Abstract
The effect of 2,2′-pyridylisatogen tosylate (PIT) on the human P2Y1 receptor and on other recombinant P2Y receptors has been studied. We first examined the modulation by PIT of the agonist-induced accumulation of inositol phosphates. PIT blocked 2-methylthio-ADP (2-MeSADP)-induced accumulation of inositol phosphates in 1321N1 astrocytoma cells stably expressing human P2Y1 receptors in a non-competitive and concentration-dependent manner. The IC50 for reduction of the maximal agonist effect was 0.14 μM. In contrast, MRS2179, a competitive P2Y1 receptor antagonist, parallel-shifted the agonist concentration–response curve to the right. PIT also concentration-dependently blocked the P2Y1 receptor signaling induced by the endogenous agonists, ADP and ATP. A simple structural analogue of PIT was synthesized and found to be inactive as a P2Y1 receptor antagonist, suggesting that the nitroxyl group of PIT is a necessary structural component for P2Y1 receptor antagonism. We next examined the possible modulation of the binding of the newly available antagonist radioligand for the P2Y1 receptor, [3H] MRS2279. It was found that PIT (0.01–10 μM) did not inhibit [3H] MRS2279 binding to the human P2Y1 receptor. PIT (10 μM) had no effect on the competition for [3H] MRS2279 binding by agonists, ADP and ATP, suggesting that its antagonism of the P2Y1 receptor may be allosteric. PIT had no significant effect on agonist activation of other P2Y receptors, including P2Y2, P2Y4, P2Y6, P2Y11 and P2Y12 receptors. Thus, PIT selectively and non-competitively blocked P2Y1 receptor signaling without affecting nucleotide binding.
Keywords: P2Y receptors, Allosteric modulation, ADP, ATP, PIT, GPCR, Purines, Nucleotides
1. Introduction
P2 receptors exist in most tissues [1–3]. Physiological responses to extracellular nucleotides occur via both ion channel-coupled P2X receptors and G protein-coupled P2Y receptors. The P2Y1 receptor was the first member in the P2Y receptor family to be identified through cloning and is widely distributed. Its occurrence in mammalian brain, heart, vascular, kidney, liver, prostate, pulmonary and connective tissues has been described [1]. The activation of P2Y1 receptors induces the activation of phospholipase Cβ (PLCβ) leading to the formation of inositol trisphosphate and mobilization of intracellular Ca2+ as well as diacylglycerol and subsequent activation of protein kinase C (PKC). ADP and its more potent analogue 2-methylthio-ADP (2-MeSADP) are agonists at P2Y1 receptors, while the degree of intrinsic efficacy of ATP and 2-MeSATP are controversial [4–6].
2,2′-Pyridylisatogen (PIT, as tosylate form) is one of the isatogen analogues that were initially designed to be antagonists for P2 receptors [7]. PIT had been used to differentiate P1 (adenosine) and P2 (nucleotide) receptors [8]. It was later demonstrated that PIT is an allosteric modulator of the chicken P2Y1 receptor expressed in Xenopus oocytes [9]. Over a narrow concentration range (0.1–3 μM), PIT caused a potentiation (two to five-fold) of responses to ATP. However, PIT failed to potentiate inward currents induced by 2-MeSATP and it inhibited this agonist-induced response. PIT has diverse effects, including neuroprotective effects [10], and acts as a spin trapping agent [11].
Allosteric modulation of GPCRs is of increasing interest for possible therapeutic application [12]. In this study, we further evaluated the possible modulatory effects of PIT on agonist-induced accumulation of inositol phosphates in 1321N1 astrocytoma cells stably expressing P2Y1, P2Y2, P2Y4, P2Y6, and P2Y11 receptors, in Chinese hamster ovary (CHO) cells expressing the P2Y12 receptor, and on the binding of the newly available antagonist radioligand, [3H] MRS2279 [13], for the P2Y1 receptor. We found that PIT selectively and non-competitively blocked P2Y1 receptor signaling without affecting the binding of ADP and [3H] MRS2279. In contrast to previous findings with the avian subtype [9], PIT did not enhance human P2Y1 receptor activity.
2. Materials and methods
2.1. Materials
myo-[3H] Inositol (20 Ci/mmol) was obtained from American Radiolabeled Chemicals (St. Louis, MO). ADP, ATP, 2-MeSADP and MRS2179 (N6-methyl-2′-deoxyadnosine-3′,5′-bisphosphate), amiloride (3,5-diamino-N-(aminoiminomethyl)-6-chloro-pyrazinecarboxamide) hydrochloride, amiloride analogues, and agmatine (N-(4-aminobutyl)guanidine) sulfate were purchased from Sigma (St. Louis, MO, USA). PIT (2-(2-pyridinyl)-(3H)-indol-3-one-1-oxide 4-methylbenzenesulfonate, >99% purity by HPLC), PPADS (pyridoxal phosphate-6-azobenzene-2,4-disulfonic acid), suramin, and SCH-202676 were from Tocris (Ellisville, MO, USA). Pharmacological substances were stored as DMSO stock solutions at 4 °C. The radioligand [3H] MRS2279 (2-chloro-N6-methyl-(N)-methanocarba-2′-deoxyadenosine-3′,5′-bisphosphate) was prepared as described [13].
