Abstract
Lung transplantation has the worst outcome compared to all solid organ transplants due to chronic rejection known as obliterative bronchiolitis (OB). Pathogenesis of OB is a complex interplay of alloimmune-dependent and -independent factors, which leads to the development of inflammation, fibrosis, and airway obliteration that have been resistant to therapy. The alloimmune-independent inflammatory pathway has been the recent focus in the pathogenesis of rejection, suggesting that targeting this may offer therapeutic benefits. As a potent anti-inflammatory agent, epigallo-catechin-galleate (EGCG), a green tea catechin, has been very effective in ameliorating inflammation in a variety of diseases, providing the rationale for its use in this study in a murine heterotopic tracheal allograft model of OB. Mice treated with EGCG had reduced inflammation, with significantly less neutrophil and macrophage infiltration and significantly reduced fibrosis. On further investigation into the mechanisms, inflammatory cytokines keratinocyte (KC), interleukin-17 (IL-17), and tumor necrosis factor-α (TNF-α), involved in neutrophil recruitment, were reduced in the EGCG-treated mice. In addition, monocyte chemokine monocyte chemoattractant protein-1 (MCP-1) was significantly reduced by EGCG treatment. Antifibrotic cytokine interferon-γ –inducible protein-10 (IP-10) was increased and profibrotic cytokine transforming growth factor-β (TGF-β) was reduced, further characterizing the antifibrotic effects of EGCG. These findings suggest that EGCG has great potential in ameliorating the development of obliterative airway disease.
Keywords: cytokine, heterotopic tracheal allograft, neutrophil, obliterative airway disease
Lung transplantation is the only treatment for many end-stage lung diseases, but graft survival is limited despite modern immunosuppressive regimens. The major obstacle to long-term survival is chronic rejection of the graft, which is not amenable to standard immunosuppressive therapies used to prevent acute rejection. Chronic rejection, known as obliterative bronchiolitis (OB), leads to inflammation and luminal obliteration of the bronchioles. Clinically, OB manifests as progressive loss of lung function and decline in the forced expiratory volume due to airflow obstruction characteristic of bronchiolitis obliterans syndrome (BOS) resulting in respiratory failure and death [1–3]. Lack of knowledge about the etiology, pathophysiology, and risk factors makes it difficult to treat OB. Graft rejection has long been thought to be alloimmune mediated, thus therapeutics have mainly focused on acquired immunity. Recent research has emphasized an additional role for the innate immunity in chronic rejection, which may explain why OB is not ameliorated by immunosuppressive therapies alone [2, 3]. In addition to the known role of immunological injury, over the past few years an important role for cytokines, inflammatory cells such as polymorphonuclear neutrophils (PMNs), and macrophages from the innate immune system is being increasingly recognized [2, 3]. PMNs have been shown to cause parenchymal damage and increased rejection in heart transplants [4]. PMNs are among the first inflammatory cells to be detected in the bronchoalveolar lavage and lung biopsy specimens of patients with OB. PMNs and their chemoattractant interleukin-8 (IL-8) is elevated in the bronchoalveolar lavage fluid in BOS [5–7] and this is the subset of patients that have shown superior graft survival with macrolide treatment, which reduces PMNs and IL-8 in the graft. In addition, macrophages also play an important role in the pathogenesis of chronic rejection, as depletion of macrophages ameliorates OB [8]. Several cytokines and chemokines that are chemoattractive for the neutrophils and macrophages are known to play a role in the murine heterotopic tracheal transplant model of OB. The inflammatory phase is characterized by expression of vast array of cytokines, including the CXCR2 ligands keratinocyte-derived chemokine (KC; CXCL1), macrophage inflammatory protein-2 (MIP-2; CXCL2); and LIX (CXCL5) [9]. In addition, monocytes and cytokines chemotactic for them, the CCR2 chemokines such as monocyte chemoattractant protein-1 (MCP-1; CCL2) [10] and CCR5 cytokines MIP-1α and -1β (CCL3/4) [11], have also been reported.
OB has 2 major components, the prolonged allograft rejection and the resulting fibrosis of the small airways. Fibrotic diseases in general are hard to treat because the pathways that lead to fibrosis are not well known and once fibrosis is established it seems to be irreversible [12]. Transforming growth factor-β (TGF-β) is important in the fibroproliferative phase of OB, as it is required for the differentiation of myofibroblasts [13] and may promote fibrous luminal obliteration. All these observations suggest that activation of the innate immunity is either required or at least amplifies the rejection mediated by the alloimmune system.
Epigallo-catechin-galleate (EGCG), the main green tea catechin, has sparked great interest, as it has antioxidant [14], anti-inflammatory, antitumor, and antifibrotic [15] properties. It has already been shown to ameliorate disease progression in many animal models of chronic and acute lung disease, such as acute respiratory distress syndrome (ARDS), lung fibrosis [16], and cigarette smoke–induced emphysema [17]. The role of EGCG in rejection post lung transplantation is not known. This study was designed to test the hypothesis that EGCG ameliorates the development of chronic graft rejection. Thus, we used the established murine heterotopic airway transplant model of OB [18], to test the benefits of EGCG in ameliorating injury chronic rejection. In this model, tracheal allografts develop fibrous obliteration, also known as obliterative airway disease, which is histologically similar to OB found in humans.
MATERIALS AND METHODS
Animals and Reagents
C57BL/6 and BALB/c mice (6 to 10 weeks old) were purchased from Charles River Laboratories (Wilmington, MA). The mice were housed in the Children’s Hospital Boston animal facility under specific pathogen-free conditions. Epigallo-catechin-3-galleate (EGCG) was purchased from Sigma-Aldrich (St. Louis, MO).
Heterotopic Airway Transplant Model
We used an established model of obliterative airway disease involving heterotopic tracheal transplant with major hisotcompatibility complex (MHC)-mismatched combinations of C57BL/6 (H-2b) and BALB/c (H-2d) mice as described previously [18]. Tracheas from wild-type (WT) BALB/c mice were transplanted subcutaneously (s.c.) into the backs of WT C57BL/6 (phosphate-buffered saline [PBS]-control or EGCG-treatment group) mice. The mice were injected daily intraperitoneally (i.p.) with 0.1 mL PBS or EGCG (dissolved in PBS, 50 or 75 mg/kg), and no other drugs were administered to the mice during the experiments. Preliminary experiments determined that 75 mg/kg was optimal in our tracheal transplant model. At 2 days, 1 week, 2 weeks, and 4 weeks, the transplanted mice were euthanized and the tracheas were harvested. All animal protocols were approved by the Children’s Hospital Animal Care and Use Committee.
Immunohistochemistry
Airways were fixed in formalin-free zinc fixative (BD Biosciences Pharmingen, San Diego, CA), cut in half, and paraffin-embedded with the cut side down. Cross-sections of tracheal tissues (5 µm) were subjected to hematoxylin and eosin (H&E) staining, Masson’s trichrome staining, or various specific antibody staining. Cytokeratin-positive epithelium was stained with rabbit polyclonal antibodies against mouse cytokeratin 5 (Abcam, Cambridge, MA). Polymorphonuclear neutrophils (PMNs) and macrophages were stained with rat anti-mouse antibodies to granulocyte marker Ly-6C/6G(Gr-1) and Mac-3, respectively (BD Biosciences Pharmingen). Blood vessels were stained with rabbit antivon Willebrand factor (vWF) antibodies (Millipore Temecula, CA). A standard ABC vector kit (Vector Laboratories, Burlingame, CA) was used. Specific staining was confirmed by using isotype control antibody. All PMNs and blood vessels were counted manually in the entire section (outside and inside of the tracheal lumen) of the allograft, and macrophages were counted in 5 random high-power fields under a microscope.
