Abstract
Agonist activation of the δ-opioid receptor leads to internalization via Gβγ recruitment of G protein coupled receptor kinase-2, which phosphorylates the receptor at several sites, including Ser363, allowing β-arrestin binding and localization to clathrin coated pits. Using HEK cells expressing a δ-opioid receptor we tested the hypothesis that prevention of receptor coupling to G protein by treatment with pertussis toxin (PTX) will block these processes. PTX treatment did not reduce phosphorylation of δ-opioid receptor Ser363 in response to the agonist DPDPE, or recruitment of β-arrestin 2-GFP to the membrane and only slowed, but did not prevent, DPDPE-induced internalization. Similarly PTX treatment only partially prevented the ability of the δ-opioid peptide agonists deltorphin II and [Met5]enkephalin and the non-peptide agonist BW373U86 to induce receptor internalization. No internalization was seen with morphine, oxymorphindole or the putative δ1-opioid agonist TAN-67 in the presence or absence of PTX, even though TAN-67 showed a strong activation of G protein, as measured by [35S]GTPγS binding. The ability of an agonist to stimulate phosphorylation at Ser363 was predictive of its capacity to induce internalization. The results suggest a role for G protein in δ-opioid receptor internalization, but show that alternative G protein independent pathways exist.
Keywords: β-arrestin, δ-opioid receptor, G protein coupled receptor kinase, internalization, pertussis toxin, phosphorylation
The δ-opioid receptor belongs to the family of 7-transmembrane domain receptors coupled to heterotrimeric G proteins (GPCRs). In addition to antinociceptive effects (Narita and Suzuki 2004), δ-opioid receptor agonists have been shown to have antidepressant-like (Broom et al. 2002a) and antiparkinson-like activities (Hudzik et al. 2000). However, the potential beneficial actions of δ-opioid agonists are hindered by their propensity to induce rapid and prolonged tolerance (Brandt et al. 2001; Broom et al. 2002c; Broom et al. 2002b; Jutkiewicz et al. 2005). Internalization of the δ-opioid receptor has long been thought to play a role in tolerance (Eisinger and Schulz 2005).
After activation by agonist, the δ-opioid receptor undergoes phosphorylation by G protein coupled receptor kinase 2 (GRK) (Pei et al. 1995), followed by interaction with β-arrestin to block further coupling to G proteins leading to desensitization (Kovoor et al. 1997) and internalization (Zhang et al. 1999) via clathrin coated pits (Trapaidze et al. 1996). Recruitment of GRK2 to the membrane requires the βγ subunits of the G protein heterotrimer (Li et al. 2003). However, the necessity of G protein activation for δ-opioid receptor internalization is unclear. An early report (Law et al. 1985) showed that pretreatment of NG108-15 cells (rat neuroblastoma x mouse glioma hybridoma) with pertussis toxin (PTX), to inhibit δ-opioid receptor coupling to Gαi/o proteins, had no effect on the ability of the δ-opioid agonist DADLE ([D-Ala2,D-Leu5]enkephalin) to induce receptor internalization. Similarly, in mouse neuroblastoma Neuro2A cells, stably expressing a δ-opioid receptor, PTX failed to block internalization by the selective agonist DPDPE ([D-Pen2,D-Pen5]enkephalin) (Chakrabarti et al. 1997). In contrast, in human embryonic kidney (HEK) 293 cells stably expressing the mouse δ-opioid receptor, PTX pretreatment inhibited by 60 % the ability of the δ-opioid agonist DTLET ([DThr2]-Leu-enkephalin-Thr6) to mediate internalization (Kramer et al. 2000b).
Whether G proteins are required or not, there is evidence that GRKs of some type, possibly including those not recruited by Gβγ such as GRK5 and GRK6 (Ferguson 2001; Lodowski et al. 2006) are involved in δ-opioid receptor desensitization (Willets and Kelly 2001) and internalization (Hasbi et al. 2000). Certainly, mutagenesis data from several groups suggests that preventing phosphorylation of serine and/or threonine residues in the C-terminus of the δ-opioid receptor blocks receptor internalization across a variety of cell lines, although this effect is not always complete (Trapaidze et al. 1996; Zhao et al. 1997; Murray et al. 1998; Kouhen et al. 2000; Whistler et al. 2001; Zhang et al. 2005; Navratilova et al. 2007). These data thus indicate that the δ-opioid receptor may be able to participate in both G protein dependent and independent phosphorylation and internalization.
Several studies have suggested that δ-opioid receptor agonists are capable of stimulating distinct fates of the receptor. For example, preincubation of HEK 293 cells stably expressing the mouse δ-opioid receptor with the peptide agonists DPDPE or DSLET ([D-Ser2,D-Leu5]enkephalin-Thr6), or the alkaloid etorphine resulted in receptor desensitization and internalization, but the alkaloid levorphanol, which induced receptor desensitization failed to stimulate internalization (Bot et al. 1997). In human neuroblastoma SK-N-BE cells DPDPE and deltorphin I stimulated internalization and subsequent lysosomal degradation of the δ-opioid receptor, whereas etorphine stimulated internalization followed by recycling of receptor to the cell surface (Marie et al. 2003). However, activation of a pathway for degradation of the δ-opioid receptor is not specific to peptides, as the endogenous peptides, [Leu5]enkephalin and [Met5]enkephalin, caused recycling after internalization, but the synthetic δ-opioid, SNC80, led to degradation (Lecoq et al. 2004). Obviously, whether the δ-opioid receptor is recycled or degraded appears to be agonist and cell specific.
In the present study we test the hypothesis that the δ-opioid receptor undergoes agonist specific Gi/o-protein dependent and independent phosphorylation and internalization. To answer this question we have studied these events in HEK 293 cells expressing a FLAG-tagged δ-opioid receptor (HEKδ) in the presence or absence of PTX and with a variety of δ-opioid agonists. PTX treatment had no effect on δ-opioid receptor phosphorylation or translocation of β-arrestin 2-GFP to the cell surface, but did reduce receptor internalization. There was agreement between the ability of different agonists to stimulate G protein and cause internalization with the exception of the non-peptide δ-opioid agonist, TAN-67, which caused a level of G protein activation equal to that of DPDPE, but did not cause internalization.
