Abstract
The gastrointestinal mucosa has proven to be an interesting tissue for which to investigate disease-related metabolism. In this review, we outline some evidence that implicates metabolic signaling as important features of barrier in the healthy and disease. Studies from cultured cell systems, animal models and human patients have revealed that metabolites generated within the inflammatory microenvironment are central to barrier regulation. These studies have revealed a prominent role for hypoxia and hypoxia-inducible factor (HIF) at key steps in adenine nucleotide metabolism and within the creatine kinase pathway. Results from animal models of intestinal inflammation have demonstrated an almost uniformly beneficial influence of HIF stabilization on disease outcomes and barrier function. Studies underway to elucidate the contribution of immune responses will provide additional insight into how metabolic changes contribute to the complexity of the gastrointestinal tract and how such information might be harnessed for therapeutic benefit.
Keywords: Colitis, creatine, epithelium, inflammation, metabolism, mucosa, murine model, nucleoside, nucleotidase, neutrophil, nucleotide, phosphocreatine
Abbreviations: AMP, adenosine monophosphate; ChIP, chromatin immunoprecipitation; CK, creatine kinase; HIF, hypoxia-inducible factor; PHD, prolyl hydroxylase; PMN, polymorphonuclear leukcoyte, neutrophil; TJ, tight junction; VASP, vasodilator-stimulated; ZO-1, zonula occludens-1
Introduction
Ongoing inflammatory responses are associated with profound shifts in tissue metabolism.1 These changes include local depletion of nutrients, increased oxygen consumption and the generation of large quantities of reactive nitrogen and oxygen intermediates.2 Such shifts in tissue metabolism result, at least in part, from profound recruitment of inflammatory cell types, particularly myeloid cells such as neutrophils (PMN). The vast majority of inflammatory cells are recruited to, as opposed to resident at inflammatory lesions.3 By stark contrast, adaptive immune responses are characterized by high rates of local T and B cell proliferation and have significantly different metabolic demands.4,5 Herein, it is important to understand the interactions between microenvironmental metabolic changes (e.g. glucose, oxygen, ATP) as they relate to metabolic triggers and molecular mechanisms of immune cell recruitment / activation into these areas. Additionally, it is imperative to define whether mechanisms initiated by such metabolic shifts might serve as relevant therapeutic targets.
The intestinal mucosae are characterized by a uniquely dynamic oxygenation profile, experiencing multiple profound fluctuations in blood perfusion and metabolism per day.2 Even in the basal state, component epithelial cells lining the mucosa exist in a relatively low oxygen tension environment, described as ‘physiologic hypoxia’. Classically, in the small intestine, this has been attributed to a countercurrent oxygen exchange mechanism, whereby oxygen from arterial blood supplying the villi diffuses to adjacent venules, traveling from villous tip to base, resulting in graded hypoxia.6 However, a steep oxygen gradient has also been documented in more distal, colonic regions of the gastrointestinal (GI) tract, spanning from the anaerobic lumen, across the epithelium to the richly vascularized sub-epithelial mucosa. Given the high-energy requirement of the gut and the integral role of the epithelium in maintaining intestinal homeostasis, it is pertinent that these cells have evolved a number of molecular mechanisms to cope with such challenging metabolic conditions. Indeed, the intestinal epithelium has proven to be remarkably resistant to hypoxia, and even very low levels of oxygenation within this cell layer may provide a regulatory adaptation for maintenance of barrier function and integrity.7
A role for epithelial barrier dysregulation in inflammatory bowel disease (IBD) is supported by observations of increased intestinal permeability in a subset of first-degree relatives of patients with Crohn's disease (CD).8 Barrier function of the epithelial monolayer is mediated by a number of specialized anatomical features that confer selective permeability to luminal contents.9 Epithelia are polarized, with apical surface functions optimized for luminal interaction and enteric bacterial exclusion (e.g., intercellular junctions, vectorial membrane transport systems and mucus secretion) and basolateral surfaces adapted for interface with the underlying mucosa and immune cell repertoire. Notably, both absorptive and barrier epithelial functions are physiologically regulated by oxygen.10 Intestinal epithelia also actively participate as innate immune sensors of microbial pathogens and commensal organisms.11,12 In fact, a state of low-grade inflammation at the GI mucosal surface is sustained by omnipresent luminal antigens, and is central both to development of oral tolerance and to priming of the mucosal immune system should antigenic material penetrate the epithelial barrier. Studies with gnotobiotic mice have further revealed that enteric microbiota themselves influence epithelial cell metabolism, barrier function and survival.13 As increased epithelial permeability and resultant mucosal inflammation underlie2 the pathology of IBD, a fundamental understanding of microenvironmental metabolic factors that influence initiation, perpetuation and resolution of overt disease is central to defining potential therapeutic targets. Here, we will discuss how such barrier is dynamically regulated, particularly related to metabolic changes associated with inflammation.
The Contribution of HIF and Barrier Function
Given the significant shifts in metabolism and oxygen availability during inflammation, a number of studies have shown that hypoxia-inducible factor (HIF) triggers the expression of genes that enable intestinal epithelial cells to function as an effective barrier.14,17 HIF is a member of the Per-ARNT-Sim (PAS) family of basic helix-loop-helix (bHLH) transcription factors and is considered one of the central regulators of tissue metabolism.18 HIF activation is dependent upon stabilization of an O2-dependent degradation domain (ODD) domain of the α-subunit and subsequent nuclear translocation to form a functional complex with HIF-1β and cofactors such as CREB-binding protein and its ortholog p300.19 Under conditions of adequate oxygen supply, iron and O2-dependent hydroxylation of 2 prolines (Pro564 and Pro402) within the ODD of HIF-α initiates the association with the von Hippel-Lindau tumor suppressor protein (pVHL) and rapid degradation via ubiquitin-E3 ligase proteasomal targeting.20,21 A second hypoxic switch operates in the carboxy terminal transactivation domain of HIF-α( Here, hypoxia blocks the hydroxylation of Asp80, thereby facilitating the recruitment of CBP/p300.22
The importance of HIF to epithelial barrier function was originally shown by microarray analysis of intestinal epithelial cells cultured in hypoxia.16 These studies have subsequently been validated in animal models of intestinal inflammation23,28 and in inflamed human intestinal tissues.29,31 The functional proteins encoded by hypoxia-induced, HIF-dependent mRNAs localize primarily to the most luminal aspect of polarized epithelia. Molecular studies of these hypoxia-elicited pathway(s) have shown a dependence on HIF-mediated transcriptional responses. In extension of these original studies to the in vivo setting, Karhausen, et al. generated mice expressing either mutant Hif1a (causing constitutive repression of Hif1a) or mutant von Hippel-Lindau (causing constitutive overexpression of HIF) targeted to the intestinal epithelial cells.25 Loss of epithelial HIF-1α resulted in a more severe colitic phenotype than wild-type animals, with increased weight loss, decreased colon length and importantly, increased intestinal permeability. Conversely, constitutively active intestinal epithelial HIF was protective for each of the parameters studied. These findings were model-dependent, since epithelial HIF-based signaling has also been shown to promote inflammation in another study.28 However, the findings confirmed that intestinal epithelial cells can adapt to hypoxia and that HIF may contribute such adaptation.
