Abstract
Endothelial cells (EC) form a semi-permeable barrier between the interior space of blood vessels and the underlying tissues. In acute lung injury (ALI) the EC barrier is weakened leading to increased vascular permeability. It is widely accepted that EC barrier integrity is critically dependent upon intact cytoskeletal structure and cell junctions. Edemagenic agonists, like thrombin or endotoxin lipopolysaccharide (LPS), induced cytoskeletal rearrangement, and EC contractile responses leading to disruption of intercellular contacts and EC permeability increase. The highly clinically-relevant cytoskeletal mechanisms of EC barrier dysfunction are currently under intense investigation and will be described and discussed in the current review.
Keywords: acute lung injury, barrier function, cytoskeleton, endothelial junctions, thrombin, pulmonary endothelium
Abbreviations: AJ, adherens junction; ALI, Acute Lung Injury; ARDS, Acute Respiratory Distress Syndrome; CaD, caldesmon; CPI-17, PKC potentiated inhibitory protein of 17 kDa; EC, endothelial cells; GJ, gap junction; HSP-27, small heat shock actin-capping protein of 27 kDa; IL, interleukin; LPS, lipopolysaccharide; MLC, myosin light chain; MLCK, Ca2+/calmodulin (CaM) dependent MLC kinase; MLCP, myosin light chain phosphatase; MT, microtubules; MYPT1, myosin phosphatase targeting subunit 1; PKA, protein kinase A; PKC, protein kinase C; SM, smooth muscle; TLR4, toll-like receptor 4; TNFα, tumor necrosis factor α; TJ, tight junction
Introduction
Lung endothelium forms a semi-permeable barrier between the blood and the interstitial space.1 Disruption of endothelial barrier results in the movement of fluid and macromolecules into the interstitium and pulmonary air spaces causing pulmonary edema which is a common feature of Acute Lung Injury (ALI) and its more severe form Acute Respiratory Distress Syndrome (ARDS). The integrity of pulmonary EC monolayer is a critical requirement for tissue and organ homeostasis. EC barrier is heavily dependent upon the EC cytoskeleton network primarily microfilaments and microtubules which tightly linked to cell junction proteins.1-4 This review will describe the cytoskeletal mechanisms of EC permeability increase, induced by various inflammatory conditions focusing on edemagenic agonists, like LPS and thrombin.
Clinical and physiological importance of the lung vascular barrier
The alveolar-capillary barrier is formed by the microvascular endothelium, the alveolar epithelium and the basement membrane. Direct or indirect injuries of the lung caused by inflammatory or toxic mediators can lead to pathophysiological syndromes such as severe pneumonia and ALI/ARDS. Despite recent therapeutic advances, these conditions still have high (30–40%) rates of patient mortality.5 The acute phase of lung injury is characterized by a massive and rapid flood of protein rich edema fluid into the alveolar spaces as a consequence of increased endothelial permeability5 (Fig. 1). Neutrophils are adhering to the injured endothelium and migrating through the interstitium into the alveoli,6,7 whereas the macrophages are secreting cytokines (IL-1, 6, 8 and 10) and TNFα.8 ALI/ARDS leads to impaired gas exchange and may cause respiratory failure.9 It is widely accepted that EC barrier dysfunction, a prominent feature of these clinical syndromes is tightly linked to agonist-induced cytoskeletal remodeling resulting in the disruption of cell-cell contacts, paracellular gap formation and EC barrier compromise.3,4 Apart from ventilation strategies there is no standard treatment for pulmonary edema, making the investigation of regulatory mechanisms of endothelial barrier dysfunction highly clinically important.5
Figure 1.

Endothelial activation in ALI. Edemagenic agents like bacterial toxins (LPS) or inflammatory mediators (thrombin) disrupt endothelial barrier leading to EC permeability increase accompanying by inflammatory response
Endothelial barrier properties
The vascular endothelium serves as a semi-selective barrier lining in the vessel walls (Fig. 1). It dynamically regulates the liquid and macromolecule transport between the blood and the interstitial space.10 The vasculature is lined by heterogeneous population of endothelial cells. This heterogeneity is derived from the origin of endothelial cells in the vascular tree. The barrier function, surface biochemistry, and morphology of confluent monolayers of microvascular and macrovascular endothelial cells are different for these 2 cell types.11 In general, microvascular EC form a tighter barrier, compared to macrovascular one. It was found that permeability is ∼16-fold less for sucrose and to ∼2-fold less for albumin in microvascular EC compared to macrovascular EC monolayers.12 Conversely, primary cultures of microvascular EC produced 10 times higher transmonolayer electrical resistance (TER) compared to macrovascular one.13 Although the precise mechanisms that regulate this variability are still under investigation, microarray analysis showed a significant variation in microvascular and macrovascular gene expression patterns.14 Extracellular matrix proteins, collagen 4α1, collagen 4α2, and laminin were associated with microvessel endothelia, while fibronectin, collagen 5α1, and collagen 5α2 were seen with the large vessel endothelia.14 Furthermore, electron microscopy revealed that microvascular EC have more developed intercellular junctions with more focal membrane adhesion sites per junction than the macrovascular cells.12 Pulmonary artery endothelial cells (macrovascular EC) participate in blood homeostasis, blood-tissue exchange regulation under various conditions.15 They share similarities in cell characteristics and in physiological properties with pulmonary microvascular EC. However, in vivo models of pulmonary edema suggest that most fluid filtration occurs in the microcirculation.16
Endothelial permeability pathways
A variety of physical, inflammatory and bioactive stimuli alter the EC barrier leading to gap formation, increasing vessel permeability and compromising organ function. Permeability across endothelial and epithelial cell monolayers can involve transcellular, paracellular or the combination of both pathways (Fig. 2). The transcellular transport involves membrane-attached cytosolic caveolae that migrate through the endothelial cells and transfer macromolecules from the blood to the interstitium.10 The main player in this process is the Src kinase, which can phosphorylate caveolin-1 on tyrosine residues inducing the migration of the vesicles across the endothelium.17 Recent studies demonstrated that transcellular permeability increase precedes and may trigger paracellular permeability increase via signaling involved Src-mediated phosphorylation of caveolin-1.18 However, majority of trafficking occurs through the paracellular route,19 which will be described in this review in more details.
Figure 2.
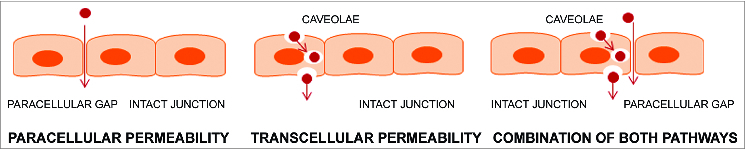
EC permeability pathways. Edemagenic agonists can increase endothelial permeability via caveolae-mediated transcellular route or (and) via increased intercellular gaps (paracellular route).
External stimuli leading to EC barrier compromise
The capillary endothelium is impermeable to macromolecules under basal conditions. This is due to the network of cytoskeletal and cell-junction elements which protect the endothelial barrier integrity. In state of acute or chronic inflammation, sepsis, diabetes, angiogenesis, or excessive level of mechanical alterations (stretch or shear stress), the EC barrier integrity is compromised. Inflammatory mediators such as LPS, thrombin, pro-inflammatory cytokines, or reactive oxygen species induce the loss of endothelial barrier function leading to permeability increase to solute and plasma proteins.20-23
LPS, a pro-inflammatory mediator and constituent of Gram-negative bacterial cell wall, directly disrupts macro- and microvascular EC barrier function in vitro and in vivo.20,24,25 LPS primarily acts through the activation of toll-like receptor 4 (TLR4).26 LPS-induced EC barrier dysfunction is correlated with actin reorganization and caspase-mediated cleavage of cytoskeletal proteins that participate in cell-cell and cell-matrix adhesion.25 Signal transduction mechanisms for LPS-induced EC permeability are not completely clear yet, but likely involve Tyr kinase(s), protein kinase C (PKC) as well as Rho signaling.27-30 Murine lung injury induced by LPS is a model that has been shown to be largely consistent with sepsis-induced ALI.31 Specifically, the injury elicited is characterized by neutrophil infiltration into the lung in association with increased inflammatory mediators including TNFα and NF-kB.31
Thrombin is a serine protease generated by injured endothelial cells by the cleavage of circulating prothrombin, participating in the prothrombinase complex which also contains factors X and V, Ca2+ and membrane phospholipids.32 Thrombin not only induces coagulation, but also affects endothelial barrier function by releasing of inflammatory mediators and growth factors as well as inducing leukocyte adhesion on EC surface.33 The cellular responses of EC to thrombin are mainly mediated through a thrombin-specific protease-activated receptor, PAR1.34,35,36 In vitro, thrombin produces rapid, reversible, concentration-dependent increases in EC permeability as measured by the clearance rate of Evans blue dye-labeled albumin across EC monolayers37,38 or by changes in transendothelial electrical resistance.21,39,40 Thrombin infusion in animals resembles that seen after LPS administration in several respects, including pulmonary hypertension and increased pulmonary vascular permeability.41,42 Interestingly, thrombin inhibitor, anti-thrombin III (AT III) prevents LPS-induced pulmonary vascular injury suggesting the involvement of thrombin in LPS-induced permeability response.43
Contractile mechanisms of EC permeability
Endothelial barrier integrity is maintained by the precisely regulated balance between actomyosin contractile forces and adhesive cell-cell, cell-matrix tethering forces.4 Both competing forces are generated by the cytoskeleton comprising actin microfilaments, microtubules and intermediate filaments.3,4 Therefore, the complex network of cytoskeleton is critical in the EC barrier regulation. Disruption of either intact actin or microtubule network leads to formation of paracellular gaps and permeability increase.44,45 Under quiescent conditions, when the balance is tilted toward tethering forces, a thick cortical actin ring can be observed, where endothelial cells can maintain tight connections with each other and the underlying matrix.3,4 Due to the effect of barrier-compromising agents like thrombin or LPS, the balance is shifted toward contractile forces (Fig. 3).
