Abstract
In the beating heart, mechanical stretch triggers the production of reactive oxygen or nitrogen species that target Ca2+-signaling proteins. Termed mechano-chemo transduction, this pathway “tunes” the calcium release machinery in the healthy heart; when dysregulated, it contributes to disease. In this issue of Science Signaling, Jian et al. used a “cell-in-gel” method to show that contractions in healthy heart cells elicit a steep, viscosity-dependent increase in mechano-chemo transduction in which nitric oxide synthase (NOS), NADPH oxidase 2 (Nox2), and Ca2+/calmodulin-dependent kinase II (CaMKII) contribute. These authors provide evidence for a role of neuronal NOS (nNOS) over endothelial NOS; they supported their findings with super-resolution microscopy, which localized nNOS nearest to the Ca2+ release sites. In a disease model, signaling through nNOS and CaMKII rather than through Nox2 was enhanced, supporting the independent mechano-activation of these enzymes. The coupling of these quantitative approaches will provide a new understanding of mechano-chemo transduction.
More than 60 years ago, Sandow coined the term excitation-contraction (EC) coupling and proposed cytosolic calcium as the critical activator of muscle contraction (1); it has now become clear that contraction itself provides important feedback in shaping this calcium signal. In this issue of Science Signaling, Jian et al. extend the concept of mechano-chemo transduction, a term that encompasses how mechanical stress dynamically modulates calcium handling through chemical effectors (2). Jian et al. showed the utility of a tool in which they embedded cardiomyocytes in a variable-viscosity gel that interacts with the cell membrane. The “cell-in-gel” contracts under viscoelastic load with stress transmitted in three dimensions (3). Using this technique, they identified neuronal nitric oxide synthase (nNOS), NADPH oxidase 2 (Nox2), and Ca2+/calmodulin-dependent kinase II (CaMKII) as key enzymes involved in the mechanical modulation of EC coupling and raise intriguing questions about the mechanism involved. Here, we offer a mechanistic discussion and testable hypotheses for future work aimed at defining the interplay of cell mechanics and calcium signaling in healthy and diseased myocardium.
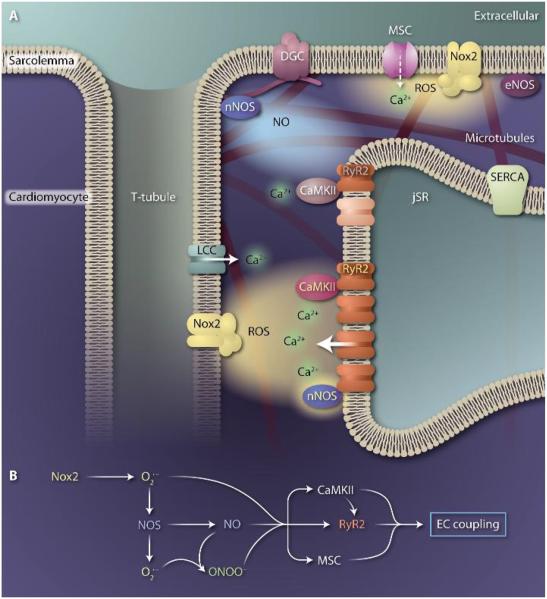
Key players in cardiomyocyte mechano-chemo transduction are located in the dyad (Fig. 1), the intersection between a junctional sarcoplasmic reticulum (jSR) and transverse tubule. The type 2 ryanodine receptors (RyR2s) cluster in the jSR membrane, directly opposed to L-type calcium channels in the t-tubule, and are activated by calciuminduced calcium release. The importance of RyR2 regulation is underscored not simply by its role in releasing Ca2+ stores to drive contraction, but also because abnormal RyR2 activity contributes to Ca2+-dependent arrhythmias, hypertrophy, and heart failure. At the dyad, previously established regulators of RyR2 function include nNOS and endothelial NOS (eNOS), both of which produce NO; Nox2, which produces superoxide that is rapidly dismuted to hydrogen peroxide, resulting in ROS in the dyadic space; and CaMKII.
Fig. 1. Mechano-chemo transduction in the heart.