It is noteworthy that epithelial barrier protective pathways driven by HIF tend not to be the classical regulators of barrier function, such as the tight junction proteins occludin or claudins. Rather, the HIF-regulated pathways are more to do with overall tissue integrity, including increased mucin production,32 molecules that modify mucins (e.g. intestinal trefoil factor,14) xenobiotic clearance by P-glycoprotein,15 nucleotide metabolism / signaling (by ecto-5’-nucleotidase and CD73)16,17 and most recently creatine metabolism33 (see later).
Nucleotide Metabolism in Barrier Regulation
As depicted in Figure 1, circulating or locally released nucleotides are rapidly metabolized by ecto-enzymes localized on the cell surface. Sources of extracellular nucleotides are numerous, and include the active release of nucleotides in the form of ADP and ATP by most any cell type and bystander release from dying or apoptotic cells.34 During mucosal inflammation, neutrophils and platelets are rich sources of adenine nucleotides,35 which when delivered to the luminal surface are enzymatically hydrolyzed to adenosine and function to promote electrogenic chloride secretion (and concomitant water transport) as well as barrier restitution.
Figure 1.

Sources of adenine nucleotides and metabolic steps to adenosine formation in the mucosa. During active inflammation, sources of adenine nucleotides include the active release of ATP from both PMN and platelets as well as epithelial cells. In areas of ongoing inflammation, diminished oxygen supply (inflammatory hypoxia) coordinates the metabolism of nucleotides to adenosine and subsequent signaling to adenosine receptors. ATP is selectively metabolized to adenosine by a 2-step enzymatic reaction involving ecto-apyrase (CD39) and ecto-nucleotidase (CD73). Adenosine binding to apical adenosine A2B receptors results in activation of intracellular cyclic AMP, the phosphorylation of VASP and the resealing junctions following PMN transmigration.
Ecto-5′-nucleotidase (CD73) is a glycosyl phosphatidylinositol (GPI)-linked, membrane-bound glycoprotein that hydrolyzes extracellular nucleoside monophosphates into bioactive nucleoside intermediates.36 Surface-bound CD73 metabolizes adenosine 5′-monophosphate (AMP) to adenosine, which when released can activate one of 4 types of G-protein coupled, 7 transmembrane spanning adenosine receptors (AdoR) or can be internalized through dipyridamole-sensitive carriers.37 Adenosine receptors are expressed on a wide variety of cells, and many cell types have been shown to express more than one isoform of the receptor. Likewise, activation of surface AdoR has been shown to regulate diverse physiologic endpoints. In the recent years, our understanding of nucleotide metabolic pathways has benefited from the development of genetically manipulated animals, particularly mice deficient in Cd73 or a second nucleotide metabolizing enzyme, Cd39 (ecto-apyrase), that catalyzes the phosphohydrolysis of ATP and ADP to AMP.
A number of studies have implicated CD39 and CD73 in the control of tissue barrier function, particularly in during hypoxia. Successful transmigration of leukocytes, especially polymorphonuclear (PMN, neutrophil) leukocytes across endothelia and epithelia is accomplished by temporary self-deformation with localized widening of the inter-junctional spaces,38 a process with the potential to disturb endothelial and epithelial barrier function. Original studies by Lennon et al. revealed that the prominent signaling pathway for closing inter-endothelial gaps during neutrophil transmigration involved adenosine-induced “restitution” of barrier.39 Until recently, only limited information existed regarding the biochemical events that regulate cellular barriers in the setting of either PMN activation or transmigration.40,41 These studies showed that inhibition of CD73 using either APCP or anti-CD73 monoclonal antibody 1E9 inhibited the resealing of endothelial and epithelial barriers by as much as 85%,39 suggesting the necessity for extracellular nucleotide metabolism in this pathway. Subsequent studies revealed that adenosine produced from neutrophil-derived AMP was responsible for enhanced barrier function via activation of the adenosine A2B receptor (AA2BR) coupling to cytoskeletal links.42 More recently, it was shown that in addition to (or rather than) releasing AMP, neutrophils actively release ATP following receptor-mediated stimulation.43 Such ATP is hydrolyzed to adenosine at the endothelial cell surface through the coordinated actions of CD39 and CD73. It is not clear exactly how neutrophils and / or endothelial cells release ATP, although several mechanisms have been proposed, including direct transport through ATP-binding cassette (ABC) proteins, transport through connexin hemichannels, as well as vesicular release.44
CD73 lies central to the regulation of tissue barriers during episodes of hypoxia (Fig. 1). Studies have revealed that CD73 is a strongly HIF-regulated gene and is critically important for the generation of extracellular adenosine in hypoxia.16 Studies in mouse models of increased intestinal permeability revealed that oral delivery the CD73 inhibitor APCP promotes movement of inert tracers, such as FITC-labeled dextran, across the intestinal epithelium.16 Likewise, to investigate hypoxia-induced changes in tissue permeability in Cd73−/− mice,45 we have used Evan's blue dye, which binds tightly to plasma albumin.46 Quantification of formamide-extractable Evan's blue from individual tissues can then be interpreted as a function of vascular leak.47 In general, hypoxia increases vascular permeability 2- to fold4- over normoxia, depending on the tissue.17 Pharmacologic interventions have suggested that CD73 is protective under such circumstances, and most studies have implicated a protective role for adenosine AA2AR and AA2BR in maintaining barrier function.48,49 These studies have defined CD39 and CD73 as the pacemakers for the fine-tuning of epithelial and endothelial permeability. These innate protective pathways share the common strategy of increasing extracellular adenosine concentrations and promoting adenosine signaling from the cell surface through the cytoskeleton and ultimately to dynamic regulation of tight junctions.
Mechanisms of Barrier Regulation by Adenosine
Tight junctions (TJs) constitute the backbone for the structural integrity of the barrier and provide a physical basis for the epithelial barrier to ions and solutes. Contributing to the polarized phenotype of epithelia, they furthermore prevent lipid diffusion between apical and basolateral membrane domains, the so called “fence function” of tight junctions.50 The tight junction is comprised of both transmembrane and peripheral membrane proteins, which are linked to the actin-based cytoskeleton50 where both complex assembly and transcriptional control of its components is tightly regulated by a variety of physiological and pathophysiological stimuli. Ischemia dramatically affects TJ integrity resulting in loss of transepithelial electrical resistance which has been observed both in ATP depletion models51 and in vitro hypoxia models.14,52,53 Some of the permeability changes are attributed to a alterations in distribution of occludin, zonula occludens-1 (ZO-1), ZO-2 and cingulin.51,54 Furthermore, vascular endothelial growth factor (VEGF) is a generally accepted to be a hypoxia compensatory mechanism initiating increased angiogenesis in regions of reduced oxygen supply. The underlying mechanism involves the loss of occludin organization apparently through the activation of extracellular signal regulated kinases (ERK1/2)55 and through nitric oxide (NO)-dependent mechanisms.56 Furthermore, TJ integrity is influenced by perturbations of the interaction with the actin-based cytoskeleton57,58 and by the degradation of such membrane-cytoskeletal proteins as ankyrin and fodrin both in ATP depletion models59 and in endothelial hypoxia.60
Prompted by the observation that hypoxic cells have a lessened capacity to generate cyclic nucleotides,61 a number of studies have addressed the role of cyclic adenosine monophosphate (cAMP) on TJ permeability. In general, changes in cAMP do not significantly impact TJ permeability of the intact epithelium.62 Restitution, the re-establishment of disrupted epithelia, is however, significantly dependent on adequate generation of cAMP. For example, it was shown that post-hypoxic epithelia fail to normally re-develop barrier following either physiologic disruption (e.g., neutrophil migration, see Fig. 1) or modeled disruption (e.g. calcium depletion), and that such defects were at least in part attributed to diminished cAMP generation.53 At present, it is unclear why cAMP may be so critical to restitution. Recent studies have suggested that increases in intracellular cAMP levels may promote barrier function through different mechanisms including increased expression of TJ proteins ZO-1 and occludin63 and an increase of mean number of TJ strands.64 Furthermore increases in cAMP levels are accompanied by an increase in polymerized actin and increased phosphorylation of intermediate filaments, suggesting that cAMP-mediated changes in permeability may be due to alterations in cellular cytoskeleton.65 Because of the actin-binding and cross linking functions of vasodilator-stimulated phosphoprotein (VASP), its protein kinase A (PKA) mediated phosphorylation may be crucial in this pathway. This work revealed that VASP localizes with ZO-1 at the TJ and appears as phospho-VASP at the junction following adenosine simulation (Fig. 2).66 Apart from the increasing insights that link cAMP signal transduction to tight junctional organization, cAMP affects permeability through modulation of the junctional expression of adherens junction proteins VE-cadherin and PECAM-163 and through cAMP-dependent phosphorylation of VE-cadherin.67 Finally, compelling evidence indicates that oxidative stress, commonly associated with hypoxia-reoxygenation, directly influences the structure of the endothelial55 and epithelial TJ.68,69
Figure 2.

