Abstract
In the present paper, we report that PLA2G4A (Group IVA phospholipase A2) is important in the development and function of rodent testes. Interstitial cells of rat testes had high PLA2 (phospholipase A2) activity that was very sensitive to the PLA2G4A-preferential inhibitor AACOCF3 (arachidonyl trifluoromethyl ketone). PLA2G4A protein was expressed primarily in the interstitial cells of wild-type mouse testes throughout maturation. Although Pla2g4a knockout (Pla2g4a−/− ) male mice are fertile, their sexual maturation was delayed, as indicated by cauda epididymal sperm count and seminal vesicle development. Delayed function of Pla2g4a−/− mice testes was associated with histological abnormalities including disorganized architecture, swollen appearance and fewer interstitial cells. Basal secretion of testosterone was attenuated significantly and steroidogenic response to hCG (human chorionic gonadotropin) treatment was reduced in Pla2g4a−/− mice compared with their Pla2g4a+/+ littermates during the sexual maturation period. Chemical inhibition of PLA2G4A activity by AACOCF3 or pyrrophenone significantly reduced hCG-stimulated testosterone production in cultured rat interstitial cells. AACOCF3 inhibited forskolin- and cAMP analogue-stimulated testosterone production. These results provide the first evidence that PLA2G4A plays a role in male testes physiology and development. These results may have implications for the potential clinical use of PLA2G4A inhibitors.
Keywords: arachidonic acid, Group IVA phospholipase A2 (PLA2G4A), Leydig cell, sexual maturation, testosterone
INTRODUCTION
PLA2s (phospholipases A2) are a growing family of enzymes that function in lipid digestion, microbial degradation, membrane remodelling and signal transduction [1]. More than 20 isoforms of mammalian PLA2s with different primary structure, Ca2+ dependence and substrate specificity have been identified and are divided into five groups: the sPLA2s (secretory PLA2s), the cPLA2s (cytosolic PLA2s), the iPLA2s (Ca2+ -independent PLA2s), PAF-AHs (platelet-activating factor acetylhydrolases) and the lysosomal PLA2s. These PLA2s have highly diverse regulatory mechanisms and different expression, distribution and biochemical actions [1].
PLA2s hydrolyse a fatty acid from the sn-2 position of membrane phospholipids, a site enriched in AA (arachidonic acid). AA is as an important intracellular signalling molecule [2] and serves as a precursor of eicosanoids that are pleiotropic lipid mediators [3,4]. AA is metabolized primarily by two different groups of enzymes, PTGSs (cyclo-oxygenases) and ALOXs (lipoxygenases). Metabolic products include PGs (prostaglandins), thromboxanes, hydroxyeicosatetraenoic acids and leukotrienes. PLA2G4A (Group IVA PLA2) is considered very important for reasons that include its preference for AA-containing phospholipids and the fact that physiological increases in cytosolic free Ca2+ cause translocation of PLA2G4A to the membrane compartments (in particular the nuclear envelope, endoplasmic reticulum and Golgi body) [1,5], where PTGS-2 and ALOX5 also preferentially localize [6,7].
The varied and important roles for PGs in mammalian reproduction have been widely investigated in females. Extensive studies with genetic and pharmacological ablation of PG synthases or receptors have clearly demonstrated the essential or crucial roles of PGs in early pregnancy (ovulation, fertilization, implantation and decidualization) and in parturition [4,8]. Even though PGs were first identified in human seminal plasma [9], the roles of PLA2s and their metabolites in male reproduction has remained largely undefined until very recently. A significant amount of PLA2 is secreted into seminal plasma [10–12]. Phospholipid membrane degradation and other signalling mechanisms elaborated through PLA2s have been implicated in the capacitation [13,14], acrosome reaction [15–17] and fertilization [18,19] functions of sperm. In previous studies the expression of several sPLA2s (PLA2G2C, PLA2G2F and PLA2G12) [20–22] and a iPLA2 (PLA2G6) [23,24] have been identified in the testes. A comprehensive and well-defined immunohistochemical study by Masuda et al. [25] revealed that PLA2G2C, G2D, G2E, G2F, G5 and G10 were diversely expressed in spermatogenic cells within the seminiferous tubules, and that PLA2G2F, G5 and G10 were localized in the interstitial Leydig cells of mouse testes. More recent studies using gene-knockout techniques have clearly identified the PLA2 isoforms with indispensable roles in male reproduction. Spermatogenesis within the testis is severely impaired by the absence of PLA2G13B [26–28]. Sperm maturation during the epididymal transit required the action of epithelial cell-derived PLA2G3 [29]. Also in released sperm cells, PLA2G6A and PLA2G10 [30,31] contributed profoundly to motility, capacitation and the acrosome reaction, all of which are essential for the sperm to fertilize eggs. However, our knowledge regarding the functional significance of many other PLA2 enzymes in male reproduction remains incomplete. It remains unclear whether PLA2G4A is present in testes or has functional significance in male reproduction. This contrasts with the well-defined roles of PLA2G4A in the female reproductive system [32–34].
In the present study, we examined the role of PLA2G4A in male reproduction by using Pla2g4a knockout (Pla2g4a−/− ) and littermate Pla2g4a+/+ mice [33]. To complement these studies in genetically engineered mice, we carried out studies in normal rats. We show the presence of PLA2G4A activity in rodent testes and provide the first in vivo evidence of its functional significance in the male reproductive system. The present paper reports that PLA2G4A is necessary for the normal time sequence of sexual maturation and also defines the pathway by which PLA2G4A regulates testicular steroidogenic activity.
EXPERIMENTAL
Chemicals and antibodies
The specific antibodies against human PLA2G4A used in the Western blot analysis and immunohistochemistry were provided by Dr Andrey Cybulsky (McGill University, Montreal, Quebec, Canada) and Wyeth respectively. The radiolabelled PC (phosphatidylcholine) (1-stearoyl-2-[5,6,8,9,11,12,14,15-3H]arachidonyl PC) and [1,2,6,7-3H]testosterone were obtained from DuPont-NEN and Amersham Biosciences respectively. AACOCF3 (arachidonyl trifluoromethyl ketone; an inhibitor of PLA2G4A [1]), BEL (bromoenol lactone; an inhibitor of iPLA2 [1]) and EIA (enzyme immunoassay) kits for PGE2 and testosterone were purchased from Cayman Chemical. Pyrrophenone, the highly specific inhibitor of PLA2G4A [1], was donated by Dr Takashi Ono (Shionogi Research Laboratory, Osaka, Japan) [35]. Forskolin, dbcAMP (dibutyryl cAMP), AA, indomethacin and NDGA (nordihydroguaiaretic acid) were purchased from Sigma. hCG (human chorionic gonadotropin) which has LH (luteinizing hormone) activity was purchased from Sigma and Sankyo. All reagents were of analytical grade.
Animals
Genetically engineered mice were generated by gene targeting in C57BL/6J mouse embryonic cells to disrupt an exon of the Pla2g4 gene that resulted in creation of a null allele [33]. Heterozygotes were interbred to produce homozygous null mice. Mice were genotyped by PCR analysis of genomic DNA isolated from tail biopsies. All experiments comparing wild-type (Pla2g4a+/+ ), heterozygous (Pla2g4a+/− ) and knockout (Pla2g4a−/− ) mice used littermates or age-matched animals derived from the same breeding colony. Adult male rats of the Wistar–Imamichi strain used in the present study were 3–5 months old and ranged from 400 to 500 g in body mass. Animal handling and all procedures employed in this study were carried out following the Guidelines of the Animal Care and Use Committee of Massachusetts General Hospital and Harvard Medical School, and of Kitasato University School of Veterinary Medicine.
Analyses of male reproductive functions of Pla2g4a−/− mice
Male mice usually experience puberty during 4–6 weeks of age and become competent to breed in the laboratory by 8 weeks of age. To evaluate the impact of the Pla2g4a gene on the development of the male reproductive system, mice of the three genotypes were studied at peripubertal (38–48 days old), mature (70–90 days old) and fully mature (119–141 days old) stages. Under isoflurane anaesthesia, blood was taken via cardiac puncture. Following killing of the mice by an overdose of pentobarbitol, pairs of testes, epididymides and seminal vesicles were removed rapidly and weighed. Tissues were fixed with Bouin’s fixative for standard histology or kept frozen (at −20°C) for subsequent analyses. Spermatogenesis was determined by counting cauda epididymal sperm. Each pair of cauda epididymides was cut into pieces in DMEM (Dulbecco’s modified Eagle’s medium) to release the sperm. Sperm were inactivated by a 1:10 dilution with 4% NaCl solution and their numbers were counted using a haemocytometer.
