Abstract
In pubertal male Syrian hamsters, exposure to anabolic/androgenic steroids (AAS) during adolescence facilitates a high level of offensive aggression modulated by the enhanced development and activity of the vasopressin (AVP) and dopamine (DA) neural systems within the latero-anterior hypothalamus (LAH), i.e., a brain region implicated in the control of aggression. The present studies provide a detailed report of the pharmacologic interactions between AVP and DA D2 receptor signaling within the LAH in the control of adolescent AAS-induced offensive aggression. Male Syrian hamsters were treated with AAS throughout adolescence and tested for aggression after local infusion of the DA D2 receptor antagonist eticlopride (ETIC) alone, or in combination with AVP in the LAH in an effort to determine the influence of DA D2 receptors relative to AVP-receptor mediated aggression mechanisms. As previously shown, ETIC infusion into the LAH suppressed adolescent AAS-induced aggressive responding; however, the AAS-induced aggressive phenotype was rescued by the co-infusion of AVP into the LAH. These behavioral data indicate that interactions between AVP and DA neural systems within the LAH modulate the control of aggression following adolescent exposure to AAS and that DA D2 receptor signaling functions upstream of AVP in the LAH to control this behavioral response.
Key Terms: anterior hypothalamus, latero-anterior hypothalamus, eticlopride, vasopressin, dopamine, D2 antagonist, microinjection, anabolic-androgenic steroids, aggression, adolescence, Syrian hamster
Introduction
In male Syrian hamsters (Mesocricetus auratus), exposure to anabolic/androgenic steroids (AAS) during adolescent development produces a mature and escalated aggressive phenotype (DeLeon, Grimes, & Melloni, 2002; Grimes, Ricci, & Melloni, 2007; Harrison, Connor, Nowak, Nash, & Melloni, 2000; Melloni & Ricci, 2010). Relatedly, in a series of studies, we have shown that adolescent AAS exposure alters several neurotransmitter systems implicated in the control of aggression within the latero-anterior subdivision of the hypothalamus (LAH) (Carrillo et al., 2011; Harrison et al., 2000; Schwartzer & Melloni, 2010a, 2010b); namely the vasopressin (AVP) (Carrillo, Ricci, & Melloni, 2011; Grimes, Ricci, & Melloni, 2006; Grimes et al., 2007; Harrison et al., 2000; Melloni & Ricci, 2010), serotonin (5HT) (Grimes & Melloni, 2002, 2005; Ricci, Rasakham, Grimes, & Melloni, 2006), and γ-aminobutyric acid (GABA) (Grimes, Ricci, & Melloni, 2003; Schwartzer, Ricci, & Melloni, 2009) neural systems. Similarly, dopamine (DA) neural signaling through several receptors has also been linked to aggression. In particular the DA D2-like receptor has been implicated in aggressive responding in mice (Arregui, Azpiroz, Brain, & Simon, 1993) and hamsters (Schwartzer et al., 2009). Accordingly, tyrosine hydroxylase (TH) containing neurons, afferent fibers, and DA D2 receptors are present within regions associated with aggressive behavior that encompass the hypothalamic neural circuit that controls offensive aggression in hamsters (i.e., the bed nucleus of the stria terminalis, lateral septum, medial amygdala, and ventrolateral hypothalamus) (Delville, De Vries, & Ferris, 2000; Ricci, Schwartzer, & Melloni, 2009; Schwartzer & Melloni, 2010b; Schwartzer et al., 2009) suggesting that DA activity modulates aggression through DA D2 receptors in these brain regions. Interestingly, hypothalamic DA neurons are responsive to androgens in a fashion consistent with the generation of the aggressive phenotype (Thiblin, Finn, Ross, & Stenfors, 1999), so alterations in this neural system may play a role in adolescent AAS-induced offensive aggression.
Recently, we showed that direct application of DA D2 antagonists to the LAH dose dependently suppresses adolescent AAS-induced offensive aggression in hamsters (Schwartzer & Melloni, 2010a). Relatedly, we also found that adolescent AAS administration increases TH afferent development and DA D2 receptor localization/expression in the LAH of male Syrian hamsters. Importantly, these alterations were confined to the LAH brain region as similar adolescent AAS-induced changes were not observed in any other nuclei comprising the hypothalamic neural circuit that controls aggression in hamsters (Ricci et al., 2009; Schwartzer & Melloni, 2010b). In particular, adolescent AAS-treated hamsters have more TH neurons and afferent fibers within the nucleus circularis (NC) and medial supraoptic nucleus (mSON) subregions of the LAH (Melloni & Ricci, 2010; Ricci et al., 2009). Taken together, these data suggest that adolescent AAS exposure increases DA development and DA D2 receptor density in the LAH, and this increase in LAH DA activity facilitates aggression through activation of LAH DA D2 receptor signaling.
A functional relationship exists between AVP and DA in the anterior hypothalamus (AH), such that we recently observed that AVP and TH are co-expressed in neurons in the NC and mSON, i.e., AVP and DA containing neuronal compartments that project AVP and DA afferent streams into the LAH in hamsters (Melloni & Ricci, 2010). These data indicate that adolescent AAS-induced aggression may be modulated by enhanced release/activity of AVP and DA within the LAH, and that DA acts (through DA D2 receptors) independently of AVP within this brain site to facilitate offensive aggression. The nature of the functional interaction between AVP and DA within the LAH and how this interaction relates to adolescent AAS-induced aggressive behavior is largely unknown. It is possible that DA D2 receptors modulate aggression as a result of downstream attenuation of AH AVP activity.
To elucidate the relationship of DA/AVP neural signaling in the LAH and adolescent AAS-induced aggression we treated male hamsters throughout adolescent development with AAS then tested them for aggressive and social behaviors after microinjection of the DA D2 antagonist eticlopride alone, or in combination with AVP, directly into the LAH in an effort to establish the functional arrangement and the influence of DA D2 receptors relative to AVP-receptor mediated aggression mechanisms.
