Abstract
In this brief account, we review work from our lab with a focus on late-stage introduction of fluorine and fluorinated functional groups into small molecules. We attempt to highlight practical developments, which we believe may have potential for industrial applications, and critically reflect on developments that may not yet meet the bar for practical use.
1. Introduction
With regard to developing “process-relevant” chemistry, we feel that there are two general types of contributions that academic chemists can strive to make. The first is the development of known chemical reactivity into methods that hold promise for scalability and sustainability in large-scale manufacturing. The second is the discovery of previously unappreciated synthetic transformations that may, with appropriate development, enable practical access to desirable products. In this review, we aim to highlight work from our own lab that we hope could eventually become contributions to process chemistry. In particular we will describe recently developed methods for the late-stage introduction of fluorine and fluorinated functional groups into small molecules, which enable the synthesis of fluorinated compounds of interest in pharmaceuticals and agrochemicals, and provide for access to 18F-labeled tracers for positron emission tomography (PET). This brief account is not meant to serve as an exhaustive literature review; for a more comprehensive survey of contemporary advances in late-stage fluorination, the interested reader is encouraged to explore the references provided below.
2. Late-Stage Fluorination
Fluorine-containing organic molecules are of high importance due to the unique properties that fluorination can impart upon a molecule.1 Despite the paucity of fluorinated natural products,2−5 fluorinated compounds are common in modern society—approximately 20% of commercial pharmaceuticals and 30% of agrochemicals contain fluorine.6−9 Introduction of fluorine into pharmaceuticals can increase lipophilicity and metabolic stability, which can enhance the efficacy and bioavailability of a drug compound.6 Additionally, the isotope 18F is the preferred positron-emitting isotope for positron emission tomography (PET), and the widespread use of 2-[18F]fluoro-2-deoxyglucose ([18F]FDG) in PET has had an impact in oncology.10 For these reasons, the synthesis of fluorinated organic molecules has received considerable attention.11−13 Many conventional fluorination reactions are well-established, using either electrophilic fluorine gas (F2) or nucleophilic displacement reactions with fluoride, such as the Halex process.14 While these established methods have been successful on an industrial scale, such reactions often require harsh reaction conditions that limit their substrate scope and selectivity. As a result, fluorination reactions are often conducted on simple substrates that are subsequently used as building blocks for elaboration.