Isolation of testicular interstitial cells and seminiferous tubules
Fractions of interstitial cells and seminiferous tubules were prepared from rats by modification as described previously [36]. Adult rats were killed and the testes were removed, decapsulated and dispersed by shaking (160 cycles/min at 34°C, for 15 min) in a polypropylene tube containing enzyme solution (two testes in 5.0 ml of solution containing 1 mg/ml collagenase and 1 mg/ml BSA in Ca2+ and Mg2+ -free PBS solution). The dispersed tissues were diluted with Buffer A (25 mM Hepes, 0.7 mM Na2HPO4, 5 mM KCl and 140 mM NaCl), and the seminiferous tubules were allowed to settle. The remaining supernatant (interstitial cell fraction) and pellet (seminiferous tubule fraction) were collected separately, centrifuged (200 g for 5 min at 20°C) and resuspended in 0.25 M sucrose, 0.25 mM EDTA and 0.05 M Tris/HCl (pH 9.0). The tissues and cells were disrupted by sonication (output 25 W for 10 s at 4°C, 3 times). After centrifugation (10000 g for 20 min at 4°C), the supernatant was processed immediately for the analysis of PLA2 activity. Interstitial cell fractions of testes of 70-day-old wild-type mice were also prepared.
Western blot analysis
Western blot analysis of testicular PLA2G4A was performed as described previously [37]. Briefly, tissue samples were homogenized in lysis buffer [20 mM Hepes (pH 7.4), 2 mM EGTA, 1 mM DTT (dithiothreitol), 1 mM Na3VO4, 1% Triton X-100, 10% glycerol, 2 μM leupeptin, 400 μM PMSF and 50 μM 2-glycerophosphate] and stored at −80°C until processing. Lysates were spun for 5 min at 10000 g at 4°C and 15 μg of supernatant proteins were separated by electrophoresis on SDS/PAGE (10% gel) and transferred on to Immobilon-P membranes. The membrane was incubated with the anti-PLA2G4A antibody (1:500) followed by horseradish peroxidaseconjugated anti-rabbit antibody (1:2000). Spleen samples from the same animals served as positive and negative controls. PLA2G4A was detected by enhanced chemiluminescence (PerkinElmer Life Sciences).
Testosterone secretion in vivo
Plasma testosterone levels of 70–90-day-old mice were determined under basal and stimulated conditions. In the latter case, mice received an single dose of an LH mimic, hCG (5 IU/head, intraperitoneally) and were killed 1 h later. Blood was collected via cardiac puncture and between 10:00 and 14:00 h to reduce possible circadian variation of the hormone level. Additional experiments were conducted using normal rats (3–5 months old) to examine the effect of pre-treatment with indomethacin on the hCG-induced testosterone secretory response. Blood was first taken via cardiac puncture and then the rats were treated with vehicle (0.3 ml of DMSO) or indomethacin (1.0 mg, subcutaneously). At 30 min later, the rats received a single dose of hCG (20 IU, intravenously) and blood was sampled again 1 h after hCG treatment.
Testosterone secretion in vitro
As it was not feasible to prepare a sufficient amount of interstitial cells from mice, we used rat interstitial cells for a detailed study of the steroidogenic mechanisms in vitro. Interstitial cells were prepared from fully mature rats as described above, and placed into primary culture as described previously [36] with some modifications. After separation of interstitial cells from seminiferous tubules, the cell pellet was washed with deionized water and the osmolarity immediately restored with 5× Hanks balanced salt solution. The cell pellet was centrifuged (200 g for 5 min at 20°C) and resuspended in 5% foetal bovine serum (Gibco) in DMEM and filtered through a cell strainer (Beckton Dickinson). The filtrate was counted for cell concentration and viability. The cell preparation had < 3% sperm contaminants and >80% viability. The interstitial cells prepared were plated (2.5 × 105 cells/0.5 ml per well) in 24-well culture plates and pre-incubated at 37°C for 90 min. The medium was then replaced with DMEM and cells were used for experiments.
Interstitial cells were treated with different doses of either AACOCF3 or pyrrophenone (PLA2G4A inhibitors), indomethacin (a PTGS inhibitor) or NDGA (an ALOX inhibitor). Following treatment with an inhibitor or vehicle for 5–10 min, the cells were exposed to hCG (100 m-units/ml). In an additional experiment, the effect of AA supplementation (0.5, 5 and 50 μM) was tested in cells where hCG stimulation was inhibited by 10 μM AACOCF3. The effects of exogenously added AA (0.5, 5 and 50 μM) were also examined in the presence or absence of hCG (100 m-units/ml). The effects of AACOCF3 on testosterone production enhanced by an adenylate cyclase activator, forskolin or the cAMP analogue dbcAMP were also examined. The culture media were collected after 3 h of incubation and stored at −20°C until assayed for testosterone levels.
PLA2 activity assay
PLA2 activities in the cytosolic fraction of whole testes and seminal vesicles of 70–90-day-old mice were measured as described previously [38]. The activities in lysates of interstitial cells of adult rat and mouse and of rat seminiferous tubules were also measured. Briefly, tissues were homogenized in 0.25 M sucrose, 0.25 mM EDTA and 0.05 M Tris/HCl (pH 9.0). The homogenates were centrifuged at 105000 g for 1 h at 4°C, and the supernatants (cytosol) were assayed for protein concentration using a Bio-Rad Laboratories assay kit. Liposomal substrate was prepared with radiolabelled and non-radiolabelled PC at a molar ratio of 1:4. The assay mixtures (200 μl in total volume) contained 0.1 M Tris/HCl (pH 9.0), 4 mM CaCl2, 1 mg/ml fatty-acid-free BSA, 2 μM phospholipid substrate and cytosol. In some experiments, inhibitors were used to characterize PLA2 activity. The reactions were performed at 37°C for 1 h and stopped by adding 1 ml of Dole’s reagent. The released fatty acid was extracted and measured for radioactivity by liquid scintillation counting.
Assay of PGE2 and testosterone
PGE2 levels in seminal vesicles were determined using a commercial EIA kit as described previously [38]. The tissue was homogenized in 0.05 M Tris/HCl (pH 7.4) containing 0.9% NaCl, 0.01% Triton X-100 and 0.0057% thimerosal and then centrifuged at 10000 g for 20 min at 4°C. The supernatant was assayed directly and normalized for protein concentration. Testosterone in the mouse blood plasma was extracted with diethyl ether and assayed using a commercial EIA kit according to the manufacturer’s protocol. Testosterone in the rat blood plasma and culture medium of rat interstitial cells was extracted and measured by RIA.
Histology and immunohistochemistry
The testes, prostate, epididymides and seminal vesicles from Pla2g4a+/+ and Pla2g4a−/− mice of 44–78, 119 and 122 days in age were fixed with Bouin’s fixative overnight, which is known to be suitable for mouse testes to maintain tissue integrity [39]. Tissues were dehydrated with graded ethanol baths and embedded in paraffin wax. Blocks were serially cut at 6–8 μm in thickness. Sections were stained with haematoxylin and eosin.
Testicular expression of immunoreactive PLA2G4A in mature (90-day-old) Pla2g4a+/+ mice was analysed as described previously [38]. The issues were processed and sections were made as described above. Endogenous peroxidase was blocked by pre-treatment with 0.3% H2O2 in methanol for 30 min. Anti-human PLA2G4A antibody (1:500) was applied at room temperature (20°C) for 90 min. Any antigen–antibody complexes were visualized with the Vectastain Elite ABC staining kit (Vector Laboratories) and 3,3′-diaminobenzidine tetrahydrochrolide as peroxidase substrate. Controls were performed with non-specific mouse IgG. Slides were counter-stained with haematoxylin.
Statistical analysis
Results are presented as means ± S.E.M. with the number of experiments indicated. The means among different groups were analysed by one-way ANOVA, Student’s t test or Tukey–Kramer multiple comparison test as appropriate. A P value less than 0.05 was considered significant.