Methods
Animals
Male Syrian hamsters exposed to one of two possible experimental treatments (N=84) were obtained from Charles River (Wilmington, MA) and individually housed in polycarbonate cages, and maintained at ambient room temperature (22–24 °C, with 55% relative humidity) on a reverse light–dark cycle (14L:10 D; lights off at 08:00) as previously described (Grimes & Melloni, 2002). Food and water were provided ad libitum. For aggression testing, stimulus (intruder) males of equal size and weight to the experimental animals were obtained from Charles River Laboratories one week prior to the behavioral test, group-housed (five animals per cage) in large polycarbonate cages, and maintained as above to acclimate to the animal facility. All studies were preapproved by the Northeastern University Institutional Animal Care and Use Committee and all methods employed are consistent with guidelines provided by the National Institutes of Health for the scientific treatment of animals.
Drugs
Testosterone cypionate, nandrolone decanoate, and boldenone undecylenate were purchased from Steraloids Inc (Newport, RI) and prepared in sesame oil (SO). Eticlopride hydrochloride (ETIC) and arginine vasopressin (AVP) were purchased from Sigma Aldrich (St. Louis, MO). The ETIC was dissolved in 0.9% (wt/vol) normal saline and the AVP was dissolved in ddH2O.
Experimental Treatment
On Postnatal day (P) 27, hamsters (n=42) received daily subcutaneous (SC) injections (0.1–0.2 ml) of an AAS mixture consisting of 2 mg/kg testosterone cypionate, 2 mg/kg nandrolone decanoate, and 1 mg/kg boldenone undecylenate dissolved in SO, for 30 consecutive days during adolescent development (P27-P56). This treatment regimen has been shown repeatedly to produce highly aggressive animals in greater than 85% of the treatment pool (DeLeon, Grimes, & Melloni, 2002; Grimes & Melloni, 2005). As a nonaggressive control, a separate set of animals received SC injections of vehicle (SO; n=42).
Surgical Procedure
One week prior to aggression testing (P50), all animals used for behavioral pharmacology experiments (n=42 SO; n=42 AAS; total n=84) were anesthetized with a ketamine/xylazine cocktail (80 mg/kg:12 mg/kg) and placed into a stereotaxic device for unilateral implantation of a 26 gauge guide cannula aimed at the LAH. An incision was made to expose the dorsal surface of the skull. The skull surface was then wiped clean to reveal the position of lambda and bregma landmarks. A small hole was drilled into the skull at the coordinate position necessary to gain access to the anterior hypothalamus, specifically directed to the lateral subdivision (i.e., 0.7 mm anterior to bregma, 0.6 mm lateral to the midsagittal suture, 6.8 mm ventral from dura). The cannula was placed in the brain angled at 8 degrees and anchored to the skull using dental screws and acrylic. The head-wound was then sutured closed and topical antibiotic ointment applied to the wound area. To verify cannula placement, animals were anesthetized with carbon-dioxide one day following behavioral testing and transcardially perfused with 4% paraformaldehyde. Brains were removed, postfixed for 90 min in perfusion fixative, and cryoprotected overnight in 30% sucrose at 4 °C. Brains were cut at 45 μm on a freezing microtome in serial coronal sections and mounted on gelatin-coated slides. Sections were stained with cresyl violet, dehydrated through a series of alcohols, cleared with xylene, and coverslipped with Permount (Fischer Scientific, Pittsburgh, PA). Only animals with correctly placed cannula tips into the LAH were included in the statistical analysis.
Experimental Treatment and Behavioral Testing
For behavioral pharmacology studies, one day following the last injection of AAS or SO (P57), hamsters were randomly assigned to one of three treatment groups (n = 28/group) and tested for offensive aggression following two consecutive microinjections into the LAH (0.5 μL each) using a 2-μl Hamilton syringe (as described previously in Schwartzer & Melloni, 2010b) of the following substances: saline, ETIC (i.e., DA D2 antagonist; 0.5 mM), or AVP (0.9 μM). The following drugs or drug combinations were administered: saline, ETIC, ETIC+AVP. Each drug was delivered over 3 min in a volume of 500 nl, followed by a 2 minute time period to allow drug to diffuse away from the syringe tip. Consecutive microinjections were separated by 5 min. Doses of ETIC and AVP were selected based on their behavioral effects reported previously (Beyer & Steketee, 2000; Ferris et al., 1997; Ferris, Albers, Wesolowski, Goldman, & Luman, 1984; Schwartzer & Melloni, 2010b; Sweidan, Edinger, & Siegel, 1991). Following the microinjection regimen, animals were placed back in their home cages for 5-10 min, and then tested for offensive aggression using the resident-intruder paradigm, i.e., a well-characterized and ethologically valid model of offensive aggression in Syrian hamsters (Floody & Pfaff, 1974; Lerwill & Makings, 1971). Briefly, a novel intruder of similar size and weight was introduced into the home cage of the experimental animal (resident) and the resident was scored for general (composite, bites, attack latency) and targeted (frontal attacks, lateral attacks, and flank bites), aggressive responses as previously described (Grimes et al., 2003; Morrison, Ricci, & Melloni, 2014; Ricci et al., 2006). An attack was scored each time the resident animal would pursue and then either lunge toward and/or confine the intruder by upright and sideways threat; each generally followed by a direct attempt to bite the intruder's face/head (frontal attack) or lateral or dorsal flank/rump (lateral attack) target area(s). A composite aggression score, used as a general measure of offensive aggression, was defined as the total number of attacks (i.e., frontal and lateral attacks) and bites (i.e., flank/rump bites) during the behavioral test period. Attack latency was recorded and defined as the period of time between the beginning of the behavioral test and the first attack the residents made toward an intruder. In the case of no attacks, attack latency was assigned the maximum latency (i.e., 600 seconds). Each aggression test lasted for 10 min and was videotaped and scored manually by two observers blind to experimental treatment. Inter-rater reliability was set at 95%. No intruder was used for more than one behavioral test, and all subjects were tested during the first 4 hours of the dark cycle under dim red illumination to control for circadian influences on behavioral responding. In addition to aggressive behaviors, residents were measured for social interest toward intruders defined as the period of time during which the resident deliberately initiated contact with the intruder (i.e., total contact time between the intruder and resident [TCT]). Animals were also scored for measures of motility/escape (i.e., wall climbing; Lumley et al., 2000; Shettleworth, 1975) and stereotypy (i.e., self-grooming; Berridge, 1999) to account for non-specific effects of drugs during the 10 min agonistic encounter.
