Abstract
Nearly 20% of all breast cancer cases are ductal carcinoma in situ (DCIS), with over 60,000 cases diagnosed each year. Many of these cases would never cause clinical symptoms or threaten the life of the woman; however, it is currently impossible to distinguish which lesions will progress to invasive disease from those that will not. DCIS is generally associated with an excellent prognosis regardless of treatment pathway, but there is variation in treatment aggressiveness that appears to exceed the medical uncertainty associated with DCIS management. Therefore, it would seem that a significant proportion of women with DCIS receive more extensive treatment than is needed. This overtreatment of DCIS is a growing concern among the breast cancer community and has implications for both the patient (via adverse treatment-related effects, as well as out-of-pocket costs) and society (via economic costs and the public health and environmental harms resulting from healthcare delivery). This paper discusses DCIS treatment pathways and their implications for patients and society, and calls for further research to examine the factors that are leading to such wide variation in treatment decisions.
Introduction
In 2014, an estimated 232,000 new breast cancer cases will be diagnosed in the U.S. (1), of which approximately 20% will be ductal carcinoma in situ (DCIS) (2, 3). DCIS is the earliest form of breast cancer; it is a non-invasive disease and estimates suggest that without treatment, up to 70% of cases would never become clinically relevant (4-6). However, clinicians cannot differentiate lesions that are likely to progress to invasive, potentially lethal disease from those that could be spared treatment. A diagnosis of DCIS carries an excellent prognosis with a 15-year breast cancer mortality rate of 3%, regardless of the treatment received (7, 8). Treatment of DCIS varies widely in aggressiveness and may include some combination of breast conserving surgery (BCS) with or without radiation, unilateral or bilateral total mastectomy, contralateral prophylactic mastectomy, breast reconstruction, and anti-estrogen hormone therapy (9, 10). Aggressive treatment may be an appropriate therapeutic approach in certain high-risk cases, but an increasing number of studies have shown institutional, regional, and national-level variation in the use of mastectomy that exceeds the medical uncertainty pertaining to DCIS management (9, 11-13). This variation has led to widespread concern in the medical community that DCIS is being overtreated (14). In many cases, aggressive treatment may not be in the best long-term interest of the patient given potential treatment complications, the adequate cancer control provided by less aggressive treatments, and the increased risk of long term complications posed by some treatments. The objectives of this review are to discuss the range of impacts that may result from the overtreatment of DCIS, and to call for further research to determine the factors that have led to the current wide variation in treatment decisions.
DCIS treatment pathways and patient health outcomes
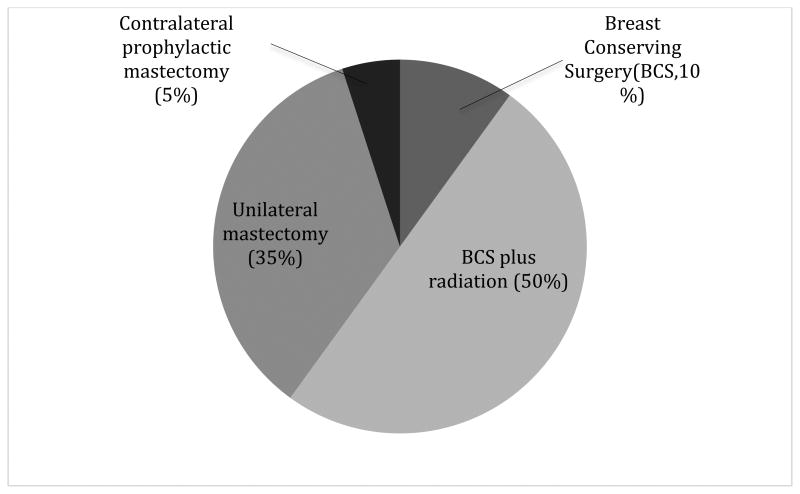
About half of DCIS cases receive BCS with radiation (Figure 1), and approximately 40% of all patients, regardless of surgical treatment pathway, receive hormone therapy (e.g., Tamoxifen) as an adjuvant therapy for lesions that are estrogen receptor (ER)-positive (9, 10). While these treatments result in a very high survival rate, DCIS diagnosis and treatment in general has been associated with adverse health effects among patients. DCIS survivors report decreased physical activity, high rates of weight gain, and elevated use of anti-depressants following DCIS treatment (15), as well as reduction in social functioning, mental health, and vitality (16, 17).