2.2. Cell culture and membrane preparation
Human astrocytoma cells stably expressing human P2Y1, P2Y2, P2Y4, P2Y6, and P2Y11 and rat P2Y6 receptors were cultured in Dulbecco’s modified Eagle’s medium (DMEM, JRH Biosciences Inc., Lenexa, KS, USA) and F12 (1:1) supplemented with 10% fetal bovine serum, 100 units penicillin per ml, 100 μg streptomycin/ml, 2 μmol glutamine per ml, and 500 μg geneticin per ml. After harvesting, the cells were homogenized and suspended and then centrifuged at 100 × g for 5 min at room temperature. The pellet was resuspended in 50 mM tris(hydroxymethyl)aminomethane (Tris)–HCl buffer (pH 7.4). The suspension was homogenized with a Polytron electric homogenizer (Brinkmann, NY, USA) for 10 s and was then re-centrifuged at 20,000 × g for 20 min at 4 °C. The resultant pellets were resuspended in buffer, and the suspension was stored at −80 °C until the binding experiments. The protein concentration was measured with the Bradford assay [14].
2.3. Radioligand binding assays
P2Y1 receptor binding experiments were performed as previously described [13]. Briefly, membranes (40 μg protein per tube) from astrocytoma cells stably expressing human P2Y1 receptors were incubated with [3H] MRS2279 (8 nM) for 30 in at 4 °C in a total assay volume of 200 μl. The radiolabeled ligand concentration used in the assay approximated the Kd value in binding to the receptor. Binding reactions were terminated by filtration through Whatman GF/B glass-fiber filters under reduced pressure with a MT-24 cell harvester (Brandel, Gaithersburg, MD, USA), and radioactivity was determined with a Packard liquid scintillation counter (Perkin-Elmer, Downers Grove, IL, USA).
2.4. Functional assays of receptor activation
The assay of PLC activation was carried out as previously described [15]. 1321N1 astrocytoma cells stably expressing human P2Y receptors were harvested by trypsinization and grown in six-well plates (−106 cells per well; Costar, Cambridge, MA) in DMEM culture medium supplemented with 2 μCi/ml of myo-[3H] inositol. After a 24-h labeling period, cells were pre-incubated with 10 mM LiCl and for 20 min at room temperature. Because IP3, the initial product of PLC activity and the relevant second messenger downstream of the P2Y receptors, is rapidly converted to the di-and monophosphate, which is later metabolized to myo-inositol, lithium chloride was added to inhibit myo-inositol 1-phosphatase [16]. Thus, measurement of myo-inositol 1-phosphate (IP) as end product of this cascade represented PLC activity. The mixtures were swirled to ensure uniformity. Following the addition of agonists, the cells were incubated for 30 min at 37° and 5% CO2. The supernatants were removed by aspiration, and 800 μl of cold 20 mM formic acid was added to each well. Cell extracts were collected after a 30 min incubation at 4 °C and neutralized with 300 μl of 60 mM NH4OH. The inositol monophosphate fraction was then isolated by anion exchange chromatography. The content of each well was applied to a small anion exchange column (AG-1-X8; Bio-Rad, Hercules, CA) that had been pretreated with 15 ml of 0.1 M formic acid/3 M ammonium formate, followed by 15 ml of water. The columns were then washed with 15 ml of a solution containing 5 mM sodium borate and 60 mM sodium formate. [3H] Inositol phosphates were eluted twice with 5 ml of 0.1 M formic acid/ 0.2 M ammonium formate, and radioactivity was quantified by liquid scintillation counting (LKB Wallace 1215 Rackbeta scintillation counter).
A functional assay of stimulation of adenylate cyclase via the hP2Y12 receptor stably expressed in CHO cells was carried out in the presence of 10 μM forskolin by methods previously described [17].
2.5. Synthesis of MRS3461
N-Pyridin-2-yl-phthalimide (MRS3461) was synthesized from equimolar amounts of pyridine-2-yl-amine and phthaloyl dichloride in N,N-dimethylformamide at room temperature [18]. The solution was concentrated and poured into water, which was then extracted with diethyl ether. The ether extracts were concentrated, and the product was purified on preparative thin layer chromatography, eluting with chloroform/methanol (98/2, by volume), and re-crystallized from ethyl acetate. Mass spectra and nuclear magnetic resonance spectra confirmed the white crystals to be the desired compound. 1H NMR (CDCI3, 300 MHz) δ8.75–8.65 (m, 1H), 7.99–7.79 (m, 4H), 7.46–7.43 (m, 1H), 7.38–7.35 (m, 1H), 7.26 (s, 1H). MS (fast atom bombardment, positive mode) m/z = 225 (M + 1).