Figure 3.

EC cytoskeletal rearrangement in response to edemagenic agonists. Thrombin or LPS activates their receptors (PAR-1 and TLR4, respectively) leading to activation of pro-inflammatory intracellular cascades (intracellular Ca2+ increase, activation of Rho, PKC and Src signaling) following by microtubule dissolution, increased MLC phosphorylation (MLCK activation, MLCP inhibition) and phosphorylation of regulatory cytoskeletal proteins, CaD and HSP-27 (via p38 MAPK activation). These events result in actomyosin contraction, actin rearrangement and disruption of intercellular contacts, following by EC permeability increase.
Thrombin cleaves and activates its G-protein-coupled receptor (PAR-1). Engagement of Gq protein leads to activation of phospholipase C resulting in intracellular [Ca2+] increase.46 Ca2+ elevation activates the Ca2+/calmodulin (CaM) dependent myosin light chain (MLC) kinase (MLCK) that phosphorylates MLC and, consequently, actomyosin interaction and cell contraction will be evoked.47,48 Beside the Ca2+/CaM-induced activation, endothelial (non-muscle) MLCK can be activated by Src-mediated Tyr phosphorylation on its unique N-terminal fragment, which is absent in smooth muscle (SM) MLCK.49 Thrombin was shown to increase EC permeability in a Src/MLCK-dependent manner via MLC-mediated contractile mechanism.37,50
Additionally, thrombin and LPS induced MLC-mediated EC contractile response and permeability via activation of Rho signaling pathway.21,29 The Ras homologous small GTPase Rho acts as molecular switch, cycling between an active GTP-bound and inactive GDP-bound state.51 Rho activity is positively regulated by guanosine nucleotide exchange factors (GEFs) and inhibited by GTPase-activating proteins (GAPs), and GDP-dissociation inhibitors (GDIs).52 Thrombin induced Rho activation involved G12/13-mediated activation of p115RhoGEF, GEF-H1 activation, as well as PKC-mediated inhibition of GDI-1.21,53,54 LPS-induced Rho activation dependent upon the activity of Src family kinases and direct nitration of RhoA at a Tyr side chain.34,29,55 GTP-bound Rho activates its downstream effector, Rho-kinase, which increases MLC phosphorylation by 2 mechanisms: directly, via phosphorylation of MLC at Ser19 and indirectly, via phosphorylation of the targeting subunit (MYPT1) of the myosin phosphatase (MLCP). Phosphorylation of MYPT1 at the inhibitory Thr686 and Thr850 sites leads to the inhibition of MLCP, accumulation of phospho-MLC resulting in cell contraction.56,57,21
Inhibition of MLCP also can be achieved through activation of CPI-17 (PKC potentiated inhibitory protein of 17 kDa). This soluble globular protein was first identified in SM cells, and later was found in several non-muscle cells including microvascular EC.58,59 Phosphorylation of CPI-17 at Thr38 by PKC increases its inhibitory potency toward MLCP ∼1000-fold.60,61 Histamine and thrombin (to a lesser extent) activate CPI-17 in PKC-dependent manner in ECs.58 CPI-17 depletion significantly attenuates histamine-induced microvascular permeability increase implicating CPI-17-mediated mechanism of MLCP inhibition in EC barrier regulation58 (Fig. 3).
EC barrier dysfunction and cytoskeletal rearrangement are not always associated with triggering contraction by an increase in MLC phosphorylation. Some agonists, like direct PKC activators induced EC permeability without increasing MLC phosphorylation at Ser19/Thr18.62,63 Phorbol ester-induced EC barrier dysfunction is accompanied by increased phosphorylation of a cytoskeletal protein, caldesmon (CaD).63-65 CaD contains distinct binding sites for actin and myosin, thereby potentially regulating actomyosin interactions and promoting actin filament formation in the absence of MLC phosphorylation.66-68 Phorbol ester-induced phosphorylation of CaD correlates with contraction and has been postulated as an on/off switch regulating actomyosin interactions in smooth muscle.67 It is clear that CaD is directly involved in EC cytoskeletal arrangement and migration,69 however, the functional significance of CaD phosphorylation in the regulation of EC barrier function have not been fully investigated.
Interestingly, PKC does not directly phosphorylate CaD. Phorbol ester-induced EC barrier dysfunction includes complex signaling involving sequential activation of Ras, Raf-1 and MEK resulting in activation of ERK1/2 MAP kinases,70 which phosphorylate CaD and are responsible for CaD-mediated contractile response in smooth muscle.67 Aside of ERK 1/2, another MAPK family member, p38 kinase is also directly involved in EC cytoskeletal remodeling and permeability.71-73 p38, but not ERKs, is involved in thrombin-induced EC barrier compromise73 and p38 signaling is involved in several in vivo models of lung injury including the LPS model of ALI.74-76 p38 MAPK downstream targets contain several cytoskeletal proteins such as CaD and HSP-27.77,78
Small heat shock actin-capping protein, HSP-27, is phosphorylated by MAP kinase-activated protein kinase 2 (MAPKAP kinase 2), that is in turn phosphorylated by p38 MAPK.77,79 Phosphorylation of HSP-27 promotes F-actin formation, membrane blebbing and mediates actin reorganization and cell migration in human endothelium.89,80-82 However, the role of HSP-27 in the regulation of EC permeability remains controversial. For example, pertussis toxin-induced EC permeability is temporally linked to p38 MAPK activation and phosphorylation of HSP-27 in EC72; and LPS-induced endothelial barrier dysfunction correlates with HSP-27 phosphorylation in vivo.76 In contrary, depletion of HSP-27 did not prevent p38-mediated TGFβ-induced EC barrier dysfunction.83 Therefore, the exact cytoskeletal targets of p38 MAPK in endothelium remain undetermined. The putative targets include ezrin/radixin/moesin (ERM) proteins, which may be phosphorylated through p38-dependent mechanisms,84 but apparently, the role of ERM phosphorylation in EC barrier regulation is agonist-specific.84-87 A few studies implicated the involvement of p38 activity in the activation of Rho/Rho kinase pathway and EC barrier dysfunction induced by TGFβ and Staphylococcus aureus-derived toxins.83,88 In contrast, inhibition of p38 has no effect on thrombin-induced MLC phosphorylation, which involves Rho activation.21,73 Finally, recent study supports the cross-talk between p38 and Rho pathways in the regulation of microvascular permeability.88
Crosstalk Between Microtubules and Microfilaments in EC Permeability Regulation
Paracellular gap formation evoked by barrier-disruptive agents resulting in increased endothelial permeability is governed by the coordinated communication among cytoskeletal elements. Disruption of microtubule (MT) structure leads to an increase in transendothelial permeability associated with a characteristic loss of the peripheral actin band as well as an increase in the density of actin stress fibers, increased levels of MLC phosphorylation, consistent with actomyosin contraction, and paracellular gap formation.45,89 Further, microtubule dissolution increased vascular permeability in murine model.75 Vice versa, stabilization of microtubules protects EC monolayer in vitro and in vivo.75,90,91 Edemagenic agonists like thrombin, LPS, TNFα and TGFβ induce partial microtubule dissolution accompanied by activation of EC contraction and permeability increase.91-94 The effect of microtubule dissolution on actin reorganization is attributed to stimulation of Rho and p38 MAPK pathways, but not to an increase in [Ca2+], neither to MLCK or ERK1/2 activation.45,93,95
In the thrombin model of EC permeability microtubule disassembly precedes actin stress fiber formation.96 Thrombin may induce microtubule dissolution via stimulation of G12/13/p115RhoGEF cascade, followed by Rho/Rho kinase activation, resulting in phosphorylation of the microtubule-associated protein, tau.93 In its unphosphorylated form, tau promotes assembly of microtubules and inhibits the rate of depolymerization.97-99 Phosphorylation of tau decreases its capacity to bind microtubules and promotes MT assembly.99,100 Interestingly, p38 MAPK is also able to phosphorylate tau in vitro.101 Inhibition of p38 attenuates microtubule dissolution and permeability increase induced by various agonists94,102 suggesting that thrombin-induced p38 activation may also be involved in MT destabilization via tau phosphorylation.
Thrombin may also destabilize microtubules via Rho kinase-mediated phosphorylation and activation of LIM kinase (LIMK).103 In quiescent conditions, LIM kinase is associated with microtubules. Thrombin treatment or ectopic expression of Rho kinase leads to dissociation of LIM kinase from microtubules accompanied by MT destabilization, phosphorylation/inhibition of cofilin, an actin depolymerization factor, resulting in F-actin assembly.103
It was also recently reported that thrombin may destabilize microtubules via dephosphorylation of stathmin, a MT-associated protein, which in its phosphorylated form stabilizes the microtubules.104 However, the thrombin-induced phosphatase, which is able to dephosphorylate stathmin and is involved in thrombin-induced permeability increase, is not known yet.
Thrombin-induced microtubule dissolution may further activate Rho pathway via GEF-H1, which has been recently characterized as a Rho-specific GEF localizing on microtubules.105 In its MT-bound state, GEF-H1 is inactive, whereas GEF-H1 release caused by MT disassembly stimulates its activity toward Rho.106 Importantly, GEF-H1 is directly involved in thrombin-induced permeability increase.53
Microtubule dissolution may also affect cellular localization and activity of cytoskeletal regulatory proteins like CaD, which can be involved in EC barrier regulation. CaD co-purifies with microtubules from brain and potentiates tubulin polymerization.107,108 Phosphorylation of CaD by cell cycle-dependent cdc2 kinase (Pro-directed kinase, similar to MAPK) eliminates MT-binding activity of CaD, and also decreases CaD-mediated inhibition of actomyosin ATPase, consistent with contraction.107,108 Ectopic expression of CaD in fibroblasts eliminates the increase in focal adhesions and microfilament bundles induced by MT dissolution and Rho activation.109
Current findings describing the role of microtubule/microfilament crosstalk in thrombin permeability model are summarized on Figure 4. Thrombin may induce cytoskeletal reorganization leading to permeability increase in 2 phases. In the initial phase thrombin-induced engagement of heterotrimeric G-proteins activates Rho (via p115RhoGEF) and p38 MAPK pools associated with microtubules, resulting in phosphorylation/activation of MT-associated proteins, like LIMK, tau and CaD. In addition, thrombin destabilizes microtubules by dephosphorylation of stathmin. In the final stage MT dissolution releases MT-associated protein complexes, further activating Rho (via GEF-H1) and p38 MAPK pathways, leading to increased phosphorylation of cytoskeletal targets, stress fiber formation, and barrier compromise.
