Abstract
The epithelial tight junction determines the paracellular water and ion movement in the intestine and also prevents uptake of larger molecules, including antigens, in an uncontrolled manner. Claudin-2, one of the 27 mammalian claudins regulating that barrier function, forms a paracellular channel for small cations and water. It is typically expressed in leaky epithelia like proximal nephron and small intestine and provides a major pathway for the paracellular transport of sodium, potassium, and fluid. In intestinal inflammation (Crohn's disease, ulcerative colitis), immune-mediated diseases (celiac disease), and infections (HIV enteropathy), claudin-2 is upregulated in small and large intestine and contributes to diarrhea via a leak flux mechanism. In parallel to that upregulation, other epithelial and tight junctional features are altered and the luminal uptake of antigenic macromolecules is enhanced, for which claudin-2 may be partially responsible through induction of tight junction strand discontinuities.
Keywords: celiac disease, claudin-2, Crohn's disease, HIV, IFNγ, inflammatory bowel disease, TNFα, tight junction, ulcerative colitis
Abbreviations: AP, activator protein; CARD15, caspase recruitment domain-containing protein 15; DSS, dextran sodium sulfate; ECL, extracellular loop; ERK, extracellular-regulated kinase; gp, glycoprotein; HIV, human immunodeficiency virus; HNF, hepatocyte nuclear factor; IBD, inflammatory bowel disease; IFN, interferon; IL, interleukin; JAM, junctional adhesion molecule; JNK, c-jun N-terminal kinase; LPS, lipopolysaccharides; MAPK, mitogen-activated protein kinase; MDCK, Madine Darby canine kidney; MLC, myosin light chain; NFκB, nuclear factor kappa B; NOD2, nucleotide-binding oligomerization domain-containing protein 2; PI3K, phosphatidyl-inositol-3-kinase; Rho, ras homolog; ROCK, Rho kinase; STAT, signal transducers and activators of transcription; Tat, trans-activator of transcription; TEER, transepithelial electrical resistance; TJ, tight junction; TNBS, 2,4,6-trinitrobenzene sulfonic acid; TNF, tumor necrosis factor; Vpr, viral protein r; ZO, zonula occludens
Introduction
The function of the epithelial barrier as part of the innate immunity of the small and large intestine depends on the apical plasma membranes of enterocytes and the junctional complexes between them.1 The main diffusion barrier within this junctional complex is formed by the tight junction (TJ) which contains transmembrane proteins. These TJ proteins are organized as heteropolymers forming strands at the apical pole of the basolateral membrane that interact in trans with TJ proteins of adjacent cells.
The expression pattern of the TJ protein family claudins with 27 members in mammals is both organ- and segment-specific.2 The majority of these, including claudin-1, -3, -4, -5, -7, and -8, confer barrier properties and are often found in tight epithelia like the distal regions of the intestine (Table 1). Others, such as claudin-2, induce channel formation within the TJ, and are mostly expressed in leaky epithelia like the proximal intestine.
Table 1.
Expression of predominant claudins in human intestine
| small intestine | large intestine | Ref. | |
|---|---|---|---|
| Claudin-1 | ++ | ++ | 30,51,116,115 |
| Claudin-2 | ++ | + | 23,30,51,116 |
| Claudin-3 | + | ++ | 23,30,51,116 |
| Claudin-4 | + | ++ | 23,30,51,116,115 |
| Claudin-5 | ++ | 30,116 | |
| Claudin-7 | + | ++ | 23,30,51 |
| Claudin-8 | + | ++ | 23,30 |
| Claudin-12 | ++ | ++ | 23 |
| Claudin-15 | ++ | + | 23 |
+ mild expression; ++ strong expression.
However, whether they are more tightening or more channel-forming, functional consequences should be assumed to be more significant in an intermediate tight epithelium like the colon and rectum than in a leaky epithelium as the small intestine.
During intestinal inflammation, the protein pattern of TJ strands can undergo rapid changes. A frequent regulatory event during inflammation is the increased expression of claudin-2 and its insertion into TJ strands. Starting in the lower crypt, upregulation extends the expression of claudin-2 toward the surface epithelium. The functional role of this modified expression and the consequential increase in leakiness, however, is less clear so far.
Aim of this review article is to summarize our present knowledge about the channel-forming TJ protein claudin-2 in the inflamed intestine in different diseases, especially in regard to function, regulation and therapeutic aspects.
Structure and Function of the Cation and Water Channel Claudin-2
Claudin-2, which is predominantly expressed in leaky epithelia, is known as a channel-forming TJ protein permeable to small cations and water.3,4 Claudin-2 is a 24.5 kDa integral membrane protein of 230 amino acids which, like all claudins, consists of 4 transmembrane helices, 2 extracellular loops (ECL), a small intracellular loop, a short intracellular NH2 terminus and a longer intracellular COOH terminus.5 The COOH-terminus contains a PDZ-binding motif through which claudin-2 is able to interact with the scaffolding proteins MAGUKs (membrane-associated guanylate kinases), Zonula occludens-1 (ZO-1), -2, and -3.6,7 Trans-interactions have been demonstrated for claudin-2 with neighboring claudin-2 (homodimeric) or with claudin-3 (heterodimeric), but not with claudin-1.8 Lim and coworkers showed by using single-molecule force spectroscopy that trans-interactions in claudin-2 are ECL1-ECL1-mediated and not ECL2-ECL2- or ECL1-ECL2-mediated.9
The ECL1 includes the claudin signature sequence (WGLWCC) and is formed by amino acids 29–81, making it larger than ECL2.10 The ECL1 is discussed as the pore-lining domain being critical for the channel formation of claudin-2, which is estimated to have a diameter of 6.5 Å at its narrowest site.11,12 Two highly conserved cysteines in the ECL1, C54 and C64, are connected intramolecularly by a disulfide bond and necessary for lining the pore by stabilizing the fold in ECL1, but have no influence on claudin-2 trafficking.13,14 Additional pore-lining residues were identified in a study using cysteine-scanning mutagenesis and thiol group modification including, from the narrowest to the widest part of the pore, S68, S47, T62/I66, T56, T32/G45, and M52.15 Pore-lining residues from D65 to S68 in the ECL1 are believed to be the most important ones for the paracellular functions of claudin-2, and the negatively charged D65 is already known to be determinative for cation selectivity.11,12 Additionally, through electrostatic interactions with D6512 and weaker interactions with negatively charged π-electrons of the Y67 aromatic residue16, cations are able to permeate the pore in a dehydrated or partially dehydrated state.
The channel formed by claudin-2 is selectively permeable to small cations without significant discrimination between Li+, Na+, and K+ and in addition it is permeable to water molecules (diameter of 2.8 Å).3,4,12 Water and cation transport appear to be coupled with paracellular Na+ flux inducing paracellular water flux and in turn, osmotically-induced water flux driving paracellular Na+ movement. As a consequence of the selectivity and dimensions of the claudin-2-based channel, anions, uncharged oligomers like mannitol and lactulose, and macromolecules like 4 kDa FITC-dextran are not able to pass through.3,12,17 Thus, different paracellular pathways have been postulated for small ions and for macromolecules.18 Small ions predominantly pass through claudin-based channels of the bicellular TJ (high-capacity, charge- and size-selective “pore pathway”). This means that the uptake of antigens and pathogens during inflammation and/or infection is not mediated by the claudin-2 channel itself. Instead, it is suggestive that concomitant alterations of other TJ proteins and possibly the change in TJ ultrastructure during claudin-2 upregulation could contribute to the leaky gut under pathologic conditions.
Using freeze-fracture electron microscopy to investigate TJ ultrastructure (TJ strand count and meshwork depth) it was revealed that claudin-2 incorporation into the TJ network does not affect the number, but it could affect the pattern of TJ strands. In mouse fibroblasts transfection with claudin-2 resulted in discontinuous strands on the P-face, whereas continuous strands were found after transfection with claudin-1.5,19,20 The same was observed in MDCK I cells transfected with claudin-2 and in MDCK II cells with high endogenous claudin-2, TJ strands were discontinuous on the P-face, and on the E-face intramembranous particles were scattered within the grooves.19 In contrast, in MDCK C7 cells, no changes in TJ ultrastructure could be observed after incorporation of claudin-2 into the TJ.4
Claudin-2 shows a tissue-specific distribution pattern, with higher expression of claudin-2 in leaky epithelia like in the proximal nephron and small intestine and less in tight epithelia like in the collecting duct and colon21–23 Additionally, in the human small intestine, claudin-2 is expressed along the crypt-villus axis, whereas in the fetal colon the expression is limited to the crypt base and in the adult colon tissue it is even absent. Similarly, in Caco-2 cells claudin-2 is expressed only in undifferentiated stages with high permeability.24
Claudin-2 KO mice show a decreased transepithelial conductance in the small intestine due to a decrease in Na+ permeability.25 Interestingly, claudin-2 loss did not change the intestinal homeostasis of Na+ and K+, presumably as the result of an expression pattern of claudin-2 restricted only to crypts. This is in contrast to the other known cation channel of the intestine, claudin-15, which is expressed in both, villi and crypts in mice.25 Claudin-15 is indispensable for luminal Na+ homeostasis, a prerequisite for efficient glucose absorption in the small intestine. The crystal structure of claudin-15 has recently been described and revealed a characteristic β-sheet fold which is anchored to a transmembrane 4-helix bundle by a consensus motif. The extramembrane β-sheet domain consists of 5 β-strands, 4 of which build the ECL1 and one the ECL2. The negatively charged amino acids responsible for cation selectivity are located on one edge of the β-sheet domain.26 Together with the crystal structure of claudin-2, which is still unknown, our molecular understanding of paracellular ion channels will be further advanced.