Materials and methods
Materials
[35S]GTPγS (guanosine-5’-O-(3-[35S]thio)triphosphate) and [3H]diprenorphine were from Perkin Elmer Life and Analytical Sciences (Boston, MA). Morphine sulphate, (+)BW373U86, deltorphin II, naltrindole, oxymorphindole (OMI), naloxone and TAN-67 were obtained through the Narcotic Drug and Opioid Peptide Basic Research Center at the University of Michigan (Ann Arbor, MI). DPDPE, [Met5]enkephalin, 100 x protease inhibitor cocktail, GDP (guanosine diphosphate), p-nitrophenyl phosphate (pNPP), M1 mouse anti-FLAG antibody and M2 mouse anti-FLAG antibody conjugated to alkaline phosphatase were from Sigma-Aldrich (St. Louis, MO). Goat-anti-rabbit or mouse antibodies conjugated to horse radish peroxidase (HRP) were from Santa Cruz Biotechnology (Santa Cruz, CA) and rabbit anti-phospho Ser363-δ-opioid receptor antibody from Cell Signaling (Danvers, MA). The following were gifts: bovine phosducin-GFP (green fluorescent protein) cDNA (Rüdiger Schulz, University of Munich, Germany), FLAG-δ-opioid receptor cDNA coding for human δ-opioid receptor fused with FLAG epitope on the N-terminus (Lee-Yuan Liu-Chen, Temple University, Philadelphia, PA), β-arrestin 2-GFP cDNA (Marc Caron, Duke University, Durham, NC). Poly-D-Lysine coated 24-well plates and poly-D-Lysine coated 12 mm, No. 1 coverslips were from BD Biosciences (San Jose, CA). PTX was from List Biological Laboratories Inc. (Campbell, CA) and gallein from TCI America (Portland, OR). EcoLume scintillation cocktail and ultrapure formaldehyde were obtained from MP Biomedicals (Aurora, OH) and Polysciences Inc. (Warrington, PA), respectively. ProLong Gold antifade reagent, Alexa 594 goat anti-mouse IgG and Lipofectamine 2000 were from Invitrogen (Carlsbad, CA).
Cell culture and transfection
To prepare cells stably expressing FLAG-δ-opioid receptor, cDNA was transfected into HEK 293 cells using Lipofectamine 2000 reagent according to the manufacturer's instructions. Cells expressing FLAG-δ-opioid receptor were selected in the presence of 0.8 mg/ml geneticin and receptor number was determined using a [3H]diprenorphine binding assay (described below). Cells were grown in Dulbecco's modified Eagle medium (DMEM) supplemented with 10% fetal bovine serum, 90 units/ml penicillin, and 90 μg/ml streptomycin and 0.8 mg/ml geneticin (for stable FLAG-δ-opioid receptor cells only). For transient transfection HEK 293 cells were seeded onto poly-D-Lysine coated 24-well plates containing the appropriate cDNA (plasmids and amounts are given in the Figure legends) in complex with Lipofectamine 2000 reagent and the cells were used 48 h after transfection.
Ligand binding assay
Membranes (15 – 20 μg), prepared as described previously (Clark et al. 2003), were incubated in 50 mM Tris-HCl, pH 7.4 with a saturating concentration of [3H]diprenorphine (2 - 4 nM) in the presence or absence of 50 μM naloxone for 60 min in a shaking water bath at 25 °C. Samples were filtered through glass fiber filters mounted on a Brandel cell harvester (Gaithersburg, MD) and rinsed three times with 4 °C 50 mM Tris-HCl. EcoLume scintillation cocktail was added to the filters and the radioactivity counted by liquid scintillation counting. The FLAG δ-opioid receptor cell line used in this study expressed 12 pmol receptors/mg protein.
Second Messenger Assays
Measurement of cyclic AMP levels, MAP kinase phosphorylation and release of intracellular calcium in response to 10 μM DPDPE or 10 μM UK14304 where indicated, were measured in HEK 293 cells stably expressing the δ-opioid receptor as described previously (Clark et al. 2003). Cyclic AMP accumulation during a 10 min incubation with 10 μM DPDPE was measured. In the intracellular calcium assay, cells were incubated with or without 10 μM gallein for 10 min prior to the addition of DPDPE.
Confocal microscopy
HEK 293 cells stably expressing FLAG-δ-opioid receptors (0.6 × 106 cells/well) were added to 24-well plates containing poly-D-Lysine 12 mm cover slips. After overnight incubation, cells were treated with or without 100 ng/ml PTX. The following day cells were incubated for 60 min in the presence or absence of 10 μM DPDPE with or without PTX. Cells were fixed in 4% formaldehyde in PBS for 20 min, followed by permeabilization with 0.1% Triton X-100 for 10 min. FLAG-δ-opioid receptor was detected with 1:1000 dilution of M1 mouse anti-FLAG antibody and 1:1000 Alexa 594 goat anti-mouse antibody. The coverslips were mounted onto slides using ProLong Gold antifade reagent according to the manufacturer's instructions. For β-arrestin 2-GFP transiently transfected cells, β-arrestin 2-GFP cDNA (0.4 μg) in complex with Lipofectamine 2000 was added to 24-well plates containing poly-D-Lysine 12 mm cover slips. HEK 293 cells stably expressing FLAG-δ-opioid receptors (0.6 × 106 cells/well) were then added immediately to the wells. After overnight incubation, cells were treated with or without 100 ng/ml PTX. The following day cells were incubated for 5 min in the presence or absence of 10 μM drug with or without PTX. Cells were stained as described above except that the cells were not permeabilized. Images were collected using an Olympus Fluoview 500 confocal microscope with argon or helium-neon lasers.
Internalization assay
Transiently or stably transfected cells were seeded (0.75 × 106 cells per well) onto poly-D-Lysine coated 24-well plates. After overnight incubation, cells were treated with or without 100 ng/ml PTX and the next day incubated with drug in the presence or absence of PTX. For the gallein experiments, cells were preincubated with 10 μM gallein in DMSO (dimethyl sulfoxide) or DMSO for 10 min prior to the addition of DPDPE. At the end of the incubation period, the cells were fixed with 3.7% formaldehyde in TBS (tris buffered saline, 25 mM Tris-HCl, pH 7.4, 2.7 mM KCl, 140 mM NaCl) for 5 min at room temperature. The cells were washed three times with TBS, blocked with 1% non-fat dry milk for 1 h at room temperature and washed two times with TBS and incubated with monoclonal anti-FLAG M2 alkaline phosphatase antibody for 1 h at room temperature. Cells were washed five times and incubated with pNPP for 30 min at room temperature. 0.2 ml aliquots were added to 0.05 ml 3 N NaOH in a 96-well plate. Absorbance at 405 nm was measured using a VERSAmax tunable microplate reader (Molecular Devices, Sunnyvale, CA). The percentage of receptors internalized was calculated using the following equation: [1–(Drug O.D.–Background O.D./ Control O.D.–Background O.D.) x 100]. Background was defined as the absorbance of untransfected HEK 293 cells and control as absorbance from untreated FLAG δ-opioid receptor expressing cells.