(A) Simplified model of key players at the calcium release unit of a cardiomyocyte. Upon mechanical stress, mechanosensitive enzymes generate local signals that target calcium handling proteins. These include Nox2, which produces ROS in the sarcolemmal and T-tubule membrane, and NOS, which produces NO and is localized to the SR and sarcolemma membranes as part of the dystrophin-dystroglycan complex (DGC). ROS and NO can directly regulate RyR2 activity through oxidation and nitrosylation, respectively, or can activate CaMKII, which modulates RyR2 through phosphorylation. Superoxide in the subspace can uncouple nNOS, resulting in superoxide rather than NO production. Superoxide and NO can also interact to form peroxynitrite. Additional targets of ROS and NO include the mechanosensitive channel (MSC), the L-type Ca2+ channel (LCC), and the sarcoplasmic/endoplasmic reticulum Ca2+ ATPase (SERCA). ROS and NO preferentially modify target proteins in their immediate vicinity, as depicted by the sensitized RyR2 and CaMKII (in darker colors). Although mechano-chemo coupling tunes EC coupling in the healthy heart, altered localization or expression of key players underscores the destabilized calcium signaling seen with increased mechanical stress and in disease. (B) Potential linear reaction schemes involved in mechano-chemo transduction.
The principal finding of Jian et al. is that compared to myocytes contracting under little or no load, cell-in-gel contractions increased global calcium release and led to a high frequency of Ca2+ sparks (4) without detectably changing SR calcium content. Although this finding is consistent with enhanced activation of RyR2, simple pump-leak balance (5) would suggest that SR Ca2+ content should decrease, thereby implicating additional factors. The authors also propose that the enhanced RyR2 activation contributes to the Anrep effect, the slow augmentation of calcium release and contractility that occurs with an increase in cell stress (6).
Furthermore, the authors identified NOS as a key regulator of the augmented response. Although this result extends the work of the Solott group and others (7), the use of pharmacologic agents and genetic ablation revealed that nNOS rather than eNOS was the dominant chemoeffector, and super-resolution imaging showed colocalization of nNOS near the RyR2s. This combination of live-cell and super-resolution imaging sets a new standard for examining the micro-domain signaling of these molecules.
Nox2 or CaMKII inhibition were equally effective to nNOS inhibition in blocking mechanically induced activation of RyR2 during cell-in-gel contractions. This confirms reports from our group and others showing that Nox2-generated ROS (X-ROS) is a potent mechano-chemoeffector of RyR2, but also raises the question of apparently redundant signaling.
One possibility is that Nox2 and NOS are triggered by separate mechanical stimuli that are each activated during cell-in-gel contractions. X-ROS signaling is activated by longitudinal and axial stresses through the microtubule cytoskeleton (2, 8, 9), whereas NOS is modulated by membrane shear stress through yet-unidentified elements. As modeled (10), a cell-in-gel contraction results in both longitudinal and axial stress as well as three-dimensional shear stress at the membrane. This provides a parsimonious explanation for dual activation of NOS and Nox2 in the cell-in-gel experiments, yet incompletely explains their redundant roles.
Another nuance of the cell-in-gel system that may contribute to augmented signaling is the steep, nonlinear dependence of RyR2 activation on gel density. In fact, healthy cells contracting in the denser gel exhibited very high Ca2+ spark frequencies consistent with destabilized RyR2s more commonly seen in disease models. Although we speculate that the stiffer gel may more closely approximate supraphysiologic stress, the signaling generated in these experiments has resulted in important new findings. A critical next step is to define the viscoelastic environment of the healthy and diseased heart in situ; with these parameters, the gel can be adjusted to evaluate cardiomyocytes under pathophysiologic and physiologic stresses. An additional advancement would be the ability to apply diastolic stretch to the cell-in-gel system to separate the effects of preload and afterload stress, as each may have different effects on downstream signaling.
Jian et al.'s investigation into an arrhythmia model arising from a troponin T (TnT) mutation reveals that similar stresses may elicit different signaling depending on the pathophysiological background. In contrast to healthy cells, the cell-in-gel enhancement of RyR2 activation in TnT mutant myocytes was dependent on NOS and CaMKII but in dependent of Nox2—a finding that supports independent activation pathways. However, in a dystrophin-deficient cardiomyopathy model, the microtubule cytoskeleton is stiffened and Nox2 abundance is increased; consistently, microtubule-dependent Nox2 hyperactivation underlies the Ca2+ instability induced by preload stress (2). Results in disease states will undoubtedly correlate with the abundance and regulation of the key mechanosensitive enzymes and transducers; without this information, it is difficult to place the TnT mutant results in context.
