Abstract

Mechanistic investigations of a Rh(I)-catalyzed direct C–H alkylation of benzylic amines with alkenes, formally an C(sp3)–H activation, reveal this reaction to proceed via imine intermediates and, hence, via C(sp2)–H activation. The reaction shows a primary kinetic isotope effect of 4.3 at the benzylic C–H position together with a reversible H–D exchange at the same position, which indicates that there are at least two distinct steps in which the corresponding C–H bonds are broken. The imine intermediates are shown to be converted to the final product under the reaction conditions, and a time course analysis of the alkylated imine intermediate shows that it is formed before the final amine product in the course of the reaction.
Keywords: heterogeneous reaction, Rh(I) catalysis, C−H activation, cyclometalation, direct alkylation, kinetic isotope effects, reaction mechanism
1. Introduction
In recent years, the development of catalytic C–H activation methods to selectively functionalize organic molecules at unsubstituted carbon atoms has emerged tremendously.1 Especially, C–H activation of sp2-hybridized C–H bonds is now quite common and well established in the field.2 On the other hand, C–H activation of sp3-hybridized C–H bonds still remains difficult and is therefore of special interest.3 To gain a better understanding of these reactions and overcome the difficulties, detailed mechanistic investigations are important. They provide insight into the intrinsic problems and indicate in which step of the catalytic cycle optimization is necessary. A very useful and general method to achieve catalytic C–H activation is cyclometalation, which employs the aid of a nearby coordinating group to direct the catalyst selectively to one specific C–H bond to be activated and functionalized.4 Our group recently reported several useful methods for the C–H activation of benzylic sp3 C–H bonds directed by 3-substituted pyridin-2-yls, including Ru(0)-catalyzed arylation employing arylboronates5 and Ru(II)-catalyzed arylation of benzylic amines with aryl halides.6 Herein, we report detailed mechanistic investigations into a Rh(I)-catalyzed method to alkylate benzylic amines using either alkyl bromides or alkenes. It should be noted that the alkylation of 1 using alkenes catalyzed by Ru(0) was already reported by the group of Jun in 1998;7 however, to the best of our knowledge, there have not yet been any published reports using Rh(I) catalysis or contributions dedicated to detailed mechanistic investigations of such a transformation.
The initial idea behind this research was to use alkyl halides in direct alkylation reactions of benzylamines carrying a directing group on the amine function. We had developed several direct arylation protocols and gained significant experience and mechanistic understanding with this type of substrate. Therefore, we were confident that we could use the same system for direct alkylation using alkyl halides, a coupling between two sp3-hybridized carbon atoms.
2. Results and Discussion
2.1. Screening of Direct C–H Alkylation Reactions
We started the development of direct alkylation of 1 using the conditions optimized for our direct arylation reactions but employing alkyl bromides as the alkyl source (Table 1: entry 1, conditions derived from the aryl chloride protocol; entry 2, conditions derived from the aryl bromide protocol).6 Initial experiments using 1-bromoheptane as the alkyl source showed promising conversion of substrate 1 of ∼20% to the alkylated product 2 (Table 1, entries 1 and 2), but significant amounts of unreacted 1 (∼40%) remained. After intensively screening reaction conditions employing the [RuCl2(p-cymene)]2 catalyst without any significant improvements (not shown),8 it turned out that [RhCl(cod)]2 gave better conversion (Table 1, entry 3). Further optimizing the reaction conditions showed that the addition of ligands had no beneficial influence, and cyclohexanol proved to be unnecessary (Table 1, entries 5 and 6).9 The best results were obtained when raising the temperature to 160 °C and changing the solvent to toluene (Table 1, entry 6).
Table 1. Selected Screening Results for Direct C–H Alkylation of 1 Using Alkyl Bromides.

| entry | catalysta | base | additive | solvent | tempb | time | conversion of 1 | conversion to 2 |
|---|---|---|---|---|---|---|---|---|
| 1 | [RuCl2(p-cymene)]2 (0.1 equiv) | K2CO3 (3 equiv) | PPh3 (0.05 equiv) cyclohexanol (1 equiv) | o-xylene | 140 °C | 24 h | 56% | 23% |
| 2 | [RuCl2(p-cymene)]2 (0.1 equiv) | K2CO3 (3 equiv) | KOPiv (0.3 equiv) cyclohexanol (1 equiv) | o-xylene | 140 °C | 24 h | 60% | 20% |
| 3 | [RhCl(cod)]2 (0.1 equiv) | K2CO3 (3 equiv) | cyclohexanol (1 equiv) | o-xylene | 140 °C | 24 h | 70% | 31% |
| 4 | [RhCl(cod)]2 (0.1 equiv) | K2CO3 (3 equiv) | cyclohexanol (1 equiv) | toluene | 140 °C | 24 h | 73% | 37% |
| 5 | [RhCl(cod)]2 (0.1 equiv) | K2CO3 (3 equiv) | cyclohexanol (1 equiv) | toluene | 160 °C | 24 h | 97% | 70% (56%)d |
| 6 | [RhCl(cod)]2 (0.1 equiv) | K2CO3 (3 equiv) | toluene | 160 °C | 24 h | 98% | 70% |
Equivalents based on monomer unit.
Reaction block temperatures and not inside temperatures of the reaction mixtures.
Based on GC analysis of the reaction mixture with dodecane as internal standard.
Isolated yield.
After our optimization efforts, we started investigating the substrate scope with respect to alkyl bromides. We were especially interested in using secondary alkyl bromides because they should lead to branched products. However, when conducting the reaction with 2-bromobutane, we found that alkylation had taken place at the terminal carbon, and the same product was obtained as in the reaction with 1-bromobutane (Scheme 1).
Scheme 1. Direct Alkylation of Benzylic Amine 1 Using Either 1-Bromobutane or 2-Bromobutane.
