Abstract
Vascular permeability is a vital function of the circulatory system that is regulated in large part by the limited flux of solutes, water, and cells through the endothelial cell layer. One major pathway through this barrier is via the inter-endothelial junction, which is driven by the regulation of cadherin-based adhesions. The endothelium also forms attachments with surrounding proteins and cells via 2 classes of adhesion molecules, the integrins and IgCAMs. Integrins and IgCAMs propagate activation of multiple downstream signals that potentially impact cadherin adhesion. Here we discuss the known contributions of integrin and IgCAM signaling to the regulation of cadherin adhesion stability, endothelial barrier function, and vascular permeability. Emphasis is placed on known and prospective crosstalk signaling mechanisms between integrins, the IgCAMs- ICAM-1 and PECAM-1, and inter-endothelial cadherin adhesions, as potential strategic signaling nodes for multipartite regulation of cadherin adhesion.
Keywords: adhesion, endothelial barrier function, integrins, ICAM-1, permeability, PECAM-1, transendothelial migration, VE-cadherin
Abbreviations: IgCAM, immunoglobulin superfamily cell adhesion molecule; JAM, junctional adhesion molecule; ICAM-1, intercellular adhesion molecule 1; PECAM-1, platelet endothelial cell adhesion molecule 1; VCAM-1, vascular cell adhesion molecule 1; VEGF, vascular endothelial growth factor; LPS, lipopolysaccharide; TNF-α, tumor necrosis factor α; PKC, protein kinase C; VE-PTP, Receptor-type tyrosine-protein phosphatase β; SHP-2, Src homology region 2 domain-containing phosphatase; RDG, arginine-aspartic acid- glutamine; TGF-β, transforming growth factor-β; S1P, sphingosine 1 phosphate; fMLP, f-Met-Leu-Phe; eNOS, endothelial nitric oxide synthase; iNOS, inducible nitric oxide synthase
Introduction
Vascular permeability is an innate function of the circulatory system that regulates the flux of fluid, protein, and immune cells from blood to tissue. In most non-inflamed tissues, vascular permeability is controlled by the “barrier” comprised by the microvascular wall, which includes the endothelial glycocalyx, the endothelium, basement membrane, and any accessory cells (i.e. pericytes or smooth muscle cells) wrapped around the outer surface of the vessel. Though each of these components contributes to the permeability of the vascular wall, most studies have focused on the role of the endothelium, which forms an effective barrier to the movement of protein and cells from blood to tissue.1,2
The endothelium occupies a unique physiological niche, receiving soluble signals from both the blood and tissue, and interacting, directly and indirectly, with cells from both compartments. Direct interactions between the endothelium and its immediate physical environment are mediated by adhesion receptors, which in addition to the cadherins, include the integrins, immunoglobulin-cell adhesion molecules (IgCAMs), junctional adhesion molecules (JAMs), claudins, and occludin. While direct regulation of cadherin adhesion is known to occur downstream of permeability-promoting soluble signals, recent evidence suggests that adhesion events mediated by other adhesion receptors, notably the integrins and IgCAMs, can modify cadherin signaling to effect vascular permeability. In this review, we discuss what is known about regulation of VE-cadherin based inter-endothelial junctions by endothelial adhesion signaling, what affect this has on the modification of vascular permeability in vivo (if known), and identify remaining questions that are critical to understanding the complex crosstalk between endothelial adhesion receptors.
Regulation of Inter-Endothelial Adherens Junctions Under Resting Conditions
The path which solutes, fluid, and cells take through the endothelial barrier is divided into 2 routes. Transcellular permeability occurs via clathrin- and caveaolae-mediated vesicular transport, whereas paracellular permeability occurs via dynamic regulation of inter-endothelial junctions. Regulation of inter-endothelial junctions is controlled at the level of homotypic VE-cadherin adhesion between neighboring cells.3 VE-cadherin expression on the plasma membrane is promoted and stabilized by the expression of cytoplasmic adaptor proteins p120-catenin and β−catenin which bind to the juxtamembrane and C-terminal portion of the VE-cadherin cytoplasmic domain, respectively. β−catenin also mediates the connection between VE-cadherin and the actin cytoskeleton via adaptor proteins such as α−catenin4,5; this connection is absolutely required for junction maintenance. In addition, VE-cadherin adhesion is regulated by actin cytoskeletal dynamics. Confluent endothelial monolayers, as we would expect to find in vivo, exhibit predominantly circumferential actin fibers (also termed cortical actin) and few radial stress fibers. Many stimuli that affect vascular permeability also induce actin cytoskeletal turnover via the activation of RhoA, which is accompanied by loss of cortical actin and increased radial stress fibers (for review, see 6). In unstimulated endothelial cells, vascular permeability is maintained at basal levels by the low level action of Rac1 or Cdc42 GTPases. Under resting conditions, these enzymes stabilize the circumferential actin fibers that support robust VE-cadherin adhesions. Activation of Rac1 or Cdc42 have been noted downstream of S1P7 and cAMP/Rap1 signaling,8,9 both of which are known to stabilize endothelial cell junctions.
Regulation of Inter-Endothelial Adherens Junctions During Inflammation
Much of our knowledge of endothelial junction regulation comes from the study of inflammation. During inflammation the endothelium responds to a complex array of signals and acquires new capacities, i.e. the endothelium becomes “activated.” Endothelial activation is marked by localized leakage of protein-rich fluid and recruitment and activation of circulating leukocytes, accompanied by a breakdown of intercellular junctions and a decrease in barrier function.1,10 These events are also characteristic of the endothelial dysfunction seen in many disease states, including arthritis and atherosclerosis, and are even observed during the abnormal formation of new vessels.11 Studying the regulation of vascular permeability during the inflammatory response has led to many breakthroughs in the field, however it is important to remember that permeability in and of itself is not pathological. Basal permeability is an essential function of the vascular system. Care should be taken to represent inflammation-induced permeability in terms of increased permeability over baseline, or, as some have termed it, as “hyper-permeability.”
Studies of inflammatory hyper-permeability have revealed that VE-cadherin adhesion is primarily down-regulated by mechanisms that induce the disassembly of the cadherin-catenin adhesion complex. Internalization of the VE-cadherin receptor can occur via clathrin- or cavaeolae- mediated pathways, where it can be targeted for either degradation or recycling. VEGF stimulation, for example, promotes the endocytosis of VE-cadherin downstream of active Src, which promotes the activation of p21-activated-kinase (PAK) and subsequent phosphorylation of VE-cadherin on Ser665. Phosphorylation of this residue promotes the recruitment of β−arrestin-2 and clathrin-mediated internalization.12 Short-term stimulation with LPS also stimulates clathrin-mediated internalization, however longer treatment with LPS promotes the association of VE-cadherin with caveolin-1, and siRNA mediated knockdown of caveolin-1 rescues VE-cadherin plasma membrane localization.13
Junction disassembly can also induce phosphorylation of VE-cadherin or its associated adaptor proteins, p120- and β−catenin. Phosphorylation of VE-cadherin at Y658 or Y731, leads to dissociation of p120 and β−catenin respectively.14 Other phosphorylation sites on VE-cadherin that have been reported to affect junction stability include Y733, Y645, and Y685.15 Phosphorylation of p120 at S879 by PKC-α inhibits its association with the VE-cadherin cytoplasmic domain, and increases VE-cadherin internalization.16 β−catenin is phosphorylated on multiple residues; Y654 and Y489 are targeted by Src/RTK17 and Abl,18 respectively, and disrupt binding to VE-cadherin, whereas phosphorylation of Y142 disrupts binding to α−catenin.19 Phosphorylation of cadherin complex proteins is itself downregulated by the presence of junctional phosphatases, including VE-PTP,20 SHP-2,21 and DEP1,22 thus increased expression or activation of these phosphatases can also regulate endothelial permeability.
Integrin signaling in endothelial cells
Integrins form a well-characterized class of adhesion molecules that bind to components of the extracellular matrix (ECM) and IgCAMs. Integrins are expressed as a heterodimer of α and β subunits, which form a calcium-dependent interface required for ligand binding. Integrin adhesion can be induced in a bidirectional manner, e.g., by activating the integrin molecule through intracellular signal transduction, or by ligand binding to the extracellular domains. Subsequent signaling can promote further integrin activation, in addition to many other signaling events. In epithelial monolayers, the occurrence of crosstalk signaling between cadherin and integrin-based adhesions is well established, where assembly of adherens junctions limits integrin expression to the basal surface in epithelial monolayers.23,24 Integrins can be observed within epithelial cell-cell contacts, but integrin activation is restricted in close proximity to the adherens junction due to local down-regulation of integrin expression25 and depletion of phosphatidylinositol-4,5-bisphosphate (PtdIns(4,5,)P2,PIP2).26 While endothelial cell-cell contacts lack the strict segregation of epithelial layers, the same cadherin-adhesion dependent crosstalk mechanisms are assumed to occur in the endothelium.
Quiescent endothelial cells express a wide array of integrins, including modest levels of α1β1, α2β1, α5β1, and α6β1, as well as low levels of α3β1, α6β4, and αvβ5.27 During angiogenesis, these cells highly up-regulate α5β1 and αvβ3, which have been considered targets for anti-angiogenic therapy. In endothelial cells, integrins are required for cell proliferation, migration, and sprouting angiogenesis, among other functions.27-29
Regulation of the Endothelial Barrier and Vascular Permeability by Integrin Adhesion
β3 integrins have been known to indirectly regulate VE-cadherin adhesion and endothelial permeability via increasing VEGF signaling.30,31 However, β3 knockout mice exhibit increased endothelial leak (Miles assay) in response to LPS or VEGF, when compared to wildtype animals.32,33 Curtis, et al34 demonstrated that blocking β1 and β3 adhesion using a soluble peptide containing the β1/β3 integrin-binding motif RGD also weakened the transendothelial resistance of unstimulated cells. Application of the RGD peptide also blocked Rac activity and decreased the appearance of cadherin complexes.34 These data suggest that integrin adhesion could promote endothelial barrier function, perhaps by promoting the stability of circumferential actin.
