Abstract
Mutations in the KRAS oncogene represent one of the most prevalent genetic alterations in colorectal cancer (CRC), the third leading cause of cancer-related death in the US. In addition to their well-characterized function in driving tumor progression, KRAS mutations have been recognized as a critical determinant of the therapeutic response of CRC. Recent studies demonstrate that KRAS-mutant tumors are intrinsically insensitive to clinically-used epidermal growth factor receptor (EGFR) targeting antibodies, including cetuximab and panitumumab. Acquired resistance to the anti-EGFR therapy was found to be associated with enrichment of KRAS-mutant tumor cells. However, the underlying molecular mechanism of mutant-KRAS-mediated therapeutic resistance has remained unclear. Despite intensive efforts, directly targeting mutant KRAS has been largely unsuccessful. This review summarizes the recent advances in understanding the biological function of KRAS mutations in determining the therapeutic response of CRC, highlighting several recently developed agents and strategies for targeting mutant KRAS, such as synthetic lethal interactions.
Keywords: Colorectal cancer, EGFR, KRAS, Synthetic lethality, Targeted therapy
Introduction
Despite recent advances in early detection and therapeutic intervention, colorectal cancer (CRC) remains one of the most deadly cancers in the United States.1 It is estimated that nearly 137,000 people will be diagnosed, and 50,000 will die from the disease in 2014.2 While surgical removal leads to high cure rates of localized disease, metastatic CRCs are typically associated with a poor prognosis with the majority of patients dying within two years upon diagnosis, resulting in a five-year survival rate of just 11%.3, 4
Colorectal tumorigenesis often begins with precursor lesions in the colonic epithelium, and is driven by a series of genetic and epigenetic alterations.5, 6 One of the earliest genetic changes is the loss of the APC tumor suppressor gene, which antagonizes β-catenin signaling and is mutated in the majority of CRCs.7 Another key early event is the activation of the oncogene KRAS, which is mutated in over one-third of CRCs.5, 8 Genetic instability has also been implicated to contribute to colorectal tumorigenesis.9, 10 KRAS mutations, although not a significant prognostic factor, have been shown to play a critical role in CRC treatment. Over the years, activation of this oncogene has been linked to resistance to the agents utilized in front-line therapy for CRC.11, 12 Intensive efforts have been devoted to understanding how KRAS mutations affect CRC therapy, in particular targeted therapy, and how to overcome mutant-KRAS-mediated therapeutic resistance. The National Cancer Institute (NCI) has recently established the RAS Program to explore innovative ways to attack the proteins encoded by mutant RAS genes or other vulnerabilities as a way to treat key types of cancer such as CRC. In this review, we summarize the current understanding of KRAS biology and how the mutational status of KRAS affects the response to CRC therapy, as well as recent advances in developing novel therapeutic strategies and agents for targeting KRAS-mutant cancers.
KRAS biology
RAS proteins represent prototypical members of a large family of small GTP-binding proteins.13 The human RAS superfamily consists of more than 100 members, which can be divided into six subfamilies.14 Three prototypical RAS proteins include HRAS, NRAS, and KRAS.15 While they are highly homologous in amino acid sequence and ubiquitously expressed, KRAS is the only one that is essential for normal development as shown by mouse genetic studies.16, 17, 18 KRAS can be expressed as two different splice variants, referred to as 4A and 4B, through alternative splicing within exon 4.15 The 4B variant is the dominant form commonly known as KRAS.8
KRAS is a membrane-bound GTPase that cycles between an active GTP-bound form and an inactive GDP-bound form due to the hydrolysis of the bound GTP (Fig. 1A).14, 19 The switches between these two states are controlled by two classes of proteins: guanosine nucleotide exchange factors (known as GEFs) and GTPase-activating proteins (known as GAPs). As their names suggest, GEFs assist with the exchange of bound GDP with GTP, whereas GAPs stimulate the hydrolytic ability of RAS to convert bound GTP to GDP.13 The proper membrane localization and function of the RAS proteins are regulated by several post-translational modifications in the C-terminal “CAAX” motif, including farnesylation of the cysteine residue, proteolytic removal of the terminal three residues (AAX), as well as methylation of the cysteine residue.15, 19 In addition, the plasma membrane localization of KRAS also requires a basic poly-lysine region located immediately upstream of the C-terminus.19, 20
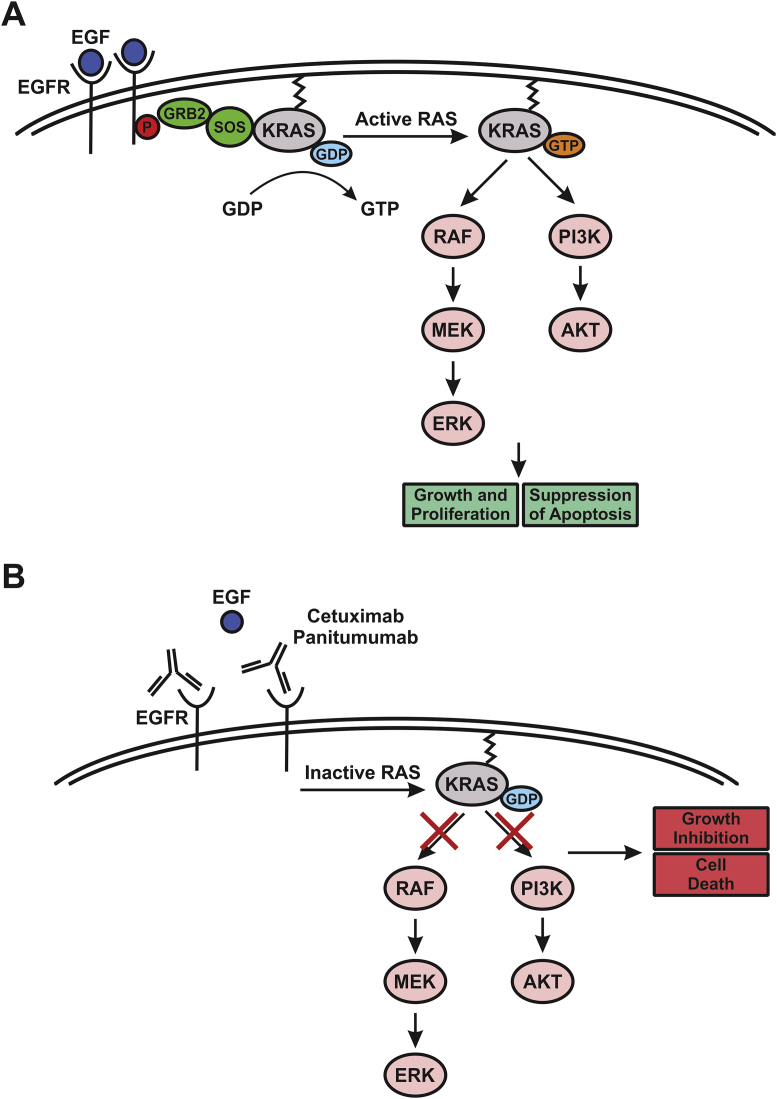
Figure 1.