[35S]GTPγS binding assay
As described previously (Traynor and Nahorski 1995), membranes (15 – 20 μg) were incubated in 20 mM Tris-HCl, pH 7.4, 1 mM EDTA (ethylenediamine tetraacetic acid), 5 mM MgCl2, 100 mM NaCl, 2.2 mM dithiothreitol (prepared freshly), 30 μM GDP, 0.1 nM [35S]GTPγS, and 10 μM drug or Super Q H2O for 60 min in a shaking water bath at 25 °C. Samples were filtered through GF/C glass-fiber filtermats mounted on a Brandel cell harvester and rinsed four times with 4 °C 50 mM Tris-HCl, pH 7.4, 5 mM MgCl2, and 100 mM NaCl. After drying, EcoLume scintillation cocktail was added to the filtermats, which were counted in a Wallac 1450 MicroBeta Liquid Scintillation and Luminescence Counter (Perkin Elmer, Boston, MA).
Western blot analysis
FLAG-δ-opioid receptor cells were seeded on plates and treated with 100 ng/ml PTX overnight, then incubated with 10 μM drug for 1 h at 37 °C. The cells were rinsed with PBS (phosphate buffered saline) and lysates collected with RIPA (radioimmuno precipitation assay) buffer (50 mM Tris, pH 7.4, 150 mM NaCl, 1% Triton X-100, 1% sodium deoxycholic acid, 0.1% sodium dodecyl sulfate (SDS)) plus protease inhibitor, 2 mM EDTA, 100 μM NaF, 100 μM phenylmethanesulfonyl fluoride, and 10 μM sodium orthovanadate. Lysates were sonicated briefly and centrifuged at 10,000 x g for 10 min. For each sample, equal protein amounts (measured by bicinchoninic acid (BCA) assay) diluted in SDS sample buffer (62.5 mM Tris-HCl, pH 6.8, 2% SDS, 10% glycerol, 0.0008% bromophenol blue) and β-mercaptoethanol were loaded onto 10% polyacrylamide gels. After transfer to nitrocellulose membranes and blocking for 1 h with 5% non-fat dried milk, the membrane was incubated with 1:1000 dilution of rabbit anti-phosphorylated δ-opioid receptor antibody overnight. After washing, the membrane was incubated with 1:10,000 HRP-goat anti-rabbit IgG for 1 h. To probe for FLAG-δ-opioid receptor, the membrane was stripped (2% SDS, 0.1 M β-mercaptoethanol, 63 mM Tris, pH 6.8 at 55° C for 45 min), washed, incubated with 5% non-fat dried milk overnight and then with 1:2000 mouse-anti-FLAG for 1 h in 5% non-fat dried milk made up in TBS-Tween containing 1 mM CaCl2. After washing, the membrane was treated with 1:10,000 HRP-goat-anti-mouse IgG for 1 h. Membranes were treated with LumiGLO and bands were detected using the EpiChemi3 darkroom (UVP, Upland, CA). Bands were quantitated using the LabWorks program.
Data and statistical analysis
All graphs were created in GraphPad Prism 4.02. Points and error bars represent the mean of three independent experiments ± standard error of the mean except (SEM) where indicated in figure legends. Two way analysis of variance (ANOVA) without matching and the Bonferroni post test or the Student's t-test (unpaired) were used to assess statistical significance.
Results
δ-opioid receptor internalization
DPDPE treatment afforded internalization of FLAG-tagged δ-opioid receptors in HEK293 cells (HEKδ) pretreated with or without PTX (Fig. 1a). At 1 h internalization was consistently greater in the absence of PTX treatment (60.3 ± 3.0%) than following PTX treatment (37.7 ± 2.7%, p < 0.0001, n = 26). Shorter time points (Fig. 1b, inset) did not show a significant difference. However, over a longer period DPDPE-induced internalization (Fig. 1b) showed a significant difference between PTX treated and untreated cells (p < 0.01) such that internalization occurred at a slower rate in cells pretreated with PTX than control cells, (t1/2 = 0.25 ± 0.034 h in control cells and 0.52 ± 0.12 h in PTX-treated cells). However, the same maximum degree of internalization was obtained at 6 h in the absence (82 ± 5%) or presence of PTX (79 ± 5%). EC50 values obtained at 1 h for DPDPE-induced internalization in cells treated with or without PTX were 22.7 ± 4.2 and 26.6 ± 12.0 nM, respectively (Fig. 2a). Based on the results in Fig. 2a, 10 μM DPDPE was used to obtain maximal internalization in subsequent experiments.
Fig. 1.
DPDPE induced FLAG δ-opioid receptor internalization in the presence or absence of PTX. (a) HEKδ cells were treated overnight with, or without, 100 ng/ml PTX, then incubated with 10 μM DPDPE ± 100 ng/ml PTX for 60 min at 37 °C. Visualization of FLAG δ-opioid receptor by confocal microscopy was as described in Materials and methods. Bar represents 10 μm. (b) HEKδ cells were treated overnight with, or without, 100 ng/ml PTX, then incubated with 10 μM DPDPE ± 100 ng/ml PTX for either short (inset) or longer time periods at 37 °C. Measurement of cell surface FLAG δ-opioid receptor was as described in Materials and methods. Internalization (%) is expressed as the mean of three or four experiments each performed in triplicate. The effect of PTX (0.5 h to 6h) was significant p = 0.0079.
Fig. 2.
DPDPE concentration response curves as measured by internalization or [35S]GTPγS binding and the effect of hypertonic sucrose on DPDPE induced internalization. (a) HEKδ cells were treated overnight with or without 100 ng/ml PTX, then incubated with differing concentrations of DPDPE for 1h at 37 °C. FLAG δ-opioid receptor at the cell surface was measured as described in Materials and methods. Percent internalization is expressed as the average of two independent experiments, each performed in triplicate. EC50 = 22.7 ± 4.2 nM and EC50 = 26.6 ± 12.1 nM for control or PTX treated cells, respectively. The interaction between PTX treatment and log [DPDPE] is significant, ***p < 0.001, compared to control (2-way ANOVA). (b) [35S]GTPγS binding to HEKδ cell membranes in response to varying concentrations of DPDPE was measured as described in Materials and methods. Data are expressed as percentage of [35S]GTPγS binding stimulated by SNC80 and are from 3 separate experiments, each in duplicate. EC50 = 12.0 ± 3.3 nM. (c) HEKδ cells were treated overnight with or without 100 ng/ml PTX, then incubated with 10 μM DPDPE in the absence or presence of 0.45 M sucrose or PTX for 1h at 37 °C. Measurement of cell surface FLAG δ-opioid receptor was as described in Materials and methods. Internalization (%) is expressed as the average of three independent experiments, each performed in triplicate.