On the other hand, studies performed using either function blocking antibodies to αvβ5, or β5 knockout animals, have shown a decrease in vascular permeability induced by VEGF, TGFβ, thrombin or LPS in vitro, or by mechanical ventilation in vivo.35 β5 knockout mice also showed increased survival in a mouse model of sepsis.36 In addition, an interesting study by Alghisi, et al interrogated the effect of the αvβ3 antagonist cilengitide on endothelial leak. Cilengitide blocked αvβ3 adhesion to matrix, but unlike studies performed in β3 knockouts, induced a redistribution of the integrin to cell edges. There, αvβ3 remained active, and induced phosphorylation of VE-cadherin on Y658 and Y731, causing a loss of VE-cadherin from cell contacts and an increase in monolayer leak.37 Thus activation of β5 or αvβ3 integrins appears to down-regulate endothelial barrier function, whereas activation of β1 integrins promotes endothelial barrier function.
Additional clues can be gathered from the effects of extracellular matrix proteins on vascular permeability. In vivo, one could expect that changes in extracellular matrix expression and availability as ligand could change during vascular injury or in response to an inflammatory stimulus. Indeed, concentrations of fibronectin, a large ECM protein both expressed by the endothelium and a major plasma protein, are increased following vascular injury and during vascular development.38-42 Though fibronectin is a ligand for α5β1 and αvβ3, addition of fibronectin to endothelial cell culture blocked TNF−α-induced endothelial leak.34 As TNF−α inhibits β1 integrin activation, this effect is likely due to an increase in β1 integrin activity. Similarly, porcine coronary venules treated with an RGD containing peptide that inhibits fibronectin-integrin binding displayed a 2-3-fold increase in permeability to albumin.43 In contrast, treatment of cultured endothelial cells with purified vitronectin or an integrin binding fragment of fibrinogen, both ligands for αvβ3, increased VE-cadherin internalization via activation of αvβ3.43 In support, vitronectin knockout animals exhibit a blunted increase in permeability in response to ischemic injury,44 however full-length fibrinogen, which binds to the endothelium via ICAM-1 and α5β1, also induces endothelial leak.45 Thus the outcome of endothelial integrin-based adhesion is likely determined by the specific integrins and integrin-ligands expressed in the vascular environment.
Integrin-Adhesion Dependent Regulation of Cadherin Adhesion
Though endothelial integrin adhesion can clearly modulate VE-cadherin based adhesions, the mechanism or mechanisms by which this occurs remain obscure. Multiple signaling pathways are activated downstream of integrin ligation, including many also known to regulate vascular permeability downstream of inflammatory stimuli. These signaling mechanisms can be grouped into 2 large categories, those that regulate cadherin phosphorylation and junction disassembly, and those that mediate changes in the interaction of cadherin-based adhesions with the actin cytoskeleton. While these broad categories are imperfect, we will use these as a framework to briefly discuss what is known about the signals downstream of integrins that regulate vascular permeability.
Regulation of junction disassembly by integrin adhesion
Integrin adhesion activates a number of kinases, including Src,46 focal adhesion kinase (FAK),47 and integrin-linked kinase (ILK).48 Src family kinases phosphorylate residues on VE-cadherin and β−catenin that are important for the cadherin/β−catenin interaction. Though the direct evidence is limited, activation of Src downstream of αvβ3 activation has been shown to induce phosphorylation of VE-cadherin at Y658 and Y731.49 Integrin adhesion also induces activation of the Src family kinases Fer50 and Fyn,51 which can mediate phosphorylation of Y654 and Y142 of β−catenin. However, in the absence of direct evidence, it is unclear whether Fer or Fyn activation downstream of integrins significantly regulates inter-endothelial junction stability.
Integrin-mediated Src activation also promotes activation of FAK, which traditionally promotes the turnover of integrin-based adhesions. FAK contains a kinase, and multiple protein-protein interaction domains, which are considered key to the temporal regulation of Rho and Rac GTPase. FAK is involved in TGFβ−induced vascular permeability along with its upstream regulator, Src.52 An extensive study by Jean, et al found that in tumor-associated endothelial cells, human lung endothelial cells, and human umbilical vein endothelial cells, FAK inhibition decreases VE-cadherin Y658 phosphorylation. FAK can also be activated downstream of growth factor receptors. FAK activation is required for VEGF-dependent phosphorylation of VE-cadherin Y658 and recent studies have demonstrated that FAK can directly phosphorylate this residue (Fig. 1). Pulmonary endothelial cells isolated from FAK kinase-dead knock-in mice showed decreased monolayer leak and decreased Y658 phosphorylation.53
Figure 1.
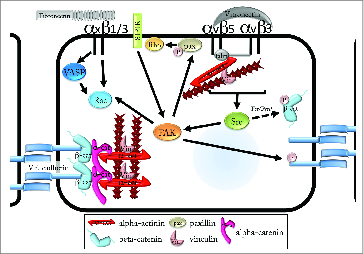
Intracellular regulation of VE-cadherin by integrins. Activation of αvβ5 or αvβ3 causes activation of c-Src (Src) and subsequent activation of focal adhesion kinase (FAK). FAK phosphorylates tyrosine 658 on VE-cadherin, prompting loss of cadherin adhesion. FAK also phosphorylates paxillin (pax), which leads to the activation of Rho GTPase and the formation of radial stress fibers. Alternatively, the Src-family kinases Fer and Fyn phosphorylate tyrosines 654 and 142 on β−catenin. While Fer and Fyn can be activated downstream of integrin ligation, no evidence yet exists for integrin-mediated phosphorylation of β−catenin. Activation of β1 and β3 integrins conversely promotes cadherin adhesion. Activation of FAK downstream of sphingosine-1-phosphate recptor-1 (S1P1R) as well as integrin-dependent activation of vasodilator-stimulated phosphoprotein (VASP) triggers activation of Rac1 GTPase. Rac then promotes the stability of cortical actin and of cadherin adhesions.
On the other hand, numerous studies have linked FAK activation to barrier-promoting effects. Embryonic endothelial cells derived from FAK knockout mice exhibit increased permeability compared with wild type.54 Loss of FAK expression in pulmonary artery endothelial cells, results in extended barrier disruption after thrombin stimulation.55 In addition, tyrosine phosphorylation of FAK in pulmonary artery endothelial cells is associated with barrier enhancement,56-58 and occurs downstream of the barrier-promoting activity of S1P59 (Fig. 1). Parsing the barrier-promoting and -disrupting effects of FAK activation is a significant challenge, not the least because of the diversity of downstream signals modulated by FAK. It is extremely likely that integrin-mediated activation of FAK does play a role in regulation of the vascular barrier, however lack of direct evidence, and the absence of models in which the relative contribution of integrin-activated FAK can be measured, limits our ability to define its precise role.
Regulation of endothelial actin cytoskeletal turnover by integrin adhesion
Changes in actin turnover are a well-characterized outcome of integrin adhesion signaling. Integrin adhesion can activate Arp2/3 to promote actin polymerization and branching. Integrin signaling also activates multiple members of the Rho GTPase family, including RhoA, Rac1 and 2, and Cdc42. As previously discussed, Cdc42 and Rac activation are important for formation of circumferential actin bundles that are associated with stable cell-cell contacts. Heightened Rac activity can also block RhoA activation, which is associated with increased actin-myosin contractility and is generally believed to negatively regulate endothelial cell-cell contact and promote vascular permeability. However, RhoA-mediated activation of the formin mDia inhibits VEGF-stimulated permeability by sequestering Src and preventing VE-cadherin internalization,60 suggesting that there may be conditions in which RhoA activation may not exert negative regulatory pressure on endothelial permeability.
In addition, many proteins that regulate actin filament dynamics and are important for endothelial barrier regulation are also components of the integrin cytoplasmic adhesion complex. For example, phosphorylation of paxillin via an Src-FAK-ERK mechanism stimulates increased permeability in HPAEC and is associated with activation of RhoA.61 Vasodilator stimulated phosphoprotein (VASP) is classically associated with actin filament polymerizaton downstream of integrin activaton. VASP also mediates cAMP-mediated Rac1 activation during cell-cell contact formation.62 VASP null endothelial cells exhibit impaired β1 integrin dependent adhesion and VE-cadherin adhesion, though cadherin expression and localization appears normal. VASP deficiency also correlates with reduced actomyosin contractility and Rac dependent formation of cortical actin, which likely contributes to the loss of integrin and cadherin mediated adhesion.8
α−actinin is an actin crosslinking protein involved in the maturation of integrin-based adhesions. α−actinin can also link the VE-cadherin complex to the actin cytoskeleton by simultaneously binding α−catenin and actin and promoting cortical actin rearrangement.63 Vinculin is a scaffolding protein that interacts with multiple proteins in the integrin cytoplasmic adhesion complex.64 Vinculin is associated with mature focal adhesions where it stabilizes integrin-based adhesions and permits the transmission of actin-generated mechanical forces across the cell membrane.65 Vinculin is also recruited to cadherin-based adhesions by α−catenin where it reinforces the connection of the actin cytoskeleton with VE-cadherin66 (Fig. 1). The association of α−actinin and vinculin with both integrin- and cadherin-based adhesions suggests the possibility that there could be a competition-based crosstalk between the 2 types of adhesion receptors. This would depend on α−actinin and vinculin concentrations, as well as the number and stability of integrin and cadherin adhesions. Both α−actinin and vinculin are highly abundant, as are integrins and cadherins, in most cell types. However, local control of protein concentrations could be sufficient to exert negative regulatory pressure due to competition for these cytoplasmic actin-binding proteins. This hypothesis has yet to be explored to any significant extent, and parsing the individual and communal roles of α−actinin and vinculin in integrin and cadherin signaling will require future studies.
Endothelial IgCAM-Mediated Regulation of Endothelial Cell-Cell Contact and Vascular Permeability
Endothelial immunoglobulin superfamily cell adhesion molecules (IgCAMs) are key regulators of leukocyte-endothelial interaction. In the vascular endothelium, intercellular adhesion molecule -1 (ICAM-1) is expressed constitutively at low levels and acts as a ligand for β2 integrins expressed on circulating leukocytes. ICAM-1 ligation by leukocyte integrins stimulates the firm adhesion required for extravasation. VCAM-1 is induced by cytokine stimulation of the endothelium, and is a ligand for α4β1 and α4β7 integrins expressed on leukocytes. VCAM-1 expression is important for leukocyte adhesion and rolling,67-70 and to some extent leukocyte extravasation,71-73 but its involvement in the regulation of vascular permeability is unclear. PECAM-1 is highly expressed by endothelial cells, as well as on the surface of platelets, monocytes, neutrophils, and some types of T-cells. PECAM-1 expression is concentrated at endothelial cell-cell contacts, where it mediates leukocyte diapedesis via homotypic PECAM-1 interactions with the transmigrating cell.74 Similar to PECAM-1, junctional adhesion molecules (JAMs) are also found at endothelial cell-cell contacts, and mediate both endothelial-endothelial and endothelial-leukocyte interactions (reviewed in 75). A thorough review of IgCAM adhesion signaling in inflammation was published earlier this year,76 so our treatment of this topic will be brief.