Figure 1.
Survivors who receive Tamoxifen as part of their treatment regimen for ER-positive lesions experience an increased risk of developing endometrial cancer and are more likely to experience thromboembolic and cerebrovascular events, hot flashes, irregular menses, and vaginal discharge than those who do not receive this hormone therapy (18-23). Radiation therapy also has a number of associated side effects including an increased risk of cardiac toxicity, secondary cancers, pneumonitis, and most commonly lymphedema and radiation-induced fatigue (22, 24-26). Survivors who elect to undergo mastectomy report lower rates of physical activity than those who choose a less aggressive treatment (27). After mastectomy, DCIS patients have reported lower body image and sexual functioning than those treated with BCS (28). Our preliminary data from the Wisconsin In Situ Cohort suggests that DCIS patients treated with BCS may have better physical function and fewer physical role limitations, compared to women treated with mastectomy. These outcomes suggest that overly aggressive treatment of DCIS may have unnecessary long-term impacts on the health and well being of women.
The question of overtreatment
The medical community has responded to DCIS overtreatment concerns in a number of ways. Some have proposed changing the terminology used to describe DCIS since many women overestimate the risk of a DCIS diagnosis (17, 29). Others have suggested active surveillance, or “watchful waiting,” as a reasonable approach to treating DCIS (30) since estimates suggest that, in the absence of treatment, only one in five low-grade DCIS cases would progress to invasive disease over a period of up to 40 years (31, 32). In a state-of-the-science consensus conference, a panel convened by the National Cancer Institute concluded that research seeking to understand treatment decision-making for DCIS patients is a high priority topic, with a goal of enabling more appropriate DCIS care (33). A better understanding of treatment decision-making will be required to reduce overtreatment and create conditions under which new approaches to patient care such as watchful waiting can be tested.
Economic, Public Health, and Environmental Impacts of DCIS Treatment
In general, the provision of medical care is associated with a number of societal trade-offs; such trade-offs have not been characterized for DCIS. In this section, we provide an overview of the costs of medical care and call for an investigation to determine the specific costs associated with DCIS.
The economic costs of healthcare are perhaps the most recognized impacts and range from the burden of out-of-pocket costs on individual patients and families (34, 35), to the societal level costs associated with national medical expenditures (36, 37). In 2010, the overall cost of care for female breast cancer was estimated to be $16.5 billion in the U.S. (38). As a result of increasingly intensive treatments and the introduction of new cancer therapies, total breast cancer treatment costs are projected to increase by 32% in the next 10 years (38). DCIS treatment represents only a portion of these costs, but the implication that DCIS may often be overtreated suggests that the proportion of these economic costs associated with DCIS treatment could be much lower.
The economic costs of medical care represent a vast array of goods and services that have further inter- related public health and environmental impacts. Medical care relies upon a vast infrastructure of workers and natural resource inputs to support everyday delivery of care (39). Each of the materials used in this infrastructure has an environmental legacy starting with its extraction as a natural resource, continuing through manufacture, distribution, and transport, through the eventual disposal of part or all of the material as waste. Throughout the life cycles of these material commodity chains, workers and communities may be exposed to hazardous substances that may ultimately lead to negative health outcomes.