2.6. Statistical analysis
Binding and functional parameters were calculated using the Prism software (GraphPAD, San Diego, CA, USA). IC50 values obtained from competition curves were converted to Ki values using the Cheng–Prusoff equation [19]. Data were expressed as mean ± standard error.
3. Results
3.1. Effects of PIT on agonist-induced accumulation of inositol phosphates in 1321N1 astrocytoma cells stably expressing human P2Y1 receptors
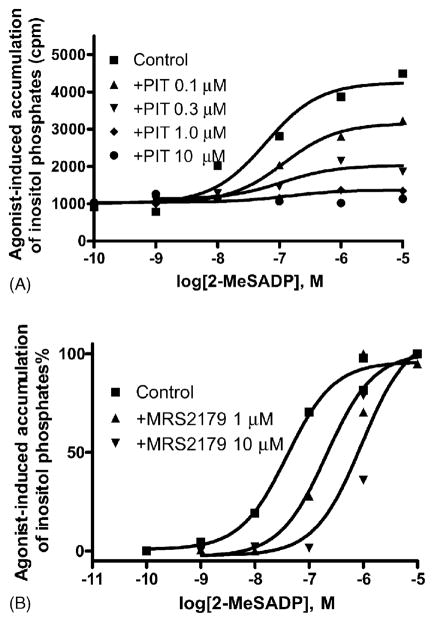
The P2Y1 receptor agonist 2-MeSADP-induced accumulation of inositol phosphates with an EC50 value of 36± 14 nM (n = 3). PIT (0.1–10 μM) diminished human P2Y1 receptor signaling in a non-competitive, concentration-dependent manner. The maximal agonist effect was reduced progressively by increasing concentrations of PIT, with an IC50 of 0.14 μM. PIT (10 μM) completely blocked the agonist activity of 2-MeSADP (Fig. 1A). As a control, MRS2179, a known competitive P2Y1 receptor antagonist [20], right-shifted the agonist concentration–response curve in parallel (Fig. 1B). PIT itself (0.1–10 μM) failed to induce accumulation of inositol phosphates directly in 1321N1 astrocytoma cells stably expressing human P2Y1 receptors (data not shown); thus, it was not an agonist at this receptor.
Fig. 1.
Effects of PIT and MRS2179, a competitive P2Y1 receptor antagonist, on agonist-induced accumulation of IP, as a measure of inositol phosphates in 1321N1 astrocytoma cells stably expressing the human P2Y1 receptor. Data were from one experiment performed in duplicate, which represents at least three independent experiments of similar results. The EC50 value of 2-MeSADP listed in the text was calculated from three independent experiments performed in duplicate.
Based on the finding that PIT antagonized activation of the P2Y1 receptor, we prepared a structural analogue MRS3461, which contained a carbonyl group in place of the nitroxyl group of PIT (Fig. 2). In contrast to PIT, which concentration-dependently inhibited accumulation of inositol phosphates induced by the agonist 2-MeSADP, MRS3461 had no significant effect (Fig. 3a). PIT also blocked the P2Y1 receptor signaling induced by the endogenous agonist ADP in a concentration-dependent manner (Fig. 3b).
Fig. 2.

Chemical structures of pyridylisatogen and MRS3461.
Fig. 3.
(A) Effects of PIT and a structural analogue MRS3461 on agonist 2-MeSADP (100 nM)-induced accumulation of IP, as a measure of inositol phosphates in 1321N1 astrocytoma cells stably expressing P2Y1 receptors (n = 3). (B) Effect of PIT on agonist ADP (1 μM)-induced accumulation of inositol phosphates.