Regardless of the activation stress, signaling interactions among nNOS, Nox2, and CaMKII likely contribute to their apparently equal enhancement of RyR2 activation (Fig. 1B). Nox2-generated superoxide, which becomes H2O2, can directly oxidize and activate RyR2, as can NO. However, superoxide can also interact with NO to form peroxynitrite, which also modifies target proteins such as RyR2. CaMKII is a likely player in this pathway because it can be activated by oxidation by Nox2-ROS (11) and NO (12). Synergism among Nox2, NOS, and CaMKII has become increasingly evident. We find that with frequent passive stretches, X-ROS production dominates RyR2 modulation initially (within seconds), but NOS and CaMKII signaling arise after a delay (tens of seconds) (13). Consistent with this finding, Dries et al. reported that Nox2-dependent activation of CaMKII likely underlies RyR2 modulation during high-frequency mechanical stress (14).
An additional target of this synergistic interplay not addressed by Jian et al. is the mechanosensitive channel (MSC) (Fig. 1). MSCs are modulated by both ROS and NO and are implicated in the Anrep effect. X-ROS activation of MSCs contributes to increased cytosolic calcium during mechanical stress (9) and may contribute to the RyR2 activation reported here. The role of NO is more complex: Kass and co-workers have demonstrated that activation of protein kinase G, which is driven by NO, suppresses MSC activity to blunt the Anrep effect (15). Initially, this appears in opposition to the results presented by Jian et al., which suggest that NO augments the Anrep effect through modulation of RyR2; how can these seemingly disparate results be reconciled?
First, bear in mind that mechano-chemo transduction depends on the nature of the mechanical stress and abundance of key players. Studies using various methods of stress in complex disease models require both independent and unified interpretation. Although mechanotransduction in the cell-in-gel system is currently uncalibrated and complex, it is a methodological approach that makes measuring mechanotransduction accessible to the scientific community. However, there are still two issues to reconcile: (i) the conflicting role of NO as a modulator of the Anrep effect, and (ii) the seemingly redundant effects of Nox2, CaMKII, and nNOS on EC coupling. We thus propose a conceptual hypothesis to unify both unresolved issues (Fig. 1).
In the presence of superoxide, nNOS becomes “uncoupled” and switches from NO to superoxide production (16). We hypothesize that healthy cells contracting in a stiff viscous gel will generate substantially more Nox2-ROS, which can uncouple nearby nNOS, leading to an additional source of superoxide. Both superoxide sources may then directly modify RyR2 and synergistically effect EC coupling through CaMKII (Fig. 1). This would explain the redundant effects seen by Jian et al., while also separating the influence of NO from uncoupled nNOS (and thus ROS) on the Anrep effect. New work with the cell-in-gel and complementary approaches will enable direct testing of this and alternative hypotheses in healthy and diseased heart.
Regardless of the nature or magnitude of stress, the study of Jian et al. highlights the importance of conducting experiments under mechanical load. Mechanical stress clearly influences calcium signaling, and studies in unloaded myocytes present a valuable but incomplete story. The new tools and questions stemming from the work of Jian et al. show that exciting discoveries await those who study mechanochemo transduction.
