Abstract
As previous studies suggested that IL-9 may control intestinal barrier function, we tested the role of IL-9 in experimental T cell-mediated colitis induced by the hapten reagent 2,4,6-trinitrobenzenesulfonic acid (TNBS). The deficiency of IL-9 suppressed TNBS-induced colitis and led to lower numbers of PU.1 expressing T cells in the lamia propria, suggesting a regulatory role for Th9 cells in the experimental TNBS colitis model. Since IL-9 is known to functionally alter intestinal barrier function in colonic inflammation, we assessed the expression of tight junction molecules in intestinal epithelial cells of TNBS-inflamed mice. Therefore we made real-time PCR analyses for tight junction molecules in the inflamed colon from wild-type and IL-9 KO mice, immunofluorescent stainings and investigated the expression of junctional proteins directly in intestinal epithelial cells of TNBS-inflamed mice by Western blot studies. The results demonstrated that sealing proteins like occludin were up regulated in the colon of inflamed IL-9 KO mice. In contrast, the tight junction protein Claudin1 showed lower expression levels when IL-9 is absent. Surprisingly, the pore-forming molecule Claudin2 revealed equal expression in TNBS-treated wild-type and IL-9-deficient animals. These results illustrate the pleiotropic functions of IL-9 in changing intestinal permeability in experimental colitis. Thus, modulation of IL-9 function emerges as a new approach for regulating barrier function in intestinal inflammation.
Keywords: IL-9, TNBS colitis, Th9 cells, intestinal epithelial cells, tight junctions, claudins
Abbreviations: CD, Crohn´s disease; IBD, Inflammatory bowel disease; IL-9, Interleukin-9; KO, knockout; Th9, T-helper cell type 9; TNBS, 2,4,6-trinitrobenzenesulfonic acid; RT-PCR, reverse transcription-polymerase chain reaction; UC, ulcerative colitis; WT, wild-type
Introduction
Inflammatory bowel disease (IBD) is a disorder of the gastrointestinal tract that is caused by an uncontrolled inflammatory immune reaction. IBD consists of 2 subtypes, ulcerative colitis (UC) and Crohn´s disease (CD). The symptoms, mucosal inflammation and ulcerations in both forms of IBD, are driven by activated immune cells particularly T lymphocytes producing large amounts of cytokines and inducing tissue damage in IBD patients.1 Whereas CD4+ T cells in Crohn's disease express the T helper cell 1 (TH1)-associated key regulator T-bet and produce IFN-γ, T cells in ulcerative colitis are known to produce TH2 associated cytokines such as IL-5 and IL-13.2 Recent studies identified a new subset of T helper cells named Th9 cells, which develop from naïve T cells under the influence of IL-4 together with TGFβ and are characterized by the production of Interleukin-9.3 The IL-9 gene transcription is mainly controlled by PU.1, an ETS family transcription factor that is induced by TGFβ stimulation in T cells. Consistently, histone modifications associated with the Th9 phenotype were found to be dependent on PU.1 strongly suggesting that this transcription factor controls Th9 T cell differentiation.4 The cytokine IL-9 is pleiotropic and has many biological effects on a number of distinct cell types. Beyond the first description as a T-cell and mast cell growth factor IL-9 affects lymphocytes, mast cells and epithelial cells that may all contribute to the development of colitis.5
An essential function of the intestinal epithelium is to maintain tissue integrity while regulating gut permeability. This depends on the coordinate expression and interaction of proteins complexes like tight junction molecules. The tight junction follows from the apical to the basal side of the cell and is the most effective guard to contribute to the maintaining selective permeability. Proteins of the tight junction are claudins, occludins and cytoplasmic cytoskeletal linker proteins; whereas occludins and the family of claudins make up the transmembrane proteins.6 Occludin is a 65 kDa protein containing 4 transmembrane domains and is thought to play a functional and structural role in defining the paracellular barrier. The claudins are a gene family with molecular weights ranging from 20 to 26 kDa. So far, 24 members of the protein family have been identified. Each family protein member exerts a different function in determining tissue-specific variations depending on the cell type and the host organism.7 The dynamic nature of tight junctions is well demonstrated by the ability of cytokines to modulate the expression of tight junctions proteins, regulate the assembly and change the epithelial permeability.8 In chronic colitis, this barrier gets compromised and harmful substances can enter from the intestinal lumen and initiate disease. Alterations in the tight junction structure and function have been seen in both human IBD patients and models of intestinal inflammation.9,10 Recently, it has been shown that IL-9 regulates barrier function through the regulation of Claudin2 in an experimental model of ulcerative colitis. In contrast, expression levels of occludin and Claudin3 were rather unaffected.11
Here, we have studied the expression level of regulatory tight junction molecules in an experimental model of Th1 T cell-mediated TNBS-colitis. We found that in contrast to oxazolone colitis the expression of Claudin2 is not influenced by IL-9 in this model; whereas we noted changes in Claudin1 and occludin expression. Thus, in different inflammatory conditions there may be multiple IL-9-dependent regulatory inputs affecting intestinal permeability via the regulation of tight junction molecules.