VASP localization at epithelial tight junctions. In the upper panels, ZO-1 (red) and VASP (green) were stained and analyzed by confocal microscopy. Staining at the level of the TJ for ZO-1 was observed as the characteristic “chicken-wire” staining pattern. Staining for VASP resulted in a nearly identical staining pattern, with specific co-localized areas shown in yellow. The lower panels depict localization of phospho-VASP at TJs in response to adenosine receptor stimulation. Epithelial cells were stimulated with the adenosine analog NECA for indicated periods of time (range 0–10 min) and stained with anti-phospho-VASP. Phospho-VASP is prominently localized to the TJ under conditions in which cAMP is elevated (arrows). Adapted from Lawrence, et. al.66
Creatine Metabolism and Barrier Regulation
HIF has been implicated as the master regulator of metabolism.18 Given the strong association between HIF and colitis,23,27,70-76 we recently examined the contribution of HIF to metabolic changes in intestinal epithelia. For these purposes, we performed chromatin immunoprecipitation (ChIP) with HIF-1α and HIF-2α antibodies followed by hybridization to a promoter microarray.33 The log2 ratio (input-Cy3/HIF-ChIP-Cy5) was analyzed to identify sequences specific for HIF-1α and HIF-2α binding in hypoxia. Highly enriched subsets in the HIF-1α ChIP hits included multiple enzymes of the glycolytic pathway and jumonji domain (JmjC) containing histone demethylases. In addition, this analysis revealed prominent changes associated with autophagy, immunity, transcription and metabolism. Notably, promoter sequences for the cytosolic creatine kinase (CK) genes CKB (brain) and CKM (muscle) emerged as high fidelity HIF-2 specific targets. Likewise, 2 mitochondrial isoforms of CK (MTA1 and MT2) were significantly enriched in HIF-2 ChIP. Morphologic analysis revealed that both the CKM and CKB isoforms prominently localize to junctional regions of confluence intestinal epithelia (Fig. 3).
Figure 3.
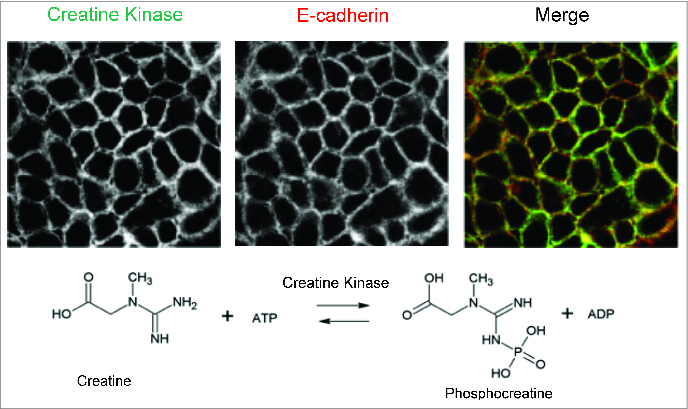
Localization of creatine kinase at epithelial junctions. In the upper panels, creatine kinase (muscle isoform) and E-cadherin were co-stained and analyzed by confocal microscopy. Staining at the level of the adherens junction revealed nearly complete co-localization (merged image). The lower reaction depicts the enzymatic reaction catalyzed by creatine kinase. Adapted from Glover et al.33
Surprisingly little is known about CK function in the mucosa. The creatine kinase pathway is often neglected in energy metabolism, as it is assumed that high-energy phosphate transport between sites of ATP production (mitochondria / glycolysis) and ATP consumption (ATPases) relies solely on the diffusion of ATP and ADP. While this may hold true in tissues devoid of CK and PCr (such as the liver), it is clearly inadequate for CK-containing tissues with high and fluctuating energy demands (e.g., skeletal and cardiac muscle, brain, retina, and spermatozoa).77 In these latter tissues, 4 distinct types of CK subunits are expressed in a developmental, species and tissue-specific manner.77 Our studies demonstrated that each of these CK subunits are expressed in cultured intestinal epithelial cell lines as well as murine colonic epithelial cells in vivo.33 All CK isoenzymes catalyze the reversible transfer of the γ-phosphate group of ATP to the guanidino group of Cr to yield ADP and PCr (Fig. 3). In high CK expressing tissues, a large pool of PCr is available for rapid regeneration of ATP hydrolyzed during high energy expenditure. During periods of high energy demand, CK reaction remains in a near-equilibrium state, keeping the concentrations of ATP and ADP constant, and thus, CK “buffers” the cytosolic phosphorylation potential that seems to be crucial for the proper functioning of a variety of cellular ATPases.77
An excellent example of high-energy expenditure within the mucosa (and therefore a need for an ATP buffering system such as Cr-PCr) is the restitution of intestinal epithelial cells following insult. Indeed, the dynamic regulation of epithelial junctions is tightly linked to the circumferential F-actin belt.78 Such F-actin contractility is highly dependent on adequate availability of high-energy phosphates and a ready supply of ATP. The dynamic nature of this F-actin-ATP interaction has been elegantly demonstrated using epithelial models of ATP depletion,78 mutagenesis of myosin light chain kinase79 as well as pharmacologically targeting actin polymerization (e.g. phalloidin).80 G-actin monomers assemble into filaments with distinct ends, a barbed, fast growing end and a pointed, slow growing end.78 Each monomer contains one nucleotide binding cleft where ATP is transformed into ADP and inorganic phosphate (Pi) via 2 processes: ATP cleavage, which produces ADP = Pi-actin, and Pi release, which leads to ADP-actin. ATP cleavage is extremely slow for actin monomers but is strongly increased after the monomer has been incorporated into a filament and turned into a protomer (or filament subunit). This process requires a surprisingly large amount of cellular energy. For example, it is estimated that endothelial cells expend nearly 20%81 and platelets expend as much as 50% of cellular ATP82 in actin-myosin-related regulation of the cytoskeleton. From this perspective, it is not surprising that Cr-PCr may well be critical for energy homeostasis and tissue barrier function during episodes of mucosal inflammation.