ICAM-1
Activation of ICAM-1 signaling leads to increased leukocyte transmigration, increased vascular permeability and loss of the endothelial barrier, and rearrangement of the actin cytoskeleton. ICAM-1 activation also stimulates the downregulation of endothelial junction proteins VE-cadherin, occludin, and the tight junction adaptor proteins ZO-1 and ZO-2.77 Most studies have examined the effect of ICAM-1 mediated signaling following the activation of inflammatory signaling pathways. However, recent evidence suggests that ICAM-1 signaling can be activated by ligation in the absence of inflammatory stimulus. Sumagin, et al78 demonstrated that rolling leukocytes or antibody-mediated ICAM-1 crosslinking could stimulate an ICAM-1 dependent increase in vascular permeability in unstimulated mouse cremaster arterioles. This is supported by other work showing that over-expression ICAM-1 in unstimulated endothelial cells decreases endothelial barrier function.79 Crosslinking ICAM-1 with ICAM-1 coated beads also promotes endothelial leak, as well as recruiting VCAM to sites of ICAM-1 clustering.80 Treatment of cells with TNF−α or VEGF81 leads to an up-regulation of ICAM-1 expression after as little as 4 hours of treatment. Increased ICAM-1 expression is subsequently necessary for endothelial permeability and leukocyte transmigration. TNF−α stimulation has also been shown to induce a redistribution of ICAM-1 in vivo, where the heterogeneous distribution of ICAM-1 regulates the location of leukocyte adhesion.82
Functionally, ICAM-1 activity appears to require both the extracellular and intracellular domains. Blocking antibodies directed against the extracellular domain of ICAM-1 reduce leukocyte adhesions and vascular permeability in both mouse pial and cremaster vessels.83,84 Studies employing a truncation mutant of ICAM-1 lacking the cytoplasmic domain have demonstrated that this domain is required for ICAM-1-mediated leukocyte transmigration, but not leukocyte-endothelial adhesion.85,86 The studies by Greenwood, et al and Sumagin, et al utilized a cell permeable cytoplasmic domain construct that inhibits endogenous ICAM-1 activity. As in the truncation experiments, treatment of brain microvascular cells or mouse cremaster venules with penetratin-ICAM-1 peptide strongly inhibited transendothelial migration but did not affect leukocyte adhesion.85,87 Furthermore, treatment with this inhibitory peptide decreased the number of gaps in VE-cadherin staining observed in venules in response to fMLP.87 However, in human dermal microvascular cells, the absence of the cytoplasmic domain was not able to inhibit ICAM-1-induced loss of endothelial barrier function.79 Based on the relatively small number of studies, and the concept that transendotheilal migration is accompanied by an increase in solute translocation across the endothelium, it is not appropriate at present to conclude that cytoplasmic ICAM-1 signaling is uninvolved in the regulation of vascular permeability. However, just how the relatively short ICAM-1 cytoplasmic domain would mediate such regulation is not abundantly clear.
The ICAM-1 cytoplasmic domain has been reported to bind to several actin binding proteins, including α−actinin,88 cortactin,89 and ezrin.90 Recent evidence suggests that binding to some of these partners is independent,88 indicating that several distinct pools of ICAM-1 receptor may be present in the cell. Ligation of ICAM-1 transduces a signal that causes rearrangement of the actin cytoskeleton, likely via increasing cytoplasmic calcium levels,91 though the exact mechanism remains unclear. ICAM-1 ligation has been shown to activate Rac1, RhoA, PKC, Src family kinases, and the docking protein p130Cas.92 Inhibition of RhoA in ICAM-1 over-expressing cells mimics the effects of truncating the cytoplasmic domain on leukocyte transmigration and adhesion,86 suggesting that RhoA signaling is a major regulator of ICAM-1-mediated leukocyte-endothelial interactions. Src activation downstream of ICAM-1 ligation induces phosphorylation of caveolin-1, which is required for transcellular vascular permeability following neutrophil adhesion.93 Rac1 activation downstream of ICAM-1 mediates the activation of NADPH oxidase (Nox) and the production of reactive oxygen species (ROS). The rise in ROS levels promotes activation of the kinases Src and Pyk2, which can phosphorylate VE-cadherin on Y658 and Y731.94 Interestingly, crosslinking VCAM-1 also stimulates calcium signaling and activation of Nox.95 Analogous to ICAM-1 signaling, Vockel, et al demonstrated that VCAM-1-mediated ROS production also leads to activation of Pyk296 (Fig. 2). They show that this pathway stimulates the dissociation of VE-cadherin and VE-PTP to disrupt endothelial cell contacts, leaving open the question of whether VCAM-driven Pyk2 also stimulates the phosphorylation of VE-cadherin.
Figure 2.
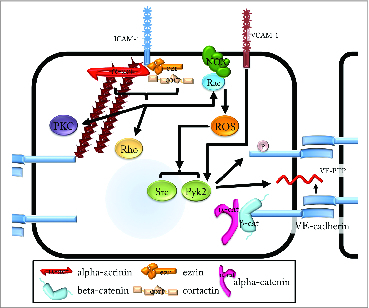
Intercellular regulation of VE-cadherin by ICAM-1 and VCAM-1. Ligation of ICAM-1 recruits the actin binding proteins α-actinin (α−act), ezrin (ezr), and cortactin (cort). ICAM-1 activity leads to activation of Rac, which is a regulatory component of NADPH oxidases (NOX). Nox activation following binding of GTP-bound Rac stimulates the formation of reactive oxygen species (ROS). ROS can subsequently activate Src kinase and protein tyrosine kinase 2β (Pyk2). Src and Pyk2 then phosphorylate Y658 on VE-cadherin and stimulate junction disassembly. In addition, ICAM-1 activates Rho GTPase, leading to stress fiber formation, as well as PKCs (PKC), which mediate ICAM-1 dependent increased vascular permeability in the absence of an inflammatory stimulus. Ligation of VCAM-1 also activates Pyk2 to promote the loss of interaction between VE-PTP and VE-cadherin, increasing phosphorylation of junction proteins and stimulating junction disassembly.
PECAM-1
PECAM-1 expression, on the other hand, blocks neutrophil migration in response to TNF−α.97 PECAM-1 expression is increased by barrier promoting substances like statins,97 and reduced by barrier disrupting agents like TNF−α and ionizing radiation.98 Knockdown of PECAM in endothelial cells in culture increases monolayer leak.99 Blocking PECAM-1 using a function-blocking antibody decreases neutrophil-endothelial interactions and decreases permeability in multiple disease models.100,101 PECAM-1 knockout mice demonstrate pulmonary fibrosis preceded by enhanced vascular leakiness and deposition of hemosiderin in the lung.102 PECAM knockouts also exhibit increased survival when challenged with endotoxin,103 and an earlier incidence of experimental autoimmune encephalitis.104 In addition, endothelial cells isolated from PECAM deficient animals demonstrate increased leak in response to LPS103 and histamine.104 While the intracellular pathways regulated by PECAM-1 are not completely defined, PECAM-1 mediated interactions can lead to activation of PLC–γ,105 Akt and upregulation of eNOS via STAT3,106 or increased Gαq/11 signaling.107 One interesting signaling mechanism involves the Immunoreceptor Tyrosine-based Inhibitory Motif, or ITIM domain, in the PECAM-1 cytoplasmic tail. Phosphorylation of tyrosines 663 and 686 in the ITIM domain promotes binding of the tyrosine phosphatase SHP-2.108 PECAM-1 can also bind to β−catenin. However, in contrast to SHP-2 binding, β−catenin tyrosine phosphorylation -rather than PECAM phosphorylation- was required for this association. This suggests that PECAM, β−catenin and SHP-2 form a ternary complex, which brings tyrosine-phosphorylated β−catenin in close proximity to a phosphatase. In fact, expression of an ITIM-defective PECAM-1 mutant severely increases β−catenin tyrosine phosphorylation, supporting the idea that PECAM-1 coordinates the dephosphorylation of β−catenin, and perhaps the subsequent stabilization of β−catenin/VE-cadherin association, via its interaction with SHP-2.
Regulation of Vascular Permeability by Cadherin Adhesion
Finally, we would like to quickly pay attention to a rather overlooked facet of cadherin signaling that may also play a significant role in the regulation of vascular permeability. Dissociation of p120 and β−catenin from VE-cadherin can stimulate feedback mechanisms to both promote and disrupt cadherin adhesion. As our understanding of endothelial junctions moves past the concept of junctions as static structural elements to one that encompasses the highly dynamic nature of the cadherin adhesion, it becomes clear that p120 and β−catenin likely function to modulate cadherin adhesion when both bound to- and dissociated from- the receptor.
p120 regulates local RhoA activation by recruiting the RhoA specific GTPase activating protein, p190RhoGAP, to the cadherin cytoplasmic complex.109 However, in fibroblasts, cytoplasmic p120 can bind directly to RhoA and inhibit its intrinsic ability to dissociate guanosine diphosphate,110 similar to the action of RhoGDI. In endothelial cells, overexpression of p120 blocks neutrophil transmigration.111 However, this study from the Luscinskas laboratory determined that this was not due to the binding of p120 and RhoA, but rather to the ability of cytoplasmic p120 to inhibit the association of active Src with VE-cadherin. Others have shown that in Chinese hamster ovary cells, p120 can bind to Vav2, an exchange factor for Rac1, Cdc42, and RhoA, and mediate Rac1 activation.112 These data suggest that conditions that increase cytoplasmic p120 levels would limit RhoA activation and perhaps impair actin cytoskeletal reorganization. However, loss of p120 from the cadherin adhesion complex can also lead to the nuclear localization of p120.113 How p120 is translocated into the nucleus is unknown. What is known is that once there, association of p120 with the transcription factor Kaiso leads to increased expression of Rho in corneal endothelial cells.114 In bovine pulmonary artery endothelial cells and human brain endothelial cells, Kaiso and p120 co-immunoprecipitate. Knockdown of p120 increased the expression of a Kaiso reporter construct 2-fold but also significantly reduced Kaiso protein levels.115 The study by O’Donnell et al in brain microvascular and pulmonary artery endothelial cells supports the idea that p120 depletion increases the transcriptional activity of Kaiso but also suggests that loss of p120 activates NFκB and AP-1116. Further studies are needed to fully understand the role of nuclear p120 signaling in the endothelium. However, in epithelial cancers, loss of cadherin function is associated with an increase in nuclear Kaiso and a worse prognosis. This suggests the possibility that loss of VE-cadherin mediated adhesion could trigger changes in endothelial cell morphology or gene expression due to an increase in the cytoplasmic or nuclear localization of p120, respectively. More information is needed about the levels of p120 required to gain effective Rho inhibition, how those levels relate to VE-cadherin protein expression, and what genes are regulated by nuclear p120, before a realistic model of p120 regulation of vascular permeability, incorporating all cellular pools of p120 catenin, can be formed. Feel free to speculate wildly.