EGFR-induced and KRAS-mediated signaling pathways. (A) Activation of EGFR upon ligand binding and its subsequent auto-phosphorylation create a docking site for the SOS/GRB2 complex, resulting in nucleotide exchange by SOS and the GTP-bound form of KRAS. KRAS then signals through the RAF/MEK/ERK and PI3K/AKT cascades to promote cell growth and suppress apoptosis. (B) Anti-EGFR antibodies, including cetuximab and panitumumab, bind to EGFR and prevent ligand binding and subsequent KRAS activation, leading to growth suppression and cell death due to the inhibition of the RAF/MEK/ERK and PI3K/AKT pathways. Mutant KRAS can override the effect of anti-EGFR antibodies leading to cell growth and survival.
Once properly localized, KRAS mediates a myriad of intracellular signaling events through its numerous effector pathways. Signaling by receptor tyrosine kinases (RTKs), in particular the epidermal growth factor receptor (EGFR), is a widely-utilized and well-understood model for studying KRAS activation (Fig. 1A).16, 21 The activation of EGFR upon ligand binding and its subsequent auto-phosphorylation create a docking site for the adaptor protein growth-factor-receptor-bound protein 2 (GRB2), which binds to the GEF Son of Sevenless (SOS) in the cytosol. The recruitment of this protein complex to the phosphorylated receptor enables SOS to function as the exchange factor for KRAS, resulting in nucleotide exchange and the GTP-bound form of KRAS (Fig. 1A).16, 21, 22
Among numerous downstream effectors of KRAS, the best characterized include RAF and phosphoinositide-3 kinase (PI3K), as well as the GEFs for the RAS-like (Ral) small GTPases (RalGEFs).23, 24 The major axes of RAS signaling through the RAF/MEK/ERK and PI3K/AKT cascades ultimately control processes such as cell growth and survival (Fig. 1A).16 This is accomplished in part by ERK-regulated activation of transcription factors that promote cell cycle progression, and by AKT-mediated inactivation of pro-apoptotic proteins for apoptosis suppression.16, 25 In addition, a number of alternate effectors of KRAS have been described in an extensive body of literature, which regulate processes such as cell migration, endocytosis, changes in cytoskeleton, and calcium signaling.19, 23, 25, 26
KRAS mutations and role in CRC
Nearly 30% of human cancers possess activating RAS mutations,27 85% of which are KRAS mutations.28 In CRCs, KRAS mutations are found in approximately 35%–40% of cases, and those of HRAS and NRAS in <5%.4, 29 The vast majority of KRAS mutations are located in codons 12 and 13, and the remainder in codons 61, 146, and other residues.29, 30 While the hotspot codon 12 and 13 mutations of KRAS do not interfere with its ability to associate with GAPs, they alter the position of a catalytic glutamate residue at codon 61.13, 15 This results in the reduced GTPase activity of KRAS and decreased rate of GTP hydrolysis by 3–9 fold compared to wild-type (WT) KRAS.15 Using isogenic knock-out and knock-in CRC cell lines with either WT KRAS or activating mutations, recent studies have illustrated that these mutations play a significant role in tumor cell survival and tumor progression.31, 32, 33, 34 The functional consequences of KRAS mutations include increased cellular proliferation, suppression of apoptosis, altered cell metabolism, and changes in the tumor microenvironment.13, 27 For example, GTP-bound KRAS enables the upregulation of growth factors and transcription factors known to promote cell cycle progression, such as c-JUN and c-FOS.13 Recent studies identified the Yes-associated protein 1 (YAP1) transcriptional co-activator as a key mediator of the oncogenic effect of KRAS.35, 36 From a therapeutic standpoint, suppression of apoptosis is probably one of the most important consequences of KRAS mutations.27 Activated KRAS can inhibit the apoptotic signaling cascade through its effector PI3K, which in turn activates AKT,25, 27 a potent pro-survival kinase that inhibits apoptosis via several mechanisms, such as the phosphorylation and subsequent inactivation of the pro-apoptotic Bcl-2 family protein BAD, and the inhibitory phosphorylation of the initiator caspase-9.25
Current treatments and targeted therapy
CRC patients are often treated with conventional cytotoxic chemotherapy and recently developed targeted therapy. Cytotoxic chemotherapy typically entails the use of pyrimidine analogs such as 5-fluorouracil (5-FU),37 which inhibits thymidylate synthase required for nucleotide synthesis.38 Other cytotoxic drugs commonly used for CRC treatment include the topoisomerase I inhibitor irinotecan, the platinum drug oxaliplatin, and the oral pro-drug capecitabine (metabolized into fluorouracil).38, 39 Front-line treatment involves combinations of these agents, such as FOLFOX (fluorouracil, leucovorin, and oxaliplatin) and FOLFIRI (fluorouracil, leucovorin, and irinotecan),12, 38 which have been shown to improve response rates and overall survival of CRC patients over single agents.39
Targeted therapy, referred to as treatments that aim to inhibit the processes that cancer cells rely on for survival and proliferation, has recently moved to the forefront of CRC treatment.40 Several targeted drugs have been approved by the US Food and Drug Administration (FDA) for CRC treatment, including the anti-vascular endothelial growth factor (VEGF) antibody bevacizumab, which suppresses tumor angiogenesis, the anti-EGFR antibodies cetuximab and panitumumab, and more recently, the multi-kinase inhibitor regorafenib, which targets RTKs involved in angiogenesis and mitogenic signaling.41, 42 The utilization of targeted therapy has increased significantly. For example, it was estimated that roughly 44% of all CRC patients treated in the US received anti-EGFR antibody therapy in 2008.43
EGFR is a member of the ERBB family of RTKs that is frequently overexpressed in CRC.44 Much akin to KRAS signaling, EGFR transduces signals from extracellular stimuli through key intracellular pathways, such as the RAS/RAF/MEK/ERK and PI3K/AKT cascades.45 The anti-EGFR antibodies used for CRC treatment, including cetuximab and panitumumab, both can inhibit ligand binding and EGFR homo-dimerization or hetero-dimerization with other ERBB family members (Fig. 1B), which are necessary for its subsequent auto-phosphorylation and full activation.42, 46 Cetuximab is a chimeric human-murine antibody of the IgG1 isotype, and panitumumab is a humanized antibody of the IgG2 isotype.47, 48 In addition to their effect on ligand binding, cetuximab and panitumumab can promote EGFR internalization and subsequent degradation, thus decreasing the cell surface level of EGFR.48 Interestingly, cetuximab can also block EGFR nuclear translocation whereby it can act as a transcription factor.42 Both cetuximab and panitumumab have become part of first-, second-, and third-line therapy for CRC.12, 48, 49 Data from clinical trials have shown the therapeutic benefit of anti-EGFR antibody therapy either as a stand-alone agent or in combination with chemotherapy regimens.50, 51, 52, 53 However, primary and secondary resistance to this therapy has become a significant issue.
