Abstract
Background
Early accurate acute kidney injury (AKI) diagnosis is needed to pursue AKI treatment trials. We evaluated Cystatin C (CysC) as an early biomarker of serum creatinine (SCr)-AKI and as an alternative to define AKI.
Methods
160 non-cardiac children admitted to our intensive care unit (ICU) were prospectively studied. We measured daily CysC and SCr. AKI was staged according to the Kidney Disease: Improving Global Outcomes (KDIGO) SCr criteria and by similarly applied criteria, using CysC (CysC-AKI) We calculated area under the curve (AUC) for 1) neutrophil gelatinase associated lipocalin (NGAL), interleukin-18 (IL-18), kidney injury molecule-1 (KIM-1) and urine cystatin C (uCysC) to diagnose SCr- and CysC-AKI and 2) for CysC to diagnose SCr-AKI. We evaluated the relation of each AKI definition with length of stay and mechanical ventilation duration.
Results
44% of patients developed SCr-AKI; 32% developed CysC-AKI. Whether AKI was defined by SCr or CysC, NGALwas associated with AKI severity. CysC-AKI was not more strongly associated with clinical outcomes. However, early ICU admission CysC predicted SCr-AKI development within 48 hours (AUC=0.70 [95% CI 0.53 – 0.89]).
Conclusions
Our findings do not support replacing SCr by CysC to define AKI. However, CysC may be used for predicting AKI development when obtained early in ICU admission.
Keywords: diagnostic testing, urine biomarkers, acute renal failure, pediatric intensive care unit
INTRODUCTION
Acute kidney injury (AKI) is a frequent condition associated with poor outcome in children admitted to the pediatric intensive care unit (PICU). Therapeutic interventions have failed to reduce AKI morbidity and mortality because they relied on a delayed AKI biomarker, serum creatinine (SCr) [1]. Human studies have attempted to validate novel biomarkers of early AKI diagnosis identified in animal models [2-8]. Although these studies were encouraging, recent publications have revealed only fair to good diagnostic accuracy of new AKI biomarkers [2, 7, 9-11].
A possible reason for lack of successful early urinary AKI biomarkers is that the “gold standard” used for AKI diagnosis is SCr rise, as applied in the Risk, Injury, Failure, End-Stage Renal Disease (RIFLE)[12], Acute Kidney Injury Network (AKIN)[13] and the Kidney Disease: Improving Global Outcomes (KDIGO) definitions for AKI[14]. SCr concentrations vary with age, gender, muscle mass, medication and intravascular volume status[15]. This likely leads to misclassification of AKI status and poorer estimated performance of new AKI diagnostic biomarkers.
Serum Cystatin C (CysC), a low molecular-weight protein measurable in blood and freely filtered by the glomerulus, is purported to be less affected by age and gender[16]. In children with chronic kidney disease, CysC is a more accurate marker of glomerular filtration rate (GFR) [17, 18]. Acute CysC changes may therefore provide more accurate AKI diagnosis; this would increase validity of novel biomarker and of AKI-outcome studies. Some studies have suggested that serum CysC rise with AKI occurs before rise in SCr, leading to evaluation of CysC as an “early AKI biomarker” [7, 19]. However, malignancy, corticosteroids and thyroid dysfunction may lead to CysC variability [20-23].
We sought to evaluate the use of CysC from two perspectives, in children admitted to the PICU as 1) an alternative to SCr for defining AKI and 2) an early biomarker of SCr-defined AKI. We hypothesized that if CysC is a more accurate marker of AKI, then the relationship between CysC-defined AKI with clinical outcomes and urine injury biomarkers should be stronger than that of SCr-defined AKI with biomarkers.
MATERIALS and METHODS
Design, Setting, Subject Recruitment
This was a prospective cohort study performed in children between 1 month and 18 years old admitted to the Montreal Children's Hospital (MCH) PICU, Canada between November, 2007 and September, 2010. Children between 1 month and 18 years old admitted to the PICU were eligible. We focused on children who were not immediately post-operative from cardiac surgery. Exclusion criteria were: known end stage renal disease, having received a renal transplant, a high likelihood of death in the following 48 hours (determined by the PICU attending staff) and availability of <0.25 CysC and <0.25 SCr values per PICU day (determined by dividing number of available daily values by PICU admission days). Research ethics board approval was obtained (Montreal Children’s Hospital) before initiating study activities, which adhered to the Declaration of Helsinki. Prents provided informed consent before enrolment.
Overall Study Procedure
In subjects recruited within the first 48 hours of PICU admission, daily clinical data, urine (up to 30 cc, freshly voided from a urinary catheter or from diapers using cotton balls, at approximately 8 AM) and blood were collected for up to 14 days of PICU admission. Blood was collected during routine morning PICU bloodwork, with extraction of an additional 1 to 2 cc of blood for this study. If blood work was not planned by the PICU team, no blood was obtained. The MCH biochemistry laboratory stores serum from all routine blood draws for 2 weeks at −20°C. We obtained this serum for SCr and CysC measurements from the days before recruitment (e.g. PICU day 1, if recruited PICU day 2). In subjects recruited after 48 hours of PICU admission, the protocol only included blood collection for CysC and SCr measurement (not urine for biomarkers). All biospecimens were centrifuged at 1000 g for 15 minutes at room temperature. Urine and serum were aliquoted and stored at −80°C until biomarker measurement, performed every 2 to 5 months.
Clinical data collection
The following clinical variables were collected: age, gender, primary PICU diagnosis, variables required for the Pediatric Risk of Mortality (PRISM) Score[24] and Pediatric Logistic Organ Dysfunction (PELOD) Score[25], requirement of mechanical ventilation, urinary catheter, vasopressors or renal replacement therapy, and treatment with steroids or thyroid medications. The following clinical outcomes were collected: duration of PICU and hospital stay, duration of mechanical ventilation, and mortality. Baseline estimated GFR was calculated by the Schwartz formula[26], using the lowest SCr in the 3 months before PICU admission (“baseline” SCr). When no baseline SCr was available, a normal baseline estimated GFR of 120 ml/min/1.73m2 was assumed (reasonable, due to the extremely low prevalence of chronic kidney disease in children[27]).