Results
Deficiency of IL-9 leads to protection in the TNBS colitis model
TNBS colitis is a model that mimicks some aspects of Crohn´s disease in humans. In particular, it is associated with a Th1 related immune response. To investigate whether IL-9, which mainly is produced by Th9 cells, also plays a role in this disease by regulation of the barrier function, we started to subject wild-type and IL-9 deficient animals to TNBS-induced colitis model. The manifestation of the TNBS related inflammation in the colon was controlled by mini-endoscopic analysis. It was found that IL-9-deficient mice were almost completely protected from TNBS colitis (Fig. 1A). Scoring of colitis activity during high resolution mini-endoscopy demonstrated a significantly lower activity of mucosal inflammation in IL-9 KO mice. Additionally, IL-9 knockout mice lost significantly less body weight than wild-type mice upon administration of TNBS, suggesting that IL-9 deficiency protects from the development of acute colitis. Consistently, in vivo bioluminescence imaging of reactive oxygen species revealed more pronounced inflammation in wild-type mice compared to IL-9-deficient animals. Finally, the histopathological staining confirmed significant suppression of TNBS-induced colitis in IL-9 KO mice as compared to wild-type mice. Marked signs of inflammation such as goblet cell depletion, ulcers, and accumulation of mononuclear cells were noted in WT mice, whereas IL-9 KO mice showed little or no evidence of colitis (Fig. 1A). Collectively, these findings underline the important effect of IL-9 for the development of murine colitis induced by TNBS. As these data suggested that IL-9 plays a role in TNBS-induced colitis, we next determined the importance of the Th9 related transcription factor PU.1. Therefore immunofluorescence staining for PU.1 was performed from cryosections of colonic tissue from wild-type and IL-9 deficient TNBS treated animals. It was found that the number of PU.1 expressing cells was significantly increased in the mucosa of TNBS inflamed wild-type species as compared to IL-9 deficient mice. To exclude the possibility that this increase was mainly due to B cells expressing PU.1, double staining analysis for PU.1 and T cells was performed. These studies revealed a significant up regulation of the number of PU.1/CD4+-expressing cells in the colonic tissue of wild-type mice (Fig. 1B). Taken together, these findings suggested the presence of PU.1 expressing Th9 cells and a regulatory role of IL-9 in TNBS colitis model.
Figure 1.
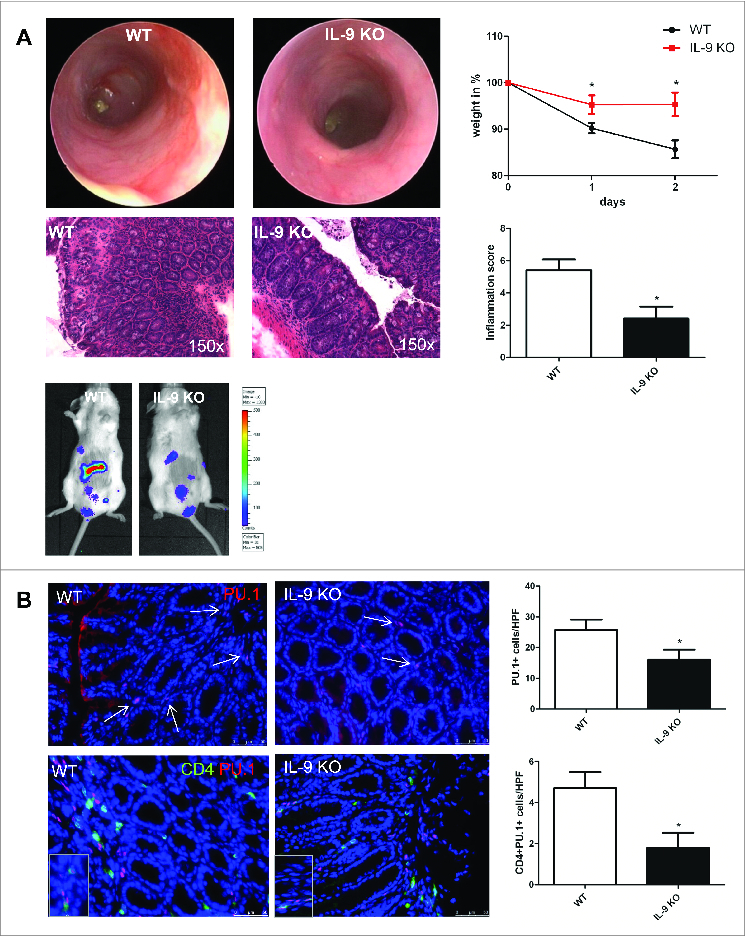
Regulatory role of Interleukin-9 in TNBS-mediated colitis model. Wild-type and IL-9-deficient animals were subjected to the TNBS colitis model (A). The manifestation of colitis was monitored using mini-endoscopy and endoscopic scoring of colitis activity. Endoscopic score was calculated regarding 5 parameters of inflammation (fibrin, translucent, granularity, vascularity and stool). Analysis of the body weight was done at indicated time points and significant differences are marked. IVIS analysis with Luminol detected the free radical production during inflammation in WT and IL-9 KO mice. Finally, histopathologic sections revealed the assessment of colitis in both groups. Data represent results of 3 independent experiments. Cryosections from TNBS-treated WT and IL-9 KO mice were stained with a specific anti PU.1 antibody. Cell nuclei were counterstained with HOECHST dye (B). Representative stainings and statistical analysis are shown. In addition, double staining with anti CD4 (green) and anti PU.1 (red) was performed and significant changes are indicated. For this analysis 4 different animals per group were used.