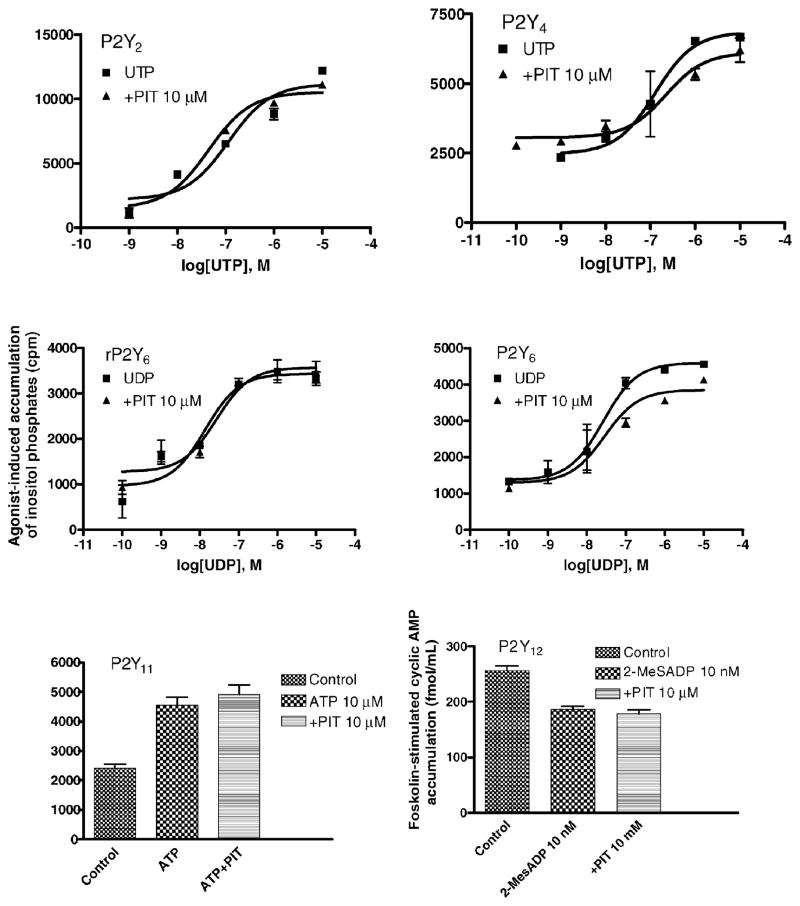
3.2. Effects of PIT on other P2Y receptors
PIT (10 μM) had no significant effects on UTP-induced P2Y2 and P2Y4 receptor signaling, ATP-induced P2Y11 receptor signaling, or 2-MeSADP-induced P2Y12 receptor signaling (Fig. 4). PIT also had no effect on rat P2Y6 receptor signaling at concentrations up to 10 μM. At concentrations ≤1 μM, PIT had no effect on the activation of the human P2Y6 receptor, and at a higher concentration (10 μM) PIT induced only a modest decrease of the signaling (Fig. 4).
Fig. 4.
Effects of PIT on activation of human P2Y2, P2Y4, P2Y6, P2Y11 and P2Y12 receptors and rat P2Y6 receptors. Inhibition by 2-MeSADP of forskolin (10 μM)-stimulated activation of adenylate cyclase was the indicator of activity at the P2Y12 receptor (receptor stably expressed in CHO cells). At all of the other receptors, stimulation of PLC was measured (receptors expressed in 1321N1 astrocytoma cells), using as agonists: UTP (P2Y2, P2Y4), UDP (P2Y6), and ATP (P2Y11). Data were from one experiment performed in duplicate, which represents three independent experiments of similar results.
3.3. Affinity of PIT and other potential allosteric modulators, and known P2Y ligands for human P2Y1 receptors stably expressed in human astrocytoma cells
Fig. 5 shows that the competitive antagonist MRS2179 displaced the binding of [3H] MRS2279 with a Ki value of 103 ± 37 nM (n = 3) PIT at concentrations ranging from 0.1 to 100 μM had no effect on [3H] MRS2279 binding; thus, it appeared to antagonize the P2Y1 receptor through an allosteric mechanism. Similarly various broad spectrum GPCR modulators such as the thiadiazole derivative SCH-202676, amiloride and its analogues, and agmatine [21–27] also did not compete for the binding of [3H] MRS2279. The potency of known agonists and antagonists and potential allosteric modulators were summarized in Table 1.
Fig. 5.
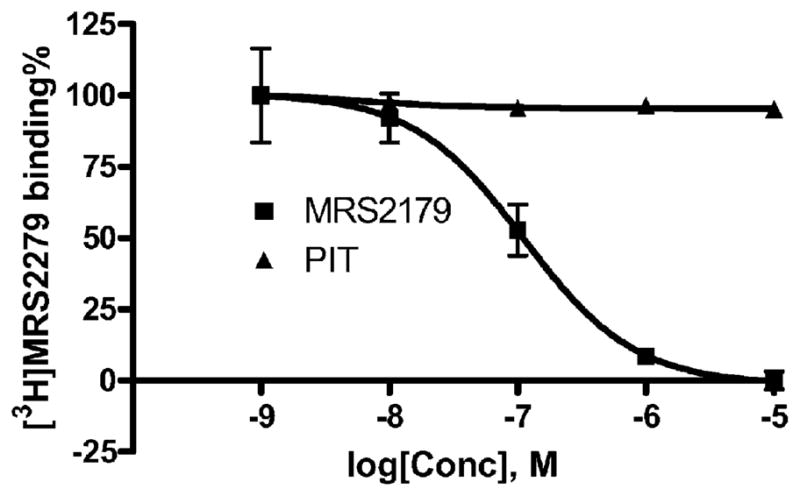
Effect of PIT and MRS2179 on the binding of [3H] MRS2279 to membranes from astrocytoma cells stably expressing the human P2Y1 receptor. Membranes (40 μg protein per tube) from 1321N1 astrocytoma cells stably expressing the human P2Y1 receptor were incubated with [3H] MRS2279 (8 nM) for 30 min at 4 °C. Results were expressed as mean ± S.E.M. from three independent experiments performed in duplicate. The potencies of PIT and MRS2179 are listed in Table 1.
