Abstract
Disruption to dopamine homeostasis during brain development has been implicated in a variety of neuropsychiatric disorders, including depression and schizophrenia. Inappropriate expression or activity of GABAergic interneurons are common features of many of these disorders. We discovered a persistent upregulation of GAD67+ and parvalbumin+ neurons within the anterior cingulate cortex of dopamine D2 receptor knockout mice, while other GABAergic interneuron markers were unaffected. Interneuron distribution and number were not altered in the striatum or in the dopamine-poor somatosensory cortex. The changes were already present by postnatal day 14, indicating a developmental etiology. D2eGFP BAC transgenic mice demonstrated the presence of D2 receptor expression within a subset of parvalbumin-expressing cortical interneurons, suggesting the possibility of a direct cellular mechanism through which D2 receptor stimulation regulates interneuron differentiation or survival. D2 receptor knockout mice also exhibited decreased depressive-like behavior compared with wild-type controls in the tail suspension test. These data indicate that dopamine signaling modulates interneuron number and emotional behavior and that developmental D2 receptor loss or blockade could reveal a potential mechanism for the prodromal basis of neuropsychiatric disorders.
Keywords: Inhibitory, knockout, Drd2-eGFP, parvalbumin, depression, GABA
Psychiatric disorders such as schizophrenia, bipolar disorder, depression, and attention deficit disorder have a neurodevelopmental basis, despite the fact that symptoms are not apparent until later in life, sometimes not emerging until adulthood. Brain regions such as the striatum (STR) and frontal cortex, particularly the anterior cingulate cortex (ACC), are key regions of neuropathology in psychiatric disorders. During sensitive periods of development, these regions are highly influenced by neurotransmitters, such as dopamine (DA), which alter neuronal migration, differentiation, and signaling, contributing to the development of these disorders.1−3 Drugs targeting DA receptors are also commonly used to treat these disorders.4
This DAergic signaling is part of a complex circuitry that is mediated in part by aspiny interneurons. These interneurons comprise a small portion of the overall cell population and are either cholinergic (STR) or GABAergic (STR and cerebral cortex).5,6 Neurons containing DA receptors, on the contrary, are quite populous in these regions. Disruption of DAergic signaling early in development induces abnormal GABA neuron development.7−11 In particular, the DA D2 receptor (D2R) is important in proper neuronal development. Neonatal or embryonic treatment with D2R agonists produces long-term cognitive deficits and locomotor dysfunction.12,13 Overexpression of D2R in the striatum not only produces increased D1 receptor activation in the prefrontal cortex and deficits in working memory, a task correlated to prefrontal activity,14 but also decreases dendritic arborization while increasing membrane excitability.15 Genetic loss of D2R results in fewer DAergic neurons during development16 and altered GABAergic transmission in adulthood.17 Indeed, proper D2R signaling is necessary to maintain appropriate interneuron migration in the cortex, indicating that the D2R is capable of influencing GABAergic interneuron development.9
While the aforementioned findings indicate that D2R and GABAergic expression and function are linked, the underlying mechanisms are not clear, nor is it understood which GABAergic interneuron subclasses are most affected or whether developmental trajectory later normalizes. In vitro data indicate that D2Rs alter neuronal differentiation in the cerebral cortex, particularly of parvalbumin (PV)+ interneurons,18 one such class of these GABAergic interneurons. D2R-mediated changes to specific subsets of GABAergic interneurons are of great interest, because alterations to various subsets are key features in many neuropsychiatric disorders also involving D2Rs.19−23 The purpose of this study was to determine the alterations in subsets of GABAergic interneurons within the STR and ACC in constitutive D2R knockout mice, based on the hypothesis that specific interneuron markers would be altered in select brain regions of the knockout mice.
Results and Discussion