Alterations of KRAS status and the response to targeted therapy
A major challenge in anti-cancer therapy, especially targeted therapy, is the emergence of drug resistance. This has been exemplified by the finding that alterations in the KRAS gene render anti-EGFR antibodies ineffective for CRC treatment. An initial study published in 2006 showed the lack of response to cetuximab in patients with KRAS-mutant metastatic CRCs,54 followed by another study confirming the causal role of KRAS mutations.55 A host of subsequent studies, including further clinical trials and retrospective analyses of tumor samples, verified the notion that KRAS mutations underlie the lack of response to anti-EGFR therapy.56, 57, 58, 59 These findings led to the issuance of a provisional clinical opinion by the American Society of Clinical Oncology in 2009, stating that metastatic CRC patients eligible to receive an anti-EGFR antibody therapy should have their KRAS mutational status profiled; and those with a codon 12 or 13 mutation in KRAS should be excluded from this treatment.60 Collectively, these studies have established the KRAS status as a key factor in choosing treatment options of metastatic CRC patients, as well as a major biomarker of resistance to anti-EGFR antibody therapy.61
A critical question that remains is how resistance to anti-EGFR therapy emerges. Several recent studies have characterized the mechanism of secondary resistance to anti-EGFR therapy by assessing the temporal alterations of KRAS mutational status. Cell culture studies using CRC cells that are exquisitely sensitive to anti-EGFR antibodies have shown that prolonged treatment with anti-EGFR antibodies selects for resistant clones that harbor KRAS mutations.62, 63 The results of these studies suggest that KRAS mutations can pre-exist in a small proportion of a population of cancer cells, and can also be continuously generated de novo due to genomic instability. Thus, the pressure of anti-EGFR treatment enriches cell populations harboring KRAS mutations, resulting in acquired resistance to the treatment.64 Further supporting their role in mediating resistance to anti-EGFR therapy, KRAS mutations were detected in serum samples and metastases from treated patients, with the levels of these mutations correlated with relapse and disease progression.63, 65, 66 In addition to point mutations, frank amplification of KRAS, although an extremely rare event in CRC, can also lead to resistance to anti-EGFR therapy.67
The molecular mechanisms by which KRAS mutations lead to resistance to anti-EGFR therapy have remained unresolved. Given the role of KRAS as a key downstream effector of EGFR signaling, it is perhaps not surprising that constitutive activation of KRAS by mutations bypasses the upstream effect of EGFR inhibition. However, players that are responsible for circumventing the response to anti-EGFR therapy in a KRAS-mutant background remain elusive.68 Recent studies indicate that KRAS-mutant cells heavily rely on the RAF/MEK/ERK cascade for survival, which provides a clue for dissecting the precise signaling pathways underlying the resistance phenotype.62 Analysis of cell line models and CRC samples indicates that oncogenic KRAS plays a role in the maintenance of high expression levels of Bcl-XL, an anti-apoptotic Bcl-2 family protein, whose overexpression alone is sufficient to confer resistance to anti-EGFR antibodies in CRC cells.69 This study suggests that suppression of apoptosis is a mechanism of mutant-KRAS-mediated resistance to anti-EGFR therapy.
Targeting mutant KRAS for anti-cancer therapy
The high mutation frequency and its role in therapeutic resistance make KRAS an ideal target for anti-cancer therapy. The proof-of-principle for targeting KRAS has been demonstrated by the profound phenotypes of KRAS ablation in mice and human cancer cells.45, 70 A variety of strategies have been designed to target mutant KRAS, including direct targeting by inhibiting its GTPase activity and indirect targeting by suppressing its post-translational modifications or downstream effectors (Table 1).84
Table 1.
Recently developed agents for targeting mutant KRAS.
| Target | Mechanism of action | References | |
|---|---|---|---|
| Direct targeting agents | |||
| Small molecules from fragment-based screen | KRAS; WT, G12V, G12D; HRAS | Inhibition of SOS binding | 71 |
| 4,6-dichloro-2-methyl-3-aminoethyl-indole (DCAI) | KRAS; G12D | Inhibition of SOS binding | 72 |
| Hits from fragment-based screen | KRAS; G12C | Inhibition of SOS binding | 73 |
| SML-8-73-1 | KRAS; G12C | GDP analog | 74 |
| Indirect targeting agents | |||
| Farnesyltransferase inhibitors | Farnesyltransferase; post-translational modification | Inhibition of prenylation by farnesyl group | 75, 76 |
| Geranylgeranyltransferase I inhibitors | Geranylgeranyltransferase I; post-translational modification | Inhibition of prenylation by geranylgeranyl group | 77 |
| Inhibitors of KRAS and PDEδ interaction | KRAS and PDEδ interaction | Inhibition of prenylation | 78 |
| Inhibitors of RAF, MEK, and PI3K | RAF, MEK, and PI3K | Inhibition of KRAS effector pathways | 41, 79, 80 |
| Cetuximab and Pimasertib | EGFR and MEK; upstream and downstream KRAS pathway components | Dual inhibition of EGFR and MEK signaling | 62 |
| NVP-BEZ235-AN and AZD6244 | PI3K and MEK; upstream and downstream KRAS pathway components | Dual inhibition of PI3K and MEK signaling | 81 |
| siRNA | Human and mouse KRAS pathway genes | Inhibition of protein expression | 82 |
| RAF265 and Selumetinib | RAF1/BRAF and MEK | Dual inhibition of RAF and MEK signaling | 83 |