The binary signaling properties of β−catenin, on the other hand, have been well established, if not well mapped, in endothelial cells. In addition to its role as a cytoplasmic adapter for cadherin adhesion, β−catenin is the key second messenger in canonical Wnt (Wingless and INT-1) signaling. In this pathway, Wnt binding to the cell surface receptor Frizzled (Fzd) and co-receptor LRP5/6 causes intracellular association of disheveled (Dvl) with Fzd. Activation of Dvl causes inactivation of the constitutive β−catenin destruction complex (axin/adenomatous polyposis coli (APC)/glycogen synthase kinase 3β (GSK3β)) that normally targets cytoplasmic β−catenin for proteosomal degradation.117,118 β−catenin then is free to translocate into the nucleus, where it interacts with the TCF/LEF family of transcription factors (reviewed in118). Wnt signaling in microvascular endothelial cells increases the proportion of cytoplasmic β−catenin, TCF/LEF transcriptional activity, proliferation and tube formation.119,120 Expression of Wnt-1 in human umbilical vein endothelial cells stimulates proliferation and survival and promotes the expression of IL-8.121 In resting adult vasculature, β−catenin is rarely seen in the cytoplasm or nucleus.122 However, β−catenin is targeted to the nucleus by inflammatory stimuli,113,123 suggesting nuclear β−catenin could play a role in the loss of the endothelial barrier and vascular permeability.
Disassembly of adherens junctions, such as after endothelial exposure to activated neutrophils, is accompanied by the release of β−catenin.14,124 Some inflammatory mediators, such as PGE2125 and thrombin,113 can inhibit GSK3β, thus blocking cytoplasmic β−catenin degradation. Others, such as VEGF, can increase nuclear β−catenin activity by stimulating nitrosylation of β−catenin.126 Nitrosylation of β−catenin by VEGF-induced eNOS, or β−catenin nitration by macrophage derived NO,127 leads to the shuttling of β−catenin into the nucleus. There, it can activate transcription by binding to the TCF/LEF family of transcriptional repressors. Interestingly β−catenin nitration appears to also promote the interaction of nuclear β−catenin with NFκB p65.127 However, β−catenin nuclear localization following loss of VE-cadherin adhesion does not necessarily require inactivation of the degradation complex or additional post-translational modification of β−catenin. Taddei, et al showed that VE-cadherin null cells exhibit higher levels of nuclear β−catenin.128 Our lab has shown that loss of the junctional scaffolding protein KRIT1 leads to loss of VE-cadherin adhesion and increased nuclear β−catenin in vitro, and increased vascular permeability in vivo.129,130 Thus modification of inter-endothelial cell contacts is capable of switching the predominant mechanism of β−catenin signaling from junctional to nuclear.
β−catenin dependent transcriptional activity encompasses the up- and down-regulation of a wide variety of genes, in a cell context-dependent manner. Taddei et al showed that β−catenin, in concert with FOXO1, suppressed the expression of the tight junction protein claudin 1128. In KRIT1 deficient endothelial cells, nuclear β−catenin promotes the expression of cyclin dD1, fibronectin, and VEGF-A130. Other β−catenin target genes (e.g., c-myc, cyclinD1, cox-2, IL-8) have been linked to inflammation.121,131,132 The β−catenin dependent transcriptome in endothelial cells is likely to vary significantly from that in epithelial cells, as well as between in vitro and in vivo conditions. However, there appears to be sufficient evidence to consider nuclear β−catenin signaling when examining the regulation of vascular permeability, particularly under pathological or stimulated conditions.
Concluding Remarks
Endothelial cells in vivo mediate their attachment to their environment through a panoply of adhesion receptors. Some of these are active only during inflammation, whereas others function in both stimulated and unstimulated conditions. Even in vitro, endothelial cells plated on tissue culture plastic are exposed to extracellular matrix molecules found in calf serum, and secrete an extensive matrix of their own within 24 hrs of plating (Dr. Glading, unpublished observation). Cell-cell contacts of cultured endothelial cells contain plentiful PECAM-1, and resting cells express low levels of ICAM-1. Thus the components necessary for “other” adhesion receptors to modify the responses measured in the laboratory are present and accounted for. The absence of an extensive body of literature describing in detail how other adhesion molecules regulate inter-endothelial cell contacts, endothelial barrier function, and vascular permeability rather underscores the inattention this area has received. When one considers how to approach this problem, the presence of common signaling nodes in inflammatory signaling pathways, integrin signaling, and IgCAM signaling spark a certain automatic focus. Activation of Src family kinases, alterations to the connection between cadherins and the actin cytoskeleton, and cytoskeletal regulation by RhoA and Rac1 appear to be key regulatory checkpoints in the regulation of endothelial cell-cell contact and vascular permeability. Fully understanding how each of these fundamental signaling nodes is regulated in vitro, whether in the context of adhesion receptor signaling, or during inflammation, may be the necessary basis needed to address the complexities of an in vivo system. Issues that should be considered when approaching these questions include variation in signaling due to endothelial cell origin, which should not be underestimated. Rather than being treated as unwanted sources of error, these variations likely provide vital clues to the physiological differences between vascular beds. In addition, cells may use different mechanisms to regulate basal permeability vs. inflammation-mediated hyper-permeability, thus the unstimulated condition should not be overlooked.
All cellular functions operate in the context of expression of a specific subset of genes. Stimulation of endothelial cells or intact vessels clearly triggers significant changes in the cellular transcriptome. While the nuclear functions of p120- and β−catenin have been known for quite some time, the impact of these binary signaling molecules on the endothelial barrier and vascular permeability has been artificially restricted to their function at cytoskeletal adaptors. Recent evidence suggests that nuclear functions of p120- and β−catenin could underlie a novel regulatory mechanism for vascular permeability.130 Recent technological advances should make it possible to investigate nuclear p120- and β−catenin contributions to vascular permeability on a large scale. Nevertheless, the complexity of signaling mechanisms regulating the endothelial barrier and vascular permeability appear to be expanding exponentially, meaning that more and more sophisticated techniques, perhaps merging in vivo physiological measurements with computational biology, may be needed to assess this emerging regulatory system.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- 1.Pober JS, Sessa WC. Evolving functions of endothelial cells in inflammation. Nat Rev Immunol 2007; 7:803-15; PMID:; http://dx.doi.org/ 10.1038/nri2171 [DOI] [PubMed] [Google Scholar]
- 2.Kumar P, Shen Q, Pivetti CD, Lee ES, Wu MH, Yuan SY. Molecular mechanisms of endothelial hyperpermeability: implications in inflammation. Expert Rev Mol Med 2009; 11:e19; PMID:; http://dx.doi.org/ 10.1017/S1462399409001112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dejana E, Tournier-Lasserve E, Weinstein BM. The control of vascular integrity by endothelial cell junctions: molecular basis and pathological implications. Dev Cell 2009; 16:209-21; PMID:; http://dx.doi.org/ 10.1016/j.devcel.2009.01.004 [DOI] [PubMed] [Google Scholar]
- 4.Pokutta S, Choi HJ, Ahlsen G, Hansen SD, Weis WI. Structural and thermodynamic characterization of cadherin.beta-catenin.alpha-catenin complex formation. J Biol Chem 2014; 289:13589-601; PMID:; http://dx.doi.org/ 10.1074/jbc.M114.554709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Desai R, Sarpal R, Ishiyama N, Pellikka M, Ikura M, Tepass U. Monomeric alpha-catenin links cadherin to the actin cytoskeleton. Nat Cell Biol 2013; 15:261-73; PMID:; http://dx.doi.org/ 10.1038/ncb2685 [DOI] [PubMed] [Google Scholar]
- 6.Heemskerk N, van Rijssel J, van Buul JD. Rho-GTPase signaling in leukocyte extravasation: An endothelial point of view. Cell Adh Migr 2014; 8:67-75; PMID:; http://dx.doi.org/ 10.4161/cam.28244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang L, Dudek SM. Regulation of vascular permeability by sphingosine 1-phosphate. Microvasc Res 2009; 77:39-45; PMID:; http://dx.doi.org/ 10.1016/j.mvr.2008.09.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schlegel N, Waschke J. VASP is involved in cAMP-mediated Rac 1 activation in microvascular endothelial cells. Am J Physiol Cell Physiol 2009; 296:C453-62; PMID:; http://dx.doi.org/ 10.1152/ajpcell.00360.2008 [DOI] [PubMed] [Google Scholar]