HIF Prolyl-Hydroxylase Inhibitors as a Common Target for Metabolic Barrier Regulation
In the past decade, the mechanisms of HIF stabilization have been clarified. Three HIF prolyl hydroxylases (PDH), termed PHD1–3, have been demonstrated to be important in the hypoxic regulation of the HIF pathway.83 Each of these hydroxylases are encoded by different genes and their gene product enzymes demonstrate tissue specific expression patterns.83 All 3 PHD's are found in the intestinal epithelium.23,27,84 Significantly, different phenotypes in mice genetically lacking individual isoforms of the hydroxylases exist. Studies in PHD1−/− mice, for example, have revealed a reprogrammed basal metabolism, decreased exercise performance85,86 and increased intestinal barrier function due to decreased epithelial apoptosis.87 PHD2 homozygous knockout is embryonic lethal due to abnormal developmental angiogenesis.88,89 PHD2 heterozygous knockout animals show enhanced tumor angiogenesis but decreased metastasis.88 PHD3 homozygous knockout mice demonstrate reduced neuronal apoptosis, abnormal sympathoadrenal development and reduced blood pressure.90. These diverse phenotypes strongly suggest distinct isoform-specific functions in vivo.
The discovery of HIF-selective PHD's as control points of HIF expression has provided the basis for the development of PHD-based molecular tools and therapies.91,92. Pharmacological inactivation of the PHDs by 2-oxoglutarate analogs is sufficient to stabilize HIF-1α,91 but is nonspecific for individual PHD isoforms. In vitro studies suggest some significant differences in substrate specificity. For example, PHD3 does not hydroxylate proline 564 on HIF-α, and comparison of enzyme activity in vitro showed that the oxygen-dependent degradation sequence is hydroxylated most efficiently by PHD2.93,94 These observations have generated significant interest in identifying enzyme-modifying therapeutics. Indeed, a number of PHD inhibitors have been described, including direct inhibitors of the prolyl-hydroxylases,95 analogs of naturally occurring cyclic hydroxamates,96 as well as antagonists of α-keto-glutarate.91 As such, we have hypothesized that pharmacologic activation of HIF would afford protection in murine colitic disease. For these purposes, we and others have used prolyl hydroxylase inhibitors that stabilize HIF-1α and induce the expression of downstream HIF target genes. These results show that the PHD inhibition provides an overall beneficial influence on clinical symptoms (weight loss, colon length, tissue TNF α / IFNγ) in murine TNBS or DSS colitis models, most likely due to their barrier protective function.23,27
More recently, we and others have utilized a AKB-4924, a relatively HIF-1-selective PHD inhibitor in mucosal inflammation models.97,98 The basis for HIF-1 over HIF-2 selectivity is not known at the present time. Administration of AKB-4924 in models of murine colitis augmented epithelial barrier function and led to an approximately 50-fold reduction in serum endotoxin during colitis. AKB-4924 also decreased cytokines involved in pyrogenesis and hypothermia, significantly reducing serum levels of IL-1β, IL-6 and TNF-α, while increasing IL-10. AKB-4924 offered no protection against colitis in epithelial-specific HIF-1α deficient mice, strongly implicating epithelial HIF-1α as the tissue target for AKB-4924-mediated protection. Such findings emphasize the role of epithelial barrier function during inflammatory diseases in the colon and may provide the basis for a therapeutic use of PHD inhibitors in inflammatory mucosal disease.
Conclusion
The low baseline O2 tension and profoundly dynamic energy demands of gastrointestinal mucosae provide a unique setting to study metabolic signaling in health and disease. Restitution of the epithelial barrier and assembly of apical epithelial junctions defines a pivotal determinant of resolution after inflammatory insult. Hypoxia, and specifically HIF-regulated pathways, are strongly associated with barrier function in colitis, wherein altered nucleotide signaling and cellular bioenergetics contribute fundamentally to inflammatory resolution. Studies in animal models of IBD have demonstrated an overall protective and anti-inflammatory influence of HIF stabilizing agents (esp. prolyl hydroxylase inhibition), identifying this mucosal surface as a strong candidate for targeted HIF-based therapy. Throughout these in vitro and in vivo studies, the metabolism of adenine nucleotides, and more recently creatine metabolism, have proven relevant to multiple signaling pathways in the epithelium. Overall, the endogenous adaptive metabolic pathways activated in response to hypoxia represent potentially important new windows of therapeutic opportunity in mucosal inflammation.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Funding
This work was supported by NIH grants DK50189 / HL60569 / DK095491, VA Merit Award 1I01BX002182, and by the Crohn's and Colitis Foundation of America.
References
- 1.Kominsky DJ, Campbell EL, Colgan SP. Metabolic shifts in immunity and inflammation. J Immunol 2010;184:4062-8; PMID:; http://dx.doi.org/ 10.4049/jimmunol.0903002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Colgan SP, Taylor CT. Hypoxia: an alarm signal during intestinal inflammation. Nat Rev Gastroenterol Hepatol 2010;7:281-287; PMID:; http://dx.doi.org/ 10.1038/nrgastro.2010.39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lewis JS, Lee JA, Underwood JC, Harris AL, Lewis CE. Macrophage responses to hypoxia: relevance to disease mechanisms. J Leukoc Biol 1999;66:889-900; PMID: [DOI] [PubMed] [Google Scholar]
- 4.Fox CJ, Hammerman PS, Thompson CB. Fuel feeds function: energy metabolism and the T-cell response. Nat Rev Immunol 2005;5:844-52; PMID:; http://dx.doi.org/ 10.1038/nri1710 [DOI] [PubMed] [Google Scholar]
- 5.Sitkovsky M, Lukashev D. Regulation of immune cells by local-tissue oxygen tension: HIF1 a and adenosine receptors. Nat Rev Immunol 2005;5:712-21; PMID:; http://dx.doi.org/ 10.1038/nri1685 [DOI] [PubMed] [Google Scholar]
- 6.Shepherd AP.Metabolic control of intestinal oxygenation and blood flow. Fed Proc 1982;41:2084-9; PMID: [PubMed] [Google Scholar]