Statistics
Behavioral data were compared between AAS and vehicle-treated animals for a given microinjection regimen and also between saline-treated controls within a given pretreatment condition (i.e., AAS or SO) using a priori planned comparison t-tests following a significant main effect from a one-way analysis of variance (ANOVA). The α level for all tests was set at 0.05.
Results
There were 56 animals with correctly placed LAH cannula included in the final analysis (Saline: n = 20; ETIC: n = 18; ETIC+AVP: n = 18). Consistent with a number of our previous studies (see Melloni & Ricci, 2010 for review), data from AAS-treated animals microinjected with saline alone (AAS-saline controls) showed that hamsters exposed to AAS throughout adolescent development displayed greater overall offensive aggression. These animals had significantly higher composite aggression scores (t(18) = 3.28 p < 0.01) and shorter latencies to attack (t(18) = 3.05, p < 0.01) compared to SO-treated animals administered saline (SO-saline controls) (Figure 1). On average, AAS-saline control animals were 3-times quicker to attack and their composite aggression scores were greater than 3-fold higher than their SO-saline treated counterparts. In contrast, there was no significant difference in the frequency of total bites between AAS- and SO- treated saline controls (p > 0.05; see Figure 1).
Figure 1.
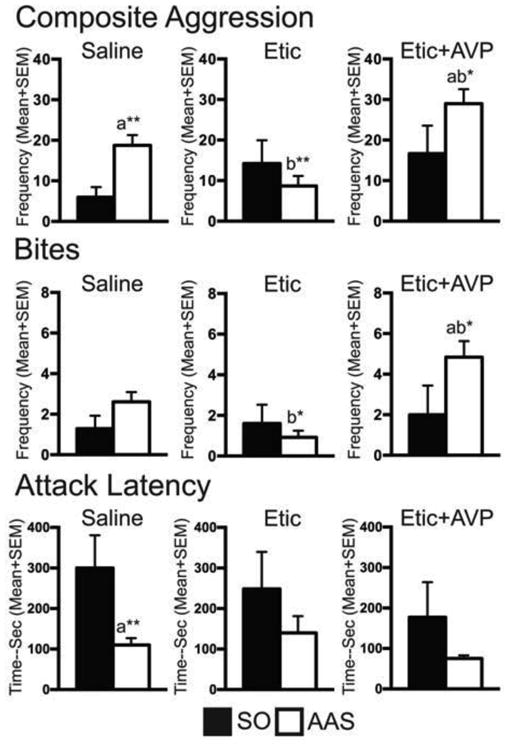
Composite aggression score, total bites, and attack latency of anabolic/androgenic steroid (AAS), or sesame oil (SO) treated animals after microinjection of saline, eticlopride (Etic), or a combination of Etic with vasopressin (AVP) into the latero-anterior hypothalamus (LAH). Generally, when compared to saline-treated controls or SO-treated controls, the combination of Etic and AVP enhanced aggressive behavior in AAS-treated animals. Consistent with previous findings, Etic microinjections inhibited aggressive behavior in AAS treated animals to levels similar to those in SO-treated controls, and also below those observed in saline-treated AAS animals. a: Significantly different from the drug regimen's SO-treated control group; b: Significantly different from saline-treated controls; * P < 0.05; ** P < 0.01, *** P < 0.001.
Similar to our previous findings (Schwartzer & Melloni, 2010a, 2010b), DA D2 antagonism in the LAH suppressed adolescent AAS-induced aggressive responding. Specifically, AAS-treated animals administered ETIC (AAS-ETIC animals), displayed significantly lower composite aggression scores (t(24) = 2.82, p < 0.01) and directed fewer total bites onto intruders (t(24) = 2.70, p < 0.01) than did AAS animals administered saline (AAS-saline controls) (Figure 1). Despite these findings, attack latency times between AAS-ETIC and AAS-saline control animals were not significantly different (p > 0.05). Similarly, when compared with SO-treated controls (i.e., SO-ETIC animals), AAS-ETIC treated animals showed no significant differences in composite aggression score, attack latency, or total bites. Nor were there any significant differences across any measure of aggressive behavior between SO-saline and SO-ETIC animals (p > 0.05 for all).
Further investigation revealed that the DA D2 suppression of adolescent AAS-induced offensive aggression could be reversed by co-infusions of AVP into the LAH. Indeed, AAS-treated animals microinjected with ETIC+AVP (AAS-ETIC+AVP animals) had significantly higher composite aggression scores (t(16) = 2.19, p < 0.05) and directed more total bites (t(16) = 2.35, p < 0.05) onto intruders than SO-treated hamsters exposed to the same microinjection regimen (i.e., SO-ETIC+AVP animals). AAS-ETIC+AVP animals also had significantly higher composite aggression scores (t(23) = 2.27, p < 0.05) and directed more total bites (t(23) = 2.30, p < 0.05) than AAS-saline control animals. In the most extreme instance, AAS-ETIC+AVP directed nearly 5-times the number of total bites onto intruders as did AAS-saline controls. There were no differences in attack latency between AAS-ETIC+AVP animals and SO-ETIC+AVP controls or between AAS-ETIC+AVP animals and AAS-saline controls (p > 0.05 for all). Nor was there any difference between SO-ETIC+AVP and SO-saline animals across any measure of aggressive behavior (p > 0.05 for all)
Examination of specific aggressive behaviors showed that DA and DA/AVP neural signaling have significant effects on targeted aggressive responses in adolescent AAS-treated animals. For instance, similar to that observed in our prior studies (see Melloni & Ricci, 2010 for review), AAS-saline control animals directed nearly >3-times the number of frontal- and lateral- attacks onto intruders than did their SO-saline treated counterparts (Figure 2). These differences were statistically significant (i.e., frontal- (t(18) = 2.127, p < 0.05) and lateral- (t(18)=2.72, p < 0.05) attacks, respectively). In contrast, there were no significant differences in the frequency of flank bites between AAS- and SO- treated saline controls (p > 0.05, Figure 2).