Laboratory measurements
SCr was measured at our central biochemistry laboratory using an IDMS-traceable assay. Urine NGAL and IL-18 and serum CysC were measured at the Cincinnati Children's Hospital Medical Center Biomarker Laboratory, Ohio (PD, MB, QM) [7, 28] and KIM-1 was measured at Brigham & Women's Hospital, Harvard, Massachusetts (JV, VS)[29]. NGAL was measured using a commercial ELISA kit (Bioporto, Gentofte, Denmark) per manufacturer's instructions. The average intra-assay and inter-assay coefficient of variations (CV, verified) was between 2.5% and 6.3%. IL-18 was measured in the same laboratory using an IL-18 ELISA kit (Medical & Biological Laboratories, Nagoya, Japan) per manufacturer’s instructions. The inter- and intra-assay CV’s range between 3.01% and 10%. KIM-1 was measured using a microbead based assay on luminex system[29]. Inter- and intra-assay CV’s were <15%. CysC was measured using a nephelometer (Siemens BN-II, Siemens, AG; www.Siemens.com) (coefficient of variation <1.1%). Individuals performing biomarker measurements were blinded to clinical data.
SCr-based definition (KDIGO-SCr)
Traditional AKI definition was based on SCr criteria of the KDIGO definition. Peak SCr value during PICU admission was divided by the baseline SCr. Subjects with a ≥50% SCr increase within 7 days or 26.5 μmol/l SCr rise from baseline within 48 hours were classified as SCr-AKI Stage 1. Subjects with SCr doubling were classified as Stage 2 and those with SCr tripling, dialysis requirement or an eGFR < 35 ml/min/1.73m2 at any time were classified as Stage 3.
CysC-based AKI definitions
We defined AKI according to change in CysC (hereafter CysC-AKI). We applied the KDIGO definition in a similar way to KDIGO-SCr criteria, to the extent that this was feasible (including the timing of rise criterion, the eGFR criterion of Stage 3, but excluding the equivalent of “0.3 mg/dL rise from baseline” of the Stage 1 SCr criterion). It was necessary to determine a reasonable way to estimate baseline (pre-PICU) CysC which could be easily reproducible in other studies and in clinical care, when pre-PICU baseline CysC would not be available. We used the Schwartz formula-estimated baseline eGFR to back-calculate baseline CysC concentrations, using a validated CysC GFR formula [18, 26].
Statistical Analysis
Level of agreement between the different AKI definitions using SCr and CysC was evaluated using the Kappa-statistic.
CysC as an alternative method to define AKI
Associations between urine AKI biomarkers and SCr-AKI and CysC-AKI definitions
Subjects with urine available were included in these analyses. As a crude method to evaluate the relationship between SCr- and CysC-AKI and urine AKI biomarkers, peak PICU biomarker concentrations were compared across SCr and CysC-AKI severity strata using Kruskall-Wallis tests. AKI was defined by 4 categories: 1) no SCr and no CysC-AKI, 2) SCr-AKI only, 3) CysC-AKI only and 4) SCr and CysC-AKI. Peak urine biomarker concentrations were compared between the categories. Receiver operating characteristic (ROC) curves with calculation of area under the curve (AUC) was used to evaluate biomarkers for AKI diagnosis when obtained on: 1) the day of AKI attainment (discrimination) and 2) in the first 48 hours of PICU (early PICU AKI diagnosis).
Comparing associations between SCr-AKI versus CysC-AKI and outcomes
All subjects were included. Multivariate Cox proportional hazards regression was used to evaluate and compare whether SCr-AKI and CysC-AKI were associated with increased risk for longer length of PICU stay and for mechanical ventilation (LOV). Age, gender, PRISM, trauma and neurological primary diagnosis, presence/absence of infection (respiratory and non-respiratory), and vasopressor requirement were controlled for. Unadjusted and adjusted hazard ratio’s (and 95% confidence intervals) were calculated, with a HR<1 indicating AKI as a risk factor for longer length of stay or ventilation (i.e. AKI reduces the “risk” of discharge or extubation).
CysC as an early biomarker of SCr-AKI
All subjects were included. The day of SCr-AKI attainment was identified. The AUC for CysC drawn 2 days before, 1 day before and on the day of first SCr-AKI attainment were calculated to diagnose the presence of AKI. The first PICU CysC concentrations (if drawn within 48 hours of PICU admission) was used to calculate AUC for future AKI development, in children who did not yet have AKI at PICU admission. As a secondary analysis, the continuous net reclassification improvement (NRI) and integrated discrimination improvement (IDI) of early PICU CysC were calculated to evaluate improvement in predicting AKI development, compared to using a predictive model of age and admission PRISM score alone[30, 31]. Analyses were performed using STATA SE 10®, College Station, Texas, USA.
RESULTS
Study population
Figure 1 describes the study flow. There were 160 subjects available for analysis. Table 1 displays that PELOD score, length of PICU stay and of mechanical ventilation and proportion receiving vasopressors were significantly higher in AKI versus non-AKI subjects using both definitions. PRISM score, duration of hospital stay, and renal replacement therapy were only significantly higher in subjects with SCr-defined AKI. Only 1 subject received thyroxin during PICU admission and 77 (48%) subjects were treated with steroids at some time during admission (47% of patients with and 49% of patients without SCr-AKI, p=0.8). The distribution of steroid treatment did not differ between patients with versus without CysC-AKI (52% versus 46%, p=0.5).
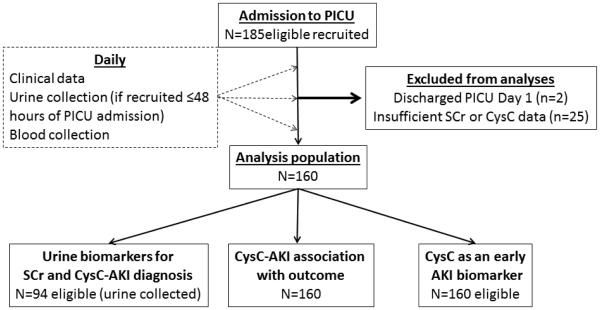
Figure 1. Study flow chart.
Displays the flow, beginning with the total number recruited, exclusions to final analysis populations.
PICU: pediatric intensive care unit; CysC: Cystatin C; SCr: serum creatinine; AKI: acute kidney injury; SCr-AKI: AKI defined by SCr rise criteria; CysC-AKI: AKI defined by CysC rise criteria.
Table 1.