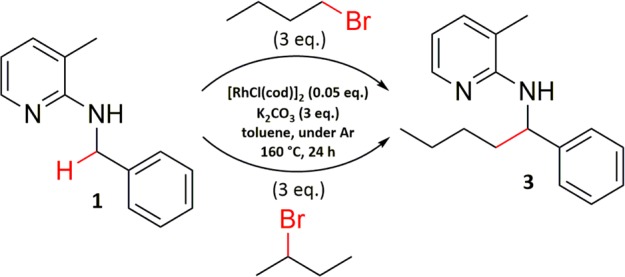
Because β-H-elimination is very common in alkyl complexes of transition metals, we suspected the reaction proceeds via the corresponding terminal alkene.10 Hence, it was tested whether alkenes would react under these conditions to form the corresponding alkylated products. The first experiments using hex-1-ene gave the corresponding alkylated product 4 in 44% isolated yield. In addition, in experiments using either 1-bromododecane or 1-bromo-2-phenylethane, the corresponding alkenes could be detected in low amounts by means of GC/MS in the reaction mixture. In the absence of [RhCl(cod)]2, no alkene formation was observed, confirming a crucial role of the catalyst and excluding simple thermal elimination.
Because the conditions applied in the first experiments with alkenes stemmed from optimizing the alkyl bromide protocol, further screening experiments were carried out. Initial experiments showed that the alkylation reaction using alkenes proceeded considerably faster; therefore, the temperature could be reduced to 150 °C. In addition, using degassed toluene accelerated the reaction significantly. The optimized reaction conditions are shown in Scheme 2.
Scheme 2. Optimized Reaction Conditions for Direct C–H Alkylation of Benzylic Amine 1 Using Alkenes.

Yields are calibrated GC yields.
There are several features to be noted here. First, as can be seen in Scheme 2, we observed significant formation of two byproducts, 5 and 6. At first sight, they seem rather surprising; however, their formation can be explained by C–C activations.11 We conducted several experiments, discussed later (vide infra), to investigate their formation. Second, small amounts (below 1%) of the corresponding imines of 1 and 4 were detected in the reaction mixture throughout the whole reaction time. Third, K2CO3 proved to be absolutely crucial in this reaction because experiments without K2CO3 did not show any conversion to the corresponding alkylated product. In the reaction employing alkyl bromides, K2CO3 is conceptually needed to catch the HBr formed and, therefore, to drive the reaction to the products. In the case of alkenes, however, there is no formation of an acidic byproduct because the reaction toward 1 is formally an addition of a C–H bond across a double bond. Finally, only 79% of the initial amount of 1 is found in the products. Because we were not able to detect any other byproducts and the Rh catalyst used is capable of breaking C–C bonds, obviously, we suspect that there is significant decomposition of material to compounds that cannot easily be detected in the reaction mixture.
2.2. Kinetic Profile of the Direct Alkylation of 1 Using Hex-1-ene
As entry point to our mechanistic investigations, we determined the kinetic profile of the olefin alkylation protocol (Figure 1).12
Figure 1.
Determination of the kinetic time course of direct C–H alkylations of benzylic amine 1 using hex-1-ene.
There are some features to be noted. First, the reaction is finished after 2 h, and the amounts of 4, 5, and 6 remain constant thereafter. This can be rationalized either by inhibition or decomposition of the active catalyst under these conditions or by a reversible reaction among 4, 5, and 6. Second, both 4 and 6 are formed more or less from the beginning, and no significant induction period is observed, except for the time the reaction mixture needs to reach a constant temperature.13 This is very important because it allows us to easily determine kinetic parameters by using the method of initial rates (vide infra). From this kinetic profile, the initial reaction rate under the optimized reaction conditions was determined (See the Supporting Information for details).
2.3. Side Product Studies
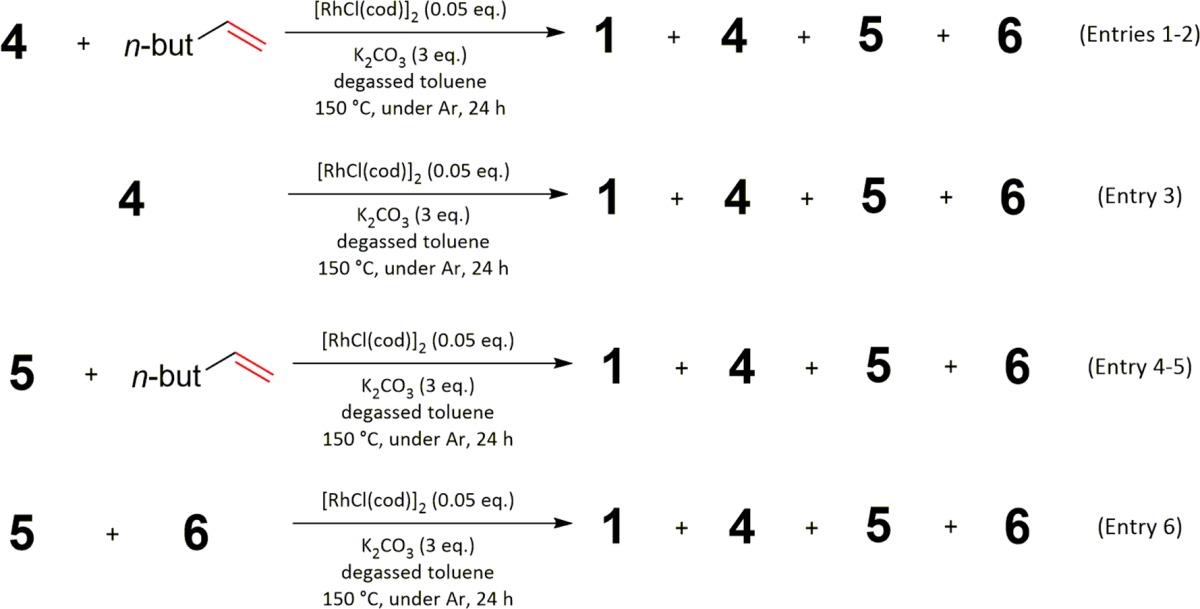
To gain more information about the side products observed in the reaction using alkenes, several experiments that subjected these side products to the reaction conditions were conducted (Table 2).