Regulation of Claudin-2
Expression of claudin-2 is changed under different pathologic conditions, such as cancer, infectious diseases, and inflammation.27 In inflammatory bowel diseases (IBD), an increase in claudin-2 is associated with a decrease and/or redistribution of sealing TJ proteins including claudin-1, -3, -4, -5, and/or -8. These changes in conjunction with the induction of epithelial apoptosis lead to barrier dysfunction and increased ion and water permeability, which in turn results in leak-flux diarrhea.28–30
Under inflamed conditions, cytokines such as tumor necrosis factor-α (TNFα) and interleukin (IL)-13 are increased and induce an upregulation of claudin-2 as shown in cell culture studies.29,31–33 For example, the pro-inflammatory cytokine TNFα caused an increase in claudin-2 expression via phosphatidylinositol-3-kinase (PI3K) signaling in the colonic HT-29/B6 cell line32,34, while IL-13 did the same in T84 colon cells (Fig. 1).29 Additionally, IL-17 increased claudin-2 expression in T84 cells via the MEK-ERK pathway35 and IL-6 induced an upregulation in Caco-2 cells and mouse colon through MEK-ERK and PI3K signaling (Fig. 1).33 Activation of these pathways resulted in an increased expression of the transcription factor Cdx2, thereby enhancing claudin-2 promoter activity.33
Figure 1.
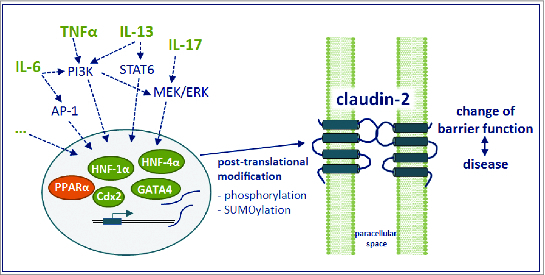
Summary of claudin-2 regulation. Figure is based on references.24,29,32–36,38–46 See text for detailed description.
Cdx2 and Cdx1 possessing the Cdx homeodomain are the most important transcription factors involved in claudin-2 expression that have been identified in human intestinal epithelial cells so far. The Cdx2-induced claudin-2 promoter activation is augmented by hepatocyte-nuclear factor (HNF)-1α, an organ-specific regulator of claudin-2 expression that is essential in the ileum and liver, but not in the kidney.36 Functional crosstalk between Cdx-related transcriptional activation and Wnt signaling is involved in the regulation of promoter-mediated claudin-2 gene expression, and is thought to be essential for the differentiation of epithelial cells.37 In human intestine, it was shown that in addition to Cdx2 and HNF-1α, expression of the transcription factor GATA4 correlated with claudin-2 abundance (Fig. 1). In line with decreased claudin-2 expression, no GATA4 is expressed in the colon and in differentiating intestinal Caco-2 cells.24 GATA4 appears to interact functionally with Cdx2 and HNF-1α to modulate transcription of different intestinal genes38, and cotransfection of Cdx2, GATA4 and HNF-1α increased claudin-2 promoter activity in the intestinal Caco-2 cell line.24 Furthermore, in Caco-2 cells and mouse intestine, claudin-2 is also upregulated by the transcription factor complex activator protein 1 (AP-1) induced by IL-6 and JNK (c-Jun N-terminal kinase) activation39, and by signal transducers and activators of transcription 6 (STAT6), which is induced by IL-13 in ulcerative colitis (Fig. 1).40 Inhibition of STAT6 phosphorylation or transfection with STAT6 siRNA prevented claudin-2 upregulation in T84 and HT-29 cells.40–42 On the other hand, the peroxisome proliferator-activated receptor α (PPARα) appears to downregulate claudin-2 (Fig. 1) as PPARα knockout mice exhibit enhanced intestinal claudin-2 expression and increased lactulose/mannitol uptake ratio in experimental colitis, probably by indirect means.43
Claudin-2 is also regulated on posttranslational level (Fig. 1), e.g. by altering the phosphorylation levels. By prediction software, Gonzáles-Mariscal and coworkers identified 10 potential phosphorylation sites for the carboxyl tail of human claudin-2.44 So far, the phosphorylation site S208 has been demonstrated to have a regulatory effect by promoting the localization of claudin-2 in the plasma membrane of MDCK cells and reducing its trafficking to lysosomes.45 The same group demonstrated that claudin-2 is downregulated in MDCK cells by modification of residue K218 by small ubiquitin-like modifier-1 (SUMO-1).46 Finally, claudin-2 can be regulated by other claudins. For example, insertion of claudin-8 in MDCK II cells displaced endogenous claudin-2 from the TJ, downregulated its expression, and tightened the paracellular barrier.47,48
Claudin-2 in Diseases of the Small and Large Intestine
Impaired barrier function is a central finding in several intestinal diseases presenting with inflammation and/or diarrhea. These barrier defects often include an increase in claudin-2 expression, which occurs in the IBDs, Crohn's disease and ulcerative colitis, infectious diseases including human immunodeficiency virus (HIV) infection, and gluten-sensitive enteropathy (Table 2).30,49–52 However, the barrier disturbances in these diseases are differentially mediated, and while alterations in the expression and/or localization of TJ proteins predominate in some, induction of epithelial apoptosis and/or leaks/erosions play an important role in others. Furthermore, claudin-2 is not the only TJ protein dysregulated in intestinal diseases, and changes in barrier-strengthening and other channel-forming TJ proteins must also be considered in the overall picture of barrier modification processes.
Table 2.
Altered claudin expression profile in intestinal barrier defects
| Disease | claudin-1 | claudin-2 | claudin-3 | claudin-4 | claudin-5 | claudin-7 | claudin-8 | claudin-15 | occludin | Ref. # |
|---|---|---|---|---|---|---|---|---|---|---|
| Crohn's disease | = | ↑ | ↓ | = | ↓ | = | ↓ | not detected | ↓ | 30 |
| ulcerative colitis | ↓, = | ↑ | = | ↓ | ↓ | ↓ | 50,51 | |||
| celiac disease | = | ↑ | ↓ | = | ↓ | = | ↑ | ↓ | 52 | |
| HIV | ↓ | ↑ | = | = | 100 |
In addition, claudin-11, -12, -14 and -16 were not detected, neither in Crohn's disease patients nor in controls.30 Claudin-2 was not detectable in controls of all diseases.↑ increased, ↓ decreased, and = unchanged expression of tight junction protein versus control.
Crohn's Disease
The importance of an intact epithelial TJ network as a central part of epithelial barrier integrity and disease pathogenesis is especially evident in Crohn's disease. Inflammatory areas can be seen throughout the whole gastrointestinal tract but are most frequent in the distal small intestine and in the large intestine. Mucosal immune stimulation through a leaky barrier is a fundamental event in Crohn's disease and an important pathomechanism in the onset, recurrence, and persistence of disease activity. In addition, clinical symptoms such as leak flux diarrhea result from a leaky barrier, for which the increased claudin-2 expression is a predominant feature.
This upregulation is an early and functionally important alteration in the inflamed mucosa of Crohn's disease patients, while TJ proteins with sealing properties, including claudins-3, -4, -5, and -8 and occludin, are downregulated and redistributed off the TJ domain (Table 2).29,30,53 Functional consequences of dysregulated TJ protein expression were found in endoscopic biopsies from patients with mild to moderate Crohn's disease, which, in addition to altered TJ protein composition, displayed changes on ultrastructural level; decreased TJ strand numbers, increased strand discontinuities and pearl string-like strands, as well as impaired barrier function.30 The extent to which the increase in claudin-2 expression contributed to the appearance of strand discontinuities is not clear, yet. However, the fact that strand breaks appear in inflamed tissues with high claudin-2 content and claudin-2 transfection into L-fibroblasts–in contrast to claudin-1 or -3 transfection–induces the formation of discontinuous strands, points to an important role of claudin-2 in this context.5
Enhanced epithelial endocytosis in Crohn's disease could be an additional mechanism by which not only bacterial translocation is enhanced but also TJ strands are removed. So far, this has been studied for occludin in response to TNFα.54 Which TJ proteins are also concerned by this mechanism is still not fully understood but claudin-5 and -8 are possible candidates, since they are found to be internalized in Crohn's disease. Whether or not claudin-2 is endocytosed in parallel as well, is not known but even under inflammatory conditions most of the immune reactivity of claudin-2 has been detected in the TJ and not in subapical cellular compartments.30
TNFα and interferon-γ (IFNγ) are important effector cytokines in Crohn's disease, and have been originally presented as the typical Th1-cytokine profile of the disease.55 Additional cytokines contributing to impaired epithelial function have been identified, including the Th2-cytokines IL-4 and -13, IL-17, IL-1β, and IL-6, and even mast cell released mediators such as histamine have been found to play a role.56,57 However, it remains to be seen whether its pro- or anti-inflammatory effects on the intestinal epithelium will prevail.58,59 Notably, these cytokines induce intestinal barrier defects in cultured epithelial cells and animal models that resemble those found in Crohn's disease patients, including TJ changes and epithelial apoptosis induction.60 Cytokines can influence TJs through either transcriptional regulation or reorganization of their subcellular distribution within the enterocyte. For example, TNFα stimulates claudin-2 protein expression via the PI3K pathway37 and, in combination with IFNγ, also downregulates the barrier-forming claudins -1, -5, and -7.61 In addition, induction of myosin light chain kinase (MLCK), an important regulator of general TJ physiology and involved in pathologic changes seen in IBD62, by TNFα or IL-1β leads to reduction in barrier function.63,64 As shown for TNFα, an increase in MLC phosphorylation by MLCK results in reorganisation of perijunctional F-actin, leading to the translocation of occludin into the cytosol by a caveolin-1-dependent endocytic process.58,65 Endocytosis-mediated barrier changes are also induced by IFNγ, which stimulates the macropinocytosis of occludin, JAM-A, and claudin-1 via Rho/Rock signaling.66
Barrier disturbances have long been postulated as a significant cofactor in the etiology of Crohn's disease. Elevated intestinal permeability precedes relapse or even onset of the disease in some patients.67,68 Genetic predispositions to barrier defects could lead to inappropriate immune stimulation and chronic intestinal inflammation. For example, polymorphisms in caspase recruitment domain 15 nucleotide-binding oligomerization domain 2 (CARD15/NOD2), a sensor of bacterial cell wall components and immune system stimulator, are associated with higher mucosal permeability and an increased susceptibility to Crohn's disease.69–71 The precise mechanisms linking CARD15/NOD2 mutations and increased intestinal permeability are still unknown. One possibility is that impaired production of antimicrobial defensins associated with CARD15/NOD2 mutations could facilitate bacterial translocation into the mucosa, leading to inflammation and cytokine-mediated TJ changes.72–76 In support of this thesis, CARD15/NOD2 knockout mice, which are more susceptible to TNBS colitis, show intestinal changes that include enhanced bacterial translocation, elevated TNFα, IFNγ, and IL-4, decreased ZO-1 and -2, and increased permeability.77 Although potential changes in the expression of claudin proteins were not reported, all 3 cytokines are known to have significant effects on TJ protein expression, including the regulation of claudin-2.30,32,34,78 A more direct link between claudin-2 and the induction of colitis is found in SAMP1/YitFc mice, which show an increase in claudin-2 expression and intestinal permeability that precedes the onset of a spontaneous Crohn's disease-like ileitis.79
Ulcerative Colitis