Study population characteristics, comparing AKI versus non-AKI subjects, using SCr and CysC AKI criteria.
| Characteristic | No SCr-AKI (n=90) | SCr-AKI (n=70) | No CysC-AKI (n=109) | CysC-AKI(n=51) |
|---|---|---|---|---|
| Continuous variables | Mean (Standard deviation), Median | |||
| Age (years) | 5.3 (5.8), 2.9 | 4.0 (5.5), 0.8 | 5.4 (5.6), 2.9 | 3.5 (5.6), 0.3 § |
| Baseline eGFR (ml/min/1.73m2) | 119.4 (36.7), 120.0 | 121.1 (37.4), 120.0 | 116.1 (31.6), 120.0 | 129.0 (45.4), 120.0 |
| PRISM score | 6.0 (4.2), 4.0 | 8.4 (5.1), 7.5 £ | 6.6 (4.5), 6.0 | 7.9 (5.3), 7.0 |
| PELOD score | 26.3 (7.6), 21.0 | 32.1 (8.7), 31.0 § | 27.3 (8.1), 22.0 | 32.1 (8.7), 31.0 § |
| Length of PICU stay (days) | 7.8 (7.4), 5.0 | 11.8 (9.2), 9.5 § | 8.7 (7.9), 6.0 | 11.4 (9.4), 9.0 £ |
| Length of hospital stay (days) | 30.5 (52.5), 15.5 | 31.8 (26.9), 20.5 £ | 32.8 (49.4), 18.0 | 27.4 [25.2], 17.0 |
| Length of ventilation (days) | 4.1 (4.0), 3.0 | 6.7 (4.7), 7.0 § | 4.8 (4.4), 3.0 | 6.2 (4.4), 6.0 £ |
| Categorical variables | Column N (%) | |||
| Male | 58 (64) | 38 (54) | 65 (60) | 31 (61) |
| Primary Admission Diagnoses | ||||
| Non-respiratory infection | 9 (10) | 7 (10) | 11 (10) | 5 (9.8) |
| Respiratory infection | 22 (24) | 13 (19) | 22 (20) | 13 (25) |
| Trauma | 3 (3) | 3 (4) | 5 (5) | 1 (2) |
| Neurological | 21 (23) | 8 (11) | 25 (23) | 4 (8)£ |
| Malignancy | 5 (6) | 5 (7) | 9 (8) | 1 (2) |
| Other | 30 (33) | 34 (49) | 37 (34) | 27 (53) |
| Ventilated | 71 (79) | 59 (84) | 87 (80) | 43 (84) |
| Received vasopressors | 26 (29) | 37 (53)£ | 36 (33) | 27 (53)£ |
| Urinary catheter | 66 (73) | 61 (87)£ | 84 (77) | 43 (84) |
| Renal replacement therapy | 0 (0) | 3 (4) £ | 1 (1) | 2 (4) |
| Death | 2 (2) | 3 (4) | 4 (4) | 1 (2) |
p<0.001 between AKI and non-AKI groups
p<0.05 between AKI and non-AKI groups
AKI by SCr and CysC
Seventy (44%) subjects developed SCr-AKI and 51 (32%) developed CysC-AKI. There was 44% agreement in the KDIGO staging classification by SCr-AKI and CysC-AKI (Kappa = 0.29, p<0.001) and there was 52% agreement between the presence or absence of any AKI by both methods (Kappa = 0.33, p<0.001). The disagreement between the two methods manifested largely by SCr classifying subjects as having worse KDIGO AKI Stages than CysC (Table 2). Pre-PICU serum was available in 20 subjects for CysC measurement. Lowest pre-PICU CysC concentrations fairly but significantly correlated with estimated baseline CysC (r = 0.45, p = 0.04). There was no significant difference between baseline CysC obtained from available pre-PICU CysC versus estimated baseline CysC concentrations (0.88±0.43 mg/l versus 0.87±0.49 mg/l, respectively, p = 0.95).
Table 2.
Comparison and level of agreement between AKI-SCr and AKI-CysC1.
| AKI by SCr AKI by CysC |
Stage 0 | Stage 1 | Stage 2 | Stage 3 | Row Totals |
|---|---|---|---|---|---|
| Stage 0 | 74 | 22 | 12 | 1 | 109 |
| Stage 1 | 11 | 11 | 3 | 2 | 27 |
| Stage 2 | 5 | 2 | 7 | 6 | 20 |
| Stage 3 | 0 | 0 | 0 | 4 | 4 |
| Column Totals | 90 | 35 | 22 | 13 | 160 |
Grey boxes represent subjects in whom there was agreement in the AKI severity classification between SCr-AKI and CysC-AKI.
Relationship between urine biomarkers and AKI by SCr and CysC
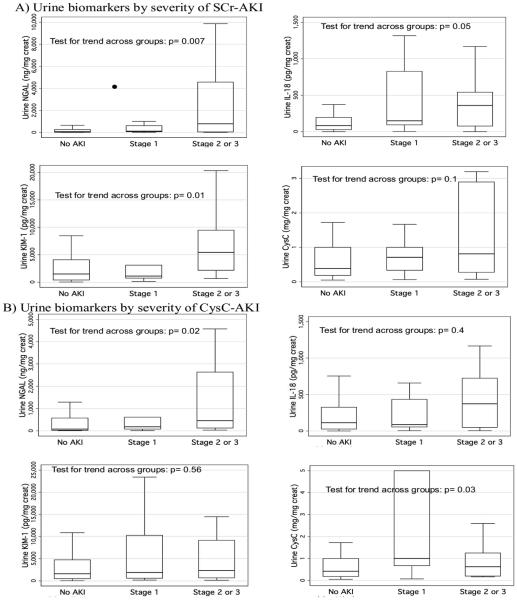
Worsening SCr-AKI severity was significantly associated with increasing peak urine NGAL, IL-18 and KIM-1 concentrations, whereas CysC-AKI severity was only significantly associated with increasing peak urine NGAL and urine CysC concentrations (Figure 2).
Figure 2. Peak pediatric intensive care unit biomarker concentrations by acute kidney injury severity stratum, using SCr-AKI and CysC-AKI definitions.
A) Demonstrates box plots (middle line is the median; upper and lower box bordes are the 75th and 25th percentile values, respectively) of biomarker concentrations for urine neutrophil gelatinase-associated lipocalin, interleukin-18, kidney injury molecule-1 and urine cystatin C across AKI severity strata (no AKI, Stage 1 AKI and Stages 2 or 3 AKI). P-values are from performance of a non-parametric test for trend across groups. B) Represents identical analyses but with AKI defined by rise in CysC from baseline (CysC-AKI).
SCr: serum creatinine; SCR-AKI: acute kidney injury defined by rise in serum creatinine from baseline; NGAL: neutrophil gelatinase-associated lipocalin; AKI: acute kidney injury; IL-18: interleukin-18; KIM-1: kidney injury molecule-1; CysC: Cystatin C; CysC-AKI: acute kidney injury defined by rise in serum cystatin C from baseline.