Table 2. Investigation of the Formation of the By-Products 5 and 6 in the Direct C–H Alkylation Using Hex-1-ene.
| compd amt, %a |
||||||
|---|---|---|---|---|---|---|
| entry | starting compd(s) | hex-1-ene amt, equiv | 1 | 4 | 5 | 6 |
| 1 | 4 (1 equiv) | 3 | <0.1 | 62 | 5 | 11 |
| 2 | 4 (1 equiv) | 6 | <0.1 | 72 | 4 | 12 |
| 3 | 4 (1 equiv) | 0 | 2 | 76 | 2 | 3 |
| 4 | 5 (1 equiv) | 3 | <0.1 | 4 | 68 | <0.1 |
| 5 | 5 (1 equiv) | 6 | <0.1 | 6 | 58 | <0.1 |
| 6 | 5 (0.5 equiv) + 6 (0.5 equiv)b | 0 | <0.1 | 1 | 42 | 46 |
Determined at the end of the reaction; calibrated GC yields.
Sum of amounts of 5 and 6 was considered as 1 equiv.
Entries 1 and 2 show that the initial product, 4, can be converted to the two side products 5 and 6 by subjecting it to the reaction conditions. Without any alkene present (entry 3), compound 4 is even converted back to 1, showing that its formation is reversible under the reaction conditions. Entries 4 and 5 indicate that compound 5 can be converted to 4 under the reaction conditions, showing that its formation is also reversible. Finally, mixing 5 and 6 in the absence of alkene leads to formation of 4 (entry 6). Overall, these experiments demonstrate that the formation of 5 and 6 starting from 4 and the formation of 4 from 1 are, in principle, reversible and that C–C bond cleavages are occurring in the reaction mixture to a significant extent.
Interestingly, no side product deriving from bisalkylation of the benzylic position was detected in any experiment. One possible explanation is that formation of a quaternary carbon is disfavored because of steric hindrance. Alternatively, if the alkylation does not occur on the amine substrate 1 but proceeds via an imine intermediate thereof, formation of a quaternary carbon is impossible. This can be considered as a first finding, pointing toward an imine mechanism. In addition, in a similar system using imines with the same directing group and also employing Rh(I) catalysis, similar C–C activation reactions are already known.14
2.4. Base Studies
In the reaction using alkenes, a very pronounced dependence of the initial rate on the adsorbed water content of K2CO315 was found, which is depicted in Figure 2. The higher the content of adsorbed water in the base, the higher the observed initial reaction rate.
Figure 2.
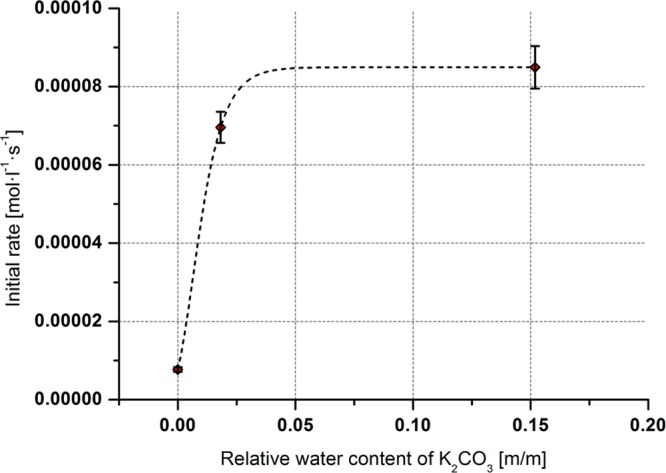
Dependence of the initial reaction rate on the adsorbed water content of K2CO3 for the direct C–H alkylation using hex-1-ene in dry toluene.
That water is beneficial for C–H activation reactions is already known in some cases.16 However, to the best of our knowledge, there has been no report on using a base effectively insoluble in the reaction solvent containing superficially adsorbed water to increase the reaction rate. At present, this significant dependence on the adsorbed water content cannot be satisfactorily interpreted, and further investigations need to be carried out.
2.5. Kinetic Isotope Effect Studies
To gain more information about the rate-determining step of the reaction, kinetic isotope effect (KIE) studies were conducted. In C–H activation reactions, one major question is always whether the oxidative addition of the metal catalyst into the C–H bond is rate-determining. Therefore, in a first study, the KIE of the benzylic C–H bonds was determined (Scheme 3) by measuring the initial rate of the reaction with the deuterated compound 1a and comparing it with the initial rate we determined for 1 under the same reaction conditions.
Scheme 3. Determination of the KIE of the Benzylic C–H Bonds in the Direct C–H Alkylation of 1 Using Hex-1-ene.
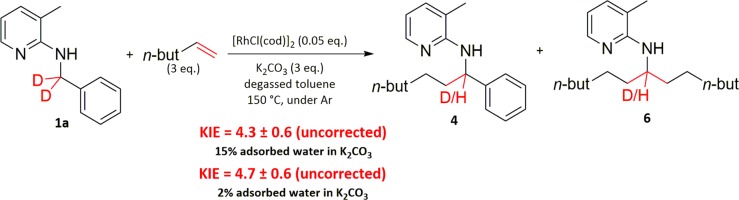
The large observed value indicates a primary KIE and suggests that the benzylic C–H bond is broken in the rate-determining step; that is, oxidative addition into the C–H bond is rate-determining. There are several things to notice. First, the KIE is independent of the adsorbed water content of K2CO3. This is very important so that the result can be compared with the KIE study of the N–H bond (vide infra). Second, there is significant H–D exchange observed in unreacted 1a during the reaction. Actually, the H–D exchange is higher than the total amount of products formed (details in the Supporting Information). This seems contradictory to the primary KIE observed because this would suggest a (compared with the overall reaction rate) fast and reversible C–H activation. However, it makes perfect sense if the reaction proceeds over the corresponding imine via a fast, reversible amine-to-imine interconversion, which would account for the H–D exchange observed. The consecutive oxidative addition into the sp2-hybridized C–H bond would then be rate-determining, accounting for the large primary KIE.