The potency of DPDPE to cause internalization was similar to the potency (12 ± 3 nM) obtained when measuring DPDPE activation of G protein in membranes from cells that were not treated with PTX using the [35S]GTPγS binding assay (Fig. 2b). The effectiveness of PTX to block Gα coupling was confirmed by measuring DPDPE-stimulation of [35S]GTPγS binding, DPDPE-induced phosphorylation of mitogen-activated protein (MAP) kinase or DPDPE-inhibition of cyclic AMP production in HEKδ cells, after overnight treatment with 100 ng/ml PTX. Stimulation of [35S]GTPγS binding and phosphorylation of MAP kinase were blocked completely by pretreatment with PTX (data not shown), while inhibition of cyclic AMP production dropped from 89 ± 4 % to 28 ± 3%, a decrease similar to that reported previously (Bot et al. 1997; Selley et al. 1998).
The above results suggest that there is an efficient G protein independent mechanism of internalization that appears to occur at a slightly slower rate. To determine if this alternate pathway requires clathrin coated pits for internalization, HEKδ cells pre-incubated with or without PTX were treated with hypertonic sucrose in order to inhibit clathrin coated pit formation (Heuser and Anderson 1989). In both cases, hypertonic sucrose blocked internalization (Fig. 2c).
Effect of Gβγ inhibitors on δ-opioid receptor internalization
To address the possibility that the PTX insensitive Go/i protein, Gz, is coupling with δ-opioid receptors in PTX-treated HEKδ cells and supplying the Gβγ subunits necessary to recruit GRK, we examined the effects of gallein, a compound that prevents the interaction of Gβγ with effectors (Lehmann et al. 2008). Gallein (10 μM) had no effect on DPDPE-induced internalization (DMSO (vehicle): 35.4 ± 0.6 % versus gallein: 31.3 ± 1.9 %). In these same HEKδ cells gallein was able to reduce Gβγ-mediated release of intracellular calcium afforded by DPDPE (10 μM) by 58 ± 10 % or the adrenergic α2 receptor agonist, UK14304 (10 μM), by 84 ± 16 % (data not shown). These results were supported by the finding that transient co-expression of the Gβγ scavenger phosducin-GFP (Schulz et al. 2002) or GFP alone, together with the FLAG-δ-opioid receptor in HEK293 cells, did not change the extent of DPDPE-induced internalization (GFP expressing cells: 30.0 ± 0.9 % versus phosducin-GFP expressing cells: 35.5 ± 2.2 %). In addition, we were unable to find evidence of Gαz expression in these cells by western blot (data not shown). Together these data indicate that Gβγ subunits from PTX insensitive G proteins are not providing an alternative route for δ-opioid receptor internalization in HEKδ cells.
Ligand dependency of δ-opioid receptor internalization
It is possible that internalization of the δ-opioid receptor in HEKδ cells in PTX-treated cells is specific to DPDPE and not other δ-opioid receptor ligands. Consequently, we examined internalization of the δ-opioid receptor by a series of ligands at a maximal (10 μM) concentration over 1 h. Following pretreatment with PTX the synthetic peptidic agonists DPDPE and deltorphin II, the endogenous peptide agonist [Met5]enkephalin and the non-peptidic agonist (+)BW373U86 were able to afford internalization of the δ-opioid receptor in HEKδ cells (Fig. 3a), although there was a significant reduction following PTX treatment. The δ-opioid agonists, oxymorphindole and TAN-67 as well as the μ-opioid agonist, morphine, caused no significant internalization in the presence or absence of PTX pretreatment and the δ-opioid receptor antagonist naltrindole was also ineffective. In contrast, the δ-opioid ligands stimulated [35S]GTPγS binding to membranes from HEKδ cells in the order of TAN-67 = deltorphin II = DPDPE > [Met5]enkephalin = (+)BW373U86 = oxymorphindole = morphine. (Fig. 3b). No stimulation was observed with naltrindole. For each agonist, activation of [35S]GTPγS binding was blocked completely by pretreatment of the cells with PTX.
Fig. 3.
Internalization of FLAG δ-opioid receptor and stimulation of [35S]GTPγS binding in response to incubation with different ligands. (a) HEKδ cells were treated overnight with, or without, 100 ng/ml PTX, then incubated with 10 μM ligand in the presence or absence of PTX for 1h at 37 °C. Measurement of cell surface FLAG δ-opioid receptor was as described in Materials and methods. Internalization (%) is expressed as the average of three independent experiments, each performed in triplicate ***p < 0.001, **p < 0.01, *p < 0.05 compared to control. (b) Membranes were prepared from HEKδ cells cells treated with, or without, 100 ng/ml PTX overnight. [35S]GTPγS binding to these membranes in response to 10 μM ligand was measured as described in Materials and methods. Data are presented as a percentage of maximal DPDPE stimulation in the absence of PTX and are from at least 3 separate experiments, each in triplicate.
Phosphorylation of δ-opioid receptor Ser363
Although there are several potential phosphorylation sites within the C-terminus of the δ-opioid receptor, phosphorylation at Ser363 has been reported to occur first in response to agonist binding (Kouhen et al. 2000). The various agonists (10 μM, 1h) were examined for their ability to induce phosphoryaltion of Ser363 as detected by western blot with anti-phospho Ser363-δ-opioid receptor antibody (Fig. 4). DPDPE, deltorphin II, and (+)BW373U86 caused a marked phosphorylation of Ser363, TAN-67 caused a lesser degree of phosphorylation while there was no evidence of phosphorylation with oxymorphindole or morphine. Treatment of HEKδ cells with PTX increased the level of δ-opioid receptor expression as measured by the FLAG epitope (see FLAG blot in Fig. 4a). However, there remained a high level of phosphorylation by DPDPE, deltorphin II, and (+)BW373U86 in the presence of PTX, which was similar to that observed in the absence of PTX (Fig. 4b).
Fig. 4.
Phosphorylation of Ser363 of FLAG δ-opioid receptor relative to total receptor. HEKδ cells were treated overnight with, or without, 100 ng/ml PTX, then incubated with 10 μM DPDPE in the presence or absence of PTX for 1h at 37 °C. Detection of phosphorylated δ-opioid receptor and FLAG were performed as described in Materials and methods. The phospho-Ser363 antibody was shown to be specific as agonist treatment of untransfected HEK 293 cells did not induce a 55 kDa band (data not shown). Figure 4a shows a representative western blot. In figure 4b data are represented as the ratio of phosphorylated δ-opioid receptor mean density to FLAG mean density for three independent experiments.