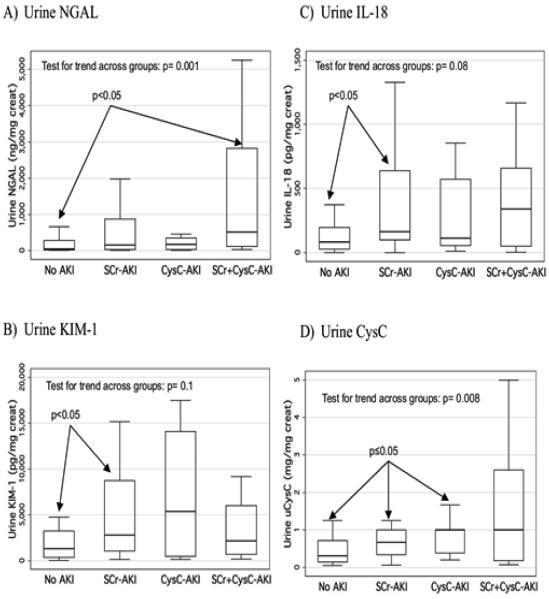
Subjects who had both SCr-AKI and CysC-AKI had the highest peak urine biomarker concentrations for all biomarkers (versus patients with no AKI, SCr-AKI only or CysC-AKI only), but this association was only significant with NGAL and urine CysC (Figure 3).
Figure 3. Comparison of peak biomarker concentrations across 4 AKI categories: No AKI, SCr-AKI only, CysC-AKI only, both SCr + CysC AKI.
For all 4 studied biomarkers, biomarker concentrations are compared between 4 groups: patients with no AKI by SCr or CysC rise; patients who only have acute kidney injury by SCr rise; patients who only have acute kidney injury by CysC rise; patients who have acute kidney injury using both methods. The p-values within the graphs are from performance of a non-parametric test for trend across groups.
NGAL: neutrophil gelatinase-associated lipocalin; AKI: acute kidney injury; SCR-AKI: acute kidney injury defined by rise in serum creatinine from baseline; CysC-AKI: acute kidney injury defined by rise in serum cystatin C from baseline; SCr+CysC-AKI: acute kidney injury defined by rise in serum creatinine and Cystatin C from baseline; IL-18: interleukin-18; KIM-1: kidney injury molecule-1; CysC: cystatin C.
Biomarker diagnostic characteristics for SCr and CysC-AKI
Whether AKI was defined by SCr or CysC, urine NGAL obtained on the day of AKI diagnosis had similar AUC for discriminating for presence of AKI (AUC=0.68 and 0.70 respectively, Table 3). IL-18 diagnostic characteristics were substantially higher using SCr-AKI (AUC=0.68 versus 0.54). The 85% specificity and sensitivity biomarker concentration cut-offs for AKI diagnosis by SCr and CysC methods were fairly similar (Table 3).
Table 3.
Area under the receiver operating characteristic curve for biomarkers to discriminate for the presence of a) SCr-AKI and b) CysC-AKI1 when biomarkers were obtained on the day of AKI attainment.
| Biomarker on 1st AKI day |
SCrAKIAUC2
(95% CI) |
Biomarker cut-off for 85%) Specificity3 (associated sensitivity) 85%) Sensitivity4 (associated specificity) |
CysC AKI AUC2
(95% CI) |
Biomarker cut-off for 85%) Specificity3 (associated sensitivity) 85%) Sensitivity4 (associated specificity) |
|---|---|---|---|---|
| Urine NGAL (ng/mg creat) |
0.68* (0.55 – 0.81) | 196 ng/mg (39%) sensitivity) 12 ng/mg (24% specificity) |
0.70# (0.58-0.83) | 276 (27% sensitivity) 23 (47% specificity) |
| Urine IL-18 (pg/mg creat) | 0.68* (0.55 – 0.80) | 255 pg/mg (32% sensitivity) 23 pg/mg (35%) specificity) |
0.54 (0.40 - 0.69) | 274 (18% sensitivity) 17 (27% specificity) |
| Urine KIM-1 (pg/mg creat) |
0.61 (0.48 - 0.74) | 3356 pg/mg (17%) sensitivity) 180 pg/mg (29% specificity) |
0.61 (0.47 – 0.75) | 4479 (15% sensitivity) 195 (28% specificity) |
| Urine CysC (mg/mg creat) |
0.52(0.38 – 0.66) | 0.62 mg/mg (14%) sensitivity) 0.76 mg/mg (26%) specificity) |
0.52(0.38 – 0.66) | 0.62 (10% sensitivity) 0.88 (27% specificity) |
Abbreviations: SCr: serum creatinine; SCr-AKI: acute kidney injury defined by SCr rise., CysC: Cystatin C; CysC-AKI: acute kidney injury defined by CysC rise; AUC: area under the receiver operating characteristic curve; NGAL: neutrophil gelatinase-associated lipocalin; IL-18: interleukin-18; KIM-1: kidney injury molecule-1.
Area under the curve for biomarkers to discriminate for the presence of SCr-AKI (left) and CysC-AKI (right).
biomarker concentrations which resulted in 85% specificity to diagnose AKI were chosen; the sensitivity at that concentration is provided.
biomarker concentrations which resulted in 85% sensitivity to diagnose AKI were chosen; the specificity at that concentration is provided.
AUC for NGAL and IL-18 were significantly higher for SCr-AKI diagnosis than KIM-1 and urine CysC (p<0.05)
AUC for NGAL was significantly higher for CysC-AKI diagnosis than IL-18, KIM-1, and urine CysC (p<0.05)
SCr and CysC-AKI diagnosis using biomarkers at PICU admission
The best AUC for predicting CysC-AKI development was for early PICU NGAL (AUC=0.69, Table 4); early PICU IL-18 had the best AUC for predicting SCr-AKI (AUC=0.69, Table 4). Overall, early PICU biomarker AUCs did not differ substantially between the two AKI definition methods (Table 4). When the outcome was Stage 2 AKI or worse, NGAL and IL-18 AUCs were significantly higher for predicting SCr-AKI compared to other biomarkers (lower half Table 4, AUC’s = 0.76 and 0.69, respectively); NGAL AUC was significantly higher than other biomarkers to predict CysC-AKI Stage 2 or worse compared to other biomarkers (lower half of Table 4, AUC=0.78).
Table 4.