Figure 2.
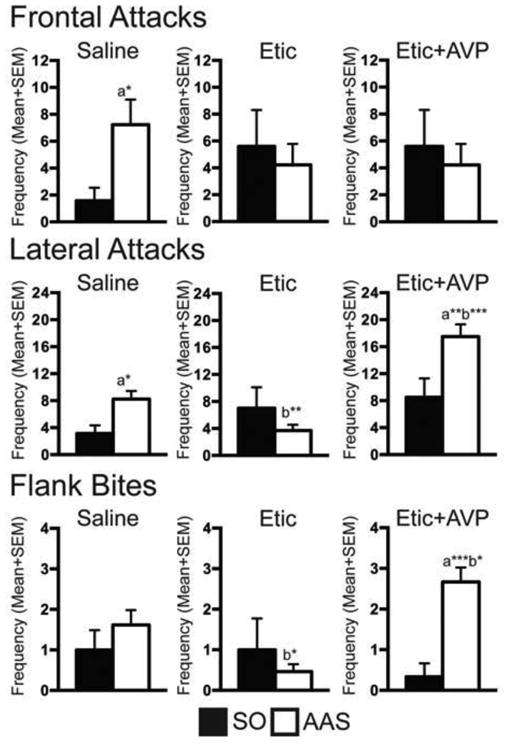
Frontal attacks, lateral attacks, and flank bites of anabolic/androgenic steroid (AAS), or sesame oil (SO) treated animals after microinjection of saline, eticlopride (Etic), or a combination of Etic with vasopressin (AVP) into the latero-anterior hypothalamus (LAH). When compared to saline-treated controls or SO-treated controls, the combination of Etic and AVP enhanced components of the aggressive phenotype in AAS-treated animals; specifically lateral attacks and flank bites, but not frontal attacks. Conversely, Etic microinjections inhibited lateral attacks and flank bites in AAS-treated animals compared to saline controls, but not their SO-treated counterparts. a: Significantly different from the drug regimen's SO-treated control group; b: Significantly different from saline-treated controls; * P < 0.05; ** P < 0.01, *** P < 0.001.
Similar, in part, to that observed previously (Schwartzer & Melloni, 2010a, 2010b), DA D2 antagonism in the LAH suppresses select aspects of the adolescent AAS-induced aggressive response. For instance, AAS-treated animals administered ETIC (AAS-ETIC animals) directed significantly fewer lateral attacks (t(24)= 2.76, p < 0.01) and flank bites (t(24) = 2.47, p < 0.05) onto intruders than did AAS-saline control animals. In fact, AAS-ETIC animals directed only about half the number of lateral attacks and flank bites onto intruders as AAS-Saline controls. However, no difference in frontal attacks existed between AAS-ETIC and AAS-saline controls, nor were there any significant differences in lateral attacks, frontal attacks, and flank bites between AAS-ETIC and SO-ETIC treated animals (p > 0.05 for all).
Co-injection of AVP into the LAH reversed the specific aggression-suppressing effects of DA D2 receptor antagonists. AAS-treated animals microinjected with ETIC+AVP (AAS-ETIC+AVP animals) directed significantly more lateral attacks (t(16) = 3.39, p < 0.01) and flank bites (t(16) = 3.81, p < 0.001) onto intruders when compared to SO-treated hamsters exposed to the same microinjection regimen. AAS-ETIC+AVP animals also directed significantly more lateral attacks (t(23) = 4.35, p < 0.001), and flank bites (t(23) = 2.14, p < 0.05) onto intruders than did AAS-saline controls. In each condition, AAS-ETIC+AVP animals directed nearly 2-fold the number of lateral attacks and flank bites as did SO-ETIC+AVP or AAS-saline controls. As above, no difference in frontal attacks existed between AAS-ETIC+AVP and AAS-saline or SO-ETIC+AVP control animals (p > 0.05 each comparison).
Table 1 summarizes the effects of drug regimen on social behavior, motor activity, and stereotypy in hamsters treated throughout adolescence with AAS and SO. Differences in drug regimen had no effect on total contact time, grooming, wall climbing behavior, or line crosses. Additionally, there was no effect of microinjection regimen over flank marking behavior with the exception of AAS-ETIC+AVP animals who performed significantly more flank marks than AAS-saline controls (t(23)=2.41, p < 0.05).
Table 1.
Effects of Microinjection of Saline, Eticlopride, or Combinations of Eticlopride with AVP into the LAH of Adolescent AAS- or Sesame Oil- (SO) Treated Hamsters on Social Contact, Locomotor (Line Crosses), and Stereotypy Measures.