- 9.Spindler V, Peter D, Harms GS, Asan E, Waschke J. Ultrastructural analysis reveals cAMP-dependent enhancement of microvascular endothelial barrier functions via Rac1-mediated reorganization of intercellular junctions. Am J Pathol 2011; 178:2424-36; PMID:; http://dx.doi.org/ 10.1016/j.ajpath.2011.01.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Alom-Ruiz SP, Anilkumar N, Shah AM. Reactive oxygen species and endothelial activation. Antioxid Redox Signal 2008; 10:1089-100; PMID:; http://dx.doi.org/ 10.1089/ars.2007.2007 [DOI] [PubMed] [Google Scholar]
- 11.Kim YW, West XZ, Byzova TV. Inflammation and oxidative stress in angiogenesis and vascular disease. J Mol Med (Berl) 2013; 91:323-8; PMID:; http://dx.doi.org/ 10.1007/s00109-013-1007-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gavard J, Gutkind J. VEGF controls endothelial-cell permeability by promoting the beta-arrestin-dependent endocytosis of VE-cadherin. Nat Cell Biol 2006; 8:1223-34; PMID:; http://dx.doi.org/ 10.1038/ncb1486 [DOI] [PubMed] [Google Scholar]
- 13.Zhang Y, Zhang L, Li Y, Sun S, Tan H. Different contributions of clathrin- and caveolae-mediated endocytosis of vascular endothelial cadherin to lipopolysaccharide-induced vascular hyperpermeability. PLoS One 2014; 9:e106328; PMID:; http://dx.doi.org/ 10.1371/journal.pone.0106328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Potter MD, Barbero S, Cheresh DA. Tyrosine phosphorylation of VE-cadherin prevents binding of p120- and beta-catenin and maintains the cellular mesenchymal state. J Biol Chem 2005; 280:31906-12; PMID:; http://dx.doi.org/ 10.1074/jbc.M505568200 [DOI] [PubMed] [Google Scholar]
- 15.Sidibe A, Polena H, Pernet-Gallay K, Razanajatovo J, Mannic T, Chaumontel N, Bama S, Maréchal I, Huber P, Gulino-Debrac D, et al. VE-cadherin Y685F knock-in mouse is sensitive to vascular permeability in recurrent angiogenic organs. Am J Physiol Heart Circ Physiol 2014; 307:H455-63; PMID:; http://dx.doi.org/ 10.1152/ajpheart.00774.2013 [DOI] [PubMed] [Google Scholar]
- 16.Vandenbroucke St Amant E, Tauseef M, Vogel SM, Gao XP, Mehta D, Komarova YA, Malik AB. PKCalpha activation of p120-catenin serine 879 phospho-switch disassembles VE-cadherin junctions and disrupts vascular integrity. Circ Res 2012; 111:739-49; PMID:; http://dx.doi.org/ 10.1161/CIRCRESAHA.112.269654 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Roura S, Miravet S, Piedra J, Garcia de Herreros A, Dunach M. Regulation of E-cadherinCatenin association by tyrosine phosphorylation. J Biol Chem 1999; 274:36734-40; PMID:; http://dx.doi.org/ 10.1074/jbc.274.51.36734 [DOI] [PubMed] [Google Scholar]
- 18.Rhee J, Mahfooz NS, Arregui C, Lilien J, Balsamo J, VanBerkum MF. Activation of the repulsive receptor Roundabout inhibits N-cadherin-mediated cell adhesion. Nat Cell Biol 2002; 4:798-805; PMID:; http://dx.doi.org/ 10.1038/ncb858 [DOI] [PubMed] [Google Scholar]
- 19.Piedra J, Miravet S, Castano J, Palmer HG, Heisterkamp N, Garcia de Herreros A, Duñach M. p120 Catenin-associated Fer and Fyn tyrosine kinases regulate beta-catenin Tyr-142 phosphorylation and beta-catenin-alpha-catenin Interaction. Mol Cell Biol 2003; 23:2287-97; PMID:; http://dx.doi.org/ 10.1128/MCB.23.7.2287-2297.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nawroth R, Poell G, Ranft A, Kloep S, Samulowitz U, Fachinger G, Golding M, Shima DT, Deutsch U, Vestweber D. VE-PTP and VE-cadherin ectodomains interact to facilitate regulation of phosphorylation and cell contacts. Embo J 2002; 21:4885-95; PMID:; http://dx.doi.org/ 10.1093/emboj/cdf497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wessel F, Winderlich M, Holm M, Frye M, Rivera-Galdos R, Vockel M, Linnepe R, Ipe U, Stadtmann A, Zarbock A, et al. Leukocyte extravasation and vascular permeability are each controlled in vivo by different tyrosine residues of VE-cadherin. Nat Immunol 2014; 15:223-30; PMID:; http://dx.doi.org/ 10.1038/ni.2824 [DOI] [PubMed] [Google Scholar]
- 22.Holsinger LJ, Ward K, Duffield B, Zachwieja J, Jallal B. The transmembrane receptor protein tyrosine phosphatase DEP1 interacts with p120(ctn). Oncogene 2002; 21:7067-76; PMID:; http://dx.doi.org/ 10.1038/sj.onc.1205858 [DOI] [PubMed] [Google Scholar]
- 23.Nejsum LN, Nelson WJ. A molecular mechanism directly linking E-cadherin adhesion to initiation of epithelial cell surface polarity. J Cell Biol 2007; 178:323-35; PMID:; http://dx.doi.org/ 10.1083/jcb.200705094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shaw RM, Fay AJ, Puthenveedu MA, von Zastrow M, Jan YN, Jan LY. Microtubule plus-end-tracking proteins target gap junctions directly from the cell interior to adherens junctions. Cell 2007; 128:547-60; PMID:; http://dx.doi.org/ 10.1016/j.cell.2006.12.037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fujito T, Ikeda W, Kakunaga S, Minami Y, Kajita M, Sakamoto Y, Monden M, Takai Y. Inhibition of cell movement and proliferation by cell-cell contact-induced interaction of Necl-5 with nectin-3. J Cell Biol 2005; 171:165-73; PMID:; http://dx.doi.org/ 10.1083/jcb.200501090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sakamoto Y, Ogita H, Komura H, Takai Y. Involvement of nectin in inactivation of integrin alpha(v)beta(3) after the establishment of cell-cell adhesion. J Biol Chem 2008; 283:496-505; PMID:; http://dx.doi.org/ 10.1074/jbc.M704195200 [DOI] [PubMed] [Google Scholar]
- 27.Stupack D, Cheresh D. ECM remodeling regulates angiogenesis: endothelial integrins look for new ligands. Sci STKE 2002; 2002:PE7; PMID: [DOI] [PubMed] [Google Scholar]
- 28.Weis SM, Cheresh DA. alphaV integrins in angiogenesis and cancer. Cold Spring Harb Perspect Med 2011; 1:a006478; PMID:; http://dx.doi.org/ 10.1101/cshperspect.a006478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rüegg C, Mariotti A. Vascular integrins: pleiotropic adhesion and signaling molecules in vascular homeostasis and angiogenesis. Cell Mol Life Sci 2003; 60:1135-57; PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mahabeleshwar GH, Feng W, Reddy K, Plow EF, Byzova TV. Mechanisms of integrin-vascular endothelial growth factor receptor cross-activation in angiogenesis. Circ Res 2007; 101:570-80; PMID:; http://dx.doi.org/ 10.1161/CIRCRESAHA.107.155655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zaric J, Ruegg C. Integrin-mediated adhesion and soluble ligand binding stabilize COX-2 protein levels in endothelial cells by inducing expression and preventing degradation. J Biol Chem 2005; 280:1077-85; PMID:; http://dx.doi.org/ 10.1074/jbc.M410006200 [DOI] [PubMed] [Google Scholar]
- 32.Su G, Atakilit A, Li JT, Wu N, Bhattacharya M, Zhu J, Shieh JE, Li E, Chen R, Sun S, et al. Absence of integrin alphavbeta3 enhances vascular leak in mice by inhibiting endothelial cortical actin formation. Am J Respir Crit Care Med 2012; 185:58-66; PMID:; http://dx.doi.org/ 10.1164/rccm.201108-1381OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Robinson SD, Reynolds LE, Wyder L, Hicklin DJ, Hodivala-Dilke KM. Beta3-integrin regulates vascular endothelial growth factor-A-dependent permeability. Arterioscler Thromb Vasc Biol 2004; 24:2108-14; PMID:; http://dx.doi.org/ 10.1161/01.ATV.0000143857.27408.de [DOI] [PubMed] [Google Scholar]
- 34.Curtis TM, McKeown-Longo PJ, Vincent PA, Homan SM, Wheatley EM, Saba TM. Fibronectin attenuates increased endothelial monolayer permeability after RGD peptide, anti-alpha 5 beta 1, or TNF-alpha exposure. Am J Physiol 1995; 269:L248-60; PMID: [DOI] [PubMed] [Google Scholar]
- 35.Su G, Hodnett M, Wu N, Atakilit A, Kosinski C, Godzich M, Huang XZ, Kim JK, Frank JA, Matthay MA, et al. Integrin alphavbeta5 regulates lung vascular permeability and pulmonary endothelial barrier function. Am J Respir Cell Mol Biol 2007; 36:377-86; PMID:; http://dx.doi.org/ 10.1165/rcmb.2006-0238OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Su G, Atakilit A, Li JT, Wu N, Luong J, Chen R, Bhattacharya M, Sheppard D. Effective treatment of mouse sepsis with an inhibitory antibody targeting integrin alphavbeta5. Crit Care Med 2013; 41:546-53; PMID:; http://dx.doi.org/ 10.1097/CCM.0b013e3182711b1e [DOI] [PubMed] [Google Scholar]
- 37.Alghisi GC, Ponsonnet L, Ruegg C. The integrin antagonist cilengitide activates alphaVbeta3, disrupts VE-cadherin localization at cell junctions and enhances permeability in endothelial cells. PLoS One 2009; 4:e4449; PMID:; http://dx.doi.org/ 10.1371/journal.pone.0004449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Milner R, Hung S, Erokwu B, Dore-Duffy P, LaManna JC, Del Zoppo GJ. Increased expression of fibronectin and the alpha 5 beta 1 integrin in angiogenic cerebral blood vessels of mice subject to hypobaric hypoxia. Mol Cell Neurosci 2008; 38:43-52; PMID:; http://dx.doi.org/ 10.1016/j.mcn.2008.01.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ment LR, Stewart WB, Scaramuzzino D, Madri JA. An in vitro three-dimensional coculture model of cerebral microvascular angiogenesis and differentiation. In Vitro Cell Dev Biol Anim 1997; 33:684-91; PMID:; http://dx.doi.org/ 10.1007/s11626-997-0126-y [DOI] [PubMed] [Google Scholar]
- 40.Gnanaguru G, Brunken WJ. The cell-matrix interface: a possible target for treating retinal vascular related pathologies. J Ophthalmic Vis Res 2012; 7:316-27; PMID: [PMC free article] [PubMed] [Google Scholar]