Despite numerous attempts, developing small-molecule inhibitors of KRAS has proven to be extremely challenging. Several recent reports have described novel small molecules that interfere with GEF binding to lock KRAS in an inactive state.85 For example, in silico and NMR-based screens were used to identify small molecules that bind to a distinct pocket on KRAS and inhibit SOS-mediated nucleotide exchange to prevent the activation of WT or mutant KRAS.52, 71, 72 More specific approaches have been utilized to identify small-molecule inhibitors that covalently bind to mutant KRAS. A disulphide-fragment-based screening approach was used to identify electrophilic compounds that can form a disulfide bond with the cysteine residue in the G12C mutant of KRAS.73 While these compounds do not affect WT KRAS, they can preferentially bind the G12C mutant, disrupt SOS binding, and increase its affinity for GDP to prevent the activation of mutant KRAS. A similar approach identified a GDP analog as a covalent inhibitor of the G12C mutant.74
Another approach for targeting KRAS is to inhibit its post-translational modifications using farnesyltransferase (FTase) inhibitors (FTIs), which inhibit the prenylation of KRAS that is required for proper plasma membrane attachment.75 FTIs were shown to suppress cancer cell growth in pre-clinical studies, but unfortunately, they did not exhibit clinical efficacy as single agents.77, 86 The lack of clinical response to FTIs was explained by the prenylation of KRAS through alternative mechanisms, such as those involving geranylgeranyltransferase I (GGTase I).76, 77 Dual inhibitors of GGTase and FTase, such as L778,123, were therefore developed. However, its toxicities in clinical trials prohibited further clinical development.87 Another strategy illustrated by a recent study is to inhibit the interaction between KRAS and cyclic GMP phosphodiesterase δ (PDEδ), which mediates correct localization and signaling by farnesylated KRAS.78
Targeting KRAS-mutant cancer can also be achieved by inhibiting other components of its key signaling axes or through combination therapies. For example, the RAF inhibitor regorafenib has been recently approved by the FDA for the treatment of metastatic CRCs.41 A number of PI3K and MEK pathway inhibitors have been developed and tested in clinical trials.79, 80 A small interfering RNA (siRNA) library against RAS pathway genes has also been created, and transfection of this library into CRC cells could suppress cell growth both in vitro and in vivo.82 Utilizing CRC cell lines that were selected for resistance to anti-EGFR antibodies, a recent study revealed that dual inhibition of both EGFR and MEK resulted in a pronounced reduction of cell viability.62 Concomitant blockade of RAF1 and MEK in CRC cells with activating KRAS mutations was also shown to drastically reduce cell viability.83 Furthermore, the combination of PI3K and MEK inhibitors suppressed murine lung tumors with the G12D mutant KRAS.81
Despite the promises, targeting KRAS-driven cancers remains one of the most difficult challenges in anti-cancer therapy due to several obstacles, including limited understanding of RAS-mediated signaling transduction feedback loops, pathway redundancy, tumor heterogeneity, unclear mechanisms of how RAS proteins activate their downstream targets, as well as unresolved structures of protein complexes formed by RAS oncoproteins.88 It is essential to fill in these knowledge gaps in order to develop more effective agents for targeting KRAS-mutant cancers.
Synthetic lethality of KRAS mutations
An emerging theme for targeting oncogenic mutations, including those of KRAS, exploits the concept of synthetic lethality, a genetic term referring to loss of viability resulting from a combination of two separate non-lethal mutations.89 While synthetic lethality has been extensively studied in lower organisms such as yeast, its applications have only recently been extended into anti-cancer therapy. In the case of mutant KRAS, synthetic lethality has been used loosely to indicate selective killing of KRAS-mutant cells as the result of another mutation, treatment with a pharmacological agent, a change in gene expression, or other perturbations (Fig. 2). Another good example is the sensitivity of BRCA1- or BRCA2-mutant breast tumors to poly (ADP-ribose) polymerase (PARP) inhibitors.90 It is attractive to use this approach to target oncogenic mutations because pharmacologically more tractable targets can be explored to develop more efficacious small-molecule inhibitors.
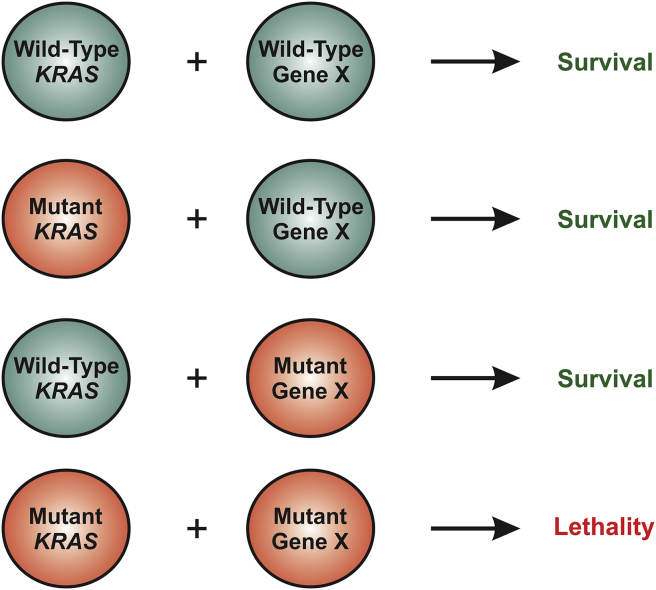
Figure 2.
Mutant-KRAS-mediated synthetic lethality. While a mutation in KRAS or gene X alone is insufficient to kill cells, a combination of mutations in these two genes can lead to cell death, resulting in synthetic lethality. Therefore, specific targeting of mutant KRAS can be achieved indirectly by targeting gene X.
A powerful approach for uncovering synthetic lethal interactions is large-scale screening based on RNA interference (RNAi).91 For KRAS-mutant cancers, a host of synthetic lethal interactions have been uncovered and characterized (Table 2). A recent study employing a small hairpin RNA (shRNA) library screen on isogenic WT and KRAS-mutant CRC cells identified several synthetic lethal interactions with mutant KRAS, including depletions of the mitotic protein polo-like kinase-1 (PLK1), anaphase-promoting complex/cyclosome (APC/C) subunits, as well as components of the proteasome.92 An RNAi-based screen was also used to identify the depletion of TANK-binding kinase 1 (TBK1), a kinase involved in NF-κB signaling, to be synthetically lethal with mutant KRAS.93 This finding also provided a mechanistic link between suppression of TBK1 expression with the downregulation of Bcl-XL through NF-κB signaling.93 Other synthetic lethal partners with mutant KRAS include the transforming growth factor beta-activated kinase 1 (TAK1), the Snail2 transcriptional repressor, the serine/threonine protein kinase STK33, the GATA2 transcription factor, and the dual inhibition of Bcl-XL and MEK.94, 95, 96, 97, 98 These examples illustrate the usefulness of genetic approaches for uncovering novel synthetic lethal interactions, which can potentially be translated into new therapeutic agents to selectively target KRAS-mutant cancers.
Table 2.