RESULTS
Characterization of testicular PLA2G4A
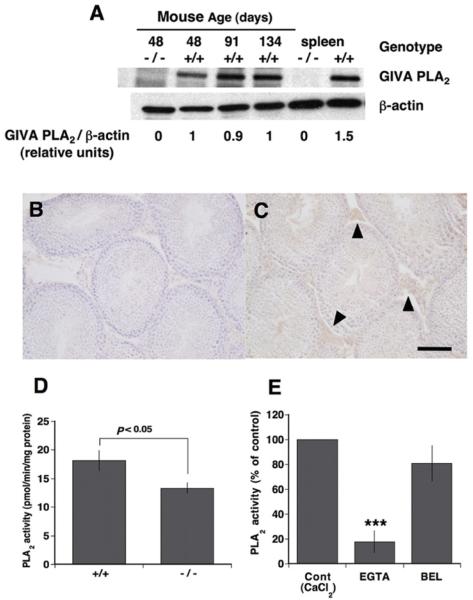
Western blot analyses were performed to determine whether PLA2G4A is expressed by normal mouse testes. A band corresponding to PLA2G4A was apparent in Pla2g4a+/+ testes and spleen, but absent from Pla2g4a−/− organs (Figure 1A). Pla2g4a+/+ testes showed little age-related change in the expression level of PLA2G4A. Immunohistochemistry revealed that immunoreactive signals for PLA2G4A were localized mainly to interstitial tissues and very faintly to the seminiferous tubules of a 90-day-old mouse (Figure 1C) with no reactions in control samples stained using normal IgG (Figure 1B). The PLA2 activity in the cytosol of 70–90-day-old Pla2g4a+/+ mice testes was 18.2 ± 1.8 pmol/min per mg of protein (n = 6). This value was significantly (P < 0.05) higher than that in age-matched Pla2g4a−/− testes (Figure 1D), but the level of PLA2 activity in the knockout mouse indicates that there are other forms of PLA2 present in the mouse testes. The PLA2 activity in Pla2g4a−/− testes was reduced to 17.7% and 80.1% of baseline values by treatment with EGTA and BEL respectively (Figure 1E), indicating that most of the residual PLA2 activity is Ca2+ -dependent.
Figure 1. Mouse testicular expression of PLA2G4A and characterization of enzymatic activity.
(A) Western blot analysis of mouse testicular expression of PLA2G4A. Testes from 48-day-old Pla2g4a−/− mice and from 48-, 91- and 134-day-old Pla2g4a+/+ mice were analysed for expression of PLA2G4A protein. Spleen protein extracts from Pla2g4a−/− and Pla2g4a+/+ mice served as negative and positive controls. β-Actin was used as a loading control. The relative amount of PLA2G4A as determined by densitometry is shown for each condition and does not change with age in the wild-type mice. (B and C) Immunohistochemistry of PLA2G4A in mature (90-day-old) Pla2g4a+/+ mice testis. Immunoreactivity for PLA2G4A was localized to interstitial cells (arrowheads) (C) and was absent from non-specific mouse IgG-applied tissue sections (B). Scale bar, 50 μm. (D) PLA2 activity was measured in cytosolic fractions of total tissue (testes) homogenates of 70–90-day-old Pla2g4a+/+ and Pla2g4a−/− mice (n = 6). The activity of Pla2g4a+/+ testes was significantly higher than that of Pla2g4a−/− testes. (E) Effects of 4 mM CaCl2, 1 mM EGTA or 50 mM BEL on PLA2 activity of Pla2g4a−/− testicular homogenate. Results are means ± S.E.M. (n = 3). ***P < 0.001 compared against the control.
PLA2 activity in lysates of interstitial cells and seminiferous tubules of normal, 3–5-month-old rat testes was determined. Interstitial cells had 4.2-fold higher activity than seminiferous tubule fractions (P < 0.05) (Figure 2A). AACOCF3 and EGTA treatments reduced PLA2 activity in interstitial cells to 42.1% and 62.4% of control cell activity respectively (Figure 2B). By comparison AACOCF3 treatment reduced the PLA2 activity in 70-day-old mice interstitial cells to 37.8% of control activity (results not shown), indicating that the relative amount of AACOCF3-inhibited PLA2s are similar in mouse and rat cell preparations. Treatment of rat seminiferous tubule lysate with EGTA reduced PLA2 activity to 59.7% of control activity, similar to the relative reduction seen in interstitial cells (Figure 2B), whereas the reduction of PLA2 activity by AACOCF3 treatment was less than that seen in interstitial cells (results not shown). These results indicate that the relative amounts of Ca2+ -dependent PLA2s are similar in interstitial cells and seminiferous tubules.
Figure 2. Characterization of PLA2 activity in rat testicular fractions.
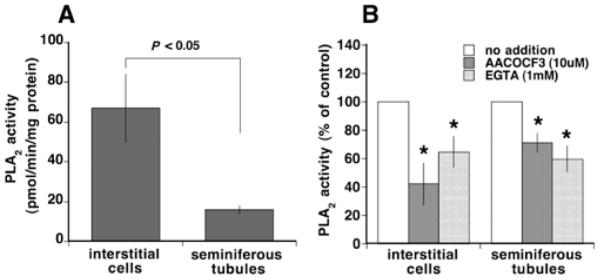
(A) PLA2 activity in extracts of interstitial cells and seminiferous tubules of adult (3–5 months old) rat testes (n = 3). (B) Effects of AACOCF3 (10 μM) or EDTA (1 mM) on PLA2 activities in rat interstitial cells and seminiferous tubules (n = 3). *P < 0.05 compared against the control vehicle-treated group. Results are means ± S.E.M.
Because the seminal vesicle is the principal source of seminal plasma and PGs, we evaluated the impact of Pla2g4a deletion on PLA2 activity and PGE2 levels in seminal fluid of 70–90-day-old mice. The PLA2 activity in Pla2g4a+/+ seminal fluid was significantly higher than that of Pla2g4a−/− seminal fluid (3.4 ± 0.5 compared with 1.7 ± 0.5 pmol/min per mg of protein, n = 6, P < 0.05). PGE2 levels in Pla2g4a+/+ and Pla2g4a−/− seminal fluids were 2.1 ± 1.0 and 1.1 ± 0.3 ng/mg of protein (n = 6) respectively, a difference that did not reach statistical significance.
Reproductive development of Pla2g4a−/− male mice
Given the biochemical evidence for the expression of PLA2G4A in normal murine testes, we evaluated the effects of Pla2g4a deficiency on testes development and function in vivo. Gross examination of Pla2g4a−/− testes revealed no abnormalities in any stages (results not shown), and testicular weight showed age-associated increases in all genotypes without any significant differences between genotypes of any stages (Figure 3A).
Figure 3. Pla2g4a−/− mice have delayed sexual maturation.
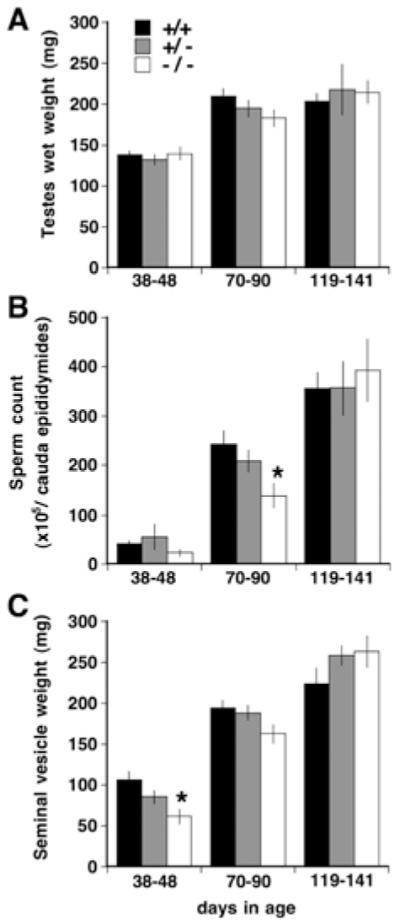
Testicular weight (A), cauda epididymal sperm count (B) and seminal vesicle weight (C) were determined for Pla2g4a+/+ , Pla2g4a+/− and Pla2g4a−/− mice at juvenile (38–48 days old, n = 4–6), maturing (70–90 days old, n = 10–12) and fully mature (119–141 days old, n = 3–5) stages. All results are means ± S.E.M. *P < 0.05 compared with the age-matched Pla2g4a+/+ group. If we combine Pla2g4a+/+ and Pla2g4a+/− mice at 70–90 days old in (C), the difference between animals completely lacking PLA2G4A and those having two or one allele is statistically significant (P = 0.049).