Alterations in brain DA signaling result in atypical neuronal development, which can have long-lasting cellular and functional consequences and lead to the prodromal bases of cognitive and psychiatric disorders. D2 receptors have previously been shown to modulate GABAergic interneuron expression and function, thus suggesting that D2R activity alters the development of inhibitory circuitry. The purpose of this study was to determine which subsets of GABAergic interneurons, and in which brain regions, would be affected by genetic loss-of-function of the D2R.
GABAergic Neuronal Expression in the Adult D2R KO Mouse
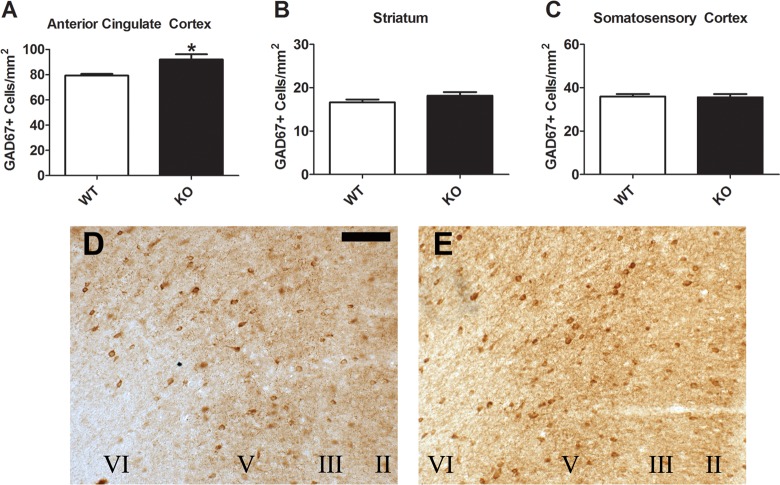
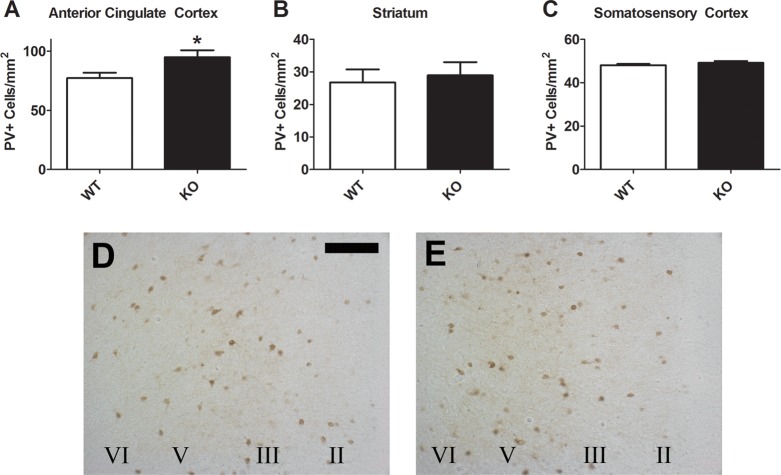
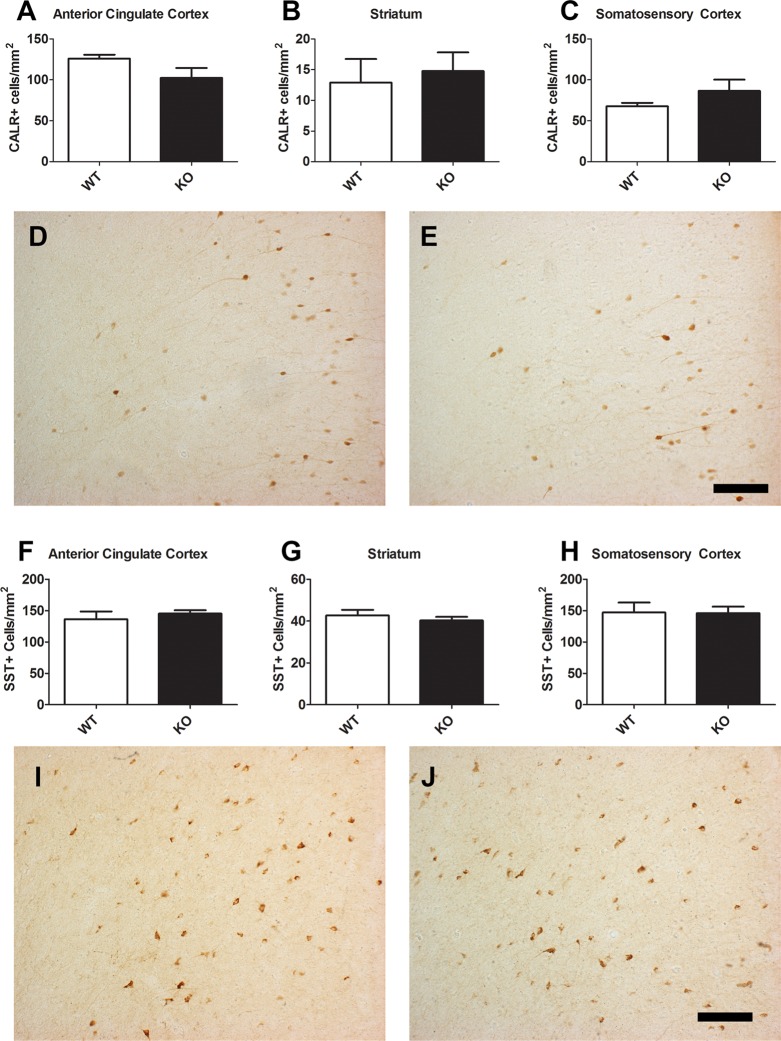
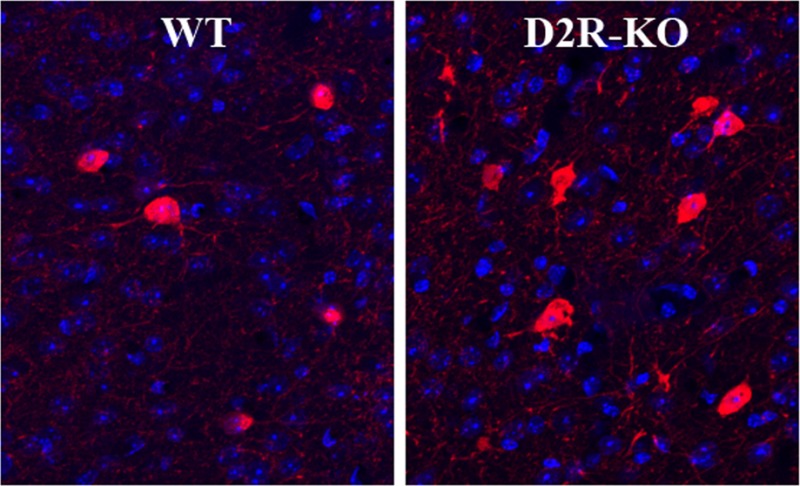
Coronal brain sections containing the ACC, STR, and primary somatosensory cortex (SSC) of adult mice were immunostained for GAD67. There was a significant increase in the number of GAD67+ cells in the ACC of knockout (KO) mice relative to wild-type (WT) controls (Figure 1A,D,E; t(12) = 2.568, p < 0.05). No differences were found in either the STR (Figure 1B; p = 0.185) or SSC (Figure 1C; p = 0.869). This is perhaps not surprising given that PV does not colocalize with D2eGFP in the STR, where PV+ neurons are of relatively low density, or in the SSC, which has very low expression of D2R. The increase in GAD67+ neurons was fairly uniform across cortical layers; that is, it did not appear to be limited to only superficial or deep layers. We then sought to determine whether this effect in the ACC was specific to all GABAergic markers or encompassed only a subset. Similar to the GAD67 staining, the ACC of KO mice showed increased parvalbumin (PV)-expressing cells than in the WT brain (Figure 2A,D,E; t(12) = 2.236, p < 0.05), with again no significant differences found in either the STR (Figure 2B; p = 0.707) or SSC (Figure 2C; p = 0.333). In contrast, calretinin (CALR; Figure 3A–E) and somatostatin (SST; Figure 3F–J) staining did not differ between the genotypes in any of the regions examined.
Figure 1.
GAD67 staining in the adult D2R knockout mouse. Increased levels were found in the ACC of the KO (N = 8) relative to the WT (A; N = 6). No such change was apparent in either the STR (B) or SSC (C). Representative micrographs in the ACC show expression of GAD67 in the WT (D) and KO (E) mice. Scale bar = 100 μm. Cortical layers are indicated by Roman numerals. *p < 0.05.
Figure 2.
PV cell density in the ACC (A), STR (B), and SSC (C) of the adult D2R knockout mouse. Increased PV+ cells were found in the ACC of KO mice [E; N = 8 (N = 11 for STR counts)] relative to the WT mice [D; N = 6 (N = 10 for STR counts)]. Scale bar = 100 μm. *p < 0.05.
Figure 3.
Cell counts of CALR (A–C) and SST (F–H) in the ACC (A, F), STR (B, G), and SSC (C, H) of the adult D2R KO knockout mouse (N = 6 for WT; N = 8, KO). Values did not differ for either of these two cell types between genotypes. Representative images at 20× magnification for CALR (D, E) and SST (I, J) within the ACC are shown in WT (D, I) and KO (E, J) tissue. Scale bar = 100 μm.
Previous studies have demonstrated that alterations to DA homeostasis during development alter the expression of GABAergic interneurons.18 For example, prenatal cocaine exposure in rabbits changes GABA levels and the dendritic morphology of PV+ GABAergic interneurons.8,24,25 Bhide and colleagues have demonstrated that prenatal cocaine exposure in mice decreases GAD67+ neurons within the prefrontal cortex,26 specifically those expressing PV.27 Interestingly, application of a D2R agonist to embryonic slice cultures decreased the total number of GABAergic cells that migrated to the cerebral cortex, and cultures derived from D2R KO mice exhibited increased migration.9 However, the lack of changes in SST and CALR in D2R KO mice is not unexpected. PV, SST, and CALR originate from progenitor cells within the medial ganglionic eminence, with some populations of CALR+ cells also derived from the caudal ganglionic eminence.5,28 However, these progenitor cells are functionally diverse and transcriptionally distinct,29−31 owing to the diversity of expression and localization of interneuron cell types.32−35 Ablation of the D2R could be affecting upstream factors that result in aberrant expression of PV but not SST or CALR (see ref (36)).