- 41.Mustafa DAN, Burgers PC, Dekker LJ, Charif H, Titulaer MK, Smitt PAES, Luider TM, Kros JM. Identification of glioma neovascularization-related proteins by using MALDI-FTMS and nano-LC fractionation to microdissected tumor vessels. Mol Cell Proteomics 2007; 6:1147-57; PMID:; http://dx.doi.org/ 10.1074/mcp.M600295-MCP200 [DOI] [PubMed] [Google Scholar]
- 42.Chiu C-H, Chou C-W, Takada S, Liu Y-W. Development and fibronectin signaling requirements of the zebrafish interrenal vessel. PLoS One 2012; 7:e43040; PMID:; http://dx.doi.org/ 10.1371/journal.pone.0043040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wu MH, Ustinova E, Granger HJ. Integrin binding to fibronectin and vitronectin maintains the barrier function of isolated porcine coronary venules. J Physiol 2001; 532:785-91; PMID:; http://dx.doi.org/ 10.1111/j.1469-7793.2001.0785e.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Li R, Ren M, Chen N, Luo M, Zhang Z, Wu J. Vitronectin increases vascular permeability by promoting VE-cadherin internalization at cell junctions. PLoS One 2012; 7:e37195; PMID:; http://dx.doi.org/ 10.1371/journal.pone.0037195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tyagi N, Roberts AM, Dean WL, Tyagi SC, Lominadze D. Fibrinogen induces endothelial cell permeability. Mol Cell Biochem 2008; 307:13-22; PMID:; http://dx.doi.org/ 10.1007/s11010-007-9579-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hanks SK, Polte TR. Signaling through focal adhesion kinase. Bioessays 1997; 19:137-45; PMID:; http://dx.doi.org/ 10.1002/bies.950190208 [DOI] [PubMed] [Google Scholar]
- 47.Infusino GA, Jacobson JR. Endothelial FAK as a therapeutic target in disease. Microvasc Res 2012; 83:89-96; PMID:; http://dx.doi.org/ 10.1016/j.mvr.2011.09.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ghatak S, Morgner J, Wickstrom SA. ILK: a pseudokinase with a unique function in the integrin-actin linkage. Biochem Soc Trans 2013; 41:995-1001; PMID:; http://dx.doi.org/ 10.1042/BST20130062 [DOI] [PubMed] [Google Scholar]
- 49.Orsenigo F, Giampietro C, Ferrari A, Corada M, Galaup A, Sigismund S, Ristagno G, Maddaluno L, Koh GY, Franco D, et al. Phosphorylation of VE-cadherin is modulated by haemodynamic forces and contributes to the regulation of vascular permeability in vivo. Nat Commun 2012; 3:1208; PMID:; http://dx.doi.org/ 10.1038/ncomms2199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sangrar W, Gao Y, Scott M, Truesdell P, Greer PA. Fer-mediated cortactin phosphorylation is associated with efficient fibroblast migration and is dependent on reactive oxygen species generation during integrin-mediated cell adhesion. Mol Cell Biol 2007; 27:6140-52; PMID:; http://dx.doi.org/ 10.1128/MCB.01744-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yang X, Dutta U, Shaw LM. SHP2 mediates the localized activation of Fyn downstream of the alpha6beta4 integrin to promote carcinoma invasion. Mol Cell Biol 2010; 30:5306-17; PMID:; http://dx.doi.org/ 10.1128/MCB.00326-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lee YH, Kayyali US, Sousa AM, Rajan T, Lechleider RJ, Day RM. Transforming growth factor-beta1 effects on endothelial monolayer permeability involve focal adhesion kinaseSrc. Am J Respir Cell Mol Biol 2007; 37:485-93; PMID:; http://dx.doi.org/ 10.1165/rcmb.2006-0439OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Jean C, Chen XL, Nam JO, Tancioni I, Uryu S, Lawson C, Ward KK, Walsh CT, Miller NL, Ghassemian M, et al. Inhibition of endothelial FAK activity prevents tumor metastasis by enhancing barrier function. J Cell Biol 2014; 204:247-63; PMID:; http://dx.doi.org/ 10.1083/jcb.201307067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zhang G, Xu S, Qian Y, He P. Sphingosine-1-phosphate prevents permeability increases via activation of endothelial sphingosine-1-phosphate receptor 1 in rat venules. Am J Physiol Heart Circ Physiol 2010; 299:H1494-504; PMID:; http://dx.doi.org/ 10.1152/ajpheart.00462.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Mehta D, Tiruppathi C, Sandoval R, Minshall RD, Holinstat M, Malik AB. Modulatory role of focal adhesion kinase in regulating human pulmonary arterial endothelial barrier function. J Physiol 2002; 539:779-89; PMID:; http://dx.doi.org/ 10.1113/jphysiol.2001.013289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Birukova AA, Chatchavalvanich S, Oskolkova O, Bochkov VN, Birukov KG. Signaling pathways involved in OxPAPC-induced pulmonary endothelial barrier protection. Microvasc Res 2007; 73:173-81; PMID:; http://dx.doi.org/ 10.1016/j.mvr.2006.12.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Birukova AA, Arce FT, Moldobaeva N, Dudek SM, Garcia JG, Lal R, Birukov KG. Endothelial permeability is controlled by spatially defined cytoskeletal mechanics: atomic force microscopy force mapping of pulmonary endothelial monolayer. Nanomedicine 2009; 5:30-41; PMID:; http://dx.doi.org/ 10.1016/j.nano.2008.07.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Birukova AA, Cokic I, Moldobaeva N, Birukov KG. Paxillin is involved in the differential regulation of endothelial barrier by HGF and VEGF. Am J Respir Cell Mol Biol 2009; 40:99-107; PMID:; http://dx.doi.org/ 10.1165/rcmb.2008-0099OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Singleton PA, Dudek SM, Ma SF, Garcia JG. Transactivation of sphingosine 1-phosphate receptors is essential for vascular barrier regulation. Novel role for hyaluronan and CD44 receptor family. J Biol Chem 2006; 281:34381-93; PMID:; http://dx.doi.org/ 10.1074/jbc.M603680200 [DOI] [PubMed] [Google Scholar]
- 60.Gavard J, Patel V, Gutkind JS. Angiopoietin-1 prevents VEGF-induced endothelial permeability by sequestering Src through mDia. Dev Cell 2008; 14:25-36; PMID:; http://dx.doi.org/ 10.1016/j.devcel.2007.10.019 [DOI] [PubMed] [Google Scholar]
- 61.Gawlak G, Tian Y, O’Donnell JJ, 3rd, Tian X, Birukova AA, Birukov KG. Paxillin mediates stretch-induced Rho signaling and endothelial permeability via assembly of paxillin-p4244MAPK-GEF-H1 complex. Faseb J 2014; 28:3249-60; PMID:; http://dx.doi.org/ 10.1096/fj.13-245142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Call GS, Chung JY, Davis JA, Price BD, Primavera TS, Thomson NC, Wagner MV, Hansen MD. Zyxin phosphorylation at serine 142 modulates the zyxin head-tail interaction to alter cell-cell adhesion. Biochem Biophys Res Commun 2011; 404:780-4; PMID:; http://dx.doi.org/ 10.1016/j.bbrc.2010.12.058 [DOI] [PubMed] [Google Scholar]
- 63.Nieset JE, Redfield AR, Jin F, Knudsen KA, Johnson KR, Wheelock MJ. Characterization of the interactions of alpha-catenin with alpha-actinin and beta-cateninplakoglobin. J Cell Sci 1997; 110 (Pt 8):1013-22; PMID: [DOI] [PubMed] [Google Scholar]
- 64.Humphries JD, Wang P, Streuli C, Geiger B, Humphries MJ, Ballestrem C. Vinculin controls focal adhesion formation by direct interactions with talin and actin. J Cell Biol 2007; 179:1043-57; PMID:; http://dx.doi.org/ 10.1083/jcb.200703036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Mierke CT, Kollmannsberger P, Zitterbart DP, Smith J, Fabry B, Goldmann WH. Mechano-coupling and regulation of contractility by the vinculin tail domain. Biophys J 2008; 94:661-70; PMID:; http://dx.doi.org/ 10.1529/biophysj.107.108472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Huveneers S, Oldenburg J, Spanjaard E, van der Krogt G, Grigoriev I, Akhmanova A, Rehmann H, de Rooij J. Vinculin associates with endothelial VE-cadherin junctions to control force-dependent remodeling. J Cell Biol 2012; 196:641-52; PMID:; http://dx.doi.org/ 10.1083/jcb.201108120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Davenpeck KL, Sterbinsky SA, Bochner BS. Rat neutrophils express alpha4 and beta1 integrins and bind to vascular cell adhesion molecule-1 (VCAM-1) and mucosal addressin cell adhesion molecule-1 (MAdCAM-1). Blood 1998; 91:2341-6; PMID: [PubMed] [Google Scholar]
- 68.Barringhaus KG, Phillips JW, Thatte JS, Sanders JM, Czarnik AC, Bennett DK, Ley KF, Sarembock IJ. Alpha4beta1 integrin (VLA-4) blockade attenuates both early and late leukocyte recruitment and neointimal growth following carotid injury in apolipoprotein E (–) mice. J Vasc Res 2004; 41:252-60; PMID:; http://dx.doi.org/ 10.1159/000078646 [DOI] [PubMed] [Google Scholar]
- 69.Huo Y, Hafezi-Moghadam A, Ley K. Role of vascular cell adhesion molecule-1 and fibronectin connecting segment-1 in monocyte rolling and adhesion on early atherosclerotic lesions. Circ Res 2000; 87:153-9; PMID:; http://dx.doi.org/ 10.1161/01.RES.87.2.153 [DOI] [PubMed] [Google Scholar]
- 70.Sanz MJ, Marinova-Mutafchieva L, Green P, Lobb RR, Feldmann M, Nourshargh S. IL-4-induced eosinophil accumulation in rat skin is dependent on endogenous TNF-alpha and alpha 4 integrinVCAM-1 adhesion pathways. J Immunol 1998; 160:5637-45; PMID: [PubMed] [Google Scholar]
- 71.Madri JA, Graesser D, Haas T. The roles of adhesion molecules and proteinases in lymphocyte transendothelial migration. Biochem Cell Biol 1996; 74:749-57; PMID:; http://dx.doi.org/ 10.1139/o96-082 [DOI] [PubMed] [Google Scholar]