IL-9 regulates barrier function in a model of TNBS colitis
To elucidate potential mechanisms for the protection against TNBS colitis provided by IL-9 deficiency, we assessed the expression of molecules, which are important for intestinal barrier function in subsequent studies. Initially, we analyzed mRNA levels of specific tight junction molecules. Total mRNA was isolated from inflamed colonic tissue from WT and IL-9 KO animals and as a control from the colon of untreated WT and IL-9 KO animals and cDNA synthesis was done. Quantitative RT-PCR revealed that levels of the sealing protein Claudin1 in the TNBS treated colon were significantly lower in the absence of IL-9. Contrarily, the mRNA expression of the tight junction protein occludin was significantly upregulated in the colon of IL-9 KO mice compared to wild-type mice, suggesting that protection of IL-9 KO mice from TNBS colitis is associated with selective differences in expression of barrier proteins. In contrast to those tight junction molecules the relative expression of Claudin2 was unchanged in both species. Additionally, Claudin4, Claudin7 and JAM-A were significantly up regulated during TNBS inflammation in IL-9 KO mice in contrast to WT mice. The mRNA levels of all tight junction molecules showed no differences in the colon of WT and IL-9 KO mice, pointing out the relevance of IL-9 on the regulation of intestinal barrier permeability during TNBS-mediated inflammation (Fig. 2A). Thus, IL-9-deficient mice were relatively protected from barrier alterations during experimental colitis. Furthermore protein levels from TNBS treated WT and IL-9 KO mice were investigated. For this, we isolated epithelial cells from wild-type and IL-9 deficient animals and checked for protein expression via Western Blot. It turned out, that protein levels were consistent with the mRNA levels of tight junction molecules. Protein levels of Claudin1 were reduced in epithelial cells from TNBS treated IL-9 KO mice in contrast to wild-type animals. Consistently with the mRNA levels above occludin protein expression was higher expressed in TNBS-treated IL-9 deficient mice. Protein levels of the pore-forming molecule Claudin2, however, were equal in both strains (Fig. 2B). These findings implicate a role for IL-9 in the control of genes that affect barrier function in primary intestinal epithelial cells. As these findings were consistent with the idea that IL-9 deficiency influences tight junction molecules we further explored the expression of these proteins directly in the colon of TNBS treated wild-type and IL-9 KO mice. Claudin1 is mainly expressed as a network structure between the individual crypts of the colon. Immunofluorescence staining for Claudin1 clearly showed stronger expression in the colon of IL-9 deficient animals than in wild-type mice (Fig. 3A), suggesting that the diminished expression of the sealing factor Claudin1 in the colon of IL-9 KO mice is important for the protection in the TNBS colitis model. Additionally, Claudin2 showed a rather network-like structure between the crypts. The expression of the pore forming Claudin2, however, was not different in both TNBS inflamed species (Fig. 3B). Furthermore, the staining of claudin4 illustrated a closed protein structure in IL-9-deficient mice with TNBS-induced inflammation, whereas this network-like structure is interrupted and destroyed in the colon of WT TNBS-inflamed mice (Fig. 3C). Additionally, tight junction proteins like Claudin7 and JAM-A yielded a stronger fluorescent signal when IL-9 was absent (Fig. 3D+E). The findings of the immunofluorescence stainings showed the localization and structure of the tight junction molecules in the colon and were consistent with the results obtained from RT PCR analysis and Western Blot quantification.
Figure 2.