D2R-modulation of PV expression was also region-specific, because no regulation of GAD67 or PV was found in the STR or SSC. It has been demonstrated that D2R overexpression within the STR alters working memory, a functional output correlated with the prefrontal cortex.14 Moreover, PV within the frontal cortex has been shown to contribute to this behavior.37 While D2R ablation in this model had no significant effect on PV expression within the STR itself, we cannot rule out that loss of striatal D2R might alter PV-mediated cortical output. The lack of effect in SSC is not unexpected, because this region has little or no DA innervations.24,38
Interneuron Expression in the Developing Forebrain
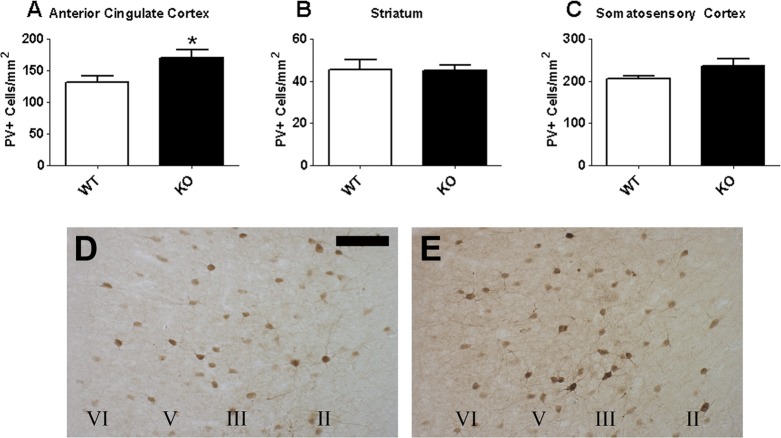
To determine whether the differences in PV expression originated during development or were confined to adulthood, we next examined PV expression in animals at postnatal day (P)14, the period during which PV expression rapidly increases in the rodent brain.39−41 Again, we found that PV cell density was significantly increased throughout the ACC of P14 D2R KO mice (approximately +30%, Figure 4A,D,E; t(8) = 2.341, p < 0.05). Once again, no differences were found between genotypes in either the STR (Figure 4B; p = 0.936) or SSC (Figure 4C; p = 0.196).
Figure 4.
PV cellular expression in the ACC (A), STR (B), and SSC (C) of the D2R KO transgenic mouse at P14 (N = 5/genotype). PV cell density is elevated in the ACC of KO mice (E) relative to the WT mice (D). Scale bar = 100 μm. *p < 0.05.
Colocalization of D2R and PV
Several previous studies have suggested a significant degree of colocalization of PV and D2R gene transcripts,42−45 but others have seen much less D2R in cortical PV+ interneurons.46,47 We observed moderate colocalization of Pvalb and Drd2 within the ACC via fluorescent double-label in situ hybridization, but levels of Drd2 were too low to allow for accurate quantification (data not shown). Poor selectivity of existing D2R antibodies has hampered colocalization studies at the protein level. We therefore stained for PV in D2eGFP BAC transgenic mice.48 Approximately 24.3% ± 1.7% (67.1 ± 4.6 neurons/mm2) of PV+ cells within the ACC coexpressed eGFP (Figure 5), demonstrating that a considerable number of PV+ neurons in the mouse ACC express D2R. Moreover, we did not observe any significant colocalization of PV with D2eGFP within the STR and SSC (data not shown). These data are consistent with a potential direct cellular mechanism through which D2 receptor stimulation can regulate interneuron differentiation or survival.
Figure 5.
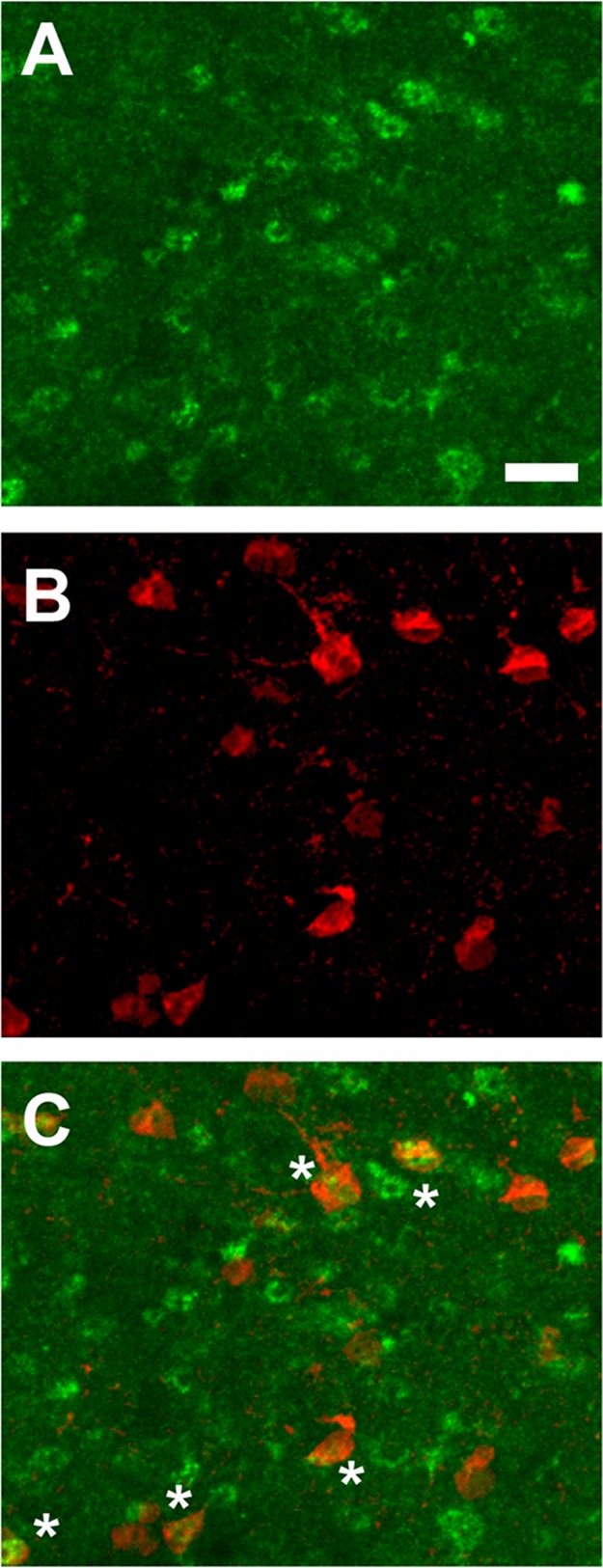
Representative micrographs of colocalization of GFP (indicating the D2R; A, green) and PV (B, red) in the ACC of D2eGFP BAC transgenic mice (N = 5). Colocalized neurons are apparent in the merged image (C), as denoted by asterisks (*). Scale bar = 25 μm.