In a second study, the KIE of the N–H bond was determined (Scheme 4) to find out whether this hypothesis could be supported. The KIE was determined by comparing the initial reaction rate of deuterated compound 1b and the initial rate obtained for the undeuterated compound 1 under the same reaction conditions. It should be noted that this is not an easy experiment to perform because the N–D readily exchanges with any H2O that is introduced into the reaction mixture. The concomitant extent of H–D exchange has to be minimized to obtain a meaningful result.
Scheme 4. Determination of the KIE of the N–H Bond in the Direct C–H Alkylation of 1 Using Hex-1-ene.

Before the value is discussed, it is important to mention that because of the adsorbed water introduced with K2CO3, the H–D exchange in this experiment series was high.17 Using K2CO3 with 2% of adsorbed water resulted in an H–D exchange low enough (even though still 56%) to determine the KIE reliably by correcting the observed initial rate (see the Supporting Information for details). The small KIE value observed for the N–H bond indicates that the N–H bond is not broken in the rate-determining step, excluding a mechanism in which one of the benzylic C–H bonds and the N–H bond are broken simultaneously.18 However, it rather suggests a secondary KIE, which would support the previously established hypothesis of a fast, reversible imine formation prior to rate-determining oxidative addition into the C–H bond.
2.6. Substrate Scope Investigation: Tertiary Amines
The simplest experiment series to support or exclude an imine mechanism is to test whether tertiary benzylic amines are converted to the corresponding alkylated products under the reaction conditions (Scheme 5). If the formation of the corresponding products was observed at a similar rate as compared with secondary benzylic amines, this would be evidence against an imine mechanism because the formation of the corresponding iminium ion is not expected to be occurring at a similar rate, if possible at all.19
Scheme 5. Attempted Alkylation of Tertiary Benzylic Amines 7a and 7b.

In neither experiment were any alkylated products observed, even after 24 h of reaction time, again supporting the hypothesis of the reaction proceeding over the corresponding imines.
2.7. Imine studies
On the basis of our previous results, the hypothesis is that amine 1 is converted to imine 9 and gets alkylated and the alkylated imine 10 is then converted back to alkylated amine 4 (Scheme 6). Of course, these interconversions would occur on a rhodium complex. It should be noted that a similar amine-to-imine oxidation prior to alkylation has been proposed previously in the literature.20 However, in that example, the final reaction product is a ketone, which is formed after hydrolysis of the imine, so the amine-to-imine interconversion was not reversed.
Scheme 6. Hypothetical General Reaction Course of the Direct C–H Alkylation of Benzylic Amine 1.

It was hypothesized that if imines 9 and 10 were intermediates in the reaction, they should be converted to the products of the reaction under the reaction conditions. However, subjecting only imine 9 as starting material to the reaction conditions resulted in only a very low conversion (see the Supporting Information for more details). Therefore, crossover experiments were performed using 0.05 equiv of imines 9 and 10, respectively, together with 1 equiv of amine 1c as starting materials (Scheme 7).
Scheme 7. Crossover Experiments Investigating Whether Imines 9 and 10 Are Converted to the Corresponding Products of the Direct C–H Alkylation Using Hex-1-ene Together with Benzylic Amine 1c.
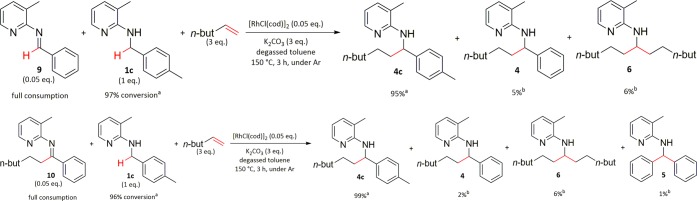
Based on GC analysis of the reaction mixture with dodecane as internal standard, assuming a conversion factor of 1 (not calibrated).
Calibrated GC yields.
In the first crossover experiment, amine 1c was alkylated in the presence of imine 9, which was completely converted to the alkylated amine 4. In the second, the same amine, 1c, was reacted in the presence of alkylated imine 10. Full consumption of 10 was observed, and it was converted to products 4 (2%), 5 (1%), and 6 (1%) with a total recovery of 80% (4% found from 5% used). These results show that both imine 9 and alkylated imine 10 are transformed to the corresponding amine products under the reaction conditions.
2.8. Investigation of Imine Intermediate Kinetics
All previous experiments are in agreement with a reaction mechanism proceeding via the imines 9 and 10. Still, a reaction mechanism in which the alkylation takes place at the amine and the imines are formed in off-cycle reactions cannot yet be excluded. All three mechanistic scenarios depicted in Scheme 8 are possible. Either imines 9 and 10 are formed as off-cycle products from 1 and 4, respectively (scenario 1), or the reaction mechanism is proceeding through imines 9 and 10 as intermediates, with the alkylation taking place at the imine (scenario 2). Scenario 3 is a combination of those two cases in which both pathways occur at a similar rate.
Scheme 8. Possible Mechanistic Scenarios for the Direct C–H Alkylation of Benzylic Amine 1 Using Alkenes.
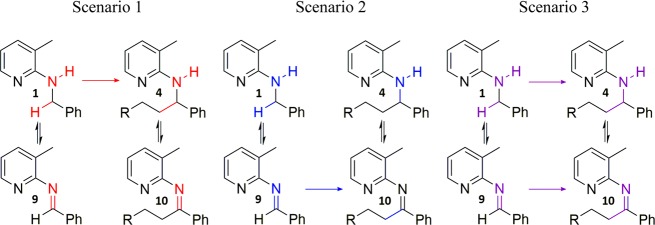
For simplicity, schemes are not fully stoichiometrically balanced.