| Behavior | Drug Regimen | ||
|---|---|---|---|
|
| |||
| Saline | Eticlopride | Eticlopride +AVP | |
| Contact time (Sec) | |||
| SO | 408 ± 36 | 420 ± 67 | 388 ± 40 |
| AAS | 463 ± 25 | 427 ± 25 | 392 ± 21 |
| Self grooming | |||
| SO | 4 ± 1.1 | 2.2 ± 1 | 4.8 ± 1.1 |
| AAS | 7.1 ± 1.3 | 7.6 ± 2.6 | 3.6 ± 0.5 |
| Wall climbing | |||
| SO | 12.6 ± 3.8 | 27.6 ± 14.3 | 13.3 ± 3.3 |
| AAS | 12.5 ± 2.1 | 13.1 ± 1.6 | 15 ± 2.6 |
| Line crosses | |||
| SO | 26.4 ± 3.5 | 39.2 ± 13.4 | 30.8 ± 3.4 |
| AAS | 45.5 ± 4.8 | 40.2 ± 5.5 | 36.0 ± 3.0 |
Data represent Mean±SEM
Significantly different from the drug regimen's SO-treated control group; p < 0.05
Significantly different from saline-treated control group; p < 0.05
Discussion
The pharmacological manipulation of DA neural signaling has been shown to modulate aggressive behavior in various species and animal models of aggression (Aguilar, Minarro, Perez-Iranzo, & Simon, 1994; Arregui et al., 1993; Garmendia, Sanchez, Azpiroz, Brain, & Simon, 1992; Navarro & Manzaneque, 1997; Navarro, Minarro, & Simon, 1993; Navarro, Velasco, & Manzaneque, 2000), including hamsters administered AAS during adolescent development (Schwartzer and Melloni, 2010a,b). In recent studies we have shown that the central blockade of DA D2 receptor signaling within the LAH brain region effectively suppresses the high levels of offensive aggression observed in hamsters administered moderate doses of AAS during adolescence, while leaving other social, comfort, and locomotor behaviors unaffected (Schwartzer and Melloni, 2010a,b). We have also shown that treatment with AAS throughout adolescence increases DA D2 receptor localization/expression within the LAH (Ricci et al., 2009; Schwartzer et al., 2009), supporting the notion that increased DA D2 receptor signaling in the LAH acts to facilitate offensive aggression. Given that activation of DA D2 receptors produces neuronal inhibition (Wachtel, Hu, Galloway, & White, 1989), the increases in DA D2 receptor localization/expression found in the LAH of aggressive, adolescent AAS-treated animals suggests that hypothalamic DA enhances aggressive responding through increased neuronal inhibition within the LAH. From a functional standpoint, our prior studies suggested that DA D2 receptor activity in the LAH likely increased offensive aggression through a dis-inhibitory mechanism, perhaps by disinhibiting downstream neuronal populations that facilitate aggression (i.e., AVP). Accordingly, we hypothesized that AVP co-infusion into the LAH along with DA D2 receptor antagonists should reverse the aggression-suppressing influence of DA D2 receptor blockade, restoring aggression to the high level normally observed in adolescent AAS-treated animals. To test this hypothesis, in the present study we used local microinfusion techniques to directly target AVP and DA D2 receptor populations within the LAH brain region. The behavioral data from the present study replicate our previous findings that the central blockade of the DA D2 receptors selectively within the LAH suppresses adolescent AAS-induced aggressive behavior without altering other social, comfort, or locomotive behaviors, as both general and targeted measures of offensive aggression where significantly reduced by the central infusion of eticlopride into the LAH brain region. In addition, here we extend the findings of Schwartzer et al. by showing that the aggression-suppressing effects of D2 antagonists are specific to animals administered AAS during adolescence since we observed no differences in aggression measures between SO-treated animals microinjected with saline versus those microinjected with eticlopride. Moveover, to determine the influence of DA D2 receptors relative to AVP-receptor mediated aggression mechanisms, we furthered previous findings by showing that co-infusion of AVP along with a DA D2 antagonist restores and enhances the highly aggressive phenotype normally observed in adolescent AAS-treated animals. This finding suggests that the DA D2 receptor suppression of offensive aggression is independent of AVP activity in AAS-treated animals and supports the hypothesis that AVP is acting downstream of DA D2 receptor signaling within the LAH.
In our animal model of adolescent AAS-induced offensive aggression, the majority of AAS-treated adolescents target their offensive responses to the lateral flank/rump regions of intruders (Morrison et al., 2014; Ricci, Morrison, & Melloni, 2013; Schwartzer & Melloni, 2010a, 2010b), i.e., a hallmark characteristic of the adult aggressive phenotype (Taravosh-Lahn & Delville, 2004; Wommack & Delville, 2003). Thus AAS-treated animals display the adult form of offensive aggression in the absence of social experience, indicating that adolescent exposure to AAS may circumvent the learning of mature fighting behavior. In the current study, analysis of the effects of eticlopride and eticlopride/AVP combinations on individual types of aggressive acts revealed a receptor-specific suppression, and then behavioral rescue of only certain aggressive behaviors which delineate mature forms of aggression (Pellis & Pellis, 1988; Wommack & Delville, 2003). Specifically, DA D2 receptor antagonism within the LAH decreased the number of lateral attacks and flank bites, but not frontal attacks in adolescent AAS-treated animals. In fact, AAS-treated hamsters administered eticlopride into the LAH displayed a similar frequency of frontal attacks as aggressive, AAS-treated animals infused with saline into the LAH (Figure 2), indicating that DA D2 receptor blockade has no suppressive effect on this aspect of the aggressive response pattern in adolescent AAS-treated animals. Further, SO-treated animals administered eticlopride into the LAH also showed a similar frequency of frontal attacks as aggressive, AAS-treated animals infused with saline (Figure 2), indicating that DA D2 receptor blockade may actually enhance this aspect of the aggressive response pattern in control animals. Interestingly, co-infusion of AVP completely reversed the suppressive effects of DA D2 receptor antagonists on both lateral attacks and flank bites, but had no effect on the frontal attacks. In fact, co-application of AVP to AAS-treated animals significantly increased the number of lateral attacks and flank bites (but not frontal attacks) directed onto intruders as compared to AAS-treated animals infused with saline or eticlopride alone, supporting the notion that AVP activity in the LAH brain region acts independently of DA D2 receptor signaling and, that it alone is a strong activator of the mature adult aggressive response pattern. Together, these data suggest blocking DA neural signaling through DA D2 receptors within the LAH of aggressive, AAS-treated animals may function to simultaneously reduce mature forms of offensive aggression and enhance juvenile play behaviors, and that activation of AVP neural signaling within this brain region acts downstream of DA D2 receptor signaling within the LAH to facilitate the expression/display of highly escalated and mature forms of offensive aggression in hamsters.