Area under the receiver operating characteristic curve for biomarkers in the first 24-48 hours of intensive care unit (ICU1) admission to predict AKI and Stage 2 or worse AKI.
| Biomarker on Day 1 or 2 of ICU admission | SCr-AKI AUC (95% CI)2 | CysC-AKI AUC (95% CI)2 |
|---|---|---|
| Urine NGAL (ng/mg creatinine) | 0.61 (0.46 – 0.76) | 0.69 (0.54 - 0.84) |
| Urine IL-18 (pg/mg creatinine) | 0.69 (0.55 - 0.82) | 0.55 (0.37 – 0.73) |
| Urine KIM-1 (pg/mg creatinine) | 0.49 (0.33 – 0.64) | 0.54 (0.37 – 0.72) |
| Urine CysC (mg/mg creatinine) | 0.59 (0.44 – 0.74) | 0.61 (0.45 – 0.76) |
| Biomarker on Day 1 or 2 of ICU admission |
SCr-AKI Stage 2 AUC3
(95% CI) |
CysC-AKI Stage 2 AUC3
(95% CI) |
| Urine NGAL (ng/mg creatinine) | 0.76 (0.59 – 0.94)* | 0.78 (0.65 - 0.92) # |
| Urine IL-18 (pg/mg creatinine) | 0.69 (0.52 – 0.86)* | 0.62 (0.39 – 0.84) |
| Urine KIM-1 (pg/mg creatinine) | 0.59 (0.43 – 0.75) | 0.53 (0.29 – 0.77) |
| Urine CysC (mg/mg creatinine) | 0.63 (0.45 – 0.82) | 0.50 (0.31 – 0.69) |
Abbreviations: ICU: intensive care unit; SCr-AKI: acute kidney injury defined by SCr rise; CysC: Cystatin C; AUC: area under the receiver operating characteristic curve; CysC-AKI: acute kidney injury defined by CysC rise; NGAL: neutrophil gelatinase-associated lipocalin; IL-18: interleukin-18; KIM-1: kidney injury molecule-1; CI: confidence interval..
Area under the receiver operating characteristic curve for biomarkers obtained within the first 24-48 hours of intensive care unit admission to predict future development of SCr-AKI (left), CysC-AKI (right).
In these analyses, the outcome was development of Stage 2 (doubling or SCr or worse, or dialysis) AKI.
AUC for NGAL and IL-18 were significantly higher for predicting SCr-AKI Stage 2 compared to KIM-1 (p<0.05)
AUC for NGAL was significantly higher for predicting CysC-AKI Stage 2 than KIM-1 and urine CysC (p<0.05)
AKI associations with outcomes
SCr-AKI predicted longer PICU length of stay (LOS) and longer LOV (adjusted hazard ratio’s = 0.56 [95% CI 0.39-0.79] and 0.49 [95% CI 0.33-0.72], respectively) when adjusted for other variables. CysC-AKI was associated with these outcomes in univariate analyses (not shown) but not in adjusted analyses (adjusted hazard ratio’s = 0.91 [95% CI 0.64-1.29] and 1.11 [95% CI 0.74-1.65], respectively).
CysC as an “early biomarker” of AKI
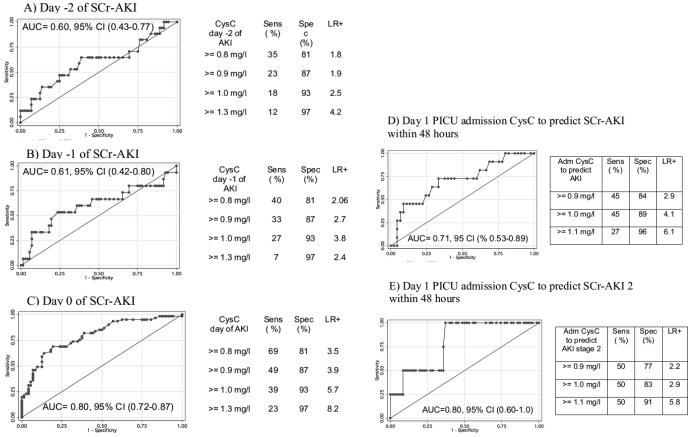
As shown in Figure 4, AUC for SCr-AKI diagnosis by CysC increased progressively the closer the CysC sampling was to the day of SCr-AKI attainment. AUC of CysC was highest and statistically significant when measured on the day of AKI attainment (Figure 4c). The best CysC cut-off for SCr- AKI diagnosis, favouring high specificity and likelihood ratio, was 1.3 mg/l, regardless of CysC sampling day (Figures a to c). Figures 4 d and e show that CysC measured within the first 48 PICU hours predicted SCr-AKI development with AUC = 0.71 (Figure 4d) and predicted Stage 2 SCr-AKI development with AUC = 0.80 (Figure 4e). The NRI of first 48 hours PICU CysC to predict AKI development over a simple clinical model including age and PRISM score was 0.61 (standard error 0.34, p=0.05); IDI was 0.06 (standard error = 0.04, p=0.1).
Figure 4. Prediction of SCr-AKI using CysC from 2 to 0 days before SCr-AKI (a to c) and from early PICU admission (d and e).
The figures represent receiver operating characteristic curves for CysC concentrations to diagnose SCr-AKI. Figure A) represents the receiver operating characteristic curve for CysC obtained 2 days before AKI diagnosis; B) for CysC from 1 day before diagnosis; C) for CysC from the day of AKI diagnosis; D) for CysC obtained in early PICU admission to predict SCr-AKI development within 48 hours; E) for CysC obtained in early PICU admission to predict SCr-AKI stage 2 development within 48 hours. Within each graph, the area under the curve (with associated 95% confidence intervals) is shown. To the right of each curve, the sensitivity, specificity and likelihood ratio for selected CysC concentrations cutoffs are shown in association with each graph.
SCR-AKI: acute kidney injury defined by rise in serum creatinine from baseline; AUC: area under the curve; CI: confidence interval; CysC: Cystatin C; AKI: acute kidney injury; Sens: sensitivity; Spec: specificity; LR+: positive likelihood ratio; SCr-AKI 2: acute kidney injury stage 2.
DISCUSSION
CysC has been proposed as an alternative to SCr for estimating GFR, for years. However, SCr continues to be used by most laboratories worldwide, since no studies have really addressed the extent to which incorporating CysC into clinical care will actually lead to change in management or change in health care costs. Our study therefore attempted to perform an initial evaluation of the extent to which CysC in the PICU (where AKI is most common in children’s hospitals) might potentially serve to indicate acute renal dysfunction. This is the first prospective study evaluating serum CysC for AKI diagnosis in non-cardiac children admitted to the PICU. We evaluated CysC: 1) to define AKI and 2) as an early diagnostic biomarker of SCr-AKI. Urine biomarkers and morbidity outcomes were not more associated with CysC-AKI. However, CysC predicted AKI development when obtained early in PICU admission.