Perhaps for similar reasons by which fluorine has not been used extensively in Nature, selective formation of carbon–fluorine bonds has remained particularly challenging for synthetic chemists. For many applications, especially the synthesis of 18F PET tracers, it is crucial to perform fluorination on advanced intermediates that already bear complex functionality. In the synthesis of fluorinated pharmaceutical candidates, it is often preferable to be able to selectively and reliably fluorinate at any desired point in the synthetic route, rather than being restricted to the use of simple, commercially available fluorinated building blocks. This challenge has inspired many laboratories, including our own, to work toward developing mild, selective reactions for the installation of fluorine and fluorinated functional groups in complex small molecules.15−24
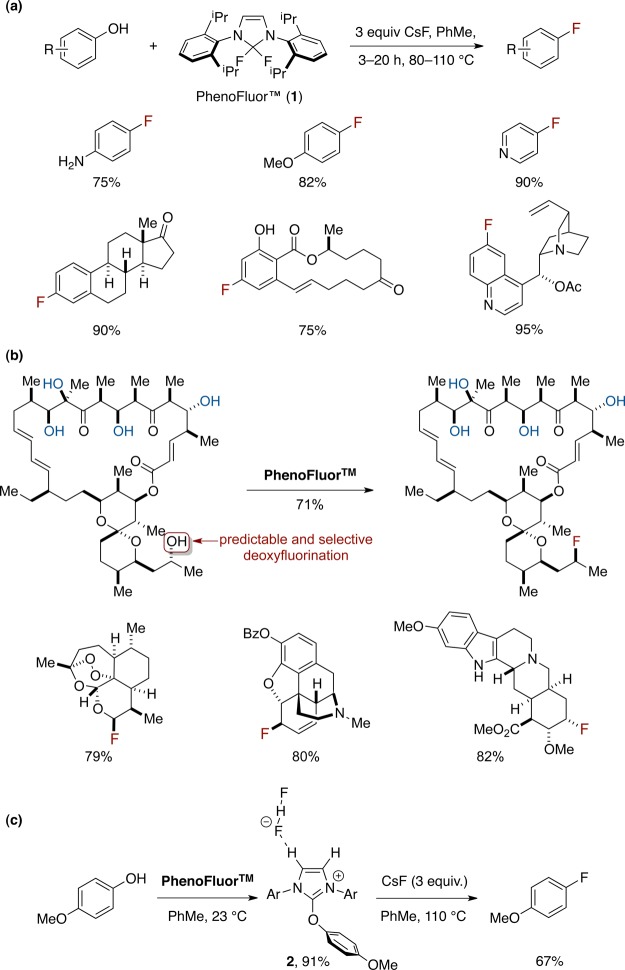
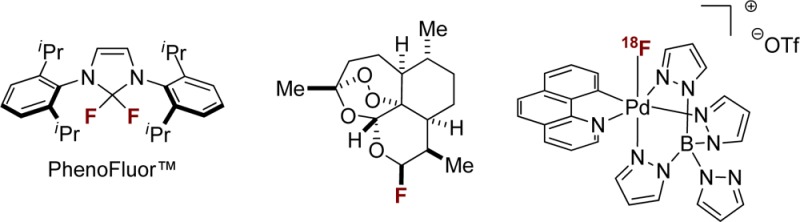
Our desire to develop practical, operationally simple fluorination reactions of readily available starting materials led to the development of the new deoxyfluorination reagent PhenoFluor (1), currently commercially available from Sigma-Aldrich and Strem Chemicals Inc. ($0.80/mg). In 2011 we reported the use of PhenoFluor for one-step synthesis of aryl fluorides via ipso-substitution of phenols (Figure 1a).25 The deoxyfluorination reaction is effective for a wide variety of aryls, including electron-rich phenols that cannot be fluorinated using conventional nucleophilic aromatic substitution reactions. Other commercially available deoxyfluorination reagents such as DAST, Xtalfluor, and DEOXYFLUOR did not afford product for electron-rich substrates such as 4-methoxyphenol.25 We have also demonstrated that PhenoFluor can be used for selective late-stage fluorination of complex small molecules by deoxyfluorination of aliphatic alcohols (Figure 1b).26 The proposed mechanism for fluorination with PhenoFluor involves formation of a 2-phenoxyimidazolium bifluoride salt, which affords fluorinated arene and the urea byproduct upon nucleophilic attack by fluoride. When 4-methoxyphenol was mixed with Phenofluor, salt 2 could be isolated in 91% yield (Figure 1c). X-ray crystallographic analysis of 2 revealed a hydrogen bond between one hydrogen atom of the imidazolium heterocycle and the bifluoride counteranion. On the basis of 1H NMR evidence, we postulate that a similar hydrogen bond exists in solution, and we believe that the ability of salts such as 2 to participate in hydrogen bonding with bifluoride is important to the success of PhenoFluor as a deoxyfluorination reagent. In accordance with this hypothesis, derivatives of imidazolium salt 2 that lack the ability to hydrogen bond, such as the structurally analogous 2-phenoxyimidazolinium salt (3), do not produce aryl fluoride upon heating. While PhenoFluor would likely not be suitable for large-scale use due to the reagent’s mass, catalytic turnover should be conceptually possible. The development of a catalytic reagent with reactivity analogous to that of PhenoFluor would be a major advance.
Figure 1.
(a) Deoxyfluorination of phenols with PhenoFluor. (b) Late-stage deoxyfluorination of complex aliphatic alcohols with PhenoFluor. (c) Intermediate 2 observed upon treatment of phenols with PhenoFluor, with hydrogen-bonding interaction observed via X-ray crystallography and 1H NMR spectroscopy.