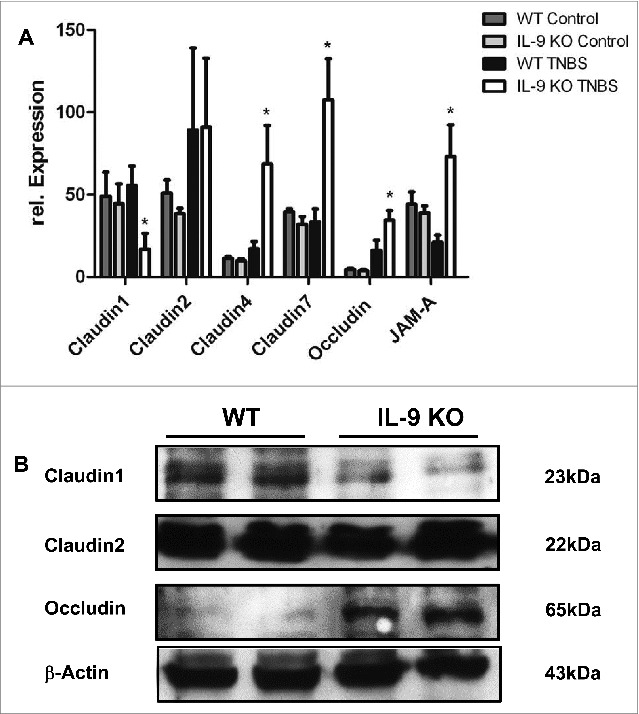
TNBS-induced inflammation led to different expression levels of Claudin1, Claudin4, Claudin7, JAM-A and occludin in WT and IL-9 KO mice. Analysis of tight junction proteins was done in the TNBS-inflamed colon from wild-type and IL-9 deficient animals (A). Total mRNA was isolated from the TNBS inflamed colon and from control WT and IL-9 KO mice and RT PCR quantification was done. Relative expression of Claudin1, Claudin2, Claudin4, Claudin7, JAM-A and occludin was normalized to the house-keeping gene 18sRNA and significant differences were indicated. For statistics 3–9 animals per group were included. Untreated WT and IL-9 KO showed no difference in the expression of all tight junction proteins. Up regulation of Claudin1 mRNA was observed in TNBS-treated WT animals, in contrast to IL- 9 KO mice, whereas Claudin2 levels were unchanged. Claudin4, Claudin7 and JAM-A mRNA expression was significantly down regulated in inflamed WT mice. Occludin mRNA was downregulated in TNBS-inflamed WT mice in comparison to IL-9 deficient mice. Western Blot quantification of tight junction proteins was done in protein extract from isolated epithelial cells from TNBS treated WT and IL-9 KO animals (B). Each lane contained proteins from 0.25 x 106 epithelial cells. Protein bands of β-actin served as loading control. Claudin1 showed more protein in epithelial cells from TNBS inflamed WT mice, contrarily, occludin levels were lower. Protein levels of Claudin2 were unchanged in both groups. Western Blot analysis was done with 4 mice per group. A representative set of 2 animals per group are shown.
Figure 5.
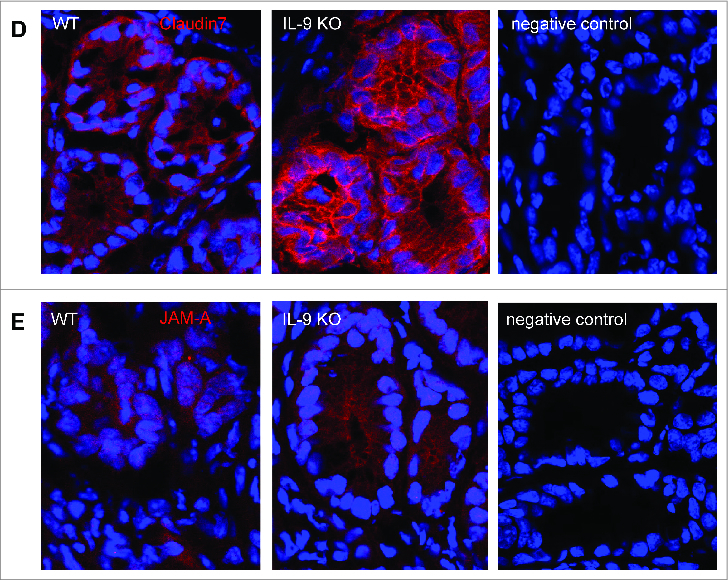
Different protein expression of Claudin1, Claudin2, Claudin4, Claudin7 and JAM-A in the colon of TNBS-treated WT and IL-9 KO mice. Analysis of tight junction proteins was done in TNBS-treated wild-type and IL-9 KO mice by immunofluorescence staining of cryosections with specific antibodies against Claudin1 (A). Cells were counterstained with HOECHST dye. Representative stainings as well as negative control from 4 mice per group are shown. Immunofluorescence microscopy of cryosections of colon tissues from TNBS-treated wild-type and IL-9-deficient mice, as well as negative control samples, with staining of the tight junction proteins Claudin2 (B), Claudin4 (C), Claudin7 (D) or JAM-A (E) were shown. Cell nuclei were stained with HOECHST dye. One representative staining per group and negative controls are shown.