- 7.Furuta GT, Turner JR, Taylor CT, Hershberg RM, Comerford K, Narravula S, et al. Hypoxia-inducible factor 1-dependent induction of intestinal trefoil factor protects barrier function during hypoxia. J Exp Med 2001;193:1027-34; PMID:; http://dx.doi.org/ 10.1084/jem.193.9.1027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hollander D, Vadheim CM, Brettholz E, Petersen GM, Delahunty T, Rotter JI. Increased intestinal permeability in patients with Crohn's disease and their relatives. A possible etiologic factor. Ann Intern Med 1986;105:883-5; PMID:; http://dx.doi.org/ 10.7326/0003-4819-105-6-883 [DOI] [PubMed] [Google Scholar]
- 9.Laukoetter MG, Nava P, Nusrat A. Role of the intestinal barrier in inflammatory bowel disease. World J Gastroenterol 2008;14:401-7; PMID:; http://dx.doi.org/ 10.3748/wjg.14.401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Taylor CT, Colgan SP. Hypoxia and gastrointestinal disease. J Mol Med 2007;85:1295-300; PMID:; http://dx.doi.org/ 10.1007/s00109-007-0277-z [DOI] [PubMed] [Google Scholar]
- 11.Clavel T, Haller D. Molecular interactions between bacteria, the epithelium, and the mucosal immune system in the intestinal tract: implications for chronic inflammation. Curr Issues Intest Microbiol 2007;8:25-43; PMID: [PubMed] [Google Scholar]
- 12.Kagnoff MF, Eckmann L. Epithelial cells as sensors for microbial infection. J Clin Invest 1997;100:6-10; PMID:; http://dx.doi.org/ 10.1172/JCI119522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Madsen K, Cornish A, Soper P, McKaigney C, Jijon H, Yachimec C, et al. Probiotic bacteria enhance murine and human intestinal epithelial barrier function. Gastroenterology 2001;121:580-91; PMID:; http://dx.doi.org/ 10.1053/gast.2001.27224 [DOI] [PubMed] [Google Scholar]
- 14.Furuta GT, Turner JR, Taylor CT, Hershberg RM, Comerford KM, Narravula S, et al. Hypoxia-inducible factor 1-dependent induction of intestinal trefoil factor protects barrier function during hypoxia. J. Ex. Med. 2001;193:1027-1034; PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Comerford KM, Wallace TJ, Karhausen J, Louis NA, Montalto MC, Colgan SP. Hypoxia-inducible factor-1-dependent regulation of the multidrug resistance (MDR1) gene. Cancer Res 2002;62:3387-94; PMID: [PubMed] [Google Scholar]
- 16.Synnestvedt K, Furuta GT, Comerford KM, Louis N, Karhausen J, Eltzschig HK, et al. Ecto-5'-nucleotidase (CD73) regulation by hypoxia-inducible factor-1 (HIF-1) mediates permeability changes in intestinal epithelia. J. Clin. Invest. 2002;110:993-1002; PMID:; http://dx.doi.org/ 10.1172/JCI0215337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Eltzschig HK, Ibla JC, Furuta GT, Leonard MO, Jacobson KA, Enjyoji K, et al. Coordinated adenine nucleotide phosphohydrolysis and nucleoside signaling in posthypoxic endothelium: role of ectonucleotidases and adenosine A2B receptors. J. Ex. Med. 2003;198:783-796; PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Semenza GL.Regulation of Metabolism by Hypoxia-Inducible Factor 1. Cold Spring Harb Symp Quant Biol 2011;76:347-53; PMID: [DOI] [PubMed] [Google Scholar]
- 19.Semenza GL.HIF-1, O(2), and the 3 PHDs: how animal cells signal hypoxia to the nucleus. Cell 2001;107:1-3; PMID:; http://dx.doi.org/ 10.1016/S0092-8674(01)00518-9 [DOI] [PubMed] [Google Scholar]
- 20.Maxwell PH, Wiesener MS, Chang GW, Clifford SC, Vaux EC, Cockman ME, et al. The tumour suppressor protein VHL targets hypoxia-inducible factors for oxygen-dependent proteolysis. Nature 1999;399:271-5; PMID:; http://dx.doi.org/ 10.1038/20459 [DOI] [PubMed] [Google Scholar]
- 21.Tanimoto K, Makino Y, Pereira T, Poellinger L. Mechanism of regulation of the hypoxia-inducible factor-1 a by the von Hippel-Lindau tumor suppressor protein. Embo J 2000;19:4298-309; PMID:; http://dx.doi.org/ 10.1093/emboj/19.16.4298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lando D, Peet DJ, Whelan DA, Gorman JJ, Murray LW. Asparagine hydroxylation of the HIF transactivation domain: a hypoxic switch. Sci 2002;295:858-861; PMID:; http://dx.doi.org/ 10.1126/science.1068592 [DOI] [PubMed] [Google Scholar]
- 23.Cummins EP, Seeballuck F, Keely SJ, Mangan NE, Callanan JJ, Fallon PG, Taylor CT. The hydroxylase inhibitor dimethyloxalylglycine is protective in a murine model of colitis. Gastroenterology 2008;134:156-65; PMID:; http://dx.doi.org/ 10.1053/j.gastro.2007.10.012 [DOI] [PubMed] [Google Scholar]
- 24.Han IO, Kim HS, Kim HC, Joe EH, Kim WK. Synergistic expression of inducible nitric oxide synthase by phorbol ester and interferon-gamma is mediated through NF-kappaB and ERK in microglial cells. J Neurosci Res 2003;73:659-69; PMID:; http://dx.doi.org/ 10.1002/jnr.10706 [DOI] [PubMed] [Google Scholar]
- 25.Karhausen JO, Furuta GT, Tomaszewski JE, Johnson RS, Colgan SP, Haase VH. Epithelial hypoxia-inducible factor-1 is protective in murine experimental colitis. J Clin Invest 2004;114:1098-1106; PMID:; http://dx.doi.org/ 10.1172/JCI200421086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Morote-Garcia JC, Rosenberger P, Nivillac NM, Coe IR, Eltzschig HK. Hypoxia-inducible factor-dependent repression of equilibrative nucleoside transporter 2 attenuates mucosal inflammation during intestinal hypoxia. Gastroenterology 2009;136:607-18; PMID:; http://dx.doi.org/ 10.1053/j.gastro.2008.10.037 [DOI] [PubMed] [Google Scholar]
- 27.Robinson A, Keely S, Karhausen J, Gerich ME, Furuta GT, Colgan SP. Mucosal protection by hypoxia-inducible factor prolyl hydroxylase inhibition. Gastroenterology 2008;134:145-55; PMID:; http://dx.doi.org/ 10.1053/j.gastro.2007.09.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shah YM, Ito S, Morimura K, Chen C, Yim SH, Haase VH, Gonzalez FJ. Hypoxia-inducible factor augments experimental colitis through an MIF-dependent inflammatory signaling cascade. Gastroenterology 2008;134:2036-48, 2048e1-3; PMID:; http://dx.doi.org/10.1053/j.gastro.2008.03.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Giatromanolaki A, Sivridis E, Maltezos E, Papazoglou D, Simopoulos C, Gatter KC, Harris AL, Koukourakis MI. Hypoxia inducible factor 1alpha and 2alpha overexpression in inflammatory bowel disease. J Clin Pathol 2003;56:209-13; PMID:; http://dx.doi.org/ 10.1136/jcp.56.3.209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mariani F, Sena P, Marzona L, Riccio M, Fano R, Manni P, Gregorio CD, Pezzi A, Leon MP, Monni S, et al. Cyclooxygenase-2 and Hypoxia-Inducible Factor-1alpha protein expression is related to inflammation, and up-regulated since the early steps of colorectal carcinogenesis. Cancer Lett 2009;279:221-9; PMID:; http://dx.doi.org/ 10.1016/j.canlet.2009.02.001 [DOI] [PubMed] [Google Scholar]