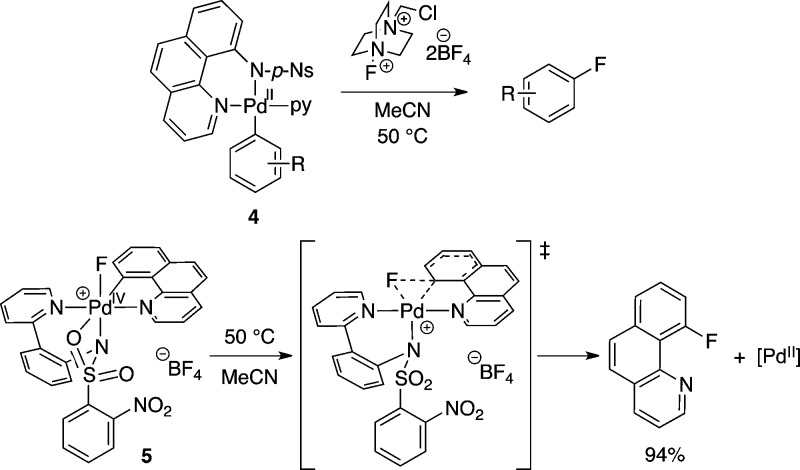
PhenoFluor provides for a practical synthesis of aryl fluorides, but is currently applicable only to substrates that contain the requisite phenol functionality. A transition metal cross-coupling approach, on the other hand, has the potential to expand the possible substrate scope to also include aryl halides, arylboronic acids, and aryl stannanes. Therefore, transition metal-mediated or -catalyzed C–F bond formation is a promising route for the late-stage fluorination of complex small molecules and for the synthesis of 18F-labeled PET tracers. However, C–F reductive elimination from a transition metal complex is challenging due to the strength and high polarization of metal–fluorine bonds.27 In 2008, we reported that a range of arylboronic acids could be converted into the corresponding aryl fluorides in a regiospecific reaction sequence via palladium complexes 4 and electrophilic fluorinating reagent Selectfluor (Figure 2).28,29 We demonstrated the first example of well-defined carbon–fluorine reductive elimination to form aryl fluorides from a transition metal complex, Pd(IV) fluoride 5.30,31 Experimental and computational investigations support a concerted C–F reductive elimination mechanism from 5; dissociation of one oxygen atom of the tridenate pyridyl-sulfonamide ligand gives a five-coordinate Pd(IV) complex that readily undergoes C–F reductive elimination (Figure 2).32
Figure 2.
Pd-mediated electrophilic fluorination of arenes, and well-defined C–F reductive elimination from Pd(IV) fluoride 5 featuring a hemilabile pyridyl-sulfonamide ligand to promote reductive elimination via a five-coordinate transition state.
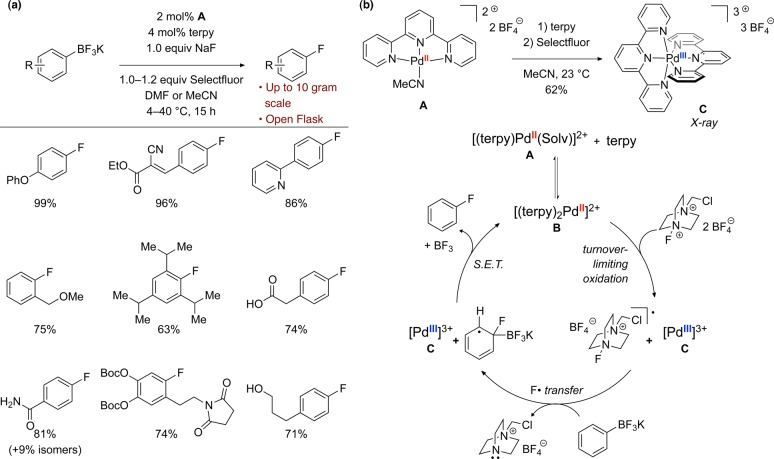
Having established the viability of palladium-mediated C–F bond formation, we sought to incorporate this reactivity into a practical, catalytic reaction. Our work in this area has recently led to the development of a palladium-catalyzed fluorination of arylboronic acid derivatives, using commercially available fluorinating reagent Selectfluor.33 Arylboronic acid derivatives are desirable building blocks due to their synthetic accessibility, stability, and low toxicity. Our palladium-catalyzed reaction can be performed in an open flask and is effective for milligram- to at least multigram-scale synthesis of aryl fluorides, which are readily isolated. Inseparable side products from protodeborylation were not observed for the majority of substrates. As shown in Figure 3a, a wide variety of aryl trifluoroborates can be fluorinated, including both electron-rich and electron-poor arenes. Ketones, primary amides, carboxylic acids, esters, alcohols, basic heterocycles, aryl bromides, and ortho,ortho′-disubstitution are tolerated in the reaction. Arylboronic acids, pinacol boronic esters, and electron-rich MIDA boranates can also be fluorinated using the palladium-catalyzed reaction. Limitations of the reaction include the inability to fluorinate heterocycles and the formation of constitutional isomers for some electron-poor substrates. Contrary to our initial expectations, kinetic studies suggest a mechanism distinct from other known arene fluorination reactions, which proceeds through a single-electron-transfer (SET) pathway involving an unusual Pd(III) intermediate that has been isolated and characterized (Figure 3b). While C–F bond formation is proposed to occur via reductive elimination from an aryl–metal fluoride complex in the palladium-mediated fluorination of arylboronic acids (vide supra), the palladium-catalyzed fluorination seems to proceed without the formation of organopalladium intermediates yet provides high levels of selectivity.
