Inspired by distraction: Mind wandering facilitates creative incubation. Psychol Sci. 2012;23(10):1117–1122. doi: 10.1177/0956797612446024. [DOI] [PubMed] [Google Scholar]
- 8.Mooneyham BW, Schooler JW. The costs and benefits of mind-wandering: A review. Can J Exp Psychol. 2013;67(1):11–18. doi: 10.1037/a0031569. [DOI] [PubMed] [Google Scholar]
- 9.Andrews-Hanna JR, Smallwood J, Spreng RN. The default network and self-generated thought: Component processes, dynamic control, and clinical relevance. Ann N Y Acad Sci. 2014;1316(1):29–52. doi: 10.1111/nyas.12360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Christoff K, Gordon AM, Smallwood J, Smith R, Schooler JW. Experience sampling during fMRI reveals default network and executive system contributions to mind wandering. Proc Natl Acad Sci USA. 2009;106(21):8719–8724. doi: 10.1073/pnas.0900234106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mason MF, et al. Wandering minds: The default network and stimulus-independent thought. Science. 2007;315(5810):393–395. doi: 10.1126/science.1131295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Stawarczyk D, Majerus S, Maquet P, D’Argembeau A. Neural correlates of ongoing conscious experience: Both task-unrelatedness and stimulus-independence are related to default network activity. PLoS ONE. 2011;6(2):e16997. doi: 10.1371/journal.pone.0016997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Allen M, et al. The balanced mind: The variability of task-unrelated thoughts predicts error monitoring. Front Hum Neurosci. 2013;7:743. doi: 10.3389/fnhum.2013.00743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mittner M, et al. When the brain takes a break: A model-based analysis of mind wandering. J Neurosci. 2014;34(49):16286–16295. doi: 10.1523/JNEUROSCI.2062-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kucyi A, Salomons TV, Davis KD. Mind wandering away from pain dynamically engages antinociceptive and default mode brain networks. Proc Natl Acad Sci USA. 2013;110(46):18692–18697. doi: 10.1073/pnas.1312902110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Miller EK, Cohen JD. An integrative theory of prefrontal cortex function. Annu Rev Neurosci. 2001;24(1):167–202. doi: 10.1146/annurev.neuro.24.1.167. [DOI] [PubMed] [Google Scholar]
- 17.Dumontheil I, Gilbert SJ, Frith CD, Burgess PW. Recruitment of lateral rostral prefrontal cortex in spontaneous and task-related thoughts. Q J Exp Psychol (Hove) 2010;63(9):1740–1756. doi: 10.1080/17470210903538114. [DOI] [PubMed] [Google Scholar]
- 18.Dixon ML, Fox KC, Christoff K. A framework for understanding the relationship between externally and internally directed cognition. Neuropsychologia. 2014;62:321–330. doi: 10.1016/j.neuropsychologia.2014.05.024. [DOI] [PubMed] [Google Scholar]
- 19.Schooler JW, et al. Meta-awareness, perceptual decoupling and the wandering mind. Trends Cogn Sci. 2011;15(7):319–326. doi: 10.1016/j.tics.2011.05.006. [DOI] [PubMed] [Google Scholar]
- 20.McVay JC, Kane MJ. Does mind wandering reflect executive function or executive failure? Comment on Smallwood and Schooler (2006) and Watkins (2008) Psychol Bull. 2010;136(2):188–197, discussion 198–207. doi: 10.1037/a0018298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Filmer HL, Dux PE, Mattingley JB. Applications of transcranial direct current stimulation for understanding brain function. Trends Neurosci. 2014;37(12):742–753. doi: 10.1016/j.tins.2014.08.003. [DOI] [PubMed] [Google Scholar]
- 22.Nitsche MA, et al. Transcranial direct current stimulation: State of the art 2008. Brain Stimulat. 2008;1(3):206–223. doi: 10.1016/j.brs.2008.06.004. [DOI] [PubMed] [Google Scholar]
- 23.Stagg CJ, Nitsche MA. Physiological basis of transcranial direct current stimulation. Neuroscientist. 2011;17(1):37–53. doi: 10.1177/1073858410386614. [DOI] [PubMed] [Google Scholar]
- 24.Robertson IH, Manly T, Andrade J, Baddeley BT, Yiend J. ‘Oops!’: performance correlates of everyday attentional failures in traumatic brain injured and normal subjects. Neuropsychologia. 1997;35(6):747–758. doi: 10.1016/s0028-3932(97)00015-8. [DOI] [PubMed] [Google Scholar]
- 25.Smallwood J, McSpadden M, Luus B, Schooler J. Segmenting the stream of consciousness: The psychological correlates of temporal structures in the time series data of a continuous performance task. Brain Cogn. 2008;66(1):50–56. doi: 10.1016/j.bandc.2007.05.004. [DOI] [PubMed] [Google Scholar]
- 26.Stagg CJ, et al. Polarity and timing-dependent effects of transcranial direct current stimulation in explicit motor learning. Neuropsychologia. 2011;49(5):800–804. doi: 10.1016/j.neuropsychologia.2011.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nitsche MA, et al. Shaping the effects of transcranial direct current stimulation of the human motor cortex. J Neurophysiol. 2007;97(4):3109–3117. doi: 10.1152/jn.01312.2006. [DOI] [PubMed] [Google Scholar]