Previous research on the environmental and public health impacts of cancer care suggests that the largest impacts of medical care result from the cumulative costs that increase throughout the life cycle of medical supply chains (40, 41). The life-cycle consequences of plastics illustrate the range of impacts from medical supply chains, and the ubiquity of plastics in medical care makes their cumulative social and ecological impacts directly applicable to DCIS. As a petroleum-derived product, plastic production is associated with habitat destruction, soil erosion, chemical contamination of land and water, human health and safety risks for oil workers and neighboring communities, and social justice issues related to oil drilling and extraction (42). Emissions from the long-distance transport of commodities such as plastic are a major contributor to the environmental and human health impacts of climate change (43, 44). At the point-of-care, the leaching of bisphenol A (BPA) and bis(2-ethylhexyl) phthalate (DEHP) from plastic tubing, intravenous bags, and other supplies pose health risks to patients (45). Finally, disposal of plastic waste poses a number of health risks, including the consequences of dioxin emissions from medical waste incineration, which has led to an ongoing campaign to remove polyvinyl chloride (PVC) from materials used in healthcare (46).
Other currently understudied areas of concern related to DCIS treatment are the public and occupational health outcomes of ionizing radiation exposure that result from breast cancer diagnosis and treatment. Exposures to ionizing radiation related to the management of DCIS occur during screening mammography used for diagnosis, radiation therapy as part of treatment, and subsequent screening and surveillance mammography. This exposure risk involves patients as well as trained medical staff. Current conservative estimates suggest that 86 cancers will be induced and 11 deaths will occur per 100,000 women due to mammography in women who receive annual mammograms from age 40 to 55, and biennial mammograms thereafter to age 74 (47). Though the risk of screening mammography is generally considered small in relation to the number of lives saved (1 per 1,339 50 to 59 year old women screened; Nelson 2009), this trade-off must be seen in light of increasing concern regarding the over diagnosis and overtreatment of breast cancer (48, 49). Likewise, whereas whole-breast radiation following BCS is associated with reduced incidence of recurrence among DCIS patients, there is no evidence that this treatment reduces breast cancer mortality, likely because breast cancer death after DCIS is a relatively rare event regardless of treatment (50). In addition, therapeutic radiation is associated with a number of adverse health impacts as described above. When combined, the evidence of risks and benefits to patients from radiation used in DCIS imaging and treatment suggests that radiation poses harms that deserve serious consideration. The same is true of occupational exposures to ionizing radiation among medical workers. There is a paucity of data regarding average annual doses, time trends, organ doses of radiation, and associated lifetime cancer risks among medical radiation workers (51). Though the health risks of such radiation exposure are greatly minimized in the U.S. thanks to radiation-specific occupational health and safety standards, they are cumulative over time and therefore merit continued surveillance.
Conclusions
Two separate DCIS management issues appear to be significant factors in the overtreatment of this non-invasive disease. First, there are many cases of DCIS that could be effectively managed with minimal treatment, but our current inability to clinically differentiate lesions that could be spared treatment from those that require more aggressive intervention causes many women to undergo treatment that would otherwise be considered unnecessary. Second, DCIS patients often choose treatments that are more aggressive than the medical community believes are necessary, for example mastectomy when BCS is considered appropriate. Regardless of cause, DCIS overtreatment leads to a number of potentially adverse implications for patient health and society. In addition to improved prognostic tools, efforts to improve our understanding of patient, physician, and other factors in the treatment decision-making process are needed. Qualitative and quantitative research evaluating the decision-making process and the downstream impacts on patients and society will help inform policies and develop strategies for patients and providers to make evidence-based decisions regarding treatment choices. Lessons learned from DCIS overtreatment may be applicable to other diseases, especially when they incur a combination of uncertainty and intense emotions. In addition, the life-cycle analysis approach suggested here can be widely applied to other medical interventions to clarify the societal trade-offs associated with current medical practices and complex decision-making.
Acknowledgments
This work was supported in part by grant U54 CA163303 from the National Cancer Institute.
Contributor Information
Christine M. Vatovec, Rubenstein School of Environment and Natural Resources & College of Medicine, Vermont Cancer Center, University of Vermont.