Grading
Sections with H&E stains were graded by 2 independent observers who were blinded for the groups. A scale from 0 (no luminal obliteration) to 4 (maximal obliteration) was used to grade luminal obliteration. Cytokeratin 5–stained epithelium was graded by the percent coverage of the luminal surface.
Blood Leukocyte Analysis
Blood was collected retro-orbitally from mice of either group in EDTA tubes (Microvette, Sarstedt, Germany). Total leukocyte count along with a differential count of the leukocyte subset from blood was obtained by the ADVIA 120 hematology analyzer (Siemens Healthcare Diagnostics, Deerfield, IL). Total leukocyte count, absolute neutrophil count, absolute mononuclear cell count, and absolute eosinophil count in blood were compared between mice of aforementioned groups.
Bone Marrow Analysis
Bone marrow was obtained from mice of either group by flushing femur and tibia. Red blood cells (RBCs) in the bone marrow sediment were lysed using 0.2 N hypotonic saline and then neutralized with 1.6 N saline. Total numbers of leukocytes from bone marrow of the mice were counted with a hemacytometer using trypan blue dye exclusion. Cytospins were prepared from the bone marrow cell suspension and stained with Wright-Giemsa for manual differential count.
Analysis of Hydroxyproline Content
Tracheal hydroxyproline content was measured as an indicator of collagen deposition using the method outlined by Woessner [19]. Briefly, explanted tracheas were manually homogenized in Hanks’ balanced salt solution (HBSS) and dried in a speed-vac. Samples were then hydrolyzed in 6 M HCl for 18 hours at 110°C. Aliquots (0.1 mL) were analyzed for hydroxyproline content by mixing with chloramine-T and Ehrlich’s reagent to produce a hydroxyproline chromophore that was quantified at 550 nm spectrophotometrically. Standard curve was generated using hydroxyproline (Sigma) for each experiment in a 96-well plate.
Myeloperoxidase Assay
The myeloperoxidase (MPO) activity, an enzyme specific for polymorphonuclear cells [20], was used as an index of tracheal neutrophil accumulation. Explanted tracheas were manually homogenized in 2 mL HBSS. MPO activity was measured as described previously [21]. Briefly, the samples were centrifuged for 10 minutes at 3200 rpm and supernatants were kept in −20°C for cytokine assays. The pellets were first resuspended in 2 mL of 0.2% NaCl for about 30 seconds and then additional 2 mL of 1.6% NaCl was added to the mixture. After centrifugation at 3200 rpm for 10 minutes at 4°C, the pellets were resupended in 1 mL of HBSS containing 1% hexadecyltrimethylammonium bromide, 100 mM NaAc, and 20 mM EDTA (1% HTAB buffer). The samples were subjected to freeze and thaw for 3 times using liquid nitrogen, and were centrifuged at 10,000 rpm for 10 minutes at 4°C. Samples (75 µL each) were mixed with 100 µL TMB Substrate Reagent (BD Biosciences) in a 96-plate well. Reactions were stopped with 50 µL of 2 M H2SO4 and absorbance was read at 460 nm. Standard curve was generated in each experiment using a mouse neutrophil preparation aliquot with known cell numbers.
Cytokine and Enzyme-Linked Immunosorbent Assays
Tracheal explants were homogenized on ice and Complete Protease Inhibitor Cocktail Tablets (Roche) were used in tissue lysate preparation. Protein concentration was determined according to the bicinchoninic acid (BCA) assay (Pierce) and analyzed with SoftMax Pro v5 (Molecular Devices). The tissue lysates were aliquoted and kept in −80°C until experiments. Lysis buffer was used as blank in both assays. MILLIPLEX MAP Mouse Cytokine/Chemokine 96-well plate assay kit was purchased from Millipore, and the following cytokines were analyzed: granuclocyte-macrophage colony-stimulating factor (GM-CSF), interferon-γ (IFN-γ), IL-10, IL-17, IL-1β, IFN-γ -inducible protein-10 (IP-10), KC, LIX,MCP-1, MIG, MIP-1α, MIP-1β, MIP-2, tumor necrosis factor-α (TNF-α), and vascular endothelial growth factor (VEGF). The standards and controls were provided in the Millipore kit and the cytokine assays were performed with a Luminex 200 (Luminex), according to the manufacturer’s instruction. TGF-β was assayed using conventional enzyme-linked immunosorbent assay (ELISA) with Mouse TGF-β1 Duo Set including a sample activation step per manufacturer’s protocol (http://www.rndsystems.com/pdf/dy1679.pdf). Results were normalized against total protein input.