To distinguish between scenarios 1 and 2, we developed a method based on simplified kinetic models. Because compounds 10 and 4 are interconvertible, the change of the ratio of these two compounds over time can deliver the desired information. If the ratio of the concentrations of 10 and 4 is decreasing over time in an initial reaction period, it would mean that 10 is formed before 4 in the course of the reaction, supporting the imine mechanism. If the same ratio is increasing, the opposite is true.21 The complete mathematical background of this method is given in the Supporting Information. However, it is also shown in the Supporting Information that experimentally, the third mechanistic scenario is very hard if not impossible to exclude by this method.
Plotting the ratio of imine 10 to amine 4 over time, the following result was obtained (Figure 3). The decreasing ratio of imine 10 to amine 4 over time is in accordance with mechanistic scenario 2 in which the reaction occurs at the imine, not at the amine, and excludes scenario 1. However, as already mentioned, mechanistic scenario 3 in which both pathways occur at a similar rate cannot be excluded. Because mechanistic scenario 2 is the significantly simpler explanation for all our observations, we prefer it over scenario 3. Either way, the reaction would proceed (at least to a significant extent) over the corresponding imines.
Figure 3.
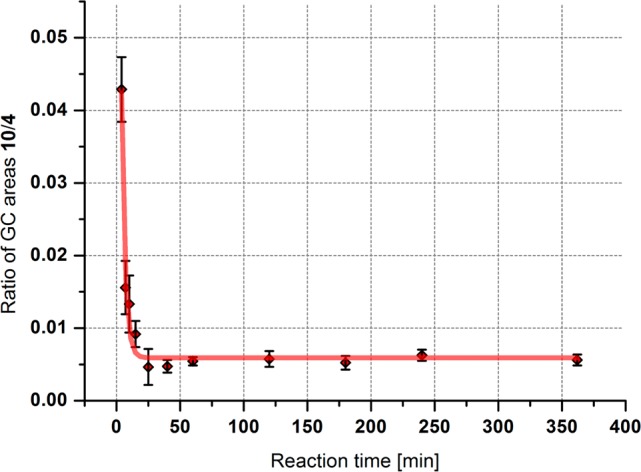
Time course of the ratio of the GC areas under the peaks of imine 10 and amine 4.
2.9. Mechanistic proposal
On the basis of our experimental results, we propose the following general mechanistic outline for the direct C–H alkylation using alkenes (Scheme 9).
Scheme 9. Mechanistic Proposal for the Direct C–H Alkylation of Benzylic Amines Using Alkenes.

The rate-determining step is indicated by a significantly shorter forward reaction arrow. “H2” emphasizes that it is not known how the two hydrogen atoms are bonded to the catalyst.
The catalytic cycle starts with coordination of 1 to a rhodium species (I in Scheme 9), then the corresponding amine complex II is reversibly interconverted to the corresponding imine (III), which is supported by the H–D exchange observed in unreacted 1 in the KIE studies of the benzylic C–H bond. Imine 9 can then be released from this complex (not depicted in Scheme 9) or it reacts in the rate-determining oxidative addition into the sp2-hybridized C–H bond to form a cyclometalated intermediate, IV, which is supported by the large KIE of about 4–5 observed for the benzylic C–H bond. Then, alkylation of intermediate IV with alkene yields the rhodium complex V of the alkylated imine 10. This imine can now be reversibly released (not depicted in Scheme 9) or it is converted to the alkylated amine coordinated to Rh (VI), which is released as the main product of the reaction. Since the whole reaction is shown to be in principle reversible, all mechanistic steps are also depicted to be reversible.
It should be noted that additional ligands coordinated to Rh were omitted because nothing is known about them on the basis of our experiments. In addition, it is not known how the two hydrogen atoms are bonded to the catalyst. If they would be bonded directly to Rh, the oxidative addition into the C(sp2)–H bond of the imine would result in a Rh(V) species that is not very common in literature but was reported on previous occasions, although on different complex systems.22 Alternatively, the interconversion of amine and imine could also proceed by transfer hydrogenations with a ligand on Rh. Transfer hydrogenations employing rhodium catalysis are quite common in the literature.23
Altogether, there are several important issues that remain open in this mechanistic investigation. First, almost nothing is known about the intermediate Rh species involved in the catalytic cycle. Second, the mechanism of the amine-to-imine interconversion is not known, either. Third, the role of K2CO3 and the water introduced with it need to be investigated. The last major unresolved issue is how the exchange between H and Ph in the interconversions of 4 and byproducts 5 and 6 actually occurs. These points certainly need to be addressed in follow-up studies, which are under way in our lab to get more insight into the reaction mechanism.
3. Conclusion
Benzylic amines were alkylated using both alkyl bromides and alkenes, and the latter transformation was investigated in detail. Kinetic and mechanistic evidence shows that the reaction does not proceed directly over the amines but instead over the corresponding imines, showing that the formal C(sp3)–H activation reaction proceeds mechanistically over an C(sp2)–H activation pathway. Additional experiments need to be performed to investigate the role of K2CO3 in the reaction and gain insight into the nature of the rhodium complexes involved in the catalytic cycle. Furthermore, the alkylation using alkyl bromides needs to be investigated and exploited further because gaseous short-chain alkenes could be avoided using the corresponding alkyl bromides.
Acknowledgments
We acknowledge the Austrian Science Foundation (FWF, Project P21202-N17) for financial support of this work. We also thank Fabian Glatz for proof-reading of mathematical derivations.
Supporting Information Available
The following file is available free of charge on the ACS Publications website at DOI: 10.1021/cs501924c.
Full experimental details, spectroscopic data for all new compounds, data points from kinetic experiments and full mathematical details (PDF)
Author Present Address
† Department of Chemistry, The Scripps Research Institute, 10550 North Torrey Pines Road, La Jolla, California 92037, United States
The authors declare no competing financial interest.