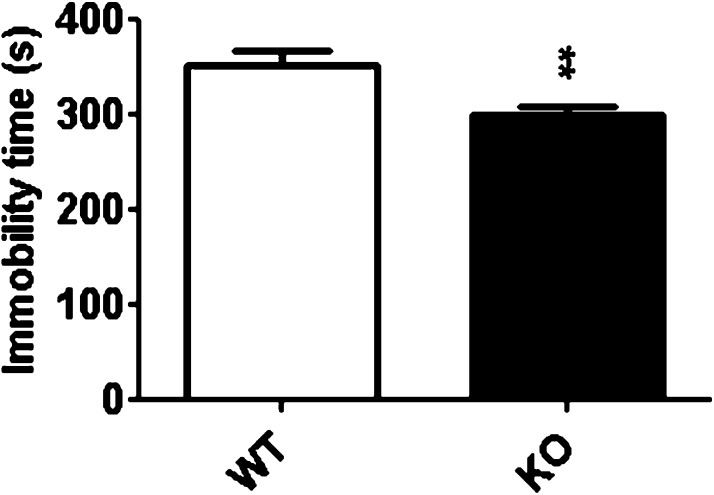
Reduced Depressive-Like Behavior in D2R KO Mice
D2 receptors have been implicated in the onset of depression, and disturbances in GABAergic function and expression are characteristics of depression.49 Thus, the tail suspension test (TST) was used to measure depressive-type behavior in a separate cohort of mice. D2R KO mice spent significantly less time immobile than WT mice under basal conditions (Figure 6; t(23) = 3.425, p < 0.01). Of note, this response goes against the well documented and dramatic hypoactivity exhibited by this line in open field/locomotor assays;50 therefore, if anything, this assessment in the TST would underestimate any antidepressant-like phenotype in this line. Alterations both to GABAergic interneuron expression and activity as well as to D2R have been implicated in the onset of depression. For instance, depression has been associated with decreased levels of cortical GABA in humans.49,51 More specifically, decreases in PV expression or activity within frontal cortex have been correlated with depression in humans23 and depressive-like behaviors in rodents.52,53 Dopamine also plays a key role in the mechanisms underlying depression.54 D2 receptors, including those within the ACC, are involved in the development of depression,55,56 and D2R ligands can be used to treat the disorder.54 Others have suggested that it is through the actions of the polysialylated form of the neural cell adhesion molecule (PSA-NCAM) that the D2R mediates its effects upon PV,57 effects that contribute to the onset of neuropsychiatric diseases such as depression.58 Alternatively, the loss of D2R within other regions, such as the striatum, could also contribute to this phenotype. Recent studies have shown that VTA DA projections to the nucleus accumbens contribute to depressive-like behaviors59−61 and that the D2R specifically is involved in this phenotype.62,63 While our data cannot directly link the D2R-mediated increase in cortical PV+ cells, or the loss of D2R within the striatum, to the antidepressant behavioral phenotype, these results suggest that these mechanisms potentially contribute to depressive behaviors.
Figure 6.
D2R-mediated changes in depressive-like behavior. D2R KO mice (N = 16) spend significantly less time immobile in the tail suspension test relative to WT mice (N = 9). **p < 0.01 vs WT.
We note that alterations both to GABAergic interneuron expression and activity as well as to D2R have been implicated in the onset of depression. For instance, depression has been associated with decreased levels of cortical GABA in human,49,51 and decreases in PV expression or activity within frontal cortex have been correlated with depression in humans23 and depressive-like behaviors in rodents.52,53 Perhaps most strikingly, Disc1 transgenic mice, an animal model of schizophrenia-related phenotypes, display both reduced PV interneurons in the frontal cortex and increased immobility in depression-related tests,64 the converse of the responses we found in the D2R nulls. Dopamine also plays a key role in the mechanisms underlying depression.54 D2 receptors, including those within the ACC, are involved in the development of depression,55,56 and D2R ligands can be used to treat the disorder.54 While our data are only correlative at this time and do not directly link the D2R-mediated increase in PV+ cells to the antidepressant behavioral phenotype, these results suggest that these mechanisms potentially contribute to depressive behaviors. The recent creation of D2R floxed mice65 will allow specific inactivation of D2R within PV+ neurons, and it will be of interest to examine such mice for these phenotypes.
Summary and Conclusions
Disruptions to DA homeostasis have been implicated in a number of neuropsychiatric and neurological disorders. Many of these disorders, such as schizophrenia, autism, Rett syndrome, dystonia, and depression, are also associated with GABAergic dysfunction.19,66−69 Indeed, interneuron transplantation has recently been proposed as a potential therapy for such conditions,70 as has targeting the GABAergic system to treat neuropsychiatric disorders during adolescence.71 Moreover, developmental alterations to PV expression and function, in particular, can lead to disrupted excitation and inhibitory balance that underlies many of these disorders.19,36,69,72−77
In the current report, we demonstrate that D2R can regulate interneuron expression and likely function in the ACC, as loss of D2R results in elevated GAD67+ and PV+ cell density. This effect is limited to this particular subset of GABAergic interneurons, because no changes were seen in other subclasses, and is long-lasting. Given the relatively high expression of D2R in the ACC, it is likely that the D2R plays an inhibitory role upon cortical circuits, including GABAergic PV+ interneurons. These alterations in cell number suggest that increased GAD67+ and PV+ cells contribute to increased inhibitory control and altered microcircuitry within this region. Taken together, our data indicate that alterations to the D2R during development result in long-term consequences for interneuron number and functional circuits.
Methods
Animals
D2R knockout (KO) mice on a C57Bl/6J background were obtained from Jackson Laboratories (Bar Harbor, ME).78 WT and KO littermates were used for each assay. D2eGFP BAC transgenic mice, created by the GENSAT project,48 were obtained via the MMRRC (UC Davis). These mice, originally on a FVB/N background, were backcrossed onto a C57Bl6/J background for more than 10 generations. Adult mice were housed 3–5/cage and were provided with rodent chow and tap water ad libitum, while younger animals (P14 mice) remained with their dam and sire until experimentation. Mice were housed in a temperature- and humidity-controlled AAALAC-approved facility that is maintained on a 12:12 h light/dark cycle (lights on 0600–1800 h). WT and KO mice were attained by breeding heterozygotic pairs, while BAC transgenic mice were produced by breeding hemizygotic and WT mice, with only the hemizygotic mice being used for experimentation. Both males and females were utilized for the histological assays, while only males were used for behavioral testing. All protocols were approved by the Vanderbilt University Institutional Animal Care and Use Committee, and all studies were performed in accordance with the recommendations in the National Institute of Health’s Guide for the Care and Use of Laboratory Animals. Genotypes were confirmed by polymerase chain reaction (PCR) analysis of tail tissue. The following forward and reverse primers were used to identify the presence of a neomycin (neo) cassette, which is present in D2R KO mice: 5′-CTT GGG TGG AGA GGC TAT TC-3′ and 5′-AGG TGA GAT GAC AGG AGA TC-3′. The following primer set was used to identify WT mice (forward and reverse primers, respectively): 5′-TGT GAC TGC AAC ATC CCA CC-3′ and 5′-GCG GAA CTC AAT GTT GAA GG-3′. Touchdown PCR with primers at 1 μM final concentration was then performed. Samples were incubated at 94 °C for 8 min. Samples were then kept at 94 °C for 20 s, cooled to 64 °C (−0.5 °C × cycle number) for 30 s, and then heated to 72 °C for 35 s for 12 cycles. After the 12th cycle, samples were heated to 94 °C for 20 s, cooled to 58 °C for 30 s, and then reheated to 72 °C for 35 s for 25 cycles. Afterward, samples were held at 72 °C for 2 min, and then cooled to 4 °C. Bands were visualized by gel electrophoresis (neo band at ∼270 bp and WT band at 108 bp). BAC transgenic mice were genotyped using the following primer sets against the eGFP gene (forward and reverse, respectively): 5′-CCT ACG GCG TGC AGT GCT TCA GC-3′ and 5′-CGG CGA GCT GCA CGC TGC GTC CTC-3′. Dopamine transporter primers (forward, 5′-CCC GTC TAC CCA TGA GTA AAA-3′; reverse, 5′-CTC CAC CTT CCT AGC ACT AAC-3′) were run simultaneously with each sample to identify WT (i.e., nonhemizygotic mice) in lieu of relying upon the absence of the eGFP band to determine the genotype. With 1 μM primers (final concentration), samples were incubated at 94 °C for 3 min, followed by 30 cycles at 94 °C (30 s), 61 °C (45 s), and 72 °C (45 s). Afterward, samples were heated to 72 °C for 10 min, and then cooled to 4 °C. Bands were visualized by gel electrophoresis (DA transporter band at 565 bp and eGFP band at ∼300 bp).