Upregulated claudin-2 expression and increased intestinal permeabilities are also characteristic of ulcerative colitis, the other major IBD, which affects the large intestine. As for Crohn's disease, intestinal barrier defects in ulcerative colitis are multifactorial, and elevated claudin-2 levels are accompanied by a downregulation and relocalization of barrier forming TJ proteins, including claudin-1, -4, and -7, and occludin (Table 2)50,51, a higher rate of apoptosis, and epithelial lesions.80 Furthermore, the role of claudin-2 in structural TJ strand defects leading to discontinuous and pearl string-like strands is complicated by the fact that there are higher levels of claudin-2 in ulcerative colitis than in Crohn's disease, but less strand breaks.29,81
The key effector cytokine in ulcerative colitis, IL-13, is considered to be an important factor in epithelial barrier dysfunction.50 Epithelial permeability is increased by IL-13 through claudin-2 upregulation, and the induction of epithelial apoptosis and inhibition of epithelial restitution processes, which lead to the development of microerosions and lesions.50,82,83 Mechanisms regulating IL-13-induced claudin-2 expression include both PI3K and STAT6 signaling pathways.29,40 Notably, in STAT6-deficient mice, attenuation of oxazolone-induced colitis is associated with a decrease in claudin-2 expression.41
Genetic predisposition to ulcerative colitis via barrier dysfunction could arise from mutations in transcription factors that regulate TJ protein expression. Hepatocyte nuclear factor-4α (HNF-4α), a transcriptional regulator of TJ proteins, was recently identified as a susceptibility locus for ulcerative colitis in a genome-wide association study, and intestinal expression is decreased in ulcerative colitis patients and dextran sodium sulfate (DSS)-treated mice.77,84 In the absence of HNF-4α, deregulation of TJ protein expression, including an upregulation of claudin-2 and downregulation of claudin-4 and -7, and increased intestinal permeability occurs, and a chronic IBD-like inflammation spontaneously develops in mice.38,84,85 HNF-4α likely regulates claudin-2 expression in a manner similar to HNF-1α, which enhances claudin-2 transcription through HNF-1-binding sites in the promoter region.36 Thus, claudin-2 expression in ulcerative colitis may be increased as the result of a HNF-4α mutation.
Celiac disease
Celiac disease is an autoimmune enteropathy triggered by the gliadin fraction of dietary gluten in genetically prone individuals. In celiac disease, the small intestine is affected with a longitudinal gradient of more involved proximal and less altered distal segments, where the gliadin or its cleavage products have already been completely degraded or absorbed. As in IBD, barrier disturbances are a key feature of celiac disease, and play a key role in disease pathogenesis.86 Compromised barrier integrity is a component of disease induction by facilitating the unwanted access of immunogenic gliadin peptides to the mucosal immune system, which triggers innate and adaptive immune responses. The aberrant immune response mediates intestinal barrier changes, including a characteristic mucosal transformation toward villus atrophy, that are reflected in the typical clinical presentation–chronic diarrhea, malabsorption, and weight loss.
Both paracellular and transcellular mechanisms contribute to the transport of gliadin. During early stages of the disease, endocytotic uptake and apical-to-basolateral transcytosis is a common route for gliadin passage87,88 while deregulation of the paracellular pathway by TJ alterations is more typically associated with advanced stages.89–91 However, the occurrence of increased intestinal permeability in healthy relatives of patients92 and the discovery of disease susceptibility genes encoding for proteins involved in TJ assembly (MYO9B, PAR-3, MAGI-2) suggest that a primary barrier defect could facilitate the onset and/or perpetuation of the disease. Whether primary defects can contribute to the uptake of gliadin or its cleavage products and trigger disease activation is yet to be diagnosed, as a role for the paracellular pathway in gliadin transport–at least in early stages of the disease–is controversial.87,93
TJs in celiac disease show significant structural defects, including less and more discontinuous strands, and changes in protein composition and localization (Fig. 2).94,95 Duodenal biopsies from children with celiac disease revealed an upregulation of claudin-2 that correlates with disease severity.91 In a detailed analysis of TJs in advanced celiac disease, the expression and localization of several proteins were altered, and these changes, rather than apoptosis or lesions, were responsible for functional paracellular leaks.90 Expression of claudin-2 and also -15 was increased in patient biopsies. However, in contrast to the TJ-localization of claudin-2, claudin-15 was primarily found in intracellular vesicles. Thus, claudin-15 likely contributes little to the barrier disturbances; rather, considering its central role in intestinal glucose absorption25, it may be more functionally relevant for glucose malabsorption in celiac disease. Notably, claudin-15 knock-out mice present a megaintestine morphology with hypertrophic villi, indicating a role in epithelial growth and differentiation regulation.25 Thus, claudin-15 could possibly be involved in the mucosal architecture changes in celiac disease, too.
Figure 2.
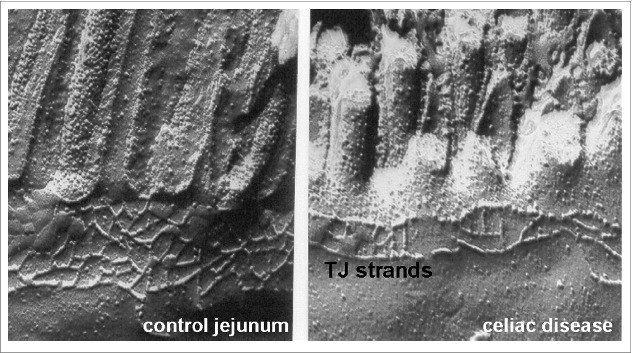
Freeze fracture electron microscopy of epithelial tight junctions from the jejunum of controls (left) and acute celiac disease patients with villus atrophy (right) obtained from Schulzke et al.95 Significant reduction in the number or horizontally oriented strands and the depth of the tight junctional strand network was observed. Additionally, strand discontinuities appeared in celiac disease.
Also contributing to the barrier defect, barrier-forming proteins, claudin-3, -5, -7, and occludin were concomitantly downregulated (Table 2).90 Expression of both claudin-3 and -5 was not only decreased, but also distributed off the TJ domain and into intracellular vesicle-like compartments, possibly due to changes in intracellular trafficking (e.g., altered endocytosis or disturbed movement to the plasma membrane). While experimental data on trafficking of TJ proteins in celiac disease is not available, IFN-γ, a key effector cytokine in celiac disease, is an important regulator of TJ endocytosis.96 IFN-γ upregulated claudin-2 in celiac disease if disease activity is very pronounced as in Marsh IIIc (complete villus atrophy).88 Additionally, TGFß2 seems to be involved in epithelial-mesenchymal-transition (EMT) with a concomitant change in cell polarity that also causes elevated epithelial permeability due to altered expression and assembly of TJ proteins claudin-2, -3, -5, -7 and ZO-1.52
HIV-enteropathy
Untreated HIV infection is characterized by loss of CD4-positive T lymphocytes as well as by systemic immune activation which are linked to each other via HIV-associated immune activation as a driving force for T-helper cell depletion.97,98 Elevated serum concentrations of microbiota lead to hyperimmune activation. Based on this, a defect of the intestinal mucosal barrier–so far mainly studied in the small intestine but presumably also affecting the large intestine–has been proposed to allow for increased translocation of gut microbiota. Evidence for this comes from studies combining a quantitative analysis of barrier function with structural data which suggest epithelial apoptosis and altered TJ protein expression as structural correlates of the HIV-associated barrier defect.99,100 Intestinal expression of claudin-2 was found to be increased in untreated HIV-infected patients100 which probably contributes to increased transepithelial cation and water fluxes and to the onset of diarrhea (via a leak flux mechanism). Thus, it is reasonable to conclude that changes in TJ ultrastructure are responsible for the increase in epithelial permeability for small-molecular weight solutes. However, according to our present understanding these alterations contribute most likely only in part to the accelerated macromolecule translocation. The latter could e.g. also be due to epithelial apoptosis with a parallel increase in perforin-expressing CD8+ lymphocytes in the mucosa.99 TJ changes and epithelial apoptosis are due to the increased production and release of pro-inflammatory cytokines like IL-2, IL-4, and TNFα during HIV-infection of the mucosa.100 An increased production of inflammatory cytokines such as TNFα, IL-6, IL-8 after incubation with HIV-1 was indeed found in cultured intestinal epithelial cells.101
Even the mucosa of patients with primary HIV infection showed increased expression of genes related to immune activation and decreased expression of genes related to epithelial repair.102 Thus, the mucosal barrier defect develops already during primary HIV infection. An additional effect may be exerted by HIV itself, which during primary infection actively replicates within the intestinal mucosa.103,104 As shown in cell culture studies, the viral (glyco)proteins gp120, Tat and Vpr can directly affect epithelial barrier function by inducing alterations of TJ protein expression and epithelial apoptosis.105,106
After termination of the immunological events of primary infection, HIV infection enters the chronic stage which is characterized by continual viral replication, chronic hyperimmune activation and a progressive decline of peripheral CD4 positive T-lymphocytes. During this stage of infection, perforin-expressing CD8 T cells cannot be detected anymore in the mucosa of the gastrointestinal tract.99,107,108 Despite this, mucosal barrier remains compromised–as indicated by an increase in permeability for ions and small-molecular weight solutes as well as a higher rate of epithelial apoptosis in chronic disease99–indicating additional immunological trigger mechanisms for the induction of barrier dysfunction. The mechanisms responsible for this include TJ protein expression changes for claudin-1 (decreased) and claudin-2 (increased) (Table 2).99
The question however, which mechanisms maintain the increased mucosal production of inflammatory cytokines in chronic HIV infection, has not been fully resolved, since there are no measurements on endocytotic antigen uptake so far. Another putative factor is IL-22 which was shown to play a crucial role in mucosal repair and regeneration and which is downregulated by the influence of macrophages in HIV-infected patients.109
Therapeutic Aspects
For inhibiting claudin-2 upregulation in intestinal diseases several therapeutic options could be discussed. In general, all strategies which inhibit the increase in production of cytokines involved in claudin-2 alterations are a target for therapeutic intervention. It has been shown that anti-TNFα therapies for patients with IBD cause “mucosal healing” which suggests restitution of the intestinal barrier function.110 The underlying mechanisms were identified by an in vitro study on 2 intestinal cell models, Caco-2 and T-84. The anti-TNFα antibody adalimumab antagonized the decrease in transepithelial electrical resistance (TEER) and the appearance of irregular membrane undulations and prevented internalization of TJ proteins upon combined exposure to IFNγ and TNFα. Adalimumab inhibited the TNFα induced changes in expression of claudin-1, -2, -4, and occludin and the activation of the PI3K signaling which was observed in T-84 cells. In both cell models, the cytokine-induced increase in phosphorylation of myosin light chain and the activation of p38 MAPK and NFκB signaling which accompanies the decline in TEER is inhibited by adalimumab. Thus, adalimumab prevents barrier dysfunction induced by TNFα, on the levels of function and structure as well as signaling cascades.