Mujde Z. Erten, Department of Surgery, College of Medicine, Vermont Cancer Center, University of Vermont.
Jane Kolodinsky, Department of Community Development and Applied Economics, University of Vermont.
Phil Brown, Department of Sociology and Anthropology and Department of Health Sciences, Northeastern University.
Marie Wood, Department of Medicine, College of Medicine, Vermont Cancer Center, University of Vermont
Ted James, Department of Surgery, University of Vermont, Vermont Cancer Center.
Brian L. Sprague, Department of Surgery, Vermont Cancer Center, University of Vermont.
References
- 1.NCI. SEER Stat Fact Sheets: Breast Cancer. National Cancer Institute; [Accessed 12 May 2014]. [Google Scholar]
- 2.Society AC. Cancer Facts & Figures 2009. Atlanta GA USA: American Cancer Society; 2009. [Google Scholar]
- 3.Howlader N, N A, Krapcho M, Neyman N, Aminou R, Waldron W, Altekruse SF, Kosary CL, Ruhl J, Tatalovich Z, Cho H, Mariotto A, Eisner MP, Lewis DR, Chen HS, Feuer EJ, Cronin KA, Edwards BK, editors. SEER Cancer Statistics Review, 1975-2008. Bethesda, MD: National Cancer Institute; 2011. [Google Scholar]
- 4.Eusebi V, Foschini MP, Cook MG, Berrino F, Azzopardi JG. Long-term follow-up of in situ carcinoma of the breast with special emphasis on clinging carcinoma. Seminars in diagnostic pathology. 1989;6(2):165–73. [PubMed] [Google Scholar]
- 5.Page DL, Dupont WD, Rogers LW, Landenberger M. Intraductal carcinoma of the breast: follow-up after biopsy only. Cancer. 1982;49(4):751–8. doi: 10.1002/1097-0142(19820215)49:4<751::aid-cncr2820490426>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- 6.Rosen PP, Braun DW, Jr, Kinne DE. The clinical significance of pre-invasive breast carcinoma. Cancer. 1980;46(4 Suppl):919–25. doi: 10.1002/1097-0142(19800815)46:4+<919::aid-cncr2820461311>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 7.Ernster VL, Barclay J, Kerlikowske K, Wilkie H, Ballard-Barbash R. Mortality among women with ductal carcinoma in situ of the breast in the population-based surveillance, epidemiology and end results program. Archives of internal medicine. 2000;160(7):953–8. doi: 10.1001/archinte.160.7.953. [DOI] [PubMed] [Google Scholar]
- 8.Wapnir IL, Dignam JJ, Fisher B, Mamounas EP, Anderson SJ, Julian TB, et al. Long-term outcomes of invasive ipsilateral breast tumor recurrences after lumpectomy in NSABP B-17 and B-24 randomized clinical trials for DCIS. Journal of the National Cancer Institute. 2011;103(6):478–88. doi: 10.1093/jnci/djr027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Greenberg CC, Lipsitz SR, Hughes ME, Edge SB, Theriault R, Wilson JL, et al. Institutional variation in the surgical treatment of breast cancer: a study of the NCCN. Annals of surgery. 2011;254(2):339–45. doi: 10.1097/SLA.0b013e3182263bb0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sprague BL, McLaughlin V, Hampton JM, Newcomb PA, Trentham-Dietz A. Disease-free survival by treatment after a DCIS diagnosis in a population-based cohort study. Breast cancer research and treatment. 2013;141(1):145–54. doi: 10.1007/s10549-013-2670-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Farrow DC, Hunt WC, Samet JM. Geographic variation in the treatment of localized breast cancer. The New England journal of medicine. 1992;326(17):1097–101. doi: 10.1056/NEJM199204233261701. [DOI] [PubMed] [Google Scholar]