Figure 4.

Hypothetic mechanism of thrombin-induced microtubule-mediated EC barrier compromise. Thrombin activates its receptor (PAR-1) leading to activation of trimeric G-proteins (G12/13 and Gq), following by initial activation (phase 1) of Rho and p38 MAPK signaling and resulting in disruption of microtubule structure via activation of MT-binding proteins (phosphorylation of tau, CaD and LIMK and dephosphorylation of stathmin). At phase 2 MT dissolution leads to further activation of Rho (via release and activation of GEF-H1) and p38 MAPK followed by additional phosphorylation of cytoskeletal targets and their relocation to actin cytoskeleton resulting in actin rearrangement and permeability increase. MT stab: MT stabilization, MT inh: microtubule inhibition.
Endothelial cell junctions and barrier regulation
The vascular endothelium is constantly exposed to hemodynamic stimuli, such as shear stress, contraction or dilation of the vessels. The continuous reorganization of cell junctions and the cytoskeleton have key importance in the maintenance of the endothelial barrier integrity. Reshaping of the cells allows the endothelial monolayer to adapt to the dynamic conditions to which it is exposed.110 Inter-endothelial communicating structures mainly comprise of adherens junctions (AJ), tight junctions (TJ) and gap junctions (GJ) (Fig. 5).
Figure 5.

Schematic representation of major intercellular contacts.
AJs are critical in the maintenance of endothelial integrity providing connection between neighboring ECs, thus regulating endothelial barrier function. AJs represent the majority of cell junctions comprising the endothelial barrier, in contrast with epithelial cells where tight junctions dominate.10 AJs are composed of VE-cadherin and its cytoplasmic binding partners: α-, β- γ-, p120 catenins, which link AJs to the actin cytoskeleton. The assembly of the VE-cadherin-catenin complex is regulated by phosphorylation, and their dissociation leads to EC barrier dysfunction.111
VE-cadherin is a transmembrane protein that mediates hemophilic binding of adjacent cells in a Ca2+-dependent manner.111 The extracellular region contains 5 repeating domains which coordinate with calcium ions and form a rod-like structure. The intracellular tail of VE-cadherin has 2 domains, the juxtamembrane domain (JMD) and the C-terminal domain (CTD). JMD binds p120 catenin, while CTD binds β-catenin or plakoglobin (γ-catenin) which attach α-catenin to link the cadherin-catenin complex to the actin cytoskeleton. α-catenin also interacts with other actin-binding proteins, specifically, α-actinin, vinculin, TJ zonula occludin proteins: ZO-1, ZO-2, ZO-3 and possibly spectrin. VE-cadherin is critical for the proper assembly of AJs, and for normal endothelial barrier function.112 VE-cadherin impairing results in interstitial edema and inflammation in lung and heart microvasculature.113
Catenins also play an important role in the regulation of AJ assembly. β-catenin has a dual role in cells. First it was identified as a component of AJs in the late '80s. Kemler and colleagues were able to isolate β-catenin together with α-catenin and plakoglobin.114 Later genetic and embryogenic studies revealed β-catenin as a component of the Wnt signaling pathway playing an important role in embryonic development and tumorogenesis.115 Recent study implicates the involvement of Wnt signaling in EC barrier regulation.116
Plakoglobin plays an important role in cadherin/catenin complex assembly, as a linker between this complex and F-actin cytoskeleton.117 Plakoglobin is an intracellular binding partner for VE-cadherin in ECs and its main function is to stabilize the AJ complex.117,118 Through α-catenin, plakoglobin is in connection with actin-binding proteins, like α-actinin and ZO-1.119 Plakoglobin is closely related to β-catenin, sharing 80% sequence identity120 and can bind the cytoplasmic domains of the classical cadherins. Both β-catenin and plakoglobin were shown to stabilize the linkage between VE-cadherin and the actin cytoskeleton, thus regulating endothelial barrier function.10 Thrombin-induced release of β-catenin and p120 catenin from the cell membrane has been described recently in human endothelium.121 Interestingly, recent studies implicated the involvement of p120 catenin in inhibition of Rho signaling in ECs.122
Regulation of AJs assembly and junctional permeability by reversible phosphorylation
The dynamic assembly and disassembly of AJs depends on protein-protein interactions regulated by reversible phosphorylation. Histamine, tumor necrosis factor (TNF) and vascular endothelial growth factor induced tyrosine phosphorylation of VE-cadherin, β-catenin and p120 thus increasing endothelial barrier permeability.123 For instance, tyrosine phosphorylation on Tyr860 of VE-cadherin and Tyr654 on β-catenin leads to disassembly of the catenin-cadherin complex.124 G12 binding to VE cadherin stimulates Src-mediated VE-cadherin phosphorylation at Tyr658 leading to AJ disassembly.125 Recent studies revealed the possibility of AJ regulation by Ser/Thr phosphorylation as well. For example, activation of PKCα leads to phosphorylation of p120 catenin at Ser879 resulting in AJs disassembly.126 The cytoplasmic domain of VE-cadherin is phosphorylated at Ser684,-686,-692 creating more interaction sites for β-catenin binding.111 Huber and Weis identified 2 residues in cadherin (Ser684 and Ser699) which are phosphorylated by casein kinase 2 (CK-2) and glycogen synthase kinase 3 (GSK-3). This phosphorylation of cadherin could stabilize and strengthen the catenin-cadherin complex by several hundred folds.111,127,128 However, there are some reports indicating that cadherin phosphorylation can be a negative factor for binding to β-catenin.129 E-cadherin phosphorylation mediated by CK-2 leads to the disruption of AJs in keratinocytes.130,131
Multiple kinases are involved in β-catenin phosphorylation such as casein kinase I (CK-I) and GSK-3 β.132,133 These kinases induce the phosphorylation of β-catenin on Se33,37 and Thr,41 respectively, leading to its ubiquitination and proteosomal degradation.132,133 Wnt and other stimuli lead to the inactivation of GSK-3β, thus decreasing β-catenin phosphorylation, translocation into the nucleus and binding to transcription factors.115 In contrary, Ser552 phosphorylation of β-catenin is not implicated in the Wnt signaling. In quiescent cells the phosphorylation level of this serine residue is very low and phospho-β-catenin Ser552 could be detected at the cell periphery of adjacent ECs. Phosphorylation of β-catenin at Ser552 by AKT leads to its dissociation from cell contacts.134,135 Finally, the inhibition of Ser/Thr phosphatases caused hyperphosphorylation of β-catenin on Ser/Thr residues and resulted in the loss of cell-cell contacts131 implicating the involvement Ser/Thr phosphatases in AJ assembly.
TJs regulate the transport of ions and solutes through the paracellular pathway.136 They comprise of 2 families of transmembrane proteins, occludins and claudins as well as their cytoplasmic partners, zonula occludens (ZO) proteins, which connect TJs to actin cytoskeleton.137 Compared to AJs, mechanisms regulating TJs are far less understood. AJs assembly precedes tight junction formation and in some in vivo cases cadherin is required for the formation of TJs, as it controls the recruitment of ZO-1 to TJ complexes.138 Up-regulation of EC-specific claudin-5 isoform is involved in EC barrier enhancement in some, but not all models.139,140 Conversely, edemagenic agonists decreased claudin-5 and ZO-1 expression accompanied by translocation of ZO-1 from the cytoskeleton to the membrane/nuclear fractions.141,142 Recent study implicated the involvement of PKCε/Erk1/2 MAPK axis in phosphorylation of ZO-1 at Thr770,772.143 This phosphorylation is accompanied by dissociation of ZO-1 from occludin resulting in EC barrier dysfunction.143 In contrary, cyclic-strain-induced enhancement of EC barrier function involved increased PKC-dependent ZO-1-occludin association.144 In addition, Tyr phosphorylation of ZO-2 is involved in its dissociation from TJs and barrier dysfunction.145
Gap junctions (GJ) form intercellular channels involving in the passages of ions and macromolecules between neighboring cells. They also present in ECs and play an important role in endothelial functions; however, information regarding the involvement of GJ in EC permeability regulation is limited and somewhat controversial. Recent studies on pulmonary EC demonstrated that the expression of TJ protein, connexin 43, is involved in LPS-induced permeability increase.146 Consistent with these observations, connexin 43 inhibition blocked thrombin-induced permeability increase in lung capillaries.147 In contrary, other report demonstrated that thrombin-induced permeability is accompanied by internalization (inhibition) of TJ communications in vascular endothelium.148 Further studies are needed to define the involvement of TJ in the EC permeability regulation.
Conclusion
Molecular basis of ALI and ARDS is still poorly understood. Based on the existing literature we proposed complex mechanisms involving crosstalk between microtubule and microfilaments accompanied by activation/phosphorylation cytoskeletal proteins following by re-arrangement of cell junctions. Further studies are needed to define cytoskeletal-specific structure/function relationships and enhance our understanding of the lung vascular barrier regulation.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Funding
This manuscript was supported by grant PO1HL0101902 from the National Institute of Health and Extramural Success Award from the Georgia Regents University.