Supplementary Material
References
- For selected recent reviews on C–H activation, see the following:; a Shi G.; Zhang Y. Adv. Synth. Catal. 2014, 356, 1419–1442 10.1002/adsc.201400028. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Gao K.; Yoshikai N. Acc. Chem. Res. 2014, 47, 1208–1219 10.1021/ar400270x. [DOI] [PubMed] [Google Scholar]; c Tsurugi H.; Yamamoto K.; Nagae H.; Kaneko H.; Mashima K. Dalton Trans. 2014, 43, 2331–2343 10.1039/c3dt52758a. [DOI] [PubMed] [Google Scholar]; d Thirunavukkarasu V. S.; Kozhushkov S. I.; Ackermann L. Chem. Commun. 2014, 50, 29–39 10.1039/c3cc47028h. [DOI] [PubMed] [Google Scholar]; e Xie J.; Jin H.; Xu P.; Zhu C. Tetrahedron Lett. 2014, 55, 36–48 10.1016/j.tetlet.2013.10.090. [DOI] [Google Scholar]; f Gritsch P. J.; Leitner C.; Pfaffenbach M.; Gaich T. Angew. Chem., Int. Ed. 2014, 53, 1208–1217 10.1002/anie.201307391. [DOI] [PubMed] [Google Scholar]; g Shul’pin G. B. Dalton Trans. 2013, 42, 12794–12818 10.1039/c3dt51004b. [DOI] [PubMed] [Google Scholar]; h Rouquet G.; Chatani N. Angew. Chem., Int. Ed. 2013, 52, 11726–11743 10.1002/anie.201301451. [DOI] [PubMed] [Google Scholar]; i Baillie R. A.; Legzdins P. Acc. Chem. Res. 2014, 47, 330–340 10.1021/ar400108p. [DOI] [PubMed] [Google Scholar]; j Wang C. Synlett 2013, 24, 1606–1613 10.1055/s-0033-1339299. [DOI] [Google Scholar]; k Webb J. R.; Burgess S. A.; Cundari T. R.; Gunnoe T. B. Dalton Trans. 2013, 42, 16646–16665 10.1039/c3dt52164h. [DOI] [PubMed] [Google Scholar]; l Yan G.; Wu X.; Yang M. Org. Biomol. Chem. 2013, 11, 5558–5578 10.1039/c3ob40652k. [DOI] [PubMed] [Google Scholar]; m Wencel-Delord J.; Glorius F. Nat. Chem. 2013, 5, 369–375 10.1038/nchem.1607. [DOI] [PubMed] [Google Scholar]; n Sun X.; Li J.; Huang X.; Sun C. Curr. Inorg. Chem. 2012, 2, 64–85 10.2174/1877944111202010064. [DOI] [Google Scholar]; o Mousseau J. J.; Charette A. B. Acc. Chem. Res. 2013, 46, 412–424 10.1021/ar300185z. [DOI] [PubMed] [Google Scholar]; p Li B.-J.; Shi Z.-J. Chem. Soc. Rev. 2012, 41, 5588–5598 10.1039/c2cs35096c. [DOI] [PubMed] [Google Scholar]; q Colby D. A.; Tsai A. S.; Bergman R. G.; Ellman J. A. Acc. Chem. Res. 2012, 45, 814–825 10.1021/ar200190g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- For selected recent reviews on sp2 C–H bond functionalization, see the following:; a Kapdi A. R. Dalton Trans. 2014, 43, 3021–3034 10.1039/c3dt52737a. [DOI] [PubMed] [Google Scholar]; b Rossi R.; Bellina F.; Lessi M.; Manzini C. Adv. Synth. Catal. 2014, 356, 17–117 10.1002/adsc.201300922. [DOI] [Google Scholar]; c Zhou L.; Lu W. Chem.—Eur. J. 2014, 20, 634–642 10.1002/chem.201303670. [DOI] [PubMed] [Google Scholar]; d Wencel-Delord J.; Colobert F. Chem.—Eur. J. 2013, 19, 14010–14017 10.1002/chem.201302576. [DOI] [PubMed] [Google Scholar]; e Julia-Hernandez F.; Simonetti M.; Larrosa I. Angew. Chem., Int. Ed. 2013, 52, 11458–11460 10.1002/anie.201306425. [DOI] [PubMed] [Google Scholar]; f Okamoto K.; Zhang J.; Housekeeper J. B.; Marder S. R.; Luscombe C. K. Macromolecules 2013, 46, 8059–8078 10.1021/ma401190r. [DOI] [Google Scholar]; g Li B.; Dixneuf P. H. Chem. Soc. Rev. 2013, 42, 5744–5767 10.1039/c3cs60020c. [DOI] [PubMed] [Google Scholar]; h Shang X. S.; Liu Z.-Q. Chem. Soc. Rev. 2013, 42, 3253–3260 10.1039/c2cs35445d. [DOI] [PubMed] [Google Scholar]
- For selected recent reviews on sp3 C–H bond functionalization, see the following:; a Qin Y.; Lv J.; Luo S. Tetrahedron Lett. 2014, 55, 551–558 10.1016/j.tetlet.2013.11.051. [DOI] [Google Scholar]; b Dastbaravardeh N.; Christakakou M.; Haider M.; Schnürch M. Synthesis 2014, 46, 1421–1439 10.1055/s-0033-1338625. [DOI] [Google Scholar]; c Girard S. A.; Knauber T.; Li C.-J. Angew. Chem., Int. Ed. 2014, 53, 74–100 10.1002/anie.201304268. [DOI] [PubMed] [Google Scholar]; d Cavaliere V. N.; Mindiola D. J. Chem. Sci. 2012, 3, 3356–3365 10.1039/c2sc20530k. [DOI] [Google Scholar]; e Ramirez T. A.; Zhao B.; Shi Y. Chem. Soc. Rev. 2012, 41, 931–942 10.1039/c1cs15104e. [DOI] [PubMed] [Google Scholar]
- For selected papers on cyclometalation, see the following:; a Granell J.; Martinez M. Dalton Trans. 2012, 41, 11243–11258 10.1039/c2dt30866e. [DOI] [PubMed] [Google Scholar]; b Han Y.-F.; Jin G.-X. Chem. Soc. Rev. 2014, 43, 2799–2823 10.1039/c3cs60343a. [DOI] [PubMed] [Google Scholar]; c Omae I. J. Organomet. Chem. 2011, 696, 1128–1145 10.1016/j.jorganchem.2010.11.023. [DOI] [Google Scholar]; d Albrecht M. Chem. Rev. 2010, 110, 576–623 10.1021/cr900279a. [DOI] [PubMed] [Google Scholar]; e Engle K. M.; Mei T.-S.; Wasa M.; Yu J.-Q. Acc. Chem. Res. 2012, 45, 788–802 10.1021/ar200185g. [DOI] [PMC free article] [PubMed] [Google Scholar]; f van der Boom M. E.; Milstein D. Chem. Rev. 2003, 103, 1759–1792 10.1021/cr960118r. [DOI] [PubMed] [Google Scholar]; g Johnson K. R. D.; Hayes P. G. Chem. Soc. Rev. 2013, 42, 1947–1960 10.1039/c2cs35356c. [DOI] [PubMed] [Google Scholar]