- 12.Nattinger AB, Gottlieb MS, Veum J, Yahnke D, Goodwin JS. Geographic variation in the use of breast-conserving treatment for breast cancer. The New England journal of medicine. 1992;326(17):1102–7. doi: 10.1056/NEJM199204233261702. [DOI] [PubMed] [Google Scholar]
- 13.Tuttle TM, Jarosek S, Habermann EB, Arrington A, Abraham A, Morris TJ, et al. Increasing rates of contralateral prophylactic mastectomy among patients with ductal carcinoma in situ. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2009;27(9):1362–7. doi: 10.1200/JCO.2008.20.1681. [DOI] [PubMed] [Google Scholar]
- 14.Esserman LJ, Thompson IM, Jr, Reid B. Overdiagnosis and overtreatment in cancer: an opportunity for improvement. JAMA : the journal of the American Medical Association. 2013;310(8):797–8. doi: 10.1001/jama.2013.108415. [DOI] [PubMed] [Google Scholar]
- 15.Sprague BL, Trentham-Dietz A, Nichols HB, Hampton JM, Newcomb PA. Change in lifestyle behaviors and medication use after a diagnosis of ductal carcinoma in situ. Breast cancer research and treatment. 2010;124(2):487–95. doi: 10.1007/s10549-010-0869-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Claus EB, Petruzella S, Carter D, Kasl S. Quality of life for women diagnosed with breast carcinoma in situ. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2006;24(30):4875–81. doi: 10.1200/JCO.2005.05.2290. [DOI] [PubMed] [Google Scholar]
- 17.Partridge A, Adloff K, Blood E, Dees EC, Kaelin C, Golshan M, et al. Risk perceptions and psychosocial outcomes of women with ductal carcinoma in situ: longitudinal results from a cohort study. Journal of the National Cancer Institute. 2008;100(4):243–51. doi: 10.1093/jnci/djn010. [DOI] [PubMed] [Google Scholar]
- 18.Arimidex TAoiCTG. Buzdar A, Howell A, Cuzick J, Wale C, Distler W, et al. Comprehensive side-effect profile of anastrozole and tamoxifen as adjuvant treatment for early-stage breast cancer: long-term safety analysis of the ATAC trial. The lancet oncology. 2006;7(8):633–43. doi: 10.1016/S1470-2045(06)70767-7. [DOI] [PubMed] [Google Scholar]
- 19.Group EBCTC. Tamoxifen for early breast cancer: an overview of the randomised trials. Early Breast Cancer Trialists' Collaborative Group. Lancet. 1998;351(9114):1451–67. [PubMed] [Google Scholar]
- 20.Braithwaite RS, Chlebowski RT, Lau J, George S, Hess R, Col NF. Meta-analysis of vascular and neoplastic events associated with tamoxifen. Journal of general internal medicine. 2003;18(11):937–47. doi: 10.1046/j.1525-1497.2003.20724.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cuzick J, Powles T, Veronesi U, Forbes J, Edwards R, Ashley S, et al. Overview of the main outcomes in breast-cancer prevention trials. Lancet. 2003;361(9354):296–300. doi: 10.1016/S0140-6736(03)12342-2. [DOI] [PubMed] [Google Scholar]
- 22.Shapiro CL, Recht A. Side effects of adjuvant treatment of breast cancer. The New England journal of medicine. 2001;344(26):1997–2008. doi: 10.1056/NEJM200106283442607. [DOI] [PubMed] [Google Scholar]
- 23.Wysowski DK, Honig SF, Beitz J. Uterine Sarcoma Associated with Tamoxifen Use. New England Journal of Medicine. 2002;346(23):1832–3. doi: 10.1056/NEJM200206063462319. [DOI] [PubMed] [Google Scholar]