References
- 1.Bazzoni G, Dejana E. Endothelial cell-to-cell junctions: molecular organization and role in vascular homeostasis. Physiol Rev 2004; 84:869-901; PMID:; http://dx.doi.org/ 10.1152/physrev.00035.2003 [DOI] [PubMed] [Google Scholar]
- 2.Pugin J. Sepsis and the immune response. Intensive Care Med 1999; 25:1027-8; PMID:; http://dx.doi.org/ 10.1007/s001340051003 [DOI] [PubMed] [Google Scholar]
- 3.Bogatcheva NV, Verin AD. The role of cytoskeleton in the regulation of vascular endothelial barrier function. Microvasc Res 2008; 76:202-7; PMID:; http://dx.doi.org/ 10.1016/j.mvr.2008.06.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dudek SM, Garcia JG. Cytoskeletal regulation of pulmonary vascular permeability. J Appl Physiol 2001; 91:1487-500; PMID: [DOI] [PubMed] [Google Scholar]
- 5.Ware LB, Matthay MA. The acute respiratory distress syndrome. N Engl J Med 2000; 342:1334-49; PMID:; http://dx.doi.org/ 10.1056/NEJM200005043421806 [DOI] [PubMed] [Google Scholar]
- 6.Anderson WR, Thielen K. Correlative study of adult respiratory distress syndrome by light, scanning, and transmission electron microscopy. Ultrastruct Pathol 1992; 16:615-28; PMID:; http://dx.doi.org/ 10.3109/01913129209023751 [DOI] [PubMed] [Google Scholar]
- 7.Bachofen M, Weibel ER. Structural alterations of lung parenchyma in the adult respiratory distress syndrome. Clin Chest Med 1982; 3:35-56; PMID: [PubMed] [Google Scholar]
- 8.Matthay MA. Conference summary: acute lung injury. Chest 1999; 116:119S-26S; PMID:; http://dx.doi.org/ 10.1378/chest.116.suppl_1.119S [DOI] [PubMed] [Google Scholar]
- 9.Lewis JF, Jobe AH. Surfactant and the adult respiratory distress syndrome. Am Rev Respir Dis 1993; 147:218-33; PMID:; http://dx.doi.org/ 10.1164/ajrccm/147.1.218 [DOI] [PubMed] [Google Scholar]
- 10.Mehta D, Malik AB. Signaling mechanisms regulating endothelial permeability. Physiol Rev 2006; 86:279-367; PMID:; http://dx.doi.org/ 10.1152/physrev.00012.2005 [DOI] [PubMed] [Google Scholar]
- 11.Stevens T. Functional and molecular heterogeneity of pulmonary endothelial cells. Proc Am Thor Soc 2011; 8:453-7; PMID:; http://dx.doi.org/ 10.1513/pats.201101-004MW [DOI] [PubMed] [Google Scholar]
- 12.Schnitzer JE, Siflinger-Birnboim A, Del Vecchio PJ, Malik AB. Segmental differentiation of permeability, protein glycosylation, and morphology of cultured bovine lung vascular endothelium. Biochem Biophys Res Commun 1994; 199:11-9; PMID:; http://dx.doi.org/ 10.1006/bbrc.1994.1185 [DOI] [PubMed] [Google Scholar]
- 13.Blum MS, Toninelli E, Anderson JM, Balda MS, Zhou J, O'Donnell L, Pardi R, Bender JR. Cytoskeletal rearrangement mediates human microvascular endothelial tight junction modulation by cytokines. Am J Phys 1997; 273:H286-94; PMID: [DOI] [PubMed] [Google Scholar]
- 14.Chi JT, Chang HY, Haraldsen G, Jahnsen FL, Troyanskaya OG, Chang DS, Wang Z, Rockson SG, van de Rijn M, Botstein D, et al. Endothelial cell diversity revealed by global expression profiling. Proc Natl Acad Sci U S A 2003; 100:10623-8; PMID:; http://dx.doi.org/ 10.1073/pnas.1434429100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Terramani TT, Eton D, Bui PA, Wang Y, Weaver FA, Yu H. Human macrovascular endothelial cells: optimization of culture conditions. In Vitro Cell Dev Biol Anim 2000; 36:125-32; PMID:; http://dx.doi.org/ 10.1290/1071-2690(2000)036%3c0125:HMECOO%3e2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- 16.Saguil A, Fargo M. Acute respiratory distress syndrome: diagnosis and management. Am Fam Physician 2012; 85:352-8; PMID: [PubMed] [Google Scholar]
- 17.Rothberg KG, Heuser JE, Donzell WC, Ying YS, Glenney JR, Anderson RG. Caveolin, a protein component of caveolae membrane coats. Cell 1992; 68:673-82; PMID:; http://dx.doi.org/ 10.1016/0092-8674(92)90143-Z [DOI] [PubMed] [Google Scholar]
- 18.Sun Y, Hu G, Zhang X, Minshall RD. Phosphorylation of caveolin-1 regulates oxidant-induced pulmonary vascular permeability via paracellular and transcellular pathways. Circ Res 2009; 105:676-85, 15 p following 85; PMID:; http://dx.doi.org/ 10.1161/CIRCRESAHA.109.201673 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Majno G, Palade GE. Studies on inflammation. 1. The effect of histamine and serotonin on vascular permeability: an electron microscopic study. J Biophys Biochem Cytol 1961; 11:571-605; PMID:; http://dx.doi.org/ 10.1083/jcb.11.3.571 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chatterjee A, Snead C, Yetik-Anacak G, Antonova G, Zeng J, Catravas JD. Heat shock protein 90 inhibitors attenuate LPS-induced endothelial hyperpermeability. Am J Physiol Lung Cell Mol Physiol 2008; 294:L755-63; PMID:; http://dx.doi.org/ 10.1152/ajplung.00350.2007 [DOI] [PubMed] [Google Scholar]
- 21.Birukova AA, Smurova K, Birukov KG, Kaibuchi K, Garcia JG, Verin AD. Role of Rho GTPases in thrombin-induced lung vascular endothelial cells barrier dysfunction. Microvasc Res 2004; 67:64-77; PMID:; http://dx.doi.org/ 10.1016/j.mvr.2003.09.007 [DOI] [PubMed] [Google Scholar]
- 22.Petrache I, Verin AD, Crow MT, Birukova A, Liu F, Garcia JG. Differential effect of MLC kinase in TNF-alpha-induced endothelial cell apoptosis and barrier dysfunction. Am J Physiol Lung Cell Mol Physiol 2001; 280:L1168-78; PMID: [DOI] [PubMed] [Google Scholar]
- 23.Garcia JG, Schaphorst KL, Verin AD, Vepa S, Patterson CE, Natarajan V. Diperoxovanadate alters endothelial cell focal contacts and barrier function: role of tyrosine phosphorylation. J Appl Physiol 2000; 89:2333-43; PMID: [DOI] [PubMed] [Google Scholar]
- 24.Bannerman DD, Goldblum SE. Direct effects of endotoxin on the endothelium: barrier function and injury. Lab Invest 1999; 79:1181-99; PMID: [PubMed] [Google Scholar]
- 25.Bannerman DD, Sathyamoorthy M, Goldblum SE. Bacterial lipopolysaccharide disrupts endothelial monolayer integrity and survival signaling events through caspase cleavage of adherens junction proteins. J Biol Chem 1998; 273:35371-80; PMID:; http://dx.doi.org/ 10.1074/jbc.273.52.35371 [DOI] [PubMed] [Google Scholar]
- 26.Gong P, Angelini DJ, Yang S, Xia G, Cross AS, Mann D, Bannerman DD, Vogel SN, Goldblum SE. TLR4 signaling is coupled to SRC family kinase activation, tyrosine phosphorylation of zonula adherens proteins, and opening of the paracellular pathway in human lung microvascular endothelia. J Biol Chem 008; 283:13437-49; PMID:; http://dx.doi.org/ 10.1074/jbc.M707986200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bannerman DD, Goldblum SE. Endotoxin induces endothelial barrier dysfunction through protein tyrosine phosphorylation. Am J Physiol 1997; 273:L217-26; PMID: [DOI] [PubMed] [Google Scholar]
- 28.Barabutis N, Handa V, Dimitropoulou C, Rafikov R, Snead C, Kumar S, Joshi A, Thangjam G, Fulton D, Black SM, et al. LPS induces pp60c-src-mediated tyrosine phosphorylation of Hsp90 in lung vascular endothelial cells and mouse lung. Am J Physiol Lung Cell Mol Physiol 2013; 304:L883-93; PMID:; http://dx.doi.org/ 10.1152/ajplung.00419.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Joshi AD, Dimitropoulou C, Thangjam G, Snead C, Feldman S, Barabutis N, Fulton D, Hou Y, Kumar S, Patel V, et al. Heat Shock Protein 90 Inhibitors Prevent LPS-Induced Endothelial Barrier Dysfunction by Disrupting RhoA Signaling. Am J Respir Cell Mol Biol 2014; 50:170-9; PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhao Y, Davis HW. Endotoxin causes phosphorylation of MARCKS in pulmonary vascular endothelial cells. J Cell Biochem 2000; 79:496-505; PMID:; http://dx.doi.org/ 10.1002/1097-4644(20001201)79:3%3c496::AID-JCB140%3e3.0.CO;2-5 [DOI] [PubMed] [Google Scholar]
- 31.Chatterjee A, Dimitropoulou C, Drakopanayiotakis F, Antonova G, Snead C, Cannon J, Venema RC, Catravas JD. Heat shock protein 90 inhibitors prolong survival, attenuate inflammation, and reduce lung injury in murine sepsis. Am J Respir Crit Care Med 2007; 176:667-75; PMID:; http://dx.doi.org/ 10.1164/rccm.200702-291OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Grand RJ, Turnell AS, Grabham PW. Cellular consequences of thrombin-receptor activation. Biochem J 1996; 313 (Pt 2):353-68; PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bogatcheva NV, Garcia JG, Verin AD. Molecular mechanisms of thrombin-induced endothelial cell permeability. Biochem (Mosc) 2002; 67:75-84; PMID:; http://dx.doi.org/ 10.1023/A:1013904231324 [DOI] [PubMed] [Google Scholar]