Another possibility for protection of the intestinal barrier includes the treatment with butyrate, a short-chain fatty acid produced by microbial fermentation of carbohydrates in the colon. A study employing a mouse model of DSS colitis revealed an attenuation of inflammation and mucosal lesions after oral administration of sodium butyrate.111 It was shown by a microarray study, that in the human colonic epithelial cell line HT-29 the expression of 19,400 genes was changed in response to butyrate. Among these, 221 genes specifically associated with the processes of proliferation, differentiation, and apoptosis were identified, 59 of these genes were upregulated and 162 downregulated, in accordance with the known butyrate effects. The claudin-2 gene belongs to the genes which were downregulated after butyrate treatment.112 This downregulation depends on a reduced binding affinity of transcription factors within the claudin-2 promoter.113 In response to butyrate treatment, many different parameters could be involved in the protective action on intestinal barrier function, such as upregulation of barrier-forming TJ proteins claudin-1, occludin, ZO-1, ZO-2, anti-inflammatory effects, and regulation of cell proliferation, differentiation and apoptosis.
For another natural compound, the plant alkaloid berberine, barrier protective effects have recently been revealed.34 Berberine is used in traditional Eastern medicine for the treatment of diarrhea and gastroenteritis. The alkaloid has been shown to cause a downregulation of claudin-2 which is associated with the antidiarrheal effect. Additionally, it was found to completely antagonize the TNFα-mediated barrier defects in the intestinal cell model HT-29/B6 and in rat colon. TNFα induced a decrease in TEER and an increase in permeability for the paracellular marker fluorescein. This was due to the removal of claudin-1 from the TJ and the increase in claudin-2 expression. Berberine prevented TNFα-induced claudin-1 disassembly and upregulation of claudin-2. The effects of berberine seem to be due to inhibition of the PI3K/Akt signaling pathway which is involved in claudin-2 expression and the src-kinase and NFκB pathways responsible for claudin-1 assembly within the TJ. It has been shown that berberine exerts a protective effect on intestinal injury in rats challenged with lipopolysaccharides (LPS).114 In rats, pretreatment with berberine prior to the administration of LPS significantly suppressed the increased levels of TNFα, IL-1ß and nitric oxide in the plasma as well as the activation of toll-like receptor 4 and NFκB in the ileum.
However, one has to keep in mind that none of the possible therapeutic interventions discussed here exert an effect which was exclusively specific for claudin-2. Nevertheless, since all these compounds affect different components contributing to intestinal barrier function, they may be suitable for multimodal therapies. Additionally, compounds which prevent production, release or action of pro-inflammatory cytokines such as TNFα, IFNγ, IL-2, IL-4 and IL-13 exert protective effects on intestinal diseases associated with epithelial barrier dysfunction.
Conclusions
Claudin-2 is a structural component of tight junction strands which forms cation channels. However, the functional consequence for epithelial barrier function depends on the background of all other claudins in the TJ. In small and large intestine, claudin-2 expression allows for a better passage of sodium and water by formation of paracellular channels being permeable to these. Claudin-2 seems to contribute also to TJ strand discontinuities, although it can not be the only determinator of this phenomenon. Therefore, claudin-2 distinctively upregulated in most inflammatory and infectious diseases of the intestine is rather a contributor or even only an indicator than the reason for an increased macromolecule passage through the intestinal wall. Though, claudin-2 itself contributes to diarrhea in many intestinal diseases via a leak flux mechanisms.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- 1.Anderson JM. Molecular structure of tight junctions and their role in epithelial transport. News Physiol Sci 2001; 16:126-30; PMID: [DOI] [PubMed] [Google Scholar]
- 2.Markov AG, Voronkova MA, Volgin GN, Yablonsky PK, Fromm M, Amasheh S. Tight junction proteins contribute to barrier properties in human pleura. Respir Physiol Neurobiol 2011; 175:331-5; PMID:; http://dx.doi.org/ 10.1016/j.resp.2010.12.012 [DOI] [PubMed] [Google Scholar]
- 3.Amasheh S, Meiri N, Gitter AH, Schoneberg T, Mankertz J, Schulzke JD, Fromm M. Claudin-2 expression induces cation-selective channels in tight junctions of epithelial cells. J Cell Sci 2002; 115:4969-76; PMID:; http://dx.doi.org/ 10.1242/jcs.00165 [DOI] [PubMed] [Google Scholar]
- 4.Rosenthal R, Milatz S, Krug SM, Oelrich B, Schulzke JD, Amasheh S, Gunzel D, Fromm M. Claudin-2, a component of the tight junction, forms a paracellular water channel. J Cell Sci 2010; 123:1913-21; PMID:; http://dx.doi.org/ 10.1242/jcs.060665 [DOI] [PubMed] [Google Scholar]
- 5.Furuse M, Fujita K, Hiiragi T, Fujimoto K, Tsukita S. Claudin-1 and -2: novel integral membrane proteins localizing at tight junctions with no sequence similarity to occludin. J Cell Biol 1998; 141:1539-50; PMID:; http://dx.doi.org/ 10.1083/jcb.141.7.1539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Itoh M, Furuse M, Morita K, Kubota K, Saitou M, Tsukita S. Direct binding of three tight junction-associated MAGUKs, ZO-1, ZO-2, and ZO-3, with the COOH termini of claudins. J Cell Biol 1999; 147:1351-63; PMID:; http://dx.doi.org/ 10.1083/jcb.147.6.1351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Stiffler MA, Chen JR, Grantcharova VP, Lei Y, Fuchs D, Allen JE, Zaslavskaia LA, MacBeath G. PDZ domain binding selectivity is optimized across the mouse proteome. Science 2007; 317:364-9; PMID:; http://dx.doi.org/ 10.1126/science.1144592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Furuse M, Sasaki H, Tsukita S. Manner of interaction of heterogeneous claudin species within and between tight junction strands. J Cell Biol 1999; 147:891-903; PMID:; http://dx.doi.org/ 10.1083/jcb.147.4.891 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lim TS, Vedula SR, Hunziker W, Lim CT. Kinetics of adhesion mediated by extracellular loops of claudin-2 as revealed by single-molecule force spectroscopy. J Mol Biol 2008; 381:681-91; PMID:; http://dx.doi.org/ 10.1016/j.jmb.2008.06.009 [DOI] [PubMed] [Google Scholar]
- 10.Van Itallie CM, Colegio OR, Anderson JM. The cytoplasmic tails of claudins can influence tight junction barrier properties through effects on protein stability. J Membr Biol 2004; 199:29-38; PMID:; http://dx.doi.org/ 10.1007/s00232-004-0673-z [DOI] [PubMed] [Google Scholar]
- 11.Angelow S, Yu AS. Cysteine mutagenesis to study the structure of claudin-2 paracellular pores. Ann N Y Acad Sci 2009; 1165:143-7; PMID:; http://dx.doi.org/ 10.1111/j.1749-6632.2009.04038.x [DOI] [PubMed] [Google Scholar]