Mechanistically, results from these studies support the notion that AAS-induced increased DA D2 receptor signaling in the LAH acts to facilitate offensive aggression, perhaps by enhancing downstream AVP neural signaling within the LAH. This notion is supported by our prior studies that show that adolescent AAS-treated animals displaying a mature and highly escalated aggressive phenotype express more DA D2 receptors and have more DA and AVP-containing afferent terminals within the LAH (Harrison et al., 2000; Ricci et al., 2009; Schwartzer et al., 2009). Recently, we found that DA D2 receptors are expressed on GABAergic neurons within the LAH and that adolescent AAS exposure increases the number of GABA-containing neurons within the LAH brain region (Schwartzer et al., 2009). Interestingly, DA D2 receptor signaling reduces inhibitory postsynaptic currents in AH GABA neurons that synapse onto hypothalamic magnocellular neurons (Azdad, Piet, Poulain, & Oliet, 2003), and DA and GABA neural signaling has been shown to regulate the release of AVP (Forsling & Williams, 1984; Knepel, Nutto, & Hertting, 1980). Given that hypothalamic AVP release is directly linked to the control of offensive aggression (Ferris et al., 1997; Melloni and Ricci, 2010), it is possible that DA facilitates downstream LAH AVP activity by inhibiting GABA neurons within the LAH, essentially disinhibiting aggression. Indeed, the current findings support the existence of a DA D2 receptor-regulated disinhibitory aggression circuit since, in the presence of a DA D2 receptor-blocking agent, exogenous AVP rescued the highly escalated and mature AAS-induced aggressive phenotype and also enhanced dominant flank marking activity. Moreover, this aggression enhancing effect of eticlopride+AVP was not observed in vehicle treated animals and did not alter ancillary behaviors between groups, further supporting the hypothesis that DA D2 receptors are an important component to AVP mechanisms that regulate the display of mature forms of AAS-induced aggressive behavior. Further, the recovery of AAS-induced heightened aggression after AVP administration following eticlopride indicates that aggression-stimulating neurons are directly activated by AVP and must then lie separate and downstream of a plausible DA D2 disinhibitory gating mechanism. Indeed, AAS exposure increases aggressive behaviors primarily by enhancing AVP signaling within the LAH (Grimes et al., 2007; Melloni & Ricci, 2010), and microinjection of AVP into the AH region enhances aggressive behavior and flank marking even in the absence of AAS-altered DA neural systems within the LAH (Ferris et al., 1997, 1984). Speculation aside, the interaction between DA and AVP (and possibly GABA) in the LAH must be further investigated to better understand the network of neural systems responsible for heightened aggressive response after adolescent exposure to AAS.
In summary, the present study shows that DA D2 receptor blockade suppresses the highly escalated and mature form of offensive aggression induced by repeated exposure to AAS during adolescent development. In AAS-treated animals, microinjection of AVP following eticlopride microinjection significantly enhanced the expression and display of the mature aggressive phenotype beyond levels typically observed in adolescent AAS-exposed animals. These findings add to the compendium of research that describes how AAS alters aggressive behavior at the level of specific local neurochemical interactions further extending our understanding of the aggression-stimulating properties of adolescent exposure to AAS. The current data strongly suggest a mechanism whereby DA D2/AVP relationships facilitate mature AAS-induced aggressive acts indirectly and likely through a disinhibitory circuit (e.g., D2 inhibition of GABA release) in the LAH; this notion is currently under investigation in our laboratory.
Acknowledgments
The authors would also like to thank Jillian Joyce and Courtney Davis for their technical support in the completion of the experimental procedures. This study was supported by research grant (R01) DA10547 from NIH to R.H.M. Its contents are solely the responsibility of the authors and do not necessarily represent the official view of NIH.
References
- Aguilar MA, Minarro J, Perez-Iranzo N, Simon VM. Behavioral Profile of Raclopride in Agonistic Encounters Between Male Mice. Pharmacology, Biochemistry, and Behavior. 1994;47(3):753–756. doi: 10.1016/0091-3057(94)90185-6. [DOI] [PubMed] [Google Scholar]
- Arregui A, Azpiroz A, Brain PF, Simon V. Effects of two selective dopaminergic antagonists on ethologically-assessed encounters in male mice. General Pharmacology. 1993;24(2):353–6. doi: 10.1016/0306-3623(93)90316-p. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/8482519. [DOI] [PubMed] [Google Scholar]
- Azdad K, Piet R, Poulain DA, Oliet SHR. Dopamine D4 receptor-mediated presynaptic inhibition of GABAergic transmission in the rat supraoptic nucleus. Journal of Neurophysiology. 2003;90(2):559–65. doi: 10.1152/jn.00226.2003. [DOI] [PubMed] [Google Scholar]
- Berridge KC. Comparative Fine Structure of Action : Rules of Form and Sequence in the Grooming Patterns of Six Rodent Species. Behaviour. 1999;113(1/2):21–56. [Google Scholar]
- Beyer CE, Steketee JD. Intra-medial prefrontal cortex injection of quinpirole, but not SKF 38393, blocks the acute motor-stimulant response to cocaine in the rat. Psychopharmacology. 2000;151(2-3):211–8. doi: 10.1007/s002139900345. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/10972467. [DOI] [PubMed] [Google Scholar]
- Carrillo M, Ricci La, Melloni RH. Glutamate-vasopressin interactions and the neurobiology of anabolic steroid-induced offensive aggression. Neuroscience. 2011;185:85–96. doi: 10.1016/j.neuroscience.2011.03.056. [DOI] [PubMed] [Google Scholar]