Figure 3.
(a) Palladium-catalyzed fluorination of aryl trifluoroborates. (b) Proposed mechanism for palladium-catalyzed fluorination, involving a single-electron-transfer (SET) pathway and isolated Pd(III) intermediate C.
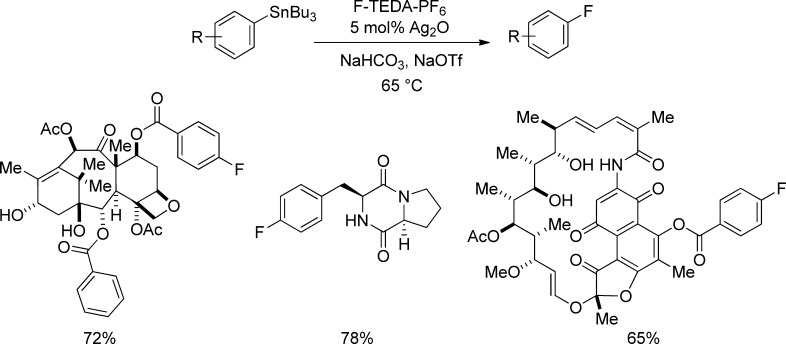
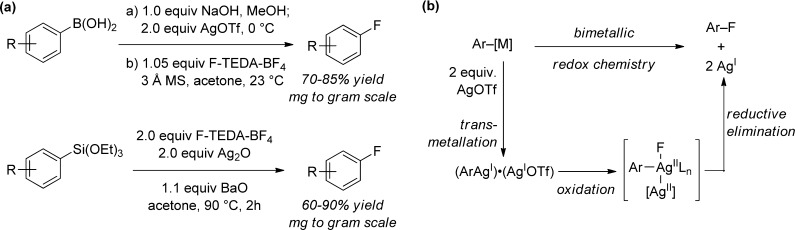
The palladium-catalyzed fluorination of arylboronic acid derivatives allows for practical access to aryl fluorides from readily available starting materials but displays limitations with respect to substrate scope, which will require further development in the future. For late-stage fluorination of highly functionalized molecules, our group’s silver-catalyzed fluorination of aryl stannanes has proven exceptionally effective for complex substrates (Figure 4).34,35 The silver-catalyzed method tolerates a variety of functional groups including heterocycles and protic functionality such as alcohols. The functional group tolerance and substrate scope observed for the silver-catalyzed reaction have not been demonstrated for any other fluorination reaction to date. A disadvantage is the use of aryl stannanes as starting materials, which are toxic and require an additional synthetic step for preparation from more readily available aryl halides. Less toxic arylboronic acids and aryl silanes can also be fluorinated if silver is used in stoichiometric quantities (Figure 5a),36,37 and preliminary mechanistic investigation suggests the involvement of a multinuclear arylsilver complex, in which metal–metal redox cooperation may facilitate the transformation (Figure 5b).34,35,38
Figure 4.
Silver-catalyzed late-stage fluorination of complex small molecules.
Figure 5.
(a) Silver-mediated fluorination of arylboronic acids and aryl silanes. (b) Proposed bimetallic mechanism for Ag-mediated C–F bond formation ([M] = SnBu3, B(OH)2, Si(OEt)3).