- 28.Nitsche MA, Boggio PS, Fregni F, Pascual-Leone A. Treatment of depression with transcranial direct current stimulation (tDCS): A review. Exp Neurol. 2009;219(1):14–19. doi: 10.1016/j.expneurol.2009.03.038. [DOI] [PubMed] [Google Scholar]
- 29.Brunoni AR, Vanderhasselt M-A. Working memory improvement with non-invasive brain stimulation of the dorsolateral prefrontal cortex: A systematic review and meta-analysis. Brain Cogn. 2014;86:1–9. doi: 10.1016/j.bandc.2014.01.008. [DOI] [PubMed] [Google Scholar]
- 30.Zmigrod S, Colzato LS, Hommel B. Evidence for a role of the right dorsolateral prefrontal cortex in controlling stimulus-response integration: A transcranial direct current stimulation (tDCS) study. Brain Stimulat. 2014;7(4):516–520. doi: 10.1016/j.brs.2014.03.004. [DOI] [PubMed] [Google Scholar]
- 31.Mungee A, et al. Transcranial direct current stimulation of the prefrontal cortex: A means to modulate fear memories. Neuroreport. 2014;25(7):480–484. doi: 10.1097/WNR.0000000000000119. [DOI] [PubMed] [Google Scholar]
- 32.Fregni F, et al. Anodal transcranial direct current stimulation of prefrontal cortex enhances working memory. Exp Brain Res. 2005;166(1):23–30. doi: 10.1007/s00221-005-2334-6. [DOI] [PubMed] [Google Scholar]
- 33.Wagner S, et al. Investigation of tDCS volume conduction effects in a highly realistic head model. J Neural Eng. 2014;11(1):016002. doi: 10.1088/1741-2560/11/1/016002. [DOI] [PubMed] [Google Scholar]
- 34.Peña-Gómez C, et al. Modulation of large-scale brain networks by transcranial direct current stimulation evidenced by resting-state functional MRI. Brain Stimulat. 2012;5(3):252–263. doi: 10.1016/j.brs.2011.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Keeser D, et al. Prefrontal transcranial direct current stimulation changes connectivity of resting-state networks during fMRI. J Neurosci. 2011;31(43):15284–15293. doi: 10.1523/JNEUROSCI.0542-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Christoff K, Gabrieli JD. The frontopolar cortex and human cognition: Evidence for a rostrocaudal hierarchical organization within the human prefrontal cortex. Psychobiology (Austin Tex) 2000;28(2):168–186. [Google Scholar]
- 37.Nitsche MA, Paulus W. Excitability changes induced in the human motor cortex by weak transcranial direct current stimulation. J Physiol. 2000;527(Pt 3):633–639. doi: 10.1111/j.1469-7793.2000.t01-1-00633.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Meinzer M, et al. Transcranial direct current stimulation and simultaneous functional magnetic resonance imaging. J Vis Exp. 2014;(86):e51730–e51730. doi: 10.3791/51730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Vanderhasselt M-A, et al. tDCS over the left prefrontal cortex enhances cognitive control for positive affective stimuli. PLoS ONE. 2013;8(5):e62219. doi: 10.1371/journal.pone.0062219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Teasdale JD, et al. Stimulus-independent thought depends on central executive resources. Mem Cognit. 1995;23(5):551–559. doi: 10.3758/bf03197257. [DOI] [PubMed] [Google Scholar]
- 41.McVay JC, Kane MJ. Conducting the train of thought: Working memory capacity, goal neglect, and mind wandering in an executive-control task. J Exp Psychol Learn Mem Cogn. 2009;35(1):196–204. doi: 10.1037/a0014104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Martin DM, Liu R, Alonzo A, Green M, Loo CK. Use of transcranial direct current stimulation (tDCS) to enhance cognitive training: Effect of timing of stimulation. Exp Brain Res. 2014;232(10):3345–3351. doi: 10.1007/s00221-014-4022-x. [DOI] [PubMed] [Google Scholar]
- 43.Fertonani A, Brambilla M, Cotelli M, Miniussi C. The timing of cognitive plasticity in physiological aging: A tDCS study of naming. Front Aging Neurosci. 2014;6:131. doi: 10.3389/fnagi.2014.00131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pirulli C, Fertonani A, Miniussi C. The role of timing in the induction of neuromodulation in perceptual learning by transcranial electric stimulation. Brain Stimulat. 2013;6(4):683–689. doi: 10.1016/j.brs.2012.12.005. [DOI] [PubMed] [Google Scholar]
- 45.Brainard DH. The Psychophysics Toolbox. Spat Vis. 1997;10(4):433–436. [PubMed] [Google Scholar]
- 46.Reis J, et al. Noninvasive cortical stimulation enhances motor skill acquisition over multiple days through an effect on consolidation. Proc Natl Acad Sci USA. 2009;106(5):1590–1595. doi: 10.1073/pnas.0805413106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cohen Kadosh R, Soskic S, Iuculano T, Kanai R, Walsh V. Modulating neuronal activity produces specific and long-lasting changes in numerical competence. Curr Biol. 2010;20(22):2016–2020. doi: 10.1016/j.cub.2010.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.