Mouse age and genotype had significant effects on spermatogenesis (Figure 3B). During the pubertal period (38–48 days), the sperm counts in Pla2g4a+/+ and Pla2g4a+/− mice were (42.1 ± 6.2)×105 and (55.8 ± 26.1)×105 (n = 4 or 5) respectively, whereas in Pla2g4a−/− mice it was (23.2 ± 7.1)×105 (n = 5). In mature mice (70–90 days in age), sperm counts in Pla2g4a+/+ and Pla2g4a+/− animals exceeded 200×105. However, sperm counts in the Pla2g4a−/− mice were only (139.7 ± 25.1)×105, a 42.4% reduction compared with Pla2g4a+/+ mice (P < 0.05). In Pla2g4a−/− mice over 119 days old, the sperm count approached 400×105 and was comparable with those of other genotypes.
The growth of the seminal vesicles is dependent on testicular testosterone secretion and therefore seminal vesicle weight is an index of sexual maturation. The seminal vesicle weight of the youngest (38–48 days) Pla2g4a−/− mice was significantly lower than that of Pla2g4a+/+ mice (P < 0.05) (Figure 3C). In mice between 70 and 90 days old, the tissue weight trended lower in the Pla2g4a−/− animals when compared with Pla2g4a+/+ and Pla2g4a+/− animals, but the weights were not statistically different at this age interval. By 119–141 days of age, there were no differences in tissue weight among the three groups.
Taken together these results indicate that PLA2G4A contributes to the timely progression of sexual maturation in male mice. Pla2g4a deletion causes delays in maturation that may be related to decreased levels of AA or eicosanoids in the male testicular apparatus.
Histology of Pla2g4a−/− mice testes
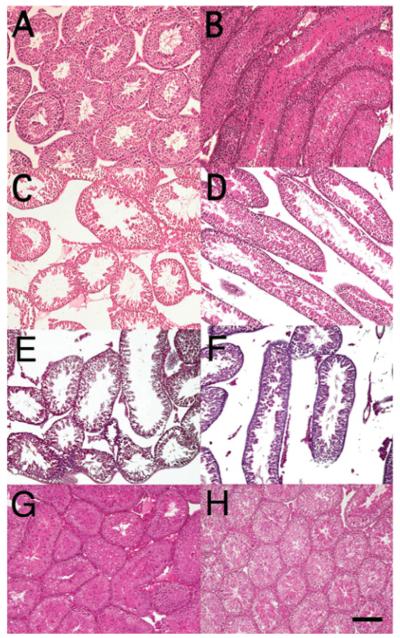
The functional evidence for retardation of spermatogenesis and seminal vesicle growth in Pla2g4a−/− animals led us to investigate the histology of the testes and other reproductive organs. We examined testes from Pla2g4a+/+ and Pla2g4a−/− mice 44–78 days old (peripubertal to young mature periods, n = 7 for each genotype) and from fully mature mice between 119 and 122 days old (n = 2 for each genotype). The Pla2g4a+/+ testes showed a highly integrated seminiferous tubule structure, and within almost all of them a normal complement of the various germ cell types, including elongated spermatids (Figures 4A and 4B). Pla2g4a−/− testes also had elongated spermatids within most seminiferous tubules (Figures 4C and 4D). Pla2g4a−/− testes had marked histological abnormalities including loose and disorganized junctions within and among seminiferous tubules, a reduction in interstitial tissues, and decreased heights of the seminiferous epithelium (Figures 4C–4F). Of the seven Pla2g4a−/− specimens examined, two testes were severely (Figures 4E and 4F) and three were moderately (Figures 4C and 4D) abnormal. In contrast, among seven Pla2g4a+/+ testes, only one testis showed a mild abnormality in seminiferous epithelium structure. The testes of two fully mature (119- and 122-day-old) Pla2g4a−/− mice had sperm counts and seminal vesicle growth comparable with those of age-matched Pla2g4a+/+ and Pla2g4a+/− mice, and showed intact histology with no differences (Figures 4G and 4H). Other reproductive tissues (epididymides, seminal vesicles and prostate) that have simple glandular structures appeared histologically normal in young (~70-day-old) Pla2g4a−/− mice (results not shown).
Figure 4. Histological abnormalities of Pla2g4a−/− testes.
Histological analysis of testes from juvenile 44-day-old Pla2g4a+/+ (A and B) and Pla2g4a−/− (C–F) mice, and fully mature 119-day-old Pla2g4a+/+ (G) and 122-day-old Pla2g4a−/− (H) mice. Representative cross-sections (A, C, E, G and H) and parallel sections (B, D and F) of haematoxylin and eosin-stained seminiferous tubules are shown. Juvenile wild-type testes show a highly integrated structure of seminiferous tubules with normal spermatogenesis within them. In the testes of juvenile Pla2g4a−/− mice, elongated spermatids were also seen. However, the structure of the seminiferous epithelium tends to be disorganized and loose and disrupted junctions are evident among tubules. Pla2g4a−/− testes (H) as well as Pla2g4a+/+ testes (G) of approximately 120-day-old mice appear histologically normal. Scale bar is 100 μm and is applicable for all panels.
Testosterone secretory activity in vivo
To explore the possible cause for the retarded spermatogenesis and seminal vesicle growth seen in pubertal and maturing Pla2g4a−/− mice, we analysed plasma testosterone levels in mice 70–90 days old (Figure 5A). The basal level of testosterone in Pla2g4a+/+ mice was 1.23 ± 0.33 ng/ml, whereas in Pla2g4a−/− mice it was significantly lower (P < 0.05) at 0.36 ± 0.14 ng/ml (n = 7) (Figure 5A). We tested the testicular steroidogenic response to hCG. The Pla2g4a+/+ and Pla2g4a+/− mice responded to a pharmacological dose of hCG with large increases in testosterone levels of >30 ng/ml after 1 h. The Pla2g4a−/− mice also exhibited a significant testosterone response following hCG, but the absolute value was below 20 ng/ml (P = 0.139 compared with the Pla2g4a+/+ group, P = 0.030 compared with the combined Pla2g4a+/+ and Pla2g4a+/− groups) (Figure 5B). These results strongly suggest that delayed sexual maturation in Pla2g4a−/− mice is due, in part, to a defect in testicular testosterone production.
Figure 5. Testosterone secretory activity in vivo is impaired by Pla2g4a deficiency and indomethacin pre-treatment.
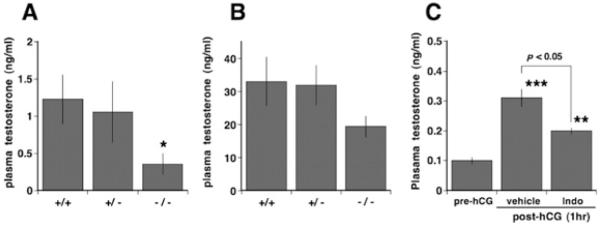
Plasma testosterone was determined in Pla2g4a+/+ , Pla2g4a+/− and Pla2g4a−/− mice of 70–90 days old in basal (untreated) conditions (A) and 1 h after hCG (5 IU/head, intraperitoneally) challenge (B) in vivo. *P < 0.05 compared with Pla2g4a+/+ littermate controls. If we combine Pla2g4a+/+ and Pla2g4a+/− mice in (B), the difference between animals completely lacking Pla2g4a and those having two or one allele is statistically significant (P = 0.03). (C) Blood testosterone levels were also measured in adult rats before and 1 h after treatment with either vehicle (0.3 ml of DMSO) or indomethacin (1 mg, subcutaneously) and subsequent hCG (20 IU, intravenously) 30 min later. Plasma testosterone levels were determined by EIA or RIA. Results are means ± S.E.M. (n = 5–7). **P < 0.01, ***P < 0.001 compared with pre-hCG controls.