- 12.Yu AS, Cheng MH, Angelow S, Gunzel D, Kanzawa SA, Schneeberger EE, Fromm M, Coalson RD. Molecular basis for cation selectivity in claudin-2-based paracellular pores: identification of an electrostatic interaction site. J Gen Physiol 2009; 133:111-27; PMID:; http://dx.doi.org/ 10.1085/jgp.200810154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Angelow S, Ahlstrom R, Yu AS. Biology of claudins. Am J Physiol Renal Physiol 2008; 295:F867-76; PMID:; http://dx.doi.org/ 10.1152/ajprenal.90264.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li J, Angelow S, Linge A, Zhuo M, Yu AS. Claudin-2 pore function requires an intramolecular disulfide bond between two conserved extracellular cysteines. Am J Physiol Cell Physiol 2013; 305:C190-6; PMID:; http://dx.doi.org/ 10.1152/ajpcell.00074.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li J, Zhuo M, Pei L, Rajagopal M, Yu AS. Comprehensive cysteine-scanning mutagenesis reveals claudin-2 pore-lining residues with different intrapore locations. J Biol Chem 2014; 289:6475-84P; PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li J, Zhuo M, Pei L, Yu AS. Conserved aromatic residue confers cation selectivity in claudin-2 and claudin-10b. J Biol Chem 2013; 288:22790-7; PMID:; http://dx.doi.org/ 10.1074/jbc.M113.484238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Inai T, Sengoku A, Hirose E, Iida H, Shibata Y. Comparative characterization of mouse rectum CMT93-I and -II cells by expression of claudin isoforms and tight junction morphology and function. Histochem Cell Biol 2008; 129:223-32; PMID:; http://dx.doi.org/ 10.1007/s00418-007-0360-0 [DOI] [PubMed] [Google Scholar]
- 18.Anderson JM, Van Itallie CM, Fanning AS. Setting up a selective barrier at the apical junction complex. Curr Opin Cell Biol 2004; 16:140-5; PMID:; http://dx.doi.org/ 10.1016/j.ceb.2004.01.005 [DOI] [PubMed] [Google Scholar]
- 19.Furuse M, Furuse K, Sasaki H, Tsukita S. Conversion of zonulae occludentes from tight to leaky strand type by introducing claudin-2 into Madin-Darby canine kidney I cells. J Cell Biol 2001; 153:263-72; PMID:; http://dx.doi.org/ 10.1083/jcb.153.2.263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Singh AB, Sugimoto K, Dhawan P, Harris RC. Juxtacrine activation of EGFR regulates claudin expression and increases transepithelial resistance. Am J Physiol Cell Physiol 2007; 293:C1660-8; PMID:; http://dx.doi.org/ 10.1152/ajpcell.00274.2007 [DOI] [PubMed] [Google Scholar]
- 21.Enck AH, Berger UV, Yu AS. Claudin-2 is selectively expressed in proximal nephron in mouse kidney. Am J Physiol Renal Physiol 2001; 281:F966-74; PMID: [DOI] [PubMed] [Google Scholar]
- 22.Kiuchi-Saishin Y, Gotoh S, Furuse M, Takasuga A, Tano Y, Tsukita S. Differential expression patterns of claudins, tight junction membrane proteins, in mouse nephron segments. J Am Soc Nephrol 2002; 13:875-86; PMID: [DOI] [PubMed] [Google Scholar]
- 23.Lameris AL, Huybers S, Kaukinen K, Makela TH, Bindels RJ, Hoenderop JG, Nevalainen PI. Expression profiling of claudins in the human gastrointestinal tract in health and during inflammatory bowel disease. Scand J Gastroenterol 2013; 48:58-69; PMID:; http://dx.doi.org/ 10.3109/00365521.2012.741616 [DOI] [PubMed] [Google Scholar]
- 24.Escaffit F, Boudreau F, Beaulieu JF. Differential expression of claudin-2 along the human intestine: implication of GATA-4 in the maintenance of claudin-2 in differentiating cells. J Cell Physiol 2005; 203:15-26; PMID:; http://dx.doi.org/ 10.1002/jcp.20189 [DOI] [PubMed] [Google Scholar]
- 25.Tamura A, Hayashi H, Imasato M, Yamazaki Y, Hagiwara A, Wada M, Noda T, Watanabe M, Suzuki Y, Tsukita S. Loss of claudin-15, but not claudin-2, causes Na+ deficiency and glucose malabsorption in mouse small intestine. Gastroenterology 2011; 140:913-23; PMID:; http://dx.doi.org/ 10.1053/j.gastro.2010.08.006 [DOI] [PubMed] [Google Scholar]
- 26.Suzuki H, Nishizawa T, Tani K, Yamazaki Y, Tamura A, Ishitani R, Dohmae N, Tsukita S, Nureki O, Fujiyoshi Y. Crystal structure of a claudin provides insight into the architecture of tight junctions. Science 2014; 344:304-7; PMID:; http://dx.doi.org/ 10.1126/science.1248571 [DOI] [PubMed] [Google Scholar]
- 27.Gunzel D, Fromm M. Claudins and other tight junction proteins. Compr Physiol 2012; 2:1819-52; PMID: [DOI] [PubMed] [Google Scholar]
- 28.Amasheh S, Dullat S, Fromm M, Schulzke JD, Buhr HJ, Kroesen AJ. Inflamed pouch mucosa possesses altered tight junctions indicating recurrence of inflammatory bowel disease. Int J Colorectal Dis 2009; 24:1149-56; PMID:; http://dx.doi.org/ 10.1007/s00384-009-0737-8 [DOI] [PubMed] [Google Scholar]
- 29.Prasad S, Mingrino R, Kaukinen K, Hayes KL, Powell RM, MacDonald TT, Collins JE. Inflammatory processes have differential effects on claudins 2, 3 and 4 in colonic epithelial cells. Lab Invest 2005; 85:1139-62; PMID:; http://dx.doi.org/ 10.1038/labinvest.3700316 [DOI] [PubMed] [Google Scholar]
- 30.Zeissig S, Burgel N, Gunzel D, Richter J, Mankertz J, Wahnschaffe U, Kroesen AJ, Zeitz M, Fromm M, Schulzke JD. Changes in expression and distribution of claudin 2, 5 and 8 lead to discontinuous tight junctions and barrier dysfunction in active Crohn's disease. Gut 2007; 56:61-72; PMID:; http://dx.doi.org/ 10.1136/gut.2006.094375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Heller F, Fromm A, Gitter AH, Mankertz J, Schulzke JD. Epithelial apoptosis is a prominent feature of the epithelial barrier disturbance in intestinal inflammation: effect of pro-inflammatory interleukin-13 on epithelial cell function. Mucosal Immunol 2008; 1 Suppl 1:S58-61; PMID:; http://dx.doi.org/ 10.1038/mi.2008.46 [DOI] [PubMed] [Google Scholar]
- 32.Mankertz J, Amasheh M, Krug SM, Fromm A, Amasheh S, Hillenbrand B, Tavalali S, Fromm M, Schulzke JD. TNFalpha up-regulates claudin-2 expression in epithelial HT-29/B6 cells via phosphatidylinositol-3-kinase signaling. Cell Tissue Res 2009; 336:67-77; PMID:; http://dx.doi.org/ 10.1007/s00441-009-0751-8 [DOI] [PubMed] [Google Scholar]
- 33.Suzuki T, Yoshinaga N, Tanabe S. Interleukin-6 (IL-6) regulates claudin-2 expression and tight junction permeability in intestinal epithelium. J Biol Chem 2011; 286:31263-71; PMID:; http://dx.doi.org/ 10.1074/jbc.M111.238147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Amasheh M, Fromm A, Krug SM, Amasheh S, Andres S, Zeitz M, Fromm M, Schulzke JD. TNFalpha-induced and berberine-antagonized tight junction barrier impairment via tyrosine kinase, Akt and NFkappaB signaling. J Cell Sci 2010; 123:4145-55; PMID:; http://dx.doi.org/ 10.1242/jcs.070896 [DOI] [PubMed] [Google Scholar]
- 35.Kinugasa T, Sakaguchi T, Gu X, Reinecker HC. Claudins regulate the intestinal barrier in response to immune mediators. Gastroenterology 2000; 118:1001-11; PMID:; http://dx.doi.org/ 10.1016/S0016-5085(00)70351-9 [DOI] [PubMed] [Google Scholar]
- 36.Sakaguchi T, Gu X, Golden HM, Suh E, Rhoads DB, Reinecker HC. Cloning of the human claudin-2 5'-flanking region revealed a TATA-less promoter with conserved binding sites in mouse and human for caudal-related homeodomain proteins and hepatocyte nuclear factor-1alpha. J Biol Chem 2002; 277:21361-70; PMID:; http://dx.doi.org/ 10.1074/jbc.M110261200 [DOI] [PubMed] [Google Scholar]
- 37.Mankertz J, Hillenbrand B, Tavalali S, Huber O, Fromm M, Schulzke JD. Functional crosstalk between Wnt signaling and Cdx-related transcriptional activation in the regulation of the claudin-2 promoter activity. Biochem Biophys Res Commun 2004; 314:1001-7; PMID:; http://dx.doi.org/ 10.1016/j.bbrc.2003.12.185 [DOI] [PubMed] [Google Scholar]
- 38.Boudreau F, Rings EH, van Wering HM, Kim RK, Swain GP, Krasinski SD, Moffett J, Grand RJ, Suh ER, Traber PG. Hepatocyte nuclear factor-1 alpha, GATA-4, and caudal related homeodomain protein Cdx2 interact functionally to modulate intestinal gene transcription. Implication for the developmental regulation of the sucrase-isomaltase gene. J Biol Chem 2002; 277:31909-17; PMID:; http://dx.doi.org/ 10.1074/jbc.M204622200 [DOI] [PubMed] [Google Scholar]