We and others have shown that serum CysC is a more accurate marker of GFR than SCr is, in children with chronic kidney disease [17, 18, 32]. It is therefore reasonable to hypothesize that acute CysC change might more accurately detect acute GFR reduction than SCr does. To evaluate this, it is imperative to define a reasonable baseline CysC. Some proposed methods have included using CysC concentration at least 3 days before SCr-AKI occurs or using lowest CysC concentrations during ICU admission [19, 33]. These methods are rational for retrospective analysis of previously performed studies, where CysC values throughout the study will already be available at the time of analysis; however, in the ICU setting, where AKI timing is unclear and true baseline CysC will most often not be available, defining a baseline is much more challenging. Our baseline CysC method was rational though not ideal. Two studies of adults used admission CysC as baseline [34, 35]. Both studies found that AKI - hospital outcomes associations were not different whether AKI was defined by SCr or CysC. In a multi-centre study of children undergoing cardiac surgery, pre-operative CysC was used as baseline [36]. Thus, when the exact timing of potential injury (e.g. cardiac surgery) is known, defining baseline CysC is simple. Although we only had 20 patients to evaluate, our estimated baseline CysC was similar to true pre-ICU concentrations.
Several studies have used CysC rise as a renal outcome [37-39]. Given the lack of understanding of CysC rise as a valid AKI outcome, it is difficult to interpret these study findings. We attempted to address this issue. First, there was substantial disagreement between SCr-AKI and CysC-AKI diagnosis, suggesting that these two definitions provide distinct information in several patients. We found that CysC was not more strongly associated with urine injury biomarkers relative to SCr. Conversely, patients with both CysC-AKI and SCr-AKI have the highest biomarker concentrations of the whole group, confirming some important concordance between both methods. Overall, our findings do not support the use of CysC-AKI to “replace” SCr-AKI; however, in studies aiming for highest AKI diagnosis accuracy, both markers might be used to define AKI outcome together. Future studied should address that question.
Most AKI CysC studies have evaluated CysC for early SCr-AKI diagnosis, similar to how urine AKI biomarkers might be used [40-42]. These studies have been reviewed in a meta-analysis [43], of which only 1 was performed in children. The pooled AUC for SCr-AKI diagnosis using CysC was 0.96, which decreased to 0.86 when one study with strong diagnostic characteristics was eliminated, suggesting that CysC is useful for early SCr-AKI diagnosis. The best CysC concentration cut-offs for SCr-AKI diagnosis in these studies varied greatly, from 0.8 to 2.04 mg/l, due to differences in CysC collection times relative to AKI attainment. Krawczeski et al evaluated early post-operative CysC for AKI diagnosis in children having cardiac surgery; a concentration of 1.16 mg/l had the best diagnostic performance, similar to our evaluated cut-offs[28]. Contrary to these encouraging studies, Wald et al found that early post-operative CysC predicted AKI occurrence with AUC of only 0.60-0.68, depending on timing of CysC sampling [42]. We found that when CysC is measured on the first day of PICU admission, in children who do not already have SCr-AKI, CysC concentration demonstrates good prediction (AUC 0.71-0.80). However, these AUC’s are not excellent, as has been found with other proposed AKI biomarkers. Thus CysC may be beneficial in future trials for AKI prevention in the PICU, particularly if combined with data from other urine AKI biomarkers. Based on contradictory results from previous studies, such findings must be confirmed in diagnostic and age-group specific populations.
There are limitations to this study. This study was single-centre, limiting generalizability. The sample size limited our ability to perform subgroup analyses, as evidenced by our rather wide confidence intervals around AUC estimates, and prevented us from more extensively evaluating added benefit of early PICU serum CysC for AKI prediction, over other factors alone (i.e. NRI, IDI). We only included age and PRISM in the clinical model (decided a priori) and though our point estimate for the NRI was quite high (61%), it barely attained statistical significance (p=0.05); the IDI was not statistically significant. There are still unknown factors which may affect CysC in critically ill patients. An important limitation was that our baseline CysC had to be estimated. Until CysC is routinely measured in the hospital setting (if it ever occurs), this limitation will be impossible to address. Future studies using CysC change as an AKI outcome might attempt to obtain blood from prior to PICU admission in order to better estimate baseline CysC and improve accuracy of CysC-AKI outcome diagnosis.
In conclusion, CysC can be used for early AKI diagnosis when obtained early in PICU admission. Future studies evaluating CysC as an AKI outcome should determine whether obtaining “true” baseline CysC improves performance over use of estimated baseline CysC.
ACKNOWLEDGEMENTS
Dr. Zappitelli received institutional funding from the McGill University Health Centre Research Institute, the Kidney Research Scientist Core Education and National Training Program and the Fonds de Recherches en Santé du Quebec to support this work.
Footnotes
DISCLOSURES
PD is a co-inventor on patents submitted for the use of NGAL as a biomarker of kidney injury. JB is a co-inventor on patents involving KIM-1.
REFERENCES
- 1.Nephrology ASo. American Society of Nephrology Renal Research Report. J Am Soc Nephrol. 2005;16:1886–1903. doi: 10.1681/ASN.2005030285. [DOI] [PubMed] [Google Scholar]
- 2.Al-Ismaili Z, Palijan A, Zappitelli M. Biomarkers of acute kidney injury in children: discovery, evaluation, and clinical application. Pediatr Nephrol. 2011;26:29–40. doi: 10.1007/s00467-010-1576-0. [DOI] [PubMed] [Google Scholar]
- 3.Devarajan P. Cellular and molecular derangements in acute tubular necrosis. Curr Opin Pediatr. 2005;17:193–199. doi: 10.1097/01.mop.0000152620.59425.eb. [DOI] [PubMed] [Google Scholar]