Synthetic lethal interactions in KRAS-mutant cancers.
| Genes or proteins | Cancer types | Methods of discovery | References |
|---|---|---|---|
| PLK1 | Colorectal | shRNA screen | 92 |
| Anaphase-Promoting Complex/Cyclosome (APC/C) | Colorectal | shRNA screen | 92 |
| Proteasome | Colorectal | shRNA screen | 92 |
| TBK1 | Lung | shRNA screen | 93 |
| STK33 | Colorectal, breast, and leukemia | shRNA screen | 94 |
| SNAIL | Colorectal | shRNA screen | 95 |
| TAK1 | Colorectal | Gene expression profiling and knockdown | 96 |
| GATA2 | Lung | shRNA screen | 97 |
| Bcl-XL/MEK | Colorectal, pancreatic, and lung | shRNA screen | 98 |
Conclusions
KRAS mutations are not only one of the most frequent genetic alterations in CRC, but also a critical determinant of response to EGFR-targeted therapy. A better understanding of how the KRAS oncoprotein activates its downstream targets, coupled with targeting mutant KRAS using novel agents and approaches, such as synthetic lethal interactions, may afford new ways of curing KRAS-driven cancers including CRC.
Conflicts of interest
The authors have no conflict of interest to disclose.
Acknowledgments
The authors would like to thank Dr. Jian Yu and Rochelle Fletcher for critical reading. K.K. is supported in part by the graduate student fellowship from the Department of Pharmacology & Chemical Biology. Research in L.Z.'s lab is supported by the National Institute of Health grants R01CA106348 and R01CA172136.
Footnotes
Peer review under responsibility of Chongqing Medical University.
References
- 1.Cunningham D., Atkin W., Lenz H.J. Colorectal cancer. Lancet. 2010;375:1030–1047. doi: 10.1016/S0140-6736(10)60353-4. [DOI] [PubMed] [Google Scholar]
- 2.Society AC . American Cancer Society; 2014. Colorectal Cancer Facts & Figures. [Google Scholar]
- 3.Markowitz S.D., Dawson D.M., Willis J. Focus on colon cancer. Cancer Cell. 2002;1:233–236. doi: 10.1016/s1535-6108(02)00053-3. [DOI] [PubMed] [Google Scholar]
- 4.Brand T.M., Wheeler D.L. KRAS mutant colorectal tumors: past and present. Small GTPases. 2012;3:34–39. doi: 10.4161/sgtp.18751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vogelstein B., Kinzler K.W. Cancer genes and the pathways they control. Nat Med. 2004;10:789–799. doi: 10.1038/nm1087. [DOI] [PubMed] [Google Scholar]
- 6.Fearon E.R., Vogelstein B. A genetic model for colorectal tumorigenesis. Cell. 1990;61:759–767. doi: 10.1016/0092-8674(90)90186-i. [DOI] [PubMed] [Google Scholar]
- 7.Moran A., Ortega P., de Juan C. Differential colorectal carcinogenesis: molecular basis and clinical relevance. World J Gastrointest Oncol. 2010;2:151–158. doi: 10.4251/wjgo.v2.i3.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jancik S., Drabek J., Radzioch D. Clinical relevance of KRAS in human cancers. J Biomed Biotechnol. 2010;2010:150960. doi: 10.1155/2010/150960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lengauer C., Kinzler K.W., Vogelstein B. Genetic instability in colorectal cancers. Nature. 1997;386:623–627. doi: 10.1038/386623a0. [DOI] [PubMed] [Google Scholar]
- 10.Grady W.M., Carethers J.M. Genomic and epigenetic instability in colorectal cancer pathogenesis. Gastroenterology. 2008;135:1079–1099. doi: 10.1053/j.gastro.2008.07.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Prenen H., Tejpar S., Van Cutsem E. New strategies for treatment of KRAS mutant metastatic colorectal cancer. Clin Cancer Res. 2010;16:2921–2926. doi: 10.1158/1078-0432.CCR-09-2029. [DOI] [PubMed] [Google Scholar]
- 12.Sullivan K.M., Kozuch P.S. Impact of KRAS mutations on management of colorectal carcinoma. Patholog Res Int. 2011;2011:219309. doi: 10.4061/2011/219309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pylayeva-Gupta Y., Grabocka E., Bar-Sagi D. RAS oncogenes: weaving a tumorigenic web. Nat Rev Cancer. 2011;11:761–774. doi: 10.1038/nrc3106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Giehl K. Oncogenic Ras in tumour progression and metastasis. Biol Chem. 2005;386:193–205. doi: 10.1515/BC.2005.025. [DOI] [PubMed] [Google Scholar]
- 15.Lowy D.R., Willumsen B.M. Function and regulation of ras. Annu Rev Biochem. 1993;62:851–891. doi: 10.1146/annurev.bi.62.070193.004223. [DOI] [PubMed] [Google Scholar]
- 16.Downward J. Targeting RAS signalling pathways in cancer therapy. Nat Rev Cancer. 2003;3:11–22. doi: 10.1038/nrc969. [DOI] [PubMed] [Google Scholar]
- 17.Johnson L., Greenbaum D., Cichowski K. K-ras is an essential gene in the mouse with partial functional overlap with N-ras. Genes Dev. 1997;11:2468–2481. doi: 10.1101/gad.11.19.2468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Malumbres M., Barbacid M. RAS oncogenes: the first 30 years. Nat Rev Cancer. 2003;3:459–465. doi: 10.1038/nrc1097. [DOI] [PubMed] [Google Scholar]
- 19.Karnoub A.E., Weinberg R.A. Ras oncogenes: split personalities. Nat Rev Mol Cell Biol. 2008;9:517–531. doi: 10.1038/nrm2438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Reuther G.W., Der C.J. The Ras branch of small GTPases: Ras family members don't fall far from the tree. Curr Opin Cell Biol. 2000;12:157–165. doi: 10.1016/s0955-0674(99)00071-x. [DOI] [PubMed] [Google Scholar]
- 21.Cooper G.M. 2nd ed. ASM Press; Sinauer Associates; Washington, D.C.; Sunderland, Mass: 2000. The Cell: A Molecular Approach. [Google Scholar]
- 22.Buday L., Downward J. Epidermal growth factor regulates p21ras through the formation of a complex of receptor, Grb2 adapter protein, and Sos nucleotide exchange factor. Cell. 1993;73:611–620. doi: 10.1016/0092-8674(93)90146-h. [DOI] [PubMed] [Google Scholar]
- 23.Shields J.M., Pruitt K., McFall A. Understanding Ras: 'it ain't over 'til it's over'. Trends Cell Biol. 2000;10:147–154. doi: 10.1016/s0962-8924(00)01740-2. [DOI] [PubMed] [Google Scholar]