Translocation of β-arrestin 2-GFP
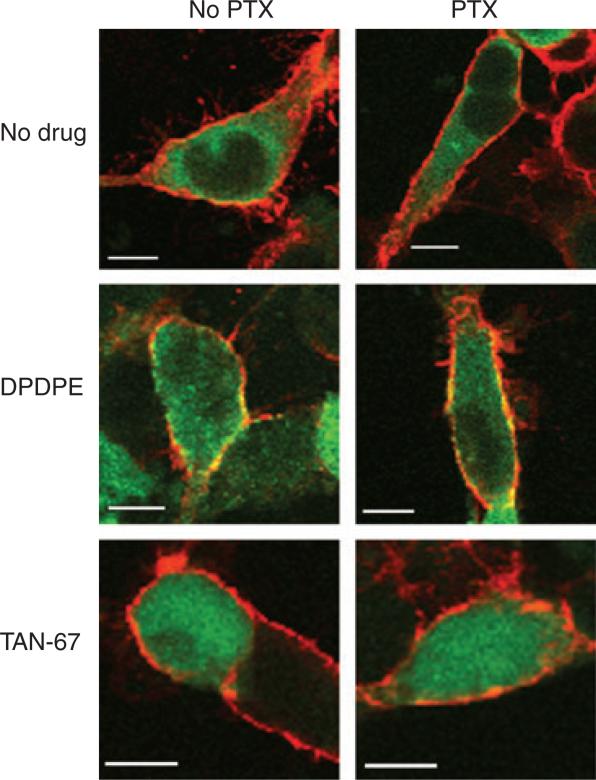
Since the δ-opioid receptor is phosphorylated at Ser363 in the presence of PTX, we hypothesized that β-arrestin 2-GFP would still be translocated to the plasma membrane. After a 5 min incubation of HEKδ cells with 10 μM DPDPE, translocation of β-arrestin 2-GFP to the plasma membrane was indistinguishable between cells pretreated with or without PTX (Fig. 5). However, TAN-67, with its reduced ability to induce receptor phosphorylation and internalization despite activating G protein strongly, failed to cause β-arrestin 2-GFP to relocate at the membrane in the absence or presence of PTX pretreatment.
Fig. 5.
DPDPE, but not TAN-67, induced translocation of β-arrestin 2-GFP. HEKδ cells were transiently transfected with 0.4 μg β-arrestin 2-GFP and grown on poly-D-Lys coated 12 mm cover slips. The transfected cells were treated overnight with, or without, 100 ng/ml PTX, then incubated with 10 μM DPDPE or TAN-67 for 5 min at 37 °C. Visualization of FLAG δ-opioid receptor and β-arrestin 2-GFP by confocal microscopy was as described in Materials and methods.
Discussion
PTX pretreatment of HEK 293 cells expressing a FLAG-δ-opioid receptor did not prevent agonist-induced δ-opioid receptor phosphorylation or the extent of receptor internalization reached after 6 h. At shorter times (1h) there was evidence for less internalization in the presence of PTX suggesting a slower time-course. The results confirm that internalization can be initiated in the absence of functional receptor-Gαi/o protein coupling (Law et al. 1985; Chakrabarti et al. 1997; Kramer et al. 2000b) and suggest that kinases other than those recruited by Gβγ are able to mediate δ-opioid receptor phosphorylation. Consequently, the degree to which agonists induce phosphorylation of Ser363 in the C-terminus of the δ-opioid receptor is more predictive of internalization than the ability of agonists to activate G protein. Since the receptor is still phosphorylated in the presence of PTX and stimulates β-arrestin 2-GFP translocation, it is likely that in the presence of PTX the receptor is internalized via the traditional pathway (Zhang et al. 1999). Indeed, internalization in the presence of PTX is sensitive to hypertonic concentrations of sucrose, suggesting a role for clathrin coated pits. It should be noted that 0.45 M sucrose affects protein phosphorylation (Junger et al. 2003) and triggers apoptosis (Friis et al. 2005) in addition to clathrin coated pit formation.
Since Gβγ subunits, as shown by the PTX-treatment, phosducin and gallein experiments are not necessary for phosphorylation or internalization, it appears that GRK2 and/or 3 are not the only kinases that can be utilized to phosphorylate the δ-opioid receptor. Likely candidates are GRK5/6, which are expressed in HEK cells (Ren et al. 2005), since they are not recruited by Gβγ (Ferguson 2001; Lodowski et al. 2006) and have been shown to be important for δ-opioid receptor desensitization (Willets and Kelly 2001) and internalization of β-adrenergic (GRK6) (Loudon and Benovic 1997) and dopamine D2 receptors (GRK5) (Ito et al. 1999). In contrast, phosphorylation of the δ-opioid receptor by Src-like protein tyrosine kinases is inhibited by PTX treatment (Kramer et al. 2000a).
The information presented in Fig. 6 compares receptor internalization with receptor phosphorylation and G protein activation in the absence of PTX in HEKδ cells. The degree of δ-opioid receptor Ser363 phosphorylation stimulated by different agonists and the ability of the agonist to induce internalization are significantly related (p = 0.02; Fig. 6a). This confirms the importance of Ser363 (Kouhen et al. 2000), in spite of the fact that other residues, specifically Thr 358 and 361 are also phosphorylated and mutation of these amino acids results in reduced internalization (Trapaidze et al. 1996; Guo et al. 2000; Kouhen et al. 2000). In contrast, figures 6 (b) and (c) demonstrate that activation of G protein as measured by [35S]GTPγS binding did not predict the ability of a ligand to invoke δ-opioid receptor phosphorylation or internalization. This confirms the finding that although PTX treatment prevented G protein activation, it did not stop receptor phosphorylation and internalization. DPDPE, deltorphin II and (+)BW373U86 showed strong activation of G protein and caused extensive phosphorylation and internalization. Yet, although, TAN-67 stimulated [35S]GTPγS binding to the same extent as the full agonists DPDPE and deltorphin II , it showed a reduced ability to phosphorylate the δ-opioid receptor , which was also reflected in an inability to recruit β-arrestin 2-GFP and a low level of receptor internalization. Morphine and oxymorphindole were able to induce [35S]GTPγS binding to almost the same extent as (+)BW373U86, but they produced almost no phosphorylation or internalization. These results agree with reports that there is not a correlation between relative efficacy, as measured by [35S]GTPγS binding and down regulation of δ-opioid receptor (Okura et al. 2003), although this has not been confirmed by other authors (Zaki et al. 2001).