Chromogenic Immunohistochemistry
Adult (P60 or older, N = 5–11/genotype) and adolescent (P14, N = 6/genotype) mice were anesthetized with sodium pentobarbital and transcardially perfused with 4% paraformaldehyde. Brains were removed and fixed overnight in paraformaldehyde at 4 °C. Following cryoprotection in a sucrose gradient (10%, 20%, and 30% in phosphate-buffered saline), brains were cut coronally into 40 μm sections on a freezing microtome and stored at −20 °C in freezing solution until further experimentation. Staining in the D2R KO and WT mice was performed as previously described using the chromogen 3,3′-diaminobenzidine to visualize proteins.7,79 Antibodies against mouse PV (1:500; Sigma-Aldrich, St. Louis, MO), mouse GAD67 (1:2000; Millipore, Billerica, MA), rat SST (1:400; Millipore), and rabbit CALR (1:4000; Swant) were used with biotinylated secondary antibodies (1:1000; Jackson ImmunoResearch, West Grove, PA). Because detergent disrupts GAD67 immunolocalization (unpublished observation), Triton X-100 was eliminated from this assay to minimize membrane rupture; thus, measured GAD67 counts are likely underreporting the total number of GABAergic cells. Sections were visualized via a Zeiss AxioImager microscope with a Zeiss AxioCam HRc camera and corresponding AxioVision 4.1 software. The ACC, STR, and SSC were imaged at 20× magnification. Sections included in analysis encompassed the range of approximately +0.98 to +1.98, relative to Bregma, with three sections (two hemispheres each) photographed and analyzed per region of interest × hemisphere by an observer blinded to genotype. For each hemisphere, one field from SCC, two fields of ACC (dorsal and ventral portions), and four fields within the STR (dorsomedial, dorsolateral, ventromedial, and ventrolateral regions) were counted. In the latter two regions, the fields were nonoverlapping. For each region examined, cells counts were summed per section and corrected for profile size using the Abercrombie correction method80 and then calculated as densities (corrected counts/mm2). Sections from WT and KO mice were always immunostained in parallel within the same experiment to ensure that any apparent differences cannot be attributable to procedural variables, such as small differences in chemical reaction time.
Fluorescent Immunohistochemistry
Hemizygotic D2eGFP adult (P60, N = 5) mice were anesthetized and perfused as described above. An antibody against mouse PV (1:200; Sigma-Aldrich) with a Cy3-tagged secondary antibody (1:1000, Jackson ImmunoResearch) was used to label PV+ neurons. An antibody against chicken GFP (1:100; Life Technologies, Grand Island, NY), in conjunction with a biotinylated secondary antibody (1:1000, Jackson ImmunoResearch), standard ABC kit (Vector Laboratories, Burlingame, CA), and AlexaFluor 488-TSA amplification kit (1:100; Invitrogen), was used to amplify endogenous GFP expression. Imaging was performed as previously mentioned, with 20× images captured of the ACC. Cells were counted based on the presence of the red (PV+) with or without green (GFP+) fluorophores. Representative images were captured via a LSM 710 META inverted confocal microscope (Zeiss, Thornwood, NY) and associated ZEN (Zeiss) software (Figure 5).
PV and D2R Fluorescent in Situ Hybridization
Clones for PV (Pvalb, accession no. BC027424) and D2R (Drd2, accession no. BC105666) were obtained commercially (Source Bioscience, Nottingham, UK, and Thermo Scientific, Pittsburgh, PA, respectively) and used for dual fluorescent in situ hybridization.81 In these experiments, DNP-labeled RNA probes were used to detect D2R mRNA followed by amplification via anti-DNP-POD (1:200, PerkinElmer) and a biotin TSA kit (1:100, PerkinElmer). Additional amplification via ABC Elite kit (Vector Laboratories) and AlexaFluor 488 TSA kit (1:100, Life Technologies) was then performed. PV probes were tagged with DIG and detected via anti-DIG-AP (1:100, Roche) and HNPP-Fast Red detection system (1:100, Roche). Slides were counterstained with DAPI, and images were visualized via a Zeiss AxioImager microscope.
Tail Suspension Test (TST)
Male mice (N = 9–16) were suspended by the tail from a vertical aluminum bar attached to the top of a box-like enclosure (33 × 33 × 32 cm3; Med Associates, St. Albans, VT). Mice were attached to the bar by tape placed ∼1.5 cm from the tip of the tail for 7 min. Force transducers and automated software (Med Associates) were used to measure immobility.
Statistical Analysis
Histological and behavioral data from the D2R KO and WT mice were analyzed via unpaired two-tailed t tests using GraphPad Prism 5 (GraphPad Software; San Diego, CA), with genotype (WT vs KO) as the main factor. Significance was set at p ≤ 0.05; data are presented as mean ± SEM.
Acknowledgments
We thank Matthew Buendia and Mary Akel for excellent technical support and Dr. Aliya Frederick, Emily Ross, and Kelli Money for reviewing the manuscript and helpful advice and conversations. Behavioral work was performed at the Vanderbilt Mouse Neurobehavioral Core, and confocal imaging was performed through the use of the VUMC Cell Imaging Shared Resource.
Author Contributions
D.L.G. and G.D.S. designed the research; D.L.G., H.H.D., J.D.G., E.L.C., and F.D.E. performed the research; D.L.G., H.H.D., F.D.E., and G.D.S. analyzed data; D.L.G. and G.D.S. wrote the paper.
This work was supported by NIH Grant R01MH086629 (G.D.S.).
The authors declare no competing financial interest.