- 72.Jahnsen FL, Haraldsen G, Aanesen JP, Haye R, Brandtzaeg P. Eosinophil infiltration is related to increased expression of vascular cell adhesion molecule-1 in nasal polyps. Am J Respir Cell Mol Biol 1995; 12:624-32; PMID:; http://dx.doi.org/ 10.1165/ajrcmb.12.6.7539273 [DOI] [PubMed] [Google Scholar]
- 73.Tsuzuki S, Toyama-Sorimachi N, Kitamura F, Tobita Y, Miyasaka M. FK506 (tacrolimus) inhibits extravasation of lymphoid cells by abrogating VLA-4VCAM-1 mediated transendothelial migration. FEBS Lett 1998; 430:414-8; PMID:; http://dx.doi.org/ 10.1016/S0014-5793(98)00703-0 [DOI] [PubMed] [Google Scholar]
- 74.Muller WA. Mechanisms of transendothelial migration of leukocytes. Circ Res 2009; 105:223-30; PMID:; http://dx.doi.org/ 10.1161/CIRCRESAHA.109.200717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Arcangeli ML, Frontera V, Aurrand-Lions M. Function of junctional adhesion molecules (JAMs) in leukocyte migration and homeostasis. Arch Immunol Ther Exp (Warsz) 2013; 61:15-23; PMID:; http://dx.doi.org/ 10.1007/s00005-012-0199-5 [DOI] [PubMed] [Google Scholar]
- 76.Marcos-Ramiro B, Garcia-Weber D, Millan J. TNF-induced endothelial barrier disruption: beyond actin and Rho. Thromb Haemost 2014; 112: 1088–1102 PMID:http://dx.doi.org/10.1160/TH14-04-0299 [DOI] [PubMed] [Google Scholar]
- 77.Patibandla PK, Tyagi N, Dean WL, Tyagi SC, Roberts AM, Lominadze D. Fibrinogen induces alterations of endothelial cell tight junction proteins. J Cell Physiol 2009; 221:195-203; PMID:; http://dx.doi.org/ 10.1002/jcp.21845 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Sumagin R, Kuebel JM, Sarelius IH. Leukocyte rolling and adhesion both contribute to regulation of microvascular permeability to albumin via ligation of ICAM-1. Am J Physiol Cell Physiol 2011; 301:C804-13; PMID:; http://dx.doi.org/ 10.1152/ajpcell.00135.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Clark PR, Manes TD, Pober JS, Kluger MS. Increased ICAM-1 expression causes endothelial cell leakiness, cytoskeletal reorganization and junctional alterations. J Invest Dermatol 2007; 127:762-74; PMID:; http://dx.doi.org/ 10.1038/sj.jid.5700670 [DOI] [PubMed] [Google Scholar]
- 80.van Buul JD, van Rijssel J, van Alphen FP, van Stalborch AM, Mul EP, Hordijk PL. ICAM-1 clustering on endothelial cells recruits VCAM-1. J Biomed Biotechnol 2010; 2010:120328; PMID:; http://dx.doi.org/ 10.1155/2010/120328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Miyamoto K, Khosrof S, Bursell SE, Moromizato Y, Aiello LP, Ogura Y, Adamis AP. Vascular endothelial growth factor (VEGF)-induced retinal vascular permeability is mediated by intercellular adhesion molecule-1 (ICAM-1). Am J Pathol 2000; 156:1733-9; PMID:; http://dx.doi.org/ 10.1016/S0002-9440(10)65044-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Sumagin R, Sarelius IH. TNF-alpha activation of arterioles and venules alters distribution and levels of ICAM-1 and affects leukocyte-endothelial cell interactions. Am J Physiol Heart Circ Physiol 2006; 291:H2116-25; PMID:; http://dx.doi.org/ 10.1152/ajpheart.00248.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Gaber MW, Yuan H, Killmar JT, Naimark MD, Kiani MF, Merchant TE. An intravital microscopy study of radiation-induced changes in permeability and leukocyte-endothelial cell interactions in the microvessels of the rat pia mater and cremaster muscle. Brain Res Brain Res Protoc 2004; 13:1-10; PMID:; http://dx.doi.org/ 10.1016/j.brainresprot.2003.11.005 [DOI] [PubMed] [Google Scholar]
- 84.Sumagin R, Lomakina E, Sarelius IH. Leukocyte-endothelial cell interactions are linked to vascular permeability via ICAM-1-mediated signaling. Am J Physiol Heart Circ Physiol 2008; 295:H969-H77; PMID:; http://dx.doi.org/ 10.1152/ajpheart.00400.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Greenwood J, Amos CL, Walters CE, Couraud PO, Lyck R, Engelhardt B, Adamson P. Intracellular domain of brain endothelial intercellular adhesion molecule-1 is essential for T lymphocyte-mediated signaling and migration. J Immunol 2003; 171:2099-108; PMID:; http://dx.doi.org/ 10.4049/jimmunol.171.4.2099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Sans E, Delachanal E, Duperray A. Analysis of the roles of ICAM-1 in neutrophil transmigration using a reconstituted mammalian cell expression model: implication of ICAM-1 cytoplasmic domain and Rho-dependent signaling pathway. J Immunol 2001; 166:544-51; PMID:; http://dx.doi.org/ 10.4049/jimmunol.166.1.544 [DOI] [PubMed] [Google Scholar]
- 87.Sumagin R, Sarelius IH. Intercellular adhesion molecule-1 enrichment near tricellular endothelial junctions is preferentially associated with leukocyte transmigration and signals for reorganization of these junctions to accommodate leukocyte passage. J Immunol 2010; 184:5242-52; PMID:; http://dx.doi.org/ 10.4049/jimmunol.0903319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Schaefer A, Te Riet J, Ritz K, Hoogenboezem M, Anthony EC, Mul FP, de Vries CJ, Daemen MJ, Figdor CG, van Buul JD, et al. Actin-binding proteins differentially regulate endothelial cell stiffness, ICAM-1 function and neutrophil transmigration. J Cell Sci 2014; 127(Pt 20):4470-82; PMID:; http://dx.doi.org/ 10.1242/jcs.154708 [DOI] [PubMed] [Google Scholar]
- 89.Vestweber D, Zeuschner D, Rottner K, Schnoor M. Cortactin regulates the activity of small GTPases and ICAM-1 clustering in endothelium: Implications for the formation of docking structures. Tissue Barriers 2013; 1:e23862; PMID:; http://dx.doi.org/ 10.4161/tisb.23862 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Barreiro O, Yanez-Mo M, Serrador JM, Montoya MC, Vicente-Manzanares M, Tejedor R, Furthmayr H, Sanchez-Madrid F. Dynamic interaction of VCAM-1 and ICAM-1 with moesin and ezrin in a novel endothelial docking structure for adherent leukocytes. J Cell Biol 2002; 157:1233-45; PMID:; http://dx.doi.org/ 10.1083/jcb.200112126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Huang AJ, Manning JE, Bandak TM, Ratau MC, Hanser KR, Silverstein SC. Endothelial cell cytosolic free calcium regulates neutrophil migration across monolayers of endothelial cells. J Cell Biol 1993; 120:1371-80; PMID:; http://dx.doi.org/ 10.1083/jcb.120.6.1371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Wang Q, Doerschuk CM. The signaling pathways induced by neutrophil-endothelial cell adhesion. Antioxid Redox Signal 2002; 4:39-47; PMID:; http://dx.doi.org/ 10.1089/152308602753625843 [DOI] [PubMed] [Google Scholar]
- 93.Hu G, Vogel SM, Schwartz DE, Malik AB, Minshall RD. Intercellular adhesion molecule-1-dependent neutrophil adhesion to endothelial cells induces caveolae-mediated pulmonary vascular hyperpermeability. Circ Res 2008; 102:e120-31; PMID:; http://dx.doi.org/ 10.1161/CIRCRESAHA.107.167486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Allingham MJ, van Buul JD, Burridge K. ICAM-1-mediated, Src- and Pyk2-dependent vascular endothelial cadherin tyrosine phosphorylation is required for leukocyte transendothelial migration. J Immunol 2007; 179:4053-64; PMID:; http://dx.doi.org/ 10.4049/jimmunol.179.6.4053 [DOI] [PubMed] [Google Scholar]
- 95.van Wetering S, van den Berk N, van Buul JD, Mul FP, Lommerse I, Mous R, ten Klooster JP, Zwaginga JJ, Hordijk PL. VCAM-1-mediated Rac signaling controls endothelial cell-cell contacts and leukocyte transmigration. Am J Physiol Cell Physiol 2003; 285:C343-52; PMID:; http://dx.doi.org/ 10.1152/ajpcell.00048.2003 [DOI] [PubMed] [Google Scholar]
- 96.Vockel M, Vestweber D. How T cells trigger the dissociation of the endothelial receptor phosphatase VE-PTP from VE-cadherin. Blood 2013; 122:2512-22; PMID:; http://dx.doi.org/ 10.1182/blood-2013-04-499228 [DOI] [PubMed] [Google Scholar]
- 97.Wei H, Fang L, Song J, Chatterjee S. Statin-inhibited endothelial permeability could be associated with its effect on PECAM-1 in endothelial cells. FEBS Lett 2005; 579:1272-8; PMID:; http://dx.doi.org/ 10.1016/j.febslet.2005.01.020 [DOI] [PubMed] [Google Scholar]
- 98.Sharma P, Templin T, Grabham P. Short term effects of gamma radiation on endothelial barrier function: uncoupling of PECAM-1. Microvasc Res 2013; 86:11-20; PMID:; http://dx.doi.org/ 10.1016/j.mvr.2012.11.007 [DOI] [PubMed] [Google Scholar]
- 99.Fernandez-Martin L, Marcos-Ramiro B, Bigarella CL, Graupera M, Cain RJ, Reglero-Real N, Jiménez A, Cernuda-Morollón E, Correas I, Cox S, et al. Crosstalk between reticular adherens junctions and platelet endothelial cell adhesion molecule-1 regulates endothelial barrier function. Arterioscler Thromb Vasc Biol 2012; 32:e90-102; PMID:; http://dx.doi.org/ 10.1161/ATVBAHA.112.252080 [DOI] [PubMed] [Google Scholar]
- 100.Wong D, Prameya R, Dorovini-Zis K. Adhesion and migration of polymorphonuclear leukocytes across human brain microvessel endothelial cells are differentially regulated by endothelial cell adhesion molecules and modulate monolayer permeability. J Neuroimmunol 2007; 184:136-48; PMID:; http://dx.doi.org/ 10.1016/j.jneuroim.2006.12.003 [DOI] [PubMed] [Google Scholar]