- 31.Matthijsen RA, Derikx JP, Kuipers D, van Dam RM, Dejong CH, Buurman WA. Enterocyte shedding and epithelial lining repair following ischemia of the human small intestine attenuate inflammation. PLoS One 2009;4:e7045; PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Louis NA, Hamilton KE, Canny G, Shekels LL, Ho SB, Colgan SP. Selective induction of mucin-3 by hypoxia in intestinal epithelia. J Cell Biochem 2006;99:1616-27; PMID:; http://dx.doi.org/ 10.1002/jcb.20947 [DOI] [PubMed] [Google Scholar]
- 33.Glover LE, Bowers BE, Saeedi B, Ehrentraut SF, Campbell EL, Bayless AJ, Dobrinskikh E, Kendrick AA, Kelly CJ, Burgess A, et al. Control of creatine metabolism by HIF is an endogenous mechanism of barrier regulation in colitis. Proc Natl Acad Sci U S A. 2013;110:19820-5; PMID:; http://dx.doi.org/ 10.1073/pnas.1302840110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Colgan SP, Eltzschig HK. Adenosine and hypoxia-inducible factor signaling in intestinal injury and recovery. Annu Rev Physiol. 2012;74:153-75; PMID:; http://dx.doi.org/ 10.1146/annurev-physiol-020911-153230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Weissmuller T, Campbell EL, Rosenberger P, Scully M, Beck PL, Furuta GT,Colgan SP. PMNs facilitate translocation of platelets across human and mouse epithelium and together alter fluid homeostasis via epithelial cell-expressed ecto-NTPDases. J Clin Invest 2008;118:3682-92; PMID:; http://dx.doi.org/ 10.1172/JCI35874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zimmermann H, Braun N. Ecto-nucleotidases–molecular structures, catalytic properties, and functional roles in the nervous system. Prog Brain Res 1999;120:371-85; PMID:; http://dx.doi.org/ 10.1016/S0079-6123(08)63570-0 [DOI] [PubMed] [Google Scholar]
- 37.Linden J.Molecular approach to adenosine receptors: receptor-mediated mechanisms of tissue protection. Annu Rev Pharmacol Toxicol 2001;41:775-87; PMID:; http://dx.doi.org/ 10.1146/annurev.pharmtox.41.1.775 [DOI] [PubMed] [Google Scholar]
- 38.Chin AC, Parkos CA. Pathobiology of neutrophil transepithelial migration: implications in mediating epithelial injury. Annu Rev Pathol 2007;2:111-43; PMID:; http://dx.doi.org/ 10.1146/annurev.pathol.2.010506.091944 [DOI] [PubMed] [Google Scholar]
- 39.Lennon PF, Taylor CT, Stahl GL, Colgan SP. Neutrophil-derived 5'-adenosine monophosphate promotes endothelial barrier function via CD73-mediated conversion to adenosine and endothelial A2B receptor activation. J Exp Med 1998;188:1433-43; PMID:; http://dx.doi.org/ 10.1084/jem.188.8.1433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dejana E, Spagnuolo R, Bazzoni G. Interendothelial junctions and their role in the control of angiogenesis, vascular permeability and leukocyte transmigration. Thromb Haemost 2001;86:308-15; PMID: [PubMed] [Google Scholar]
- 41.Ley K.Plugging the leaks. Nat Med 2001;7:1105-6; PMID:; http://dx.doi.org/ 10.1038/nm1001-1105 [DOI] [PubMed] [Google Scholar]
- 42.Comerford KM, Lawrence DW, Synnestvedt K, Levi BP, Colgan SP. Role of vasodilator-stimulated phosphoprotein in PKA-induced changes in endothelial junctional permeability. Faseb J 2002;16:583-5; PMID: [DOI] [PubMed] [Google Scholar]
- 43.Eltzschig HK, Thompson LF, Karhausen J, Cotta RJ, Ibla JC, Robson SC, Colgan SP. Endogenous adenosine produced during hypoxia attenuates neutrophil accumulation: Coordination by extracellular nucleotide metabolism. Blood 2004;104:3986-3992; PMID:; http://dx.doi.org/ 10.1182/blood-2004-06-2066 [DOI] [PubMed] [Google Scholar]
- 44.Novak I.ATP as a signaling molecule: the exocrine focus. News Physiol Sci 2003;18:12-7; PMID: [DOI] [PubMed] [Google Scholar]
- 45.Thompson LF, Eltzschig HK, Ibla JC, Van De Wiele CJ, Resta R, Morote-Garcia JC, Colgan SP. Crucial role for ecto-5'-nucleotidase (CD73) in vascular leak during hypoxia. J. Exp. Med. 2004;200:1395-1405; PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.McDonald DM, Thurston G, Baluk P. Endothelial gaps as sites for plasma leakage in inflammation. Microcirculation 1999;6:7-22; PMID:; http://dx.doi.org/ 10.1111/j.1549-8719.1999.tb00084.x [DOI] [PubMed] [Google Scholar]
- 47.Takano T, Clish CB, Gronert K, Petasis N, Serhan CN. Neutrophil-mediated changes in vascular permeability are inhibited by topical application of aspirin-triggered 15-epi-lipoxin A4 and novel lipoxin B4 stable analogues. J Clin Invest 1998;101:819-26; PMID:; http://dx.doi.org/ 10.1172/JCI1578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Eltzschig HK, Rivera-Nieves J, Colgan SP. Targeting the A2B adenosine receptor during gastrointestinal ischemia and inflammation. Expert Opin Ther Targets 2009;13:1267-77; PMID:; http://dx.doi.org/ 10.1517/14728220903241666 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Weissmueller T, Eltzschig HK, Colgan SP. Dynamic purine signalling and metabolism during neutrophil-endothelial interactions. Purinergic Signalling 2005;1:229-239; PMID:; http://dx.doi.org/ 10.1007/s11302-005-6323-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ivanov AI, Parkos CA, Nusrat A. Cytoskeletal regulation of epithelial barrier function during inflammation. Am J Pathol 2010;177:512-24. Epub 2010 Jun 25; PMID:; http://dx.doi.org/ 10.2353/ajpath.2010.100168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tsukamoto T, Nigam SK. Role of tyrosine phosphorylation in the reassembly of occludin and other tight junction proteins. Am. J. Physiol. Renal Physiol. 1999;276:F737-F750; PMID:; [DOI] [PubMed] [Google Scholar]
- 52.Taylor CT, Dzus AL, Colgan SP. Autocrine regulation of intestinal epithelial permeability induced by hypoxia: Role for basolateral release of tumor necrosis factor-a (TNF-a). Gastroenterol. 1998;114:657-668; http://dx.doi.org/ 10.1016/S0016-5085(98)70579-7 [DOI] [PubMed] [Google Scholar]