Figure 3.

(Continued)
Discussion
In the present study, we could show that PU.1 expressing T cells were present in murine experimental TNBS colitis. Studies using IL-9 knockout mice revealed a crucial role of IL-9 during inflammation. Specifically, in the lamia propria of inflamed wild-type animals higher numbers of PU.1-expressing T cells were found, suggesting that Th9 cells are important regulators for the development of TNBS colitis. As we have shown previously that Th9 cells prevent colitis in an experimental model of ulcerative colitis,11 we could additionally demonstrate that Th9 cells are involved in an experimental model that mimicks some aspect of human Crohn´s disease. The finding that IL-9 deficiency led to reduced colitis activity in the model of TNBS colitis underlines the broad relevance of IL-9 in T cell-dependent intestinal inflammation, as this model is associated, opposed to the model of ulcerative colitis, with mucosal TH1 T cell responses.12 This observation illustrates the broad diversity of IL-9 in driving T cell activation in experimental colitis models.
In order to find an explanation for the protective role of IL-9 in this experimental colitis model tight junction molecules have been investigated via RT PCR analysis and Western Blot, as those junctional proteins showed reduced expression or changes in the distribution during gut inflammation. In IBD the expression of primary sealing and integral membrane components of the tight junction is not yet well characterized. At present, occludin and the family of claudin proteins are well defined junctional molecules.13 Here, we have found that IL-9 deficiency led to a significant decrease of Claudin1 in intestinal epithelial cells of TNBS-treated IL-9 KO mice on mRNA levels and protein level, respectively. Kirschner et al. have shown before that the knockdown of Claudin1 results in increased permeability for sodium chloride ions, but it does not impair the barrier to water.14 The altered barrier function in the gastrointestinal tract during inflammation leads to loss of solutes and results in diarrhea.9 Consequently, Claudin1 is responsible for an enhanced efflux of ions in the gut leading to the disease-associated diarrhea. Additionally, erosions and ulcers in Crohn´s disease result from enhanced antigen uptake in the gut lumen and subsequent local inflammation. The accessibility for antigens is also regulated by Claudin1 as the junctional protein demonstrates a permeability function for ions between 4 to 40 kDa.14 Also Claudin1 is a transmembrane tight junction protein, its role in inflammation is unclear, and it may have additional functions that have to be defined, yet. Surprisingly, the pore-forming protein Claudin2 revealed the same expression levels in isolated intestinal epithelial cells in both TNBS-treated strains. The junctional protein Claudin2 is known to alter barrier function15 and has been previously identified as a target of IL-13 in patients with ulcerative colitis.16 As IL-13 is a key cytokine for patients suffering from ulcerative colitis this seems not to be the case for the experimental model of TNBS colitis. The equal levels of Claudin2 leads to the hypothesis that protein expression is not influenced by IL-9 alone, but in concert with other cytokines or signal transducers. Moreover, Claudin2 does not only act as a pore-forming protein, but also as trans-cellular ion transporter for Ca2+ or Mg2+, suggesting that Claudin2 has a different function in this experimental colitis model.17 A differential expression pattern with varied functions of Claudin2 in colonic inflammation opens up a new perspective in the pathogenesis of this disease, which warrants further investigated.
Occludin appears to interact, directly or indirectly, with Claudins and is accommodated in between the long strands of Claudin1 and Claudin2. The assembly of tight junctions by various proteins is important for the development of intestinal barrier function. In our study, we could show that occludin protein levels are lower in the epithelial cells associated with TNBS inflammation. The sealing protein was found to be suppressed in the inflamed mucosa of IBD patients. Therefore, it has been hypothesized that defects in the intestinal permeability might represent a general phenomenon in the gastrointestinal tract in CD, because intestinal permeability defects have been observed not only in mucosal tissue with evident intestinal alterations but also in areas lacking any macroscopic lesions.18 Our data identify a crucial regulatory role for IL-9 in an experimental model of colitis with some similarities to Crohn´s disease. The presence of PU.1 positive T cells in the inflamed TNBS mucosa of wild-type mice demonstrates that Th9 cells are the inducers of this disease. In this model an altered barrier function has been discovered with increased levels of Claudin1, but a significant loss of the sealing protein Occludin. Furthermore, IL-9 is able to modulate some other molecules involved in the tight junction, especially claudin4, claudin7 and JAM-A. The effect that claudin1 is decreased, whereas occludin is increased in hapten treated IL-9 deficient mice is in the first sight contradictory regarding barrier function. One possible explanation is, that Claudin1 is not contributing to barrier function directly but the loss of barrier function is mainly caused by occludin. The novelty is that IL-9 is able to modulate the barrier function by the regulation of Claudin1, Claudin4, Claudin7, JAM-A and occludin in this experimental colitis model. As observed by Poritz et. al. our findings postulate that there may be a difference in the pathophysiology of the tight junction complex in the two IBD diseases, regarding the results raised in the oxazolone-mediated colitis model.11,19 Thus, IL-9 plays an important role during inflammation by influencing the properties of the intestinal tissue barrier through different tight junction molecules.