- a Dastbaravardeh N.; Schnürch M.; Mihovilovic M. D. Org. Lett. 2012, 14, 1930–1933 10.1021/ol300627p. [DOI] [PubMed] [Google Scholar]; b Dastbaravardeh N.; Kirchner K.; Schnürch M.; Mihovilovic M. D. J. Org. Chem. 2013, 78, 658–672 10.1021/jo302547q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- a Dastbaravardeh N.; Schnürch M.; Mihovilovic M. D. Org. Lett. 2012, 14, 3792–3795 10.1021/ol301680v. [DOI] [PubMed] [Google Scholar]; b Dastbaravardeh N.; Schnürch M.; Mihovilovic M. D. Eur. J. Org. Chem. 2013, 14, 2878–2890 10.1002/ejoc.201300004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jun C.-H.; Hwang D.-C.; Na S.-J. Chem. Commun. 1998, 1405–1406 10.1039/a801298i. [DOI] [Google Scholar]
- The complete screening table is included in the Supporting Information.
- Cyclohexanol, which was added in our arylation protocols in order to suppress formation of significant amounts of the corresponding imine of the arylated product, could be omitted since no significant formation of imine byproduct was detected in the alkylation in its absence.
- For selected papers on β-H-eliminations in rhodium complexes, see the following:; a Wu B.; Zhang J.; Yun L.; Fu X. Dalton Trans. 2011, 40, 2213–2217 10.1039/c0dt01146k. [DOI] [PubMed] [Google Scholar]; b Zhang Q.; Yu H.-Z.; Li Y.-T.; Liu L.; Huang Y.; Fu Y. Dalton Trans. 2013, 42, 4175–4184 10.1039/c3dt31898b. [DOI] [PubMed] [Google Scholar]; c Finger M.; Reek J. N. H.; de Bruin B. Organometallics 2011, 30, 1094–1101 10.1021/om1011209. [DOI] [Google Scholar]; d Xu R.; Bittner M.; Klatt G.; Köppel H. J. Phys. Chem. A 2008, 112, 13139–13148 10.1021/jp807676n. [DOI] [PubMed] [Google Scholar]; e Zeng R.; Wu S.; Fu C.; Ma S. J. Am. Chem. Soc. 2013, 135, 18284–18287 10.1021/ja409861s. [DOI] [PubMed] [Google Scholar]; f Rosales A.; Rodriguez-Garcia I.; Lopez-Sanchez C.; Alvarez-Corral M.; Munoz-Dorado M. Tetrahedron 2011, 67, 3071–3075 10.1016/j.tet.2011.03.004. [DOI] [Google Scholar]; g van der Boom M. E.; Higgitt C. L.; Milstein D. Organometallics 1999, 18, 2413–2419 10.1021/om990073v. [DOI] [Google Scholar]; h Luo X.; Tang D.; Li M. Theochem. J. Mol. Struct. 2005, 731, 139–147 10.1016/j.theochem.2005.06.020. [DOI] [Google Scholar]
- For selected papers on C-C activation, see the following:; a Low J. J.; Goddard W. A. III J. Am. Chem. Soc. 1984, 106, 8321–8322 10.1021/ja00338a067. [DOI] [Google Scholar]; b Siegbahn P. E. M.; Blomberg M. R. A. J. Am. Chem. Soc. 1992, 114, 10548–10556 10.1021/ja00052a059. [DOI] [Google Scholar]; c Rybtchinski B.; Milstein D. Angew. Chem., Int. Ed. 1999, 38, 879–883. [DOI] [PubMed] [Google Scholar]; d Wentzel M. T.; Reddy V. J.; Hyster T. K.; Douglas C. J. Angew. Chem., Int. Ed. 2009, 48, 6121–6123 10.1002/anie.200902215. [DOI] [PubMed] [Google Scholar]; e Bowring M. A.; Bergman R. G.; Tilley T. D. J. Am. Chem. Soc. 2013, 135, 13121–13128 10.1021/ja406260j. [DOI] [PubMed] [Google Scholar]; f Evans M. E.; Li T.; Jones W. D. J. Am. Chem. Soc. 2010, 132, 16278–16284 10.1021/ja107927b. [DOI] [PubMed] [Google Scholar]; g Chaplin A. B.; Green J. C.; Weller A. S. J. Am. Chem. Soc. 2011, 133, 13162–13168 10.1021/ja2047599. [DOI] [PubMed] [Google Scholar]; h Cramer N.; Seiser T. Synlett 2011, 4, 449–460 10.1055/s-0030-1259536. [DOI] [Google Scholar]; i van der Boom M. E.; Liou S.-Y.; Ben-David Y.; Gozin M.; Milstein D. J. Am. Chem. Soc. 1998, 120, 13415–13421 10.1021/ja982345b. [DOI] [Google Scholar]
- Details about the kinetic profile and especially the calculated regression curves are given in the Supporting Information.
- All chemical reactions showing consecutive reaction kinetics like multiple step catalytic processes have in fact an induction period for the formation of the final product. However, in many cases it is too short to be determined reliably.
- Jun C.-H.; Moon C. W.; Lee D.-Y. Chem.—Eur. J. 2002, 8, 2422–2428. [DOI] [PubMed] [Google Scholar]
- The water content in K2CO3 was determined by the loss on heating by heating the solid up to about 200 °C under medium vacuum overnight assuming that only water was lost.