To evaluate whether a PG metabolite of AA might be involved in the PLA2G4A-dependent testosterone response, we examined the effect of pharmacological inhibition of the PTGS pathway on the testosterone secretory response to hCG in fully mature rats. Vehicle-treated rats showed a 3.1-fold increase in plasma testosterone level following hCG administration, but this steroidogenic response was significantly reduced by pre-treatment with indomethacin (Figure 5C).
Testosterone secretory activity in vitro
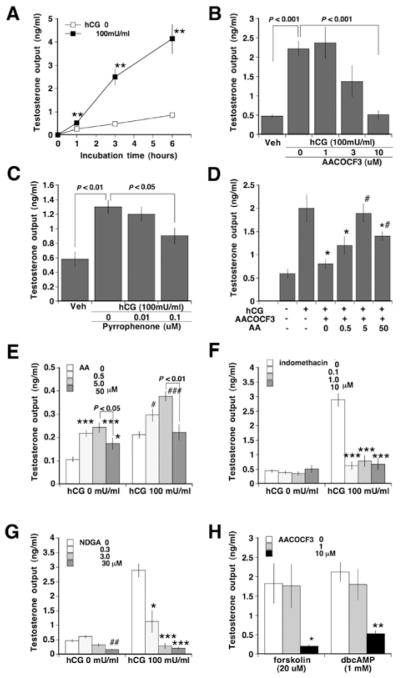
In response to hCG, primary cultured rat testicular interstitial cells showed a dose (0, 0.1, 1.0, 10 and 100 m-units/ml)-dependent increase in testosterone production (results not shown) and a linear increase in testosterone production for up to 6 h of incubation time with hCG, 100 m-units/ml (Figure 6A). hCG (100 m-units/ml) caused a 4.3-fold increase in testosterone production, which was inhibited by AACOCF3 in a dose-dependent fashion (Figure 6B). Almost complete inhibition of hCG stimulation of testosterone production was achieved by 10 μM AACOCF3. Another inhibitor of PLA2G4A, pyrrophenone (0.1 μM) diminished hCG-induced testosterone production up to 40% (Figure 6C). AACOCF3 inhibition of testosterone synthesis was significantly reversed by AA supplementation in a dose-dependent manner (Figure 6D). Exogenous AA (0.5 and 5.0 μM) stimulated testosterone production, but the highest dose (50 μM) exerted an inhibitory effect on steroidogenesis (Figure 6E). Pharmacological inhibition of the PTGS pathway or the ALOX pathway resulted in complete inhibition of hCG stimulation of steroidogenesis (Figures 6F and 6G). NDGA (30 μM) also affected basal testosterone secretion.
Figure 6. Characterization of the mechanism for the PLA2G4A/AA cascade regulation of testosterone production in vitro.
Primary cultures of rat interstitial cells were prepared and characterized. Cells were treated with or without hCG (100 m-units/ml) for up to 6 h (A). Other cells were treated with AACOCF3 (0, 1, 3 or 10 μM) and subsequent hCG (100 m-units/ml) (B). In other cells pyrrophenone (0, 0.01 or 0.1 μM) was added with hCG (100 m-units/ml) (C). AA (0.5, 5 or 50 μM) was exogenously added with hCG (100 m-units/ml) and AACOCF3 (10 μM) (D), or with or without hCG (100 m-units/ml) (E). In other experiments cells were exposed to indomethacin (0, 0.1, 1 or 10 μM) (F) or NDGA (0, 0.3, 3 or 30 μM) (G) prior to treatment with or without hCG (100 m-units/ml). In another group of experiments, the effects of AACOCF3 on forskolin- or dbcAMP-potentiated testosterone production were evaluated (H). Culture media were collected after 3 h of incubation and assayed by RIA. Results are means ± S.E.M. (n = 4–10). *P < 0.05; #P < 0.05; **P < 0.01; ###P < 0.001 compared with the respective control groups.
To study further the mechanism by which AACOCF3 inhibited testicular steroidogenesis we used two reagents that enhance the LH receptor signalling pathway. Both forskolin (0–20 μM), which activates adenylate cyclase, and dbcAMP (0–1 mM), a membrane-permeant cAMP analogue, caused a dose-dependent increase in testosterone production (results not shown). AACOCF3 (10 μM) blocked the stimulated testosterone production by both reagents (P < 0.05) (Figure 6H).
DISCUSSION
The principal findings of the present study are that PLA2G4A is expressed primarily in interstitial tissues of testes and is necessary for the normal production of testosterone and testicular development in the early phase of maturation. Over time the synthetic function, sperm count and morphology of the Pla2g4a−/− mice testes normalized. This may be due to a compensation of other PLA2s or AA-supplying enzyme over time or the entry into a PLA2G4A-independent stage of Leydig cell development [40]. The importance of several PLA2s in male reproduction has been demonstrated in the generation and function of the gamete [24–29], but to date their mechanisms of action are largely undefined. The present study reveals much greater PLA2 activity and immunoreactivity of PLA2G4A in the steroidogenic fraction than in the gametogenic fraction of testicular tissues and importantly identifies PLA2G4A as a PLA2 that contributes to LH-stimulated testosterone production.
The testes contain a large amount of polyunsaturated fatty acids. In rat testes approximately 70% of fatty acids are polyunsaturated and there is a prevalence of AA [41]. The unique AA preference of PLA2G4A distinguishes it from other PLA2s identified in Leydig cells (PLA2G2F, PLA2G3, PLA2G5 or PLA2G10) [25,29] and makes it a good candidate for steroidogenesis. Genetic inactivation of Pla2g4a caused a reduced testosterone secretion in juvenile mice (younger than 90 days old), and we speculate that testosterone insufficiency largely accounts for the reduced sperm counts and seminal vesicle growth in these mice. Retarded function is temporally matched with histological abnormalities including reduced and scattered interstitial tissues. A previous finding that AA has a mitogenic and anti-apoptotic effect on rat testicular Leydig cells in vitro [40] is consistent with our histological and hormonal results, and lends further support for PLA2G4A’s role in vivo. Pla2g4a inactivation resulted in clear abnormalities of seminiferous tubules in most mice less than 78 days old. PLA2G4A and AA regulate structure and function of cellular organelles (Golgi apparatus and rough endoplasmic reticulum) probably through modulating phospholipid metabolism [43,44]. Additionally, testosterone plays a central role in many interactions among Leydig cells, Sertoli cells, germ cells and the vasculature [45]. Testosterone also regulates the assembly and function of Sertoli cell tight junctions and thus the microenvironment of the seminiferous epithelium [46]. Thus genetic inactivation of PLA2G4A-dependent AA generation together with the altered intratesticular testosterone milieu might account for abnormal seminiferous tubules and Sertoli cell–germ cell interaction. In our initial description of the Pla2g4a−/− reproductive phenotype we noted a subtle defect in male fertility [33]. Such a phenotype may be consistent with the molecular and cellular abnormalities described.
AA and eicosanoids of the ALOX pathway are involved in testosterone secretion by Leydig cells [47–50]. It is probable that testosterone production is critically dependent upon AA that is produced primarily by PLA2G4A in juvenile Leydig cells. Over the course of maturation, the activities of other PLA2(s) may develop and compensate for the loss of Pla2g4a in the null mice. This hypothesis is supported by the fact that di-(2-ethylhexyl) phthalate causes testicular atrophy in association with a reduction in PLA2G4A activity and AA in prepubertal (1-month-old) rat testes [51]. Alternatively, the concerted action of mitochondrial acyl-CoA thioesterase and acyl-CoA synthetase may contribute to AA mobilization in testicular Leydig cells in a PLA2-independent pathway [52,53]. Nevertheless, the fact that acute PLA2G4A inhibition by two different drugs attenuated hCG-stimulated testosterone production in primary culture of rat interstitial cells may indicate that in normal circumstances PLA2G4A is an important mediator of testosterone synthesis in mature Leydig cells.