Materials and Methods
Animals
BALB/c and IL-9 deficient mice were obtained from Andrew McKenzie and were described as published before.20 Mice used in the experimental TNBS-colitis model were between 7 and 12 weeks of age and were housed under specific pathogen-free conditions. All experiments were performed in accordance with institutional guidelines.
TNBS colitis model
Induction of colitis has been done as previously described.21 For that mice were sensitized by epicutaneous application of 1% TNBS (2,4,6-Trinitrobenzenesulfonic acid) in a 4:1 aceton/oil mixture (100 μl) on day 0 followed by intrarectal administration of 3% TNBS in 50% ethanol (100 μl) 5 d later. Colitis development was monitored with a high-resolution video endoscopic system (Karl Storz GmbH) at indicated time points. Scoring of TNBS-colitis severity was performed at the end of the experiment based on 5 parameters (translucent, granularity, fibrin, vascularity and stool) according to a previously established score system.22
In vivo imaging of mice
The imaging system used was IVIS 100, which consists of a light tight chamber equipped with a cooled CCD camera. The luminescent probe L-012, which reacts with reactive oxygen species, was purchased from Wako Chemical and dissolved in sterile H2O to an end concentration of 20 mmol. 100 μl volume injection of luminol was administered intraperitoneally (i.p.). During in vivo imaging the mice were immobilized using the anesthetic isoflurane (1.5%). Image exposure times were between 1 min and 2 min, depending on the signal strength.
Immunofluorescence of murine colonic tissue
The TNBS-inflamed colon was isolated and cryosections were analyzed for several tight junction molecules. Claudins were stained with a specific anti-Claudin1 (Clone MH25; Invitrogen), anti Claudin2 (Clone MH44; Invitrogen), anti Claudin4 (Clone ZMD.306; Invitrogen) or anti Claudin7 (Clone ZMD.241; Invitrogen) antibody at a dilution of 1:50 or 1:100, respectively, followed by staining with an anti-rabbit antibody labeled with Alexa 555 (ImmunoReagents). Immunofluorescent staining for JAM-A was done with an anti-JAM-A (Clone NS0; R&D Systems) antibody at a dilution of 1:20 and secondary Cy3 labeled antibody (donkey anti goat; dianova).
Analysis of Th9 cells in the inflamed TNBS colon has been prepared with double staining for CD4 and PU.1. Staining of CD4+ cells was done using a rat monoclonal antibody to CD4 at a concentration of 1:200 and incubation for 10 hours at 4°C (BD Biosciences). Afterwards, slides were incubated with a biotinylated anti-rat secondary antibody (BioLegend), treated with streptavidin-horseradish peroxidase, and stained with TSA Cy2 system.23 For intracellular staining of the transcription factor PU.1 an anti-rabbit antibody (Thermo Scientific) was chosen, followed by a biotinylated goat-anti-rabbit antibody with subsequent staining in combination with streptavidin-Alexa555 (Invitrogen) signal amplification.
Western Blot analysis of TNBS inflamed epithelial cells
Intestinal epithelial cells were isolated from TNBS inflamed animals as previously described.24 Cells were counted and protein was extracted using the mammalian protein extraction reagent (Thermo Scientific) containing protease and phosphatase inhibitor tablets (Complete Mini Protease Inhibitor Tablets, Roche). Proteins were separated according to their molecular weight by SDS polyacrylamide gel electrophoresis and subsequently transferred to a nitrocellulose membran (BioRad). Membranes were blocked for one hour with TBS-T and 5% skimmed milk powder (Roth). Detection of Claudin1, Claudin2 and occludin was acquired by using a specific antibody (Invitrogen) in combination with an anti-rabbit HRP antibody (Cell Signaling). Incubating membranes with HRP-linked anti-actin antibody (Santa Cruz Biotechnology) for 1 hour at room temperature served as an internal control. For detection of protein bands, the Pierce Western blotting substrate ECL Plus (Thermo Scientific) was used according to the manufacturer´s recommendations.
mRNA isolation and RT PCR analysis
Total RNA was isolated from inflamed colonic tissue of TNBS-treated mice and control mice with the RNA micro-kit (Machery-Nagel). cDNA was subsequently generated with Affinity Script RT Multi-Temp RT (Agilent). Quantitative real-time PCR was performed with SensiFAST SYBR (Bioline) in combination with specific primers for CLDN1, CLDN2, CLDN4, CLDN7, F11R and Occludin (Qiagen) by use of iQ iCycler instrument (BioRad) with 18sRNA as reference gene for cDNA, the relative expression level of cytokine mRNA was calculated with the following formula: relative cytokine mRNA expression = 2^(Ct mRNA of interest − Ct mRNA 18S RNA).