The metal-mediated and -catalyzed fluorination reactions described above allow for late-stage C–F bond formation in complex small molecules, and we anticipated that such an approach could also provide for access to previously unavailable 18F-labeled fluoroarenes for PET imaging. However, formation of the C–F bond is only one challenge associated with the synthesis of 18F PET tracers. When transitioning from 19F to 18F chemistry, it is most practical to use nucleophilic [18F]fluoride, which can be produced using a cyclotron as an aqueous solution in high specific activity.10 However, the synthesis of Pd(IV)–fluoride complexes such as 5 require the use of electrophilic fluorinating reagents such as Selectfluor or XeF2 (Figure 2). It is important to note that 18F-electrophilic fluorinating reagents are generally derived from electrophilic [18F]fluorine gas ([18F]F2), which requires dilution with [19F]F2 as a carrier gas, where 19F is the natural, PET-inactive isotope.19 The need for [19F]F2 as a carrier gas results in a lower specific activity for [18F]F2 than for [18F]fluoride. High specific activity is often critical for imaging biological targets with low concentration, such as neurotransmitter receptors in the brain.10 [18F]F2 gas is also less practical to handle as compared to [18F]fluoride, due to its high reactivity and toxicity. Further challenges to the application of fluorination reactions to PET include the need for short reaction times as well as unique reaction conditions for 18F chemistry. For example, extensive drying of fluoride, as is often required for metal-mediated fluorination reactions using [19F]fluoride,15,17 can be impractical when starting from aqueous [18F]fluoride, as 18F PET tracer synthesis is typically executed on a nanomole scale. As a further consequence, the smaller ratio of fluorine to water can be problematic because hydrated fluoride has diminished nucleophilicity.39 Due to such factors, the translation of promising modern fluorination reactions to radiochemistry is often problematic.40
In order to address the challenges of developing a synthesis of 18F-labeled PET tracers using [18F]fluoride, we designed a two-step “fluoride capture/transfer” sequence (Figure 6).41 In the “capture” step, [18F]fluoride binds to a cationic Pd(IV) complex, 6, to generate [18F]Pd(IV)–fluoride complex, 7. Complex [18F]7 can behave as an electrophilic fluorinating reagent; reaction with Pd(II) aryl complex 8 results in oxidative fluorine transfer to give a [18F]Pd(IV)–fluoride complex analogous to 5, which undergoes C–F reductive elimination to provide the 18F-labeled aryl fluoride. The method can be used to synthesize 18F-labeled aryl fluorides with electron-rich arenes and a variety of functional groups, which would be otherwise challenging to access using [18F]fluoride (Figure 6). Mechanistic studies indicate that [18F]7 is formed with high rates, even at the nano- to micromolar fluoride concentrations typical for radiosyntheses with 18F, due to fast formation of an outer-sphere complex between fluoride and Pd(IV) complex 6, and that the subsequent fluorine transfer from [18F]7 to Pd(II) aryl complex 8 likely proceeds through an unusual SET/fluoride transfer/SET mechanism.42
Figure 6.
C–18F bond formation for 18F-PET tracer synthesis via two-step 18F– capture/transfer sequence.
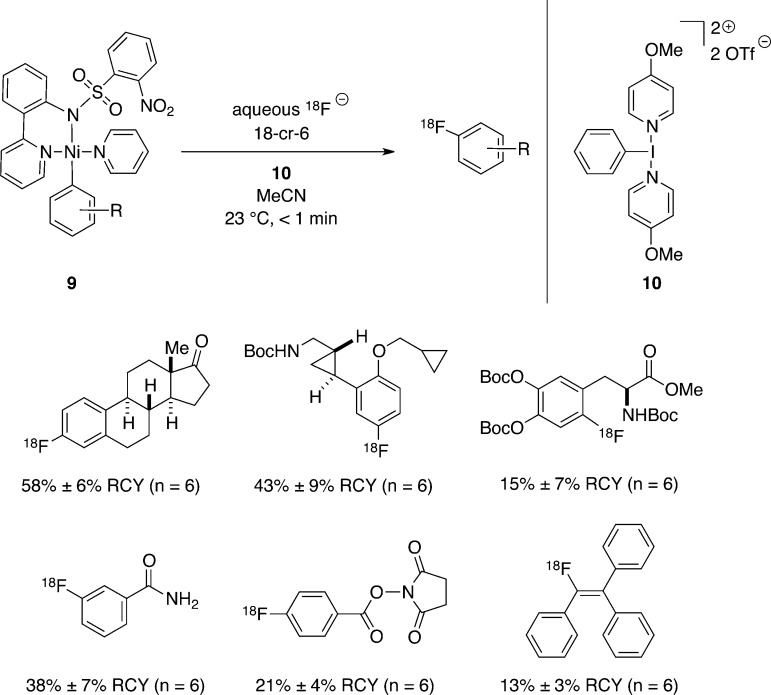
Development of improved methods for practical synthesis of PET tracer molecules using [18F]fluoride is an ongoing goal in our laboratories. We have developed a one-step nickel-mediated fluorination of arenes using aqueous [18F]fluoride, nickel complex 9, and oxidant 10 (Figure 7).43 The oxidative fluorination reaction proceeds in less than one minute for a variety of aryl– and alkenyl–nickel complexes and often provides improved radiochemical yields as compared to our two-step palladium-mediated sequence. The one-pot method involving only the nickel–aryl complex, fluoride, and oxidant circumvents the need for preparation of a separate electrophilic fluorinating reagent such as [18F]7. Additional steps result in an overall longer preparation time for the final 18F-labeled molecule; due to the 110 min half-life of 18F, the shortest possible preparation time is desirable. We hope that our palladium- and nickel-mediated 18F-fluorination reactions will ultimately find application in evaluating pharmaceutical candidates to determine biodistribution, as well as in developing new 18F-PET tracers for clinical care. Toward these aims, the palladium-mediated reaction has already enabled the synthesis of PET tracers for in vivo imaging studies in baboons.44
Figure 7.