References
- 1.Sandow A. Excitation-contraction coupling in muscular response. Yale J. Biol. Med. 1952;25:176–201. [PMC free article] [PubMed] [Google Scholar]
- 2.Prosser BL, Ward CW, Lederer WJ. X-ROS signaling: Rapid mechano-chemo transduction in heart. Science. 2011;333:1440–1445. doi: 10.1126/science.1202768. [DOI] [PubMed] [Google Scholar]
- 3.Jian Z, Han H, Zhang T, Puglisi J, Izu LT, Shaw JA, Onofiok E, Erickson JR, Chen Y-J, Horvath B, Shimkunas R, Xiao W, Li Y, Pan T, Chan J, Banyasz T, Tardiff JC, Chiamvimonvat N, Bers DM, Lam KS, Chen-Izu Y. Mechanochemotransduction during cardiomyocyte contraction is mediated by localized nitric oxide signaling. Sci. Signal. 2014;7:ra27. doi: 10.1126/scisignal.2005046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cheng H, Lederer WJ. Calcium sparks. Physiol. Rev. 2008;88:1491–1545. doi: 10.1152/physrev.00030.2007. [DOI] [PubMed] [Google Scholar]
- 5.Trafford AW, Díaz ME, Sibbring GC, Eisner DA. Modulation of CICR has no maintained effect on systolic Ca2+: Simultaneous measurements of sarcoplasmic reticulum and sarcolemmal Ca2+ fluxes in rat ventricular myocytes. J. Physiol. 2000;522:259–270. doi: 10.1111/j.1469-7793.2000.t01-2-00259.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.von Anrep G. On the part played by the suprarenals in the normal vascular reactions of the body. J. Physiol. 1912;45:307–317. doi: 10.1113/jphysiol.1912.sp001553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Petroff MG, Kim SH, Pepe S, Dessy C, Marbán E, Balligand JL, Sollott SJ. Endogenous nitric oxide mechanisms mediate the stretch dependence of Ca2+ release in cardiomyocytes. Nat. Cell Biol. 2001;3:867–873. doi: 10.1038/ncb1001-867. [DOI] [PubMed] [Google Scholar]
- 8.Iribe G, Ward CW, Camelliti P, Bollensdorff C, Mason F, Burton RAB, Garny A, Morphew MK, Hoenger A, Lederer WJ, Kohl P. Axial stretch of rat single ventricular cardiomyocytes causes an acute and transient increase in Ca2+ spark rate. Circ. Res. 2009;104:787–795. doi: 10.1161/CIRCRESAHA.108.193334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Khairallah RJ, Shi G, Sbrana F, Prosser BL, Borroto C, Mazaitis MJ, Hoffman EP, Mahurkar A, Sachs F, Sun Y, Chen Y-W, Raiteri R, Lederer WJ, Dorsey SG, Ward CW. Microtubules underlie dysfunction in duchenne muscular dystrophy. Sci. Signal. 2012;5:ra56. doi: 10.1126/scisignal.2002829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shaw J, Izu L, Chen-Izu Y. Mechanical analysis of single myocyte contraction in a 3-D elastic matrix. PLOS ONE. 2013;8:e75492. doi: 10.1371/journal.pone.0075492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Erickson JR, Joiner M-LA, Guan X, Kutschke W, Yang J, Oddis CV, Bartlett RK, Lowe JS, O'Donnell SE, Aykin-Burns N, Zimmerman MC, Zimmerman K, Ham A-JL, Weiss RM, Spitz DR, Shea MA, Colbran RJ, Mohler PJ, Anderson ME. A dynamic pathway for calcium-independent activation of CaMKII by methionine oxidation. Cell. 2008;133:462–474. doi: 10.1016/j.cell.2008.02.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gutierrez DA, Fernandez-Tenorio M, Ogrodnik J, Niggli E. NO-dependent CaMKII activation during β-adrenergic stimulation of cardiac muscle. Cardiovasc. Res. 2013;100:392–401. doi: 10.1093/cvr/cvt201. [DOI] [PubMed] [Google Scholar]
- 13.Prosser BL, Ward CW, Kerr JP, Shi G, Lederer WJ. Stretch-dependent regulation of calcium signaling in heart—Who are the key players? Biophys. J. 2014;106:322a. [Google Scholar]
- 14.Dries E, Bito V, Lenaerts I, Antoons G, Sipido KR, Macquaide N. Selective modulation of coupled ryanodine receptors during microdomain activation of CaMKII in the dyadic cleft. Circ. Res. 2013 doi: 10.1161/CIRCRESAHA.113.301896. 10.1161/CIRCRESAHA.113.301896. [DOI] [PubMed] [Google Scholar]
- 15.Seo K, Rainer PP, Lee DI, Hao S, Bedja D, Birnbaumer L, Cingolani OH, Kass DA. Hyperactive adverse mechanical-stress responses in dystrophic heart are coupled to TRPC6 and blocked by cGMP-PKG modulation. Circ. Res. 2014 doi: 10.1161/CIRCRESAHA.114.302614. 10.1161/CIRCRESAHA.114.302614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sun J, Druhan LJ, Zweier JL. Dose dependent effects of reactive oxygen and nitrogen species on the function of neuronal nitric oxide synthase. Arch. Biochem. Biophys. 2008;471:126–133. doi: 10.1016/j.abb.2008.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]