- 24.Haylock PJ, Hart LK. Fatigue in patients receiving localized radiation. Cancer nursing. 1979;2(6):461–7. [PubMed] [Google Scholar]
- 25.Smets EM, Visser MR, Willems-Groot AF, Garssen B, Oldenburger F, van Tienhoven G, et al. Fatigue and radiotherapy: (A) experience in patients undergoing treatment. British journal of cancer. 1998;78(7):899–906. doi: 10.1038/bjc.1998.599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Taunk NK, Haffty BG, Chen S, Khan AJ, Nelson C, Pierce D, et al. Comparison of radiation-induced fatigue across 3 different radiotherapeutic methods for early stage breast cancer. Cancer. 2011;117(18):4116–24. doi: 10.1002/cncr.26013. [DOI] [PubMed] [Google Scholar]
- 27.Ligibel JA, Partridge A, Giobbie-Hurder A, Golshan M, Emmons K, Winer EP. Physical activity behaviors in women with newly diagnosed ductal carcinoma-in-situ. Annals of surgical oncology. 2009;16(1):106–12. doi: 10.1245/s10434-008-0174-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Montazeri A. Health-related quality of life in breast cancer patients: a bibliographic review of the literature from 1974 to 2007. Journal of experimental & clinical cancer research : CR. 2008;27:32. doi: 10.1186/1756-9966-27-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Omer ZB, Hwang ES, Esserman LJ, Howe R, Ozanne EM. Impact of ductal carcinoma in situ terminology on patient treatment preferences. JAMA internal medicine. 2013;173(19):1830–1. doi: 10.1001/jamainternmed.2013.8405. [DOI] [PubMed] [Google Scholar]
- 30.Esserman L, Shieh Y, Thompson I. Rethinking screening for breast cancer and prostate cancer. JAMA : the journal of the American Medical Association. 2009;302(15):1685–92. doi: 10.1001/jama.2009.1498. [DOI] [PubMed] [Google Scholar]
- 31.Ozanne EM, Shieh Y, Barnes J, Bouzan C, Hwang ES, Esserman LJ. Characterizing the impact of 25 years of DCIS treatment. Breast cancer research and treatment. 2011;129(1):165–73. doi: 10.1007/s10549-011-1430-5. [DOI] [PubMed] [Google Scholar]
- 32.Sanders ME, Schuyler PA, Dupont WD, Page DL. The natural history of low-grade ductal carcinoma in situ of the breast in women treated by biopsy only revealed over 30 years of long-term follow-up. Cancer. 2005;103(12):2481–4. doi: 10.1002/cncr.21069. [DOI] [PubMed] [Google Scholar]
- 33.Allegra CJ, Aberle DR, Ganschow P, Hahn SM, Lee CN, Millon-Underwood S, et al. National Institutes of Health State-of-the-Science Conference statement: Diagnosis and Management of Ductal Carcinoma In Situ September 22-24, 2009. Journal of the National Cancer Institute. 2010;102(3):161–9. doi: 10.1093/jnci/djp485. [DOI] [PubMed] [Google Scholar]
- 34.Banthin JS, C P, Bernard DM. Financial Burden Of Health Care, 2001–2004. Health Affairs. 2008;27(1):188–95. doi: 10.1377/hlthaff.27.1.188. [DOI] [PubMed] [Google Scholar]
- 35.Davidoff AJ, Erten M, Shaffer T, Shoemaker JS, Zuckerman IH, Pandya N, et al. Out-of-pocket health care expenditure burden for Medicare beneficiaries with cancer. Cancer. 2013;119(6):1257–65. doi: 10.1002/cncr.27848. [DOI] [PubMed] [Google Scholar]
- 36.Reinhardt UE, Hussey PS, Anderson GF. U.S. health care spending in an international context. Health Aff (Millwood) 2004;23(3):10–25. doi: 10.1377/hlthaff.23.3.10. [DOI] [PubMed] [Google Scholar]