Table 1.
Effects on radioligand binding at the human P2Y1 receptor of PIT and various known, broad-spectrum allosteric modulators of GPCRs and a series of P2Y1 receptor agonists and antagonistsa
| Allosteric modulators, % inhibition (concentration) | P2Y receptor ligands, Ki (nM) | ||
|---|---|---|---|
| PITb (μM) | 0 (100) | ADP | 897 ± 163 |
| SCH-202676 (μM) | 0 (10) | ATP | 1246 ± 331 |
| Agmatine (mM) | 0 (1) | 2-MeSADP | 57 ± 12 |
| Amiloride (mM) | 0 (1) | 2-MeSATP | 66 ± 20 |
| DMAc (μM) | 0 (100) | Suramin | 3265 ± 976 |
| HMAc (μM) | 0 (100) | PPADS | 5320 ± 1238 |
| MIBAc (μM) | 0 (100 | MRS2179 | 103 ± 37 |
Membranes (40 μg protein) from 1321N1 astrocytoma cells stably expressing human P2Y1 receptors were incubated with [3H] MRS2279 (8 nM) for 30 min at 4 °C. Results were expressed as mean ± S.E.M. from three independent experiments performed in duplicate.
PIT was found to compete for binding of [3H] N6-R-(phenylisopyl)adenosine to human A1 adenosine receptors with a pKi of 5.3 [9].
Abbreviations: DMA, 5-(N,N-dimethyl)amiloride; HMA, 5-(N,N-hexamethylene)amiloride; MIBA, 5-(N-methyl-N-isobutyl)amiloride.
3.4. Effect of PIT on agonist-competition curves for [3H] MRS2279
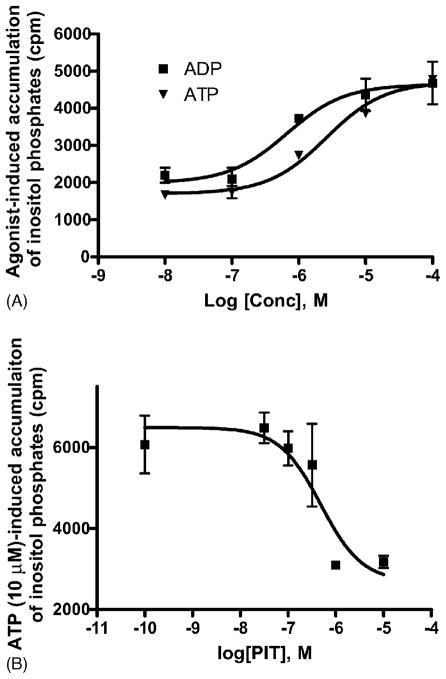
Both ADP and ATP concentration-dependently inhibited [3H] MRS2279 binding to P2Y1 receptors (Fig. 6). Since PIT blocked agonist-induced receptor activation in a concentration-dependent manner, we examined the possibility of PIT to shift the agonist competition curves using the antagonist radioligand [3H] MRS2279. However, PIT (10 μM) failed to influence the competition of ADP and ATP for the binding of [3H] MRS2279 (Fig. 6).
Fig. 6.
Effect of PIT on the competition curves of ADP and ATP for binding of the antagonist radioligand [3H] MRS2279. Membranes (40 μg protein per tube) from 1321N1 astrocytoma cells stably expressing the human P2Y1 receptor were incubated with [3H] MRS2279 (8 nM) for 30 min at 4 °C. Data were expressed as mean ± S.E.M. from three independent experiments performed in duplicate.
3.5. ADP and ATP-induced-accumulation of inositol phosphates in 1321N1 astrocytoma cells stably expressing P2Y1 receptors and the effects of PIT
The effects of ADP and ATP as agonists were compared. It was found that both ADP and ATP were fully efficacious at the human P2Y1 receptor in the current assay, with ADP being more potent (Fig. 7A). As King et al. [9] reported, PIT might enhance the effect of ATP on the chicken P2Y1 receptor expressed in Xenopus oocytes over a narrow range of concentrations; here we further examined if PIT may enhance the functional effect of ATP on the human P2Y1 receptor stably expressed in 1321N1 astrocytoma cells. However, contrary to previous findings, PIT did not enhance the ATP effect (Fig. 7B).
Fig. 7.
(A) ADP- and ATP-induced accumulation of IP, as a measure of inositol phosphates in 1321N1 astrocytoma cells stably expressing the human P2Y1 receptor. (B) Effect of PIT on ATP-induced accumulation of inositol phosphates.