- 34.Brass LF, Molino M. Protease-activated G protein-coupled receptors on human platelets and endothelial cells. Thromb Haemost 1997; 78:234-41; PMID: [PubMed] [Google Scholar]
- 35.Vu TK, Hung DT, Wheaton VI, Coughlin SR. Molecular cloning of a functional thrombin receptor reveals a novel proteolytic mechanism of receptor activation. Cell 1991; 64:1057-68; PMID:; http://dx.doi.org/ 10.1016/0092-8674(91)90261-V [DOI] [PubMed] [Google Scholar]
- 36.Vouret-Craviari V, Grall D, Van Obberghen-Schilling E. Modulation of Rho GTPase activity in endothelial cells by selective proteinase-activated receptor (PAR) agonists. J Thromb Haemost 2003; 1:1103-11; PMID:; http://dx.doi.org/ 10.1046/j.1538-7836.2003.00238.x [DOI] [PubMed] [Google Scholar]
- 37.Garcia JG, Davis HW, Patterson CE. Regulation of endothelial cell gap formation and barrier dysfunction: role of myosin light chain phosphorylation. J Cell Physiol 1995; 163:510-22; PMID:; http://dx.doi.org/ 10.1002/jcp.1041630311 [DOI] [PubMed] [Google Scholar]
- 38.Garcia JG, Siflinger-Birnboim A, Bizios R, Del Vecchio PJ, Fenton JW, 2nd, Malik AB. Thrombin-induced increase in albumin permeability across the endothelium. J Cell Physiol 1986; 128:96-104; PMID:; http://dx.doi.org/ 10.1002/jcp.1041280115 [DOI] [PubMed] [Google Scholar]
- 39.Patterson CE, Lum H, Schaphorst KL, Verin AD, Garcia JG. Regulation of endothelial barrier function by the cAMP-dependent protein kinase. Endothelium 2000; 7:287-308; PMID: [DOI] [PubMed] [Google Scholar]
- 40.Tiruppathi C, Malik AB, Del Vecchio PJ, Keese CR, Giaever I. Electrical method for detection of endothelial cell shape change in real time: assessment of endothelial barrier function. Proc Natl Acad Sci U S A 1992; 89:7919-23; PMID:; http://dx.doi.org/ 10.1073/pnas.89.17.7919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Johnson A, Tahamont MV, Malik AB. Thrombin-induced lung vascular injury. Roles of fibrinogen and fibrinolysis. Am Rev Respir Dis 1983; 128:38-44; PMID: [DOI] [PubMed] [Google Scholar]
- 42.Johnson A, Malik AB. Pulmonary transvascular fluid and protein exchange after thrombin-induced microembolism. Differential effects of cyclooxygenase inhibitors. Am Rev Respir Dis 1985; 132:70-6; PMID: [DOI] [PubMed] [Google Scholar]
- 43.Uchiba M, Okajima K, Murakami K, Okabe H, Takatsuki K. Attenuation of endotoxin-induced pulmonary vascular injury by antithrombin III. Am J Physiol 1996; 270:L921-30; PMID: [DOI] [PubMed] [Google Scholar]
- 44.Shasby DM, Shasby SS, Sullivan JM, Peach MJ. Role of endothelial cell cytoskeleton in control of endothelial permeability. Circ Res 1982; 51:657-61; PMID:; http://dx.doi.org/ 10.1161/01.RES.51.5.657 [DOI] [PubMed] [Google Scholar]
- 45.Verin AD, Birukova A, Wang P, Liu F, Becker P, Birukov K, Garcia JG. Microtubule disassembly increases endothelial cell barrier dysfunction: role of MLC phosphorylation. Am J Physiol Lung Cell Mol Physiol 2001; 281:L565-74; PMID: [DOI] [PubMed] [Google Scholar]
- 46.Dery O, Corvera CU, Steinhoff M, Bunnett NW. Proteinase-activated receptors: novel mechanisms of signaling by serine proteases. Am J Physiol 1998; 274:C1429-52; PMID: [DOI] [PubMed] [Google Scholar]
- 47.Tinsley JH, De Lanerolle P, Wilson E, Ma W, Yuan SY. Myosin light chain kinase transference induces myosin light chain activation and endothelial hyperpermeability. Am J Physiol Cell Physiol 2000; 279:C1285-9; PMID: [DOI] [PubMed] [Google Scholar]
- 48.Wysolmerski RB, Lagunoff D. Regulation of permeabilized endothelial cell retraction by myosin phosphorylation. Am J Physiol 1991; 261:C32-40; PMID: [DOI] [PubMed] [Google Scholar]
- 49.Birukov KG, Csortos C, Marzilli L, Dudek S, Ma SF, Bresnick AR, Verin AD, Cotter RJ, Garcia JG. Differential regulation of alternatively spliced endothelial cell myosin light chain kinase isoforms by p60(Src). J Biol Chem 2001; 276:8567-73; PMID:; http://dx.doi.org/ 10.1074/jbc.M005270200 [DOI] [PubMed] [Google Scholar]
- 50.Shi S, Verin AD, Schaphorst KL, Gilbert-McClain LI, Patterson CE, Irwin RP, Natarajan V, Garcia JG. Role of tyrosine phosphorylation in thrombin-induced endothelial cell contraction and barrier function. Endothelium 1998; 6:153-71; PMID:; http://dx.doi.org/ 10.3109/10623329809072202 [DOI] [PubMed] [Google Scholar]
- 51.Ridley AJ. The GTP-binding protein Rho. The Int J Biochem Cell Biol 1997; 29:1225-9; PMID:; http://dx.doi.org/ 10.1016/S1357-2725(97)00052-6 [DOI] [PubMed] [Google Scholar]
- 52.Cherfils J, Zeghouf M. Regulation of small GTPases by GEFs, GAPs, and GDIs. Physiol Rev 2013; 93:269-309; PMID:; http://dx.doi.org/ 10.1152/physrev.00003.2012 [DOI] [PubMed] [Google Scholar]
- 53.Birukova AA, Adyshev D, Gorshkov B, Bokoch GM, Birukov KG, Verin AD. GEF-H1 is involved in agonist-induced human pulmonary endothelial barrier dysfunction. Am J Physiol Lung Cell Mol Physiol 2006; 290:L540-8; PMID:; http://dx.doi.org/ 10.1152/ajplung.00259.2005 [DOI] [PubMed] [Google Scholar]
- 54.Knezevic N, Roy A, Timblin B, Konstantoulaki M, Sharma T, Malik AB, Mehta D. GDI-1 phosphorylation switch at serine 96 induces RhoA activation and increased endothelial permeability. Mol Cell Biol 2007; 27:6323-33; PMID:; http://dx.doi.org/ 10.1128/MCB.00523-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Rafikov R, Dimitropoulou C, Aggarwal S, Kangath A, Gross C, Pardo D, Sharma S, Jezierska-Drutel A, Patel V, Snead C, et al. Lipopolysaccharide Induced Lung Injury Involves the Nitration-Mediated Activation of RhoA. J Biol Chem 2014; 289:4710-22; PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Amano M, Ito M, Kimura K, Fukata Y, Chihara K, Nakano T, Matsuura Y, Kaibuchi K. Phosphorylation and activation of myosin by Rho-associated kinase (Rho-kinase). J Biol Chem 1996; 271:20246-9; PMID:; http://dx.doi.org/ 10.1074/jbc.271.34.20246 [DOI] [PubMed] [Google Scholar]
- 57.Kimura K, Ito M, Amano M, Chihara K, Fukata Y, Nakafuku M, Yamamori B, Feng J, Nakano T, Okawa K, et al. Regulation of myosin phosphatase by Rho and Rho-associated kinase (Rho-kinase). Science 1996; 273:245-8; PMID:; http://dx.doi.org/ 10.1126/science.273.5272.245 [DOI] [PubMed] [Google Scholar]
- 58.Kolosova IA, Ma SF, Adyshev DM, Wang P, Ohba M, Natarajan V, Garcia JG, Verin AD. Role of CPI-17 in the regulation of endothelial cytoskeleton. Am J Physiol Lung Cell Mol Physiol 2004; 287:L970-80; PMID:; http://dx.doi.org/ 10.1152/ajplung.00398.2003 [DOI] [PubMed] [Google Scholar]
- 59.Watanabe Y, Ito M, Kataoka Y, Wada H, Koyama M, Feng J, Shiku H, Nishikawa M. Protein kinase C-catalyzed phosphorylation of an inhibitory phosphoprotein of myosin phosphatase is involved in human platelet secretion. Blood 2001; 97:3798-805; PMID:; http://dx.doi.org/ 10.1182/blood.V97.12.3798 [DOI] [PubMed] [Google Scholar]
- 60.Dubois T, Howell S, Zemlickova E, Learmonth M, Cronshaw A, Aitken A. Novel in vitro and in vivo phosphorylation sites on protein phosphatase 1 inhibitor CPI-17. Biochemical Biophys Res Commun 2003; 302:186-92; PMID:; http://dx.doi.org/ 10.1016/S0006-291X(03)00130-X [DOI] [PubMed] [Google Scholar]
- 61.Eto M, Ohmori T, Suzuki M, Furuya K, Morita F. A novel protein phosphatase-1 inhibitory protein potentiated by protein kinase C. Isolation from porcine aorta media and characterization. J Biochem 1995; 118:1104-7; PMID: [DOI] [PubMed] [Google Scholar]
- 62.Bogatcheva NV, Verin AD, Wang P, Birukova AA, Birukov KG, Mirzopoyazova T, Adyshev DM, Chiang ET, Crow MT, Garcia JG. Phorbol esters increase MLC phosphorylation and actin remodeling in bovine lung endothelium without increased contraction. Am J Physiol Lung Cell Mol Physiol 2003; 285:L415-26; PMID: [DOI] [PubMed] [Google Scholar]
- 63.Moy AB, Blackwell K, Wang N, Haxhinasto K, Kasiske MK, Bodmer J, Reyes G, English A. Phorbol ester-mediated pulmonary artery endothelial barrier dysfunction through regulation of actin cytoskeletal mechanics. Am J Physiol Lung Cell Mol Physiol 2004; 287:L153-67; PMID:; http://dx.doi.org/ 10.1152/ajplung.00292.2003 [DOI] [PubMed] [Google Scholar]