- 24.Pruitt K., Der C.J. Ras and Rho regulation of the cell cycle and oncogenesis. Cancer Lett. 2001;171:1–10. doi: 10.1016/s0304-3835(01)00528-6. [DOI] [PubMed] [Google Scholar]
- 25.Cox A.D., Der C.J. The dark side of Ras: regulation of apoptosis. Oncogene. 2003;22:8999–9006. doi: 10.1038/sj.onc.1207111. [DOI] [PubMed] [Google Scholar]
- 26.Lambert J.M., Lambert Q.T., Reuther G.W. Tiam1 mediates Ras activation of Rac by a PI(3)K-independent mechanism. Nat Cell Biol. 2002;4:621–625. doi: 10.1038/ncb833. [DOI] [PubMed] [Google Scholar]
- 27.Downward J. Ras signalling and apoptosis. Curr Opin Genet Dev. 1998;8:49–54. doi: 10.1016/s0959-437x(98)80061-0. [DOI] [PubMed] [Google Scholar]
- 28.Matallanas D., Romano D., Al-Mulla F. Mutant K-Ras activation of the proapoptotic MST2 pathway is antagonized by wild-type K-Ras. Mol Cell. 2011;44:893–906. doi: 10.1016/j.molcel.2011.10.016. [DOI] [PubMed] [Google Scholar]
- 29.Wang H.L., Lopategui J., Amin M.B. KRAS mutation testing in human cancers: the pathologist's role in the era of personalized medicine. Adv Anat Pathol. 2010;17:23–32. doi: 10.1097/PAP.0b013e3181c6962f. [DOI] [PubMed] [Google Scholar]
- 30.Perkins G., Pilati C., Blons H. Beyond KRAS status and response to anti-EGFR therapy in metastatic colorectal cancer. Pharmacogenomics. 2014;15:1043–1052. doi: 10.2217/pgs.14.66. [DOI] [PubMed] [Google Scholar]
- 31.Shirasawa S., Furuse M., Yokoyama N. Altered growth of human colon cancer cell lines disrupted at activated Ki-ras. Science. 1993;260:85–88. doi: 10.1126/science.8465203. [DOI] [PubMed] [Google Scholar]
- 32.Yun J., Rago C., Cheong I. Glucose deprivation contributes to the development of KRAS pathway mutations in tumor cells. Science. 2009;325:1555–1559. doi: 10.1126/science.1174229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kim J.S., Lee C., Foxworth A. B-Raf is dispensable for K-Ras-mediated oncogenesis in human cancer cells. Cancer Res. 2004;64:1932–1937. doi: 10.1158/0008-5472.can-03-3862. [DOI] [PubMed] [Google Scholar]
- 34.Arena S., Isella C., Martini M. Knock-in of oncogenic Kras does not transform mouse somatic cells but triggers a transcriptional response that classifies human cancers. Cancer Res. 2007;67:8468–8476. doi: 10.1158/0008-5472.CAN-07-1126. [DOI] [PubMed] [Google Scholar]
- 35.Shao D.D., Xue W., Krall E.B. KRAS and YAP1 converge to regulate EMT and tumor survival. Cell. 2014;158:171–184. doi: 10.1016/j.cell.2014.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kapoor A., Yao W., Ying H. Yap1 activation enables bypass of oncogenic Kras addiction in pancreatic cancer. Cell. 2014;158:185–197. doi: 10.1016/j.cell.2014.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Viale P.H., Fung A., Zitella L. Advanced colorectal cancer: current treatment and nursing management with economic considerations. Clin J Oncol Nurs. 2005;9:541–552. doi: 10.1188/05.CJON.541-552. [DOI] [PubMed] [Google Scholar]
- 38.Meyerhardt J.A., Mayer R.J. Systemic therapy for colorectal cancer. N Engl J Med. 2005;352:476–487. doi: 10.1056/NEJMra040958. [DOI] [PubMed] [Google Scholar]
- 39.Venook A. Critical evaluation of current treatments in metastatic colorectal cancer. Oncologist. 2005;10:250–261. doi: 10.1634/theoncologist.10-4-250. [DOI] [PubMed] [Google Scholar]
- 40.Institute NC . National Cancer Institute; 2014. Targeted Cancer Therapies. [Google Scholar]
- 41.Grothey A., Van Cutsem E., Sobrero A. Regorafenib monotherapy for previously treated metastatic colorectal cancer (CORRECT): an international, multicentre, randomised, placebo-controlled, phase 3 trial. Lancet. 2013;381:303–312. doi: 10.1016/S0140-6736(12)61900-X. [DOI] [PubMed] [Google Scholar]
- 42.El Zouhairi M., Charabaty A., Pishvaian M.J. Molecularly targeted therapy for metastatic colon cancer: proven treatments and promising new agents. Gastrointest Cancer Res. 2011;4:15–21. [PMC free article] [PubMed] [Google Scholar]
- 43.Abrams T.A., Meyer G., Schrag D. Chemotherapy usage patterns in a US-wide cohort of patients with metastatic colorectal cancer. J Natl Cancer Inst. 2014;106:djt371. doi: 10.1093/jnci/djt371. [DOI] [PubMed] [Google Scholar]
- 44.Saif M.W. Colorectal cancer in review: the role of the EGFR pathway. Expert Opin Investig Drugs. 2010;19:357–369. doi: 10.1517/13543781003593962. [DOI] [PubMed] [Google Scholar]
- 45.Singh A., Greninger P., Rhodes D. A gene expression signature associated with “K-Ras addiction” reveals regulators of EMT and tumor cell survival. Cancer Cell. 2009;15:489–500. doi: 10.1016/j.ccr.2009.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mendelsohn J., Baselga J. The EGF receptor family as targets for cancer therapy. Oncogene. 2000;19:6550–6565. doi: 10.1038/sj.onc.1204082. [DOI] [PubMed] [Google Scholar]
- 47.Hagan S., Orr M.C., Doyle B. Targeted therapies in colorectal cancer-an integrative view by PPPM. EPMA J. 2013;4:3. doi: 10.1186/1878-5085-4-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Messersmith W.A., Hidalgo M. Panitumumab, a monoclonal anti epidermal growth factor receptor antibody in colorectal cancer: another one or the one? Clin Cancer Res. 2007;13:4664–4666. doi: 10.1158/1078-0432.CCR-07-0065. [DOI] [PubMed] [Google Scholar]
- 49.Heinemann V., Douillard J.Y., Ducreux M. Targeted therapy in metastatic colorectal cancer – an example of personalised medicine in action. Cancer Treat Rev. 2013;39:592–601. doi: 10.1016/j.ctrv.2012.12.011. [DOI] [PubMed] [Google Scholar]
- 50.Cunningham D., Humblet Y., Siena S. Cetuximab monotherapy and cetuximab plus irinotecan in irinotecan-refractory metastatic colorectal cancer. N Engl J Med. 2004;351:337–345. doi: 10.1056/NEJMoa033025. [DOI] [PubMed] [Google Scholar]