We attempted to delineate the PLA2G4A-dependent signalling pathway for testosterone synthesis using rat Leydig cells. The stimulatory effect of hCG appeared to be mediated by endogenous AA release, and exogenous AA had an additive, but not synergistic, effect on steroidogenesis in vitro. The small inhibition of steroidogenesis by the highest dose (50 μM) of AA is likely to be due to a toxic effect of this fatty acid being manifested at above 25 μM [42]. Interestingly, the blockade of either PTGS or ALOX pathway attenuated hCG stimulation of testosterone production in vitro. Moreover, indomethacin pre-treatment of intact rats reduced the steroidogenic response to hCG in vivo. It is possible that eicosanoid metabolites of both PTGS and ALOX pathways are required for maximal effects on testosterone secretion. Since the PLA2G4A-dependent PTGS/ALOX pathways are also involved in hCG-stimulated progesterone release by rat dispersed corpus luteum cells [54], PLA2G4A would be a common component of LH control of gonadal steroidogenesis. The findings that AA and its metabolites of ALOX and epoxygenase pathways regulate mRNA and protein expression of steroidogenic acute regulatory protein in MA-10 mouse Leydig cells [55–57] support this hypothesis. The results of the present study that AACOCF3 abolishes forskolinand dbcAMP-enhanced testosterone production suggests that PLA2G4A dependence is downstream from increases in cAMP and this implies that eicosanoids are not acting simply through cell-surface receptors. Further elucidation of the signalling events for LH receptor-mediated up-regulation of PLA2G4A activity, identification of the eicosanoid metabolites and their targets in testicular interstitial cells remain for further study.
The impairment of reproductive index and testicular morphology in Pla2g4a−/− mice was evident in pubertal and maturing periods (less than 70–90 days) and did not normalize until mice were fully matured (over 120 days old). We conclude that PLA2G4A is required for early sexual maturation, but becomes redundant via compensation with aging. Testicular PLA2 activity had both Ca2+ -dependent and to a lesser extent Ca2+ -independent components, and a significant PLA2G4A activity was also present in seminal vesicles. Many secreted type PLA2G2s and PLA2G6 expressed in mature mice testes [20–25,29] or several PLA2G2s present in seminal vesicle, prostate and semen [10–12,25] would compensate for the knockout of PLA2G4A in the testis and sexual accessory glands as age increases. Alternatively or additionally, the pubertal development of an endocrine network along the hypothalamus–pituitary–gonad axis may counteract the reduced synthesis of gonadal testosterone resulting from PLA2G4A deficiency in situ.
The potential impact of PLA2G4A inhibition on male testicular function needs to be evaluated carefully. Male mice usually become sexually mature at 7–8 weeks after birth and begin to breed in the laboratory. Pla2g4a−/− mice show over 40% reduction in sperm count during 10–13 weeks of age, although they are fertile. It should be noted that a reduction in gametogenic and steroidogenic function in males tends to be less easily recognized than in females owing to gender-related reproductive characteristics. The basal levels of seminal PG in rodents are very low compared with those of other animal species. This could be a reason why the Pla2g4a deletion causes only modest phenotypic effects and is consistent with the limited impact of eicosanoid pathway inhibitors [58]. The expression of PLA2 and compensation for Pla2g4a inhibition in the human reproductive system may be significantly different from those in rodents, as the Leydig cell population differs between rodents and humans [40]. Therefore the effects of Pla2g4a inhibition on male mouse fertility should not be overlooked The potential impact of this pathway in humans must be evaluated if specific PLA2G4A inhibitors are to be used safely in males.
In conclusion, PLA2G4A is expressed in the murine testes and plays a vital role in interstitial cells. This enzyme-triggered AA cascade is a critical mediator in the response of mature Leydig cells to LH stimulation and is necessary for normal development of structure and function of Leydig cells and thereby the testes. Thus PLA2G4A has an important role in sexual maturation, including spermatogenesis and growth of the male sexual accessory glands.
ACKNOWLEDGEMENTS
We thank E. O’Leary, X.-M. Sun and other members of the Bonventre laboratory for their technical assistance and useful suggestions. We also thank Y. Fujii and H. Satoh (Kitasato University) for experimental help and M. Nakata (Kitasato University) for assistance in paper preparation prior to submission.
FUNDING The work done in the laboratory of J.V.B. was supported by the National Institutes of Health National Institute of Diabetes and Digestive and Kidney Diseases [grant numbers DK39773 and DK54741]. S.K. received a fellowship for studies abroad from the Nakayama Foundation for Human Science.
Abbreviations used
- AA
arachidonic acid
- AACOCF3
arachidonyl trifluoromethyl ketone
- ALOX
lipoxygenase
- BEL
bromoenol lactone
- dbcAMP
dibutyryl cAMP
- DMEM
Dulbecco’s modified Eagle’s medium
- EIA
enzyme immunoassay
- hCG
human chorionic gonadotropin
- LH
luteinizing hormone
- NDGA
nordihydroguaiaretic acid
- PAF-AH
platelet-activating factor acetylhydrolases
- PC
phosphatidylcholine
- PG
prostaglandin
- PLA2
phospholipase A2
- iPLA2
Ca2+ -independent PLA2
- PLA2G4A
Group IVA PLA2
- PTGS
cyclo-oxygenase
- sPLA2
secretory PLA2
Footnotes
AUTHOR CONTRIBUTION Shiro Kurusu initiated this project and Joseph Bonventre directed it throughout. Shiro Kurusu, Adam Sapirstein and Joseph Bonventre designed experiments and Shiro Kurusu, Adam Sapirstein and Harumi Sawada performed them. Mitsumori Kawaminami provided experimental materials. Shiro Kurusu, Adam Sapirstein and Joseph Bonventre analysed and interpreted the data. Shiro Kurusu, Adam Sapirstein and Joseph Bonventre wrote the paper.
REFERENCES
- 1.Schaloske RH, Dennis EA. The phospholipase A2 superfamily and its group numbering system. Biochim. Biophys. Acta. 2006;1761:1246–1259. doi: 10.1016/j.bbalip.2006.07.011. [DOI] [PubMed] [Google Scholar]
- 2.Brash AR. Arachidonic acid as a bioactive molecule. J. Clin. Invest. 2001;107:1339–1345. doi: 10.1172/JCI13210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Funk CD. Prostaglandins and leukotrienes: advances in eicosanoid biology. Science. 2001;294:1871–1875. doi: 10.1126/science.294.5548.1871. [DOI] [PubMed] [Google Scholar]
- 4.Shimizu T. Lipid mediators in health and disease: enzymes and receptors as therapeutic targets for the regulation of immunity and inflammation. Annu. Rev. Pharmacol. Toxicol. 2009;49:123–150. doi: 10.1146/annurev.pharmtox.011008.145616. [DOI] [PubMed] [Google Scholar]
- 5.Kita Y, Ohto T, Uozumi N, Shimizu T. Biochemical properties and pathophysiological roles of cytosolic phospholipase A2s. Biochim. Biophys. Acta. 2006;1761:3117–3122. doi: 10.1016/j.bbalip.2006.08.001. [DOI] [PubMed] [Google Scholar]