Fig. 6.
Comparison of ligand induced stimulation of [35S]GTPγS binding, phosphorylation and internalization. Data are from internalization, [35S]GTPγS binding, and phosphorylation normalized to total receptor in the absence of PTX (Figures 3(a), 3(b) and 4(b), respectively). Panel (a) compares internalization to phosphorylation, (b) phosphorylation to [35S]GTPγS binding and (c) internalization to [35S]GTPγS binding. The r2 values are (a) 0.79, p = 0.02 and (b) 0.15, p = 0.46 and (c) 0.32, p = 0.14. Abbreviations: OMI, oxymorphindole, Mor, morphine, Delt, deltorphin II, BW, (+)BW373U86, Nalt, naltrindole, Met, [Met5]enkephalin.
The cells used in this study express a high number of δ-opioid receptors, explaining why compounds that are usually considered low efficacy partial agonists, namely morphine, oxymorphindole, and TAN-67 (Quock et al. 1999), show a robust enhancement of [35S]GTPγS binding. However, this enhancement is only seen at the level of G protein activation and is not manifested as an increased ability of these partial agonists to phosphorylate the receptor or cause internalization. There are several possible explanations for this discrepancy. Fewer numbers of receptors may need to be activated to give a [35S]GTPγS response, which is a cumulative assay, but the transient lifetime of free Gβγ may not allow for recruitment of sufficient GRK2/3 enzymes necessary for phosphorylation and internalization. On the other hand, since PTX treatment does not prevent receptor phosphorylation by the agonists, a more likely explanation is that the δ-opioid receptor conformation necessary for G protein activation is not the same as the conformation necessary to provide for efficient phosphorylation of the carboxy terminal tail. This discrepancy may be an effect of partial versus full agonists or an example of agonist-specific conformational change. Certainly, agonist specific conformations have been demonstrated at the level of δ-opioid receptor phosphorylation. Whereas SNC-80 is able to phosphorylate a δ-opioid receptor truncated after Gly338, DPDPE is not (Okura et al. 2003). Moreover, at the μ-opioid receptor it appears that the major mechanism of desensitization in the presence of the efficacious peptide agonist DAMGO is via GRK2, but for the morphine-occupied receptor desensitization involves protein kinase C (Baileyet al. 2004).
In conclusion, the results show that internalization can be mediated by at least two pathways, G protein dependent and G protein independent. Both routes require phosphorylation of Ser363 and utilize β-arrestin 2 and clathrin coated pits. Although the non-G protein mechanism appears to be somewhat less efficient it does represent a viable alternative, suggesting that internalization of the δ-opioid receptor in the presence of agonist exposure is a vital event that the cell must maintain, presumably to be in a position to respond to incoming signals. The pivotal factor appears to be whether phosphorylation occurs via recruitment of GRK2 and 3 and the ready availability of kinases that do not require Gβγ for recruitment, such as GRK5 or 6, or non GRK family members such as casein kinase I or CaM kinase II (Eisinger and Schulz 2005).
Acknowledgements
This work was supported by the National Institute of Drug Abuse grant DA04087. F.A.B. was supported by the National Institute of Drug Abuse, Biology of Drug Abuse post-doctoral training grant DA07268. We gratefully acknowledge the technical assistance of Christine Zelnik.
Abbreviations
- GPCR
G protein coupled receptor
- GRK
G protein coupled receptor kinase
- HEK
human embryonic kidney
- HEKδ
FLAG-tagged δ-opioid receptor expressed in HEK 293 cells
- DMSO
dimethyl sulfoxide
- pNPP
p-nitrophenyl phosphate
- DMEM
Dulbecco's modified Eagle medium
- SDS
sodium dodecyl sulfate
- EDTA
ethylenediamine tetraacetic acid
- HRP
horse radish peroxidase
- DPDPE
[D-Pen2,DPen5]enkephalin
- PTX
pertussis toxin
- [35S]GTPγS
guanosine-5’-O-(3-[35S]thio)triphosphate
- DADLE
[D-Ala2,D-Leu5]enkephalin
- DTLET
[D-Thr2]Leuenkephalin-Thr
- DSLET
[D-Ser2,D-Leu5]enkephalin-Thr
- PBS
phosphate buffered saline
- TBS
Tris buffered saline
- GFP
green fluorescent protein
- MAP
mitogen-activated protein
References
- Bailey CP, Kelly E, Henderson G. Protein kinase C activation enhances morphine-induced rapid desensitization of mu-opioid receptors in mature rat locus ceruleus neurons. Mol Pharmacol. 2004;66:1592–1598. doi: 10.1124/mol.104.004747. [DOI] [PubMed] [Google Scholar]
- Bot G, Blake AD, Li S, Reisine T. Opioid regulation of the mouse delta-opioid receptor expressed in human embryonic kidney 293 cells. Mol Pharmacol. 1997;52:272–281. doi: 10.1124/mol.52.2.272. [DOI] [PubMed] [Google Scholar]
- Brandt MR, Furness MS, Rice KC, Fischer BD, Negus SS. Studies of Tolerance and Dependence with the delta -Opioid Agonist SNC80 in Rhesus Monkeys Responding under a Schedule of Food Presentation. J Pharmacol Exp Ther. 2001;299:629–637. [PubMed] [Google Scholar]
- Broom DC, Jutkiewicz EM, Rice KC, Traynor JR, Woods JH. Behavioral effects of delta-opioid receptor agonists: potential antidepressants? Jpn J Pharmacol. 2002a;90:1–6. doi: 10.1254/jjp.90.1. [DOI] [PubMed] [Google Scholar]
- Broom DC, Nitsche JF, Pintar JE, Rice KC, Woods JH, Traynor JR. Comparison of Receptor Mechanisms and Efficacy Requirements for delta-Agonist-Induced Convulsive Activity and Antinociception in Mice. J Pharmacol Exp Ther. 2002b;303:723–729. doi: 10.1124/jpet.102.036525. [DOI] [PubMed] [Google Scholar]