- 51.Jonker D.J., O'Callaghan C.J., Karapetis C.S. Cetuximab for the treatment of colorectal cancer. N Engl J Med. 2007;357:2040–2048. doi: 10.1056/NEJMoa071834. [DOI] [PubMed] [Google Scholar]
- 52.Shima F., Yoshikawa Y., Ye M. In silico discovery of small-molecule Ras inhibitors that display antitumor activity by blocking the Ras-effector interaction. Proc Natl Acad Sci U S A. 2013;110:8182–8187. doi: 10.1073/pnas.1217730110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Douillard J.Y., Siena S., Cassidy J. Final results from PRIME: randomized phase III study of panitumumab with FOLFOX4 for first-line treatment of metastatic colorectal cancer. Ann Oncol. 2014;25:1346–1355. doi: 10.1093/annonc/mdu141. [DOI] [PubMed] [Google Scholar]
- 54.Lievre A., Bachet J.B., Le Corre D. KRAS mutation status is predictive of response to cetuximab therapy in colorectal cancer. Cancer Res. 2006;66:3992–3995. doi: 10.1158/0008-5472.CAN-06-0191. [DOI] [PubMed] [Google Scholar]
- 55.Benvenuti S., Sartore-Bianchi A., Di Nicolantonio F. Oncogenic activation of the RAS/RAF signaling pathway impairs the response of metastatic colorectal cancers to anti-epidermal growth factor receptor antibody therapies. Cancer Res. 2007;67:2643–2648. doi: 10.1158/0008-5472.CAN-06-4158. [DOI] [PubMed] [Google Scholar]
- 56.Banck M.S., Grothey A. Biomarkers of resistance to epidermal growth factor receptor monoclonal antibodies in patients with metastatic colorectal Cancer. Clin Cancer Res. 2009;15:7492–7501. doi: 10.1158/1078-0432.CCR-09-0188. [DOI] [PubMed] [Google Scholar]
- 57.De Roock W., Claes B., Bernasconi D. Effects of KRAS, BRAF, NRAS, and PIK3CA mutations on the efficacy of cetuximab plus chemotherapy in chemotherapy-refractory metastatic colorectal cancer: a retrospective consortium analysis. Lancet Oncol. 2010;11:753–762. doi: 10.1016/S1470-2045(10)70130-3. [DOI] [PubMed] [Google Scholar]
- 58.Karapetis C.S., Khambata-Ford S., Jonker D.J. K-ras mutations and benefit from cetuximab in advanced colorectal cancer. N Engl J Med. 2008;359:1757–1765. doi: 10.1056/NEJMoa0804385. [DOI] [PubMed] [Google Scholar]
- 59.Amado R.G., Wolf M., Peeters M. Wild-type KRAS is required for panitumumab efficacy in patients with metastatic colorectal cancer. J Clin Oncol. 2008;26:1626–1634. doi: 10.1200/JCO.2007.14.7116. [DOI] [PubMed] [Google Scholar]
- 60.Allegra C.J., Jessup J.M., Somerfield M.R. American Society of Clinical Oncology provisional clinical opinion: testing for KRAS gene mutations in patients with metastatic colorectal carcinoma to predict response to anti-epidermal growth factor receptor monoclonal antibody therapy. J Clin Oncol. 2009;27:2091–2096. doi: 10.1200/JCO.2009.21.9170. [DOI] [PubMed] [Google Scholar]
- 61.Bardelli A., Siena S. Molecular mechanisms of resistance to cetuximab and panitumumab in colorectal cancer. J Clin Oncol. 2010;28:1254–1261. doi: 10.1200/JCO.2009.24.6116. [DOI] [PubMed] [Google Scholar]
- 62.Misale S., Arena S., Lamba S. Blockade of EGFR and MEK intercepts heterogeneous mechanisms of acquired resistance to anti-EGFR therapies in colorectal cancer. Sci Transl Med. 2014;6:224ra226. doi: 10.1126/scitranslmed.3007947. [DOI] [PubMed] [Google Scholar]
- 63.Misale S., Yaeger R., Hobor S. Emergence of KRAS mutations and acquired resistance to anti-EGFR therapy in colorectal cancer. Nature. 2012;486:532–536. doi: 10.1038/nature11156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Misale S., Di Nicolantonio F., Sartore-Bianchi A. Resistance to anti-EGFR therapy in colorectal cancer: from heterogeneity to convergent evolution. Cancer Discov. 2014;4:1269–1280. doi: 10.1158/2159-8290.CD-14-0462. [DOI] [PubMed] [Google Scholar]
- 65.Bettegowda C., Sausen M., Leary R.J. Detection of circulating tumor DNA in early- and late-stage human malignancies. Sci Transl Med. 2014;6:224ra224. doi: 10.1126/scitranslmed.3007094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Diaz L.A., Jr., Williams R.T., Wu J. The molecular evolution of acquired resistance to targeted EGFR blockade in colorectal cancers. Nature. 2012;486:537–540. doi: 10.1038/nature11219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Valtorta E., Misale S., Sartore-Bianchi A. KRAS gene amplification in colorectal cancer and impact on response to EGFR-targeted therapy. Int J Cancer. 2013;133:1259–1265. doi: 10.1002/ijc.28106. [DOI] [PubMed] [Google Scholar]
- 68.Martini M., Vecchione L., Siena S. Targeted therapies: how personal should we go? Nat Rev Clin Oncol. 2012;9:87–97. doi: 10.1038/nrclinonc.2011.164. [DOI] [PubMed] [Google Scholar]
- 69.Kasper S., Breitenbuecher F., Reis H. Oncogenic RAS simultaneously protects against anti-EGFR antibody-dependent cellular cytotoxicity and EGFR signaling blockade. Oncogene. 2013;32:2873–2881. doi: 10.1038/onc.2012.302. [DOI] [PubMed] [Google Scholar]