- 39.Al-Sadi R, Ye D, Boivin M, Guo S, Hashimi M, Ereifej L, Ma TY. Interleukin-6 modulation of intestinal epithelial tight junction permeability is mediated by JNK pathway activation of claudin-2 gene. PLoS One 2014; 9:e85345; PMID:; http://dx.doi.org/ 10.1371/journal.pone.0085345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rosen MJ, Frey MR, Washington MK, Chaturvedi R, Kuhnhein LA, Matta P, Revetta FL, Wilson KT, Polk DB. STAT6 activation in ulcerative colitis: a new target for prevention of IL-13-induced colon epithelial cell dysfunction. Inflamm Bowel Dis 2011; 17:2224-34; PMID:; http://dx.doi.org/ 10.1002/ibd.21628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rosen MJ, Chaturvedi R, Washington MK, Kuhnhein LA, Moore PD, Coggeshall SS, McDonough EM, Weitkamp JH, Singh AB, Coburn LA, et al. STAT6 deficiency ameliorates severity of oxazolone colitis by decreasing expression of claudin-2 and Th2-inducing cytokines. J Immunol 2013; 190:1849-58; PMID:; http://dx.doi.org/ 10.4049/jimmunol.1201373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Weber CR, Raleigh DR, Su L, Shen L, Sullivan EA, Wang Y, Turner JR. Epithelial myosin light chain kinase activation induces mucosal interleukin-13 expression to alter tight junction ion selectivity. J Biol Chem 2010; 285:12037-46; PMID:; http://dx.doi.org/ 10.1074/jbc.M109.064808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mazzon E, Cuzzocrea S. Absence of functional peroxisome proliferator-activated receptor-alpha enhanced ileum permeability during experimental colitis. Shock 2007; 28:192-201; PMID:; http://dx.doi.org/ 10.1097/SHK.0b013e318033eb29 [DOI] [PubMed] [Google Scholar]
- 44.González-Mariscal L, Garay E, Quirós M. Regulation of claudins by posttranslational modifications and cell-signaling cascades. Curr Top Membr 65: 113-150, 2010. [Google Scholar]
- 45.Van Itallie CM, Tietgens AJ, LoGrande K, Aponte A, Gucek M, Anderson JM. Phosphorylation of claudin-2 on serine 208 promotes membrane retention and reduces trafficking to lysosomes. J Cell Sci 2012; 125:4902-12; PMID:; http://dx.doi.org/ 10.1242/jcs.111237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Van Itallie CM, Mitic LL, Anderson JM. SUMOylation of claudin-2. Ann N Y Acad Sci 2012; 1258:60-4; PMID:; http://dx.doi.org/ 10.1111/j.1749-6632.2012.06541.x [DOI] [PubMed] [Google Scholar]
- 47.Angelow S, Schneeberger EE, Yu AS. Claudin-8 expression in renal epithelial cells augments the paracellular barrier by replacing endogenous claudin-2. J Membr Biol 2007; 215:147-59; PMID:; http://dx.doi.org/ 10.1007/s00232-007-9014-3 [DOI] [PubMed] [Google Scholar]
- 48.Yu AS, Enck AH, Lencer WI, Schneeberger EE. Claudin-8 expression in Madin-Darby canine kidney cells augments the paracellular barrier to cation permeation. J Biol Chem 2003; 278:17350-9; PMID:; http://dx.doi.org/ 10.1074/jbc.M213286200 [DOI] [PubMed] [Google Scholar]
- 49.Epple HJ, Zeitz M. Intestinal mucosal barrier function in HIV infection. Ann. N.Y. Acad. Sci. 2012; 1258:19-24. [DOI] [PubMed] [Google Scholar]
- 50.Heller F, Florian P, Bojarski C, Richter J, Christ M, Hillenbrand B, Mankertz J, Gitter AH, Burgel N, Fromm M, et al. Interleukin-13 is the key effector Th2 cytokine in ulcerative colitis that affects epithelial tight junctions, apoptosis, and cell restitution. Gastroenterology 2005; 129:550-64; PMID:; http://dx.doi.org/ 10.1016/j.gastro.2005.05.002 [DOI] [PubMed] [Google Scholar]
- 51.Oshima T, Miwa H, Joh T. Changes in the expression of claudins in active ulcerative colitis. J Gastroenterol Hepatol 2008; 23 Suppl 2:S146-50; PMID:; http://dx.doi.org/ 10.1111/j.1440-1746.2008.05405.x [DOI] [PubMed] [Google Scholar]
- 52.Schumann M, Gunzel D, Buergel N, Richter JF, Troeger H, May C, Fromm A, Sorgenfrei D, Daum S, Bojarski C, et al. Cell polarity-determining proteins Par-3 and PP-1 are involved in epithelial tight junction defects in coeliac disease. Gut 2011; 61:220-8; PMID:; http://dx.doi.org/ 10.1136/gutjnl-2011-300123 [DOI] [PubMed] [Google Scholar]
- 53.Das P, Goswami P, Das TK, Nag T, Sreenivas V, Ahuja V, Panda SK, Gupta SD, Makharia GK. Comparative tight junction protein expressions in colonic Crohn's disease, ulcerative colitis, and tuberculosis: a new perspective. Virchows Arch 2012; 460:261-70; PMID:; http://dx.doi.org/ 10.1007/s00428-012-1195-1 [DOI] [PubMed] [Google Scholar]
- 54.Marchiando AM, Shen L, Graham WV, Weber CR, Schwarz BT, Austin JR, 2nd, Raleigh DR, Guan Y, Watson AJ, Montrose MH, et al. Caveolin-1-dependent occludin endocytosis is required for TNF-induced tight junction regulation in vivo. J Cell Biol 2010; 189:111-26; PMID:; http://dx.doi.org/ 10.1083/jcb.200902153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Desreumaux P, Brandt E, Gambiez L, Emilie D, Geboes K, Klein O, Ectors N, Cortot A, Capron M, Colombel JF. Distinct cytokine patterns in early and chronic ileal lesions of Crohn's disease. Gastroenterology 1997; 113:118-26; PMID:; http://dx.doi.org/ 10.1016/S0016-5085(97)70116-1 [DOI] [PubMed] [Google Scholar]
- 56.Knutson L, Ahrenstedt O, Odlind B, Hallgren R. The jejunal secretion of histamine is increased in active Crohn's disease. Gastroenterology 1990; 98:849-54; PMID:; http://dx.doi.org/ 10.1016/0016-5085(90)90006-M [DOI] [PubMed] [Google Scholar]
- 57.Zorzi F, Monteleone I, Sarra M, Calabrese E, Marafini I, Cretella M, Sedda S, Biancone L, Pallone F, Monteleone G. Distinct profiles of effector cytokines mark the different phases of Crohn's disease. PLoS One 2013; 8:e54562; PMID:; http://dx.doi.org/ 10.1371/journal.pone.0054562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Andoh A, Zhang Z, Inatomi O, Fujino S, Deguchi Y, Araki Y, Tsujikawa T, Kitoh K, Kim-Mitsuyama S, Takayanagi A, et al. Interleukin-22, a member of the IL-10 subfamily, induces inflammatory responses in colonic subepithelial myofibroblasts. Gastroenterology 2005; 129:969-84; PMID:; http://dx.doi.org/ 10.1053/j.gastro.2005.06.071 [DOI] [PubMed] [Google Scholar]
- 59.Brand S, Beigel F, Olszak T, Zitzmann K, Eichhorst ST, Otte JM, Diepolder H, Marquardt A, Jagla W, Popp A, et al. IL-22 is increased in active Crohn's disease and promotes proinflammatory gene expression and intestinal epithelial cell migration. Am J Physiol Gastrointest Liver Physiol 2006; 290:G827-38; PMID:; http://dx.doi.org/ 10.1152/ajpgi.00513.2005 [DOI] [PubMed] [Google Scholar]
- 60.John LJ, Fromm M, Schulzke JD. Epithelial barriers in intestinal inflammation. Antioxid Redox Signal 2011; 15:1255-70; PMID:; http://dx.doi.org/ 10.1089/ars.2011.3892 [DOI] [PubMed] [Google Scholar]
- 61.Amasheh M, Grotjohann I, Amasheh S, Fromm A, Soderholm JD, Zeitz M, Fromm M, Schulzke JD. Regulation of mucosal structure and barrier function in rat colon exposed to tumor necrosis factor alpha and interferon gamma in vitro: a novel model for studying the pathomechanisms of inflammatory bowel disease cytokines. Scand J Gastroenterol 2009; 44:1226-35; PMID:; http://dx.doi.org/ 10.1080/00365520903131973 [DOI] [PubMed] [Google Scholar]
- 62.Blair SA, Kane SV, Clayburgh DR, Turner JR. Epithelial myosin light chain kinase expression and activity are upregulated in inflammatory bowel disease. Lab Invest 2006; 86:191-201; PMID:; http://dx.doi.org/ 10.1038/labinvest.3700373 [DOI] [PubMed] [Google Scholar]
- 63.Al-Sadi R, Ye D, Said HM, Ma TY. Cellular and molecular mechanism of interleukin-1beta modulation of Caco-2 intestinal epithelial tight junction barrier. J Cell Mol Med 2011; 15:970-82; PMID:; http://dx.doi.org/ 10.1111/j.1582-4934.2010.01065.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ye D, Ma TY. Cellular and molecular mechanisms that mediate basal and tumour necrosis factor-alpha-induced regulation of myosin light chain kinase gene activity. J Cell Mol Med 2008; 12:1331-46; PMID:; http://dx.doi.org/ 10.1111/j.1582-4934.2008.00302.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Shen L, Black ED, Witkowski ED, Lencer WI, Guerriero V, Schneeberger EE, Turner JR. Myosin light chain phosphorylation regulates barrier function by remodeling tight junction structure. J Cell Sci 2006; 119:2095-106; PMID:; http://dx.doi.org/ 10.1242/jcs.02915 [DOI] [PubMed] [Google Scholar]
- 66.Bruewer M, Utech M, Ivanov AI, Hopkins AM, Parkos CA, Nusrat A. Interferon-gamma induces internalization of epithelial tight junction proteins via a macropinocytosis-like process. Faseb J 2005; 19:923-33; PMID:; http://dx.doi.org/ 10.1096/fj.04-3260com [DOI] [PubMed] [Google Scholar]