Statistical Analysis
The unpaired Student’s t test was used for statistical analysis. A P value of less than .05 was considered to indicate a significant difference between the 2 groups.
RESULTS
EGCG Reduces Luminal Obliteration and Improves Epithelial Regeneration
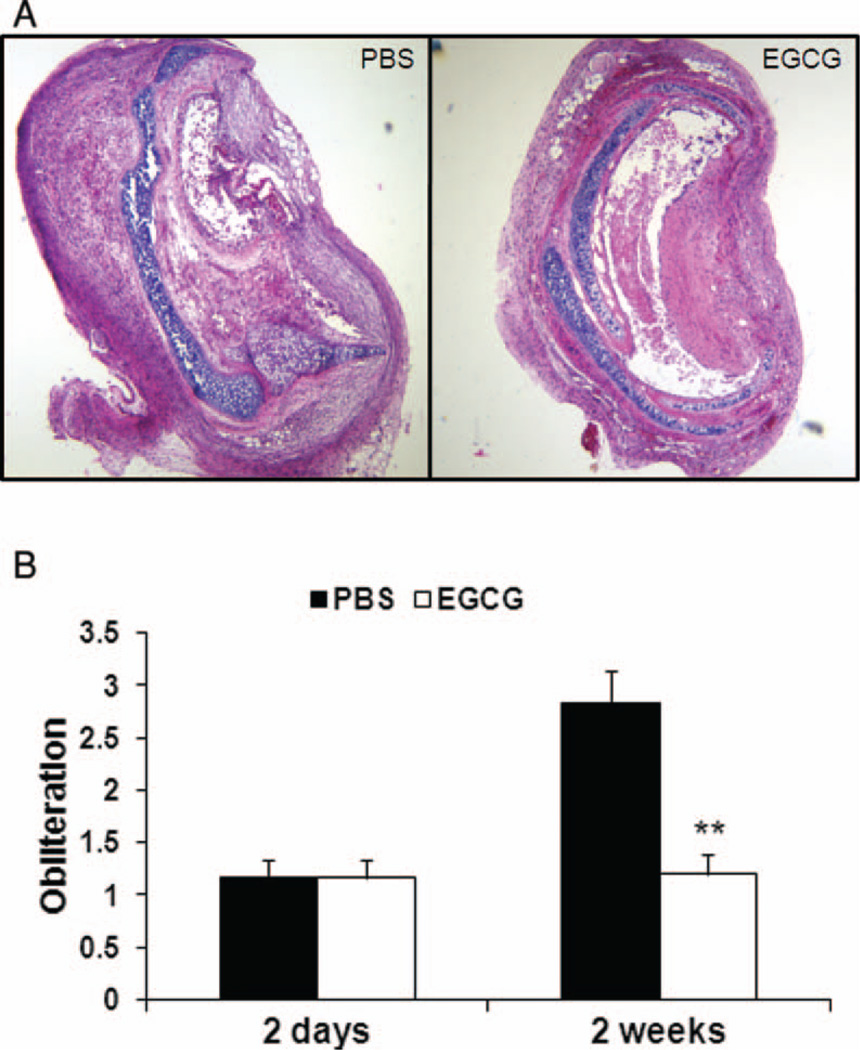
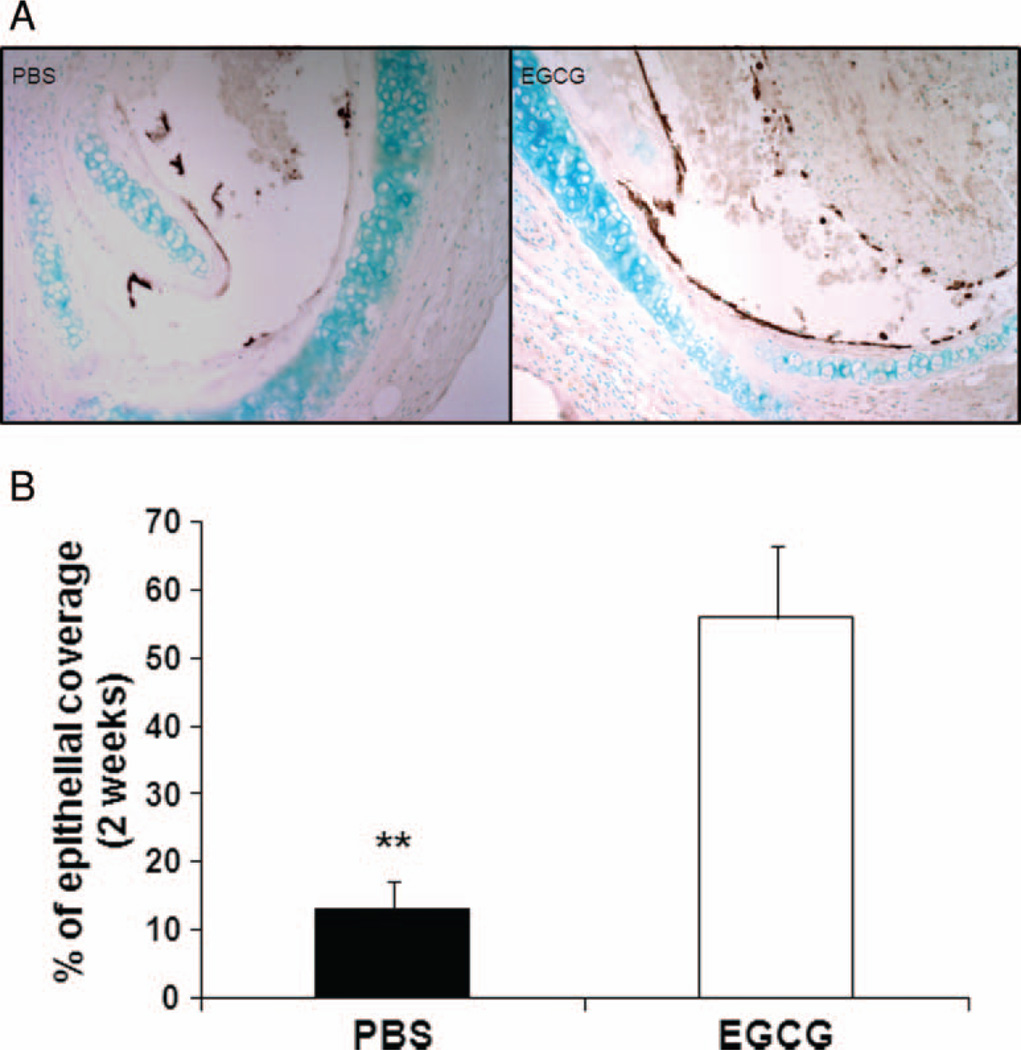
The histology grading of the H&E-stained sections revealed a significant difference between the PBS-control and EGCG-treated groups in luminal obliteration 2 weeks after transplantation. At this time point, grafts from PBS-control recipients had a grading of 2.8 compared with EGCG-treated recipients, where mean obliteration severity was 1.2 on a scale from 0 to 4 (Figure 1A and B; P < .01). There was no difference in epithelial coverage 2 days after transplantation (data not shown). However, immunohistochemical staining with antibodies against cytokeratin revealed that the overall epithelial coverage in EGCG-treated group 2 weeks post transplantation was significantly higher than that in PBS group (Figure 2A and B; 56% ± 10.61% for EGCG group versus 13.08% ± 4.08% for PBS-controls, P < .01). Interestingly, there were more submucosal glands with cytokeratin-positive cells 1 week after transplantation in EGCG-treated group (data not shown). An earlier study suggested that submucosal glands may serve as a protective niche for stem/progenitor cells in the proximal airways [22].
FIGURE 1.
Less tracheal luminal obliteration in EGCG-treated recipients. Grafts were explanted 2 days (PBS, n = 6; EGCG, n = 6) or 2 weeks (PBS, n = 5; EGCG, n = 6) after transplantation. Comparison of PBS-control and EGCG-treated groups using samples stained with H&E revealed a higher degree of luminal obliteration in the PBS-controls than in the EGCG-treated recipients 2 weeks after transplantation (A) (40× original magnification). Histological grading of H&E-stained samples indicated median obliteration was significantly lower in EGCG-treated mice (B), **P < .01. All error bars indicate SEM. (Color figure available online.)
FIGURE 2.
EGCG improves epithelial regeneration. (A and B) Immunohistochemical staining with antibodies against cytokeratin 5 (brown) revealed that tracheas in EGCG-treated group (n = 5) had significantly higher percent of epithelial coverage than those in PBS-controls (n = 6) 2 weeks post transplantation (200× original magnification), **P < .01. All error bars indicate SEM. (Color figure available online.)
EGCG Suppresses Neutrophil and Macrophage Infiltration
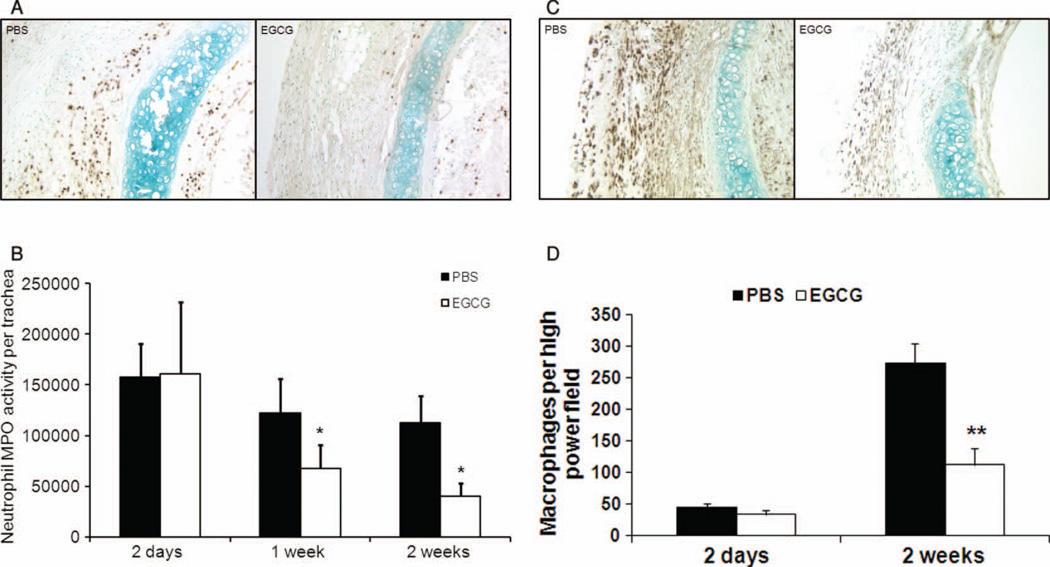
We observed significantly more neutrophils in the transplant in PBS-control mice than in EGCG-treated mice 2 weeks after transplantation (Figure 3A). We analyzed MPO activity, an index of neutrophil infiltration, in the tracheas 2 days, 1 week, and 2 weeks after transplantation. The MPO activity remained significantly lower after 1 week and 2 weeks of treatment with EGCG when compared with PBS-controls (Figure 3B; P < .05). These MPO activity data correlated with the number of Ly6C/6G–positive cells sequestered in the trachea. Similarly, we also observed that EGCG suppressed macrophage infiltration in the transplanted trachea 2 weeks after transplantation (Figure 3C and D; P < .01). Comparison of total myeloid cell numbers from blood and bone marrow showed that there is no difference between PBS-control and EGCG-treated recipients (data not shown).Hence, there is no difference in PMN myelogenesis or extravasation of PMNs from blood vessels between PBS-control and EGCG-treated groups.