- 6.Reddy ST, Herschman HR. Prostaglandin synthase 1 and prostaglandin synthase 2 are coupled to distinct phospholipases for the generation of prostaglandin D2 in activated mast cells. J. Biol. Chem. 1995;272:3231–3237. doi: 10.1074/jbc.272.6.3231. [DOI] [PubMed] [Google Scholar]
- 7.Peters-Golden M, McNish RW. Redistribution of 5-lipoxygenase and cytosolic phospholipase A2 to the nuclear fraction upon macrophage activation. Biochem. Biophys. Res. Commun. 1993;196:147–153. doi: 10.1006/bbrc.1993.2227. [DOI] [PubMed] [Google Scholar]
- 8.Sirois J, Boerboom D, Sayasith K. Prostaglandin biosynthesis and action in the ovary. In: Leung PCK, Adashi EY, editors. The Ovary. 2nd edn Elsevier Academic Press; San Diego: 2004. pp. 233–247. [Google Scholar]
- 9.Kurzrok R, Lieb CC. Biochemical studies of human semen. II. The action of semen on the human uterus. Proc. Soc. Exp. Biol. New York. 1930;28:268. [Google Scholar]
- 10.Ronkko S. Immunohistochemical localization of phospholipase A2 in human and bovine male reproductive organs. Comp. Biochem. Physiol. B. 1995;110:503–509. doi: 10.1016/0305-0491(94)00190-6. [DOI] [PubMed] [Google Scholar]
- 11.Takayama K, Hara S, Kudo I, Inoue K. Detection of 14-kDa group II phospholipase A2 in human seminal plasma. Biochem. Biophys. Res. Commun. 1991;178:1505–1511. doi: 10.1016/0006-291x(91)91064-j. [DOI] [PubMed] [Google Scholar]
- 12.Kallajoki M, Alanen KA, Nevalainen M, Nevalainen TJ. Group II phospholipase A2 in human male productive organs and genital tumors. Prostate. 1998;35:263–272. doi: 10.1002/(sici)1097-0045(19980601)35:4<263::aid-pros5>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 13.Davis BK, Byrne R, Bedigian K. Studies on the mechanism of capacitation: albumin-mediated changes in plasma membrane lipids during in vitro incubation of rat sperm cells. Proc. Natl. Acad. Sci. U.S.A. 1980;77:1546–1550. doi: 10.1073/pnas.77.3.1546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wu C, Stojanov T, Chami O, Ishii S, Shimizu T, Li A, O’Neill C. Evidence for the autocrine induction of capacitation of mammalian spermatozoa. J. Biol. Chem. 2001;276:26962–16968. doi: 10.1074/jbc.M103107200. [DOI] [PubMed] [Google Scholar]
- 15.Llanos MN, Lui CW, Meizel S. Studies of phospholipase A2 related to the hamster sperm acrosome reaction. J. Exp. Zool. 1982;22:107–117. doi: 10.1002/jez.1402210114. [DOI] [PubMed] [Google Scholar]
- 16.Bennet PJ, Moatti JP, Mansat A, Ribbes H, Cayrac JC, Pontonnier F, Chap H, Douste-Blazy L. Evidence for the activation of phospholipases during acrosome reaction of human sperm elicited by calcium ionophore A23187. Biochim. Biophys. Acta. 1987;919:255–265. doi: 10.1016/0005-2760(87)90265-7. [DOI] [PubMed] [Google Scholar]
- 17.Dominguez L, Yunes RM, Fornes MW, Burgos M, Mayorga LS. Calcium and phospholipase A2 are both required for the acrosome reaction mediated by G-proteins stimulation in human spermatozoa. Mol. Reprod. Dev. 1999;52:297–302. doi: 10.1002/(SICI)1098-2795(199903)52:3<297::AID-MRD7>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- 18.Fry MR, Ghosh SS, East JM, Franson RC. Role of human sperm phospholipase A2 in fertilization: effects of a novel inhibitor of phospholipase A2 activity on membrane perturbations and oocyte penetration. Biol. Reprod. 1992;47:751–759. doi: 10.1095/biolreprod47.5.751. [DOI] [PubMed] [Google Scholar]
- 19.Riffo MS, Parraga M. Role of phospholipase A2 in mammalian sperm–egg fusion: development of hamster oolemma fusibility by lysophosphatidylcholine. J. Exp. Zool. 1997;279:81–88. [PubMed] [Google Scholar]
- 20.Chen J, Shao C, Lazar V, Srivastava CH, Lee WH, Tischfield JA. Localization of group IIC low molecular weight phospholipase A2 mRNA to meitic cells in the mouse. J. Cell. Biochem. 1997;64:369–375. doi: 10.1002/(sici)1097-4644(19970301)64:3<369::aid-jcb3>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- 21.Gelb MH, Valentin E, Ghomashchi F, Lazdunski M, Lambeau G. Cloning and recombinant expression of a structurally novel human secreted phospholipase A2. J. Biol. Chem. 2000;275:39823–39826. doi: 10.1074/jbc.C000671200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Valentin E, Singer AG, Ghomashchi F, Lazdunski M, Gelb MH, Lambeau G. Cloning and recombinant expression of human group IIF-secreted phospholipase A2. Biochem. Biophys. Res. Commun. 2000;279:223–228. doi: 10.1006/bbrc.2000.3908. [DOI] [PubMed] [Google Scholar]
- 23.Larsson Forsell PK, Kennedy BP, Claesson HE. The human calcium-independent phospholipase A2 gene multiple enzymes with distinct properties from a single gene. Eur. J. Biochem. 1999;262:575–585. doi: 10.1046/j.1432-1327.1999.00418.x. [DOI] [PubMed] [Google Scholar]
- 24.Ma Z, Turk J. The molecular biology of the group VIA Ca2+ -independent phospholipase A2. Prog. Nucleic Acid Res. Mol. Biol. 2001;67:1–33. doi: 10.1016/s0079-6603(01)67023-5. [DOI] [PubMed] [Google Scholar]
- 25.Masuda S, Murakami M, Matsumoto S, Eguchi N, Urade Y, Lambeau G, Gelb MH, Ishikawa Y, Ishii T, Kudo I. Localization of various secretory phospholipase A2 enzymes in male reproductive organs. Biochim. Biophys. Acta. 2004;1686:61–76. doi: 10.1016/j.bbalip.2004.08.017. [DOI] [PubMed] [Google Scholar]
- 26.Koizumi H, Yamaguchi N, Hattori M, Ishikawa TO, Aoki J, Taketo MM, Inoue K, Arai H. Targeted disruption of intracellular type I platelet activating factor-acetylhydrolase catalytic subunits causes severe impairment in spermatogenesis. J. Biol. Chem. 2003;278:12489–12494. doi: 10.1074/jbc.M211836200. [DOI] [PubMed] [Google Scholar]
- 27.Nayernia K, Vauti F, Meinhardt A, Cadenas C, Schweyer S, Meyer BI, Schwandt I, Chowdhury K, Engel W, Arnold HH. Inactivation of a testis-specific Lis1 transcript in mice prevents spermatid differentiation and causes male infertility. J. Biol. Chem. 2003;278:48377–48385. doi: 10.1074/jbc.M309583200. [DOI] [PubMed] [Google Scholar]
- 28.Yan W, Assadi AH, Wynshaw-Boris A, Eichele G, Matzuk MM, Clark GD. Previously uncharacterized roles of platelet-activating factor acetylhydrolase 1b complex in mouse spermatogenesis. Proc. Natl. Acad. Sci. U.S.A. 2003;100:7189–7194. doi: 10.1073/pnas.1236145100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sato H, Taketomi Y, Isogai Y, Miki Y, Yamamoto K, Masuda S, Hosono T, Arata S, Ishikawa Y, Ishii T, et al. Group III secreted phospholipase A2 regulates epididymal sperm maturation and fertility in mice. J. Clin. Invest. 2010;120:1400–1414. doi: 10.1172/JCI40493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bao S, Miller DJ, Ma Z, Wohltmann M, Eng G, Ramanadham S, Moley K, Turk J. Male mice that do not express group VIA phospholipase A2 produce spermatozoa with impaired motility and have greatly reduced fertility. J. Biol. Chem. 2004;279:38194–38200. doi: 10.1074/jbc.M406489200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Escoffier J, Jemel I, Tanemoto A, Taketomi Y, Payre C, Coatrieux C, Sato H, Yamamoto K, Masuda S, Pernet-Gallay K, et al. Group X phospholipase A2 is released during sperm acrosome reaction and controls fertility outcome in mice. J. Clin. Invest. 2010;120:1415–1428. doi: 10.1172/JCI40494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Uozumi N, Kume K, Nagase T, Nakatani N, Ishii S, Tashiro F, Komagata Y, Maki K, Ikuta K, Ouchi Y, et al. Role of cytosolic phospholipase A2 in allergic response and parturition. Nature. 1997;390:618–622. doi: 10.1038/37622. [DOI] [PubMed] [Google Scholar]