- 67.Irvine EJ, Marshall JK. Increased intestinal permeability precedes the onset of Crohn's disease in a subject with familial risk. Gastroenterology 2000; 119:1740-4; PMID:; http://dx.doi.org/ 10.1053/gast.2000.20231 [DOI] [PubMed] [Google Scholar]
- 68.Wyatt J, Vogelsang H, Hubl W, Waldhoer T, Lochs H. Intestinal permeability and the prediction of relapse in Crohn's disease. Lancet 1993; 341:1437-9; PMID:; http://dx.doi.org/ 10.1016/0140-6736(93)90882-H [DOI] [PubMed] [Google Scholar]
- 69.Lala S, Ogura Y, Osborne C, Hor SY, Bromfield A, Davies S, Ogunbiyi O, Nunez G, Keshav S. Crohn's disease and the NOD2 gene: a role for paneth cells. Gastroenterology 2003; 125:47-57; PMID:; http://dx.doi.org/ 10.1016/S0016-5085(03)00661-9 [DOI] [PubMed] [Google Scholar]
- 70.Buhner S, Buning C, Genschel J, Kling K, Herrmann D, Dignass A, Kuechler I, Krueger S, Schmidt HH, Lochs H. Genetic basis for increased intestinal permeability in families with Crohn's disease: role of CARD15 3020insC mutation? Gut 2006; 55:342-7; PMID:; http://dx.doi.org/ 10.1136/gut.2005.065557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.D'Inca R, Annese V, di Leo V, Latiano A, Quaino V, Abazia C, Vettorato MG, Sturniolo GC. Increased intestinal permeability and NOD2 variants in familial and sporadic Crohn's disease. Aliment Pharmacol Ther 2006; 23:1455-61; PMID:; http://dx.doi.org/ 10.1111/j.1365-2036.2006.02916.x [DOI] [PubMed] [Google Scholar]
- 72.Voss E, Wehkamp J, Wehkamp K, Stange EF, Schroder JM, Harder J. NOD2/CARD15 mediates induction of the antimicrobial peptide human beta-defensin-2. J Biol Chem 2006; 281:2005-11; PMID:; http://dx.doi.org/ 10.1074/jbc.M511044200 [DOI] [PubMed] [Google Scholar]
- 73.Wehkamp J, Harder J, Weichenthal M, Schwab M, Schaffeler E, Schlee M, Herrlinger KR, Stallmach A, Noack F, Fritz P, et al. NOD2 (CARD15) mutations in Crohn's disease are associated with diminished mucosal alpha-defensin expression. Gut 2004; 53:1658-64; PMID:; http://dx.doi.org/ 10.1136/gut.2003.032805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Rosenstiel P, Fantini M, Brautigam K, Kuhbacher T, Waetzig GH, Seegert D, Schreiber S. TNF-alpha and IFN-gamma regulate the expression of the NOD2 (CARD15) gene in human intestinal epithelial cells. Gastroenterology 2003; 124:1001-9; PMID:; http://dx.doi.org/ 10.1053/gast.2003.50157 [DOI] [PubMed] [Google Scholar]
- 75.Bucker R, Troeger H, Kleer J, Fromm M, Schulzke JD. Arcobacter butzleri induces barrier dysfunction in intestinal HT-29/B6 cells. J Infect Dis 2009; 200:756-64; PMID:; http://dx.doi.org/ 10.1086/600868 [DOI] [PubMed] [Google Scholar]
- 76.Hering NA, Richter JF, Krug SM, Gunzel D, Fromm A, Bohn E, Rosenthal R, Bucker R, Fromm M, Troeger H, et al. Yersinia enterocolitica induces epithelial barrier dysfunction through regional tight junction changes in colonic HT-29/B6 cell monolayers. Lab Invest 2011; 91:310-24; PMID:; http://dx.doi.org/ 10.1038/labinvest.2010.180 [DOI] [PubMed] [Google Scholar]
- 77.Barreau F, Meinzer U, Chareyre F, Berrebi D, Niwa-Kawakita M, Dussaillant M, Foligne B, Ollendorff V, Heyman M, Bonacorsi S, et al. CARD15/NOD2 is required for Peyer's patches homeostasis in mice. PLoS One 2007; 2:e523; PMID:; http://dx.doi.org/ 10.1371/journal.pone.0000523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Wisner DM, Harris LR, 3rd, Green CL, Poritz LS. Opposing regulation of the tight junction protein claudin-2 by interferon-gamma and interleukin-4. J Surg Res 2008; 144:1-7; PMID:; http://dx.doi.org/ 10.1016/j.jss.2007.03.059 [DOI] [PubMed] [Google Scholar]
- 79.Olson TS, Reuter BK, Scott KG, Morris MA, Wang XM, Hancock LN, Burcin TL, Cohn SM, Ernst PB, Cominelli F, et al. The primary defect in experimental ileitis originates from a nonhematopoietic source. J Exp Med 2006; 203:541-52; PMID:; http://dx.doi.org/ 10.1084/jem.20050407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Gitter AH, Wullstein F, Fromm M, Schulzke JD. Epithelial barrier defects in ulcerative colitis: characterization and quantification by electrophysiological imaging. Gastroenterology 2001; 121:1320-8; PMID:; http://dx.doi.org/ 10.1053/gast.2001.29694 [DOI] [PubMed] [Google Scholar]
- 81.Schmitz H, Barmeyer C, Fromm M, Runkel N, Foss HD, Bentzel CJ, Riecken EO, Schulzke JD. Altered tight junction structure contributes to the impaired epithelial barrier function in ulcerative colitis. Gastroenterology 1999; 116:301-9; PMID:; http://dx.doi.org/ 10.1016/S0016-5085(99)70126-5 [DOI] [PubMed] [Google Scholar]
- 82.Bojarski C, Gitter AH, Bendfeldt K, Mankertz J, Schmitz H, Wagner S, Fromm M, Schulzke JD. Permeability of human HT-29/B6 colonic epithelium as a function of apoptosis. J Physiol 2001; 535:541-52; PMID:; http://dx.doi.org/ 10.1111/j.1469-7793.2001.00541.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Gitter AH, Bendfeldt K, Schulzke JD, Fromm M. Leaks in the epithelial barrier caused by spontaneous and TNF-alpha-induced single-cell apoptosis. Faseb J 2000; 14:1749-53; PMID:; http://dx.doi.org/ 10.1096/fj.99-0898com [DOI] [PubMed] [Google Scholar]
- 84.Ahn SH, Shah YM, Inoue J, Morimura K, Kim I, Yim S, Lambert G, Kurotani R, Nagashima K, Gonzalez FJ, et al. Hepatocyte nuclear factor 4alpha in the intestinal epithelial cells protects against inflammatory bowel disease. Inflamm Bowel Dis 2008; 14:908-20; PMID:; http://dx.doi.org/ 10.1002/ibd.20413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Cattin AL, Le Beyec J, Barreau F, Saint-Just S, Houllier A, Gonzalez FJ, Robine S, Pincon-Raymond M, Cardot P, Lacasa M, et al. Hepatocyte nuclear factor 4alpha, a key factor for homeostasis, cell architecture, and barrier function of the adult intestinal epithelium. Mol Cell Biol 2009; 29:6294-308; PMID:; http://dx.doi.org/ 10.1128/MCB.00939-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Visser J, Rozing J, Sapone A, Lammers K, Fasano A. Tight junctions, intestinal permeability, and autoimmunity: celiac disease and type 1 diabetes paradigms. Ann N Y Acad Sci 2009; 1165:195-205; PMID:; http://dx.doi.org/ 10.1111/j.1749-6632.2009.04037.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Matysiak-Budnik T, Candalh C, Dugave C, Namane A, Cellier C, Cerf-Bensussan N, Heyman M. Alterations of the intestinal transport and processing of gliadin peptides in celiac disease. Gastroenterology 2003; 125:696-707; PMID:; http://dx.doi.org/ 10.1016/S0016-5085(03)01049-7 [DOI] [PubMed] [Google Scholar]
- 88.Schumann M, Richter JF, Wedell I, Moos V, Zimmermann-Kordmann M, Schneider T, Daum S, Zeitz M, Fromm M, Schulzke JD. Mechanisms of epithelial translocation of the alpha(2)-gliadin-33mer in coeliac sprue. Gut 2008; 57:747-54; PMID: [DOI] [PubMed] [Google Scholar]
- 89.Ciccocioppo R, Finamore A, Ara C, Di Sabatino A, Mengheri E, Corazza GR. Altered expression, localization, and phosphorylation of epithelial junctional proteins in celiac disease. Am J Clin Pathol 2006; 125:502-11; PMID:; http://dx.doi.org/ 10.1309/DTYRA91G8R0KTM8M [DOI] [PubMed] [Google Scholar]
- 90.Schumann M, Kamel S, Pahlitzsch ML, Lebenheim L, May C, Krauss M, Hummel M, Daum S, Fromm M, Schulzke JD. Defective tight junctions in refractory celiac disease. Ann N Y Acad Sci 2012; 1258:43-51; PMID:; http://dx.doi.org/ 10.1111/j.1749-6632.2012.06565.x [DOI] [PubMed] [Google Scholar]
- 91.Szakal DN, Gyorffy H, Arato A, Cseh A, Molnar K, Papp M, Dezsofi A, Veres G. Mucosal expression of claudins 2, 3 and 4 in proximal and distal part of duodenum in children with coeliac disease. Virchows Arch 2010; 456:245-50; PMID:; http://dx.doi.org/ 10.1007/s00428-009-0879-7 [DOI] [PubMed] [Google Scholar]