FIGURE 3.
Reduced neutrophil and macrophage accumulation in EGCG-treated recipients. To assess neutrophil infiltration, 3 to 5 grafts from each treatment group were explanted 2 days, 1 week, or 2 weeks after transplantation. The tracheas in the EGCG-treated group showed significantly decreased numbers of infiltrated polymorphonuclear cells stained with antibodies against Ly-6C/6G (dark brown spots) when compared with PBS-controls 2 weeks after transplantation (A) (200× original magnification). Myeloperoxidase (MPO) activity, an index of neutrophil infiltration, was markedly reduced in EGCG-treated group both 1 week and 2 weeks after transplantation (B), *P < .05. To assess macrophage accumulation, grafts from each treatment group were explanted 2 days (PBS, n = 6; EGCG, n = 6) or 2 weeks (PBS, n = 5; EGCG, n = 6) after transplantation. Comparison of PBS-control and EGCG-treated groups using samples stained with antibodies against Mac-3 (brown) showed more macrophages in PBS-control tracheas than in EGCG-treated tracheas 2 weeks after transplantation (C) (200× original magnification). The number of macrophages per high-power field was significantly less in EGCG-treated group 2 weeks post transplantation (D), **P < .01. All error bars indicate SEM. (Color figure available online.)
EGCG Suppresses Proinflammatory Cytokines and Proangiogenic VEGF
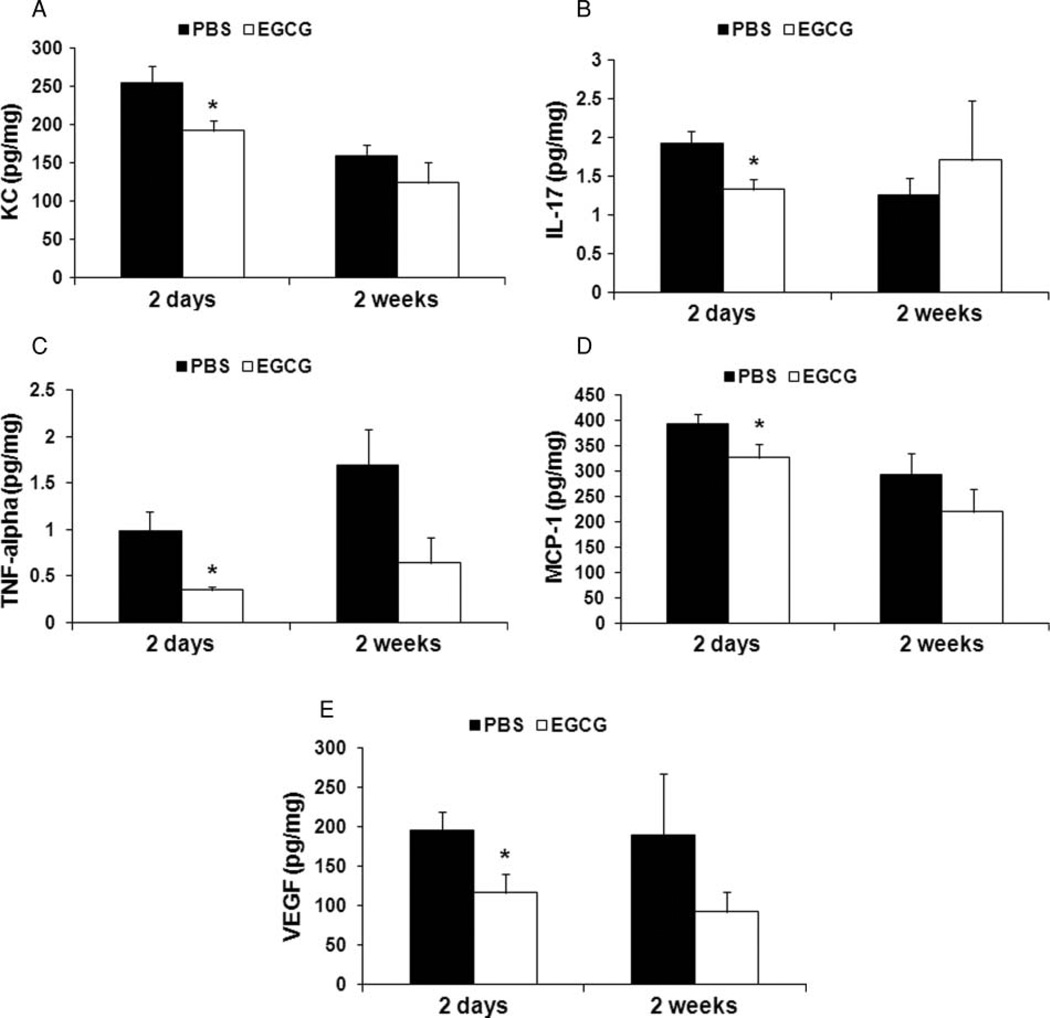
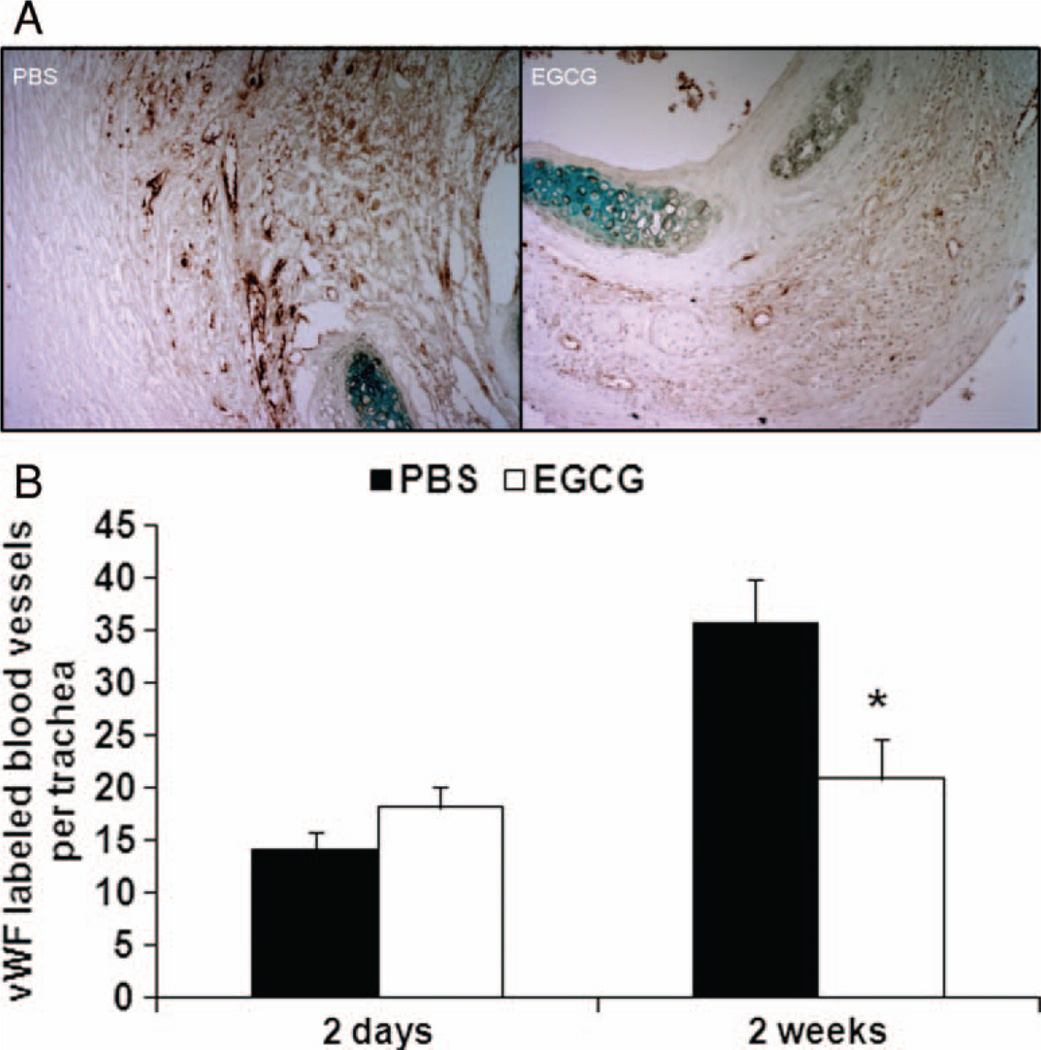
We used Luminex technology and conventional ELISA to analyze tissue expression of proinflammatory chemokine KC and proinflammatory cytokines interleukin-17 (IL-17), tumor necrosis factor-α (TNF-α), and MCP-1, which were significantly reduced 2 days after transplantation in the EGCG-treated group compared with PBS-control group (Figure 4A–D; P < .05). TNF-α also demonstrated an inhibitory trend 2 weeks after transplantation (P = .097). We then tested the expression level of anti-inflammatory cytokine IL-10. However, no difference was found between PBS-control and EGCG-treated groups at various time points. Next, in EGCG-treated group the levels of proangiogenic VEGF were significantly lower 2 days post transplantation (Figure 4E; P < .05). Indeed, the number of vessels as determined by vWF staining was significantly less in EGCG group than in PBS-controls 2 weeks post transplantation (Figure 5A and B; P < .05). In addition, IP-10 was significantly lower in EGCG-treated group 2 days post transplantation (Figure 6D; P < .05).
FIGURE 4.
Analysis of proinflammatory and proangiogenic mediators in transplants. Proinflammatory and proangiogenic mediators in both PBS-control (n = 6 for 2-day and 2-week time points) and EGCG-treated (n = 6 for 2-day and n = 5 for 2-week time points) groups were analyzed. EGCG treatment decreased levels of proinflammatory chemokine KC (A), proinflammatory cytokines IL-17 (B), TNF-α (C), MCP-1 (D), and proangiogenic VEGF (E) 2 days after transplantation, *P < .05. All error bars indicate SEM.
FIGURE 5.
Reduced angiogenesis in EGCG-treated recipients. (A) Microphotographs showing vWF-labeled blood vessels (brown) in PBS-control and EGCG-treated groups. (B) Blood vessel number per trachea (n = 5 to 6 for both PBS and EGCG groups at each time point) is also lower in EGCG-treated group 2 weeks post transplantation, *P < .05. All error bars indicate SEM. (Color figure available online.)