- 64.Stasek JE, Jr., Patterson CE, Garcia JG. Protein kinase C phosphorylates caldesmon77 and vimentin and enhances albumin permeability across cultured bovine pulmonary artery endothelial cell monolayers. J Cell Physiol 1992; 153:62-75; PMID:; http://dx.doi.org/ 10.1002/jcp.1041530110 [DOI] [PubMed] [Google Scholar]
- 65.Bogatcheva NV, Birukova A, Borbiev T, Kolosova I, Liu F, Garcia JG, Verin AD. Caldesmon is a cytoskeletal target for PKC in endothelium. J Cell Biochem 2006; 99:1593-605; PMID:; http://dx.doi.org/ 10.1002/jcb.20823 [DOI] [PubMed] [Google Scholar]
- 66.Sobue K, Sellers JR. Caldesmon, a novel regulatory protein in smooth muscle and nonmuscle actomyosin systems. J Biol Chem 1991; 266:12115-8; PMID: [PubMed] [Google Scholar]
- 67.Marston SB, Redwood CS. The molecular anatomy of caldesmon. Biochemical J 1991; 279 (Pt 1):1-16; PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Adam LP, Haeberle JR, Hathaway DR. Phosphorylation of caldesmon in arterial smooth muscle. J Biol Chem 1989; 264:7698-703; PMID: [PubMed] [Google Scholar]
- 69.Mirzapoiazova T, Kolosova IA, Romer L, Garcia JG, Verin AD. The role of caldesmon in the regulation of endothelial cytoskeleton and migration. J Cell Physiol 2005; 203:520-8; PMID:; http://dx.doi.org/ 10.1002/jcp.20244 [DOI] [PubMed] [Google Scholar]
- 70.Verin AD, Liu F, Bogatcheva N, Borbiev T, Hershenson MB, Wang P, Garcia JG. Role of ras-dependent ERK activation in phorbol ester-induced endothelial cell barrier dysfunction. Am J Physiol Lung Cell Mol Physiol 2000; 279:L360-70; PMID: [DOI] [PubMed] [Google Scholar]
- 71.Kevil CG, Oshima T, Alexander JS. The role of p38 MAP kinase in hydrogen peroxide mediated endothelial solute permeability. Endothelium 2001; 8:107-16; PMID: [DOI] [PubMed] [Google Scholar]
- 72.Garcia JG, Wang P, Schaphorst KL, Becker PM, Borbiev T, Liu F, Birukova A, Jacobs K, Bogatcheva N, Verin AD. Critical involvement of p38 MAP kinase in pertussis toxin-induced cytoskeletal reorganization and lung permeability. FASEB J 2002; 16:1064-76; PMID:; http://dx.doi.org/ 10.1096/fj.01-0895com [DOI] [PubMed] [Google Scholar]
- 73.Borbiev T, Birukova A, Liu F, Nurmukhambetova S, Gerthoffer WT, Garcia JG, Verin AD. p38 MAP kinase-dependent regulation of endothelial cell permeability. Am J Physiol Lung Cell Mol Physiol 2004; 287:L911-8; PMID:; http://dx.doi.org/ 10.1152/ajplung.00372.2003 [DOI] [PubMed] [Google Scholar]
- 74.Damarla M, Hasan E, Boueiz A, Le A, Pae HH, Montouchet C, Kolb T, Simms T, Myers A, Kayyali US, et al. Mitogen activated protein kinase activated protein kinase 2 regulates actin polymerization and vascular leak in ventilator associated lung injury. PloS One 2009; 4:e4600; PMID:; http://dx.doi.org/ 10.1371/journal.pone.0004600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Gorshkov BA, Zemskova MA, Verin AD, Bogatcheva NV. Taxol alleviates 2-methoxyestradiol-induced endothelial permeability. Vascul Pharmacol 2012; 56:56-63; PMID:; http://dx.doi.org/ 10.1016/j.vph.2011.10.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Hirano S, Rees RS, Yancy SL, Welsh MJ, Remick DG, Yamada T, Hata J, Gilmont RR. Endothelial barrier dysfunction caused by LPS correlates with phosphorylation of HSP27 in vivo. Cell Biol Toxicol 2004; 20:1-14; PMID:; http://dx.doi.org/ 10.1023/B:CBTO.0000021019.50889.aa [DOI] [PubMed] [Google Scholar]
- 77.Guay J, Lambert H, Gingras-Breton G, Lavoie JN, Huot J, Landry J. Regulation of actin filament dynamics by p38 map kinase-mediated phosphorylation of heat shock protein 27. J Cell Sci 1997; 110 (Pt 3):357-68; PMID: [DOI] [PubMed] [Google Scholar]
- 78.Hedges JC, Yamboliev IA, Ngo M, Horowitz B, Adam LP, Gerthoffer WT. p38 mitogen-activated protein kinase expression and activation in smooth muscle. Am J Physiol 1998; 275:C527-34; PMID: [DOI] [PubMed] [Google Scholar]
- 79.Rogalla T, Ehrnsperger M, Preville X, Kotlyarov A, Lutsch G, Ducasse C, Paul C, Wieske M, Arrigo AP, Buchner J, et al. Regulation of Hsp27 oligomerization, chaperone function, and protective activity against oxidative stresstumor necrosis factor alpha by phosphorylation. J Biol Chem 1999; 274:18947-56; PMID:; http://dx.doi.org/ 10.1074/jbc.274.27.18947 [DOI] [PubMed] [Google Scholar]
- 80.Huot J, Houle F, Rousseau S, Deschesnes RG, Shah GM, Landry J. SAPK2p38-dependent F-actin reorganization regulates early membrane blebbing during stress-induced apoptosis. J cell Biol 1998; 143:1361-73; PMID:; http://dx.doi.org/ 10.1083/jcb.143.5.1361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Rousseau S, Houle F, Landry J, Huot J. p38 MAP kinase activation by vascular endothelial growth factor mediates actin reorganization and cell migration in human endothelial cells. Oncogene 1997; 15:2169-77; PMID:; http://dx.doi.org/ 10.1038/sj.onc.1201380 [DOI] [PubMed] [Google Scholar]
- 82.Piotrowicz RS, Levin EG. Basolateral membrane-associated 27-kDa heat shock protein and microfilament polymerization. J Biol Chem 1997; 272:25920-7; PMID:; http://dx.doi.org/ 10.1074/jbc.272.41.25920 [DOI] [PubMed] [Google Scholar]
- 83.Lu Q, Harrington EO, Jackson H, Morin N, Shannon C, Rounds S. Transforming growth factor-beta1-induced endothelial barrier dysfunction involves Smad2-dependent p38 activation and subsequent RhoA activation. J Appl Physiol 2006; 101:375-84; PMID:; http://dx.doi.org/ 10.1152/japplphysiol.01515.2005 [DOI] [PubMed] [Google Scholar]
- 84.Koss M, Pfeiffer GR, 2nd, Wang Y, Thomas ST, Yerukhimovich M, Gaarde WA, Doerschuk CM, Wang Q. Ezrinradixinmoesin proteins are phosphorylated by TNF-alpha and modulate permeability increases in human pulmonary microvascular endothelial cells. J Immunol 2006; 176:1218-27; PMID: ; http://dx.doi.org/ 10.4049/jimmunol.176.2.1218 [DOI] [PubMed] [Google Scholar]
- 85.Adyshev DM, Dudek SM, Moldobaeva N, Kim KM, Ma SF, Kasa A, Garcia JG, Verin AD. Ezrinradixinmoesin proteins differentially regulate endothelial hyperpermeability after thrombin. Am J Physiol Lung Cell and Mol Physiol 2013; 305:L240-55; PMID:; http://dx.doi.org/ 10.1152/ajplung.00355.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Adyshev DM, Moldobaeva NK, Elangovan VR, Garcia JG, Dudek SM. Differential involvement of ezrinradixinmoesin proteins in sphingosine 1-phosphate-induced human pulmonary endothelial cell barrier enhancement. Cell Signal 2011; 23:2086-96; PMID:; http://dx.doi.org/ 10.1016/j.cellsig.2011.08.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Bogatcheva NV, Zemskova MA, Gorshkov BA, Kim KM, Daglis GA, Poirier C, Verin AD. Ezrin, radixin, and moesin are phosphorylated in response to 2-methoxyestradiol and modulate endothelial hyperpermeability. Am J Respir Cell Mol Biol 2011; 45:1185-94; PMID:; http://dx.doi.org/ 10.1165/rcmb.2011-0092OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Wu T, Xing J, Birukova AA. Cell-type-specific crosstalk between p38 MAPK and Rho signaling in lung micro- and macrovascular barrier dysfunction induced by Staphylococcus aureus-derived pathogens. Transl Res 2013; 162:45-55; PMID:; http://dx.doi.org/ 10.1016/j.trsl.2013.03.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Bogatcheva NV, Adyshev D, Mambetsariev B, Moldobaeva N, Verin AD. Involvement of microtubules, p38, and Rho kinases pathway in 2-methoxyestradiol-induced lung vascular barrier dysfunction. Am J Physiol Lung Cell Mol Physiol 2007; 292:L487-99; PMID:; http://dx.doi.org/ 10.1152/ajplung.00217.2006 [DOI] [PubMed] [Google Scholar]
- 90.Mirzapoiazova T, Kolosova IA, Moreno L, Sammani S, Garcia JG, Verin AD. Suppression of endotoxin-induced inflammation by taxol. Eur Respir J 2007; 30:429-35; PMID:; http://dx.doi.org/ 10.1183/09031936.00154206 [DOI] [PubMed] [Google Scholar]
- 91.Kratzer E, Tian Y, Sarich N, Wu T, Meliton A, Leff A, Birukova AA. Oxidative Stress Contributes to Lung Injury and Barrier Dysfunction via Microtubule Destabilization. Am J Respir Cell Mol Biol 2012; 47:688-97; PMID:; http://dx.doi.org/ 10.1165/rcmb.2012-0161OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Birukova AA, Birukov KG, Adyshev D, Usatyuk P, Natarajan V, Garcia JG, Verin AD. Involvement of microtubules and Rho pathway in TGF-beta1-induced lung vascular barrier dysfunction. J Cell Physiol 2005; 204:934-47; PMID:; http://dx.doi.org/ 10.1002/jcp.20359 [DOI] [PubMed] [Google Scholar]