Funding Statement
National Institutes of Health, United States
References
- Thompson B. L.; Levitt P.; Stanwood G. D. (2009) Prenatal exposure to drugs: Effects on brain development and implications for policy and education. Nat. Rev. Neurosci. 10, 303–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Money K. M.; Stanwood G. D. (2013) Developmental origins of brain disorders: Roles for dopamine. Front. Cell. Neurosci. 7, 260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis D. A.; Levitt P. (2002) Schizophrenia as a disorder of neurodevelopment. Annu. Rev. Neurosci. 25, 409–432. [DOI] [PubMed] [Google Scholar]
- Artigas F. (2010) The prefrontal cortex: A target for antipsychotic drugs. Acta Psychiatr. Scand. 121, 11–21. [DOI] [PubMed] [Google Scholar]
- Marin O. (2013) Cellular and molecular mechanisms controlling the migration of neocortical interneurons. Eur. J. Neurosci. 38, 2019–2029. [DOI] [PubMed] [Google Scholar]
- Kawaguchi Y. (1997) Neostriatal cell subtypes and their functional roles. Neurosci. Res. 27, 1–8. [DOI] [PubMed] [Google Scholar]
- Stanwood G. D.; Parlaman J. P.; Levitt P. (2005) Anatomical abnormalities in dopaminoceptive regions of the cerebral cortex of dopamine D1 receptor mutant mice. J. Comp. Neurol. 487, 270–282. [DOI] [PubMed] [Google Scholar]
- Stanwood G. D.; Washington R. A.; Levitt P. (2001) Identification of a sensitive period of prenatal cocaine exposure that alters the development of the anterior cingulate cortex. Cereb. Cortex 11, 430–440. [DOI] [PubMed] [Google Scholar]
- Crandall J. E.; McCarthy D. M.; Araki K. Y.; Sims J. R.; Ren J. Q.; Bhide P. G. (2007) Dopamine receptor activation modulates GABA neuron migration from the basal forebrain to the cerebral cortex. J. Neurosci. 27, 3813–3822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarthy D. M.; Zhang X.; Darnell S. B.; Sangrey G. R.; Yanagawa Y.; Sadri-Vakili G.; Bhide P. G. (2011) Cocaine alters BDNF expression and neuronal migration in the embryonic mouse forebrain. J. Neurosci. 31, 13400–13411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross E. J.; Graham D. L.; Money K. M.; Stanwood G. D. (2014) Developmental Consequences of Fetal Exposure to Drugs: What We Know and What We Still Must Learn. Neuropsychopharmacology 10.1038/npp.2014.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown R. W.; Flanigan T. J.; Thompson K. N.; Thacker S. K.; Schaefer T. L.; Williams M. T. (2004) Neonatal quinpirole treatment impairs Morris water task performance in early postweanling rats: relationship to increases in corticosterone and decreases in neurotrophic factors. Biol. Psychiatry 56, 161–168. [DOI] [PubMed] [Google Scholar]
- Brown R. W.; Gass J. T.; Kostrzewa R. M. (2002) Ontogenetic quinpirole treatments produce spatial memory deficits and enhance skilled reaching in adult rats. Pharmacol., Biochem. Behav. 72, 591–600. [DOI] [PubMed] [Google Scholar]
- Kellendonk C.; Simpson E. H.; Polan H. J.; Malleret G.; Vronskaya S.; Winiger V.; Moore H.; Kandel E. R. (2006) Transient and Selective Overexpression of Dopamine D2 Receptors in the Striatum Causes Persistent Abnormalities in Prefrontal Cortex Functioning. Neuron 49, 603–615. [DOI] [PubMed] [Google Scholar]
- Cazorla M.; Shegda M.; Ramesh B.; Harrison N. L.; Kellendonk C. (2012) Striatal D2 receptors regulate dendritic morphology of medium spiny neurons via Kir2 channels. J. Neurosci. 32, 2398–2409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S. Y.; Choi K. C.; Chang M. S.; Kim M. H.; Na Y. S.; Lee J. E.; Jin B. K.; Lee B. H.; Baik J. H. (2006) The dopamine D2 receptor regulates the development of dopaminergic neurons via extracellular signal-regulated kinase and Nurr1 activation. J. Neurosci. 26, 4567–4576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- An J. J.; Bae M. H.; Cho S. R.; Lee S. H.; Choi S. H.; Lee B. H.; Shin H. S.; Kim Y. N.; Park K. W.; Borrelli E.; Baik J. H. (2004) Altered GABAergic neurotransmission in mice lacking dopamine D2 receptors. Mol. Cell. Neurosci. 25, 732–741. [DOI] [PubMed] [Google Scholar]
- Porter L. L.; Rizzo E.; Hornung J. P. (1999) Dopamine affects parvalbumin expression during cortical development in vitro. J. Neurosci. 19, 8990–9003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marin O. (2012) Interneuron dysfunction in psychiatric disorders. Nat. Rev. Neurosci 13, 107–120. [DOI] [PubMed] [Google Scholar]
- Oh D. H.; Son H.; Hwang S.; Kim S. H. (2012) Neuropathological abnormalities of astrocytes, GABAergic neurons, and pyramidal neurons in the dorsolateral prefrontal cortices of patients with major depressive disorder. Eur. Neuropsychopharmacol. 22, 330–338. [DOI] [PubMed] [Google Scholar]
- Tripp A.; Kota R. S.; Lewis D. A.; Sibille E. (2011) Reduced somatostatin in subgenual anterior cingulate cortex in major depression. Neurobiol. Dis. 42, 116–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fung S. J.; Webster M. J.; Sivagnanasundaram S.; Duncan C.; Elashoff M.; Weickert C. S. (2010) Expression of interneuron markers in the dorsolateral prefrontal cortex of the developing human and in schizophrenia. Am. J. Psychiatry 167, 1479–1488. [DOI] [PubMed] [Google Scholar]
- Khundakar A.; Morris C.; Thomas A. J. (2011) The immunohistochemical examination of GABAergic interneuron markers in the dorsolateral prefrontal cortex of patients with late-life depression. Int. Psychogeriatr. 23, 644–653. [DOI] [PubMed] [Google Scholar]
- Stanwood G. D.; Washington R. A.; Shumsky J. S.; Levitt P. (2001) Prenatal cocaine exposure produces consistent developmental alterations in dopamine-rich regions of the cerebral cortex. Neuroscience 106, 5–14. [DOI] [PubMed] [Google Scholar]
- Wang X. H.; Jenkins A. O.; Choi L.; Murphy E. H. (1996) Altered neuronal distribution of parvalbumin in anterior cingulate cortex of rabbits exposed in utero to cocaine. Exp. Brain Res. 112, 359–371. [DOI] [PubMed] [Google Scholar]
- Crandall J. E.; Hackett H. E.; Tobet S. A.; Kosofsky B. E.; Bhide P. G. (2004) Cocaine exposure decreases GABA neuron migration from the ganglionic eminence to the cerebral cortex in embryonic mice. Cereb. Cortex 14, 665–675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarthy D. M.; Bhide P. G. (2012) Prenatal cocaine exposure decreases parvalbumin-immunoreactive neurons and GABA-to-projection neuron ratio in the medial prefrontal cortex. Dev. Neurosci. 34, 174–183. [DOI] [PubMed] [Google Scholar]