- 53.Friedman GB, Taylor CT, Parkos CA, Colgan SP. Epithelial permeability induced by neutrophil transmigration is potentiated by hypoxia: role of intracellular cAMP. J. Cell. Physiol. 1998;176:76-84; PMID: [DOI] [PubMed] [Google Scholar]
- 54.Tsukamoto T, Nigam SK. Tight junction proteins form large complexes and associate with the cytoskeleton in an ATP depletion model for reversible junction assembly. J. Biol. Chem. 1997;272:16133-16139; PMID: [DOI] [PubMed] [Google Scholar]
- 55.Kevil CG, Oshima T, Alexander B, Coe LL, Alexander JS. H(2)O(2)-mediated permeability: role of MAPK and occludin. Am J Physiol Cell Physiol 2000;279:C21-30; PMID: [DOI] [PubMed] [Google Scholar]
- 56.Wu HM, Huang QB, Yuan Y, Granger HJ. VEGF induces NO-dependent hyperpermeability in coronary venules. Am. J. Physiol. Heart Circ. Physiol. 1996;271:H2735-2739; PMID: [DOI] [PubMed] [Google Scholar]
- 57.Molitoris BA, Leiser J, Wagner MC. Role of the actin cytoskeleton in ischemia-induced cell injury and repair. Pediatr. Nephrol. 1997;11:761-767; PMID: [DOI] [PubMed] [Google Scholar]
- 58.Bacallao RA, Garginkel A, Monke S, Zampighi G, Mandel LJ. ATP depletion: a novel method to study junctional properties in epithelial tissues. I. Rearrangement of the actin cytoskeleton. J. Cell. Sci. 1994;107:3301-3313; PMID: [DOI] [PubMed] [Google Scholar]
- 59.Molitoris BA, Dahl R, Hosford M. Cellular ATP depletion induces disruption of the spectrin cytoskeletal network. Am. J. Physiol. 1996;271:f790-798; PMID: [DOI] [PubMed] [Google Scholar]
- 60.Zharikov SI, Block ER. Association of L-arginine transporters with fodrin: implications for hypoxic ihibition of arginine uptake. Am. J. Physiol. 2000;278:L111-117; PMID: [DOI] [PubMed] [Google Scholar]
- 61.Hochachka PW, Buck LT, Doll CJ, Land SC. Unifying theory of hypoxia tolerance: molecular/metabolic defense and rescue mechanisms for surviving oxygen lack. Proc Natl Acad Sci U S A 1996;93:9493-8; PMID:; http://dx.doi.org/ 10.1073/pnas.93.18.9493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Shapiro M, Matthews J, Hecht G, Delp C, Madara JL. Stablization of F-actin prevents cAMP-elicited Cl- secretion in T84 cells. J. Clin. Invest. 1991;87:1903-9; PMID:; http://dx.doi.org/ 10.1172/JCI115215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Dye JF, Leach L, Clark P, Firth JA. Cyclic AMP and acidic fibroblast growth factor have opposing efffects on tight and adherens junctions in microvascular endothelial cells in vitro. Microvasc. Res. 2001;62:94-113; PMID: [DOI] [PubMed] [Google Scholar]
- 64.Adamson RH, Liu B, Fry GN, Rubin LL, Curry FE. Microvascular permeability and number of tight junctions are modulated by cAMP. Am J Physiol 1998;274:H1885-94; PMID: [DOI] [PubMed] [Google Scholar]
- 65.Stelzner TJ, Weil JV, O'Brien RF. Role of cyclic adenosine monophosphate in the induction of endothelial barrier properties. J. Cell. Physiol. 1989;139:157-166; PMID: [DOI] [PubMed] [Google Scholar]
- 66.Lawrence DW, Comerford KM, Colgan SP. Role of VASP in reestablishment of epithelial tight junction assembly after Ca2+ switch. Am J Physiol Cell Physiol 2002;282:C1235-45; PMID:; http://dx.doi.org/ 10.1152/ajpcell.00288.2001 [DOI] [PubMed] [Google Scholar]
- 67.Dejana E.Endothelial adherens junctions: implications in the control of vascular permeability and angiogenesis. J. Clin. Invest. 1996;98:1949-1953; PMID:; http://dx.doi.org/ 10.1172/JCI118997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Cuzzocrea S, Mazzon E, De Sarro A, Caputi AP. Role of free radicals and poly(ADP-ribose) synthetase in intestinal tight junction permeability. Mol Med 2000;6:766-78; PMID: [PMC free article] [PubMed] [Google Scholar]
- 69.Meyer TN, Schwesinger C, Ye J, Denker BM, Nigam SK. Reassembly of the tight junction after oxidative stress depends on tyrosine kinase activity. J Biol Chem 2001;276:22048-55; PMID:; http://dx.doi.org/ 10.1074/jbc.M011477200 [DOI] [PubMed] [Google Scholar]
- 70.Clambey ET, McNamee EN, Westrich JA, Glover LE, Campbell EL, Jedlicka P, de Zoeten EF, Cambier JC, Stenmark KR, Colgan SP, et al. Hypoxia-inducible factor-1 a-dependent induction of FoxP3 drives regulatory T-cell abundance and function during inflammatory hypoxia of the mucosa. Proc Natl Acad Sci U S A. 2012;109:E2784-93; PMID:; http://dx.doi.org/ 10.1073/pnas.1202366109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Glover LE, Irizarry K, Scully M, Campbell EL, Bowers BE, Aherne CM, Kominsky DJ, MacManus CF, Colgan SP. IFN-gamma attenuates HIF activity in intestinal epithelial cells through transcriptional repression of HIF-1beta. J Immunol 2011;186:1790-8; PMID:; http://dx.doi.org/ 10.4049/jimmunol.1001442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Hirota SA, Fines K, Ng J, Traboulsi D, Lee J, Ihara E, et al. Hypoxia-inducible factor signaling provides protection in Clostridium difficile-induced intestinal injury. Gastroenterology 2010;139:259-69e3; PMID:; http://dx.doi.org/ 10.1053/j.gastro.2010.03.045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Hindryckx P, De Vos M, Jacques P, Ferdinande L, Peeters H, Olievier K, Bogaert S, Brinkman B, Vandenabeele P, Elewaut D, et al. Hydroxylase inhibition abrogates TNF-a-induced intestinal epithelial damage by hypoxia-inducible factor-1-dependent repression of FADD. J Immunol. 2010;185:6306-16; PMID:; http://dx.doi.org/ 10.4049/jimmunol.1002541 [DOI] [PubMed] [Google Scholar]
- 74.Keely S, Glover LE, Weissmueller T, MacManus CF, Fillon S, Fennimore B, Colgan SP. Hypoxia-inducible factor-dependent regulation of platelet-activating factor receptor as a route for gram-positive bacterial translocation across epithelia. Mol Biol Cell 2009;21:538-46; PMID:; http://dx.doi.org/ 10.1091/mbc.E09-07-0573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Louis NA, Hamilton KE, Kong T, Colgan SP. HIF-dependent induction of apical CD55 coordinates epithelial clearance of neutrophils. Faseb J 2005;19:950-959; PMID:; http://dx.doi.org/ 10.1096/fj.04-3251com [DOI] [PubMed] [Google Scholar]
- 76.Karhausen J, Furuta GT, Tomaszewski JE, Johnson RS, Colgan SP, Haase VH. Epithelial hypoxia-inducible factor-1 is protective in murine experimental colitis. J Clin Invest 2004;114:1098-106; PMID:; http://dx.doi.org/ 10.1172/JCI200421086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Wyss M, Kaddurah-Daouk R. Creatine and creatinine metabolism. Physiol Rev. 2000;80:1107-213; PMID: [DOI] [PubMed] [Google Scholar]