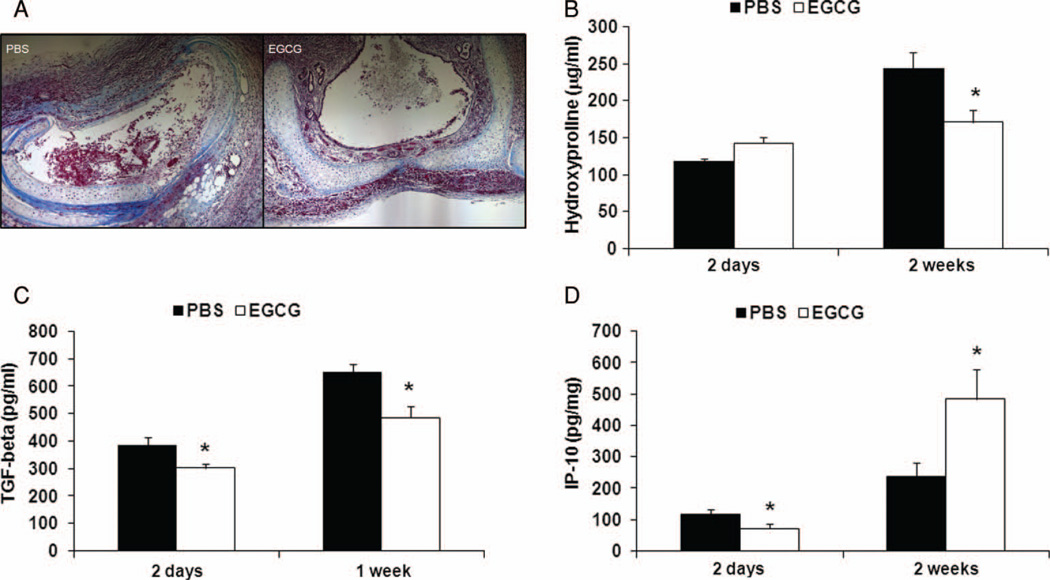
FIGURE 6.
Less fibrosis in EGCG-treated recipients. (A) Masson’s trichrome staining of collagen deposition in PBS-control and EGCG-treated groups. (B) Hydroxyproline-based quantification of collagen was performed with tracheal transplants from PBS-control (n = 5 to 6 for each time point) and EGCG-treated (n = 5 to 6 for each time point) groups. EGCG-treated mice had less collagen deposition 2 weeks after transplantation. (C) Significant inhibitory effects of EGCG on profibrotic cytokine TGF-β could be demonstrated both at 2 days (PBS, n = 6; EGCG, n = 6) and 1 week (PBS, n = 6; EGCG, n = 6) after transplantation. (D) Although initially lower at 2 days post transplantation (PBS, n = 6; EGCG, n = 5), EGCG had significantly increased antifibrotic cytokine IP-10 production 2 weeks after transplantation (PBS, n = 6; EGCG, n = 6), *P < .05. All error bars indicate SEM. (Color figure available online.)
Less Fibrosis in EGCG-Treated Recipients
We wanted to evaluate if there was a difference in the fibrosis in the trachea between PBS-controls and EGCG-treated mice. Less fibrosis was present in the airway in Masson’s trichrome staining and significantly less hydroxyproline was detected in the EGCG-treated tracheas compared with the PBS-controls 2 weeks after transplantation (Figure 6A and B; P < .05). These observations coincided with the analysis of profibrotic cytokine TGF-β, which was reduced in the EGCG group both 2 days and 1 week after transplantation (Figure 6C; P < .05). Furthermore, EGCG-treated mice expressed higher level of antifibrotic cytokine IP-10 2 weeks after transplantation (Figure 6D; P < .05).
DISCUSSION
The development of chronic rejection signified by the pathologic diagnosis of OB is the leading cause of death in lung allograft recipients. Current drugs do not prevent chronic rejection and they are associated with significant side effects such as renal impairment, hypertension, dyslipiemia, and increased risk of lymphoproliferative disorder [23, 24]. Search for effective and safe immune suppressants to improve lung transplant survival is warranted. Over the past years several experimental models of OB have been tested in animals. Single-lung transplants were performed in rats and pigs. However, the animals mostly developed acute rejection and either did not develop or infrequently developed OB [2]. Subsequently, a heterotopic tracheal transplant model was developed where the trachea from a MHC-mismatched mouse was transplanted subcutaneously into another mouse [18, 25]. In this model, epithelial injury and airway obliteration similar to human OB could be reliably reproduced. There are limitations to the heterotopic tracheal transplant model: the transplanted tracheal allograft represents large airways, has no direct blood supply, and is not functional. Despite the clear limitations to the heterotopic tracheal transplant model, its similarities to human OB pathology make it very useful for studying the airway rejection [8, 26]. More recently, murine lung transplantation was accomplished, but airway epithelial injury and chronic rejection similar to OB were not observed [27–29].