3.6. Effect of PIT on the kinetics of dissociation of [3H] MRS2279 from P2Y1 receptors
The binding of 8 nM [3H] MRS2279 to membranes from 1321N1 astrocytoma cells stably expressing human P2Y1 receptors was rapid, within 5 min reaching an apparent steady state that was maintained for at least 60 min (data not shown). The dissociation was initiated by addition of 10 μM MRS2179 in the absence and presence of 10 μM PIT. As shown in Fig. 8, PIT had no effect on [3H] MRS2279 dissociation. The k−1 values in the absence and presence of 10 μM of PIT were 0.55 ± 0.13 and 0.49 ± 04 min−1, respectively, which were not significantly different.
Fig. 8.
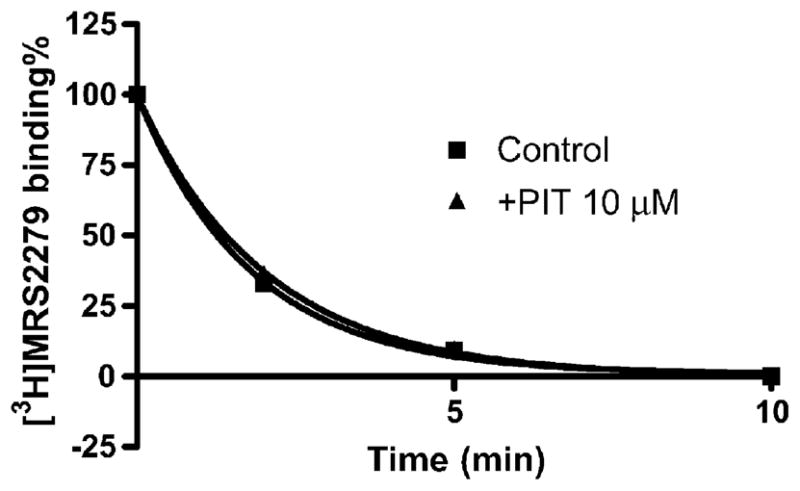
Effect of PIT on the dissociation of the antagonist radioligand [3H] MRS2279 from P2Y1 receptors. Membranes (40 μg protein per tube) from 1321N1 astrocytoma cells stably expressing the human P2Y1 receptor were incubated with [3H] MRS2279 (8 nM) for 30 min at 4 °C. The dissociation was initiated by addition of 10 μM MRS2179 in the absence and presence of PIT. Data were from a representative experiment performed in duplicate. The k−1 values listed in the text were calculated from three independent experiments performed in duplicate.
4. Discussion
Here we demonstrated that PIT selectively, non-competitively blocked human P2Y1 receptor activation in 1321N1 astrocytoma cells. PIT did not affect the binding of the antagonist [3H] MRS2279 or agonists ADP/ATP to the human P2Y1 receptor. It has been reported previously that PIT might enhance the effect of ATP but not 2-MeSATP on the chicken P2Y1 receptor expressed in Xenopus oocytes over a narrow range of concentrations (0.1–3 μM) [9]. Also, PIT enhanced purinergic vasoconstriction in the rabbit splenic artery [28]. However, in the present study PIT failed to potentiate the response induced by any of the agonists at the human P2Y1 receptor; on the contrary, it blocked the agonist-induced response. The apparent difference between the results from the present study and those previously reported [9] might be due to a number of factors, for example, species difference, different cell lines, different assay systems and differences in receptor reserve. By analogy, differences in receptor reserve may account for the observed variation in the intrinsic efficacy of ATP at P2Y1 receptors. It has been reported that ATP is an antagonist for human P2Y1 receptors in Jurkat cells and endothelial cells [4]. However, Palmer et al. [5] reported that ATP is an agonist in 1321N1 astrocytoma cells stably expressing human P2Y1 receptors. In the present study, consistent with the report by previous findings [5], ATP was as efficacious as the full agonist ADP.
In addition to PIT, which did not affect nucleotide binding, other substances known to act as general GPCR modulators also did not show any effect on [3H] MRS2279 binding to the P2Y1 receptor. These modulators include SCH-202676 [21–23], amiloride analogues [24–26], agmatine [27]. The results suggested that for the P2Y1 receptor, modes of interaction of orthosteric and allosteric binding sites might be different from that of other GPCRs.
The non-competitive antagonism of the accumulation of inositol phosphates induced by a P2Y1 receptor agonist in 1321N1 astrocytoma cells suggested that PIT might shift agonist competition curves in binding of the antagonist radioligand, [3H] MRS2279. However, this was not demonstrated in the present study. Therefore, one possible explanation of the antagonism by PIT is that it is allosteric, i.e. by acting at a site on the receptor distinct from the agonist binding site. Another explanation would be action at a protein that associates with the P2Y1 receptor, such as Gαq. The antagonism could not be purely at the level of the G protein, since other P2Y receptors, which act though the same effector system remained functional in the presence of PIT. It would be helpful to demonstrate if PIT could influence the dissociation kinetics of the agonist radioligand. However, the P2Y1 receptor agonist radioligand has not been commercially available. Also, we did not measure the possible action (or lack of effect) of PIT on another ADP-preferring nucleotide receptor, the P2Y13 subtype [17].