Statistics
Statistical differences were determined by using the Student´s t-test. P values <0.05 were considered as statistically significant and identified with asterisks [*<0.05]. Results are expressed as mean values. The error bars in histogram figures represent SEM.
Acknowledgments
The authors thank Ludmilla Sologub for excellent technical assistance.
Funding Statement
The research of B.W. and M.F.N. was supported by the Clinical Research Group KFO 257 CEDER of the DFG and UK Medical Research Council, the Wellcome Trust (100963) to A.N.J.M.
Disclosure of Potential Conflicts of Interest
There were no potential conflicts of interest.
References
- 1.Neurath MF, Finotto S, Glimcher LH. The role of Th1/Th2 polarization in mucosal immunity. Nat Med 2002; 8:567-73; PMID:; http://dx.doi.org/ 10.1038/nm0602-567 [DOI] [PubMed] [Google Scholar]
- 2.Fuss IJ, Neurath M, Boirivant M, Klein JS, de la Motte C, Strong SA, Fiocchi C, Strober W. Disparate CD4+ lamina propria (LP) lymphokine secretion profiles in inflammatory bowel disease. Crohn's disease LP cells manifest increased secretion of IFN-gamma, whereas ulcerative colitis LP cells manifest increased secretion of IL-5. J Immunol 1996; 157:1261-70; PMID: [PubMed] [Google Scholar]
- 3.Veldhoen M, Uyttenhove C, van Snick J, Helmby H, Westendorf A, Buer J, Martin B, Wilhelm C, Stockinger B. Transforming growth factor-beta 'reprograms' the differentiation of T helper 2 cells and promotes an interleukin 9-producing subset. Nat Immunol 2008; 9:1341-6; PMID:; http://dx.doi.org/ 10.1038/ni.1659 [DOI] [PubMed] [Google Scholar]
- 4.Chang HC, Sehra S, Goswami R, Yao W, Yu Q, Stritesky GL, Jabeen R, McKinley C, Ahyi AN, Han L. The transcription factor PU.1 is required for the development of IL-9-producing T cells and allergic inflammation. Nat Immunol 2010; 11:527-34; PMID:; http://dx.doi.org/ 10.1038/ni.1867 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Goswami R, Kaplan MH. A brief history of IL-9. J Immunol 2011; 186:3283-8; PMID:; http://dx.doi.org/ 10.4049/jimmunol.1003049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gonzalez-Mariscal L, Betanzos A, Nava P, Jaramillo BE. Tight junction proteins. Prog Biophys Mol Biol 2003; 81:1-44; PMID:; http://dx.doi.org/ 10.1016/S0079-6107(02)00037-8 [DOI] [PubMed] [Google Scholar]
- 7.Furuse M, Hirase T, Itoh M, Nagafuchi A, Yonemura S, Tsukita S. Occludin: a novel integral membrane protein localizing at tight junctions. J Cell Biol 1993; 123:1777-88; PMID:; http://dx.doi.org/ 10.1083/jcb.123.6.1777 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Walsh SV, Hopkins AM, Nusrat A. Modulation of tight junction structure and function by cytokines. Adv Drug Deliv Rev 2000; 41:303-13; PMID:; http://dx.doi.org/ 10.1016/S0169-409X(00)00048-X [DOI] [PubMed] [Google Scholar]
- 9.Gassler N, Rohr C, Schneider A, Kartenbeck J, Bach A, Obermuller N, Otto HF, Autschbach F, et al. . Inflammatory bowel disease is associated with changes of enterocytic junctions. Am J Physiol Gastrointest Liver Physiol 2001; 281:G216-28; PMID: [DOI] [PubMed] [Google Scholar]
- 10.Poritz LS, Garver KI, Green C, Fitzpatrick L, Ruggiero F, Koltun WA. Loss of the tight junction protein ZO-1 in dextran sulfate sodium induced colitis. J Surg Res 2007; 140:12-9; PMID:; http://dx.doi.org/ 10.1016/j.jss.2006.07.050 [DOI] [PubMed] [Google Scholar]
- 11.Gerlach K, Hwang Y, Nikolaev A, Atreya R, Dornhoff H, Steiner S, Lehr HA, Wirtz S, Vieth M, Waisman A, et al. . TH9 cells that express the transcription factor PU.1 drive T cell-mediated colitis via IL-9 receptor signaling in intestinal epithelial cells. Nat Immunol 2014; 15:676-86; PMID:; http://dx.doi.org/ 10.1038/ni.2920 [DOI] [PubMed] [Google Scholar]