- 93.Birukova AA, Birukov KG, Smurova K, Adyshev D, Kaibuchi K, Alieva I, Garcia JG, Verin AD. Novel role of microtubules in thrombin-induced endothelial barrier dysfunction. FASEB J 2004; 18:1879-90; PMID:; http://dx.doi.org/ 10.1096/fj.04-2328com [DOI] [PubMed] [Google Scholar]
- 94.Petrache I, Birukova A, Ramirez SI, Garcia JG, Verin AD. The role of the microtubules in tumor necrosis factor-alpha-induced endothelial cell permeability. Am J Respir Cell Mol Biol 2003; 28:574-81; PMID:; http://dx.doi.org/ 10.1165/rcmb.2002-0075OC [DOI] [PubMed] [Google Scholar]
- 95.Birukova AA, Birukov KG, Gorshkov B, Liu F, Garcia JG, Verin AD. MAP kinases in lung endothelial permeability induced by microtubule disassembly. Am J Physiol Lung Cell Mol Physiol 2005; 289:L75-84; PMID:; http://dx.doi.org/ 10.1152/ajplung.00447.2004 [DOI] [PubMed] [Google Scholar]
- 96.Alieva IB, Zemskov EA, Smurova KM, Kaverina IN, Verin AD. The leading role of microtubules in endothelial barrier dysfunction: disassembly of peripheral microtubules leaves behind the cytoskeletal reorganization. J Cell Biochem 2013; 114:2258-72; PMID:; http://dx.doi.org/ 10.1002/jcb.24575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Luduena RF, Fellous A, McManus L, Jordan MA, Nunez J. Contrasting roles of tau and microtubule-associated protein 2 in the vinblastine-induced aggregation of brain tubulin. J Biol Chem 1984; 259:12890-8; PMID: [PubMed] [Google Scholar]
- 98.Drechsel DN, Hyman AA, Cobb MH, Kirschner MW. Modulation of the dynamic instability of tubulin assembly by the microtubule-associated protein tau. Mol Biol Cell 1992; 3:1141-54; PMID:; http://dx.doi.org/ 10.1091/mbc.3.10.1141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Gupta RP, Abou-Donia MB. Tau phosphorylation by diisopropyl phosphorofluoridate (DFP)-treated hen brain supernatant inhibits its binding with microtubules: role of Ca2+Calmodulin-dependent protein kinase II in tau phosphorylation. Arch Biochem Biophys 1999; 365:268-78; PMID:; http://dx.doi.org/ 10.1006/abbi.1999.1165 [DOI] [PubMed] [Google Scholar]
- 100.Litersky JM, Johnson GV, Jakes R, Goedert M, Lee M, Seubert P. Tau protein is phosphorylated by cyclic AMP-dependent protein kinase and calciumcalmodulin-dependent protein kinase II within its microtubule-binding domains at Ser-262 and Ser-356. The Biochemical J 1996; 316 (Pt 2):655-60; PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Reynolds CH, Nebreda AR, Gibb GM, Utton MA, Anderton BH. Reactivating kinasep38 phosphorylates tau protein in vitro. J Neurochem 1997; 69:191-8; PMID:; http://dx.doi.org/ 10.1046/j.1471-4159.1997.69010191.x [DOI] [PubMed] [Google Scholar]
- 102.Bogatcheva NV, Adyshev D, Mambetsariev B, Moldobaeva N, Verin AD. Involvement of microtubules, p38, and Rho kinases pathway in 2-methoxyestradiol-induced lung vascular barrier dysfunction. Am J Physiol Lung Cell Mol Physiol 2007; 292:L487-99; PMID:; http://dx.doi.org/ 10.1152/ajplung.00217.2006 [DOI] [PubMed] [Google Scholar]
- 103.Gorovoy M, Niu J, Bernard O, Profirovic J, Minshall R, Neamu R, Voyno-Yasenetskaya T. LIM kinase 1 coordinates microtubule stability and actin polymerization in human endothelial cells. J Biol Chem 2005; 280:26533-42; PMID:; http://dx.doi.org/ 10.1074/jbc.M502921200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Tian X, Tian Y, Sarich N, Wu T, Birukova AA. Novel role of stathmin in microtubule-dependent control of endothelial permeability. FASEB J 2012; 26:3862-74; PMID:; http://dx.doi.org/ 10.1096/fj.12-207746 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Ren Y, Li R, Zheng Y, Busch H. Cloning and characterization of GEF-H1, a microtubule-associated guanine nucleotide exchange factor for Rac and Rho GTPases. J Biol Chem 1998; 273:34954-60; PMID:; http://dx.doi.org/ 10.1074/jbc.273.52.34954 [DOI] [PubMed] [Google Scholar]
- 106.Krendel M, Zenke FT, Bokoch GM. Nucleotide exchange factor GEF-H1 mediates cross-talk between microtubules and the actin cytoskeleton. Nature Cell Biol 2002; 4:294-301; PMID:; http://dx.doi.org/ 10.1038/ncb773 [DOI] [PubMed] [Google Scholar]
- 107.Ishikawa R, Kagami O, Hayashi C, Kohama K. The binding of nonmuscle caldesmon from brain to microtubules. Regulations by Ca(2+)-calmodulin and cdc2 kinase. FEBS Lett 1992; 299:54-6; PMID:; http://dx.doi.org/ 10.1016/0014-5793(92)80099-3 [DOI] [PubMed] [Google Scholar]
- 108.Ishikawa R, Kagami O, Hayashi C, Kohama K. Characterization of smooth muscle caldesmon as a microtubule-associated protein. Cell Motil Cytoskeleton 1992; 23:244-51; PMID:; http://dx.doi.org/ 10.1002/cm.970230404 [DOI] [PubMed] [Google Scholar]
- 109.Elbaum M, Chausovsky A, Levy ET, Shtutman M, Bershadsky AD. Microtubule involvement in regulating cell contractility and adhesion-dependent signalling: a possible mechanism for polarization of cell motility. Biochemical Soc Symp 1999; 65:147-72; PMID: [PubMed] [Google Scholar]
- 110.Dejana E, Tournier-Lasserve E, Weinstein BM. The control of vascular integrity by endothelial cell junctions: molecular basis and pathological implications. Dev Cell 2009; 16:209-21; PMID:; http://dx.doi.org/ 10.1016/j.devcel.2009.01.004 [DOI] [PubMed] [Google Scholar]
- 111.Huber AH, Weis WI. The structure of the beta-cateninE-cadherin complex and the molecular basis of diverse ligand recognition by beta-catenin. Cell 2001; 105:391-402; PMID:; http://dx.doi.org/ 10.1016/S0092-8674(01)00330-0 [DOI] [PubMed] [Google Scholar]
- 112.Stevens T, Garcia JG, Shasby DM, Bhattacharya J, Malik AB. Mechanisms regulating endothelial cell barrier function. Am J Physiol Lung Cell Mol Physiol 2000; 279:L419-22; PMID: [DOI] [PubMed] [Google Scholar]
- 113.Corada M, Mariotti M, Thurston G, Smith K, Kunkel R, Brockhaus M, Lampugnani MG, Martin-Padura I, Stoppacciaro A, Ruco L, et al. Vascular endothelial-cadherin is an important determinant of microvascular integrity in vivo. Proc Natl Acad Sci U S A 1999; 96:9815-20; PMID:; http://dx.doi.org/ 10.1073/pnas.96.17.9815 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Valenta T, Hausmann G, Basler K. The many faces and functions of beta-catenin. EMBO J 2012; 31:2714-36; PMID:; http://dx.doi.org/ 10.1038/emboj.2012.150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Kikuchi A. Tumor formation by genetic mutations in the components of the Wnt signaling pathway. Cancer Sci 2003; 94:225-9; PMID:; http://dx.doi.org/ 10.1111/j.1349-7006.2003.tb01424.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Ferreira Tojais N, Peghaire C, Franzl N, Larrieu-Lahargue F, Jaspard B, Reynaud A, Moreau C, Couffinhal T, Duplaa C, Dufourcq P. Frizzled7 controls vascular permeability through the Wnt-canonical pathway and cross-talk with endothelial cell junction complexes. Cardiovasc Res 2014; 103:291-303; PMID:; http://dx.doi.org/ 10.1093/cvr/cvu133 [DOI] [PubMed] [Google Scholar]
- 117.Aberle H, Butz S, Stappert J, Weissig H, Kemler R, Hoschuetzky H. Assembly of the cadherin-catenin complex in vitro with recombinant proteins. J Cell Sci 1994; 107 (Pt 12):3655-63; PMID: [DOI] [PubMed] [Google Scholar]
- 118.Dejana E. Endothelial cell-cell junctions: happy together. Nat Rev Mol Cell Biol 2004; 5:261-70; PMID:; http://dx.doi.org/ 10.1038/nrm1357 [DOI] [PubMed] [Google Scholar]
- 119.Weis WI, Nelson WJ. Re-solving the cadherin-catenin-actin conundrum. J Biol Chem 2006; 281:35593-7; PMID:; http://dx.doi.org/ 10.1074/jbc.R600027200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Wallez Y, Huber P. Endothelial adherens and tight junctions in vascular homeostasis, inflammation and angiogenesis. Biochim Biophys Acta 2008; 1778:794-809; PMID:; http://dx.doi.org/ 10.1016/j.bbamem.2007.09.003 [DOI] [PubMed] [Google Scholar]
- 121.Beckers CM, Garcia-Vallejo JJ, van Hinsbergh VW, van Nieuw Amerongen GP. Nuclear targeting of beta-catenin and p120ctn during thrombin-induced endothelial barrier dysfunction. Cardiovasc Res 2008; 79:679-88; PMID:; http://dx.doi.org/ 10.1093/cvr/cvn127 [DOI] [PubMed] [Google Scholar]