One-step Ni-mediated C–18F bond formation using aqueous 18F– and oxidant 10.
Along with aryl fluorides, other fluorinated functional groups are of great interest in pharmaceuticals.6 While significant advances have been made in the field of aryl trifluoromethylation,13,45 late-stage formation of aryl trifluoromethyl ethers has remained a more elusive goal. We have reported the first transition metal-mediated cross-coupling to afford aryl trifluoromethyl ethers, starting from arylboronic acids or aryl stannanes (Figure 8).46 Aryl trifluoromethoxylation reactions are challenging due to the instability of trifluoromethoxide anion in solution; above room temperature, decomposition can occur to give carbonic difluoride and fluoride.47,48 The potential for β-fluoride elimination from transition metal–trifluoromethoxide complexes poses an additional challenge to metal-mediated cross-coupling reactions.49,50 Our method consists of treatment of aryl stannanes with trifluoromethoxide 11, F-TEDA-PF6, and silver(I) hexafluorophosphate (AgPF6) at −30 °C, affording the desired aryl trifluoromethyl ethers in yields up to 88%. The trifluoromethoxylating reagent 11 is prepared in situ from trifluoromethyl trifluoromethanesulfonate, a nonfuming stable liquid that is commercially available and synthesized from triflic acid in one step, and tris(dimethylamino)sulfonium difluorotrimethylsilicate (TASF). While the TAS·OCF3 reagent (11) allows for efficient trifluoromethoxylation of a variety of functionalized arenes, the need for in situ preparation renders the reaction less practical, and development of a stable trifluoromethoxide source that is readily stored and handled would be an important development.
Figure 8.
Silver-mediated trifluoromethoxylation of aryl stannanes.
3. Conclusion
Substantial progress in fluorination methodology has been made in recent years, but the field is far from mature. We continue to work towards the development of practical, synthetically useful fluorination reactions that can enable access to a range of fluorinated products of interest in pharmaceuticals, agrochemicals, and PET imaging. Currently, there is a lack of broadly useful transition metal-catalyzed fluorination reactions; of the currently available methods, our silver-catalyzed reaction is the most effective for complex molecules but requires the use of toxic aryl stannanes, while Buchwald’s palladium-catalyzed nucleophilic fluorination approach is applicable to readily available aryl halides and pseudohalides but can result in a mixture of constitutional isomers that are difficult to separate.15,17 A handful of metal-catalyzed C–H fluorination reactions have been reported24 but are thus far limited in their functional group tolerance and substrate scope or require directing groups in the case of aromatic C–H fluorination.18 Selective C–H functionalization of arenes, without the use of coordinating directing groups, is an area of active research, and we have recently reported a palladium-catalyzed aryl C–H imidation with arene as the limiting reagent.51 Extension of this reactivity to C–H fluorination of complex substrates would be a powerful advance for the field of fluorination.
Acknowledgments
We thank the NSF (CHE-0952753), NIH-NIGMS (GM088237), NIH-NIBIB (EB013042), and AFOSR (FA9550-10-1-0170) for funding of the different research projects described herein, and the DOE SCGF for a graduate fellowship for M.G.C. The Department of Energy, Office of Science Graduate Fellowship Program (DOE SCGF) is made possible in part by the American Recovery and Reinvestment Act of 2009, administered by ORISE-ORAU under contract no. DE-AC05-06OR23100.
Biographies

Tobias Ritter was born in 1975 in Lübeck, Germany. He studied in Braunschweig, Bordeaux, Lausanne, and Stanford. After research with Prof. Barry M. Trost at Stanford, he obtained his Ph.D. working with Prof. Erick M. Carreira at ETH Zurich in 2004. He then carried out postdoctoral research with Prof. Robert H. Grubbs at Caltech. In 2006, he was appointed as Assistant Professor in the Department of Chemistry and Chemical Biology at Harvard, promoted to Associate Professor in 2010, and to Professor of Chemistry and Chemical Biology in 2012.