- Cass WA, Gerhardt GA. Direct in vivo evidence that D2 dopamine receptors can modulate dopamine uptake. Neuroscience Letters. 1994;176:259–263. doi: 10.1016/0304-3940(94)90096-5. [DOI] [PubMed] [Google Scholar]
- DeLeon KR, Grimes JM, Melloni RH. Repeated anabolic-androgenic steroid treatment during adolescence increases vasopressin V(1A) receptor binding in Syrian hamsters: correlation with offensive aggression. Hormones and Behavior. 2002;42(2):182–91. doi: 10.1006/hbeh.2002.1802. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/12367571. [DOI] [PubMed] [Google Scholar]
- Delville Y, De Vries GJ, Ferris CF. Neural connections of the anterior hypothalamus and agonistic behavior in golden hamsters. Brain, Behavior and Evolution. 2000;55(2):53–76. doi: 10.1159/000006642. doi:6642. [DOI] [PubMed] [Google Scholar]
- Ferris CF, Albers HE, Wesolowski S, Goldman B, Luman S. Vasopressin Injected into the Hypothalamus Triggers a Stereotypic Behavior in Golden Hamsters. Science. 1984;224(4648):521–523. doi: 10.1126/science.6538700. [DOI] [PubMed] [Google Scholar]
- Ferris CF, Axelson JF, Martin AM, Roberge LF. Vasopressin immunoreactivity in the anterior hypothalamus is altered during the establishment of dominant/subordinate relationships between hamsters. Neuroscience. 1989;29(3):675–683. doi: 10.1016/0306-4522(89)90140-1. [DOI] [PubMed] [Google Scholar]
- Ferris CF, Gold L, De Vries GJ, Potegal M. Evidence for a functional and anatomical relationship between the lateral septum and the hypothalamus in the control of flank marking behavior in Golden hamsters. The Journal of Comparative Neurology. 1990;293(3):476–85. doi: 10.1002/cne.902930310. [DOI] [PubMed] [Google Scholar]
- Ferris CF, Irvin RW, Potegal M, Axelson JF. Kainic Acid lesion of vasopressinergic neurons in the hypothalamus disrupts flank marking behavior in golden hamsters. Journal of Neuroendocrinology. 1990;2(2):123–9. doi: 10.1111/j.1365-2826.1990.tb00841.x. [DOI] [PubMed] [Google Scholar]
- Ferris CF, Melloni RH, Koppel G, Perry KW, Fuller RW, Delville Y. Vasopressin/serotonin interactions in the anterior hypothalamus control aggressive behavior in golden hamsters. The Journal of Neuroscience : The Official Journal of the Society for Neuroscience. 1997;17(11):4331–40. doi: 10.1523/JNEUROSCI.17-11-04331.1997. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/9151749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Floody OR, Pfaff DW. Steroid hormones and aggressive behavior: approaches to the study of hormone-sensitive brain mechanisms for behavior. Research Publications - Association for Research in Nervous and Mental Disease. 1974;52:149–85. Retrieved from http://www.safetylit.org/citations/index.php?fuseaction=citations.viewdetails&citationIds%5B%5D=citjournalarticle_435036_38. [PubMed] [Google Scholar]
- Forsling ML, Williams H. Central effects of dopamine on vasopressin release in the normally hydrated and water-loaded rat. The Journal of Physiology. 1984;346(1):49–59. doi: 10.1113/jphysiol.1984.sp015006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garmendia L, Sanchez JR, Azpiroz A, Brain PF, Simon VM. Clozapine : Strong Antiaggressive Effects With Minimal Motor Impairment. Physiology & Behavior. 1992;51:51–54. doi: 10.1016/0031-9384(92)90202-d. [DOI] [PubMed] [Google Scholar]
- Grimes JM, Melloni RH. Serotonin modulates offensive attack in adolescent anabolic steroid-treated hamsters. Pharmacology, Biochemistry, and Behavior. 2002;73(3):713–21. doi: 10.1016/s0091-3057(02)00880-8. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/12151048. [DOI] [PubMed] [Google Scholar]
- Grimes JM, Melloni RH. Serotonin-1B receptor activity and expression modulate the aggression-stimulating effects of adolescent anabolic steroid exposure in hamsters. Behavioral Neuroscience. 2005;119(5):1184–94. doi: 10.1037/0735-7044.119.5.1184. [DOI] [PubMed] [Google Scholar]
- Grimes JM, Ricci LA, Melloni RH. Glutamic acid decarboxylase (GAD65) immunoreactivity in brains of aggressive, adolescent anabolic steroid-treated hamsters. Hormones and Behavior. 2003;44(3):271–280. doi: 10.1016/S0018-506X(03)00138-7. [DOI] [PubMed] [Google Scholar]
- Grimes JM, Ricci LA, Melloni RH. Plasticity in anterior hypothalamic vasopressin correlates with aggression during anabolic-androgenic steroid withdrawal in hamsters. Behavioral Neuroscience. 2006;120(1):115–24. doi: 10.1037/0735-7044.120.1.115. [DOI] [PubMed] [Google Scholar]
- Grimes JM, Ricci LA, Melloni RH. Alterations in anterior hypothalamic vasopressin, but not serotonin, correlate with the temporal onset of aggressive behavior during adolescent anabolic-androgenic steroid exposure in hamsters (Mesocricetus auratus) Behavioral Neuroscience. 2007;121(5):941–8. doi: 10.1037/0735-7044.121.5.941. [DOI] [PubMed] [Google Scholar]
- Harrison RJ, Connor DF, Nowak C, Nash K, Melloni RH. Chronic anabolic-androgenic steroid treatment during adolescence increases anterior hypothalamic vasopressin and aggression in intact hamsters. Psychoneuroendocrinology. 2000;25(4):317–38. doi: 10.1016/s0306-4530(99)00057-8. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/10725610. [DOI] [PubMed] [Google Scholar]
- Iannazzo L, Sathananthan S, Majewski H. Modulation of dopamine release from rat striatum by protein kinase C: interaction with presynaptic D2-dopamine-autoreceptors. British Journal of Pharmacology. 1997;122(8):1561–6. doi: 10.1038/sj.bjp.0701540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knepel W, Nutto D, Hertting G. Evidence for the involvement of a GABA-mediated inhibition in the hypovolaemia-induced vasopressin release. Pflügers Archiv. 1980;388(2):177–183. doi: 10.1007/BF00584125. [DOI] [PubMed] [Google Scholar]