- 92.van Elburg RM, Uil JJ, Mulder CJ, Heymans HS. Intestinal permeability in patients with coeliac disease and relatives of patients with coeliac disease. Gut 1993; 34:354-7; PMID:; http://dx.doi.org/ 10.1136/gut.34.3.354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Menard S, Lebreton C, Schumann M, Matysiak-Budnik T, Dugave C, Bouhnik Y, Malamut G, Cellier C, Allez M, Crenn P, et al. Paracellular versus transcellular intestinal permeability to gliadin peptides in active celiac disease. Am J Pathol 2012; 180:608-15; PMID:; http://dx.doi.org/ 10.1016/j.ajpath.2011.10.019 [DOI] [PubMed] [Google Scholar]
- 94.Kohl D, Ashkenazi A, Ben-Shaul Y, Bacher A. Tight junctions of jejunal surface and crypt cells in celiac disease: a freeze-fracture study. J Pediatr Gastroenterol Nutr 1987; 6:57-65; PMID: [PubMed] [Google Scholar]
- 95.Schulzke JD, Bentzel CJ, Schulzke I, Riecken EO, Fromm M. Epithelial tight junction structure in the jejunum of children with acute and treated celiac sprue. Pediatr Res 1998; 43:435-41; PMID:; http://dx.doi.org/ 10.1203/00006450-199804000-00001 [DOI] [PubMed] [Google Scholar]
- 96.Utech M, Ivanov AI, Samarin SN, Bruewer M, Turner JR, Mrsny RJ, Parkos CA, Nusrat A. Mechanism of IFN-gamma-induced endocytosis of tight junction proteins: myosin II-dependent vacuolarization of the apical plasma membrane. Mol Biol Cell 2005; 16:5040-52; PMID:; http://dx.doi.org/ 10.1091/mbc.E05-03-0193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Brenchley JM, Price DA, Schacker TW, Asher TE, Silvestri G, Rao S, Kazzaz Z, Bornstein E, Lambotte O, Altmann D, et al. Microbial translocation is a cause of systemic immune activation in chronic HIV infection. Nat Med 2006; 12:1365-71; PMID:; http://dx.doi.org/ 10.1038/nm1511 [DOI] [PubMed] [Google Scholar]
- 98.Brenchley JM, Schacker TW, Ruff LE, Price DA, Taylor JH, Beilman GJ, Nguyen PL, Khoruts A, Larson M, Haase AT, et al. CD4+ T cell depletion during all stages of HIV disease occurs predominantly in the gastrointestinal tract. J Exp Med 2004; 200:749-59; PMID:; http://dx.doi.org/ 10.1084/jem.20040874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Epple HJ, Allers K, Troger H, Kuhl A, Erben U, Fromm M, Zeitz M, Loddenkemper C, Schulzke JD, Schneider T. Acute HIV infection induces mucosal infiltration with CD4+ and CD8+ T cells, epithelial apoptosis, and a mucosal barrier defect. Gastroenterology 2010; 139:1289-300; PMID:; http://dx.doi.org/ 10.1053/j.gastro.2010.06.065 [DOI] [PubMed] [Google Scholar]
- 100.Epple HJ, Schneider T, Troeger H, Kunkel D, Allers K, Moos V, Amasheh M, Loddenkemper C, Fromm M, Zeitz M, et al. Impairment of the intestinal barrier is evident in untreated but absent in suppressively treated HIV-infected patients. Gut 2009; 58:220-7; PMID:; http://dx.doi.org/ 10.1136/gut.2008.150425 [DOI] [PubMed] [Google Scholar]
- 101.Nazli A, Chan O, Dobson-Belaire WN, Ouellet M, Tremblay MJ, Gray-Owen SD, Arsenault AL, Kaushic C. Exposure to HIV-1 directly impairs mucosal epithelial barrier integrity allowing microbial translocation. PLoS Pathog 2010; 6:e1000852; PMID:; http://dx.doi.org/ 10.1371/journal.ppat.1000852 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Sankaran S, George MD, Reay E, Guadalupe M, Flamm J, Prindiville T, Dandekar S. Rapid onset of intestinal epithelial barrier dysfunction in primary human immunodeficiency virus infection is driven by an imbalance between immune response and mucosal repair and regeneration. J Virol 2008; 82:538-45; PMID:; http://dx.doi.org/ 10.1128/JVI.01449-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Fackler OT, Schafer M, Schmidt W, Zippel T, Heise W, Schneider T, Zeitz M, Riecken EO, Mueller-Lantzsch N, Ullrich R. HIV-1 p24 but not proviral load is increased in the intestinal mucosa compared with the peripheral blood in HIV-infected patients. Aids 1998; 12:139-46; PMID:; http://dx.doi.org/ 10.1097/00002030-199802000-00003 [DOI] [PubMed] [Google Scholar]
- 104.Mattapallil JJ, Douek DC, Hill B, Nishimura Y, Martin M, Roederer M. Massive infection and loss of memory CD4+ T cells in multiple tissues during acute SIV infection. Nature 2005; 434:1093-7; PMID:; http://dx.doi.org/ 10.1038/nature03501 [DOI] [PubMed] [Google Scholar]
- 105.Buccigrossi V, Laudiero G, Nicastro E, Miele E, Esposito F, Guarino A. The HIV-1 transactivator factor (Tat) induces enterocyte apoptosis through a redox-mediated mechanism. PLoS One 2011; 6:e29436; PMID:; http://dx.doi.org/ 10.1371/journal.pone.0029436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Clayton F, Kotler DP, Kuwada SK, Morgan T, Stepan C, Kuang J, Le J, Fantini J. Gp120-induced Bob/GPR15 activation: a possible cause of human immunodeficiency virus enteropathy. Am J Pathol 2001; 159:1933-9; PMID:; http://dx.doi.org/ 10.1016/S0002-9440(10)63040-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Quigley MA, Kamali A, Kinsman J, Kamulegeya I, Nakiyingi-Miiro J, Kiwuwa S, Kengeya-Kayondo JF, Carpenter LM, Whitworth JA. The impact of attending a behavioural intervention on HIV incidence in Masaka, Uganda. Aids 2004; 18:2055-63; PMID:; http://dx.doi.org/ 10.1097/00002030-200410210-00010 [DOI] [PubMed] [Google Scholar]
- 108.Shacklett BL, Cox CA, Quigley MF, Kreis C, Stollman NH, Jacobson MA, Andersson J, Sandberg JK, Nixon DF. Abundant expression of granzyme A, but not perforin, in granules of CD8+ T cells in GALT: implications for immune control of HIV-1 infection. J Immunol 2004; 173:641-8; PMID:; http://dx.doi.org/ 10.4049/jimmunol.173.1.641 [DOI] [PubMed] [Google Scholar]
- 109.Kim CJ, Nazli A, Rojas OL, Chege D, Alidina Z, Huibner S, Mujib S, Benko E, Kovacs C, Shin LY, et al. A role for mucosal IL-22 production and Th22 cells in HIV-associated mucosal immunopathogenesis. Mucosal Immunol 2012; 5:670-80; PMID:; http://dx.doi.org/ 10.1038/mi.2012.72 [DOI] [PubMed] [Google Scholar]
- 110.Fischer A, Gluth M, Pape UF, Wiedenmann B, Theuring F, Baumgart DC. Adalimumab prevents barrier dysfunction and antagonizes distinct effects of TNF-alpha on tight junction proteins and signaling pathways in intestinal epithelial cells. Am J Physiol Gastrointest Liver Physiol 2013; 304:G970-9; PMID:; http://dx.doi.org/ 10.1152/ajpgi.00183.2012 [DOI] [PubMed] [Google Scholar]
- 111.Vieira EL, Leonel AJ, Sad AP, Beltrao NR, Costa TF, Ferreira TM, Gomes-Santos AC, Faria AM, Peluzio MC, Cara DC, et al. Oral administration of sodium butyrate attenuates inflammation and mucosal lesion in experimental acute ulcerative colitis. J Nutr Biochem 2012; 23:430-6; PMID:; http://dx.doi.org/ 10.1016/j.jnutbio.2011.01.007 [DOI] [PubMed] [Google Scholar]
- 112.Daly K, Shirazi-Beechey SP. Microarray analysis of butyrate regulated genes in colonic epithelial cells. DNA Cell Biol 2006; 25:49-62; PMID:; http://dx.doi.org/ 10.1089/dna.2006.25.49 [DOI] [PubMed] [Google Scholar]
- 113.Ploger S, Stumpff F, Penner GB, Schulzke JD, Gabel G, Martens H, Shen Z, Gunzel D, Aschenbach JR. Microbial butyrate and its role for barrier function in the gastrointestinal tract. Ann N Y Acad Sci 2012; 1258:52-9; PMID:; http://dx.doi.org/ 10.1111/j.1749-6632.2012.06553.x [DOI] [PubMed] [Google Scholar]
- 114.Zhang Q, Piao XL, Piao XS, Lu T, Wang D, Kim SW. Preventive effect of Coptis chinensis and berberine on intestinal injury in rats challenged with lipopolysaccharides. Food Chem Toxicol 2011; 49:61-9; PMID:; http://dx.doi.org/ 10.1016/j.fct.2010.09.032 [DOI] [PubMed] [Google Scholar]
- 115.Wang N, Yu H, Ma J, Wu W, Zhao D, Shi X, Tian H, Jiang H. Evidence for tight junction protein disruption in intestinal mucosa of malignant obstructive jaundice patients. Scand J Gastroenterol 2010; 45:191-9; PMID:; http://dx.doi.org/ 10.3109/00365520903406701 [DOI] [PubMed] [Google Scholar]
- 116.Burgel N, Bojarski C, Mankertz J, Zeitz M, Fromm M, Schulzke JD. Mechanisms of diarrhea in collagenous colitis. Gastroenterology 2002; 123:433-43; PMID:; http://dx.doi.org/ 10.1053/gast.2002.34784 [DOI] [PubMed] [Google Scholar]