Michael G. Campbell received his B.S. in 2008 from Loyola University Maryland. He earned his Ph.D. in 2014 from Harvard University, where he worked on the chemistry of palladium(III) complexes with Prof. Tobias Ritter, including Pd-catalyzed fluorination. He is currently a postdoctoral fellow at MIT with Prof. Mircea Dincă.
The authors declare the following competing financial interest(s): SciFluor Life Sciences has licensed several of the technologies discussed in this review from Harvard University. T.R. has financial interest in SciFluor.
Funding Statement
National Institutes of Health, United States
References
- O’Hagan D. Chem. Soc. Rev. 2008, 37, 308–319. [DOI] [PubMed] [Google Scholar]
- Gribble G. W. In Progress in the Chemistry of Organic Natural Products; Herz W., Kirby G. W., Moore R. E., Steglich W., Tamm C., Eds.; Springer: New York, 1996; Vol. 68, pp 1–498. [Google Scholar]
- Gribble G. W. In Progress in the Chemistry of Organic Natural Products; Kinghord A. D., Falk H., Kobayashi J., Eds.; Springer: New York, 2009; Vol. 91, pp 1–613. [Google Scholar]
- O’Hagan D.; Schaffrath C.; Cobb S. L.; Hamilton J. T. G.; Murphy C. D. Nature 2002, 416, 279. [DOI] [PubMed] [Google Scholar]
- Dong C. Nature 2004, 427, 561–565. [DOI] [PubMed] [Google Scholar]
- Muller K.; Faeh C.; Diederich F. Science 2007, 317, 1881–1886. [DOI] [PubMed] [Google Scholar]
- Purser S.; Moore P. R.; Gouverneur V. Chem. Soc. Rev. 2008, 37, 320–330. [DOI] [PubMed] [Google Scholar]
- Jeschke P. ChemBioChem 2004, 5, 570–589. [DOI] [PubMed] [Google Scholar]
- Hung M. H.; Farnham W. B.; Feiring A. E. In. Fluoropolymers: Synthesis; Hougham G., Cassidy P. E., Johns K., Davidson T., Eds.; Plenum, 1999; Vol. 1, pp 51–56. [Google Scholar]
- Ametamey S. M.; Honer M.; Schubiger P. A. Chem. Rev. 2008, 37, 1501–1516. [DOI] [PubMed] [Google Scholar]
- Furuya T.; Kuttruff C. A.; Ritter T. Curr Opin Drug Discov Devel 2008, 11, 803–819. [PubMed] [Google Scholar]
- Furuya T.; Klein J.; Ritter T. Synthesis 2010, 2010, 1804–1821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furuya T.; Kamlet A. S.; Ritter T. Nature 2011, 473, 470–477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirsch P.Modern Fluoroorganic Chemistry: Synthesis, Reactivity, Applications; Wiley, 2004. [Google Scholar]
- Watson D. A.; Su M.; Teverovskiy G.; Zhang Y.; Garcia-Fortanet J.; Kinzel T.; Buchwald S. L. Science 2009, 325, 1661–1664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parsons A. T.; Buchwald S. L. Angew. Chem., Int. Ed. 2011, 50, 9120–9123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fier P. S.; Hartwig J. F. J. Am. Chem. Soc. 2012, 134, 10795–10798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hull K. L.; Anani W. Q.; Sanford M. S. J. Am. Chem. Soc. 2006, 128, 7134–7135. [DOI] [PubMed] [Google Scholar]
- Teare H.; Robins E. G.; Kirjavainen A.; Forsback S.; Sandford G.; Solin O.; Luthra S. K.; Gouverneur V. Angew. Chem., Int. Ed. 2010, 49, 6821–6824. [DOI] [PubMed] [Google Scholar]
- Teverovskiy G.; Surry D. S.; Buchwald S. L. Angew. Chem. 2011, 123, 7450–7452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalow J. A.; Doyle A. G. J. Am. Chem. Soc. 2010, 132, 3268–3269. [DOI] [PubMed] [Google Scholar]
- Katcher M. H.; Doyle A. G. J. Am. Chem. Soc. 2010, 132, 17402–17404. [DOI] [PubMed] [Google Scholar]
- Katcher M. H.; Sha A.; Doyle A. G. J. Am. Chem. Soc. 2011, 133, 15902–15905. [DOI] [PubMed] [Google Scholar]