- 4.Devarajan P. Neutrophil gelatinase-associated lipocalin: a promising biomarker for human acute kidney injury. Biomark Med. 2010;4:265–280. doi: 10.2217/bmm.10.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ferguson MA, Vaidya VS, Waikar SS, Collings FB, Sunderland KE, Gioules CJ, Bonventre JV. Urinary liver-type fatty acid-binding protein predicts adverse outcomes in acute kidney injury. Kidney Int. 2010;77:708–714. doi: 10.1038/ki.2009.422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Han WK, Waikar SS, Johnson A, Betensky RA, Dent CL, Devarajan P, Bonventre JV. Urinary biomarkers in the early diagnosis of acute kidney injury. Kidney Int. 2008;73:863–869. doi: 10.1038/sj.ki.5002715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Parikh CR, Devarajan P, Zappitelli M, Sint K, Thiessen-Philbrook H, Li S, Kim RW, Koyner JL, Coca SG, Edelstein CL, Shlipak MG, Garg AX, Krawczeski CD, Consortium T-A. Postoperative biomarkers predict acute kidney injury and poor outcomes after pediatric cardiac surgery. J Am Soc Nephrol. 2011;22:1737–1747. doi: 10.1681/ASN.2010111163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Aydoğdu M, Gürsel G, Sancak B, Yeni S, Sarı G, Taşyürek S, Türk M, Yüksel S, Senez M, Ozis TN. The use of plasma and urine neutrophil gelatinase associated lipocalin (NGAL) and Cystatin-C in early diagnosis of septic acute kidney injury in critically ill patients. Dis Markers. 2013 doi: 10.3233/DMA-130966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Du Y, Zappitelli M, Mian A, Bennett M, Ma Q, Devarajan P, Mehta R, Goldstein SL. Urinary biomarkers to detect acute kidney injury in the pediatric emergency center. Pediatr Nephrol. 2011;26:267–274. doi: 10.1007/s00467-010-1673-0. [DOI] [PubMed] [Google Scholar]
- 10.Haase M, Devarajan P, Haase-Fielitz A, Bellomo R, Cruz DN, Wagener G, Krawczeski CD, Koyner JL, Murray P, Zappitelli M, Goldstein SL, Makris K, Ronco C, Martensson J, Martling CR, Venge P, Siew E, Ware LB, Ikizler TA, Mertens PR. The outcome of neutrophil gelatinase-associated lipocalin-positive subclinical acute kidney injury: a multicenter pooled analysis of prospective studies. J Am Coll Cardiol. 2011;57:1752–1761. doi: 10.1016/j.jacc.2010.11.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zappitelli M, Washburn KK, Arikan AA, Loftis L, Ma Q, Devarajan P, Parikh CR, Goldstein SL. Urine neutrophil gelatinase-associated lipocalin is an early marker of acute kidney injury in critically ill children: a prospective cohort study. Crit Care. 2007;11:R84. doi: 10.1186/cc6089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bellomo R. Defining, quantifying, and classifying acute renal failure. Crit Care Clin. 2005;21:223–237. doi: 10.1016/j.ccc.2004.12.001. [DOI] [PubMed] [Google Scholar]
- 13.Mehta RL, Kellum JA, Shah SV, Molitoris BA, Ronco C, Warnock DG, Levin A, Network AKI. Acute Kidney Injury Network: report of an initiative to improve outcomes in acute kidney injury. Crit Care. 2007;11:R31. doi: 10.1186/cc5713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kellum JA, Lameire N, Group ftKAGW Diagnosis, evaluation, and management of acute kidney injury: a KDIGO summary (Part 1) Crit Care. 2013;17:204. doi: 10.1186/cc11454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Atiyeh BA, Dabbagh SS, Gruskin AB. Evaluation of renal function during childhood. Pediatr Rev. 1996;17:175–180. doi: 10.1542/pir.17-5-175. [DOI] [PubMed] [Google Scholar]
- 16.Laterza OF, Price CP, Scott MG. Cystatin C: an improved estimator of glomerular filtration rate? Clin Chem. 2002;48:699–707. [PubMed] [Google Scholar]
- 17.Filler G, Lepage N. Should the Schwartz formula for estimation of GFR be replaced by cystatin C formula? Pediatr Nephrol. 2003;18:981–985. doi: 10.1007/s00467-003-1271-5. [DOI] [PubMed] [Google Scholar]
- 18.Zappitelli M, Parvex P, Joseph L, Paradis G, Grey V, Lau S, Bell L. Derivation and validation of cystatin C-based prediction equations for GFR in children. Am J Kidney Dis. 2006;48:221–230. doi: 10.1053/j.ajkd.2006.04.085. [DOI] [PubMed] [Google Scholar]
- 19.Herget-Rosenthal S, Marggraf G, Hüsing J, Göring F, Pietruck F, Janssen O, Philipp T, Kribben A. Early detection of acute renal failure by serum cystatin C. Kidney Int. 2004;66:1115–1122. doi: 10.1111/j.1523-1755.2004.00861.x. [DOI] [PubMed] [Google Scholar]
- 20.Fricker M, Wiesli P, Brändle M, Schwegler B, Schmid C. Impact of thyroid dysfunction on serum cystatin C. Kidney Int. 2003;63:1944–1947. doi: 10.1046/j.1523-1755.2003.00925.x. [DOI] [PubMed] [Google Scholar]
- 21.Kos J, Stabuc B, Cimerman N, Brünner N. Serum cystatin C, a new marker of glomerular filtration rate, is increased during malignant progression. Clin Chem. 1998;44:2556–2557. [PubMed] [Google Scholar]
- 22.Manetti L, Genovesi M, Pardini E, Grasso L, Lupi I, Linda Morselli L, Pellegrini G, Martino E. Early effects of methylprednisolone infusion on serum cystatin C in patients with severe Graves' ophthalmopathy. Clin Chim Acta. 2005;356:227–228. doi: 10.1016/j.cccn.2005.01.022. [DOI] [PubMed] [Google Scholar]
- 23.Manetti L, Pardini E, Genovesi M, Campomori A, Grasso L, Morselli LL, Lupi I, Pellegrini G, Bartalena L, Bogazzi F, Martino E. Thyroid function differently affects serum cystatin C and creatinine concentrations. J Endocrinol Invest. 2005;28:346–349. doi: 10.1007/BF03347201. [DOI] [PubMed] [Google Scholar]
- 24.Akcan-Arikan A, Zappitelli M, Loftis LL, Washburn KK, Jefferson LS, Goldstein SL. Modified RIFLE criteria in critically ill children with acute kidney injury. Kidney Int. 2007;71:1028–1035. doi: 10.1038/sj.ki.5002231. [DOI] [PubMed] [Google Scholar]
- 25.Alkandari O, Eddington KA, Hyder A, Gauvin F, Ducruet T, Gottesman R, Phan V, Zappitelli M. Acute kidney injury is an independent risk factor for pediatric intensive care unit mortality, longer length of stay and prolonged mechanical ventilation in critically ill children: a two-center retrospective cohort study. Crit Care. 2011;15:R146. doi: 10.1186/cc10269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schneider J, Khemani R, Grushkin C, Bart R. Serum creatinine as stratified in the RIFLE score for acute kidney injury is associated with mortality and length of stay for children in the pediatric intensive care unit. Crit Care Med. 2010;38:933–939. doi: 10.1097/CCM.0b013e3181cd12e1. [DOI] [PubMed] [Google Scholar]