- 78.Ivanov AI, Parkos CA, Nusrat A. Cytoskeletal regulation of epithelial barrier function during inflammation. Am J Pathol. 2010;177:512-24; PMID:; http://dx.doi.org/ 10.2353/ajpath.2010.100168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Turner JR.Intestinal mucosal barrier function in health and disease. Nat Rev Immunol 2009;9:799-809; PMID:; http://dx.doi.org/ 10.1038/nri2653 [DOI] [PubMed] [Google Scholar]
- 80.Song JC, Hrnjez BJ, Farokhzad OC, Matthews JB. PKC-epsilon regulates basolateral endocytosis in human T84 intestinal epithelia: role of F-actin and MARCKS. Am J Physiol. 1999;277:C1239-49; PMID: [DOI] [PubMed] [Google Scholar]
- 81.Culic O, Gruwel ML, Schrader J. Energy turnover of vascular endothelial cells. Am J Physiol. 1997;273:C205-13; PMID: [DOI] [PubMed] [Google Scholar]
- 82.Daniel JL, Molish IR, Robkin L, Holmsen H. Nucleotide exchange between cytosolic ATP and F-actin-bound ADP may be a major energy-utilizing process in unstimulated platelets. Eur J Biochem. 1986;156:677-84; PMID:; http://dx.doi.org/ 10.1111/j.1432-1033.1986.tb09631.x [DOI] [PubMed] [Google Scholar]
- 83.Kaelin WG, Jr., Ratcliffe PJ. Oxygen sensing by metazoans: the central role of the HIF hydroxylase pathway. Mol Cell 2008;30:393-402; PMID:; http://dx.doi.org/ 10.1016/j.molcel.2008.04.009 [DOI] [PubMed] [Google Scholar]
- 84.Cummins EP, Berra E, Comerford KM, Ginouves A, Fitzgerald KT, Seeballuck F, Godson C, Nielsen JE, Moynagh P, Pouyssegur J, et al. Prolyl hydroxylase-1 negatively regulates IkappaB kinase-b, giving insight into hypoxia-induced NFkappaB activity. Proc Natl Acad Sci U S A 2006;103:18154-9; PMID:; http://dx.doi.org/ 10.1073/pnas.0602235103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Aragones J, Schneider M, Van Geyte K, Fraisl P, Dresselaers T, Mazzone M, Dirkx R, Zacchigna S, Lemieux H, Jeoung NH, et al. Deficiency or inhibition of oxygen sensor Phd1 induces hypoxia tolerance by reprogramming basal metabolism. Nat Genet 2008;40:170-80; PMID:; http://dx.doi.org/ 10.1038/ng.2007.62 [DOI] [PubMed] [Google Scholar]
- 86.Schneider M, Van Geyte K, Fraisl P, Kiss J, Aragones J, Mazzone M, et al. Loss or Silencing of the PHD1 Prolyl Hydroxylase Protects Livers of Mice Against Ischemia/Reperfusion Injury. Gastroenterology 2009; PMID: [DOI] [PubMed] [Google Scholar]
- 87.Tambuwala MM, Cummins EP, Lenihan CR, Kiss J, Stauch M, Scholz CC, Fraisl P, Lasitschka F, Mollenhauer M, Saunders SP, et al. Loss of Prolyl Hydroxylase-1 Protects Against Colitis Through Reduced Epithelial Cell Apoptosis and Increased Barrier Function. Gastroenterology 2010; 139:2093-101; PMID: [DOI] [PubMed] [Google Scholar]
- 88.Mazzone M, Dettori D, Leite de Oliveira R, Loges S, Schmidt T, Jonckx B, Tian YM, Lanahan AA, Pollard P, Ruiz de Almodovar C, et al. Heterozygous deficiency of PHD2 restores tumor oxygenation and inhibits metastasis via endothelial normalization. Cell 2009;136:839-51; PMID:; http://dx.doi.org/ 10.1016/j.cell.2009.01.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Ozolins TR, Fisher TS, Nadeau DM, Stock JL, Klein AS, Milici AJ, Morton D, Wilhelms MB, Brissette WH, Li B. Defects in embryonic development of EGLN1/PHD2 knockdown transgenic mice are associated with induction of Igfbp in the placenta. Biochem Biophys Res Commun 2009;390:372-6; PMID:;http://dx.doi.org/ 10.1016/j.bbrc.2009.08.057 [DOI] [PubMed] [Google Scholar]
- 90.Bishop T, Gallagher D, Pascual A, Lygate CA, de Bono JP, Nicholls LG, Ortega-Saenz P, Oster H, Wijeyekoon B, Sutherland AI, et al. Abnormal sympathoadrenal development and systemic hypotension in PHD3-/- mice. Mol Cell Biol 2008;28:3386-400; PMID:; http://dx.doi.org/ 10.1128/MCB.02041-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Mole DR, Schlemminger I, McNeill LA, Hewitson KS, Pugh CW, Ratcliffe PJ, Schofield CJ. Two-oxoglutarate analogue inhibitors of HIF prolyl hydroxylase. Bioorg Med Chem Lett 2003;13:2677-80; PMID:; http://dx.doi.org/ 10.1016/S0960-894X(03)00539-0 [DOI] [PubMed] [Google Scholar]
- 92.Masson N, Ratcliffe PJ. HIF prolyl and asparaginyl hydroxylases in the biological response to intracellular O(2) levels. J Cell Sci 2003;116:3041-9; PMID:; http://dx.doi.org/ 10.1242/jcs.00655 [DOI] [PubMed] [Google Scholar]
- 93.Schofield CJ, Ratcliffe PJ. Oxygen sensing by HIF hydroxylases. Nat Rev Mol Cell Biol 2004;5:343-54; PMID:; http://dx.doi.org/ 10.1038/nrm1366 [DOI] [PubMed] [Google Scholar]
- 94.Bruick RK.Oxygen sensing in the hypoxic response pathway: regulation of the hypoxia-inducible transcription factor. Genes Dev 2003;17:2614-23; PMID:; http://dx.doi.org/ 10.1101/gad.1145503 [DOI] [PubMed] [Google Scholar]
- 95.Nwogu JI, Geenen D, Bean M, Brenner MC, Huang X, Buttrick PM. Inhibition of collagen synthesis with prolyl 4-hydroxylase inhibitor improves left ventricular function and alters the pattern of left ventricular dilatation after myocardial infarction. Circulation 2001;104:2216-21; PMID:; http://dx.doi.org/ 10.1161/hc4301.097193 [DOI] [PubMed] [Google Scholar]
- 96.Schlemminger I, Mole DR, McNeill LA, Dhanda A, Hewitson KS, Tian YM, Ratcliffe PJ, Pugh CW, Schofield CJ. Analogues of dealanylalahopcin are inhibitors of human HIF prolyl hydroxylases. Bioorg Med Chem Lett 2003;13:1451-4; PMID:; http://dx.doi.org/ 10.1016/S0960-894X(03)00149-5 [DOI] [PubMed] [Google Scholar]
- 97.Keely S, Campbell EL, Baird AW, Hansbro PM, Shalwitz RA, Kotsakis A, McNamee EN, Eltzschig HK, Kominsky DJ, et al. Contribution of epithelial innate immunity to systemic protection afforded by prolyl hydroxylase inhibition in murine colitis. Mucosal Immunol 2013;22:114-123; PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Okumura CY, Hollands A, Tran DN, Olson J, Dahesh S, von Kockritz-Blickwede M, Thienphrapa W, Corle C, Jeung SN, Kotsakis A, et al. A new pharmacological agent (AKB-4924) stabilizes hypoxia inducible factor-1 (HIF-1) and increases skin innate defenses against bacterial infection. J Mol Med 2012;28:1079-1089; PMID:; http://dx.doi.org/ 10.1007/s00109-012-0882-3 [DOI] [PMC free article] [PubMed] [Google Scholar]