- 70.Fisher G.H., Wellen S.L., Klimstra D. Induction and apoptotic regression of lung adenocarcinomas by regulation of a K-Ras transgene in the presence and absence of tumor suppressor genes. Genes Dev. 2001;15:3249–3262. doi: 10.1101/gad.947701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Sun Q., Burke J.P., Phan J. Discovery of small molecules that bind to K-Ras and inhibit Sos-mediated activation. Angew Chem Int Ed Engl. 2012;51:6140–6143. doi: 10.1002/anie.201201358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Maurer T., Garrenton L.S., Oh A. Small-molecule ligands bind to a distinct pocket in Ras and inhibit SOS-mediated nucleotide exchange activity. Proc Natl Acad Sci U S A. 2012;109:5299–5304. doi: 10.1073/pnas.1116510109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ostrem J.M., Peters U., Sos M.L. K-Ras(G12C) inhibitors allosterically control GTP affinity and effector interactions. Nature. 2013;503:548–551. doi: 10.1038/nature12796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Lim S.M., Westover K.D., Ficarro S.B. Therapeutic targeting of oncogenic K-Ras by a covalent catalytic site inhibitor. Angew Chem Int Ed Engl. 2014;53:199–204. doi: 10.1002/anie.201307387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Haluska P., Dy G.K., Adjei A.A. Farnesyl transferase inhibitors as anticancer agents. Eur J Cancer. 2002;38:1685–1700. doi: 10.1016/s0959-8049(02)00166-1. [DOI] [PubMed] [Google Scholar]
- 76.Cox A.D., Der C.J. Farnesyltransferase inhibitors and cancer treatment: targeting simply Ras? Biochim Biophys Acta. 1997;1333:F51–F71. doi: 10.1016/s0304-419x(97)00011-5. [DOI] [PubMed] [Google Scholar]
- 77.Gysin S., Salt M., Young A. Therapeutic strategies for targeting ras proteins. Genes Cancer. 2011;2:359–372. doi: 10.1177/1947601911412376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Zimmermann G., Papke B., Ismail S. Small molecule inhibition of the KRAS-PDEdelta interaction impairs oncogenic KRAS signalling. Nature. 2013;497:638–642. doi: 10.1038/nature12205. [DOI] [PubMed] [Google Scholar]
- 79.Fruman D.A., Rommel C. PI3K and cancer: lessons, challenges and opportunities. Nat Rev Drug Discov. 2014;13:140–156. doi: 10.1038/nrd4204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Neuzillet C., Tijeras-Raballand A., de Mestier L. MEK in cancer and cancer therapy. Pharmacol Ther. 2014;141:160–171. doi: 10.1016/j.pharmthera.2013.10.001. [DOI] [PubMed] [Google Scholar]
- 81.Engelman J.A., Chen L., Tan X. Effective use of PI3K and MEK inhibitors to treat mutant Kras G12D and PIK3CA H1047R murine lung cancers. Nat Med. 2008;14:1351–1356. doi: 10.1038/nm.1890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Yuan T.L., Fellmann C., Lee C.S. Development of siRNA payloads to target KRAS-mutant Cancer. Cancer Discov. 2014;4:1182–1197. doi: 10.1158/2159-8290.CD-13-0900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Lamba S., Russo M., Sun C. RAF suppression synergizes with MEK inhibition in KRAS mutant cancer cells. Cell Rep. 2014;8:1475–1483. doi: 10.1016/j.celrep.2014.07.033. [DOI] [PubMed] [Google Scholar]
- 84.Vigil D., Cherfils J., Rossman K.L. Ras superfamily GEFs and GAPs: validated and tractable targets for cancer therapy? Nat Rev Cancer. 2010;10:842–857. doi: 10.1038/nrc2960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Wang Y., Kaiser C.E., Frett B. Targeting mutant KRAS for anticancer therapeutics: a review of novel small molecule modulators. J Med Chem. 2013;56:5219–5230. doi: 10.1021/jm3017706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Berndt N., Hamilton A.D., Sebti S.M. Targeting protein prenylation for cancer therapy. Nat Rev Cancer. 2011;11:775–791. doi: 10.1038/nrc3151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Friday B.B., Adjei A.A. K-ras as a target for cancer therapy. Biochim Biophys Acta. 2005;1756:127–144. doi: 10.1016/j.bbcan.2005.08.001. [DOI] [PubMed] [Google Scholar]
- 88.Stephen A.G., Esposito D., Bagni R.K. Dragging ras back in the ring. Cancer Cell. 2014;25:272–281. doi: 10.1016/j.ccr.2014.02.017. [DOI] [PubMed] [Google Scholar]
- 89.Ferrari E., Lucca C., Foiani M. A lethal combination for cancer cells: synthetic lethality screenings for drug discovery. Eur J Cancer. 2010;46:2889–2895. doi: 10.1016/j.ejca.2010.07.031. [DOI] [PubMed] [Google Scholar]
- 90.Chan D.A., Giaccia A.J. Harnessing synthetic lethal interactions in anticancer drug discovery. Nat Rev Drug Discov. 2011;10:351–364. doi: 10.1038/nrd3374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Kaelin W.G., Jr. Synthetic lethality: a framework for the development of wiser cancer therapeutics. Genome Med. 2009;1:99. doi: 10.1186/gm99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Luo J., Emanuele M.J., Li D. A genome-wide RNAi screen identifies multiple synthetic lethal interactions with the Ras oncogene. Cell. 2009;137:835–848. doi: 10.1016/j.cell.2009.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Barbie D.A., Tamayo P., Boehm J.S. Systematic RNA interference reveals that oncogenic KRAS-driven cancers require TBK1. Nature. 2009;462:108–112. doi: 10.1038/nature08460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Scholl C., Frohling S., Dunn I.F. Synthetic lethal interaction between oncogenic KRAS dependency and STK33 suppression in human cancer cells. Cell. 2009;137:821–834. doi: 10.1016/j.cell.2009.03.017. [DOI] [PubMed] [Google Scholar]
- 95.Wang Y., Ngo V.N., Marani M. Critical role for transcriptional repressor Snail2 in transformation by oncogenic RAS in colorectal carcinoma cells. Oncogene. 2010;29:4658–4670. doi: 10.1038/onc.2010.218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Singh A., Sweeney M.F., Yu M. TAK1 inhibition promotes apoptosis in KRAS-dependent colon cancers. Cell. 2012;148:639–650. doi: 10.1016/j.cell.2011.12.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Kumar M.S., Hancock D.C., Molina-Arcas M. The GATA2 transcriptional network is requisite for RAS oncogene-driven non-small cell lung cancer. Cell. 2012;149:642–655. doi: 10.1016/j.cell.2012.02.059. [DOI] [PubMed] [Google Scholar]
- 98.Corcoran R.B., Cheng K.A., Hata A.N. Synthetic lethal interaction of combined BCL-XL and MEK inhibition promotes tumor regressions in KRAS mutant cancer models. Cancer Cell. 2013;23:121–128. doi: 10.1016/j.ccr.2012.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]