- For selected papers on C–H activation reactions showing a beneficial effect of water (not including protocols employing water as solvent), see the following:; a Jiao L.; Bach T. J. Am. Chem. Soc. 2011, 133, 12990–12993 10.1021/ja2055066. [DOI] [PubMed] [Google Scholar]; b Zhao Y.-B.; Mariampillai B.; Candito D. A.; Laleu B.; Li M. Z.; Lautens M. Angew. Chem., Int. Ed. 2009, 48, 1849–1852 10.1002/anie.200805780. [DOI] [PubMed] [Google Scholar]; c Gu Z.-S.; Chen W.-X.; Shao L.-X. J. Org. Chem. 2014, 79, 5806–5811 10.1021/jo5010058. [DOI] [PubMed] [Google Scholar]; d Zheng Y.; Xiong T.; Lv Y.; Zhang J.; Zhang Q. Org. Biomol. Chem. 2013, 11, 7923–7930 10.1039/c3ob41299g. [DOI] [PubMed] [Google Scholar]
- The H–D exchange in the deuterated amine was determined by means of transmission IR spectroscopy. Details are given in the Supporting Information.
- In general, the absence of a (significant) KIE is not evidence that the corresponding bond is not broken in the rate-determining step. Since in our case a primary KIE was already observed for the benzylic C–H bonds, however, we could conclude that the N–H bond is not broken in the rate-determining step.
- Of course, this experiment would not completely exclude an imine mechanism even if the corresponding alkylation products were formed at a similar rate because the reaction could theoretically occur then via a different mechanism.
- Jo E.-A.; Lee J.-H.; Jun C.-H. Chem. Commun. 2008, 44, 5779–5781 10.1039/b814166e. [DOI] [PubMed] [Google Scholar]
- Similar methods to investigate reaction mechanisms are readily employed in the determination of biochemical reaction mechanisms:Crampin E. J.; Schnell S.; McSharry P. E. Prog. Biophys. Mol. Biol. 2004, 86, 77–112 10.1016/j.pbiomolbio.2004.04.002. [DOI] [PubMed] [Google Scholar]
- For selected papers on Rh(III) complexes undergoing oxidative addition to form Rh(V), see the following:; a Gangopadhyay S.; Basak P.; Drew M.; Gangopadhyay P. K. Chem. Commun. 2010, 46, 7436–7438 10.1039/c0cc01970d. [DOI] [PubMed] [Google Scholar]; b Esqueda A. C.; Conejero S.; Maya C.; Carmona E. Organometallics 2010, 29, 5481–5489 10.1021/om100412q. [DOI] [Google Scholar]; c McBee J. L.; Escalada J.; Tilley T. D. J. Am. Chem. Soc. 2009, 131, 12703–12713 10.1021/ja9035169. [DOI] [PubMed] [Google Scholar]; d Sunada Y.; Fujimura Y.; Nagashima H. Organometallics 2008, 27, 3502–3513 10.1021/om800151w. [DOI] [Google Scholar]; e Karshtedt D.; Bell A. T.; Tilley T. D. Organometallics 2006, 25, 4471–4482 10.1021/om060492+. [DOI] [Google Scholar]; f Brayshaw S. K.; Sceats E. L.; Green J. C.; Weller A. S. Proc. Natl. Acad. Sci. U.S.A. 2007, 104, 6921–6926 10.1073/pnas.0609824104. [DOI] [PMC free article] [PubMed] [Google Scholar]; g Nagashima H.; Tatebe K.; Ishibashi T.; Nakaoka A.; Sakakibara J.; Itho K. Organometallics 1995, 14, 2868–2879 10.1021/om00006a036. [DOI] [Google Scholar]
- For selected papers on Rh-catalyzed transfer hydrogenation reactions, see the following:; a Rafikova K.; Kystaubayeva N.; Aydemir M.; Kayan C.; Ocak Y. S.; Temel H.; Zazybin A.; Gürbüz N.; Özdemir I. J. Organomet. Chem. 2014, 758, 1–8 10.1016/j.jorganchem.2014.01.025. [DOI] [Google Scholar]; b Akinci P. A.; Gülcemal S.; Kazheva O. N.; Alexandrov G. G.; Dyachenko O. A.; Cetinkaya E.; Cetinkaya B. J. Organomet. Chem. 2014, 765, 23–30 10.1016/j.jorganchem.2014.04.033. [DOI] [Google Scholar]; c Shende V. S.; Shingote S. K.; Deshpande S. H.; Kuriakose N.; Vanka K.; Kelkar A. A. RSC Adv. 2014, 4, 46351–46356 10.1039/C4RA07964G. [DOI] [Google Scholar]; d Prakash O.; Sharma K. N.; Joshi H.; Gupta P. L.; Singh A. K. Organometallics 2014, 33, 983–993 10.1021/om401150s. [DOI] [Google Scholar]; d Prakash O.; Sharma K. N.; Joshi H.; Gupta P. L.; Singh A. K. Organometallics 2014, 33, 2535–2543 10.1021/om500149n. [DOI] [Google Scholar]; e Saleem F.; Rao G. K.; Kumar A.; Mukherjee G.; Singh A. K. Organometallics 2014, 33, 2341–2351 10.1021/om500266p. [DOI] [Google Scholar]; f Nova A.; Taylor D. J.; Blacker A. J.; Duckett S. B.; Perutz R. N.; Eisenstein O. Organometallics 2014, 33, 3433–3442 10.1021/om500519j. [DOI] [Google Scholar]; g Prakash O.; Joshi H.; Sharma K. N.; Gupta P. L.; Singh A. K. Organometallics 2014, 33, 3804–3812 10.1021/om500515z. [DOI] [Google Scholar]; h Madrigal D.; Cooksy A. L.; Somanathan R. Comput. Theor. Chem. 2012, 999, 105–108 10.1016/j.comptc.2012.08.021. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.