Green tea has been reported to possess remarkable anti-inflammatory and cancer chemopreventive effects in many animal models, cell culture systems, and epidemiological studies. These biological effects of green tea are mediated by tea polyphenols, also known as tea catechins. EGCG is the major polyphenol present in green tea [30]. Apart from its antioxidant properties, EGCG inhibits several signaling pathways that regulate the expression of proinflammatory cytokines, chemokines, and adhesion molecules [31, 32]. In this study, we found that EGCG was effective in ameliorating inflammation associated with heterotopic tracheal transplantation. EGCG treatment significantly reduced neutrophil and macrophage infiltration, and significantly decreased levels of proinflammatory and proangiogenic mediators KC, IL-17, TNF-α, MCP-1, and VEGF. Furthermore, EGCG treatment decreased fibrosis, suppressed profibrotic TGF-β, and augmented antifibrotic IP-10 tissue expression. Hence, our data demonstrate that EGCG may be beneficial in the development of obliterative airway disease through selective immunomodulatory effects.
Accumulated experience from human and animal studies indicates that both alloimmune and nonalloimmune injuries to the airway epithelium trigger an influx of inflammatory cells and the secretion of proinflammatory cytokines (e.g., IL-2, IL-6, and TNF-α) and chemokines (e.g. IL-8, RANTES, and MCP-1) by epithelial cells, T cells, and activated macrophages. The involvement of these factors results in further excessive activation of innate immunity, abnormal angiogensis, and failure of appropriate epithelial regeneration and fibroproliferative tissue remodeling [33, 34]. Moreover, Sumpter and Wilkes [35] have reported that during lung transplantation associated with injury, collagen V may be exposed to the immune system, which results in the generation of an autoimmune response. This was found to be dependent on the cytokine IL-17, as well as TNF-α and IL-1β. IL-17 is produced by Th17, a newly defined subset of T-helper cells, and is chemotactic for neutrophils. Interestingly, blocking IL-17 diminished the severity of the airway injury and fibrosis in a post–lung transplantation chronic rejection model induced by anti-MHC class I antibodies [36]. In the present study, the level of IL-17 was significantly reduced in EGCG-treated mice 2 days post transplantation. Its level had no significant increase and was the same as the PBS-control 2 weeks post transplantation. Apparently this level of IL-17 had no adverse effect on inflammatory cell recruitment. Indeed, a recent study has identified IL-17 as an active participant in the early inflammatory cascade following allograft transplantation in fully immunocompetent recipients [37].
EGCG was shown to increase stability of the triple helical structure of collagen, which renders collagen resistant to cleavage by collagenase [38]. By treating collagen with EGCG, we previously demonstrated that cleavage of collagen was important for matrix metalloproteinase-8 (MMP-8)–mediated neutrophil migration, and neutrophil collagenase MMP-8 knockout could ameliorate airway lumen obliteration and collagen deposition in the same mouse heterotopic airway transplant model of OB [39]. Thus, prevention of neutrophil recruitment, accumulation, and migration appear to be effective approaches to protect against the development of OB.
The primary basis for rejection of solid organ is host recognition of non-self-donor antigens or the alloimmune response. The immune response developed against mismatched MHC molecules of the lung allograft is initiated by a T-cell response [40]. Green tea EGCG has been shown to directly suppress T-cell proliferation through impairment of IL-2/IL-2 receptor signaling [41, 42]. Here in our heterotopic tracheal allograft model of OB, IP-10 demonstrated a bimodal response. The initial decrease in IP-10 2 days after transplantation may be helpful in reducing inflammatory damage, as it is chemoattractive for T lymphocytes [43]. Increase in IP-10 at 2 weeks may be beneficial, as it acts as an antifibrotic mediator [44]. In addition, at 2 weeks significantly lower levels of TGF-β may be beneficial in preventing the fibroproliferative response [45, 46].
Furthermore, in our study we observed lower levels of VEGF in the transplant in EGCG-treated mice 2 days post transplantation. VEGF has been associated with both acute and chronic allograft rejection, and potentiates fibrogenesis in animal models. VEGF is believed to promote obliterative airway disease. Studies suggested that VEGF may increase chemotaxis of inflammatory cells and promote luminal scarring and the progression of OB [47, 48]. Subsequently, the blockade of VEGF, along with platelet-derived growth factor, has been shown to prevent luminal occlusion of tracheal allografts [49]. In addition, we have observed decreased angiogenesis in the EGCG-treated mice. Angiogenesis-mediated fibroproliferation has been shown to be crucial in development of OB [9, 50]. Our study has shown that EGCG decreases both KC (CXCL1) and VEGF, which are proangiogenesis mediators [9, 50, 51]. In addition, the increase in IP-10 could also be beneficial, as it is angiostatic [52–54].
In summary, factors specific to the lung and its constant interactions with the environment contribute to the very high prevalence of lung allograft rejection. This study provides evidence that EGCG inhibits neutrophil and macrophage recruitment, and suppresses proinflammatory, proangiogenic, and profibrotic mediators. Thus green tea EGCG may ameliorate the development of obliterative airway disease, and may increase the overall success of lung transplantation. Targeting the precise biological mechanisms of chronic rejection is critical to ultimately improving long-term lung transplant outcomes.
Acknowledgments
This work was supported by the NIH grant 1 K08 HL084242-01 (M.S.) and Harry Shwachman Cystic Fibrosis Clinical Investigator Award SUBRAM06Q0 (M.S.). The authors thank Dr. M. Sayegh for generous support.