- Taniguchi H. (2014) Genetic dissection of GABAergic neural circuits in mouse neocortex. Front. Cell. Neurosci. 8, 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee A. T.; Gee S. M.; Vogt D.; Patel T.; Rubenstein J. L.; Sohal V. S. (2014) Pyramidal neurons in prefrontal cortex receive subtype-specific forms of excitation and inhibition. Neuron 81, 61–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kvitsiani D.; Ranade S.; Hangya B.; Taniguchi H.; Huang J. Z.; Kepecs A. (2013) Distinct behavioural and network correlates of two interneuron types in prefrontal cortex. Nature 498, 363–366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson N. R.; Runyan C. A.; Wang F. L.; Sur M. (2012) Division and subtraction by distinct cortical inhibitory networks in vivo. Nature 488, 343–348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wonders C. P.; Anderson S. A. (2006) The origin and specification of cortical interneurons. Nat. Rev. Neurosci. 7, 687–696. [DOI] [PubMed] [Google Scholar]
- Gelman D. M.; Marin O. (2010) Generation of interneuron diversity in the mouse cerebral cortex. Eur. J. Neurosci. 31, 2136–2141. [DOI] [PubMed] [Google Scholar]
- Anastasiades P. G.; Butt S. J. (2011) Decoding the transcriptional basis for GABAergic interneuron diversity in the mouse neocortex. Eur. J. Neurosci. 34, 1542–1552. [DOI] [PubMed] [Google Scholar]
- Ciceri G.; Dehorter N.; Sols I.; Huang Z. J.; Maravall M.; Marin O. (2013) Lineage-specific laminar organization of cortical GABAergic interneurons. Nat. Neurosci. 16, 1199–1210. [DOI] [PubMed] [Google Scholar]
- Powell E. M.; Campbell D. B.; Stanwood G. D.; Davis C.; Noebels J. L.; Levitt P. (2003) Genetic disruption of cortical interneuron development causes region- and GABA cell type-specific deficits, epilepsy, and behavioral dysfunction. J. Neurosci. 23, 622–631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wen L.; Lu Y. S.; Zhu X. H.; Li X. M.; Woo R. S.; Chen Y. J.; Yin D. M.; Lai C.; Terry A. V. Jr.; Vazdarjanova A.; Xiong W. C.; Mei L. (2010) Neuregulin 1 regulates pyramidal neuron activity via ErbB4 in parvalbumin-positive interneurons. Proc. Natl. Acad. Sci. U. S. A. 107, 1211–1216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lidov H. G.; Rice F. L.; Molliver M. E. (1978) The organization of the catecholamine innervation of somatosensory cortex: the barrel field of the mouse. Brain Res. 153, 577–584. [DOI] [PubMed] [Google Scholar]
- Schlosser B.; Klausa G.; Prime G.; Ten Bruggencate G. (1999) Postnatal development of calretinin- and parvalbumin-positive interneurons in the rat neostriatum: an immunohistochemical study. J. Comp. Neurol. 405, 185–198. [DOI] [PubMed] [Google Scholar]
- Solbach S.; Celio M. R. (1991) Ontogeny of the calcium binding protein parvalbumin in the rat nervous system. Anat. Embryol. (Berl) 184, 103–124. [DOI] [PubMed] [Google Scholar]
- de Lecea L.; del Rio J. A.; Soriano E. (1995) Developmental expression of parvalbumin mRNA in the cerebral cortex and hippocampus of the rat. Brain Res. Mol. Brain Res. 32, 1–13. [DOI] [PubMed] [Google Scholar]
- Le Moine C.; Gaspar P. (1998) Subpopulations of cortical GABAergic interneurons differ by their expression of D1 and D2 dopamine receptor subtypes. Brain Res. Mol. Brain Res. 58, 231–236. [DOI] [PubMed] [Google Scholar]
- Khan Z. U.; Mrzljak L.; Gutierrez A.; de la Calle A.; Goldman-Rakic P. S. (1998) Prominence of the dopamine D2 short isoform in dopaminergic pathways. Proc. Natl. Acad. Sci. U.S.A. 95, 7731–7736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan Z. U.; Gutierrez A.; Martin R.; Penafiel A.; Rivera A.; De La Calle A. (1998) Differential regional and cellular distribution of dopamine D2-like receptors: An immunocytochemical study of subtype-specific antibodies in rat and human brain. J. Comp. Neurol. 402, 353–371. [DOI] [PubMed] [Google Scholar]
- Fitzgerald M. L.; Chan J.; Mackie K.; Lupica C. R.; Pickel V. M. (2012) Altered dendritic distribution of dopamine D2 receptors and reduction in mitochondrial number in parvalbumin-containing interneurons in the medial prefrontal cortex of cannabinoid-1 (CB1) receptor knockout mice. J. Comp. Neurol. 520, 4013–4031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santana N.; Mengod G.; Artigas F. (2009) Quantitative analysis of the expression of dopamine D1 and D2 receptors in pyramidal and GABAergic neurons of the rat prefrontal cortex. Cereb. Cortex 19, 849–860. [DOI] [PubMed] [Google Scholar]
- de Almeida J.; Mengod G. (2010) D2 and D4 dopamine receptor mRNA distribution in pyramidal neurons and GABAergic subpopulations in monkey prefrontal cortex: Implications for schizophrenia treatment. Neuroscience 170, 1133–1139. [DOI] [PubMed] [Google Scholar]
- Gong S.; Zheng C.; Doughty M. L.; Losos K.; Didkovsky N.; Schambra U. B.; Nowak N. J.; Joyner A.; Leblanc G.; Hatten M. E.; Heintz N. (2003) A gene expression atlas of the central nervous system based on bacterial artificial chromosomes. Nature 425, 917–925. [DOI] [PubMed] [Google Scholar]
- Bhagwagar Z.; Wylezinska M.; Jezzard P.; Evans J.; Ashworth F.; Sule A.; Matthews P. M.; Cowen P. J. (2007) Reduction in occipital cortex gamma-aminobutyric acid concentrations in medication-free recovered unipolar depressed and bipolar subjects. Biol. Psychiatry 61, 806–812. [DOI] [PubMed] [Google Scholar]
- Kelly M. A.; Rubinstein M.; Phillips T. J.; Lessov C. N.; Burkhart-Kasch S.; Zhang G.; Bunzow J. R.; Fang Y.; Gerhardt G. A.; Grandy D. K.; Low M. J. (1998) Locomotor activity in D2 dopamine receptor-deficient mice is determined by gene dosage, genetic background, and developmental adaptations. J. Neurosci. 18, 3470–3479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanacora G.; Mason G. F.; Rothman D. L.; Behar K. L.; Hyder F.; Petroff O. A.; Berman R. M.; Charney D. S.; Krystal J. H. (1999) Reduced cortical gamma-aminobutyric acid levels in depressed patients determined by proton magnetic resonance spectroscopy. Arch. Gen. Psychiatry 56, 1043–1047. [DOI] [PubMed] [Google Scholar]
- Kigawa Y.; Hashimoto E.; Ukai W.; Ishii T.; Furuse K.; Tsujino H.; Shirasaka T.; Saito T. (2014) Stem cell therapy: A new approach to the treatment of refractory depression. J. Neural Transm. 121, 1221–1232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leussis M. P.; Freund N.; Brenhouse H. C.; Thompson B. S.; Andersen S. L. (2012) Depressive-like behavior in adolescents after maternal separation: sex differences, controllability, and GABA. Dev. Neurosci. 34, 210–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dailly E.; Chenu F.; Renard C. E.; Bourin M. (2004) Dopamine, depression and antidepressants. Fundam. Clin. Pharmacol. 18, 601–607. [DOI] [PubMed] [Google Scholar]
- Gershon A. A.; Vishne T.; Grunhaus L. (2007) Dopamine D2-like receptors and the antidepressant response. Biol. Psychiatry 61, 145–153. [DOI] [PubMed] [Google Scholar]
- Larisch R.; Klimke A.; Vosberg H.; Loffler S.; Gaebel W.; Muller-Gartner H. W. (1997) In vivo evidence for the involvement of dopamine-D2 receptors in striatum and anterior cingulate gyrus in major depression. Neuroimage 5, 251–260. [DOI] [PubMed] [Google Scholar]