Although this study did not investigate in detail the structure activity relationship of PIT and its analogues [10], a simple structural analogue MRS 3461 was synthesized and found to be inactive as a P2Y1 receptor antagonist. Thus, it appears that the nitroxyl group of PIT is a necessary structural component for antagonism of the P2Y1 receptor.
In conclusion, our data demonstrated that PIT subtype-selectively and non-competitively blocked P2Y1 receptor signaling without affecting the nucleotide binding. We found no evidence for allosteric enhancement by PIT of the recombinant human P2Y1 receptor expressed in astrocytoma cells.
Acknowledgments
We thank Professors T. Kendall Harden and Robert Nicholas of the University of North Carolina for providing 132 INI-astrocytoma cells stably transfected with human P2Y1, P2Y2, P2Y4 and P2Y6 receptors. We thank Dr. Brian King (University College, London) for helpful discussion.
Abbreviations
- ADP
adenosine 5′-diphosphate
- ATP
adenosine 5′-triphosphate
- DMEM
Dulbecco’s modified Eagle’s medium
- IP
myo-inositol 1-phosphate
- MRS2179
N6-methyl-2-deoxyadenosine-3′,5′-bisphosphate
- MRS2279
2-chloro-N6-methyl-(N)-methanocarba-2′-deoxyadenosine-3′,5′-bisphosphate
- MRS3461
N-pyridin-2-yl-phthalimide
- 2-MeSADP
2-methylthio-adenosine 5′-diphosphate
- PIT
2,2′-pyridylisatogen tosylate
- PLC
phospholipase C
- SCH-202676
N-(2,3-diphenl-l,2,4-thiadiazol-5-(2H)-ylidene)methanamine
- Tris
tris(hydroxymethyl)aminomethane
References
- 1.Ralevic V, Burnstock G. Receptors for purines and pyrimidines. Pharmacol Rev. 1998;50:13–92. [PubMed] [Google Scholar]
- 2.Jacobson KA, Jarvis MF, Williams M. Purine and pyrimidine (P2) receptors as drug targets. J Med Chem. 2002;45:4057–93. doi: 10.1021/jm020046y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lazarowski ER, Boucher RC, Harden TK. Mechanisms of release of nucleotides and integration of their action as P2X- and P2Y-receptor activating molecules. Mol Pharmacol. 2003;64:785–95. doi: 10.1124/mol.64.4.785. [DOI] [PubMed] [Google Scholar]
- 4.Hechler B, Vigne P, Leon C, Breittmayer JP, Gachet C, Frelin C. ATP derivatives are antagonists of the P2Y1 receptor: similarities to the platelet ADP receptor. Mol Pharmacol. 1998;53:727–33. [PubMed] [Google Scholar]
- 5.Palmer RK, Boyer JL, Schachter JB, Nicholas RA, Harden TK. Agonist action of adenosine triphosphates at the human P2Y1 receptor. Mol Pharmacol. 1998;54:1118–23. [PubMed] [Google Scholar]
- 6.von Kiigelgen I, Wetter A. Molecular pharmacology of P2Y-receptors. Naunyn Schmiedebergs Arch Pharmacol. 2000;362:310–23. doi: 10.1007/s002100000310. [DOI] [PubMed] [Google Scholar]
- 7.Spedding M, Menton K, Markham A, Weetman DF. Antagonists and the purinergic nerve hypothesis: 2,2′-pyridylisatogen tosylate (PIT) J Auton Nerv Syst. 2000;81:25–7. doi: 10.1016/s0165-1838(00)00142-9. [DOI] [PubMed] [Google Scholar]
- 8.Spedding M, Weetman DF. Identification of separate receptors for adenosine and adenosine 5′-triphosphate in causing relaxations of the isolated taenia of the guinea-pig caecum. Br J Pharmacol. 1976;57:305–10. doi: 10.1111/j.1476-5381.1976.tb07480.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.King BF, Dacquet C, Ziganshin AU, Weetman DF, Burnstock G, Vanhoutte PM, et al. Potentiation by 2,2′-pyridylisatogen tosylate of ATP-responses at a recombinant P2Y purinoceptor. Br J Pharmacol. 1996;117:1111–8. doi: 10.1111/j.1476-5381.1996.tb16704.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Menton K, Spedding M, Gressens P, Villa P, Williamson T, Markham A. Role of spin trapping and P2Y receptor antagonism in the neuroprotective effects of 2,2′-pyridylisatogen tosylate and related compounds. Eur J Pharmacol. 2002;444:53–60. doi: 10.1016/s0014-2999(02)01583-2. [DOI] [PubMed] [Google Scholar]
- 11.Nepveu F, Souchard JP, Rolland Y, Dorey G, Spedding M. 2-2′-Pyridylisatogen, a selective allosteric modulator of P2 receptors. Biochem Biophys Res Commun. 1998;242:272–6. doi: 10.1006/bbrc.1997.7949. [DOI] [PubMed] [Google Scholar]