Footnotes
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
REFERENCES
- 1.Christie JD, Edwards LB, Aurora P, Dobbels F, Kirk R, Rahmel AO, Taylor DO, Kucheryavaya AY, Hertz MI. Registry of the International Society for Heart and Lung Transplantation: twenty-fifth official adult lung and heart/lung transplantation report—2008. J Heart Lung Transplant. 2008;27:957–969. doi: 10.1016/j.healun.2008.07.018. [DOI] [PubMed] [Google Scholar]
- 2.Boehler A, Estenne M. Post-transplant bronchiolitis obliterans. Eur Respir J. 2003;22:1007–1018. doi: 10.1183/09031936.03.00039103. [DOI] [PubMed] [Google Scholar]
- 3.Jaramillo A, et al. Immune mechanisms in the pathogenesis of bronchiolitis obliterans syndrome after lung transplantation. Pediatr Transplant. 2005;9:84–93. doi: 10.1111/j.1399-3046.2004.00270.x. [DOI] [PubMed] [Google Scholar]
- 4.Miura M, El-Sawy T, Fairchild RL. Neutrophils mediate parenchymal tissue necrosis and accelerate the rejection of complete major histocompatibility complex-disparate cardiac allografts in the absence of interferon-gamma. Am J Pathol. 2003;162:509–519. doi: 10.1016/s0002-9440(10)63845-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.DiGiovine B, Lynch JP, 3rd, Martinez FJ, Flint A, Whyte RI, Iannettoni MD, Arenberg DA, Burdick MD, Glass MC, Wilke CA, et al. Bronchoalveolar lavage neutrophilia is associated with obliterative bronchiolitis after lung transplantation: role of IL-8. J Immunol. 1996;157:4194–4202. [PubMed] [Google Scholar]
- 6.Riise GC, Williams A, Kjellstrom C, Schersten H, Andersson BA, Kelly FJ. Bronchiolitis obliterans syndrome in lung transplant recipients is associated with increased neutrophil activity and decreased antioxidant status in the lung. Eur Respir J. 1998;12:82–88. doi: 10.1183/09031936.98.12010082. [DOI] [PubMed] [Google Scholar]
- 7.Elssner A, Jaumann F, Dobrnann S, Behr J, Schwaiblmair M, Reichenspurner H, Furst H, Briegel J, Vogelmeier C. Elevated levels of interleukin-8 and transforming growth factor-beta in bronchoalveolar lavage fluid from patients with bronchiolitis obliterans syndrome: proinflammatory role of bronchial epithelial cells. Munich Lung Transplant Group. Transplantation. 2000;70:362–367. doi: 10.1097/00007890-200007270-00022. [DOI] [PubMed] [Google Scholar]
- 8.McDyer JF. Human and murine obliterative bronchiolitis in transplant. Proc Am Thorac Soc. 2007;4:37–43. doi: 10.1513/pats.200605-107JG. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Belperio JA, Keane MP, Burdick MD, Gomperts B, Xue YY, Hong K, Mestas J, Ardehali A, Mehrad B, Saggar R, et al. Role of CXCR2/CXCR2 ligands in vascular remodeling during bronchiolitis obliterans syndrome. J Clin Invest. 2005;115:1150–1162. doi: 10.1172/JCI24233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Belperio JA, Keane MP, Burdick MD, Lynch JP, 3rd, Xue YY, Berlin A, Ross DJ, Kunkel SL, Charo IF, Strieter RM. Critical role for the chemokine MCP-1/CCR2 in the pathogenesis of bronchiolitis obliterans syndrome. J Clin Invest. 2001;108:547–556. doi: 10.1172/JCI12214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Belperio JA, Burdick MD, Keane MP, Xue YY, Lynch JP, 3rd, Daugherty BL, Kunkel SL, Strieter RM. The role of the CC chemokine, RANTES, in acute lung allograft rejection. J Immunol. 2000;165:461–472. doi: 10.4049/jimmunol.165.1.461. [DOI] [PubMed] [Google Scholar]
- 12.Wynn TA. Common and unique mechanisms regulate fibrosis in various fibroproliferative diseases. J Clin Invest. 2007;117:524–529. doi: 10.1172/JCI31487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ramirez AM, Shen Z, Ritzenthaler JD, Roman J. Myofibroblast transdifferentiation in obliterative bronchiolitis: TGF-beta signaling through Smad3-dependent and -independent pathways. Am J Transplant. 2006;6:2080–2088. doi: 10.1111/j.1600-6143.2006.01430.x. [DOI] [PubMed] [Google Scholar]
- 14.Frei B, Higdon JV. Antioxidant activity of tea polyphenols in vivo: evidence from animal studies. J Nutr. 2003;133:3275S–3284S. doi: 10.1093/jn/133.10.3275S. [DOI] [PubMed] [Google Scholar]
- 15.Meng M, Li YQ, Yan MX, Kou Y, Ren HB. Effects of epigallocatechin gallate on diethyldithiocarbamate-induced pancreatic fibrosis in rats. Biol Pharm Bull. 2007;30:1091–1096. doi: 10.1248/bpb.30.1091. [DOI] [PubMed] [Google Scholar]
- 16.Kim HR, Park BK, Oh YM, Lee YS, Lee DS, Kim HK, Kim JY, Shim TS, Lee SD. Green tea extract inhibits paraquat-induced pulmonary fibrosis by suppression of oxidative stress and endothelin-l expression. Lung. 2006;184:287–295. doi: 10.1007/s00408-005-2592-x. [DOI] [PubMed] [Google Scholar]
- 17.March TH, Wilder JA, Esparza DC, Cossey PY, Blair LF, Herrera LK, McDonald JD, Campen MJ, Mauderly JL, Seagrave J. Modulators of cigarette smoke-induced pulmonary emphysema in A/J mice. Toxicol Sci. 2006;92:545–559. doi: 10.1093/toxsci/kfl016. [DOI] [PubMed] [Google Scholar]
- 18.Hertz MI, Jessurun J, King MB, Savik SK, Murray JJ. Reproduction of the obliterative bronchiolitis lesion after heterotopic transplantation of mouse airways. Am J Pathol. 1993;142:1945–1951. [PMC free article] [PubMed] [Google Scholar]
- 19.Woessner JF., Jr The determination of hydroxyproline in tissue and protein samples containing small proportions of this imino acid. Arch Biochem Biophys. 1961;93:440–447. doi: 10.1016/0003-9861(61)90291-0. [DOI] [PubMed] [Google Scholar]
- 20.Klebanoff SJ. Myeloperoxidase: friend and foe. J Leukoc Biol. 2005;77:598–625. doi: 10.1189/jlb.1204697. [DOI] [PubMed] [Google Scholar]
- 21.Murikinati S, Juttler E, Keinert T, Ridder DA, Muhammad S, Waibler Z, Ledent C, Zimmer A, Kalinke U, Schwaninger M. Activation of cannabinoid 2 receptors protects against cerebral ischemia by inhibiting neutrophil recruitment. FASEB J. 2010;24:788–798. doi: 10.1096/fj.09-141275. [DOI] [PubMed] [Google Scholar]
- 22.Borthwick DW, Shahbazian M, Krantz QT, Dorin JR, Randell SH. Evidence for stem-cell niches in the tracheal epithelium. Am J Respir Cell Mol Biol. 2001;24:662–670. doi: 10.1165/ajrcmb.24.6.4217. [DOI] [PubMed] [Google Scholar]
- 23.Trulock EP. Lung transplantation. Am J Respir Crit Care Med. 1997;155:789–818. doi: 10.1164/ajrccm.155.3.9117010. [DOI] [PubMed] [Google Scholar]
- 24.Belperio JA, Weigt SS, Fishbein MC, Lynch JP., 3rd Chronic lung allograft rejection: mechanisms and therapy. Proc Am Thorac Soc. 2009;6:108–121. doi: 10.1513/pats.200807-073GO. [DOI] [PubMed] [Google Scholar]
- 25.Hele DJ, Yacoub MH, Belvisi MG. The heterotopic tracheal allograft as an animal model of obliterative bronchiolitis. Respir Res. 2001;2:169–183. doi: 10.1186/rr55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sato M, Keshavjee S, Liu M. Translational research: animal models of obliterative bronchiolitis after lung transplantation. Am J Transplant. 2009;9:1981–1987. doi: 10.1111/j.1600-6143.2009.02770.x. [DOI] [PubMed] [Google Scholar]