- 122.Zebda N, Tian Y, Tian X, Gawlak G, Higginbotham K, Reynolds AB, Birukova AA, Birukov KG. Interaction of p190RhoGAP with C-terminal domain of p120-catenin modulates endothelial cytoskeleton and permeability. J Biol Chem 2013; 288:18290-9; PMID:; http://dx.doi.org/ 10.1074/jbc.M112.432757 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Dejana E, Orsenigo F, Lampugnani MG. The role of adherens junctions and VE-cadherin in the control of vascular permeability. J Cell Sci 2008; 121:2115-22; PMID:; http://dx.doi.org/ 10.1242/jcs.017897 [DOI] [PubMed] [Google Scholar]
- 124.Lilien J, Balsamo J. The regulation of cadherin-mediated adhesion by tyrosine phosphorylationdephosphorylation of beta-catenin. Curr Opin Cell Biol 2005; 17:459-65; PMID:; http://dx.doi.org/ 10.1016/j.ceb.2005.08.009 [DOI] [PubMed] [Google Scholar]
- 125.Gong H, Gao X, Feng S, Siddiqui MR, Garcia A, Bonini MG, Komarova Y, Vogel SM, Mehta D, Malik AB. Evidence of a common mechanism of disassembly of adherens junctions through Galpha13 targeting of VE-cadherin. J Exp Med 2014; 211:579-91; PMID:; http://dx.doi.org/ 10.1084/jem.20131190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Vandenbroucke St Amant E, Tauseef M, Vogel SM, Gao XP, Mehta D, Komarova YA, Malik AB. PKCalpha activation of p120-catenin serine 879 phospho-switch disassembles VE-cadherin junctions and disrupts vascular integrity. Circ Res 2012; 111:739-49; PMID:; http://dx.doi.org/ 10.1161/CIRCRESAHA.112.269654 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Choi HJ, Huber AH, Weis WI. Thermodynamics of beta-catenin-ligand interactions: the roles of the N- and C-terminal tails in modulating binding affinity. J Biol Chem 2006; 281:1027-38; PMID:; http://dx.doi.org/ 10.1074/jbc.M511338200 [DOI] [PubMed] [Google Scholar]
- 128.Sampietro J, Dahlberg CL, Cho US, Hinds TR, Kimelman D, Xu W. Crystal structure of a beta-cateninBCL9Tcf4 complex. Mol Cell 2006; 24:293-300; PMID:; http://dx.doi.org/ 10.1016/j.molcel.2006.09.001 [DOI] [PubMed] [Google Scholar]
- 129.Dupre-Crochet S, Figueroa A, Hogan C, Ferber EC, Bialucha CU, Adams J, Richardson EC, Fujita Y. Casein kinase 1 is a novel negative regulator of E-cadherin-based cell-cell contacts. Mol Cell Biol 2007; 27:3804-16; PMID:; http://dx.doi.org/ 10.1128/MCB.01590-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Serres M, Filhol O, Lickert H, Grangeasse C, Chambaz EM, Stappert J, Vincent C, Schmitt D. The disruption of adherens junctions is associated with a decrease of E-cadherin phosphorylation by protein kinase CK2. Exp Cell Res 2000; 257:255-64; PMID:; http://dx.doi.org/ 10.1006/excr.2000.4895 [DOI] [PubMed] [Google Scholar]
- 131.Serres M, Grangeasse C, Haftek M, Durocher Y, Duclos B, Schmitt D. Hyperphosphorylation of beta-catenin on serine-threonine residues and loss of cell-cell contacts induced by calyculin A and okadaic acid in human epidermal cells. Exp Cell Res 1997; 231:163-72; PMID:; http://dx.doi.org/ 10.1006/excr.1996.3443 [DOI] [PubMed] [Google Scholar]
- 132.Rubinfeld B, Albert I, Porfiri E, Fiol C, Munemitsu S, Polakis P. Binding of GSK3beta to the APC-beta-catenin complex and regulation of complex assembly. Science 1996; 272:1023-6; PMID:; http://dx.doi.org/ 10.1126/science.272.5264.1023 [DOI] [PubMed] [Google Scholar]
- 133.Aberle H, Bauer A, Stappert J, Kispert A, Kemler R. beta-catenin is a target for the ubiquitin-proteasome pathway. EMBO J 1997; 16:3797-804; PMID:; http://dx.doi.org/ 10.1093/emboj/16.13.3797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Fang D, Hawke D, Zheng Y, Xia Y, Meisenhelder J, Nika H, Mills GB, Kobayashi R, Hunter T, Lu Z. Phosphorylation of beta-catenin by AKT promotes beta-catenin transcriptional activity. J Biol Chem 2007; 282:11221-9; PMID:; http://dx.doi.org/ 10.1074/jbc.M611871200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Taurin S, Sandbo N, Yau DM, Sethakorn N, Dulin NO. Phosphorylation of beta-catenin by PKA promotes ATP-induced proliferation of vascular smooth muscle cells. Am J Physiol Cell Physiol 2008; 294:C1169-74; PMID:; http://dx.doi.org/ 10.1152/ajpcell.00096.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Bazzoni G, Dejana E. Endothelial cell-to-cell junctions: molecular organization and role in vascular homeostasis. Physiol Rev 2004; 84:869-901; PMID:; http://dx.doi.org/ 10.1152/physrev.00035.2003 [DOI] [PubMed] [Google Scholar]
- 137.Harhaj NS, Antonetti DA. Regulation of tight junctions and loss of barrier function in pathophysiology. Int J Biochem Cell Biol 2004; 36:1206-37; PMID:; http://dx.doi.org/ 10.1016/j.biocel.2003.08.007 [DOI] [PubMed] [Google Scholar]
- 138.Tunggal JA, Helfrich I, Schmitz A, Schwarz H, Gunzel D, Fromm M, Kemler R, Krieg T, Niessen CM. E-cadherin is essential for in vivo epidermal barrier function by regulating tight junctions. EMBO J 2005; 24:1146-56; PMID:; http://dx.doi.org/ 10.1038/sj.emboj.7600605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Gross CM, Aggarwal S, Kumar S, Tian J, Kasa A, Bogatcheva N, Datar SA, Verin AD, Fineman JR, Black SM. Sox18 preserves the pulmonary endothelial barrier under conditions of increased shear stress. J Cell Physiol 2014; 229:1802-16; PMID:; http://dx.doi.org/ 10.1002/jcp.24633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Chen W, Sharma R, Rizzo AN, Siegler JH, Garcia JG, Jacobson JR. Role of claudin-5 in the attenuation of murine acute lung injury by simvastatin. Am J Resp Cell Mol Biol 2014; 50:328-36; PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Gillrie MR, Krishnegowda G, Lee K, Buret AG, Robbins SM, Looareesuwan S, Gowda DC, Ho M. Src-family kinase dependent disruption of endothelial barrier function by Plasmodium falciparum merozoite proteins. Blood 2007; 110:3426-35; PMID:; http://dx.doi.org/ 10.1182/blood-2007-04-084582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Yin Q, Nan H, Yan L, Huang X, Wang W, Cui G, Wei J. Alteration of tight junctions in pulmonary microvascular endothelial cells in bleomycin-treated rats. Exp Toxicol Pathol 2012; 64:81-91; PMID:; http://dx.doi.org/ 10.1016/j.etp.2010.06.010 [DOI] [PubMed] [Google Scholar]
- 143.Chattopadhyay R, Dyukova E, Singh NK, Ohba M, Mobley JA, Rao GN. Vascular endothelial tight junctions and barrier function are disrupted by 15(S)-hydroxyeicosatetraenoic acid partly via protein kinase C epsilon-mediated zona occludens-1 phosphorylation at threonine 770772. J Biol Chem 2014; 289:3148-63; PMID:; http://dx.doi.org/ 10.1074/jbc.M113.528190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Collins NT, Cummins PM, Colgan OC, Ferguson G, Birney YA, Murphy RP, Meade G, Cahill PA. Cyclic strain-mediated regulation of vascular endothelial occludin and ZO-1: influence on intercellular tight junction assembly and function. Arterioscler Thromb Vasc Biol 2006; 26:62-8; PMID:; http://dx.doi.org/ 10.1161/01.ATV.0000194097.92824.b3 [DOI] [PubMed] [Google Scholar]
- 145.Kundumani-Sridharan V, Dyukova E, Hansen DE, 3rd, Rao GN. 1215-Lipoxygenase mediates high-fat diet-induced endothelial tight junction disruption and monocyte transmigration: a new role for 15(S)-hydroxyeicosatetraenoic acid in endothelial cell dysfunction. J Biol Chem 2013; 288:15830-42; PMID:; http://dx.doi.org/ 10.1074/jbc.M113.453290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.O'Donnell JJ, 3rd, Birukova AA, Beyer EC, Birukov KG. Gap junction protein connexin43 exacerbates lung vascular permeability. PloS One 2014; 9:e100931; PMID:; http://dx.doi.org/ 10.1371/journal.pone.0100931 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Parthasarathi K, Ichimura H, Monma E, Lindert J, Quadri S, Issekutz A, Bhattacharya J. Connexin 43 mediates spread of Ca2+-dependent proinflammatory responses in lung capillaries. J Clin Invest 2006; 116:2193-200; PMID:; http://dx.doi.org/ 10.1172/JCI26605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Baker SM, Kim N, Gumpert AM, Segretain D, Falk MM. Acute internalization of gap junctions in vascular endothelial cells in response to inflammatory mediator-induced G-protein coupled receptor activation. FEBS Lett 2008; 582:4039-46; PMID:; http://dx.doi.org/ 10.1016/j.febslet.2008.10.043 [DOI] [PMC free article] [PubMed] [Google Scholar]