- 27.Pollack MM, Ruttimann UE, Getson PR. Pediatric risk of mortality (PRISM) score. Crit Care Med. 1988;16:1110–1116. doi: 10.1097/00003246-198811000-00006. [DOI] [PubMed] [Google Scholar]
- 28.Leteurtre S, Martinot A, Duhamel A, Proulx F, Grandbastien B, Cotting J, Gottesman R, Joffe A, Pfenninger J, Hubert P, Lacroix J, Leclerc F. Validation of the paediatric logistic organ dysfunction (PELOD) score: prospective, observational, multicentre study. Lancet. 2003;362:192–197. doi: 10.1016/S0140-6736(03)13908-6. [DOI] [PubMed] [Google Scholar]
- 29.Schwartz GJ, Munoz A, Schneider MF, Mak RH, Kaskel F, Warady BA, Furth SL. New equations to estimate GFR in children with CKD. J Am Soc Nephrol. 2009;20:629–637. doi: 10.1681/ASN.2008030287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fadrowski JJ, Neu AM, Schwartz GJ, Furth SL. Pediatric GFR estimating equations applied to adolescents in the general population. Clin J Am Soc Nephrol. 2011;6:1427–1435. doi: 10.2215/CJN.06460710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Krawczeski CD, Woo JG, Wang Y, Bennett MR, Ma Q, Devarajan P. Neutrophil gelatinase-associated lipocalin concentrations predict development of acute kidney injury in neonates and children after cardiopulmonary bypass. The Journal of pediatrics. 2011;158:1009–1015. doi: 10.1016/j.jpeds.2010.12.057. e1001. [DOI] [PubMed] [Google Scholar]
- 32.Vaidya VS, Waikar SS, Ferguson MA, Collings FB, Sunderland K, Gioules C, Bradwin G, Matsouaka R, Betensky RA, Curhan GC, Bonventre JV. Urinary biomarkers for sensitive and specific detection of acute kidney injury in humans. Clin Transl Sci. 2008;1:200–208. doi: 10.1111/j.1752-8062.2008.00053.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pencina MJ, D'Agostino RB, Sr., D'Agostino RB, Jr., Vasan RS. Evaluating the added predictive ability of a new marker: from area under the ROC curve to reclassification and beyond. Statistics in medicine. 2008;27:157–172. doi: 10.1002/sim.2929. discussion 207-112. [DOI] [PubMed] [Google Scholar]
- 34.Pencina MJ, D'Agostino RB, Sr., Steyerberg EW. Extensions of net reclassification improvement calculations to measure usefulness of new biomarkers. Statistics in medicine. 2011;30:11–21. doi: 10.1002/sim.4085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Grubb A, Nyman U, Bjork J, Lindstrom V, Rippe B, Sterner G, Christensson A. Simple cystatin C-based prediction equations for glomerular filtration rate compared with the modification of diet in renal disease prediction equation for adults and the Schwartz and the Counahan-Barratt prediction equations for children. Clinical chemistry. 2005;51:1420–1431. doi: 10.1373/clinchem.2005.051557. [DOI] [PubMed] [Google Scholar]
- 36.Kwon SH, Hyun J, Jeon JS, Noh H, Han DC. Subtle change of cystatin C, with or without acute kidney injury, associated with increased mortality in the intensive care unit. J Crit Care. 2011;26:566–571. doi: 10.1016/j.jcrc.2011.01.004. [DOI] [PubMed] [Google Scholar]
- 37.Lassus JP, Nieminen MS, Peuhkurinen K, Pulkki K, Siirilä-Waris K, Sund R, Harjola VP, group F-As. Markers of renal function and acute kidney injury in acute heart failure: definitions and impact on outcomes of the cardiorenal syndrome. Eur Heart J. 2010;31:2791–2798. doi: 10.1093/eurheartj/ehq293. [DOI] [PubMed] [Google Scholar]
- 38.Nejat M, Pickering JW, Walker RJ, Endre ZH. Rapid detection of acute kidney injury by plasma cystatin C in the intensive care unit. Nephrol Dial Transplant. 2010;25:3283–3289. doi: 10.1093/ndt/gfq176. [DOI] [PubMed] [Google Scholar]
- 39.Zappitelli M, Krawczeski CD, Devarajan P, Wang Z, Sint K, Thiessen-Philbrook H, Li S, Bennett MR, Ma Q, Shlipak MG, Garg AX, Parikh CR, consortium T-A. Early postoperative serum cystatin C predicts severe acute kidney injury following pediatric cardiac surgery. Kidney Int. 2011;80:655–662. doi: 10.1038/ki.2011.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Briguori C, Visconti G, Rivera NV, Focaccio A, Golia B, Giannone R, Castaldo D, De Micco F, Ricciardelli B, Colombo A. Cystatin C and contrast-induced acute kidney injury. Circulation. 2010;121:2117–2122. doi: 10.1161/CIRCULATIONAHA.109.919639. [DOI] [PubMed] [Google Scholar]
- 41.Choi YS, Shim JK, Kim JC, Kang KS, Seo YH, Ahn KR, Kwak YL. Effect of remote ischemic preconditioning on renal dysfunction after complex valvular heart surgery: a randomized controlled trial. J Thorac Cardiovasc Surg. 2011;142:148–154. doi: 10.1016/j.jtcvs.2010.11.018. [DOI] [PubMed] [Google Scholar]
- 42.Cortjens B, Royakkers AA, Determann RM, van Suijlen JD, Kamphuis SS, Foppen J, de Boer A, Wieland CW, Spronk PE, Schultz MJ, Bouman CS. Lung-protective mechanical ventilation does not protect against acute kidney injury in patients without lung injury at onset of mechanical ventilation. J Crit Care. 2012;27:261–267. doi: 10.1016/j.jcrc.2011.05.005. [DOI] [PubMed] [Google Scholar]
- 43.Haase M, Bellomo R, Devarajan P, Ma Q, Bennett MR, Möckel M, Matalanis G, Dragun D, Haase-Fielitz A. Novel biomarkers early predict the severity of acute kidney injury after cardiac surgery in adults. Ann Thorac Surg. 2009;88:124–130. doi: 10.1016/j.athoracsur.2009.04.023. [DOI] [PubMed] [Google Scholar]
- 44.Soto K, Coelho S, Rodrigues B, Martins H, Frade F, Lopes S, Cunha L, Papoila AL, Devarajan P. Cystatin C as a marker of acute kidney injury in the emergency department. Clin J Am Soc Nephrol. 2010;5:1745–1754. doi: 10.2215/CJN.00690110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wald R, Liangos O, Perianayagam MC, Kolyada A, Herget-Rosenthal S, Mazer CD, Jaber BL. Plasma cystatin C and acute kidney injury after cardiopulmonary bypass. Clin J Am Soc Nephrol. 2010;5:1373–1379. doi: 10.2215/CJN.06350909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhang Z, Lu B, Sheng X, Jin N. Cystatin C in prediction of acute kidney injury: a systemic review and meta-analysis. Am J Kidney Dis. 2011;58:356–365. doi: 10.1053/j.ajkd.2011.02.389. [DOI] [PubMed] [Google Scholar]