- Broom DC, Jutkiewicz EM, Folk JE, Traynor JR, Rice KC, Woods JH. Nonpeptidic delta-opioid receptor agonists reduce immobility in the forced swim assay in rats. Neuropsychopharmacology. 2002c;26:744–755. doi: 10.1016/S0893-133X(01)00413-4. [DOI] [PubMed] [Google Scholar]
- Chakrabarti S, Yang W, Law PY, Loh HH. The mu-opioid receptor down-regulates differently from the delta-opioid receptor: requirement of a high affinity receptor/G protein complex formation. Mol Pharmacol. 1997;52:105–113. [PubMed] [Google Scholar]
- Clark MJ, Harrison C, Zhong H, Neubig RR, Traynor JR. Endogenous RGS protein action modulates mu-opioid signaling through Galphao. Effects on adenylyl cyclase, extracellular signal-regulated kinases, and intracellular calcium pathways. J Biol Chem. 2003;278:9418–9425. doi: 10.1074/jbc.M208885200. [DOI] [PubMed] [Google Scholar]
- Eisinger DA, Schulz R. Mechanism and consequences of delta-opioid receptor internalization. Crit Rev Neurobiol. 2005;17:1–26. doi: 10.1615/critrevneurobiol.v17.i1.10. [DOI] [PubMed] [Google Scholar]
- Ferguson SS. Evolving concepts in G protein-coupled receptor endocytosis: the role in receptor desensitization and signaling. Pharmacol Rev. 2001;53:1–24. [PubMed] [Google Scholar]
- Friis MB, Friborg CR, Schneider L, Nielsen MB, Lambert IH, Christensen ST, Hoffmann EK. Cell shrinkage as a signal to apoptosis in NIH 3T3 fibroblasts. J Physiol. 2005;567:427–443. doi: 10.1113/jphysiol.2005.087130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo J, Wu Y, Zhang W, Zhao J, Devi LA, Pei G, Ma L. Identification of G protein-coupled receptor kinase 2 phosphorylation sites responsible for agonist-stimulated delta-opioid receptor phosphorylation. Mol Pharmacol. 2000;58:1050–1056. doi: 10.1124/mol.58.5.1050. [DOI] [PubMed] [Google Scholar]
- Hasbi A, Allouche S, Sichel F, Stanasila L, Massotte D, Landemore G, Polastron J, Jauzac P. Internalization and recycling of delta-opioid receptor are dependent on a phosphorylation-dephosphorylation mechanism. J Pharmacol Exp Ther. 2000;293:237–247. [PubMed] [Google Scholar]
- Heuser JE, Anderson RG. Hypertonic media inhibit receptor-mediated endocytosis by blocking clathrin-coated pit formation. J. Cell Biol. 1989;108:389–400. doi: 10.1083/jcb.108.2.389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hudzik TJ, Howell A, Payza K, Cross AJ. Antiparkinson potential of delta-opioid receptor agonists. Eur J Pharmacol. 2000;396:101–107. doi: 10.1016/s0014-2999(00)00209-0. [DOI] [PubMed] [Google Scholar]
- Ito K, Haga T, Lameh J, Sadee W. Sequestration of dopamine D2 receptors depends on coexpression of G-protein-coupled receptor kinases 2 or 5. Eur J Biochem. 1999;260:112–119. doi: 10.1046/j.1432-1327.1999.00125.x. [DOI] [PubMed] [Google Scholar]
- Junger H, Edelman DB, Junger WG. Hypertonicity promotes survival of corticospinal motoneurons via mitogen-activated protein kinase p38 signaling. J Mol Neurosci. 2003;21:111–120. doi: 10.1385/JMN:21:2:111. [DOI] [PubMed] [Google Scholar]
- Jutkiewicz EM, Kaminsky ST, Rice KC, Traynor JR, Woods JH. Differential behavioral tolerance to the delta-opioid agonist SNC80 ([(+)-4-[(alphaR)-alpha-[(2S,5R)-2,5-dimethyl-4-(2-propenyl)-1-piperazinyl ]-(3-methoxyphenyl)methyl]-N,N-diethylbenzamide) in Sprague-Dawley rats. J Pharmacol Exp Ther. 2005;315:414–422. doi: 10.1124/jpet.105.088831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kouhen OM, Wang G, Solberg J, Erickson LJ, Law PY, Loh HH. Hierarchical phosphorylation of delta-opioid receptor regulates agonist-induced receptor desensitization and internalization. J Biol Chem. 2000;275:36659–36664. doi: 10.1074/jbc.M006788200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovoor A, Nappey V, Kieffer BL, Chavkin C. Mu and delta opioid receptors are differentially desensitized by the coexpression of beta-adrenergic receptor kinase 2 and beta-arrestin 2 in xenopus oocytes. J Biol Chem. 1997;272:27605–27611. doi: 10.1074/jbc.272.44.27605. [DOI] [PubMed] [Google Scholar]
- Kramer HK, Andria ML, Esposito DH, Simon EJ. Tyrosine phosphorylation of the delta-opioid receptor. Evidence for its role in mitogen-activated protein kinase activation and receptor internalization*. Biochem Pharmacol. 2000a;60:781–792. doi: 10.1016/s0006-2952(00)00400-7. [DOI] [PubMed] [Google Scholar]
- Kramer HK, Andria ML, Kushner SA, Esposito DH, Hiller JM, Simon EJ. Mutation of tyrosine 318 (Y318F) in the delta-opioid receptor attenuates tyrosine phosphorylation, agonist-dependent receptor internalization, and mitogen-activated protein kinase activation. Brain Res Mol Brain Res. 2000b;79:55–66. doi: 10.1016/s0169-328x(00)00097-8. [DOI] [PubMed] [Google Scholar]
- Law PY, Louie AK, Loh HH. Effect of pertussis toxin treatment on the down-regulation of opiate receptors in neuroblastoma X glioma NG108-15 hybrid cells. J Biol Chem. 1985;260:14818–14823. [PubMed] [Google Scholar]
- Lecoq I, Marie N, Jauzac P, Allouche S. Different regulation of human delta-opioid receptors by SNC-80 [(+)-4-[(alphaR)-alpha-((2S,5R)-4-allyl-2,5-dimethyl-1-piperazinyl)-3-meth oxybenzyl]-N,N-diethylbenzamide] and endogenous enkephalins. J Pharmacol Exp Ther. 2004;310:666–677. doi: 10.1124/jpet.103.063958. [DOI] [PubMed] [Google Scholar]
- Lehmann DM, Seneviratne AM, Smrcka AV. Small molecule disruption of G protein beta gamma subunit signaling inhibits neutrophil chemotaxis and inflammation. Mol Pharmacol. 2008;73:410–418. doi: 10.1124/mol.107.041780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Xiang B, Su W, Zhang X, Huang Y, Ma L. Agonist-induced formation of opioid receptor-G protein-coupled receptor kinase (GRK)-G beta gamma complex on membrane is required for GRK2 function in vivo. J Biol Chem. 2003;278:30219–30226. doi: 10.1074/jbc.M302385200. [DOI] [PubMed] [Google Scholar]