- 27.Krupnick AS, Lin X, Li W, Okazaki M, Lai J, Sugimoto S, Richardson SB, Kornfeld CG, Garbow JR, Patterson GA, et al. Orthotopic mouse lung transplantation as experimental methodology to study transplant and tumor biology. Nat Protocol. 2009;4:86–93. doi: 10.1038/nprot.2008.218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Okazaki M, Gelman AE, Tietjens JR, Ibricevic A, Kornfeld CG, Huang HJ, Richardson SB, Lai J, Garbow JR, Patterson GA, et al. Maintenance of airway epithelium in acutely rejected orthotopic vascularized mouse lung transplants. Am J Respir Cell Mol Biol. 2007;37:625–630. doi: 10.1165/rcmb.2007-0257RC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Okazaki M, Krupnick AS, Kornfeld CG, Lai JM, Ritter JH, Richardson SB, Huang HJ, Das NA, Patterson GA, Gelman AE, et al. A mouse model of orthotopic vascularized aerated lung transplantation. Am J Transplant. 2007;7:1672–1679. doi: 10.1111/j.1600-6143.2007.01819.x. [DOI] [PubMed] [Google Scholar]
- 30.Yang CS, Maliakal P, Meng X. Inhibition of carcinogenesis by tea. Annu Rev Pharmacol Toxicol. 2002;42:25–54. doi: 10.1146/annurev.pharmtox.42.082101.154309. [DOI] [PubMed] [Google Scholar]
- 31.Na HK, Surh YJ. Intracellular signaling network as a prime chemopreventive target of (−)-epigallocatechin gallate. Mol Nutr Food Res. 2006;50:152–159. doi: 10.1002/mnfr.200500154. [DOI] [PubMed] [Google Scholar]
- 32.Abboud PA, Hake PW, Burroughs TJ, Odoms K, O’Connor M, Mangeshkar P, Wong HR, Zingarelli B. Therapeutic effect of epigallocatechin-3-gallate in a mouse model of colitis. Eur J Pharmacol. 2008;579:411–417. doi: 10.1016/j.ejphar.2007.10.053. [DOI] [PubMed] [Google Scholar]
- 33.Shilling RA, Wilkes DS. Immunobiology of chronic lung allograft dysfunction: new insights from the bench and beyond. Am J Transplant. 2009;9:1714–1718. doi: 10.1111/j.1600-6143.2009.02690.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Grossman EJ, Shilling RA. Bronchiolitis obliterans in lung transplantation: the good, the bad, and the future. Transl Res. 2009;153:153–165. doi: 10.1016/j.trsl.2009.01.005. [DOI] [PubMed] [Google Scholar]
- 35.Sumpter TL, Wilkes DS. Role of autoimmunity in organ allograft rejection: a focus on immunity to type V collagen in the pathogenesis of lung transplant rejection. Am J Physiol Lung Cell Mol Physiol. 2004;286:L1129–L1139. doi: 10.1152/ajplung.00330.2003. [DOI] [PubMed] [Google Scholar]
- 36.Fukarni N, Ramachandran S, Saini D, Walter M, Chapman W, Patterson GA, Mohanakumar T. Antibodies to MHC class I induce autoimmunity: role in the pathogenesis of chronic rejection. J Immunol. 2009;182:309–318. doi: 10.4049/jimmunol.182.1.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gorbacheva V, Fan R, Li X, Valujskikh A. Interleukin-17 promotes early allograft inflammation. Am J Pathol. 2010;177:1265–1273. doi: 10.2353/ajpath.2010.091106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Goo HC, Hwang YS, Choi YR, Cho HN, Suh H. Development of collagenase-resistant collagen and its interaction with adult human dermal fibroblasts. Biomaterials. 2003;24:5099–5113. doi: 10.1016/s0142-9612(03)00431-9. [DOI] [PubMed] [Google Scholar]
- 39.Khatwa UA, Kleibrink BE, Shapiro SD, Subramaniam M. MMP-8 promotes polymorphonuclear cell migration through collagen barriers in obliterative bronchiolitis. J Leukoc Biol. 2010;87:69–77. doi: 10.1189/jlb.0509361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Martinu T, Chen DF, Palmer SM. Acute rejection and humoral sensitization in lung transplant recipients. Proc Am Thorac Soc. 2009;6:54–65. doi: 10.1513/pats.200808-080GO. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wu D, Guo Z, Ren Z, Guo W, Meydani SN. Green tea EGCG suppresses T cell proliferation through impairment of IL-2/IL-2 receptor signaling. Free Radic Biol Med. 2009;47:636–643. doi: 10.1016/j.freeradbiomed.2009.06.001. [DOI] [PubMed] [Google Scholar]
- 42.Pae M, Ren Z, Meydani M, Shang F, Meydani SN, Wu D. Epigallocatechin-3-gallate directly suppresses T cell proliferation through impaired IL-2 utilization and cell cycle progression. J Nutr. 2010;140:1509–1515. doi: 10.3945/jn.110.124743. [DOI] [PubMed] [Google Scholar]
- 43.Farber JM. Mig and IP-10: CXC chemokines that target lymphocytes. J Leukoc Biol. 1997;61:246–257. [PubMed] [Google Scholar]
- 44.Frangogiannis NG. Chemokines in the ischemic myocardium: from inflammation to fibrosis. Inflamm Res. 2004;53:585–595. doi: 10.1007/s00011-004-1298-5. [DOI] [PubMed] [Google Scholar]
- 45.Weiss CH, Budinger GR, Mutlu GM, Jain M. Proteasomal regulation of pulmonary fibrosis. Proc Am Thorac Soc. 2010;7:77–83. doi: 10.1513/pats.200906-055JS. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Halwani R, Al-Muhsen S, Al-Jahdali H, Hamid Q. Role of TGF-{beta} in airway remodeling in asthma. Am J Respir Cell Mol Biol. 2011;44:127–133. doi: 10.1165/rcmb.2010-0027TR. [DOI] [PubMed] [Google Scholar]
- 47.Krebs R, Tikkanen JM, Nykanen AI, Wood J, Jeltsch M, Yla-Herttuala S, Koskinen PK, Lemstrom KB. Dual role of vascular endothelial growth factor in experimental obliterative bronchiolitis. Am J Respir Crit Care Med. 2005;171:1421–1429. doi: 10.1164/rccm.200408-1001OC. [DOI] [PubMed] [Google Scholar]
- 48.Krebs R, Hollmen ME, Tikkanen JM, Wu Y, Hicklin DJ, Koskinen PK, Lemstrom KB. Vascular endothelial growth factor plays a major role in development of experimental obliterative bronchiolitis. Transplant Proc. 2006;38:3266–3267. doi: 10.1016/j.transproceed.2006.10.087. [DOI] [PubMed] [Google Scholar]
- 49.Tikkanen JM, Hollmen M, Nykanen AI, Wood J, Koskinen PK, Lemstrom KB. Role of platelet-derived growth factor and vascular endothelial growth factor in obliterative airway disease. Am J Respir Crit Care Med. 2006;174:1145–1152. doi: 10.1164/rccm.200601-044OC. [DOI] [PubMed] [Google Scholar]
- 50.Douglas IS, Nicolls MR. Chemokine-mediated angiogenesis: an essential link in the evolution of airway fibrosis? J Clin Invest. 2005;115:1133–1136. doi: 10.1172/JCI25193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ferrara N. Vascular endothelial growth factor. Arterioscler Thromb Vasc Biol. 2009;29:789–791. doi: 10.1161/ATVBAHA.108.179663. [DOI] [PubMed] [Google Scholar]
- 52.Angiolillo AL, Sgadari C, Taub DD, Liao F, Farber JM, Maheshwari S, Kleinman HK, Reaman GH, Tosato G. Human interferon-inducible protein 10 is a potent inhibitor of angiogenesis in vivo. J Exp Med. 1995;182:155–162. doi: 10.1084/jem.182.1.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Keane MP, Arenberg DA, Lynch JP, 3rd, Whyte RI, Iannettoni MD, Burdick MD, Wilke CA, Morris SB, Glass MC, DiGiovine B, et al. The CXC chemokines, IL-8 and IP-10, regulate angiogenic activity in idiopathic pulmonary fibrosis. J Immunol. 1997;159:1437–1443. [PubMed] [Google Scholar]
- 54.Tager AM, Kradin RL, LaCamera P, Bercury SD, Campanella GS, Leary CP, Polosukhin V, Zhao LH, Sakamoto H, Blackwell TS, et al. Inhibition of pulmonary fibrosis by the chemokine IP-10/CXCL10. Am J Respir Cell Mol Biol. 2004;31:395–404. doi: 10.1165/rcmb.2004-0175OC. [DOI] [PubMed] [Google Scholar]