- Lerwill CJ, Makings P. The agonistic behaviour of the golden hamster Mesocricetus auratus (waterhouse) Animal Behaviour. 1971;19(4):714–721. doi: 10.1016/S0003-3472(71)80175-6. [DOI] [Google Scholar]
- Lumley LA, Charles RF, Charles RC, Hebert MA, Morton DM, Meyerhoff JL. Effects of social defeat and of diazepam on behavior in a resident–intruder test in male DBA/2 mice. Pharmacology Biochemistry and Behavior. 2000;67(3):433–447. doi: 10.1016/S0091-3057(00)00382-8. [DOI] [PubMed] [Google Scholar]
- Mahoney PD, Koh ET, Irvin RW, Ferris CF. Computer-Aided Mapping of Vasopressin Neurons in the Hypothalamus of the Male Golden Hamster: Evidence of Magnocellular Neurons that do not Project to the Neurohypophysis. Journal of Neuroendocrinology. 1990;2(2):113–22. doi: 10.1111/j.1365-2826.1990.tb00840.x. [DOI] [PubMed] [Google Scholar]
- Melloni RH, Ricci LA. Adolescent exposure to anabolic/androgenic steroids and the neurobiology of offensive aggression: a hypothalamic neural model based on findings in pubertal Syrian hamsters. Hormones and Behavior. 2010;58(1):177–91. doi: 10.1016/j.yhbeh.2009.11.002. [DOI] [PubMed] [Google Scholar]
- Morrison TR, Ricci LA, Melloni RH. γ-Aminobutyric acid neural signaling in the lateroanterior hypothalamus modulates aggressive behavior in adolescent anabolic/androgenic steroid-treated hamsters. Behavioural Pharmacology. 2014;25(7):673–83. doi: 10.1097/FBP.0000000000000083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navarro JF, Manzaneque JM. Acute and Subchronic Effects of Tiapride on Isolation-Induced Aggression in Male Mice. Pharmacology Biochemistry and Behavior. 1997;58(18):255–259. doi: 10.1016/s0091-3057(96)00541-2. [DOI] [PubMed] [Google Scholar]
- Navarro JF, Minarro J, Simon VM. Antiaggressive and Motor Effects of Haloperidol Show Different Temporal Patterns in the Development of Tolerance. Physiology & Behavior. 1993;53(18):1055–1059. doi: 10.1016/0031-9384(93)90359-n. [DOI] [PubMed] [Google Scholar]
- Navarro JF, Velasco R, Manzaneque JM. Acute and Subchronic Effects of Pimozide on Isolation-induced Aggression in Male Mice. Prog Neuro-Psychopharmacol & Biol Psychiat. 2000;24:131–142. doi: 10.1016/s0278-5846(99)00076-7. [DOI] [PubMed] [Google Scholar]
- O'Hara CM, Uhland-Smith A, O'Mallev KL, Todd RD. Inhibitionof Dopamine Synthesis by Dopamine D2 and D3 but Not D4 Receptors. 1996;277(1):186–192. [PubMed] [Google Scholar]
- Pellis SM, Pellis VC. Play-Fighting in the Syrian Golden Hamster Mesocricetus auratus Waterhouse, and its Relationship to Serious Fighting during Postweaning Development. Developmental Psychobiology. 1988;21(4):323–337. doi: 10.1002/dev.420210404. [DOI] [PubMed] [Google Scholar]
- Ricci LA, Morrison TR, Melloni RH. Adolescent anabolic/androgenic steroids: Aggression and anxiety during exposure predict behavioral responding during withdrawal in Syrian hamsters (Mesocricetus auratus) Hormones and Behavior. 2013;64(5):770–780. doi: 10.1016/j.yhbeh.2013.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ricci LA, Rasakham K, Grimes JM, Melloni RH. Serotonin-1A receptor activity and expression modulate adolescent anabolic/androgenic steroid-induced aggression in hamsters. Pharmacology, Biochemistry, and Behavior. 2006;85(1):1–11. doi: 10.1016/j.pbb.2006.06.022. [DOI] [PubMed] [Google Scholar]
- Ricci LA, Schwartzer JJ, Melloni RH. Alterations in the anterior hypothalamic dopamine system in aggressive adolescent AAS-treated hamsters. Hormones and Behavior. 2009;55(2):348–55. doi: 10.1016/j.yhbeh.2008.10.011. [DOI] [PubMed] [Google Scholar]
- Schwartzer JJ, Melloni RH. Anterior hypothalamic dopamine D2 receptors modulate adolescent anabolic/androgenic steroid-induced offensive aggression in the Syrian hamster. Behavioural Pharmacology. 2010a;21(4):314–22. doi: 10.1097/FBP.0b013e32833b10f1. [DOI] [PubMed] [Google Scholar]
- Schwartzer JJ, Melloni RH. Dopamine activity in the lateral anterior hypothalamus modulates AAS-induced aggression through D2 but not D5 receptors. Behavioral Neuroscience. 2010b;124(5):645–55. doi: 10.1037/a0020899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartzer JJ, Ricci LA, Melloni RH. Interactions between the dopaminergic and GABAergic neural systems in the lateral anterior hypothalamus of aggressive AAS-treated hamsters. Behavioural Brain Research. 2009;203(1):15–22. doi: 10.1016/j.bbr.2009.04.007. [DOI] [PubMed] [Google Scholar]
- Shettleworth SJ. Reinforcement and the Organization of Behavior in Golden Hamsters : Hunger, Environment, and Food Reinforcement. Journal of Experimental Psychology. 1975;104(1):56–87. [Google Scholar]
- Sweidan S, Edinger H, Siegel A. D2 dopamine receptor-mediated mechanisms in the medial preoptic-anterior hypothalamus regulate affective defense behavior in the cat. 1991;549:127–137. doi: 10.1016/0006-8993(91)90608-x. [DOI] [PubMed] [Google Scholar]
- Taravosh-Lahn K, Delville Y. Aggressive behavior in female golden hamsters: development and the effect of repeated social stress. Hormones and Behavior. 2004;46(4):428–35. doi: 10.1016/j.yhbeh.2004.03.007. [DOI] [PubMed] [Google Scholar]
- Wachtel SR, Hu X, Galloway MP, White AFJ. D1 dopamine receptor stimulation enables the postsynaptic, but not autoreceptor, effects of D2 dopamine agonists in nigrostriatal and mesoaccumbens dopamine systems. Synapse (New York, N Y) 1989;4(4):327–46. doi: 10.1002/syn.890040409. [DOI] [PubMed] [Google Scholar]
- Wommack JC, Delville Y. Repeated social stress and the development of agonistic behavior: individual differences in coping responses in male golden hamsters. Physiology & Behavior. 2003;80(2-3):303–308. doi: 10.1016/j.physbeh.2003.08.002. [DOI] [PubMed] [Google Scholar]