- 12.Christopoulos A. Allosteric binding sites on cell-surface receptors: novel targets for drug discovery. Nat Rev Drug Disc. 2002;1:198–210. doi: 10.1038/nrd746. [DOI] [PubMed] [Google Scholar]
- 13.Waldo GL, Corbitt J, Boyer JL, Ravi G, Kim HS, Ji XD, et al. Quantitation of the P2Y1 receptor with a high affinity radiolabeled antagonist. Mol Pharmacol. 2002;62:1249–57. doi: 10.1124/mol.62.5.1249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–54. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 15.Boyer JL, Adams M, Ravi RG, Jacobson KA, Harden TK. 2-Chloro N6-methyl-(N)-methanocarba-2′-deoxyadenosine-3′,5′-bisphosphate is a selective high affinity P2Y1 receptor antagonist. Br J Pharmacol. 2002;135:2004–10. doi: 10.1038/sj.bjp.0704673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Marteau F, Le Poul E, Communi D, Communi D, Labouret C, Savi P, et al. Pharmacological characterization of the human P2Y13 receptor. Mol Pharmacol. 2003;64:104–12. doi: 10.1124/mol.64.1.104. [DOI] [PubMed] [Google Scholar]
- 16.Berridge MJ, Downes CP, Hanley MR. Lithium amplifies agonist-dependent phosphatidylinositol responses in brain and salivary glands. Biochem J. 1982;206:587–95. doi: 10.1042/bj2060587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Feist S, Feist K, Schultz J. Versuche in der 2-aminopyridin reihe II. Arch Pharm. 1934;272:785–91. [Google Scholar]
- 19.Cheng Y-C, Prusoff WH. Relationship between the inhibition constant (Ki) and the concentration of inhibitor which causes 50 percent inhibition (I50) of an enzymatic reaction. Biochem Pharmacol. 1973;22:3099–108. doi: 10.1016/0006-2952(73)90196-2. [DOI] [PubMed] [Google Scholar]
- 20.Camaioni E, Boyer JL, Mohanram A, Harden TK, Jacobson KA. Deoxyadenosine-bisphosphate derivatives as potent antagonists at P2Y1 receptors. J Med Chem. 1998;41:183–90. doi: 10.1021/jm970433l. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fawzi AB, Macdonald D, Benbow LL, Smith-Torhan A, Zhan H, Weig BC, et al. SCH-202676: an allosteric modulator of both agonist and antagonist binding to G protein-coupled receptors. Mol Pharmacol. 2001;59:30–7. doi: 10.1124/mol.59.1.30. [DOI] [PubMed] [Google Scholar]
- 22.Lanzafame AA, Christopoulos A. Investigation of the interaction of a putative allosteric modulator, SCH-202676, with Mi muscarinic acetylcholine receptors. J Pharmacol Exp Ther. 2003;308:830–7. doi: 10.1124/jpet.103.060590. [DOI] [PubMed] [Google Scholar]
- 23.Gao ZG, Gross AS, Jacobson KA. Effects of SCH-202676 on adenosine and P2Y receptors. Life Sci. 2004;74:3173–80. doi: 10.1016/j.lfs.2003.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hoare SR, Strange PG. Regulation of D2 dopamine receptors by amiloride and amiloride analogs. Mol Pharmacol. 1996;50:1295–308. [PubMed] [Google Scholar]
- 25.Leppik RA, Lazareno S, Mynett A, Birdsall NJ. Characterization of the allosteric interactions between antagonists and amiloride analogues at the human α2A-adrenergic receptor. Mol Pharmacol. 1998;53:916–25. [PubMed] [Google Scholar]
- 26.Gao ZG, Melman N, Erdmann A, Kim SG, Miiller CE, Ijzerman AP, et al. Differential allosteric modulation by amiloride analogues of agonist and antagonist binding at A1 and A3 adenosine receptors. Biochem Pharmacol. 2003;65:525–34. doi: 10.1016/s0006-2952(02)01556-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li G, Regunathan S, Barrow CJ, Eshraghi J, Cooper R, Reis DJ. Agmatine: an endogenous clonidine-displacing substance in the brain. Science. 1994;263:966–9. doi: 10.1126/science.7906055. [DOI] [PubMed] [Google Scholar]
- 28.Ren LM, Burnstock G. Prominent sympathetic purinergic vasoconstriction in the rabbit splenic artery: potentiation by 2,2′-pyridylisatogen tosylate. Br J Pharmacol. 1997;120:530–6. doi: 10.1038/sj.bjp.0700933. [DOI] [PMC free article] [PubMed] [Google Scholar]