- 101.Turegun M, Gudemez E, Newman P, Zins J, Siemionow M. Blockade of platelet endothelial cell adhesion molecule-1 (PECAM-1) protects against ischemia-reperfusion injury in muscle flaps at microcirculatory level. Plast Reconstr Surg 1999; 104:1033-40; PMID:; http://dx.doi.org/ 10.1097/00006534-199909020-00021 [DOI] [PubMed] [Google Scholar]
- 102.Lishnevsky M, Young LC, Woods SJ, Groshong SD, Basaraba RJ, Gilchrist JM, Higgins DM, Gonzalez-Juarrero M, Bass TA, Muller WA, et al. Microhemorrhage is an early event in the pulmonary fibrotic disease of PECAM-1 deficient FVBn mice. Exp Mol Pathol 2014; 97:128-36; PMID:; http://dx.doi.org/ 10.1016/j.yexmp.2014.06.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Maas M, Stapleton M, Bergom C, Mattson DL, Newman DK, Newman PJ. Endothelial cell PECAM-1 confers protection against endotoxic shock. Am J Physiol Heart Circ Physiol 2005; 288:H159-64; PMID:; http://dx.doi.org/ 10.1152/ajpheart.00500.2004 [DOI] [PubMed] [Google Scholar]
- 104.Graesser D, Solowiej A, Bruckner M, Osterweil E, Juedes A, Davis S, Ruddle NH, Engelhardt B, Madri JA. Altered vascular permeability and early onset of experimental autoimmune encephalomyelitis in PECAM-1-deficient mice. J Clin Invest 2002; 109:383-92; PMID:; http://dx.doi.org/ 10.1172/JCI0213595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Pumphrey NJ, Taylor V, Freeman S, Douglas MR, Bradfield PF, Young SP, Lord JM, Wakelam MJ, Bird IN, Salmon M, et al. Differential association of cytoplasmic signalling molecules SHP-1, SHP-2, SHIP and phospholipase C-gamma1 with PECAM-1CD31. FEBS Lett 1999; 450:77-83; PMID:; http://dx.doi.org/ 10.1016/S0014-5793(99)00446-9 [DOI] [PubMed] [Google Scholar]
- 106.Fleming I, Fisslthaler B, Dixit M, Busse R. Role of PECAM-1 in the shear-stress-induced activation of Akt and the endothelial nitric oxide synthase (eNOS) in endothelial cells. J Cell Sci 2005; 118:4103-11; PMID:; http://dx.doi.org/ 10.1242/jcs.02541 [DOI] [PubMed] [Google Scholar]
- 107.Yeh JC, Otte LA, Frangos JA. Regulation of G protein-coupled receptor activities by the platelet-endothelial cell adhesion molecule, PECAM-1. Biochemistry 2008; 47:9029-39; PMID:; http://dx.doi.org/ 10.1021/bi8003846 [DOI] [PubMed] [Google Scholar]
- 108.Gratzinger D, Barreuther M, Madri JA. Platelet-endothelial cell adhesion molecule-1 modulates endothelial migration through its immunoreceptor tyrosine-based inhibitory motif. Biochem Biophys Res Commun 2003; 301:243-9; PMID:; http://dx.doi.org/ 10.1016/S0006-291X(02)02982-0 [DOI] [PubMed] [Google Scholar]
- 109.Zebda N, Tian Y, Tian X, Gawlak G, Higginbotham K, Reynolds AB, Birukova AA, Birukov KG. Interaction of p190RhoGAP with C-terminal domain of p120-catenin modulates endothelial cytoskeleton and permeability. J Biol Chem 2013; 288:18290-9; PMID:; http://dx.doi.org/ 10.1074/jbc.M112.432757 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Anastasiadis PZ, Reynolds AB. The p120 catenin family: complex roles in adhesion, signaling and cancer. J Cell Sci 2000; 113 (Pt 8):1319-34; PMID: [DOI] [PubMed] [Google Scholar]
- 111.Alcaide P, Martinelli R, Newton G, Williams MR, Adam A, Vincent PA, Luscinskas FW. p120-Catenin prevents neutrophil transmigration independently of RhoA inhibition by impairing Src dependent VE-cadherin phosphorylation. Am J Physiol Cell Physiol 2012; 303:C385-95; PMID:; http://dx.doi.org/ 10.1152/ajpcell.00126.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Noren NK, Liu BP, Burridge K, Kreft B. p120 catenin regulates the actin cytoskeleton via Rho family GTPases. J Cell Biol 2000; 150:567-80; PMID:; http://dx.doi.org/ 10.1083/jcb.150.3.567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Beckers CM, Garcia-Vallejo JJ, van Hinsbergh VW, van Nieuw Amerongen GP. Nuclear targeting of {beta}-catenin and p120ctn during thrombin-induced endothelial barrier dysfunction. Cardiovasc Res 2008; 79:679-88; PMID:; http://dx.doi.org/ 10.1093/cvr/cvn127 [DOI] [PubMed] [Google Scholar]
- 114.Zhu YT, Chen HC, Chen SY, Tseng SC. Nuclear p120 catenin unlocks mitotic block of contact-inhibited human corneal endothelial monolayers without disrupting adherent junctions. J Cell Sci 2012; 125:3636-48; PMID:; http://dx.doi.org/ 10.1242/jcs.103267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Zhang J, O’Donnell JJ, 3rd, Holian O, Vincent PA, Kim KS, Lum H. P120 catenin represses transcriptional activity through Kaiso in endothelial cells. Microvasc Res 2010; 80:233-9; PMID:; http://dx.doi.org/ 10.1016/j.mvr.2010.04.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.O’Donnell JJ, 3rd, Zhuge Y, Holian O, Cheng F, Thomas LL, Forsyth CB, Lum H. Loss of p120 catenin upregulates transcription of pro-inflammatory adhesion molecules in human endothelial cells. Microvasc Res 2011; 82:105-12; PMID:; http://dx.doi.org/ 10.1016/j.mvr.2011.04.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Kikuchi A, Kishida S, Yamamoto H. Regulation of Wnt signaling by protein-protein interaction and post-translational modifications. Exp Mol Med 2006; 38:1-10; PMID:; http://dx.doi.org/ 10.1038/emm.2006.1 [DOI] [PubMed] [Google Scholar]
- 118.Clevers H. Wntbeta-catenin signaling in development and disease. Cell 2006; 127:469-80; PMID:; http://dx.doi.org/ 10.1016/j.cell.2006.10.018 [DOI] [PubMed] [Google Scholar]
- 119.Goodwin A, Kitajewski J, D’Amore P. Wnt1 and Wnt5a affect endothelial proliferation and capillary length; Wnt2 does not. Growth Factors 2007; 25:25-32; PMID:; http://dx.doi.org/ 10.1080/08977190701272933 [DOI] [PubMed] [Google Scholar]
- 120.Wright M, Aikawa M, Szeto W, Papkoff J. Identification of a Wnt-responsive signal transduction pathway in primary endothelial cells. Biochem Biophys Res Commun 1999; 263:384-8; PMID:; http://dx.doi.org/ 10.1006/bbrc.1999.1344 [DOI] [PubMed] [Google Scholar]
- 121.Masckauchán T, Shawber C, Funahashi Y, Li C, Kitajewski J. Wntbeta-catenin signaling induces proliferation, survival and interleukin-8 in human endothelial cells. Angiogenesis 2005; 8:43-51; PMID:; http://dx.doi.org/ 10.1007/s10456-005-5612-9 [DOI] [PubMed] [Google Scholar]
- 122.Eberhart CG, Tihan T, Burger PC. Nuclear localization and mutation of beta-catenin in medulloblastomas. J Neuropathol Exp Neurol 2000; 59:333-7; PMID: [DOI] [PubMed] [Google Scholar]
- 123.Birukova AA, Malyukova I, Poroyko V, Birukov KG. Paxillin-beta-catenin interactions are involved in RacCdc42-mediated endothelial barrier-protective response to oxidized phospholipids. Am J Physiol Lung Cell Mol Physiol 2007; 293:L199-211; PMID:; http://dx.doi.org/ 10.1152/ajplung.00020.2007 [DOI] [PubMed] [Google Scholar]
- 124.Tinsley JH, Ustinova EE, Xu W, Yuan SY. Src-dependent, neutrophil-mediated vascular hyperpermeability and beta-catenin modification. Am J Physiol Cell Physiol 2002; 283:C1745-51; PMID:; http://dx.doi.org/ 10.1152/ajpcell.00230.2002 [DOI] [PubMed] [Google Scholar]
- 125.Jang MW, Yun SP, Park JH, Ryu JM, Lee JH, Han HJ. Cooperation of Epac1Rap1Akt and PKA in prostaglandin E(2) -induced proliferation of human umbilical cord blood derived mesenchymal stem cells: involvement of c-Myc and VEGF expression. J Cell Physiol 2012; 227:3756-67; PMID:; http://dx.doi.org/ 10.1002/jcp.24084 [DOI] [PubMed] [Google Scholar]
- 126.Marin N, Zamorano P, Carrasco R, Mujica P, Gonzalez FG, Quezada C, Meininger CJ, Boric MP, Durán WN, Sánchez FA. S-Nitrosation of beta-catenin and p120 catenin: a novel regulatory mechanism in endothelial hyperpermeability. Circ Res 2012; 111:553-63; PMID:; http://dx.doi.org/ 10.1161/CIRCRESAHA.112.274548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Gonzalez D, Rojas A, Herrera MB, Conlan RS. iNOS activation regulates beta-catenin association with its partners in endothelial cells. PLoS One 2012; 7:e52964; PMID:; http://dx.doi.org/ 10.1371/journal.pone.0052964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Taddei A, Giampietro C, Conti A, Orsenigo F, Breviario F, Pirazzoli V, Potente M, Daly C, Dimmeler S, Dejana E. Endothelial adherens junctions control tight junctions by VE-cadherin-mediated upregulation of claudin-5. Nat Cell Biol 2008; 10:923-34; PMID:; http://dx.doi.org/ 10.1038/ncb1752 [DOI] [PubMed] [Google Scholar]
- 129.Glading AJ, Ginsberg MH. Rap1 and its effector KRIT1CCM1 regulate beta-catenin signaling. Dis Model Mech 2010; 3:73-83; PMID:; http://dx.doi.org/ 10.1242/dmm.003293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Corr M, Lerman I, Keubel JM, Ronacher L, Misra R, Lund F, Sarelius IH, Glading AJ. Decreased Krev interaction-trapped 1 expression leads to increased vascular permeability and modifies inflammatory responses in vivo. Arterioscler Thromb Vasc Biol 2012; 32:2702-10; PMID:; http://dx.doi.org/ 10.1161/ATVBAHA.112.300115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Shtutman M, Zhurinsky J, Simcha I, Albanese C, D’Amico M, Pestell R, Ben-Ze'ev A. The cyclin D1 gene is a target of the beta-cateninLEF-1 pathway. Proc Natl Acad Sci U S A 1999; 96:5522-7; PMID:; http://dx.doi.org/ 10.1073/pnas.96.10.5522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Haertel-Wiesmann M, Liang Y, Fantl WJ, Williams LT. Regulation of cyclooxygenase-2 and periostin by Wnt-3 in mouse mammary epithelial cells. J Biol Chem 2000; 275:32046-51; PMID:; http://dx.doi.org/ 10.1074/jbc.M000074200 [DOI] [PubMed] [Google Scholar]