- 37.Poisal JA, Truffer C, Smith S, Sisko A, Cowan C, Keehan S, et al. Health Spending Projections Through 2016: Modest Changes Obscure Part D's Impact. Health Affairs. 2007;26(2):w242–w53. doi: 10.1377/hlthaff.26.2.w242. [DOI] [PubMed] [Google Scholar]
- 38.Mariotto AB, Yabroff KR, Shao Y, Feuer EJ, Brown ML. Projections of the cost of cancer care in the United States: 2010-2020. Journal of the National Cancer Institute. 2011;103(2):117–28. doi: 10.1093/jnci/djq495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jameton A, Pierce J. Environment and health: 8. Sustainable health care and emerging ethical responsibilities. CMAJ : Canadian Medical Association journal = journal de l'Association medicale canadienne. 2001;164(3):365–9. [PMC free article] [PubMed] [Google Scholar]
- 40.Vatovec C, Senier L, Bell MM. The Ecology of Dying: Commodity Chains, Governance, and the Medicalization of End-of-Life Care. In: Gislason MK, editor. Ecological Health (Advances in Medical Sociology) Vol. 15. Emerald Group Publishing Limited; 2013. pp. 195–215. [Google Scholar]
- 41.Vatovec C, Senier L, Bell M. An ecological perspective on medical care: environmental, occupational, and public health impacts of medical supply and pharmaceutical chains. EcoHealth. 2013;10(3):257–67. doi: 10.1007/s10393-013-0855-1. [DOI] [PubMed] [Google Scholar]
- 42.O'Rourke D, Connolly S. Just oil? The distribution of environmental and social impacts of oil production and consumption. Annu Rev Environ Resour. 2003;28:587–617. [Google Scholar]
- 43.Akimoto H. Global air quality and pollution. Science. 2003;302(5651):1716–9. doi: 10.1126/science.1092666. [DOI] [PubMed] [Google Scholar]
- 44.P J. Climate Change. In: F H, editor. Environmental health: From global to local. San Francisco, CA: Jossey-Bass; 2005. [Google Scholar]
- 45.Halden RU. Plastics and health risks. Annual review of public health. 2010;31:179–94. doi: 10.1146/annurev.publhealth.012809.103714. [DOI] [PubMed] [Google Scholar]
- 46.PVC and Phthalates: Health Care Without Harm. 2014 [ cited 2014 February 9]. Available from: http://www.noharm.org/us_canada/issues/toxins/pvc_phthalates/
- 47.Yaffe MJ, Mainprize JG. Risk of radiation-induced breast cancer from mammographic screening. Radiology. 2011;258(1):98–105. doi: 10.1148/radiol.10100655. [DOI] [PubMed] [Google Scholar]
- 48.Alvarado M, Ozanne E, Esserman L. Overdiagnosis and Overtreatment of Breast Cancer. American Society of Clinical Oncology educational book / ASCO American Society of Clinical Oncology Meeting. 2012;32:e40–e5. doi: 10.14694/EdBook_AM.2012.32.301. [DOI] [PubMed] [Google Scholar]
- 49.Jorgensen KJ, Keen JD, Gotzsche PC. Is mammographic screening justifiable considering its substantial overdiagnosis rate and minor effect on mortality? Radiology. 2011;260(3):621–7. doi: 10.1148/radiol.11110210. [DOI] [PubMed] [Google Scholar]
- 50.Virnig BA, S T, Tuttle TM, et al. Vol. 185. Rockville (MD): 2009. Diagnosis and Management of Ductal Carcinoma in Situ (DCIS) 4, Discussion [Evidence Reports/Technology Assessments] Available from: http://www.ncbi.nlm.nih.gov/books/NBK32578/ [PMC free article] [PubMed] [Google Scholar]
- 51.Linet MS, Slovis TL, Miller DL, Kleinerman R, Lee C, Rajaraman P, et al. Cancer risks associated with external radiation from diagnostic imaging procedures. CA: a cancer journal for clinicians. 2012 doi: 10.3322/caac.21132. [DOI] [PMC free article] [PubMed] [Google Scholar]