- 12.Strober W, Fuss I, Mannon P. The fundamental basis of inflammatory bowel disease. J Clin Invest 2007; 117:514-21; PMID:; http://dx.doi.org/ 10.1172/JCI30587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mitic LL, Van Itallie CM, Anderson JM. Molecular physiology and pathophysiology of tight junctions I. Tight junction structure and function: lessons from mutant animals and proteins. Am J Physiol Gastrointest Liver Physiol 2000; 279:G250-4; PMID: [DOI] [PubMed] [Google Scholar]
- 14.Kirschner N, Rosenthal R, Furuse M, Moll I, Fromm M, Brandner JM. Contribution of tight junction proteins to ion, macromolecule, and water barrier in keratinocytes. J Invest Dermatol 2013; 133:1161-9; PMID:; http://dx.doi.org/ 10.1038/jid.2012.507 [DOI] [PubMed] [Google Scholar]
- 15.Schulzke JD, Ploeger S, Amasheh M, Fromm A, Zeissig S, Troeger H, Richter J, Bojarski C, Schumann M, Fromm M. Epithelial tight junctions in intestinal inflammation. Ann N Y Acad Sci 2009; 1165:294-300; PMID:; http://dx.doi.org/ 10.1111/j.1749-6632.2009.04062.x [DOI] [PubMed] [Google Scholar]
- 16.Heller F, Florian P, Bojarski C, Richter J, Christ M, Hillenbrand B, Mankertz J, Gitter AH, Bürgel N, Fromm M, et al. . Interleukin-13 is the key effector Th2 cytokine in ulcerative colitis that affects epithelial tight junctions, apoptosis, and cell restitution. Gastroenterology 2005; 129:550-64; PMID:; http://dx.doi.org/ 10.1016/j.gastro.2005.05.002 [DOI] [PubMed] [Google Scholar]
- 17.Das P, Goswami P, Das TK, Nag T, Sreenivas V, Ahuja V, Panda SK, Gupta SD, Makharia GK. Comparative tight junction protein expressions in colonic Crohn's disease, ulcerative colitis, and tuberculosis: a new perspective. Virchows Arch 2012; 460:261-70; PMID:; http://dx.doi.org/ 10.1007/s00428-012-1195-1 [DOI] [PubMed] [Google Scholar]
- 18.Peeters M, Ghoos Y, Maes B, Hiele M, Geboes K, Vantrappen G, Rutgeerts P. Increased permeability of macroscopically normal small bowel in Crohn's disease. Dig Dis Sci 1994; 39:2170-6; PMID:; http://dx.doi.org/ 10.1007/BF02090367 [DOI] [PubMed] [Google Scholar]
- 19.Poritz LS, Harris LR, 3rd, Kelly AA, Koltun WA. Increase in the tight junction protein claudin-1 in intestinal inflammation. Dig Dis Sci 2011; 56:2802-9; PMID:; http://dx.doi.org/ 10.1007/s10620-011-1688-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Townsend JM, Fallon GP, Matthews JD, Smith P, Jolin EH, McKenzie NA. IL-9-deficient mice establish fundamental roles for IL-9 in pulmonary mastocytosis and goblet cell hyperplasia but not T cell development. Immunity 2000; 13:573-83; PMID:; http://dx.doi.org/ 10.1016/S1074-7613(00)00056-X [DOI] [PubMed] [Google Scholar]
- 21.Wirtz S, Neufert C, Weigmann B, Neurath MF. Chemically induced mouse models of intestinal inflammation. Nat Protoc 2007; 2:541-6; PMID:; http://dx.doi.org/ 10.1038/nprot.2007.41 [DOI] [PubMed] [Google Scholar]
- 22.Becker C, Fantini MC, Wirtz S, Nikolaev A, Kiesslich R, Lehr HA, Galle PR, Neurath MF. In vivo imaging of colitis and colon cancer development in mice using high resolution chromoendoscopy. Gut 2005; 54:950-4; PMID:; http://dx.doi.org/ 10.1136/gut.2004.061283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gerlach K, Daniel C, Lehr HA, Nikolaev A, Gerlach T, Atreya R, Rose-John S, Neurath MF, Weigmann B. Transcription factor NFATc2 controls the emergence of colon cancer associated with IL-6-dependent colitis. Cancer Res 2012; 72:4340-50; PMID:; http://dx.doi.org/ 10.1158/0008-5472.CAN-11-4155 [DOI] [PubMed] [Google Scholar]
- 24.Weigmann B, Tubbe I, Seidel D, Nicolaev A, Becker C, Neurath MF. Isolation and subsequent analysis of murine lamina propria mononuclear cells from colonic tissue. Nat Protoc 2007; 2:2307-11; PMID:; http://dx.doi.org/ 10.1038/nprot.2007.315 [DOI] [PubMed] [Google Scholar]