- Castillo-Gomez E.; Varea E.; Blasco-Ibanez J. M.; Crespo C.; Nacher J. (2011) Polysialic acid is required for dopamine D2 receptor-mediated plasticity involving inhibitory circuits of the rat medial prefrontal cortex. PLoS One 6, e29516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nacher J.; Guirado R.; Castillo-Gomez E. (2013) Structural plasticity of interneurons in the adult brain: role of PSA-NCAM and implications for psychiatric disorders. Neurochem. Res. 38, 1122–1133. [DOI] [PubMed] [Google Scholar]
- Barik J.; Marti F.; Morel C.; Fernandez S. P.; Lanteri C.; Godeheu G.; Tassin J. P.; Mombereau C.; Faure P.; Tronche F. (2013) Chronic stress triggers social aversion via glucocorticoid receptor in dopaminoceptive neurons. Science 339, 332–335. [DOI] [PubMed] [Google Scholar]
- Chaudhury D.; Walsh J. J.; Friedman A. K.; Juarez B.; Ku S. M.; Koo J. W.; Ferguson D.; Tsai H. C.; Pomeranz L.; Christoffel D. J.; Nectow A. R.; Ekstrand M.; Domingos A.; Mazei-Robison M. S.; Mouzon E.; Lobo M. K.; Neve R. L.; Friedman J. M.; Russo S. J.; Deisseroth K.; Nestler E. J.; Han M. H. (2013) Rapid regulation of depression-related behaviours by control of midbrain dopamine neurons. Nature 493, 532–536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nestler E. J.; Carlezon W. A. Jr. (2006) The mesolimbic dopamine reward circuit in depression. Biol. Psychiatry 59, 1151–1159. [DOI] [PubMed] [Google Scholar]
- Francis T. C.; Chandra R.; Friend D. M.; Finkel E.; Dayrit G.; Miranda J.; Brooks J. M.; Iniguez S. D.; O’Donnell P.; Kravitz A.; Lobo M. K. (2014) Nucleus Accumbens Medium Spiny Neuron Subtypes Mediate Depression-Related Outcomes to Social Defeat Stress. Biol. Psychiatry 10.1016/j.biopsych.2014.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lobo M. K.; Zaman S.; Damez-Werno D. M.; Koo J. W.; Bagot R. C.; DiNieri J. A.; Nugent A.; Finkel E.; Chaudhury D.; Chandra R.; Riberio E.; Rabkin J.; Mouzon E.; Cachope R.; Cheer J. F.; Han M. H.; Dietz D. M.; Self D. W.; Hurd Y. L.; Vialou V.; Nestler E. J. (2013) DeltaFosB induction in striatal medium spiny neuron subtypes in response to chronic pharmacological, emotional, and optogenetic stimuli. J. Neurosci. 33, 18381–18395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen S.; Lang B.; Nakamoto C.; Zhang F.; Pu J.; Kuan S. L.; Chatzi C.; He S.; Mackie I.; Brandon N. J.; Marquis K. L.; Day M.; Hurko O.; McCaig C. D.; Riedel G.; St Clair D. (2008) Schizophrenia-related neural and behavioral phenotypes in transgenic mice expressing truncated Disc1. J. Neurosci. 28, 10893–10904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bello E. P.; Mateo Y.; Gelman D. M.; Noain D.; Shin J. H.; Low M. J.; Alvarez V. A.; Lovinger D. M.; Rubinstein M. (2011) Cocaine supersensitivity and enhanced motivation for reward in mice lacking dopamine D2 autoreceptors. Nat. Neurosci. 14, 1033–1038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chattopadhyaya B.; Di Cristo G. (2012) GABAergic circuit dysfunctions in neurodevelopmental disorders. Front. Psychiatry 3, 51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hussman J. P. (2001) Suppressed GABAergic inhibition as a common factor in suspected etiologies of autism. J. Autism Dev. Disord. 31, 247–248. [DOI] [PubMed] [Google Scholar]
- Rubenstein J. L.; Merzenich M. M. (2003) Model of autism: Increased ratio of excitation/inhibition in key neural systems. Genes Brain Behav. 2, 255–267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gernert M.; Hamann M.; Bennay M.; Loscher W.; Richter A. (2000) Deficit of striatal parvalbumin-reactive GABAergic interneurons and decreased basal ganglia output in a genetic rodent model of idiopathic paroxysmal dystonia. J. Neurosci. 20, 7052–7058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southwell D. G.; Nicholas C. R.; Basbaum A. I.; Stryker M. P.; Kriegstein A. R.; Rubenstein J. L.; Alvarez-Buylla A. (2014) Interneurons from embryonic development to cell-based therapy. Science 344, 6180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silveri M. M.; Sneider J. T.; Crowley D. J.; Covell M. J.; Acharya D.; Rosso I. M.; Jensen J. E. (2013) Frontal lobe gamma-aminobutyric acid levels during adolescence: Associations with impulsivity and response inhibition. Biol. Psychiatry 74, 296–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis D. A.; Curley A. A.; Glausier J. R.; Volk D. W. (2012) Cortical parvalbumin interneurons and cognitive dysfunction in schizophrenia. Trends Neurosci. 35, 57–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leppa E.; Linden A. M.; Vekovischeva O. Y.; Swinny J. D.; Rantanen V.; Toppila E.; Hoger H.; Sieghart W.; Wulff P.; Wisden W.; Korpi E. R. (2011) Removal of GABA(A) receptor gamma2 subunits from parvalbumin neurons causes wide-ranging behavioral alterations. PLoS One 6, e24159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zikopoulos B.; Barbas H. (2013) Altered neural connectivity in excitatory and inhibitory cortical circuits in autism. Front. Hum. Neurosci. 7, 609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orduz D.; Bischop D. P.; Schwaller B.; Schiffmann S. N.; Gall D. (2013) Parvalbumin tunes spike-timing and efferent short-term plasticity in striatal fast spiking interneurons. J. Physiol. 591, 3215–3232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saunders J. A.; Tatard-Leitman V. M.; Suh J.; Billingslea E. N.; Roberts T. P.; Siegel S. J. (2013) Knockout of NMDA receptors in parvalbumin interneurons recreates autism-like phenotypes. Autism Res. 6, 69–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durand S.; Patrizi A.; Quast K. B.; Hachigian L.; Pavlyuk R.; Saxena A.; Carninci P.; Hensch T. K.; Fagiolini M. (2012) NMDA receptor regulation prevents regression of visual cortical function in the absence of Mecp2. Neuron 76, 1078–1090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly M. A.; Rubinstein M.; Asa S. L.; Zhang G.; Saez C.; Bunzow J. R.; Allen R. G.; Hnasko R.; Ben-Jonathan N.; Grandy D. K.; Low M. J. (1997) Pituitary lactotroph hyperplasia and chronic hyperprolactinemia in dopamine D2 receptor-deficient mice. Neuron 19, 103–113. [DOI] [PubMed] [Google Scholar]
- Stanwood G. D.; Leitch D. B.; Savchenko V.; Wu J.; Fitsanakis V. A.; Anderson D. J.; Stankowski J. N.; Aschner M.; McLaughlin B. (2009) Manganese exposure is cytotoxic and alters dopaminergic and GABAergic neurons within the basal ganglia. J. Neurochem. 110, 378–389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abercrombie M. (1946) Estimation of nuclear population from microtome sections. Anat. Rec. 94, 239–247. [DOI] [PubMed] [Google Scholar]
- Thompson B. L.; Stanwood G. D.; Levitt P. (2010) Specificity of prenatal cocaine exposure effects on cortical interneurons is independent from dopamine D1 receptor co-localization. J. Chem. Neuroanat. 39, 228–234. [DOI] [PMC free article] [PubMed] [Google Scholar]