- 33.Bonventre JV, Huang Z, Reza Taheri M, O’Leary E, Li E, Moskowitz MA, Sapirstein A. Reduced fertility and postischaemic brain injury in mice deficient in cytosolic phospholipase A2. Nature. 1997;390:622–625. doi: 10.1038/37635. [DOI] [PubMed] [Google Scholar]
- 34.Song H, Lim H, Paria BC, Matsumoto H, Swift LL, Morrow J, Bonventre JV, Dey SK. Cytosolic phospholiase A2α is crucial for ‘on-time’ embryo development that directs subsequent development. Development. 2002;129:2879–2889. doi: 10.1242/dev.129.12.2879. [DOI] [PubMed] [Google Scholar]
- 35.Ono T, Yamada K, Chikazawa Y, Ueno M, Nakamoto S, Okuno T, Seno K. Characterization of a novel inhibitor of cytosolic phospholipase A2α, pyrrophenone. Biochem. J. 2002;363:727–735. doi: 10.1042/0264-6021:3630727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Onami S, Matsuyama S, Nishihara M, Takahashi M. Splenic macrophages can modify steroidogenesis of Leydig cells. Endocr. J. 1996;43:477–485. doi: 10.1507/endocrj.43.477. [DOI] [PubMed] [Google Scholar]
- 37.Sapirstein A, Saito H, Texel SJ, Samad TA, O’Leary E, Bonventre JV. Cytosolic phospholipase A2α regulates induction of brain cyclooxygenase-2 in a mouse model of inflammation. Am. J. Physiol. 2005;288:R1774–R1782. doi: 10.1152/ajpregu.00815.2004. [DOI] [PubMed] [Google Scholar]
- 38.Kurusu S, Kaizo K, Ibashi M, Kawaminami M, Hashimoto I. Luteal phospholipase A2 activity increases during functional and structural luteolysis in pregnant rats. FEBS Lett. 1999;454:225–228. doi: 10.1016/s0014-5793(99)00389-0. [DOI] [PubMed] [Google Scholar]
- 39.Liang J-H, Sankai T, Yoshida T, Yoshikawa Y. Comparison of the effects of two fixatives for immunolocalization of testosterone in the testes of the cynomologus monkey, mouse and rat. Exp. Anim. 2000;49:301–304. doi: 10.1538/expanim.49.301. [DOI] [PubMed] [Google Scholar]
- 40.Haider SG. Cell biology of Leydig cells in the testis. Int. Rev. Cytol. 2004;233:181–241. doi: 10.1016/S0074-7696(04)33005-6. [DOI] [PubMed] [Google Scholar]
- 41.Gavazza M, Catala A. The effect of α-tocopherol on the lipid peroxidation of mitochondria and microsomes obtained from rat liver and testis. Mol. Cell. Biochem. 2001;225:121–128. doi: 10.1023/a:1012274206337. [DOI] [PubMed] [Google Scholar]
- 42.Lu ZH, Mu YM, Wang BA, Li XL, Lu JM, Li JY, Pan CY, Yanase T, Nawata H. Saturated free fatty acids, palmitic acid and stearic acid, induce apoptosis by stimulation of ceramide generation in rat testicular Leydig cell. Biochem. Biophys. Res. Commun. 2003;303:1002–1007. doi: 10.1016/s0006-291x(03)00449-2. [DOI] [PubMed] [Google Scholar]
- 43.Laposata M, Minda M, Capriotti AM, Hartman EJ, Furth EE, Iozzo RV. Reversible phenotypic modulation induced by deprivation of exogenous essential fatty acids. Lab. Invest. 1988;59:838–847. [PubMed] [Google Scholar]
- 44.Choukroun GJ, Marshansky V, Gustafson CE, McKee M, Hajjar RJ, Rosenzweig A, Brown D, Bonventre JV. Cytosolic phospholipase A2 regulates Golgi structure and modulates intracellular trafficking of membrane proteins. J. Clin. Invest. 2000;106:983–993. doi: 10.1172/JCI8914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sharpe RM, Maddocks S, Kerr JB. Cell–cell interactions in the control of spermatogenesis as studied using Leydig cell destruction and testosterone replacement. Am. J. Anat. 1990;188:3–20. doi: 10.1002/aja.1001880103. [DOI] [PubMed] [Google Scholar]
- 46.Meng J, Holdcraft RW, Shima JE, Griswold MD, Braun RE. Androgens regulate the permeability of the blood–testis barrier. Proc. Natl. Acad. Sci. U.S.A. 2005;102:16696–16670. doi: 10.1073/pnas.0506084102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Didolker AK, Sundaram K. Arachidonic acid is involved in the regulation of hCG induced steroidogenesis in rat Leydig cells. Life Sci. 1987;41:471–477. doi: 10.1016/0024-3205(87)90223-2. [DOI] [PubMed] [Google Scholar]
- 48.Abayasekara DR, Band AM, Cooke BA. Evidence for the involvement of phospholipase A2 in the regulation of luteinizing hormone-stimulated steroidogenesis in rat testis Leydig cells. Mol. Cell. Endocrinol. 1990;70:147–153. doi: 10.1016/0303-7207(90)90154-z. [DOI] [PubMed] [Google Scholar]
- 49.Romanelli F, Valenca M, Conte D, Isidori A, Negro-Vilar A. Arachidonic acid and its metabolites effects on testosterone production by rat Leydig cells. J. Endocrinol. Invest. 1995;18:186–193. doi: 10.1007/BF03347801. [DOI] [PubMed] [Google Scholar]
- 50.Mele PG, Dada LA, Paz C, Neuman I, Cymeryng CB, Mendez CF, Finkielstein CV, Cornejo Maciel F, Podesta EJ. Involvement of arachidonic acid and the lipoxygenase pathway in mediating luteinizing hormone-induced testosterone synthesis in rat Leydig cells. Endocr. Res. 1997;23:15–26. doi: 10.1080/07435809709031839. [DOI] [PubMed] [Google Scholar]
- 51.Kim HS, Ishizuka M, Kazusaka A, Fujita S. Alterations of activities of cytosolic phospholipase A2 and arachidonic acid-metabolizing enzymes in di-(2-ethylhexyl)phthalate-induced testicular atrophy. J. Vet. Med. Sci. 2004;66:119–1124. doi: 10.1292/jvms.66.1119. [DOI] [PubMed] [Google Scholar]
- 52.Cornejo Maciel F, Maloberti P, Neuman I, Cano F, Castilla R, Castillo F, Paz C, Podesta EJ. An arachidonic acid-preferring acyl-CoA synthetase is a hormone-dependent and obligatory protein in the signal transduction pathway of steroidogenic hormones. J. Mol. Endocrinol. 2005;34:655–666. doi: 10.1677/jme.1.01691. [DOI] [PubMed] [Google Scholar]
- 53.Maloberti P, Cornejo Maciel F, Castillo AF, Castilla R, Duarte A, Toledo MF, Meuli F, Mele P, Paz C, Podesta EJ. Enzymes involved in arachidonic acid release in adrenal and Leydig cells. Mol. Cell. Endocrinol. 2007;265–266:113–120. doi: 10.1016/j.mce.2006.12.026. [DOI] [PubMed] [Google Scholar]
- 54.Kurusu S, Ohkawa M, Kawaminami M. Effects of arachidonate metabolism inhibitors on basal and human chorionic gonadotropin-stimulated progesterone secretion by rat corpus luteum cells in vitro. Prostaglandins Other Lipid Mediat. 2007;83:139–145. doi: 10.1016/j.prostaglandins.2006.10.008. [DOI] [PubMed] [Google Scholar]
- 55.Wang XJ, Walsh LP, Reinhart AJ, Stocco DM. The role of arachidonic acid in steroidogenesis and steroidogenic acute regulatory (StAR) gene and protein expression. J. Biol. Chem. 2000;275:20204–20209. doi: 10.1074/jbc.M003113200. [DOI] [PubMed] [Google Scholar]
- 56.Wang XJ, Dyson MT, Mondillo C, Patrignani Z, Pignataro O, Stocco DM. Interaction between arachidonic acid and cAMP signaling pathways enhances steroidogenesis and StAR gene expression in MA-10 Leydig tumor cells. Mol. Cell. Endocrinol. 2002;188:55–63. doi: 10.1016/s0303-7207(01)00748-1. [DOI] [PubMed] [Google Scholar]
- 57.Wang X, Shen C-L, Dyson MT, Yin X, Schiffer RB, Grammas P, Stocco DM. The involvement of epoxygenase metabolites of arachidonic acid in cAMP-stimulated steroidogenesis and steroidogenic acute regulatory protein gene expression. J. Endocrinol. 2006;190:871–878. doi: 10.1677/joe.1.06933. [DOI] [PubMed] [Google Scholar]
- 58.Loscher W, Blazaki D. Effects of non-steroidal anti-inflammatory drugs on fertility of male rats. J. Reprod. Fertil. 1986;76:65–73. doi: 10.1530/jrf.0.0760065. [DOI] [PubMed] [Google Scholar]