- Liu W.; Huang X.; Cheng M. J.; Nielsen R. J.; Goddard W. A.; Groves J. T. Science 2012, 337, 1322–1325. [DOI] [PubMed] [Google Scholar]
- Tang P.; Wang W.; Ritter T. J. Am. Chem. Soc. 2011, 133, 11482–11484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sladojevich F.; Arlow S. I.; Tang P.; Ritter T. J. Am. Chem. Soc. 2013, 135, 2470–2473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grushin V. V. Acc. Chem. Res. 2010, 43, 160–171. [DOI] [PubMed] [Google Scholar]
- Furuya T.; Kaiser H. M.; Ritter T. Angew. Chem., Int. Ed. 2008, 47, 5993–5996. [DOI] [PubMed] [Google Scholar]
- Fier P. S.; Luo J.; Hartwig J. F. J. Am. Chem. Soc. 2013, 135, 2552–2559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furuya T.; Ritter T. J. Am. Chem. Soc. 2008, 130, 10060–10061. [DOI] [PubMed] [Google Scholar]
- Ball N. D.; Sanford M. S. J. Am. Chem. Soc. 2009, 131, 3796–3797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furuya T.; Benitez D.; Tkatchouk E.; Strom A. E.; Tang P.; Goddard W. A. III; Ritter T. J. Am. Chem. Soc. 2010, 132, 3793–3807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazzotti A. R.; Campbell M. G.; Tang P.; Murphy J. M.; Ritter T. J. Am. Chem. Soc. 2013, 135, 14012–14015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang P.; Furuya T.; Ritter T. J. Am. Chem. Soc. 2010, 132, 12150–12154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furuya T.; Strom A. E.; Ritter T. J. Am. Chem. Soc. 2009, 131, 1662–1663. [DOI] [PubMed] [Google Scholar]
- Furuya T.; Ritter T. Org. Lett. 2009, 11, 2860–2863. [DOI] [PubMed] [Google Scholar]
- Tang P.; Ritter T. Tetrahedron 2011, 67, 4449–4454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powers D. C.; Ritter T. Acc. Chem. Res. 2012, 45, 840–850. [DOI] [PubMed] [Google Scholar]
- Emsley J. Chem. Soc. Rev. 1980, 9, 19–124. [Google Scholar]
- Cardinale J.; Ermert J.; Kügler F.; Helfer A.; Brandt M. R.; Coenen H. H. J. Label Compd. Radiopharm 2012, 55, 450–453. [Google Scholar]
- Lee E.; Kamlet A. S.; Powers D. C.; Neumann C. N.; Boursalian G. B.; Furuya T.; Choi D. C.; Hooker J. M.; Ritter T. Science 2011, 334, 639–642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandt J. R.; Lee E.; Boursalian G. B.; Ritter T. Chem. Sci. 2014, 5, 169–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee E.; Hooker J. M.; Ritter T. J. Am. Chem. Soc. 2012, 134, 17456–17458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamlet A. S.; Neumann C. N.; Lee E.; Carlin S. M.; Moseley C. K.; Stephenson N.; Hooker J. M.; Ritter T. PLoS ONE 2013, 8, e59187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- García Monforte M.; Martínez Salvador S.; Menjón B. Eur. J. Inorg. Chem. 2012, 4945–4966,. [Google Scholar]
- Huang C.; Liang T.; Harada S.; Lee E.; Ritter T. J. Am. Chem. Soc. 2011, 133, 13308–13310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolomeitsev A. A.; Vorobyev M.; Gillandt H. Tetrahedron Lett. 2008, 49, 449–454. [Google Scholar]
- Taylor S. L.; Martin J. C. J. Org. Chem. 1987, 52, 4147–4156. [Google Scholar]
- Fout A. R.; Scott J.; Miller D. L.; Bailey B. C.; Pink M.; Mindiola D. J. Organometallics 2009, 28, 331–347. [Google Scholar]
- Kraft B. M.; Lachicotte R. J.; Jones W. D. Organometallics 2002, 21, 727–731. [Google Scholar]
- Boursalian G. B.; Ngai M.-Y.; Hojczyk K. N.; Ritter T. J. Am. Chem. Soc. 2013, 135, 13278–13281. [DOI] [PMC free article] [PubMed] [Google Scholar]