- Lodowski DT, Tesmer VM, Benovic JL, Tesmer JJ. The structure of G protein-coupled receptor kinase (GRK)-6 defines a second lineage of GRKs. J Biol Chem. 2006;281:16785–16793. doi: 10.1074/jbc.M601327200. [DOI] [PubMed] [Google Scholar]
- Loudon RP, Benovic JL. Altered activity of palmitoylation-deficient and isoprenylated forms of the G protein-coupled receptor kinase GRK6. J Biol Chem. 1997;272:27422–27427. doi: 10.1074/jbc.272.43.27422. [DOI] [PubMed] [Google Scholar]
- Marie N, Lecoq I, Jauzac P, Allouche S. Differential sorting of human delta-opioid receptors after internalization by peptide and alkaloid agonists. J Biol Chem. 2003;278:22795–22804. doi: 10.1074/jbc.M300084200. [DOI] [PubMed] [Google Scholar]
- Murray SR, Evans CJ, von Zastrow M. Phosphorylation is not required for dynamin-dependent endocytosis of a truncated mutant opioid receptor. J Biol Chem. 1998;273:24987–24991. doi: 10.1074/jbc.273.39.24987. [DOI] [PubMed] [Google Scholar]
- Narita M, Suzuki T. Delta-opioid receptor mediated antinociption/analgesia. In: Chang KJ, Porreca F, Woods JH, editors. The delta Receptor. Marcel Dekker; New York: 2004. pp. 331–354. [Google Scholar]
- Navratilova E, Waite S, Stropova D, Eaton MC, Alves ID, Hruby VJ, Roeske WR, Yamamura HI, Varga EV. Quantitative evaluation of human delta opioid receptor desensitization using the operational model of drug action. Mol Pharmacol. 2007;71:1416–1426. doi: 10.1124/mol.106.030023. [DOI] [PubMed] [Google Scholar]
- Okura T, Varga EV, Hosohata Y, Navratilova E, Cowell SM, Rice K, Nagase H, Hruby VJ, Roeske WR, Yamamura HI. Agonist-specific down-regulation of the human delta-opioid receptor. Eur J Pharmacol. 2003;459:9–16. doi: 10.1016/s0014-2999(02)02823-6. [DOI] [PubMed] [Google Scholar]
- Pei G, Kieffer BL, Lefkowitz RJ, Freedman NJ. Agonist-dependent phosphorylation of the mouse delta-opioid receptor: involvement of G protein-coupled receptor kinases but not protein kinase C. Mol Pharmacol. 1995;48:173–177. [PubMed] [Google Scholar]
- Quock RM, Burkey TH, Varga E, Hosohata Y, Hosohata K, Cowell SM, Slate CA, Ehlert FJ, Roeske WR, Yamamura HI. The delta-opioid receptor: molecular pharmacology, signal transduction, and the determination of drug efficacy. Pharmacol Rev. 1999;51:503–532. [PubMed] [Google Scholar]
- Ren XR, Reiter E, Ahn S, Kim J, Chen W, Lefkowitz RJ. Different G protein-coupled receptor kinases govern G protein and beta-arrestin-mediated signaling of V2 vasopressin receptor. Proc Natl Acad Sci U S A. 2005;102:1448–1453. doi: 10.1073/pnas.0409534102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulz R, Wehmeyer A, Schulz K. Opioid receptor types selectively cointernalize with G protein-coupled receptor kinases 2 and 3. J Pharmacol Exp Ther. 2002;300:376–384. doi: 10.1124/jpet.300.2.376. [DOI] [PubMed] [Google Scholar]
- Selley DE, Breivogel CS, Childers SR. Opioid inhibition of adenylyl cyclase in membranes from pertussis toxin-treated NG108-15 cells. J Recept Signal Transduct Res. 1998;18:25–49. doi: 10.3109/10799899809039163. [DOI] [PubMed] [Google Scholar]
- Trapaidze N, Keith DE, Cvejic S, Evans CJ, Devi LA. Sequestration of the delta opioid receptor. Role of the C terminus in agonist-mediated internalization. J Biol Chem. 1996;271:29279–29285. doi: 10.1074/jbc.271.46.29279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Traynor JR, Nahorski SR. Modulation by mu-opioid agonists of guanosine-5′-O-(3-[35S]thio)triphosphate binding to membranes from human neuroblastoma SH-SY5Y cells. Mol Pharmacol. 1995;47:848–854. [PubMed] [Google Scholar]
- Whistler JL, Tsao P, von Zastrow M. A phosphorylation-regulated brake mechanism controls the initial endocytosis of opioid receptors but is not required for post-endocytic sorting to lysosomes. J Biol Chem. 2001;276:34331–34338. doi: 10.1074/jbc.M104627200. [DOI] [PubMed] [Google Scholar]
- Willets J, Kelly E. Desensitization of endogenously expressed delta-opioid receptors: no evidence for involvement of G protein-coupled receptor kinase 2. Eur J Pharmacol. 2001;431:133–141. doi: 10.1016/s0014-2999(01)01360-7. [DOI] [PubMed] [Google Scholar]
- Zaki PA, Keith DE, Jr., Thomas JB, Carroll FI, Evans CJ. Agonist-, antagonist-, and inverse agonist-regulated trafficking of the delta-opioid receptor correlates with, but does not require, G protein activation. J Pharmacol Exp Ther. 2001;298:1015–1020. [PubMed] [Google Scholar]
- Zhang J, Ferguson SS, Law PY, Barak LS, Caron MG. Agonist-specific regulation of delta-opioid receptor trafficking by G protein-coupled receptor kinase and beta-arrestin. J Recept Signal Transduct Res. 1999;19:301–313. doi: 10.3109/10799899909036653. [DOI] [PubMed] [Google Scholar]
- Zhang X, Wang F, Chen X, Li J, Xiang B, Zhang YQ, Li BM, Ma L. Beta-arrestin1 and beta-arrestin2 are differentially required for phosphorylation-dependent and -independent internalization of delta-opioid receptors. J Neurochem. 2005;95:169–178. doi: 10.1111/j.1471-4159.2005.03352.x. [DOI] [PubMed] [Google Scholar]
- Zhao J, Pei G, Huang YL, Zhong FM, Ma L. Carboxyl terminus of delta opioid receptor is required for agonist-dependent receptor phosphorylation. Biochem Biophys Res Commun. 1997;238:71–76. doi: 10.1006/bbrc.1997.7242. [DOI] [PubMed] [Google Scholar]